Abstract
Background and Aim
Impaired lung function in early infancy is associated with later wheeze and asthma, while fetal thoracic circumference (TC) predicts severity of neonatal lung hypoplasia. Exploring fetal origins of lung function in infancy, we aimed to determine if fetal TC in mid‐pregnancy was associated with infant lung function.
Methods
From the prospective Scandinavian general population‐based PreventADALL mother–child birth cohort, all 851 3‐month‐old infants with tidal flow‐volume measurements in the awake state and ultrasound fetal size measures at 18 (min–max 16–22) weeks gestational age were included. Associations between fetal TC and time to peak tidal expiratory flow to expiratory time (t PTEF/t E) were analyzed in linear regression models. To account for gestational age variation, we adjusted TC for simultaneously measured general fetal size, by head circumference (TC/HC), abdominal circumference (TC/AC), and femur length (TC/FL). Multivariable models were adjusted for maternal age, maternal asthma, pre‐pregnancy body mass index, parity, nicotine exposure in utero, and infant sex.
Results
The infants (47.8% girls) were born at mean (SD) gestational age of 40.2 (1.30) weeks. The mean (SD) t PTEF/t E was 0.39 (0.08). The mean (SD) TC/HC was 0.75 (0.04), TC/AC 0.87 (0.04), and TC/FL 4.17 (0.26), respectively. Neither TC/HC nor TC/AC were associated with infant t PTEF/t E while a week inverse association was observed between TC/FL and t PTEF/t E ( = −0.03, 95% confidence interval [−0.05, −0.007], p = 0.01).
Conclusion
Mid‐pregnancy fetal TC adjusted for fetal head or abdominal size was not associated with t PTEF/t E in healthy, awake 3‐month‐old infants, while a weak association was observed adjusting for fetal femur length.
Keywords: femur length, fetal size, infant lung function, infant sex, pregnancy, PreventADALL, respiratory function test, thoracic circumference, tidal breathing, t PTEF/t E , tidal flow‐volume loops, tidal volume, ultrasound
Abbreviations
- AC
abdominal circumference
- BMI
body mass index
- CI
confidence interval
- FL
femur length
- GA
gestational age
- HC
head circumference
- SD
standard deviation
- TC
thoracic circumference
- TFV
tidal flow‐volume
- t PTEF /t E
the ratio of time to peak tidal expiratory flow to expiratory time
- VT
tidal volume
1. INTRODUCTION
Impaired lung function in infancy predicts lower lung function values later in life 1 , 2 , 3 , 4 and is associated with an increased risk of wheeze and asthma, 3 , 5 , 6 , 7 indicating in utero origins of aberrant lung function development.
Lung development starts with lung budding in the fourth week of fetal life. 8 , 9 At 22–24 weeks' gestational age (GA), alveolar ducts with small amount of surfactant make gas exchange possible. 10 As the alveoli grow in size and number, the lung volume and function increase until a peak in early adulthood. 8 , 9
Lung function can be measured from birth, both in the awake and sleeping state, by tidal flow‐volume (TFV) loops. The TFV ratio of time to peak tidal expiratory flow to expiratory time (t PTEF/t E) correlates with forced exhalation outcomes that usually require sedation in infants, 7 , 11 , 12 making TFV loops a suitable measure of infant lung function. Exposure to maternal smoking in utero 13 , 14 and a family history of asthma 13 , 15 increase the risk of low t PTEF/t E, and t PTEF/t E values in the lower range are associated with airway hyper‐responsiveness and asthma. 1 , 7 , 16 Infant boys tend to have lower t PTEF/t E than girls, 9 , 17 but no clear cutoff value of t PTEF/t E indicates impaired lung function.
Lower tidal volume (VT) in infancy is associated with prematurity 18 and with more severe outcome in infants with lung hypoplasia. 19 While infant t PTEF/t E values decrease during the first weeks of life, tidal volume (VT) increases with age. 20
Fetal size and growth trajectories have been associated with respiratory health. 21 In a British cohort from the general population, children who in fetal life had large first‐trimester crown‐rump‐length, had higher lung function values at 5 and 10 years of age, as well as lower risk of wheeze and asthma. 22 , 23
Fetal thoracic circumference (TC) measured by ultrasound indicates fetal lung size. 24 Fetal TC, particularly in relation to abdominal circumference (AC), as the TC/AC ratio, predicts postnatal outcome in pregnancies with increased risk of neonatal lung hypoplasia and is important for prenatal diagnosis of this disease. 25 , 26 , 27 In older children and adults, TC has been positively related to lung function. 28 , 29
We hypothesize that fetal TC may be positively associated with infant lung function and we are not aware of previous studies relating fetal TC and future lung function in healthy infants.
The aim of this study was to determine if mid‐pregnancy fetal TC was associated with infant lung function, primarily measured as t PTEF/t E and secondarily as VT at 3 months of age, and if these potential associations differ by sex.
2. MATERIAL AND METHODS
2.1. Study design and setting
Three‐month‐old infants with available measurements of lung function as well as ultrasound information on mid‐pregnancy fetal size, in the prospective general population‐based mother–child birth cohort Preventing Atopic Dermatitis and ALLergies in Children (PreventADALL), 30 were included in the present study (Figure 1). Briefly, 2394 infants were antenatally recruited to the PreventADALL study in relation to the routine ultrasound examination at approximately 18 (range 15.7–22.7) gestational weeks in Oslo and the county of Østfold, Norway, and Stockholm, Sweden. Healthy singletons and twins, born from April 2015 to April 2017 at GA of at least 35 weeks, were included at birth and the first follow‐up after birth was at 3 months of age. To ensure independence of all participants, the second twin of all twin pairs was consequently excluded from the present study.
