Abstract
Background
The aim of this study was to assess for distinct kidney function trajectories following left ventricular assist device (LVAD) placement. Cohort studies of LVAD recipients demonstrate that kidney function tends to increase early after LVAD placement, followed by decline and limited sustained improvement. Inter-individual differences in kidney function response may be obscured.
Methods
We identified continuous flow LVAD implantations in US adults (2016–17) from INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). Primary outcomes were estimated glomerular filtration rate (eGFR) trajectories pre-implantation to ~12 months. Latent class mixed models were applied to primary and validation samples. Clinical differences among trajectory groups were investigated.
Results
Among 4,615 LVAD implantations, five eGFR trajectory groups were identified. The two largest groups (Groups 1 and 2) made up >80% of the cohort, and were similar to group average trajectories previously reported, with early eGFR rise followed by decline and stabilization. Three novel trajectory groups were found: worsening followed by sustained low kidney function (Group 3, 10.1%), sustained improvement (Group 4, 3.3%), and worsening followed by variation (Group 5, 1.7%). These groups differed in baseline characteristics and outcomes. Group 4 was younger and had more cardiogenic shock and pre-implantation dialysis; Group 3 had higher rates of pre-existing chronic kidney disease, along with older age.
Conclusions
Novel eGFR trajectories were identified in a national cohort, possibly representing distinct cardiorenal processes. Type 1 cardiorenal syndrome may have been predominant in Group 4, and parenchymal kidney disease may have been predominant in Group 3.
Keywords: continuous-flow left ventricular assist device, advanced heart failure, trajectory, kidney function, cardiorenal, latent class mixed models
Introduction
Durable mechanical circulatory support with continuous-flow left ventricular assist devices (LVADs) is used for advanced heart failure (HF) refractory to medical and device therapy.1 Kidney dysfunction is common and portends worse outcomes in LVAD recipients.2 LVADs improve cardiac output and central venous pressure, macrocirculatory parameters that drive kidney dysfunction in HF.3–5 Despite these improvements, LVADs have not been shown to cause substantial sustained kidney function improvement in cohort studies.6–8 Group average eGFR changes following LVAD implantation demonstrate early increase in eGFR, peak ~1 month after implantation, followed by decline.6,8 Explanations for this lack of sustained improvement have focused on possible detrimental effects on the kidney from long-term LVAD support.9–11 However, clinical experience suggests that there are marked inter-individual differences in kidney function response. Independent of kidney function, eGFR can be affected by illness, diet, inflammation, and fluid status.12,13 As such, patterns of change may be more meaningful than static values.
Techniques enabling simultaneous unsupervised identification of trajectories and classification of individuals into distinctive trajectory groups have enabled insights in other complex longitudinal processes, including kidney transplantation and stroke.14–17 Latent class mixed models are a contemporary method for identifying subgroups with similar mean trajectory profiles while also implicitly handling missing data and irregular repeated variable measurements.18,19
To study differences in effects of continuous flow LVAD implantation on kidney health/function, we assessed for presence of distinctive eGFR trajectories in a national cohort and investigated how trajectories related to pre-implantation factors and clinical outcomes.
Methods
Cohort
Methods are summarized in Supplemental Figure S1. The INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) database from the National Heart, Lung and Blood Institute Biologic Specimen and Data Repository was used. This is a registry for people ≥19 years who received a US Food and Drug Administration-approved mechanical circulatory support device for advanced HF.20,21 We included persons who received primary implantations for advanced HF of isolated continuous flow durable LVADs, 1/1/2016–12/31/2017. The cohort was divided into primary (75%) and internal validation (25%) cohorts randomly to assess for consistency of results. This split was chosen based on the need for adequate primary cohort size to achieve precise estimates of baseline characteristics while allowing adequate validation cohort size so that the presence of small groups would be potentially identifiable. Analysis of the de-identified database was determined not to be human subjects research by the Baylor College of Medicine Institutional Review Board.
Outcome
The primary outcome was eGFR trajectory from immediately before LVAD implantation to ~12 month, censoring for transplant, device removal, or death. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) serum creatinine equation was used to calculate eGFR.22 The INTERMACS protocol collects creatinine measurements immediately before implantation, then at 1 week, and then 1, 3, 6, and 12 months. To ensure inclusion of 1-year follow-up values, we included values obtained up to 14 months. We included persons with as few as 1 creatinine measurement to enable maximal data usage. During periods of kidney replacement therapy eGFR was imputed from a uniform distribution from 2–15 ml/min/1.73m2. This was done to reflect the known low level of kidney function (for which further estimation was not possible) while avoiding numerous identical values. At low serum creatinine levels eGFR is inaccurate and small changes may result in large eGFR changes not reflective of changing kidney function.23 We minimized the impact of unstable eGFR estimates by establishing a ceiling on eGFR without enforcing identical values, replacing values ≥110 ml/min/1.73m2 with random values from 110–125 ml/min/1.73m2. We assessed how trajectory group membership related to death and heart transplantation.
Pre-implantation and implantation variables
Pre-implantation variables were collected based on the INTERMACS protocol.21 These included demographics, treatment goals, comorbidities, labs, vital signs, and INTERMACS Patient Profile (Table 1). The protocol limited collection of certain variables to specific definitional categories: baseline CKD was based on center judgment, and pre-existing diabetes was collected as center-adjudicated “severe diabetes”.24
Table 1.
