Abstract
Background
The prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing among patients with type 1 diabetes (T1D) paralleling the increasing prevalence of obesity among this population. However, little is known about the impact of intensive lifestyle intervention (ILI) on NAFLD in patients with T1D.
Methods
Using Hepatic Steatosis Index (HSI), a noninvasive surrogate predictor of NAFLD, we retrospectively evaluated 88 adult patients with T1D and obesity after one year of participating in a 12-week ILI program in real-world clinical practice. Using the NAFLD guidelines of the American Association for the Study of Liver Diseases (AASLD), we excluded 11 participants. We matched the remaining ILI cohort (age 43 ± 12 years, females 65%, diabetes duration 22 ± 9 years, A1C 8.2 ± 0.9%, body weight 101 ± 17 kg, BMI 35.3 ± 4.9 kg/m2) in 1:1 ratio with a similar cohort of patients with T1D and obesity who received standard diabetes care (SC) at the same practice and during the same period. Matching criteria included: sex, age, BMI, A1C and duration of T1D. HSI [8 + ALT/AST + BMI (+ 2 if female, + 2 if T2D)] was calculated at baseline and after 12 months of intervention.
Results
At baseline, HSI was similar between the two cohorts (46.2 ± 6.1 in the ILI cohort and 44.9 ± 5.7 in the SC cohort). After 12 months, the ILI group lost an average of 5.6 ± 2.7 kg (5.8%, p < 0.05) while the SC group maintained their baseline body weight (p < 0.001 between groups). HSI decreased significantly from baseline in the ILI group (-2.7 ± 1.1, p = 0.01), but did not change in the SC group (0.6 ± 0.9, p = 0.53, p < 0.001 between groups). Percentage of patients with high likelihood of NAFLD diagnosis decreased from 100% at baseline to 88.3% in the ILI group, and was 10.4% less compared to SC (p < 0.01). Total daily insulin dose decreased in the ILI cohort compared to the SC cohort (-6.1 ± 4.2 versus 1.34 ± 4.3 units/day, p < 0.01).
Conclusions
Twelve weeks of ILI improved HSI and decreased total daily insulin requirements in patients with T1D and obesity at one year. Short-term ILI should be implemented in the management of NAFLD for obese patients with type 1 diabetes.
Keywords: NAFLD, Type 1 Diabetes, Liver fibrosis, Obesity, Lifestyle intervention, Weight management
Introduction
Non-alcoholic fatty liver disease (NAFLD) is currently considered the most common chronic liver disease [1]. It is strongly associated with obesity, type 2 diabetes (T2D) and metabolic syndrome (MeS). However, NAFLD is also found in non-obese patients without insulin resistance [2]. Its prevalence is increasing among patients with type 1 diabetes (T1D), paralleling the increasing prevalence of obesity among this population [3]. Recent studies reported that up to 50% of patients with T1D are overweight or obese, and between 8–40% meet the MetS criteria [4]. Some studies estimated that the prevalence of NAFLD in patients with T1D is up to 44.4%. However, these studies had small sample sizes, they also showed an association between NAFLD and increased incidence of cardiovascular events, chronic kidney disease, and retinopathy [5–8]. As of now, there are no approved pharmacologic treatments for NAFLD, and weight reduction through dietary and exercise modifications remains the prime intervention tool [9].
Screening for NAFLD in the current clinical practice is frequently conducted using imaging modalities; mainly ultrasonography (US) [10], however the sensitivity of this method decreases when hepatic fat content is < 33% [11]. Other modalities include computed tomography (CT), proton magnetic resonance spectroscopy and magnetic resonance elastography [12]. However, these tools are expensive, and are not cost-effective for screening large populations. Many non-invasive biomarkers have been developed to predict NAFLD, such as the hepatic steatosis index (HSI) which is helpful for early diagnosis in order to provide opportunities for preventing and treating NAFLD and its associated complications [3, 13].
Due to scarce information on the effect of intensive lifestyle intervention (ILI) in obese patients with T1D and NAFLD, we conducted this retrospective study to evaluate the impact of ILI on hepatic steatosis. We selected a cohort of obese patients with T1D who were diagnosed with NAFLD using HSI and underwent an ILI program for 12 weeks, and followed them with a matched control for one year using HSI.
