Abstract
Background and purpose
Measures of atrophy in the whole brain can be used to reliably assess treatment effect in clinical trials of patients with multiple sclerosis (MS). Trials assessing the effect of treatment on grey matter (GM) and white matter (WM) atrophy are very informative, but hindered by technical limitations. This study aimed to measure GM and WM volume changes, using a robust longitudinal method, in patients with relapsing MS randomized to cladribine tablets 3.5 mg/kg or placebo in the CLARITY study.
Methods
We analysed T1‐weighted magnetic resonance sequences using SIENA‐XL, from 0 to 6 months (cladribine, n = 267; placebo, n = 265) and 6 to 24 months (cladribine, n = 184; placebo, n = 186). Mean percentage GM and WM volume changes (PGMVC and PWMVC) were compared using a mixed‐effect model.
Results
More GM and WM volume loss was found in patients taking cladribine versus those taking placebo in the first 6 months of treatment (PGMVC: cladribine: −0.53 vs. placebo: −0.25 [p = 0.045]; PWMVC: cladribine: −0.49 vs. placebo: −0.34 [p = 0.137]), probably due to pseudoatrophy. However, over the period 6 to 24 months, GM volume loss was significantly lower in patients on cladribine than in those on placebo (PGMVC: cladribine: −0.90 vs. placebo: −1.27 [p = 0.026]). In this period, volume changes in WM were similar in the two treatment arms (p = 0.52).
Conclusions
After a short period of pseudoatrophy, treatment with cladribine 3.5 mg/kg significantly reduced GM atrophy in comparison with placebo. This supports the relevance of GM damage in MS and may have important implications for physical and cognitive disability progression.
Keywords: brain atrophy, cladribine tablets, grey matter, multiple sclerosis, white matter
Brain atrophy measures can reliably assess the effects of treatment in patients with multiple sclerosis. Using a robust longitudinal method, we demonstrated that, after a short period of pseudoatrophy, treatment with cladribine tablets significantly reduced grey matter atrophy in comparison with placebo.
INTRODUCTION
Brain volume loss is a common and early feature in patients with multiple sclerosis (MS), mostly reflecting irreversible tissue damage and neurodegeneration, which are consistently linked to accrual of disability [1]. In recent years, there has been an increasing recognition of the importance of atrophy measures in monitoring MS worsening, especially during clinical trials [1]. Indeed, treatment effect on brain volume loss correlates with the effect on disability in relapsing–remitting (RR) MS [2], and brain atrophy has recently been used as a primary outcome measure in Phase 2 clinical trials in progressive MS [3]. Previous studies have shown important clues regarding the role of grey matter (GM) volume loss in MS progression [4, 5, 6]. Although GM atrophy measures have greater potential to show treatment effects than whole‐brain measures, they have rarely been used in clinical trials, mostly due to technical limitations [1].
A recent analysis of the CLARITY study has shown that treatment with cladribine tablets 10 mg (MAVENCLAD®; 3.5 mg/kg cumulative dose over 2 years, referred to as cladribine tablets 3.5 mg/kg) decreased whole‐brain atrophy compared with placebo, and this was associated with a lower risk of disability progression in RRMS [7]. Notably, the beneficial effect of cladribine tablets 3.5 mg/kg was evident after excluding the first 6 months of treatment, when a paradoxical reduction of brain volume following the initiation of an anti‐inflammatory therapy, a phenomenon described as ‘pseudoatrophy’, was detected [7]. However, the relative role of GM and white matter (WM) changes in the slowing of whole‐brain atrophy was not assessed, as the trial was not originally designed to evaluate compartmentalized volume changes.
Recently, a new generation of longitudinal methods that are robust in measuring volume changes have been employed in the re‐analysis of clinical trials for GM atrophy assessment [8]. Among these methods, SIENA‐XL is a recently developed segmentation‐based longitudinal method for the assessment of volume changes of tissue‐specific measures (i.e., GM and WM), which is more sensitive to compartmentalized brain changes than the traditional SIENA method [9]. Against this background, in this post hoc analysis, we used SIENA‐XL to evaluate the contribution of GM and WM volume changes to whole‐brain atrophy in patients with RRMS who were treated either with cladribine tablets 3.5 mg/kg or placebo in the CLARITY study.