Characteristics for 4,615 persons who underwent isolated, primary, continuous flow LVAD implantation in 2016 and 2017 in the US, from the INTERMACS database, by kidney function trajectory group.
| CHARACTERISTICS | 1 (n=2138) |
2 (n=1780) |
3 (n=468) |
4 (n=150) |
5 (n=79) |
P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Demographics | ||||||
|
| ||||||
| Age (years) | 63 (56–69) | 53 (41–61) | 62 (54–69) | 54 (42–63) | 55 (42–64) | <0.001 |
| Female | 21.6% | 19.9% | 23.9% | 24.0% | 21.5% | 0.31 |
| Race | ||||||
| Black | 23.1% | 27.4% | 25.6% | 30.0% | 32.9% | 0.008 |
| White | 67.0% | 59.8% | 63.9% | 56.7% | 54.4% | <0.001 |
| Asian | 1.7% | 2.0% | 1.7% | 0.7% | 2.5% | 0.73 |
| Other and unknown | 4.6% | 5.5% | 5.1% | 8.0% | 5.1% | 0.36 |
| Hispanic ethnicity | 6.6% | 8.3% | 7.3% | 14.0% | 7.6% | 0.055 |
| Vital signs | ||||||
|
| ||||||
| BMI (kg/m2) | 28.4 (24.7– 33.0) | 26.5 (23.0–31.6) | 28.6 (24.5–34.2) | 26.8 (22.5–31.8) | 27.9 (23.4–32.1) | <0.001 |
| BSA (m2) | 2.1 (1.9–2.3) | 2.0 (1.8–2.2) | 2.1 (1.9–2.3) | 2.0 (1.8–2.2) | 2.1 (1.8–2.2) | <0.001 |
| Peri-implantation factors | ||||||
|
| ||||||
| Device strategy | <0.001 | |||||
| Bridge to transplant | 23.3% | 24.8% | 24.1% | 24.0% | 19.0% | |
| Possible bridge to transplant | 21.5% | 31.1% | 22.0% | 31.3% | 33.0% | |
| Destination therapy | 54.7% | 43.4% | 53.2% | 44.7% | 45.6% | |
| INTERMACS profile | <0.001 | |||||
| Critical cardiogenic shock (1) | 13.4% | 18.3% | 27.7% | 33.3% | 36.7% | |
| Progressive decline (2) | 33.7% | 34.0% | 35.8% | 35.3% | 32.9% | |
| Stable, inotrope dependence (3) | 39.0% | 36.6% | 28.3% | 25.3% | 22.8% | |
| Resting symptoms or lesser severity (4–7) | 13.9% | 11.2% | 8.2% | 6.0% | 7.6% | |
| Interventions within 48 hours prior to implantation | ||||||
| IV inotrope | 83.6% | 84.6% | 89.1% | 92.0% | 87.3% | 0.017 |
| Dialysis | 0.1% | 0.0% | 50.0% | 7.3% | 3.8% | <0.001 |
| ECMO | 2.2% | 3.5% | 5.6% | 6.0% | 11.4% | <0.001 |
| IABP | 15.5% | 16.9% | 20.3% | 16.7% | 19.0% | 0.145 |
| Implantation surgery | ||||||
|
| ||||||
| Cardiopulmonary bypass time (min) | 84 (63–114) | 80 (61–108) | 90 (64–126) | 83 (64–125) | 96 (65–137) | <0.001 |
| Surgery Time (min) | 269 (213–351) | 255 (204–320) | 288 (219–383) | 270 (214–363) | 278 (227–332) | <0.001 |
| Aortic cross-clamp used | 17.7% | 16.4% | 21.8% | 16.7% | 16.5% | 0.37 |
| Centrifugal VAD | 34.5% | 39.2% | 42.7% | 42.0% | 48.1% | <0.001 |
| Cardiac history and presentation | ||||||
|
| ||||||
| Cardiac disease first diagnosed within 1 month prior | 69 ( 3.2) | 116 ( 6.5) | 28 ( 6.0) | 15 (10.0) | 9 (11.4) | <0.001 |
| Pulmonary hypertension | 443 (20.7) | 322 (18.1) | 88 (18.8) | 23 (15.3) | 15 (19.0) | 0.20 |
| Comorbidities | ||||||
|
| ||||||
| Documented chronic kidney disease | 593 (27.7) | 152 ( 8.5) | 193 (41.2) | 33 (22.0) | 16 (20.3) | <0.001 |
| Diabetes, “severe” classification by INTERMACS | 11.6% | 7.1% | 11.1% | 11.3% | 6.3% | <0.001 |
| Lab results | ||||||
|
| ||||||
| BUN (mg/dl) | 28 (21–38) | 19 (14–25) | 37 (27–54) | 32 (20–43) | 24 (17–40) | <0.001 |
| Creatinine, serum (mg/dl) | 1.5 (1.2–1.7) | 1.0 (0.8–1.1) | 1.9 (1.5–2.5) | 1.6 (1.3–1.9) | 1.1 (0.9–1.4) | <0.001 |
| Total bilirubin (mg/dl) | 0.9 (0.6–1.4) | 0.9 (0.6–1.6) | 1.1 (0.7–1.8) | 1.4 (0.7–2.1) | 1.3 (0.7–2.0) | <0.001 |
| Hemoglobin (g/dl) | 11.1 (9.6–12.5) | 11.4 (9.8–12.9) | 10.0 (8.7–11.5) | 10.1 (8.7–12.0) | 10.0 (8.7–12.0) | <0.001 |
| Albumin, serum (g/dl) | 3.5 (3.1–3.8) | 3.5 (3.0–3.8) | 3.3 (2.8–3.7) | 3.3 (2.9–3.7) | 3.3 (2.7–3.6) | <0.001 |
Notes: Median and interquartile range are given for continuous variables. Percentages are given for categorical variables. The primary and internal validation cohorts are combined for this table.
Abbreviations: INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; BMI, body mass index; BSA, body surface area; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; VAD, ventricular assist device; BUN, blood urea nitrogen
Statistical analysis
Identifying latent eGFR class trajectories
We used latent class mixed models to identify and model trajectories. Latent class mixed models extend linear mixed models to handle non-normally distributed outcomes and non-observed population heterogeneity.19 An assumption is made that the population is composed of a number of latent classes or groups, each characterized by a mean trajectory profile.18,19 Based on prior group level averages for eGFR following LVAD implantation,8,25 we wanted to allow non-linear eGFR trajectories, especially near time of implantation. Therefore, we chose a priori cubic splines with knots at 1, 3, and 6 months and class-specific regression parameters. We ran each model 10 times using random initial values.19,26
Finding the optimal number of trajectory groups
We evaluated latent class numbers ranging from 2 to 9. We chose the optimal number by comparing Akaike’s and Bayesian information criteria, discrimination (the ability to clearly group individuals into different classes), and entropy (the extent trajectory classes differed), along with subjective interpretability considerations (avoiding very small groups).19,27 Models were estimated separately in primary and the internal validation samples and compared for similarity. The model with the optimal number of trajectory groups was used to assign group membership to individuals.