Methods
The selected cohort for this study include obese patients with T1D who underwent the Weight Achievement and Intensive Treatment (Why WAIT) program at Joslin Diabetes Center in Boston, MA. Why WAIT is a 12-week multidisciplinary ILI program, designed for patients with diabetes and obesity in real-world clinical practice. The main components of the Why WAIT program include the following: 1) intensive medication adjustments; 2) structured modified dietary intervention; 3) individualized exercise plan; 4) cognitive behavioral support; and 5) adult group education. The program showed maintenance of weight reduction for 5 years [14]. A detailed description of the program was described elsewhere [14, 15].
Study subjects
After approval from the Institutional Review Board of the Joslin Diabetes Center, we retrospectively evaluated all adult patients with T1D and obesity who were enrolled in the Why WAIT program between September 2005 and May 2018. Eighty-eight patients met the criteria for this study. Using the NAFLD guidelines of the American Association for the Study of Liver Diseases (AASLD), we excluded 11 participants mainly due to alcohol consumption of > 21 standard alcoholic drinks per week for men and > 14 for women [16]. The remaining 77 patients were included in this analysis (ILI cohort: mean age 43 ± 12 years, females 65%, diabetes duration 22 ± 12 years, A1C 8.2 ± 1.0%, body weight 102.5 ± 17.3 kg, BMI 35.7 ± 5.0 kg/m2). We matched this ILI cohort, in 1:1 ratio, with a similar cohort of patients with T1D who received standard diabetes care (SC) at the same practice and during the same period. Matching criteria included: sex, age, BMI, A1C and duration of T1D (SC cohort: n = 77, mean age 43 ± 12 years, females 65%, diabetes duration 22 ± 9 years, A1C 8.2 ± 0.9%, body weight 100.9 ± 17.2 kg, BMI 35.3 ± 4.9 kg/m2). Diagnosis of T1D was confirmed by the presence of auto-antibodies associated with T1D development, history of diabetic ketoacidosis (DKA), very low or undetectable serum C-peptide, and additional relevant clinical criteria. HSI was calculated as [8 + ALT/AST + BMI (+ 2 if female, + 2 if T2D)] at baseline and after 12 months of intervention [17]. Other data collected at baseline and after 12 months for both cohorts included method of insulin delivery (MDI or insulin pump therapy), total insulin dose (units), weight-adjusted insulin dose (units/kg/d), blood pressure, and serum lipid profile.
Statistical analyses
All tests of group differences were based on the intent-to-treat principle using all available data. Since clinic visits during the follow-up period were not rigorously scheduled every 3 months, an approximation of each visit time to the nearest 3-month timeline was used. There was no evidence that missing data were dependent on the study group. Demographic and baseline characteristics were evaluated using descriptive statistics. Continuous variables are reported as mean ± standard deviation (SD) or standard error of the mean (SEM). Normality of the data was tested using the Kolmogorov-Smirnoff equality-of-distributions test. Categorical variables are presented as percentages. For comparisons between the two study cohorts, independent samples Student's t-test was used for continuous variables. Chi-square or Fisher’s exact tests were used to compare categorical variables. A p-value of < 0.05 was considered statistically significant. To determine the possible influence of baseline differences on the study outcomes, statistical analyses were run with and without adjusting for baseline age, BMI, T1D duration, method of insulin delivery, and HSI. We found that adjusting for these variables had no effect on the direction or the significance of study conclusions. Statistical analysis was conducted using SAS version 9.4 for Windows (SAS Institute, Cary, NC, USA 2012).
Results
Both ILI and SC cohorts were similar at baseline for age, sex, duration of diabetes, A1C, body weight, and BMI (Table 1). HSI at baseline was also comparable between the two cohorts (HSI 46.2 ± 6.1 in the ILI cohort and 44.9 ± 5.7 in the SC cohort). Total daily insulin dose, and insulin requirements per kg of body weight were not significantly different between the two cohorts. The only significant difference between them at baseline was the method of insulin delivery, where the percentage of patients using insulin infusion pumps was significantly higher in the ILI cohort compared to the SC cohort (63.3% versus 46.7%, p < 0.05).