METHODS
Subjects and imaging characteristics
We used magnetic resonance imaging (MRI) data from the CLARITY study, a Phase 3, double‐blind, placebo‐controlled, multicentre trial involving patients with RRMS, comparing those treated with cladribine tablets 3.5 mg/kg versus placebo for 2 years. Data were acquired from 2005 to 2008 [10]. MRI scans were obtained at 0, 6, 12 and 24 months. The MRI protocol for explanatory imaging endpoints included axial three‐dimensional (3D) T1‐weighted (T1W) images with the following characteristics: GE: field of view (FoV) = 230 mm, dimensions in the 1×1 plane, slice thickness = 1, repetition time (TR)/echo time (TE) = 30/minimum ms; Philips: FoV = 230 mm, dimensions in the 1 × 1 plane, slice thickness = 1, TR/TE/ = 25/4.6 ms; and Siemens: FoV = 230 mm, dimensions in the 1 × 1 plane, slice thickness = 1, TR/TE/ = 2130/4.38. Table 1 summarizes the characteristics of patients included in the analysis.
TABLE 1.
Baseline demographic, clinical and MRI lesion characteristics of the study population
| Pseudo‐atrophy (BSL‐M06) analysis | Treatment period (M06‐M24) analysis | |||
|---|---|---|---|---|
| Placebo | Cladribine tablets 3.5 mg/kg | Placebo | Cladribine tablets 3.5 mg/kg | |
| N | 265 | 267 | 186 | 184 |
| Age, years | ||||
| Mean (SD) | 39.35 (9.77) | 38.71 (10.16) | 39.9 (9.3) | 39.44 (10.3) |
| Median (min; max) | 39 (18; 64) | 39 (19; 63) | 40 (18; 64) | 39 (19; 63) |
| Female, n (%) | 172 (64.9) | 191 (71.5%) | 118 (63.4) | 138 (75) a |
| Prior use of DMT, n (%) | 71 (26.8) | 69 (25.8) | 39 (21.2) | 46 (24.7) |
| Disease duration | ||||
| Mean (SD) | 5.27 (5.46) | 4.81 (5.32) | 5.16 (5.4) | 4.48 (5.3) |
| Median (min; max) | 3.6 (0; 28) | 2.93 (0; 27) | 3.48 (0; 25) | 2.26 (0; 27) |
| EDSS score | ||||
| Mean (SD) | 2.92 (1.34) | 2.81 (1.22) | 2.85 (1.3) | 2.79 (1.2) |
| Median (min; max) | 3 (0; 6) | 2.5 (0; 6) | 3 (0; 6) | 2.5 (0; 6) |
| T2 lesions, n | ||||
| Mean (SD) | 26.75 (17.6) | 24.46 (15.6) | 25.49 (15.5) | 23.82 (15.2) |
| Median (min; max) | 22 (3; 134) | 20 (3; 89) | 22 (3; 100) | 20 (3; 79) |
| T2 lesion volume, cm3 | ||||
| Mean (SD) | 13.82 (13.1) | 13.43 (14.16) | 13.79 (12.74) | 13.01 (14.69) |
| Median (min; max) | 9.75 (0; 77) | 8.95 (0; 96) | 10.05 (0; 72) | 8.18 (0; 96) |
| T1 Gd + lesions, n | ||||
| Mean (SD) | 0.81 (2.67) | 1 (3.032) | 0.83 (2.46) | 0.92 (3.06) |
| Median (min; max) | 0 (0; 27) | 0 (0; 32) | 0 (0; 27) | 0 (0; 32) |
| GM volume, cm3 | ||||
| Mean (SD) | 766.52 (75.86) | 769.37 (77.85) | 767.18 (80.12) | 768.86 (80.28) |
| Median (min; max) | 763.29 (483.3; 975.9) | 775.36 (500.3; 950.2) | 762.21(485.3; 975.9) | 776.81 (500.3; 950.2) |
| WM volume, cm3 | ||||
| Mean (SD) | 772.52 (81.60) | 774.46 (83.13) | 774.42 (83.08) | 777.18 (86.75) |
| Median (min; max) | 766.52 (627.4; 1330.3) | 765.55 (622.6; 1326.0) | 769.81(627.4; 1319.8) | 768.22 (622.5; 1326.0) |
Abbreviations: BSL, baseline; DMT, disease‐modifying therapy; EDSS, Expanded Disability Status Scale; Gd+, gadolinium enhancing; M, month; SD, standard deviation.
The number of female participants in the two patient groups and in the treatment period was the only significant difference.
Details of the CLARITY study (ClinicalTrials.gov NCT00213135) and the results of the clinical trial have previously been reported elsewhere [10]. As a large confounding influence of pseudoatrophy was seen in a previous work assessing whole‐brain volume changes after treatment initiation with cladribine tablets [7], the analysis of the present study was performed: (i) on data from the period 0–6 months to assess presence of pseudoatrophy in GM and WM and (ii) on data from the period 6–24 months to assess treatment effects over time in these two brain compartments.