Assessing how baseline characteristics differed among trajectory groups
For univariate assessment, we used the largest trajectory group as the reference, and calculated standardized differences between each group and this reference.28 For multivariable analysis, random forest classifier models were fitted to predict group membership from baseline characteristics. The random forest approach allowed an investigation of complex, non-linear relationships. It also allowed determination of variable importance scores, so that variables could be ranked in terms of importance for trajectory group membership. We used 74 baseline variables thought to be of relevance to kidney health and with missingness <20% (Supplemental Table S1). Missing values were imputed using random forest imputation.29 Regularized random forests was used to select variables for the final model, and models were fitted on the primary sample (using stratified sampling with replacement to ensure an equal number was selected from each trajectory class, given class imbalances) and then were tested against the validation sample.30
In exploratory analyses we treated group membership as existing at time of implantation and unchanging, and evaluated relationships with death and heart transplantation using Kaplan Meier estimators and Cox proportional hazards models. Proportional hazards assumptions were tested and we used a Cox model stratifying on a covariate for which the proportional hazards assumption was violated. We plotted prevalences of dialysis utilization during follow-up for each group, censoring at death, heart transplantation, or LVAD explantation. Analyses were performed using R 4.0.2 (www.r-project.org) and the lcmm package.19
Results
Cohort characteristics
Out of 5,033 implantations, 4,615 CF-LVAD implantations met inclusion criteria. Implantations were assigned to primary (75%; 3,461) and internal validation samples (25%; 1,154). Baseline characteristics are in Table 1. Median number of serum creatinine measurements was 5 (IQR 3–5); range was 1–6. During the 14 month follow-up, 577 (12.5%) individuals died and 620 (13.4%) received heart transplants.
Trajectory groups
Identification of trajectory groups
A 5 trajectory group solution demonstrated the best characteristics (Supplemental Table S2). This was near the minimum for the BIC, maintained discrimination of 0.75 (values closer to one indicate higher posterior probability of individuals belonging to the trajectory groups they were assigned to), entropy of 0.72 (values closer to one indicate greater differences between trajectories), and avoided very small groups. The internal validation modeling characteristics are shown in Supplemental Table S2 and were similar to the primary cohort.
Trajectory descriptions
The eGFR values for the 5 trajectory groups in the primary sample and internal validation sample are presented in Figure 1. The two largest trajectory groups (Groups 1 and 2) demonstrated early eGFR increase, peak between 1–2 months, followed by decline and stabilization. These two groups comprised 84.7% of the primary cohort (85.4% of the validation cohort). These two trajectory classes differed by pre-implantation eGFR and degree of later decline. The next largest trajectory group (Group 3) demonstrated low pre-implantation eGFR with early decline and nadir between 1–2 months, followed by recovery to near pre-implantation values. Group 4 demonstrated early increase in eGFR peaking just before 2 months, with sustained eGFR improvement from the pre-implantation value. The smallest trajectory group (Group 5) demonstrated post-operative eGFR decline followed by wide variation. Supplemental Figure S2 shows the proportion of non-censored members of each trajectory group on dialysis during follow-up.
Figure 1.
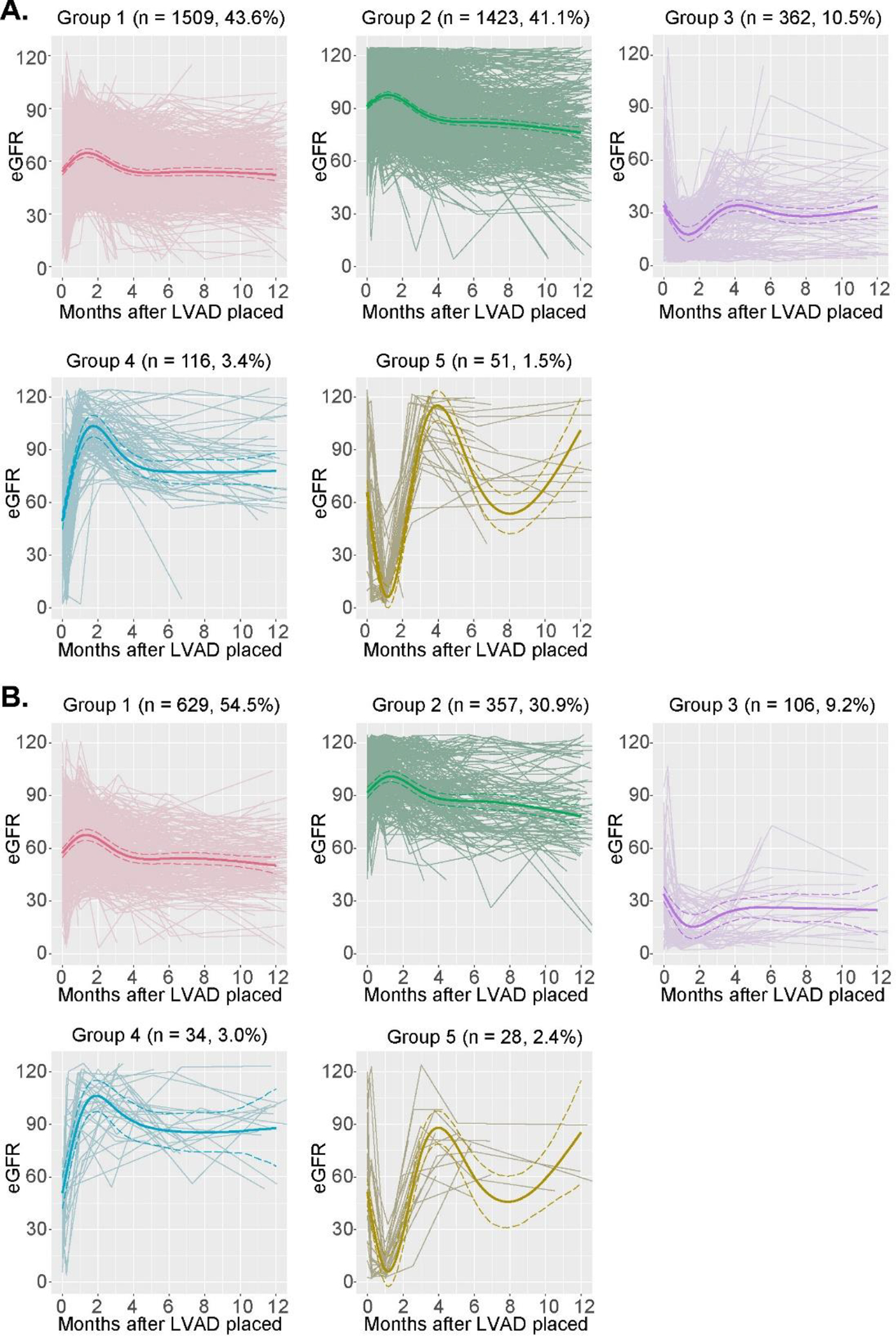
A. Estimated glomerular filtration rate (eGFR) plots for the 5 trajectory groups (primary sample, n = 3,461)
B. eGFR plots for the 5 trajectory groups (internal validation sample, n = 1,154)
Notes: Bold lines represent class-specific mean predicted trajectories for each group. Dashed lines are 95% confidence intervals for the predicted trajectories, computed by a Monte Carlo approximation of the posterior distribution of the predicted values.19 Thin lines are individual serum creatinine trajectories.