Figure 1.
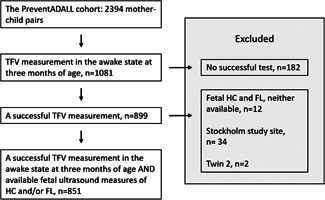
Study population. From the PreventADALL mother–child birth cohort, all 851 infants from the Oslo study site with available tidal flow‐volume (TFV) measurement in the awake state at 3 months of age as well as mid‐pregnancy ultrasound measurements of fetal head circumference (HC) and/or femur length (FL) were included. To ensure independence of all participants, twin 2 was consequently excluded.
Both parents signed an informed consent and the study was approved by the Regional Committee for Medical and Health Research Ethics in Norway (2014/518) and in Sweden (2014/2242‐31/4) and registered at clinicaltrial.gov, NCT02449850.
2.2. Participants
All 851 infants that had TFV measured in the awake state at 3 months of age and available mid‐pregnancy ultrasound measures including fetal head circumference (HC) and/or femur length (FL) were recruited in Oslo, the only PreventADALL study site measuring both TFV and fetal TC. The infants included in the present study were similar to the remaining 1543 infants in the PreventADALL cohort, except for somewhat higher frequency of breastfeeding at 3 months of age and fewer being exposed to nicotine beyond the first weeks of fetal life (Supporting Information: Table 1). Compared to the remaining infants, the mothers of included infants were older, had lower pre‐pregnancy body mass index (BMI), and were more often nulliparous and highly educated, in line with previously described study site differences in the PreventADALL study. 30
2.3. Methods
The ultrasound examination at approximately 18 weeks' GA was performed by specifically trained midwives at the participating hospitals, including HC, AC, and FL as routine measurements of general fetal size. TC was measured by tracing the bony thorax in the axial plane at the level of the four‐chamber view of the heart, using an ellipse along the ribs. One fetal medicine obstetrician trained all midwives measuring TC and ensured the quality of random samples of measurements.
Tidal flow‐volume (TFV) loops were obtained by trained study personnel at the 3‐month follow‐up. 31 Using the Eco Medics Exhalyzer® D equipment, TFV loops were collected while the infant was calm, in a supine position on caregivers' lap or in a stroller/bed. The ultrasonic flow head was connected to an appropriately sized face mask with a dead space reducer, a filtering spirette, and a CO2 adapter with capnostat in between. The mask was placed tightly over the infant's nose and mouth to avoid air leakage. After completion of all 3‐month visits, the TFV loops were visually evaluated with focus on shape and reproducibility, and technically successful loops were selected for analysis.
The mothers answered detailed electronic questionnaires on socio‐economy, lifestyle, and health, both during pregnancy and 3 months postpartum. Study personnel registered information about the delivery and the newborn infant from hospital registries, as well as infant weight and length at the 3‐month follow‐up, measured according to the study protocol.
2.4. Variables
2.4.1.
Primary outcome: The t PTEF/t E ratio as a continuous variable, and partitioned at four different cutoff values, <0.25 and below the 10th (<0.28), 25th (<0.34), and 50th (<0.39) percentiles, to identify infants with lung function in the lower ranges. See Supporting Information for further information.
Secondary outcomes: VT and VT adjusted for infant weight in kg (VT/kg).
Exposures: Fetal TC, relative to fetal HC (TC/HC), AC (TC/AC), and FL (TC/FL).
Covariates: Maternal age, maternal asthma, pre‐pregnancy BMI and parity as well as infant sex and in‐utero exposure to nicotine were found potentially relevant for analyzing the effect of fetal size on infant t PTEF/t E, identified using a Directed Acyclic Graph (DAG) 32 before statistical analyses (Supporting Information: Figure 1). To be considered as confounders of the association between fetal TC and infant lung function, variables had to potentially affect both the exposure and the outcome (Supporting Information).
All reported measures of fetal size were measured at the same ultrasound examination in mid‐pregnancy, the routine second‐trimester ultrasound at approximately 18 gestational weeks. This ultrasound examination also serves as the scan used for setting the date of pregnancy, with GA estimated by fetal HC 33 , 34 according to the clinical routines of our institution. GA estimated by FL is assumed to be equally reliable at mid‐pregnancy while it is less influenced by fetal sex. 35 Simultaneously measured, the measures of fetal size are strongly correlated (Supporting Information: Table 2), and therefore, we could not build regression models with TC alone as the main exposure, adjusting for GA at the time of ultrasound, based on either HC or FL, as a covariate. Instead, we choose to explore TC in relation to general fetal size measures, using the TC/HC, TC/AC, and TC/FL ratios as our exposures, where both TC/HC and TC/FL can be regarded as a proxy for TC adjusted for GA.
2.5. Statistical analysis
Continuous variables are presented as means with minimum–maximum (min–max) values, standard deviation (SD), or 95% confidence intervals (95% CIs). Categorical variables are presented as counts and percentages.