MRI analysis
As a first step, a quality control assessment of the scans was performed to exclude from the analysis those with artifacts, and those without full brain coverage. Further, a priori selection of the MRI was made by checking the intensity histogram of the T1W images, selecting only those with a clear peak‐distinction between GM and WM.
Pre‐gadolinium T1W MRI scans were analysed using a SIENA‐XL method [9], which allowed us to obtain percentage differences in both GM volume change (PGMVC) and WM volume change (PWMVC). Briefly, the method consisted of the following steps:
The voxel intensity within each lesional region on T1W imaging was replaced with that of the surrounding WM to avoid errors in GM and WM volume assessment due to the misclassification of voxels [11].
A subject‐specific T1W brain mask was created by merging all the T1W brain masks of that subject, each obtained with an optimized procedure for the T1W 3D images [9]. This subject‐specific brain mask was then re‐applied to each T1W image to obtain the brain image at each time point.
Finally, a pre‐segmentation intensity equalization step was performed. The intensity of all the T1W brain images of a subject were modified to impose similar histograms of pure voxels, that is, those voxels that had 100% probability of belonging to only one tissue.
The GM and WM volumes of each lesion‐filled intensity‐equalized T1W brain image were obtained by using the FAST algorithm [12] and the changes of a given tissue (i.e., GM or WM) between a pair of time points was expressed as percentage changes, that is, PGMVC or PWMVC.
Figure 1 shows an example of brain‐extracted 3D T1W images and the GM and WM segmentation maps of a patient at each time point (0, 6, 12 and 24 months).
FIGURE 1.
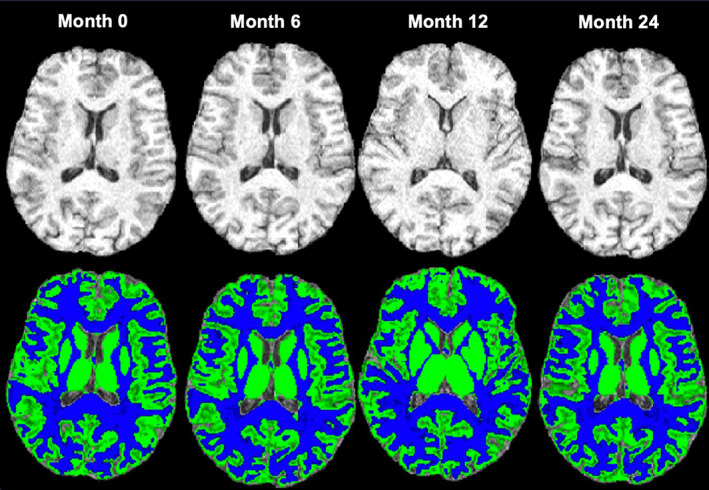
Example of brain extracted three‐dimensional T1‐weighted images (top row) and the segmentation map (bottom row) of the grey matter (GM; green) and white matter (WM; blue) of a patient at each time point (0, 6,12 and 24 months). The figure shows an example of axial T1‐weighted image quality of a patient on placebo and the segmentation maps of the GM and WM at each time point.
Statistical analysis
This was a post hoc, exploratory analysis of CLARITY in which p values were not adjusted for multiple testing and potential type I error inflation was not controlled. Differences in clinical and imaging features at baseline between the two treatment groups were assessed using a non‐parametric Mann–Whitney U‐test.
The slopes of PGMVC and PWMVC over the two interval times (0–6 months and 6–24 months) were independently compared between the two treatment arms (i.e., treated vs. placebo) with a mixed‐effect linear model. Changes over these time periods were not annualized. A treatment‐by‐time interaction term was used to assess whether the slope of changes was different according to treatment arm. A regression model with volumes as dependent variables and the interaction between treatment and other variables (i.e., baseline lesion volume, relapse rate/number in the year prior to inclusion, use of corticosteroids to treat relapses, new/enlarging lesions during follow‐up) as independent factors was used to assess the effect of these variables on volume changes in the 0–6‐month period. p values <0.05 were taken to indicate statistical significance.
Ethical standards
This post hoc study used data from the CLARITY study (NCT00213135), which was undertaken in compliance with the Declaration of Helsinki and standards of Good Clinical Practice according to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. At each centre, the relevant institutional review board or independent ethics committee reviewed and approved the trial protocol, patient information leaflet, informed consent forms, and investigator brochure. All patients provided written informed consent to participate in the trials.