Abbreviations: LVAD, left ventricular assist device
Baseline characteristics by trajectory group
Differences between trajectory groups at baseline are shown in Figure 2, with the largest trajectory group (Group 1) as the reference. Group 2 had higher heart rate, more ICDs, and markedly younger age and less CKD than Group 1. Group 3, compared to 1, had more CKD and dialysis, and lower hemoglobin. Group 4 had higher cardiogenic shock, dialysis, heart rate, and total bilirubin, in addition to younger age and more recent cardiac disease onset. Group 5 had higher total bilirubin, higher cardiogenic shock and heart rate, newer cardiac diagnosis, younger age, lower hemoglobin, and lower serum albumin.
Figure 2. Standardized differences in baseline characteristics (Group 1 is the reference). Primary sample is represented by orange dots; internal validation sample by blue dots.
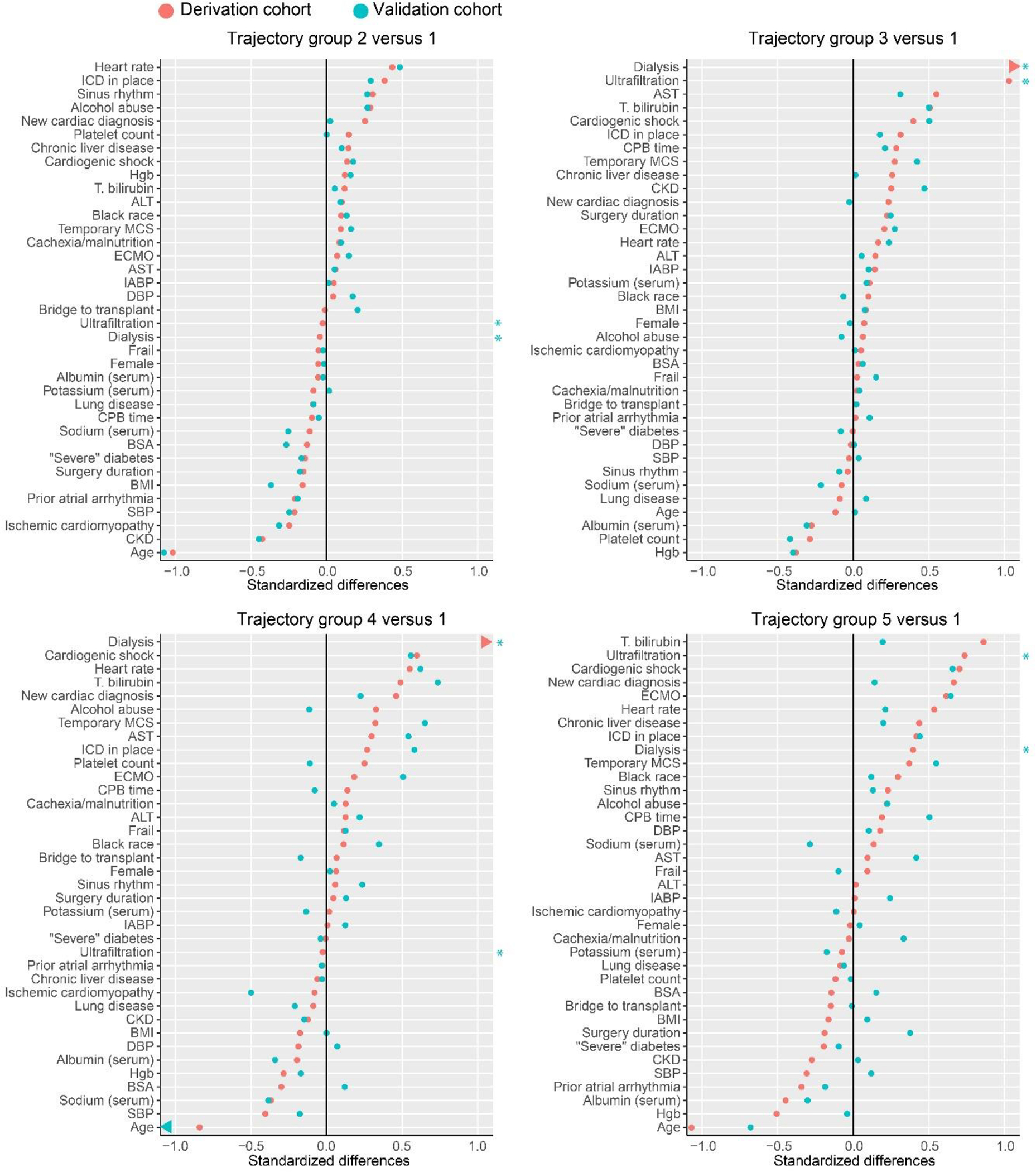
*Blue asterisks highlight that standardized differences are not available for dialysis and ultrafiltration for the internal validation sample because there were no patients with these treatments in the Group 1 in the internal validation sample.