Associations between the fetal TC ratios and infant lung function were analyzed with linear regression and are presented with regression coefficients (β estimate ()), 95% CIs, and p values. For dichotomous outcomes, we used logistic regression models, presented with odds ratios (ORs), 95% CIs, and p values. Pearson correlation was used to evaluate the relationship between continuous variables, and R 2 describes the percentage of variability explained by the particular exposure. Supplementary analyses were performed to explore if potential associations between the fetal TC ratios and the continuous lung function outcomes were different when preterm infants (born with GA of 35.0–36.9 weeks) were excluded.
As both fetal size measures and infant lung function have been shown to differ between girls and boys, possible associations between fetal size and infant's lung function were stratified for sex. Differences between the included girls and boys were tested with the independent sample t‐test. p values <0.05 were regarded as significant.
IBM SPSS statistics version 27, RStudio version 4.0.3, and Microsoft Excel 2016 were used for statistical analyses.
3. RESULTS
The 851 infants (47.8% girls) were born at a mean (min–max) GA of 40.2 (35.0–42.4) weeks with a mean (min–max) birth weight of 3.6 (1.9–4.9) kg (Table 1). The mean (min–max) GA at the time of ultrasound examination was 18.7 (16.3–22.1) weeks. At the 3‐month follow‐up, their mean (min–max) age was 93 (74–131) days, weight was 6.3 (4.4–8.9) kg, and length was 61.9 (55.5–70.9) cm.
Table 1.
Baseline characteristics of the 851 infants included in the present study
| Included infants (n = 851) | ||
|---|---|---|
| Background characteristics | n | Count (%) or mean (SD) |
| Infant characteristics | ||
| Female | 851 | 407 (47.8) |
| Age in days (3 months) | 851 | 93 (7) |
| GA at birth (weeks) | 838 | 40.2 (1.3) |
| Born with GA <37.0 weeks | 838 | 16 (1.9) |
| Weight at 3 months (kg) | 847 | 6.3 (0.8) |
| Length at 3 months (cm) | 838 | 61.9 (2.2) |
| Birth weight (kg) | 849 | 3.6 (0.5) |
| Placenta weight (g) | 824 | 668 (131) |
| BW/PW ratio | 823 | 5.5 (1.0) |
| Caesarian birth | 851 | 137 (16.1) |
| Breastfeeding at 3 months of agea | 742 | 709 (95.6) |
| Respiratory distress or cough since birth | 742 | |
| No | 701 (94.5) | |
| Yes, once | 30 (4.0) | |
| Yes, more than once | 11 (1.5) | |
| Fetal measures (mid‐pregnancy) | ||
| GA at ultrasound, based on HC (weeks) | 848 | 18.7 (0.8) |
| HC (mm) | 848 | 157.2 (10.1) |
| TC (mm) | 727 | 117.5 (9.3) |
| AC (mm) | 846 | 135.1 (10.4) |
| FL (mm) | 846 | 28.2 (2.6) |
| Maternal characteristics | ||
| Age in years | 851 | 33.0 (3.9) |
| Parity (previous deliveries) | 851 | |
| Nullipara | 542 (63.7) | |
| Pre‐gestational BMI (kg/m2) | 831 | 22.8 (3.2) |
| Hypertensive disorders of pregnancy | 848 | 71 (8.4) |
| Any use of nicotine in pregnancy (smoking and/or snus) | 851 | 87 (10.2) |
| Smoking in pregnancy | 851 | 27 (3.2) |
| Current smoking at 18 weeks GA | 851 | 1 (0.1) |
| Snus in pregnancy | 851 | 61 (7.2) |
| Current snus at 18 weeks GA | 851 | 0 (0) |
| Family history of asthma,b no. (%) | ||
| Maternal asthma | 770 | 132 (17.1) |
| Paternal asthma | 775 | 104 (13.4) |
| Sociodemographic factors, no. (%) | ||
| Education | 767 | |
| High school only or less | 39 (5.1) | |
| Higher education <4 years | 205 (26.7) | |
| Higher education ≥4 years | 503 (65.6) | |
| PhD | 20 (2.6) | |
| Country of origin – mother | 770 | |
| Norway | 677 (87.9) | |
| Sweden | 22 (2.9) | |
| Other Nordic | 6 (0.8) | |
| Rest of the world | 65 (8.4) | |
Abbreviations: AC, abdominal circumference; BMI, body mass index; BW/PW ratio, birth weight to placenta weight ratio; FL, femur length; GA, gestational age; HC, head circumference; n, number; SD, standard deviation; TC, thoracic circumference.
Partly or exclusively breastfed at 3 months of age.
Doctor diagnosed asthma.
The mean (SD) t PTEF/t E was 0.39 (0.08), the 10th percentile was 0.28, while t PTEF/t E < 0.25 was observed in 46 infants (5.4%). The mean (SD) number of TFV loops per infant was 22 (14). Fetal size proportions and correlations in mid‐pregnancy are described in Supporting Information: Table 2 and fetal size measurements for girls and boys separately in Supporting Information: Table 3.
Fetal TC relative to head (TC/HC) and abdominal (TC/AC) circumferences were not significantly associated with infant t PTEF/t E, neither in univariable models nor when adjusted for maternal age, maternal asthma, pre‐pregnancy BMI, parity, infant sex and in‐utero exposure to nicotine (Tables 2a,b). However, we observed a weak, but significant inverse association between TC relative to fetal femur length (TC/FL) and t PTEF/t E as a continuous outcome, as well as with t PTEF/t E below the 10th, 25th, and 50th percentiles, both in univariable and adjusted models (Figure 2, Table 2c).
Table 2.