RESULTS
Patient selection
From the original patient population of RRMS patients analysed in the brain volume exploratory analysis by De Stefano et al. [7], only patients treated with cladribine tablets 3.5 mg/kg (n = 336) and placebo (n = 338) were studied. From the whole group, the data of 142 patients (21%) were excluded because of poor GM/WM intensity contrast (n = 116), acquisition artifacts (n = 15), and not having full brain coverage (n = 11). In addition, 162 patients were excluded because of the missing time point at Month 24. Finally, 267 patients on treatment and 265 patients on placebo, and 184 patients on cladribine tablets 3.5 mg/kg on treatment and 186 patients on placebo were available for the analysis, respectively, from 0 to 6 months and from 6 to 24 months (Figure 2).
FIGURE 2.
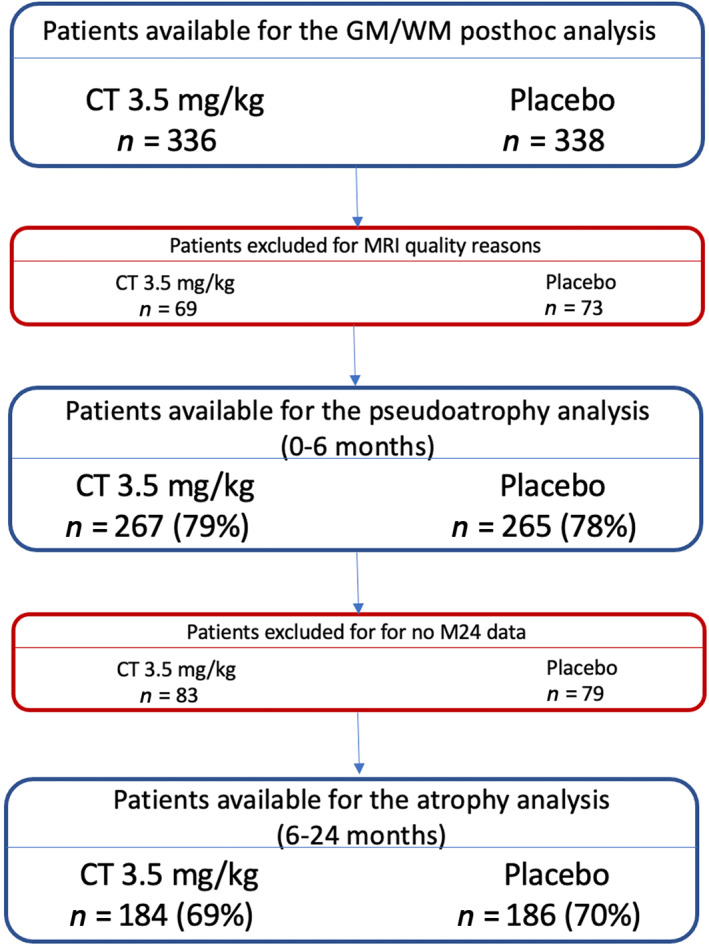
Flow diagram describing patient selection for the MRI analysis. Number of patients and the percentage of the cohort available for the analysis in the previous time interval are shown. Abbreviations: CT, cladribine tablets; GM, grey matter; M, Month; WM, white matter
Patients in the two treatment groups were uniformly distributed in both the pseudoatrophy and treatment periods. They did not differ with regard to demographic, clinical or MRI measures, except for a higher number of female patients in the treated than in the placebo group in the analysis of the the 6–24‐month period (p = 0.01).
Pseudoatrophy analysis
During the first 6 months, both GM and WM volumes were reduced in the group treated with cladribine tablets 3.5 mg/kg (PGMVC: −0.53 ± 0.10%; PWMVC: −0.49 ± 0.07%). Over the same period, the decreases in GM and WM were also observed in the placebo group (PGMVC: −0.25 ± 0.09%; PWMVC: −0.34 ± 0.07%).
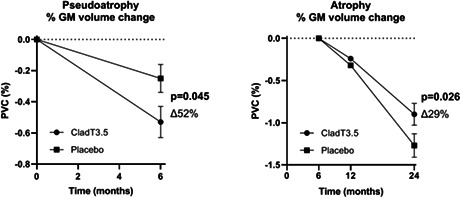
The decline in PGMVC was greater in patients treated with cladribine tablets 3.5 mg/kg than the placebo group (∆52%, p = 0.045), while no difference was found in PWMVC between the two groups (∆31%, p = 0.137; Figure 3).