Notes: Variables are ordered by standardized mean difference in the primary cohort. Group 4 vs 1 validation group age difference (represented by an arrow) was beyond the left border, with standardized difference −1.33. In the validation group, no one in group 1 received dialysis or ultrafiltration, so validation group estimates are not available.
Abbreviations: ICD, implantable cardioverter defibrillator; Hgb, hemoglobin; T, total; ALT, alanine aminotransferase; AST, aspartate aminotransferase; MCS, mechanical circulatory support; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; SBP, systolic blood pressure; DBP, diastolic blood pressure; CPB, cardiopulmonary bypass; BSA, body surface area; BMI, body mass index; CKD, chronic kidney disease
The random forests model predicting trajectory group membership using baseline variables selected 54 of 74 candidate variables (Supplemental Table S1). This model demonstrated accuracy=49.5%, kappa=0.26, and McNemar’s P-value<0.001. Age was the most important variable in determining trajectory group membership, followed by platelet count, hemoglobin, total bilirubin, and left ventricular end diastolic diameter (Supplemental Figure S3). Partial dependence plots demonstrated that increasing age increased probability of membership in Groups 1 and 3 (Supplemental Figure S4).
Heart transplantation and death by trajectory group
Survival differed between trajectory groups (Figure 3A), with Groups 3 and 5 having the lowest 1-year survival: 61.8% and 65.8% respectively. Heart transplantation over 1 year ranged from a low of 11.6% in Group 3 to a high of 27.9% in Group 4 (Figure 3B). In adjusted Cox models comparing Groups 2–5 with Group 1, Group 2 had lower risk of death with hazard ratio (HR) (95% CI) 0.63 (0.48–0.83). Groups 3 and 5 had higher risks of death than Group 1, with HR (95% CI) 3.02 (2.46–3.70) and 2.83 (1.82–4.93) respectively (Supplemental Table S3).
Figure 3. Outcomes by trajectory group over 1 year following. Survival curve for death, and cumulative distribution function for heart transplantation. Overall p-value from log-rank test: <0.001 for survival; 0.011 for heart transplantation.
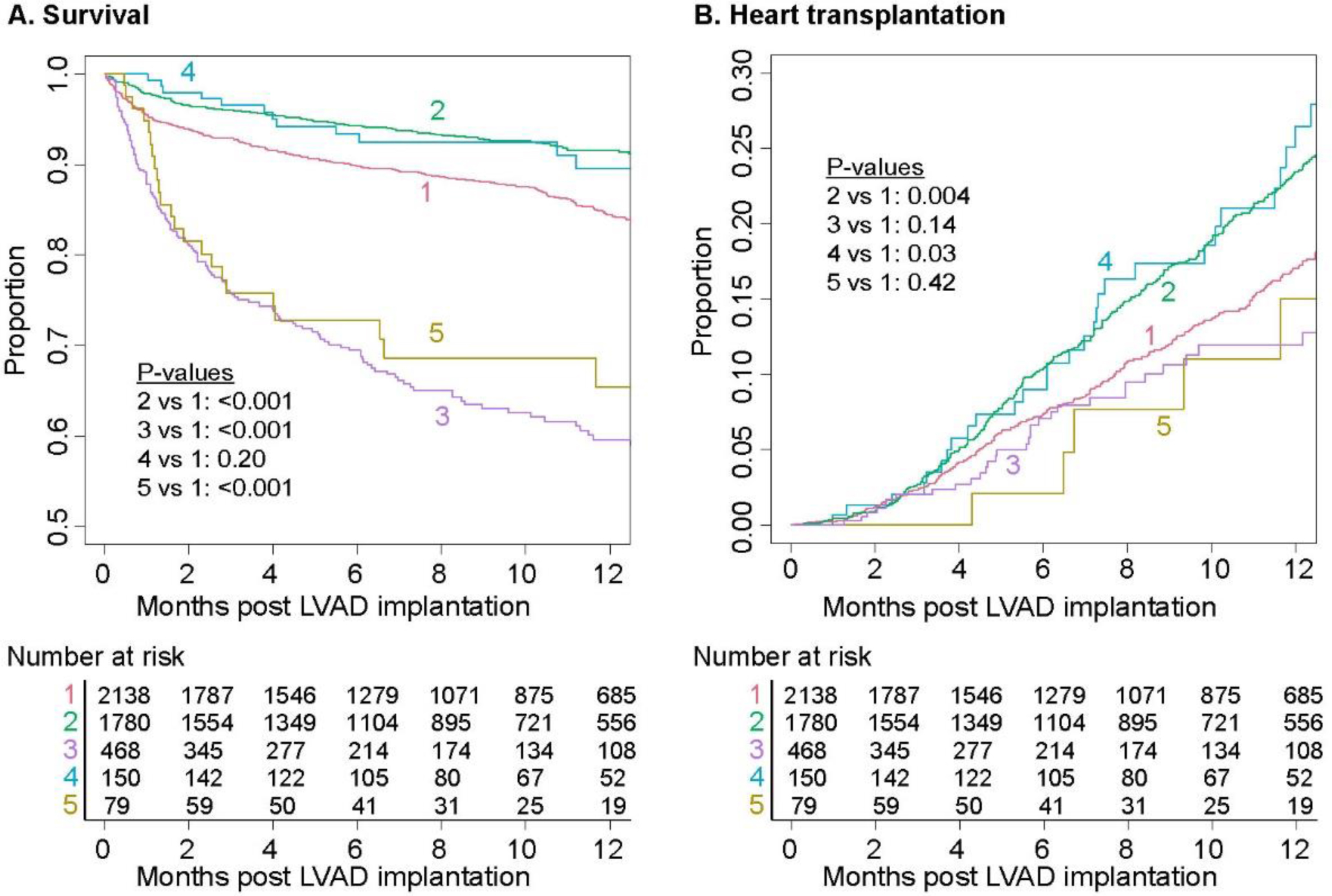
Notes: Pairwise comparison log rank test corrected for multiple comparisons using method of Benjamini and Hochberg3
Discussion
We have identified 5 distinct kidney function trajectories following LVAD implantation in a cohort of adults, including 3 groups that differ markedly from overall group averages. The two largest trajectory groups (1 and 2) had trajectories similar to group average results previously described in several reports, with early rise in eGFR, peak at one month, and later decline to near pre-implantation levels.7,8,25,31 Our analysis supports the importance of this pattern. The relationship of Group 1 and Group 2 trajectories is similar to a pattern identified in a prior study of 400 LVAD recipients, which categorized patients by pre-implantation outpatient eGFR values into CKD stages.6 The trajectory of Group 1 in the present study is very similar to the trajectory for persons with baseline CKD Stage 3A in the prior study, and Group 2 is similar to the group categorized as Stage 1 or 2 in the prior study.6 The baseline characteristics of these groups corresponded as well, with older age the most notable difference between those in the CKD Stage 3A or Group 1, compared with those in the CKD Stage 1 or 2 or Group 2.