Associations between TC, by (a) TC/HC, (b) TC/AC, and (c) TC/FL, and infant t PTEF/t E assessed in univariable and multivariable regression models
| a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| t PTEF/t E ~ TC/HC | Univariable regression (n = 726) | Multivariable regression (n = 726) | |||||||
| R 2 |
|
95% CI | p value | R 2 |
|
95% CI | p value | ||
| Continuous t PTEF/t E | 0.002 | −0.11 | −0.27 to 0.05 | 0.191 | 0.017 | −0.12 | −0.29 to 0.04 | 0.147 | |
| t PTEF/t E < 0.25 | −3.53 | −11.73 to 4.94 | 0.407 | −3.86 | −12.26 to 4.75 | 0.373 | |||
| t PTEF/t E < 10th percentile | −4.05 | −10.32 to 2.36 | 0.212 | −4.38 | −10.76 to 2.10 | 0.181 | |||
| t PTEF/t E < 25th percentile | −3.30 | −7.78 to 1.18 | 0.148 | −3.71 | −8.28 to 0.86 | 0.111 | |||
| t PTEF/t E < 50th percentile | −2.58 | −6.54 to 1.36 | 0.201 | −2.71 | −6.73 to 1.28 | 0.184 | |||
| b) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| t PTEF / t E ~ TC/AC | Univariable regression (n = 725) | Multivariable regression (n = 725) | |||||||
| R 2 |
|
95% CI | p value | R 2 |
|
95% CI | p value | ||
| Continuous t PTEF/t E | 0.001 | −0.05 | −0.19 to 0.08 | 0.449 | 0.015 | −0.05 | −0.19 to 0.09 | 0.478 | |
| t PTEF/t E < 0.25 | −1.71 | −8.60 to 5.39 | 0.631 | −1.49 | −8.42 to 5.60 | 0.676 | |||
| t PTEF/t E < 10th percentile | −3.30 | −8.53 to 2.03 | 0.221 | −3.23 | −8.53 to 2.15 | 0.235 | |||
| t PTEF/t E < 25th percentile | −2.02 | −‐5.76 to 1.72 | 0.288 | −1.94 | −5.73 to 1.87 | 0.317 | |||
| t PTEF/t E < 50th percentile | −0.87 | −4.16 to 2.41 | 0.604 | −0.77 | −4.08 to 2.54 | 0.648 | |||
| c) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| t PTEF / t E ~ TC/FL | Univariable regression (n = 724) | Multivariable regression (n = 724) | |||||||
| R 2 |
|
95% CI | p value | R 2 |
|
95% CI | p value | ||
| Continuous t PTEF/t E | 0.010 | −0.03 | −0.06 to −0.01 | 0.006 | 0.023 | −0.03 | −0.05 to −0.01 | 0.010 | |
| t PTEF/t E < 0.25 | −0.75 | −1.90 to 0.43 | 0.210 | −0.65 | −1.84 to 0.57 | 0.289 | |||
| t PTEF/t E < 10th percentile | −0.99 | −1.88 to −0.10 | 0.030 | −0.94 | −1,84 to −0.03 | 0.041 | |||
| t PTEF/t E < 25th percentile | −0.78 | −1.41 to −0.15 | 0.016 | −0.74 | −1.39 to −0.10 | 0.024 | |||
| t PTEF/t E < 50th percentile | −0.84 | −1.41 to −0.28 | 0.004 | −0.81 | −1.38 to −0.25 | 0.005 | |||
Note: All multivariable models were adjusted for maternal age, maternal asthma, pre‐pregnancy BMI, parity, infant sex, and in‐utero exposure to nicotine.
Abbreviations: AC, abdominal circumference; CI, confidence interval; FL, femur length; HC, head circumference; R 2, the percentage of variation explained by the exposure(s); TC, thoracic circumference; t PTEF/t E, the ratio of time to peak tidal expiratory flow to expiratory time; , the regression coefficient (β estimate).
Figure 2.
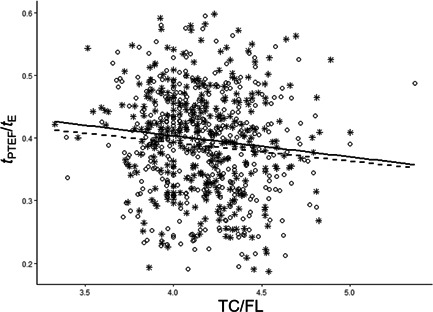
The variation in infant t PTEF/t E in relation to fetal TC/FL ratio. In the unadjusted model, the TC/FL ratio explained 1.0% of the variation in t PTEF/t E in all infants (R 2 = 0.010). R 2 for girls was 0.011 and R 2 for boys was 0.009. TC/FL, fetal thoracic circumference relative to femur length, a proxy for TC adjusted for gestational age at the time of ultrasound; t PTEF/t E, the ratio of time to peak tidal expiratory flow to expiratory time; Girls are marked with stars triangles and a whole regression line, boys are marked with dots and a broken regression line.
Fetal TC/HC and TC/FL were positively associated with infant VT, while no association was observed between TC/AC and VT (Table 3). The association between TC/HC and VT was only significant when adjusted for relevant covariates, while TC/FL was significantly associated with VT in both univariable and adjusted regression analyses. No significant associations were observed between the fetal TC ratios and VT/kg.
Table 3.