FIGURE 3.
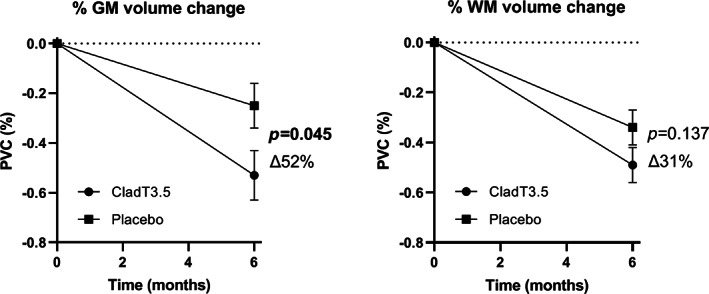
Pseudoatrophy analysis, 0–6 months, from 267 patients treated with cladribine 3.5 mg/kg and 265 patients treated with placebo. Pseudoatrophy was present both in grey matter (GM) and white matter (WM) during the first 6 months of therapy, with a significant difference in percentage volume change between cladribine and placebo for GM only. The ∆% change in the treated group and the standard errors are indicated in the figure. Abbreviations: CladT3.5, cladribine tablets 3.5 mg/kg; PVC, percentage volume change
Results did not change when considering only those patients who completed the whole duration of the trial (0–24 months; number on placebo: 186; number on cladribine treatment: 184): PGMVC: placebo −0.08%, cladribine treatment −0.51% (p = 0.008); PWMVC: placebo −0.51%, cladribine treatment −0.30% (p = 0.06).
A more evident difference of decline in GM volume at Month 6 between treatment and placebo was found in patients with higher lesion volume at baseline than in those with lower lesion volume at baseline (p for interaction = 0.049). Other variables (relapse rate/number in the year prior to inclusion, use of corticosteroids to treat relapses, new/enlarging lesions during follow‐up) did not influence the analysis.
Atrophy analysis
When considering the period from 6 to 24 months, brain volume loss was reduced in patients treated with cladribine tablets 3.5 mg/kg when compared with patients on placebo, with a significant difference in the GM only. PGMVC for cladribine tablets 3.5 mg/kg was −0.90 ± 0.13% versus placebo −1.27 ± 0.14% (∆29%, p = 0.026), while PWMVC for cladribine tablets 3.5 mg/kg was −0.32 ± 0.16% versus placebo −0.40 ± 0.13% (∆21%, p = 0.52; Figure 4).
FIGURE 4.
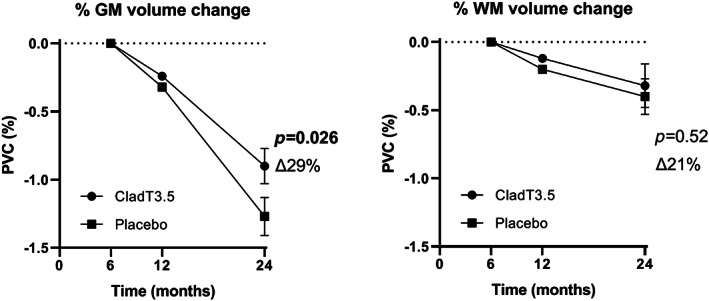
Atrophy analysis, 6–24 months, from 184 patients treated with cladribine 3.5 mg/kg and 186 patients treated with placebo. Brain volume loss from 6 to 24 months was reduced in patients treated with cladribine tablets 3.5 mg/kg body weight, with a significant difference in percentage volume change between cladribine and placebo for grey matter (GM) only. The ∆% change in the treated group and the standard errors are indicated in the figure. Abbreviations: CladT3.5, cladribine tablets 3.5 mg/kg; PVC, percentage volume change; WM, white matter
DISCUSSION
In this study, after a brief period of pseudoatrophy, GM volume loss proceeded at lower rates in RRMS patients treated with cladribine tablets than in those treated with placebo. In contrast, significant changes were not as evident in the WM compartment. These results add to previously reported data from the CLARITY study on the beneficial effect of treatment with cladribine tablets on whole‐brain volume loss [7], confirming that cladribine targets both focal inflammation and diffuse tissue damage, and pointing out that this is mostly beneficial with regard to the GM compartment and relative neurodegeneration occurring during the course of MS [13]. Indeed, a number of previous studies have shown that GM volume changes in the brain are more pronounced and clinically relevant than WM volume changes and that pronounced GM volume loss is associated with long‐term disability [4] and seems to explain physical and cognitive disability better than WM and whole‐brain atrophy [1, 4, 6]. Therefore, the predominant beneficial effect of cladribine on the GM may imply a lower risk of these patients of developing physical and cognitive disability progression.