The distinct patterns in a minority of patients (Groups 3–5) with potential pathophysiological importance, otherwise obscured by the dominant patterns, demonstrates the power of the analytic techniques to discern heterogeneity. These 3 novel trajectory groups may correspond to distinct pathophysiologic kidney health states. One trajectory group (Group 4) may correspond to pre-LVAD implantation type 1 cardiorenal syndrome (acute HF causing acute, hemodynamically-mediated kidney dysfunction), another trajectory group (Group 3) may demonstrate parenchymal kidney disease, and the smallest trajectory group (Group 5) may represent severe post-implantation AKI followed by recovery among survivors.32,33 Early and sustained improvement in eGFR is expected after LVAD implantation based on a macrocirculatory model of cardiorenal interaction, where improvement in cardiac output and central venous pressure yields sustained kidney function improvement. This fits with the trajectory observed in Group 4, and examination of baseline characteristics of this group further supports this. These were younger patients suffering from severe cardiogenic shock with recent onset cardiac disease. Thus, this group may have had isolated, severe cardiac dysfunction and healthy kidney parenchyma which responded well to hemodynamic improvement. While this is the classic cardiorenal interaction, it is striking that this group was so small. It included only about 1 in 30 LVAD implantations, suggesting that it is the exception rather than the norm and that non-macrocirculatory cardiorenal interactions predominate in most LVAD recipients.34
Group 3 (early worsening followed by sustained low kidney function) included 1 in 10 implantations, with severe cardiogenic shock, temporary MCS, and high pre-operative dialysis requirements. Many were classified as having CKD prior to implantation. Thus, this group may reflect persons with chronic parenchymal kidney disease and severe pre-operative decompensation with post-operative worsening kidney function due to lack of renal functional reserve and sensitivity to stresses. Need for pre-implantation dialysis or ultrafiltration is a known risk factor for adverse outcomes.21 Identification of persons likely to suffer from these poorer trajectories, and determining which management options are best for them, is an important goal.
Group 5, with only about 1 in 70 individuals, had individuals similar at baseline to the improvement group (Group 4) but with severe post-operative complications. This group may be the most severely acutely ill, placing them at high risk for a complicated post-operative course, with severe AKI followed by recovery (among those who survive). Development of right ventricular dysfunction and resulting high renal venous pressures may have been an important cause of the AKI in this group.11 This group, along with the persistently low kidney function group (Group 3) had the lowest survival. Among those in Group 5 who survived, kidney outcomes were favorable, likely reflecting underlying healthy kidney parenchyma.
Finally, we further examine the two largest groups (1 and 2), which make up over 8 in 10 LVAD recipients. The baseline differences between Group 1 and Group 2 are that Group 2 is substantially younger, with less CKD and less ischemic cardiomyopathy and higher cardiogenic shock. This might indicate that Group 2 represents a group like the improvement group (relatively cardiac-specific disease and less parenchymal kidney disease) although with lower severity and acuity of cardiac decompensation and less pre-LVAD implantation kidney dysfunction. The Group 2 trajectory, compared to Group 1, seems to show a more pronounced later decline, and this appears to persist over the 12 month follow-up. Several possible explanations may warrant further investigation. One is changing creatinine production, with increasing muscle mass and perhaps improved dietary intake with sustained LVAD support increasing serum creatinine. Another explanation is that long-term LVAD support has itself led to kidney damage. Potential mechanisms that have been proposed for this are endothelial damage, hemolysis, and progressive right ventricular dysfunction. Another hypothesis invokes kidney hemodynamics to explain the eGFR declines observed in Group 2, in addition to Groups 1 and 4. Kidney hyperfiltration occurs in advanced HF, with reduced cardiac output driving increased filtration fraction in an effort to maintain GFR and natriuresis.13,35 Increased perfusion following LVAD implantation may lead to a large GFR increase, subsequently moderated by kidney adaptation with reduced filtration fraction and GFR. Group 4, with a pronounced eGFR decline soon after the early peak, may be demonstrating a rapid diminution in hyperfiltration by healthy kidney parenchyma, possibly protective of kidney function.36
The most important baseline factor in predicting trajectory group membership was age, with older individuals more likely to belong to Groups 1 and 3, and younger individuals more likely to belong to Groups 2, 4, and 5. The importance of age could be driven by its relationship to cardiac disease, with younger patients having more isolated heart disease and older patients having more parenchymal kidney disease related to systemic processes (e.g., diabetes, atherosclerosis). The next two most important predictors of group membership were hematologic factors: platelet count and hemoglobin concentration. Patients with elevated platelet counts were more likely to be in the 2 trajectory groups with the highest survival (Groups 2 and 4). Low platelet counts were observed in the two groups with poor survival outcomes (Groups 3 and 5). Low hemoglobin was associated with membership in Group 4, with substantial kidney function improvement. These hematologic factors may be driven by several important processes, including hemolysis, blood loss, disseminated intravascular coagulation, and hemodilution.