Table 1.
Baseline characteristics of ILI and SC cohorts of patients with type 1 diabetes and obesity
|
ILI (n = 77) |
SC (n = 77) |
p value† | |
|---|---|---|---|
| Age (years) | 43 ± 12 | 43 ± 12 | NS |
| Female n (%) | 50 (65) | 50 (65) | NS |
| Diabetes duration (years) | 22 ± 12 | 22 ± 9 | NS |
| Weight (kg) | 102.5 ± 17.3 | 100.9 ± 17.2 | NS |
| BMI (kg/m2) | 35.7 ± 5.0 | 35.3 ± 4.9 | NS |
| ALT (U/L) | 26.8 ± 20.2 | 21.6 ± 8.5 | < 0.05 |
| AST (U/L) | 22.4 ± 9.3 | 21.7 ± 12.0 | NS |
| HbA1c (%) | 8.2 ± 1.0 | 8.2 ± 0.9 | NS |
| HSI | 46.2 ± 6.1 | 44.9 ± 5.7 | NS |
| HSI > 36 n (%) | 77 (100) | 75 (97.4) | NS |
| HSI < 30 n (%) | 0 (0) | 0 (0) | - |
| Total insulin dose (units/d) | 69.9 ± 27.5 | 70.8 ± 27.4 | NS |
| Weight-adjusted insulin dose (units/kg/d) | 0.7 ± 0.2 | 0.7 ± 0.2 | NS |
| Use of insulin pump therapy n (%) | 49 (63.6) | 36 (46.7) | < 0.05 |
| Systolic blood pressure (mmHg) | 128 ± 14 | 126 ± 15 | NS |
| Diastolic blood pressure (mmHg) | 77 ± 8 | 77 ± 9 | NS |
| Total Cholesterol (mg/dL) | 169 ± 33 | 176 ± 26 | NS |
| LDL-Cholesterol (mg/dL) | 97 ± 32 | 97 ± 27 | NS |
| HDL-Cholesterol (mg/dL) | 55 ± 16 | 58 ± 17 | NS |
| Triglycerides (mg/dL) | 114 ± 88 | 121 ± 73 | NS |
Data are mean ± SD
ILI Intensive lifestyle intervention, SC Standard diabetes care, HSI Hepatic steatosis index, NAFLD Nonalcoholic fatty liver disease, ALT Alanine aminotransferase, AST Aspartate aminotransferase
†Student’s t-test or Pearson’s chi squared between groups
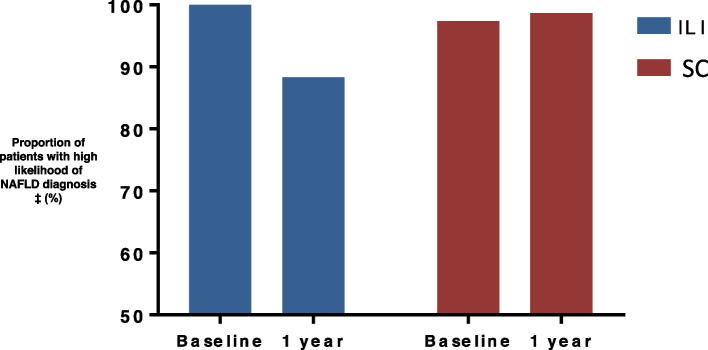
After 12 months, the ILI group lost an average of 5.6 ± 2.7 kg (5.8%, p < 0.05) while the SC group maintained their baseline body weight (p < 0.001 between groups) (Fig. 1). HSI decreased significantly from baseline in the ILI group (-2.7 ± 1.1, p = 0.01), but did not change in the SC group (0.6 ± 0.9, p = 0.53, p < 0.001 between groups) (Table 2). Changes in HSI correlated significantly with the change in body weight and the daily basal insulin dose (Fig. 2). Moreover, the percentage of patients with high likelihood of NAFLD diagnosis decreased from 100% at baseline to 88.3% at 1 year in the ILI group and was 10.4% less compared to SC (98.7%) (p < 0.01) (Fig. 3). Changes in body weight, BMI, and HSI remained significant in the ILI cohort after adjusting for age, sex, T1D duration, and method of insulin delivery.