Associations between fetal TC, by (a) TC/HC, (b) TC/AC, and (c) TC/FL, and tidal volume, by infant VT and VT/kg
| a) | ||||||
|---|---|---|---|---|---|---|
| TC/HC | ||||||
| n | R 2 |
|
95% CI | p value | ||
| V T | ||||||
| Univariable | 726 | 0.004 | 22.57 | −2.49 to 47.63 | 0.077 | |
| + infant sex | 0.016 | 26.41 | 1.34–51.47 | 0.039 | ||
| Multivariable | 0.032 | 26.39 | 1.34–51.44 | 0.039 | ||
| V T /kg | ||||||
| Univariable | 722 | 0.001 | 1.80 | −2.30 to 5.90 | 0.389 | |
| + infant sex | 0.005 | 1.45 | −2.67 to 5.57 | 0.489 | ||
| Multivariable | 0.018 | 1.37 | −2.75 to 5.50 | 0.514 | ||
| b) | ||||||
|---|---|---|---|---|---|---|
| TC/AC | ||||||
| n | R 2 |
|
95% CI | p value | ||
| V T | ||||||
| Univariable | 725 | 0.001 | 10.29 | −10.61 to 31.19 | 0.334 | |
| + infant sex | 0.011 | 11.35 | −9.48 to 32.17 | 0.285 | ||
| Multivariable | 0.027 | 10.73 | −10.07 to 31.53 | 0.311 | ||
| V T /kg | ||||||
| Univariable | 721 | <0.001 | 0.68 | −2.74 to 4.11 | 0.696 | |
| + infant sex | 0.004 | 0.56 | −2.86 to 3.99 | 0.746 | ||
| Multivariable | 0.017 | 0.43 | −3.00 to 3.85 | 0.807 | ||
| c) | ||||||
|---|---|---|---|---|---|---|
| TC/FL | ||||||
| n | R 2 |
|
95% CI | p value | ||
| V T | ||||||
| Univariable | 724 | 0.007 | 3.95 | 0.44–7.47 | 0.028 | |
| + infant sex | 0.016 | 3.87 | 0.37–7.37 | 0.030 | ||
| Multivariable | 0.031 | 3.56 | 0.06–7.05 | 0.046 | ||
| V T /kg | ||||||
| Univariable | 720 | 0.001 | 0.24 | −0.33 to 0.82 | 0.407 | |
| + infant sex | 0.006 | 0.25 | −0.32 to 0.83 | 0.389 | ||
| Multivariable | 0.019 | 0.23 | −0.35 to 0.80 | 0.440 | ||
Note: The association between fetal TC and tidal volume was assessed in univariable models, models only adjusted for infant sex, and multivariable models adjusted for maternal age, maternal asthma, pre‐pregnancy BMI, parity, infant sex, and in‐utero exposure to nicotine.
Abbreviations: AC, abdominal circumference; CI, confidence interval; FL, femur length; HC, head circumference; n, number of infants included in the respective model, R 2, the percentage of variation explained by the exposure(s); TC, thoracic circumference; VT, tidal volume; , the regression coefficient (β estimate).
As shown in Supporting Information: Table 4, the positive association between both fetal TC/HC and TC/FL and infant VT became stronger when preterm infants (i.e., GA at birth 35.0–36.9 weeks) were excluded, while the weak inverse association between TC/FL and t PTEF/t E remained similar (results not shown).
Fetal TC/HC and infant t PTEF/t E were significantly higher in girls than boys, while TC/AC and TC/FL were similar in both sexes (Supporting Information: Table 5). Infant sex was weakly (R 2 = 0.007) but significantly associated with continuous t PTEF/t E in univariable regression ( = 0.014, 95% CI [0.003, 0.03], p = 0.016), while no significant effect of infant sex was observed on the associations between the fetal TC ratios and t PTEF/t E (results not shown). When stratified for sex, the weak inverse association observed between TC/FL and t PTEF/t E remained significant among girls, although only in the univariable model, with R 2 for the adjusted model being higher in girls than in boys (Table 4).
Table 4.
The association between mid‐pregnancy fetal TC, by TC/HC (n = 726), TC/AC (n = 725), and TC/FL (n = 724), and infant t PTEF/t E in girls and boys separately
| Girls | Boys | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | R 2 |
|
95% CI | p value | n | R 2 |
|
95% CI | p value | |||
| t PTEF / t E ~ TC/HC | ||||||||||||
| Univariable | 344 | 0.005 | −0.17 | −0.41 to 0.08 | 0.178 | 382 | 0.002 | −0.09 | −0.31 to 0.13 | 0.426 | ||
| Multivariable | 0.038 | −0.17 | −0.42 to 0.08 | 0.185 | 0.017 | −0.09 | −0.32 to 0.13 | 0.422 | ||||
| t PTEF / t E ~ TC/AC | ||||||||||||
| Univariable | 344 | <0.001 | −0.004 | −0.20 to 0.19 | 0.965 | 381 | 0.003 | −0.10 | −0.29 to 0.08 | 0.267 | ||
| Multivariable | 0.033 | −0.001 | −0.20 to 0.20 | 0.989 | 0.019 | −0.10 | −0.29 to 0.09 | 0.277 | ||||
| t PTEF / t E ~ TC/FL | ||||||||||||
| Univariable | 343 | 0.011 | −0.03 | −0.07 to −0.00 | 0.048 | 381 | 0.009 | −0.03 | −0.06 to 0.00 | 0.063 | ||
| Multivariable | 0.045 | −0.03 | −0.07 to −0.00 | 0.058 | 0.023 | −0.03 | −0.06 to 0.01 | 0.105 | ||||
Note: All multivariable models were adjusted for maternal age, maternal asthma, pre‐pregnancy BMI, parity, infant sex, and in‐utero exposure to nicotine.