The finding that kidney function trajectories were associated with baseline variables known to be related to other post-implantation outcomes—age, severity of cardiac decompensation, chronicity of heart and kidney disease—further supports the importance of these factors.21,37,38 Additionally, the results show that while significant, sustained improvement can be seen following LVAD placement, this occurs in a relatively small group. Characteristics of those likely to recover kidney function were found—younger age, shorter duration of heart and kidney disease, cardiogenic shock—but further work is needed to enable prediction of kidney outcomes at the individual level. Ultimately, what is needed is therapies and management strategies to enable more LVAD recipients to achieve sustained kidney health, and this may depend in part on identifying those most at risk for adverse kidney outcomes.11,39
A limitation of the study is the use of serum creatinine-based eGFR. Accuracy of eGFR has not been assessed in LVAD recipients, and eGFR estimates provide inaccurate estimates of measured GFR in HF.40,41 Nevertheless, eGFR and creatinine, if judged in relation to outcomes rather than as physiological measurements, have demonstrated important associations with outcomes in the LVAD setting and are widely used. Additionally, the methods of this study, looking at longitudinal patterns in a biomarker score, may further reduce the importance of eGFR accuracy, as the shape of trajectories—potentially integrating changes in kidney function, body composition, and diet—rather than individual values are the primary metrics. Serum cystatin C values are not available. Use of trajectory groups as predictors in survival analyses is limited in that trajectories were not necessarily independent of the censoring caused by these outcome events. We were unable to differentiate device types in the database. We are unable to determine which factors led to patients being transplant candidates versus destination therapy candidates. Complications occurring after LVAD implantation that may have affected kidney function are not incorporated in the present analysis and warrant further study. Different choices for spline knot number and placement may have led to identification of different trajectory groups.
In conclusion, distinct kidney function trajectory groups following LVAD implantation have been identified in a national cohort, using an unsupervised algorithm, confirming the previously identified dominant trajectory patterns, and revealing other, novel trajectories. These findings demonstrate the usefulness of modern analytic techniques in investigating inter-individual differences that may be obscured in conventional analyses of heterogeneous cohorts. These trajectory groups differed in pre-implantation characteristics and may reflect distinct kidney health/function pathophysiology. To improve outcomes in this setting, further investigations into the heterogeneous causes and outcomes of kidney disease in LVAD recipients are needed.
Supplementary Material
Acknowledgement:
This manuscript was prepared using INTERMACS research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of INTERMACS or the NHLBI.
Funding:
Dr. Walther is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, (Grant / Award Number: ‘K23DK122131’).
Abbreviations:
- LVAD
left-ventricular assist device
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- eGFR
estimated glomerular filtration rate
- HF
heart failure
- BMI
body mass index
- BSA
body surface area
- ECMO
extracorporeal membrane oxygenation
- IABP
intra-aortic balloon pump
- VAD
ventricular assist device
- BUN
blood urea nitrogen
Footnotes
Disclosures:
The authors declare no conflicts of interest related to this work.
Disclaimer:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or Veterans Administration.
REFERENCES
- 1.Cook JL, Colvin M, Francis GS, et al. Recommendations for the Use of Mechanical Circulatory Support: Ambulatory and Community Patient Care: A Scientific Statement From the American Heart Association. Circulation. 2017/06/20 2017;135(25):e1145–e1158. doi: 10.1161/CIR.0000000000000507 [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. Dec 2015;34(12):1495–504. doi: 10.1016/j.healun.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 3.Morgan JA, Paone G, Nemeh HW, et al. Impact of continuous-flow left ventricular assist device support on right ventricular function. J Heart Lung Transplant. Apr 2013;32(4):398–403. doi: 10.1016/j.healun.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 4.Uriel N, Sayer G, Addetia K, et al. Hemodynamic ramp tests in patients with left ventricular assist devices. JACC: Heart Failure. 2016;4(3):208–217. [DOI] [PubMed] [Google Scholar]
- 5.Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Review Article. Nature Reviews Nephrology. 08/30/online 2016;12:610. doi: 10.1038/nrneph.2016.113 [DOI] [PubMed] [Google Scholar]
- 6.Yalcin YC, Muslem R, Veen KM, et al. Impact of Continuous Flow Left Ventricular Assist Device Therapy on Chronic Kidney Disease: A Longitudinal Multicenter Study. J Card Fail. Apr 2020;26(4):333–341. doi: 10.1016/j.cardfail.2020.01.010 [DOI] [PubMed] [Google Scholar]
- 7.Wettersten N, Estrella M, Brambatti M, et al. Kidney Function Following Left Ventricular Assist Device Implantation: An Observational Cohort Study. Kidney Med. May-Jun 2021;3(3):378–385 e1. doi: 10.1016/j.xkme.2021.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brisco MA, Kimmel SE, Coca SG, et al. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail. Jan 2014;7(1):68–75. doi: 10.1161/CIRCHEARTFAILURE.113.000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyamoto T, Karimov JH, Fukamachi K . Effects of continuous and pulsatile flows generated by ventricular assist devices on renal function and pathology. Expert review of medical devices. 2018;15(3):171–182. [DOI] [PubMed] [Google Scholar]