Fig. 1.
Changes in (A) body weight and (B) total daily insulin dose after 1 year. Data are mean±SD. ILI, Intensive lifestyle intervention; SC, Standard diabetes care. n=77 in each cohort
Table 2.
Changes after one year of ILI or SC in patients with T1D and obesity
|
ILI (n = 77) |
SC (n = 77) |
p value† | |
|---|---|---|---|
| Weight (kg) | -5.6 ± 2.7* | 1.6 ± 2.8 | < 0.001 |
| BMI (kg/m2) | -1.8 ± 0.9* | 0.6 ± 0.8 | < 0.01 |
| ALT (U/L) | -4.0 ± 2.5 | 1.9 ± 1.3 | < 0.05 |
| AST (U/L) | -0.6 ± 1.1 | 0.2 ± 1.6 | NS |
| HbA1c (%) | -0.33 ± 0.15* | -0.07 ± 0.16 | NS |
| Total daily insulin dose (units/d) | -6.1 ± 4.2 | 1.3 ± 4.3 | < 0.01 |
| Weight-adjusted insulin dose (units/kg/day) | -0.014 ± 0.020 | 0.004 ± 0.013 | NS |
| Systolic blood pressure (mmHg) | -3.6 ± 2.1 | 0.4 ± 2.5 | NS |
| Diastolic blood pressure (mmHg) | -1.9 ± 1.3 | -0.1 ± 1.4 | NS |
| Total cholesterol (mg/dL) | 0.1 ± 6.3 | 0.9 ± 5.2 | NS |
| LDL-cholesterol (mg/dL) | -4.7 ± 5.4 | 0.2 ± 3.4 | NS |
| HDL-cholesterol (mg/dL) | 1.1 ± 2.9 | -0.9 ± 1.0 | NS |
| Triglycerides (mg/dL) | -7.7 ± 13 | -0.4 ± 9.0 | NS |
| HSI | -2.7 ± 1.1* | 0.6 ± 0.9 | < 0.001 |
Date are mean ± SEM
ILI Intensive lifestyle intervention, SC Standard diabetes care, HSI Hepatic steatosis index, ALT Alanine aminotransferase, AST Aspartate aminotransferase
*p < 0.05 compared to baseline
Fig. 2.
Change in HSI, body weight, and daily basal insulin dose after 1 year. Legend. A Association between change in HSI and change in body weight after 1 year in response to ILI and SC in the total cohort. B Association between change in HSI and daily basal insulin dose after 1 year in response to ILI and SC in the total cohort. HSI, Hepatic steatosis index; ILI, Intensive lifestyle intervention; SC, Standard diabetes care. N=154
Fig. 3.
Likelihood of NAFLD diagnosis after 1 year ILI and SC. Data are %. n=77 in each cohort. NAFLD, nonalcoholic fatty liver disease. ‡ High likelihood of NAFLD diagnosis defined as having HSI>36. HSI>36 can detect NAFLD with a specificity of 92.4% and a positive likelihood ratio of 6.069
At 12 months, ALT improved in the ILI cohort compared to baseline (ALT change of -4.0 ± 2.5 U/L) with no change in the SC cohort (p < 0.05 between groups). However, the difference in AST between the two cohorts was not significant.
Total daily insulin dose decreased significantly from baseline in the ILI cohort compared to the SC cohort (-6.1 ± 4.2 versus 1.3 ± 4.3 units/day, p < 0.01). Difference in daily insulin dose remained significant after adjusting for age, sex, BMI, T1D duration and method of insulin delivery. However, weight-adjusted insulin requirements did not change in either of the two cohorts at 12 months.
A1C, blood pressure, and lipid profile showed no significant changes in either of the two cohorts at 12 months.
Discussion
Prior research showed that NAFLD develops as a possible consequence of hyperglycemia, insulin resistance, and obesity [18], however little research has been done to investigate strategies to mitigate NAFLD in patients with T1D and obesity.