Cladribine may modify the MS disease course through a variety of complex mechanisms. Although primarily considered a selective T‐ and B‐lymphocyte‐reducing agent, the influence of cladribine on the central nervous system (CNS) extends beyond lymphocyte reduction in vivo [14]. Recent in vitro studies showed that cladribine exerts immunomodulatory influences over innate and adaptive immunity, by reducing the levels of proinflammatory cytokines and decreasing immune cell infiltration into the CNS [13]. Such a mechanism of action may reduce soluble neurotoxic factors diffusing from the meningeal compartment, resulting in a beneficial anti‐inflammatory effect of the drug on the GM compartment.
The present study adds to other previous studies [15, 16, 17] showing that, in MS clinical trials, GM atrophy measures may be more informative than whole‐brain measures. However, the widespread use of GM atrophy metrics is mostly hindered by technical limitations as an accurate assessment of GM need to be based on a robust quantitative measurement of partial tissue volumes for each voxel (i.e., the amount occupied by a given tissue at each voxel). This estimation in multicentre trials is often biased by the differing quality of the acquired MRI scans, mostly due to hardware (e.g., scanner and coil) and software (e.g., image reconstruction) differences, despite the same acquisition parameter settings. In addition, this is particularly challenging when brain volume measures were not incorporated as secondary or tertiary outcomes and images with a suboptimal quality for atrophy measurement were acquired.
In line with previous observations made in the CLARITY study on whole‐brain volume loss [7], the treatment effect was observed only after the first 6 months. Indeed, patients treated with anti‐inflammatory treatments may show a rapid decrease in brain volume (i.e., pseudoatrophy) in the first months of treatment, in comparison to placebo, due to the resolution of inflammatory oedema and, probably also to changes in the volume of inflammatory cells and soluble neurotoxic factors [18, 19, 20]. A potential solution to this is to re‐baseline patients after 6 months [7, 21, 22, 23], although the assumption that pseudoatrophy occurs only during the first months of therapy is not necessarily valid, as the time course of pseudoatrophy is not completely understood and it might occur up to 1 year in some conditions [1]. Moreover, re‐baselining carries the risk of losing power because of the reduced time of observation on treatment.
Finally, in our study, the initial acceleration of volume decrease was found in both GM and WM brain compartments, but with a greater decline in GM. Although this is in line with some recent studies [18, 19], results have been contradictory in relation to the contribution of WM and GM compartments to pseudoatrophy [18]. For example, in highly active patients treated with immunoablation and autologous hematopoietic stem cell transplantation, the effect of the treatment on brain compartments over the first months was more accelerated in the GM than in the WM, resulting in a greater loss of volume over a shorter period [19]. Similarly, in RRMS patients treated with subcutaneous interferon beta‐1a 44 μg three times weekly, followed‐up with monthly MRI scans in the IMPROVE trial, pseudoatrophy was more pronounced in the GM than WM [18]. By contrast, in patients treated with natalizumab, the treatment‐associated pseudoatrophy mainly affected the WM [24]. One of the explanations for these contradictory results may be the extent of the anti‐inflammatory effect of the disease‐modifying therapies (DMTs) initiated in patients with different degrees of inflammatory activity at baseline. In addition, the distinct dynamics of the pseudoatrophy effect in patients treated with DMTs may be influenced by several factors, including the underlying immunological status of patients prior to and during treatment, disease duration and disease phenotype and the number of previously failed DMTs. Taken together, the results we present here and previous data suggest that pseudoatrophy can affect both GM and WM tissues and can be prevalent in the GM compartment in some cases.
An important limitation of the study lies in the relatively small sample size used with respect to the whole study population. This was due to the need to exclude a portion of suboptimal data (see Results). It is worth noting that this drop out was homogeneous between the two groups and driven by an a priori, rigid quality control performed on the original T1W images. However, we used the SIENA‐XL procedure, a new image‐processing method able to provide less biased and more precise estimates for GM volumes, thus overcoming some of the previous technical limitations in this measurement [9]. As reported previously [9] and confirmed here, this highly sensitive method may allow detection of subtle changes with relatively small sample sizes.