- 10.Ross DW, Stevens GR, Wanchoo R, et al. Left Ventricular Assist Devices and the Kidney. Clin J Am Soc Nephrol. Feb 7 2018;13(2):348–355. doi: 10.2215/CJN.04670417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walther CP, Civitello AB, Liao KK, Navaneethan SD. Nephrology considerations in the management of durable and temporary mechanical circulatory support. Kidney360. 2022: 10.34067/KID.0003382021. doi: 10.34067/KID.0003382021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brisco M, Hale A, Zile M, et al. Patients undergoing LVAD placement demonstrate marked sarcopenia leading to overestimation of pre-implant glomerular filtration rate. The Journal of Heart and Lung Transplantation. 2015;34(4):S165. [Google Scholar]
- 13.Krishnan A, Levin A. Laboratory Assessment of Kidney Disease: Glomerular Filtration Rate, Urinalysis, and Proteinuria. In: Yu ASL, Chertow GM, Luyckx VA, Marsden PA, Skorecki K, Taal MW, eds. Brenner & Rector’s The Kidney. 11th ed. Elsevier, Inc.; 2020:732–757:chap 23. [Google Scholar]
- 14.Raynaud M, Aubert O, Reese PP, et al. Trajectories of glomerular filtration rate and progression to end stage kidney disease after kidney transplantation. Kidney international. 2020; [DOI] [PubMed] [Google Scholar]
- 15.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. Jama. 2014;311(5):490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. New England Journal of Medicine. 2010;362(13):1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Portegies ML, Mirza SS, Verlinden VJ, et al. Mid- to Late-Life Trajectories of Blood Pressure and the Risk of Stroke: The Rotterdam Study. Hypertension. Jun 2016;67(6):1126–32. doi: 10.1161/HYPERTENSIONAHA.116.07098 [DOI] [PubMed] [Google Scholar]
- 18.Masyn KE. Latent Class Analysis and Finite Mixture Modeling. In: Little TD, ed. The Oxford Handbook of Quantitative Methods. Oxford University Press; 2013:551–611. [Google Scholar]
- 19.Proust-Lima C, Philipps V, Liquet B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. Journal of Statistical Software. June/01 2017;78(2):1 – 56. doi: 10.18637/jss.v078.i02 [DOI] [Google Scholar]
- 20.National Heart Lung and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center. Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs). National Heart, Lung, and Blood Institute. Updated May 21, 2018. Accessed September 7, 2021, https://biolincc.nhlbi.nih.gov/studies/intermacs/
- 21.Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant. Oct 2017;36(10):1080–1086. doi: 10.1016/j.healun.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. May 5 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: current status and future directions. Nature Reviews Nephrology. 2020;16(1):51–64. [DOI] [PubMed] [Google Scholar]
- 24.Society of Thoracic Surgeons. STS Intermacs Users’ Guide Version 5.0. 2018. June 28, 2018. https://www.uab.edu/medicine/intermacs/images/Intermacs-Users-Guide-v5.0-2018-06-28.pdf [Google Scholar]
- 25.Yalcin YC, Muslem R, Veen KM, et al. Impact of continuous flow left ventricular assist device therapy on chronic kidney disease: A longitudinal multicenter study. Journal of cardiac failure. 2020;26(4):333–341. [DOI] [PubMed] [Google Scholar]
- 26.Biernacki C, Celeux G, Govaert G. Choosing starting values for the EM algorithm for getting the highest likelihood in multivariate Gaussian mixture models. Computational Statistics & Data Analysis. 2003;41(3–4):561–575. [Google Scholar]
- 27.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural equation modeling: A multidisciplinary Journal. 2007;14(4):535–569. [Google Scholar]
- 28.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine. 2009;28(25):3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Buuren S Classification and Regression Trees. Flexible Imputation of Missing Data. CRC press; 2018:84–86. [Google Scholar]
- 30.Deng H, Runger G. Gene selection with guided regularized random forest. Pattern Recognition. 2013;46(12):3483–3489. [Google Scholar]
- 31.Hasin T, Topilsky Y, Schirger JA, et al. Changes in renal function after implantation of continuous-flow left ventricular assist devices. Journal of the American College of Cardiology. 2012;59(1):26–36. [DOI] [PubMed] [Google Scholar]
- 32.Rangaswami EAJ, Bhalla IV, Blair LJ, et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation. 2019;139(16):e840–e878. doi: 10.1161/CIR.0000000000000664 [DOI] [PubMed] [Google Scholar]
- 33.Neyra JA, Chawla LS. Acute Kidney Disease to Chronic Kidney Disease. Critical Care Clinics. 2021; [DOI] [PubMed] [Google Scholar]
- 34.Zannad F, Rossignol P. Cardiorenal Syndrome Revisited. Circulation. 2018;138(9):929–944. [DOI] [PubMed] [Google Scholar]
- 35.Cody RJ, Ljungman S, Covit AB, et al. Regulation of glomerular filtration rate in chronic congestive heart failure patients. Kidney Int. Sep 1988;34(3):361–7. doi: 10.1038/ki.1988.189 [DOI] [PubMed] [Google Scholar]
- 36.Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. Aug 2011;80(3):282–7. doi: 10.1038/ki.2011.79 [DOI] [PubMed] [Google Scholar]
- 37.Michaels A, Cowger J. Patient Selection for Destination LVAD Therapy: Predicting Success in the Short and Long Term. Curr Heart Fail Rep. Oct 2019;16(5):140–149. doi: 10.1007/s11897-019-00434-1 [DOI] [PubMed] [Google Scholar]
- 38.Walther CP, Winkelmayer WC, Deswal A, Niu J, Navaneethan SD. Trends in Left Ventricular Assist Device Implantation and Associated Mortality Among Patients With and Without ESRD. Am J Kidney Dis. Oct 2018;72(4):620–622. doi: 10.1053/j.ajkd.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 39.Scholz H, Boivin FJ, Schmidt-Ott KM, et al. Kidney physiology and susceptibility to acute kidney injury: implications for renoprotection. Nat Rev Nephrol. May 2021;17(5):335–349. doi: 10.1038/s41581-021-00394-7 [DOI] [PubMed] [Google Scholar]
- 40.Kervella D, Lemoine S, Sens F, et al. Cystatin C Versus Creatinine for GFR Estimation in CKD Due to Heart Failure. Am J Kidney Dis. Feb 2017;69(2):321–323. doi: 10.1053/j.ajkd.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 41.Kolsrud O, Ricksten SE, Holmberg E, et al. Measured and not estimated glomerular filtration rate should be used to assess renal function in heart transplant recipients. Nephrol Dial Transplant. Jul 2016;31(7):1182–9. doi: 10.1093/ndt/gfv338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