Abbreviations: AC, abdominal circumference; CI, confidence interval; FL, femur length; HC, head circumference; n, number; R 2, the percentage of variation explained by the exposure(s); TC, thoracic circumference; t PTEF/t E, the ratio of time to peak tidal expiratory flow to expiratory time; , the regression coefficient (β estimate).
The mean VT at 3 months of age was higher in boys, while girls had significantly higher VT/kg (Supporting Information: Table 5). Adjusting for infant sex had a significant impact on the association between TC/HC and VT, and the significant association between TC/FL and VT became somewhat stronger when infant sex was included in the model (Table 3). No significant association was observed between the fetal TC ratios and VT or VT/kg when stratified for sex (not shown).
4. DISCUSSION
In healthy 3‐month‐old infants, no significant association was observed between mid‐pregnancy fetal TC relative to head and abdominal circumferences and the t PTEF/t E ratio, while a weak inverse association between fetal TC relative to femur length and t PTEF/t E was observed. The associations between the fetal TC ratios and t PTEF/t E were similar in girls and boys. Fetal TC relative to head circumference and femur length, but not relative to abdominal circumference, were weakly associated with tidal volume at 3 months of age, while no association between the fetal TC ratios and VT/kg was observed.
The lack of association between the mid‐pregnancy fetal ratios of thoracic relative to head and abdominal circumferences and infant t PTEF/t E at 3 months of age, as well as the weak inverse association between fetal TC relative to FL and infant t PTEF/t E, are to the best of our knowledge novel findings. However, a positive correlation between TC and lung function, measured simultaneously, has been reported both in preschool children 29 and in young adults. 28 The observed inverse association between fetal TC/FL and infant t PTEF/t E implies that fetuses with smaller TC in relation to FL had higher t PTEF/t E in infancy. Fetal FL is regarded as a proxy for fetal length 36 , 37 and postnatally, body length predicts lung function. 3 , 17 , 28 , 29 However, we cannot rule out that a smaller TC contributes to the observed association, as TC/FL and t PTEF/t E appeared to be closer associated than was the case for AC/FL and t PTEF/t E (Supporting Information: Table 6). Our results are partly in line with those of Turner et al. who found no association between second trimester FL and lung function at 5 or 10 years of age, but a positive association between first‐trimester crown‐rump length and childhood lung function. 22 , 23 Similarly, in the Dutch Generation R study, higher estimated fetal weight in the second and third trimesters was associated with higher lung function at 10 years of age, while femur length, used as a proxy for fetal length, was not. 38
Although the observed association between fetal TC/FL and infant t PTEF/t E was statistically significant, the R 2 was low, indicating that fetal TC/FL alone only explains 1.0% of the total variation in infant t PTEF/t E, and 2.3% when adjusted for relevant covariates. It is therefore uncertain if the association observed between fetal TC/FL and infant t PTEF/t E is of any clinical relevance.
The lack of a clear association between the fetal thoracic size in mid‐pregnancy and infant t PTEF/t E may not be surprising. Fetal TC is a structural measure, previously mostly related to lung volume, while infant t PTEF/t E is a measure of airway function. Including infants who are generally healthy, limits the possibility to assess the potential impact of aberrant development leading to lung disease. One may therefore speculate that the room for normal variation in lung development at this early stage might be limited. The upper airways, together with fetal breathing movements, ensure expansion of the fluid‐filled fetal lungs, which is necessary for normal growth and maturation of the lungs, 8 , 39 while the more rapid growth of respiratory bronchioles and alveoli during the third trimester 9 along with increased fetal general growth may provide greater variation also in fetal thoracic size. 33 It is not clear if third‐trimester fetal TC, or TC measured at birth, might potentially have a more pronounced relation with infant lung function.
The positive association between the fetal TC ratios and infant VT, in line with fetal TC reflecting fetal lung size, 9 , 24 is to the best of our knowledge a novel finding in healthy infants. Both TC/HC and TC/FL were weakly positively associated with VT at 3 months of age, while no association was observed between TC/AC and VT in our cohort. However, the association between fetal TC/AC and postnatal outcome is well documented in neonates with conditions increasing the risk of lung hypoplasia. 25 , 26 , 27 The fetal TC ratios were not associated with VT adjusted for infant weight (VT/kg), suggesting that the effect of fetal thoracic size on VT may be mediated through the weight of the infant.
In supplementary analyses, excluding infants born before 37.0 gestational weeks revealed a somewhat stronger positive association between both TC/HC and TC/FL and VT, while the weak inverse association between TC/FL and t PTEF/t E remained similar to when all infants were included, regardless of GA at birth. Shorter time for in‐utero development of alveoli, 8 together with a smaller body size, may cause lower VT in otherwise healthy preterm compared to term infants. Therefore, including both term and preterm infants in the regression models may reduce the visibility of the association between mid‐pregnancy fetal thoracic size and infant VT.