In conclusion, treatment with cladribine tablets in patients with RRMS in the CLARITY study reduced GM and WM atrophy in comparison with placebo treatment, with a significant effect on the GM, and this may contribute to lower risks of physical and cognitive disability. Further studies are needed to better detect the effect of DMTs for MS on GM damage and its impact on disease progression.
AUTHOR CONTRIBUTIONS
Rosa Cortese: formal analysis, investigation, writing – review and editing. Marco Battaglini: methodology, software, validation, formal analysis, writing – review and editing, visualization. Maria Pia Sormani: methodology, formal analysis, writing – review and editing. Ludovico Luchetti, Giordano Gentile and Maira Inderyas: software, validation, formal analysis, writing – review and editing. Nektaria Alexandri: resources, writing – review and editing. Nicola De Stefano: conceptualization, methodology, investigation, resources, writing original draft, supervision, funding acquisition.
FUNDING INFORMATION
This study was supported by Merck (CrossRef Funder ID: 10.13039/100009945).
CONFLICT OF INTEREST
Rosa Cortese was awarded a MAGNIMS‐ECTRIMS fellowship in 2019. Marco Battaglini has nothing to disclose. Maria Pia Sormani has received consulting fees from Biogen, Genzyme, GeNeuro, MedDay, Merck, Novartis, Roche and Teva. Ludovico Luchetti, Giordano Gentile, and Maira Inderyas have nothing to disclose. NA is an employee of Merck Healthcare KGaA, Darmstadt, Germany. Nicola De Stefano is a consultant for Biogen, Merck, Novartis, Sanofi‐Genzyme, Roche and Teva, has grants or grants pending from FISM and Novartis, is on the speakers' bureaus of Biogen, Merck, Novartis, Roche, Sanofi‐Genzyme and Teva, and has received travel funds from Merck, Novartis, Roche, Sanofi‐Genzyme and Teva.
ACKNOWLEDGEMENTS
Medical writing assistance was provided by inScience Communications, Springer Healthcare Ltd, UK, and supported by Merck Healthcare KGaA, Darmstadt, Germany. Open Access Funding provided by Universita degli Studi di Siena within the CRUI‐CARE Agreement.
Cortese R, Battaglini M, Sormani MP, et al. Reduction in grey matter atrophy in patients with relapsing multiple sclerosis following treatment with cladribine tablets. Eur J Neurol. 2023;30:179‐186. doi: 10.1111/ene.15579
DATA AVAILABILITY STATEMENT
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck, Darmstadt, Germany. All requests should be submitted in writing to the data sharing portal for the healthcare business of Merck, Darmstadt, Germany https://www.merckgroup.com/en/research/our‐approach‐to‐research‐and‐development/healthcare/clinical‐trials/commitment‐responsible‐data‐sharing.html. When the healthcare business of Merck has a co‐research, co‐development, or co‐marketing or co‐promotion agreement, or when the product has been out‐licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck will endeavour to gain agreement to share data in response to requests.
REFERENCES
- 1. Sastre‐Garriga J, Pareto D, Battaglini M, et al. MAGNIMS consensus recommendations on the use of brain and spinal cord atrophy measures in clinical practice. Nat Rev Neurol. 2020;16(3):171‐182. doi: 10.1038/s41582-020-0314-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol. 2014;75(1):43‐49. doi: 10.1002/ana.24018 [DOI] [PubMed] [Google Scholar]
- 3. Tur C, Moccia M, Barkhof F, et al. Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting. Nat Rev Neurol. 2018;14(2):75‐93. doi: 10.1038/nrneurol.2017.171 [DOI] [PubMed] [Google Scholar]
- 4. Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: a 20‐year follow‐up of patients with relapse onset of multiple sclerosis. Brain. 2008;131(Pt 3):808‐817. doi: 10.1093/brain/awm329 [DOI] [PubMed] [Google Scholar]
- 5. De Stefano N, Giorgio A, Battaglini M, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74(23):1868‐1876. doi: 10.1212/WNL.0b013e3181e24136 [DOI] [PubMed] [Google Scholar]
- 6. Calabrese M, Magliozzi R, Ciccarelli O, Geurts JJ, Reynolds R, Martin R. Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci. 2015;16(3):147‐158. doi: 10.1038/nrn3900 [DOI] [PubMed] [Google Scholar]