This study showed that ILI intervention in real-world clinical practice for 12 weeks, is associated with an improvement in HSI in patients with T1D and obesity at one year in comparison to standard diabetes care that typically focuses on glycemic control. By the end of 12 months, patients who underwent ILI had a significantly lower percentage of NAFLD as diagnosed by HSI calculation, indicating possible resolution of the condition in some patients after such intervention. The study also showed improvement in ALT which is another component of HSI, indicating an improvement in liver fat. Moreover, there was a reduction in total insulin dose, which might indicate improvement in insulin sensitivity after weight reduction. Our research team previously demonstrated that ~ 7% weight reduction in patients with insulin resistance improves insulin sensitivity by ~ 57% [19]. The amount of weight loss that resulted in such improvement in this study is an average of 5.6 ± 2.7 kg (5.8%), which is significantly better than weight reduction by standard diabetes care for one year, which was 1.6 ± 2.8 kg (1.6%). Our observed results draw attention to the importance of a short period of ILI for 12 weeks as a possible modifier of NAFLD at a longer term of one year.
Other research work has demonstrated that NAFLD is potentially reversible by pharmacological intervention [20]. However, to our knowledge, this is the first study to explore the longevity of NAFLD improvement or resolution at one year after a short period of ILI. Since prior research only demonstrated the reversibility of liver fibrosis but not liver cirrhosis [21], detecting NAFLD early enough by screening susceptible patients in real-world clinical practice using simple measurements may lead to saving many cases from progressing to liver cirrhosis through implementing an effective short-term ILI.
The study has many limitations and few strengths. Perhaps the most prominent limitation is our way of diagnosing NAFLD. Although HSI is found to be reliable in determining NAFLD [22], NAFLD can only be truly diagnosed and confirmed by liver biopsy [23]. Since this study was retrospective, it was not possible for us to obtain liver biopsies for either of the two cohorts. Consequently, we cannot be certain that all included patients had NAFLD. The prevalence of NAFLD in patients with T1D is around 20–30% and might be higher in an obese population [24]. Another limitation is the fact that the BMI component of HSI is overpowered compared to that of the liver enzymes. Moreover, these indices are not validated in reflecting prospective longitudinal changes in NAFLD, fat content, and fibrosis [25–28]. However, in real-world clinical practice, a simple screening method may help in detecting more patients who may benefit from early intervention in comparison to the more costly methods. Another limitation is that patients in the ILI cohort voluntarily decided to seek a program to manage their body weight and considered more motivated for change. The retrospective nature of the study is another drawback, since included patients might have other health complications that could have impacted our findings. This warrants another well-designed prospective-controlled study to confirm our observations. Another limitation, was that the two cohorts were confined to patients with T1D and obesity, therefore our findings are not applicable to lean individuals with T1D, a few of whom may also have NAFLD. Lastly, it is hard to rule out the effects of other confounders that may affect the results, whether at baseline or at 12 months. These confounders may include: the use of insulin pump versus injections, which is higher in the ILI cohort, the use of other hypoglycemic medications, and any other medications that may affect weight or liver fat content. These drawbacks may limit the generalizability of our results to all patients with T1D and obesity. However, despite these limitations, we were able to uncover that short-term weight loss through ILI may be an effective tool for improving NAFLD parameters in patients with T1D and obesity. On the positive side, this study was conducted among patients in real-world clinical practice, which may indicate its potential value for direct application in diabetes practice, especially with its simpler screening method and available ILI programs in many clinical centers.
In conclusion, short-term ILI leads to a consequential improvement in NAFLD indices in patients with T1D and obesity and is associated with a reduction in insulin requirements. Even though HSI is not widely used for monitoring the progression of steatosis, it is a simple method for NAFLD screening and initiating ILI that results in weight reduction and potential improvement of hepatic steatosis. Therefore, we recommend a short-term ILI for obese patients with T1D and NAFLD, while calling for other prospective-controlled studies to be conducted in order to confirm our notion.
Acknowledgements
The authors would like to thank the clinical and administrative staff of the Why WAIT program at Joslin Diabetes Center. Data from this work was presented at the 79th Scientific Sessions of the American Diabetes Association in 2019.
Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- T1D
Type 1 diabetes
- T2D
Type 2 diabetes
- HSI
Hepatic steatosis index
- ILI
Intensive lifestyle intervention
- AASLD
American Association for the Study of Liver Diseases
- SC
Standard diabetes care
- MetS
Metabolic syndrome
- NASH
Nonalcoholic steatohepatitis
- US
Ultrasound
- CT
Computed tomography
- SMBG
Self-monitoring of blood glucose
- EP
Exercise psychologists
- BMI
Body-mass index
- DKA
Diabetic ketoacidosis
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- MDI
Multiple daily injections
Authors’ contributions
ST collected data, conducted statistical analysis, and drafted the manuscript. TS collected data, conducted statistical analysis and edited and reviewed the manuscript. MA and HG drafted, edited and reviewed the manuscript. SD and MWT edited and reviewed the manuscript. OH directs the WhyWAIT program, designed the study, supervised the work, and reviewed and edited the manuscript. ST and OH are guarantors of this work and take responsibility for its integrity and the accuracy of data analysis. All authors read and approved the final manuscript.
Funding
None.
Availabiltiy of data and materials
The data contained in this manuscript are held at the Joslin Diabetes Center Clinical Research Center. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The Joslin Diabetes Center, Committee on Human Studies. Consent to participate is waived by Joslin IRB for retrospective data collection.
Consent for publicaiton
Not applicable.
Competing interests
OH receives research support from National Dairy Council, Eli Lilly and Company, and Novo Nordisk; consults for Merck Inc., Sanofi-Aventis and Abbott Nutrition; is on the advisory board of Astra Zeneca Inc. and is a shareholder of Healthimation Inc. None of these entities supported this research in part or total. ST, TS, MA, SD, HG and MWT have no disclosures relevant to this work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shaheen Tomah and Tareq Salah contributed equally to this work and should be considered joint first authors.
References
- 1.Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28(4):637–653. doi: 10.1016/j.bpg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Younes R, Bugianesi E. NASH in Lean Individuals. Semin Liver Dis. 2019;39(1):86–95. doi: 10.1055/s-0038-1677517. [DOI] [PubMed] [Google Scholar]
- 3.Singh A, Le P, Lopez R, Alkhouri N. The utility of noninvasive scores in assessing the prevalence of nonalcoholic fatty liver disease and advanced fibrosis in type 1 diabetic patients. Hep Intl. 2018;12(1):37–43. doi: 10.1007/s12072-017-9840-z. [DOI] [PubMed] [Google Scholar]
- 4.Chillaron JJ, Flores Le-Roux JA, Benaiges D, Pedro-Botet J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism. 2014;63(2):181–7. doi: 10.1016/j.metabol.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Pichiri I, et al. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol. 2010;53(4):713–718. doi: 10.1016/j.jhep.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Mingolla L, Rigolon R, Pichiri I, Cavalieri V, Zoppini G, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular disease in adult patients with type 1 diabetes. Int J Cardiol. 2016;225:387–391. doi: 10.1016/j.ijcard.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Targher G, Mantovani A, Pichiri I, Mingolla L, Cavalieri V, Mantovani W, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care. 2014;37(6):1729–1736. doi: 10.2337/dc13-2704. [DOI] [PubMed] [Google Scholar]
- 8.Vendhan R, Amutha A, Anjana RM, Unnikrishnan R, Mohan V. Clinical profile of nonalcoholic Fatty liver disease among young patients with type 1 diabetes mellitus seen at a diabetes speciality center in India. Endocrine Practice. 2014;20(12):1249–1257. doi: 10.4158/EP14044.OR. [DOI] [PubMed] [Google Scholar]
- 9.Raza S, Rajak S, Upadhyay A, Tewari A, Anthony SR. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front Biosci (Landmark Ed) 2021;26(2):206–237. doi: 10.2741/4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandyarajan V, Gish RG, Alkhouri N, Noureddin M. Screening for Nonalcoholic Fatty Liver Disease in the Primary Care Clinic. Gastroenterology Hepatology. 2019;15(7):357–365. [PMC free article] [PubMed] [Google Scholar]