The different associations between the fetal TC ratios and the t PTEF/t E versus VT may not be unexpected. Postnatal airway caliber is positively associated with body length 7 , 40 and our results show that a larger TC relative to HC and FL predicts larger VT in infancy. Although the effect sizes are small, smaller fetal TC relative to FL might reflect smaller lung size relative to airway caliber, and subsequently somewhat higher t PTEF/t E in infancy. To further explore our findings suggesting that a smaller fetal TC relative to femur length may be representing relatively larger airway caliber for lung size, resulting in higher flow rates in infancy, even larger cohort studies are needed. Future studies on the PreventADALL cohort may suggest if fetuses with lower TC/FL ratio in mid‐pregnancy will have an increased risk of developing obstructive lung diseases in postnatal life.
The association between the fetal TC ratios and infant t PTEF/t E was similar in girls and boys although, when adjusted for covariates, TC/FL explained more of the total variation in t PTEF/t E in girls. While the higher TC/HC in girls may largely be explained by their smaller HC compared to boys, 34 mid‐pregnancy fetal FL is probably unrelated to sex. 33 As TC relative to AC and FL was similar among both sexes our results suggest a minimal impact of sex on fetal TC. Together with a higher t PTEF/t E in girls, in line with other studies, 3 , 4 , 9 , 17 the sex‐related bias introduced in the TC/HC ratio could possibly weaken a potential inverse association between TC/HC and t PTEF/t E.
The significantly higher VT and lower VT/kg in boys compared to girls, reflecting their larger body size, is in line with other studies. 5 , 9 , 18 While both TC/HC and TC/FL were significantly associated with VT when adjusted for sex, no association was observed when analyzed in girls and boys separately, possibly explained by reduced power.
Higher t PTEF/t E and VT/kg in girls are likely to reflect sex differences in fetal lung and airway development. Previous studies have shown larger lung volumes and more respiratory bronchioles at birth in boys, preparing for their generally larger thoracic size in adulthood, while surfactant production matures earlier in girls, enhancing small airway patency. 9 Relatively larger airways for lung size result in higher flow rates in girls compared to boys 6 , 9 , 17 and although the TC/FL ratio explained more of the total variation in t PTEF/t E in girls compared to boys, our study population was too small to conclude on potentially different associations between fetal thoracic size and lung function in girls and boys separately.
4.1. Strengths and limitations
A prospective design and the large group of healthy infants with comprehensive information on mid‐pregnancy fetal size and awake‐state lung function measurements are among the strengths of our study. Although few participants were of non‐Scandinavian origin and some maternal characteristics were related to the study site, we believe that our findings are representative for the general population, possibly limited to Caucasians. One fetal medicine obstetrician trained all midwives in measuring fetal TC and lung function measurements were analyzed according to pre‐standardized criteria.
First‐trimester ultrasound was not a routine examination in Norway at the time of recruitment and the determination of GA by second‐trimester biometric measures only, is a limitation of the study. Differently determined GA has been suggested as an explanation of inconsistent results on associations between fetal biometric measurements and respiratory outcomes. 41 As our participants at large were healthy very few infants had low t PTEF/t E values, having excluded severe fetal and/or neonatal disease and preterm birth before 35.0 gestational weeks due to exclusion criteria of the PreventADALL study. This limits the possibility to identify potential associations between fetal size and lung function during aberrant lung development involving early lung or airway pathology. On the other hand, the present study focused on exploring possible associations between lung and airway development among presumably healthy infants. As several exposures representing fetal size and outcomes reflecting infant lung function were included in our regression models, not correcting for multiple testing may also be a limitation.
5. CONCLUSION
Mid‐pregnancy fetal TC adjusted for fetal head or abdominal size was not associated with infant t PTEF/t E in healthy awake infants at 3 months of age, while a weak inverse association between fetal TC/FL and t PTEF/t E was observed. Fetal TC relative to HC and FL was positively associated with VT, probably mediated through infant weight, as the TC ratios were not associated with VT/kg. The association between fetal thoracic size and lung function was largely similar among girls and boys.
AUTHOR CONTRIBUTIONS
Hrefna K. Gudmundsdóttir: Conceptualization, investigation, writing—original draft, methodology, visualization, writing—review & editing, formal analysis, data curation. Katarina Hilde: Writing—review & editing, methodology, vsualization. Karen E. S. Bains: Investigation, writing—review & editing, data curation. Martin Färdig: Investigation, writing—review & editing, data curation. Guttorm Haugen: Conceptualization, methodology, writing—review & editing, supervision, visualization, project administration, funding acquisition. Marissa LeBlanc: Writing—review & editing, methodology, supervision, visualization. Live S. Nordhagen: Investigation, writing—review & editing. Björn Nordlund: Writing—review & editing, project administration, funding acquisition. Eva M. Rehbinder: Conceptualization, methodology, visualization, writing—review & editing, project administration, supervision, funding acquisition. Håvard O. Skjerven: Writing—review & editing, visualization, project administration. Anne C. Staff: Methodology, visualization, writing—review & editing, project administration, funding acquisition, conceptualization. Riyas Vettukattil: Software, data curation, writing—review & editing. Karin C. L. Carlsen: Conceptualization, funding acquisition, methodology, visualization, writing—review & editing, project administration, Supervision.