- 7. De Stefano N, Giorgio A, Battaglini M, et al. Reduced brain atrophy rates are associated with lower risk of disability progression in patients with relapsing multiple sclerosis treated with cladribine tablets. Mult Scler. 2018;24(2):222‐226. doi: 10.1177/1352458517690269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakamura K, Brown RA, Araujo D, Narayanan S, Arnold DL. Correlation between brain volume change and T2 relaxation time induced by dehydration and rehydration: implications for monitoring atrophy in clinical studies. NeuroImage: Clinical. 2014;6:166‐170. doi: 10.1016/j.nicl.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Battaglini M, Jenkinson M, De Stefano N. SIENA‐XL for improving the assessment of gray and white matter volume changes on brain MRI. Hum Brain Mapp. 2018;39(3):1063‐1077. doi: 10.1002/hbm.23828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giovannoni G, Comi G, Cook S, et al. A placebo‐controlled trial of oral cladribine for relapsing multiple sclerosis. New Engl J Med. 2010;362(5):416‐426. doi: 10.1056/NEJMoa0902533 [DOI] [PubMed] [Google Scholar]
- 11. Battaglini M, Jenkinson M, De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp. 2012;33(9):2062‐2071. doi: 10.1002/hbm.21344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45‐57. doi: 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]
- 13. Ceronie B, Jacobs BM, Baker D, et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J Neurol. 2018;265(5):1199‐1209. doi: 10.1007/s00415-018-8830-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacobs BM, Ammoscato F, Giovannoni G, Baker D, Schmierer K. Cladribine: mechanisms and mysteries in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2018;89(12):1266‐1271. doi: 10.1136/jnnp-2017-317411 [DOI] [PubMed] [Google Scholar]
- 15. Gaetano L, Häring DA, Radue EW, et al. Fingolimod effect on gray matter, thalamus, and white matter in patients with multiple sclerosis. Neurology. 2018;90(15):e1324‐e1332. doi: 10.1212/wnl.0000000000005292 [DOI] [PubMed] [Google Scholar]
- 16. Lee H, Nakamura K, Narayanan S, et al. Impact of immunoablation and autologous hematopoietic stem cell transplantation on gray and white matter atrophy in multiple sclerosis. Mult Scler. 2018;24(8):1055‐1066. doi: 10.1177/1352458517715811 [DOI] [PubMed] [Google Scholar]
- 17. Naismith RT, Bermel RA, Coffey CS, et al. Effects of ibudilast on MRI measures in the phase 2 SPRINT‐MS study. Neurology. 2021;96(4):e491‐e500. doi: 10.1212/wnl.0000000000011314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Stefano N, Giorgio A, Gentile G, et al. Dynamics of pseudo‐atrophy in RRMS reveals predominant gray matter compartmentalization. Ann Clin Transl Neurol. 2021;8(3):623‐630. doi: 10.1002/acn3.51302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee H, Narayanan S, Brown RA, et al. Brain atrophy after bone marrow transplantation for treatment of multiple sclerosis. Mult Scler. 2017;23(3):420‐431. doi: 10.1177/1352458516650992 [DOI] [PubMed] [Google Scholar]
- 20. Thebault S, Lee H, Bose G, et al. Neurotoxicity after hematopoietic stem cell transplant in multiple sclerosis. Ann Clin Transl Neurol. 2020;7(5):767‐775. doi: 10.1002/acn3.51045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arnold DL, Gold R, Kappos L, et al. Effects of delayed‐release dimethyl fumarate on MRI measures in the phase 3 DEFINE study. J Neurol. 2014;261(9):1794‐1802. doi: 10.1007/s00415-014-7412-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. New Engl J Med. 2016;376(3):209‐220. doi: 10.1056/NEJMoa1606468 [DOI] [PubMed] [Google Scholar]
- 23. Hauser SL, Bar‐Or A, Comi G, et al. Ocrelizumab versus interferon beta‐1a in relapsing multiple sclerosis. New Engl J Med. 2016;376(3):221‐234. doi: 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 24. Vidal‐Jordana A, Sastre‐Garriga J, Pérez‐Miralles F, et al. Brain volume loss during the first year of interferon‐beta treatment in multiple sclerosis: baseline inflammation and regional brain volume dynamics. J Neuroimaging. 2016;26(5):532‐538. doi: 10.1111/jon.12337 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck, Darmstadt, Germany. All requests should be submitted in writing to the data sharing portal for the healthcare business of Merck, Darmstadt, Germany https://www.merckgroup.com/en/research/our‐approach‐to‐research‐and‐development/healthcare/clinical‐trials/commitment‐responsible‐data‐sharing.html. When the healthcare business of Merck has a co‐research, co‐development, or co‐marketing or co‐promotion agreement, or when the product has been out‐licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck will endeavour to gain agreement to share data in response to requests.