- 11.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 12.Piazzolla VA, Mangia A. Noninvasive Diagnosis of NAFLD and NASH. Cells. 2020;9(4):1005. doi: 10.3390/cells9041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Hamdy O, Mottalib A, Morsi A, El-Sayed N, Goebel-Fabbri A, Arathuzik G, et al. Long-term effect of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: a 5-year longitudinal study. BMJ Open Diabetes Res Care. 2017;5(1):e000259. doi: 10.1136/bmjdrc-2016-000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamdy O, Carver C. The Why WAIT program: improving clinical outcomes through weight management in type 2 diabetes. Curr DiabRep. 2008;8(5):413–420. doi: 10.1007/s11892-008-0071-5. [DOI] [PubMed] [Google Scholar]
- 16.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 17.Hegazy MA, Samy MA, Tawfik A, Naguib MM, Ezzat A, Behiry ME. Abdominal subcutaneous fat thickness and homeostasis model assessment of insulin resistance as simple predictors of nonalcoholic steatohepatitis. Diabetes Metab Syndr Obes. 2019;12:1105–1111. doi: 10.2147/DMSO.S202343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell A. Tackling "diabesity" head-on. Joslin Diabetes Center's new nutrition guideline. Diabetes Self Manag. 2005;22(6):40–2-4. [PubMed] [Google Scholar]
- 19.Hamdy O, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, et al. Lifestyle Modification Improves Endothelial Function in Obese Subjects With the Insulin Resistance Syndrome. Diabetes Care. 2003;26(7):2119–2125. doi: 10.2337/diacare.26.7.2119. [DOI] [PubMed] [Google Scholar]
- 20.Perry RJ. Novel Strategies to Treat Hepatic Steatosis and Steatohepatitis. Obesity (Silver Spring). 2019;27(9):1385-7. [DOI] [PMC free article] [PubMed]
- 21.Desmet VJ, Roskams T. Cirrhosis reversal: a duel between dogma and myth. J Hepatol. 2004;40(5):860–867. doi: 10.1016/j.jhep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Sviklane L, Olmane E, Dzerve Z, Kupcs K, Pirags V, Sokolovska J. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J Gastroenterol Hepatol. 2018;33(1):270–276. doi: 10.1111/jgh.13814. [DOI] [PubMed] [Google Scholar]
- 23.Nd AM. Non-Alcoholic Fatty Liver Disease, an Overview. Integr Med (Encinitas) 2019;18(2):42–49. [PMC free article] [PubMed] [Google Scholar]
- 24.TOMAH S, SALAH T, AL-BADRI M, DHAVER SE, CURRY MP, JIANG Z, et al. 1105-P: Moderate-to-Advanced Liver Fibrosis Is Common in Patients with Type 1 Diabetes (T1D) and Nonalcoholic Fatty Liver Disease (NAFLD). Diabetes. 2022;71(Supplement_1).
- 25.Kabisch S, Bäther S, Dambeck U, Kemper M, Gerbracht C, Honsek C, et al. Liver Fat Scores Moderately Reflect Interventional Changes in Liver Fat Content by a Low-Fat Diet but Not by a Low-Carb Diet. Nutrients. 2018;10(2):157. doi: 10.3390/nu10020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabisch S, Markova M, Hornemann S, Sucher S, Pivovarova-Ramich O, Machann J, et al. Liver fat scores do not reflect interventional changes in liver fat content induced by high-protein diets. Sci Rep. 2021;11(1):8843. doi: 10.1038/s41598-021-87360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keating SE, Parker HM, Hickman IJ, Gomersall SR, Wallen MP, Coombes JS, et al. NAFLD in clinical practice: Can simple blood and anthropometric markers be used to detect change in liver fat measured by (1) H-MRS? Liver Int. 2017;37(12):1907–1915. doi: 10.1111/liv.13488. [DOI] [PubMed] [Google Scholar]
- 28.Kantartzis K, Rettig I, Staiger H, Machann J, Schick F, Scheja L, et al. An extended fatty liver index to predict non-alcoholic fatty liver disease. Diabetes Metab. 2017;43(3):229–239. doi: 10.1016/j.diabet.2016.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data contained in this manuscript are held at the Joslin Diabetes Center Clinical Research Center. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.