CONFLICTS OF INTEREST
Marissa LeBlanc reports a speaking fee from MSD unrelated to the content of this study, Eva Maria Rehbinder has received honoraria for lectures from Sanofi Genzyme, Leo Pharma, Novartis, Norwegian Psoriasis and Eczema Association, and the Norwegian Asthma and Allergy Association and Karin C. L. Carlsen reports that her institution has received honorarium and travel costs from Thermo Fisher Scientific for international symposium participation. The remaining authors have no conflicts of interest to declare.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors sincerely thank all study participants and their families, and all midwives performing mid‐pregnancy fetal ultrasound examinations at the participating hospitals. The authors thank all those who contributed in planning of the study, recruitment of participants, clinical examinations, and biological sampling, as well as those facilitating and running the study, especially: Hilde Aaneland (Division of Paediatric and Adolescent Medicine, Oslo University Hospital, Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway), Anna Asarnoj (Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden; Astrid Lindgren Children's Hospital, Karolinska University Hospital, Stockholm, Sweden), Ann Berglind (Astrid Lindgren Children's Hospital, Karolinska University Hospital, Stockholm, Sweden), Oda C. Lødrup Carlsen (Division of Paediatric and Adolescent Medicine, Oslo University Hospital, Oslo, Norway), Åshild Wik Despriée (Faculty of Health, VID Specialized University, Oslo, Norway), Kim M. A. Endre (Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway; Department of Dermatology and Vaenerology, Oslo University Hospital, Oslo, Norway), Thea Aspelund Fatnes (Division of Paediatric and Adolescent Medicine, Oslo University Hospital, Oslo, Norway), Peder A. Granlund (Division of Paediatric and Adolescent Medicine, Oslo University Hospital, Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway), Berit Granum (Department of Environmental Health, Norwegian Institute of Public Health, Oslo, Norway), Malén Gudbrandsgard (Division of Paediatric and Adolescent Medicine, Oslo University Hospital, Oslo, Norway), Sandra Götberg (Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden; Astrid Lindgren Children's Hospital, Karolinska University Hospital, Stockholm, Sweden), Gunilla Hedlin (Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden; Astrid Lindgren Children's Hospital, Karolinska University Hospital, Stockholm, Sweden), Christine M. Jonassen (Genetic Unit, Center for Laboratory Medicine, Østfold Hospital Trust, Kalnes, Norway; Faculty of Chemistry, Biotechnology and Food Science, Norwegian University of Life Sciences, Ås, Norway), Mari Rønning Kjendsli (Division of Paediatric and Adolescent Medicine, Oslo University Hospital, Oslo, Norway), Ina Kreyberg (Division of Paediatric and Adolescent Medicine, Oslo University Hospital, Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway), Linn Landro (Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway; Department of Dermatology and Vaenerology, Oslo University Hospital, Oslo, Norway), Marie Nordsletten (Division of Obstetrics and Gynecology, Oslo University Hospital, Oslo, Norway), Carina M. Saunders (Division of Paediatric and Adolescent Medicine, Oslo University Hospital, Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway), Natasha Sedergren (Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden), Cilla Söderhäll (Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden; Astrid Lindgren Children's Hospital, Karolinska University Hospital, Stockholm, Sweden), Liv Julie Sørdal (Division of Paediatric and Adolescent Medicine, Oslo University Hospital, Oslo, Norway), Sandra Ganrud Tedner (Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden; Astrid Lindgren Children's Hospital, Karolinska University Hospital, Stockholm, Sweden), Ellen Tegnerud (Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden), Magdalena R. Værnesbranden (Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway; Department of Obstetrics and Gynecology, Østfold Hospital Trust, Kalnes, Norway), Johanna Wiik (Department of Obstetrics and Gynecology, Østfold Hospital Trust, Kalnes, Norway; Department of Obstetrics and Gynecology, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden; Department of Obstetrics and Gynecology, Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden) and in memoriam Kai‐Håkon Carlsen (Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway). This study is a part of a PhD project and Hrefna Katrín Gudmundsdóttir has received funding as a doctoral research fellow from the University of Oslo, Norway. The PreventADALL study was supported by a number of public and private funding bodies with no influence on design, conduct, or analyses. The PreventADALL study has received funding from the following sources: The Regional Health Board South East, The Norwegian Research Council, Oslo University Hospital, The University of Oslo, Health and Rehabilitation Norway, The Foundation for Healthcare and Allergy Research in Sweden – Vårdalstiftelsen, The Swedish Asthma‐ and Allergy Association's Research Foundation, The Swedish Research Council – the Initiative for Clinical Therapy Research, The Swedish Heart‐Lung Foundation, SFO‐V Karolinska Institutet, Østfold Hospital Trust, by unrestricted grants from the Norwegian Association of Asthma and Allergy, The Kloster foundation, Thermo‐Fisher, Uppsala, Sweden (through supplying allergen reagents), Fürst Medical Laboratory, Oslo, Norway (through performing IgE analyses), Norwegian Society of Dermatology and Venerology, Arne Ingel's legat, Region Stockholm (ALF‐project and individual grants), Forte, Swedish Order of Freemasons Foundation Barnhuset, The Sven Jerring Foundation, The Hesselman foundation, The Magnus Bergwall foundation, The Konsul Th C Bergh's Foundation, The Swedish Society of Medicine, The King Gustaf V 80th Birthday Foundation, KI grants, The Cancer‐ and Allergy Foundation, The Pediatric Research Foundation at Astrid Lindgren Children's Hospital, The Samaritan Foundation for Pediatric research, Barnestiftelsen at Oslo University Hospital, Roche, The Frithjof Nansen Institute. The study was performed within ORAACLE (the Oslo Research Group of Asthma and Allergy in Childhood; the Lung and Environment).
Gudmundsdóttir HK, Hilde K, Bains KES, et al. Fetal thoracic circumference in mid‐pregnancy and infant lung function. Pediatric Pulmonology. 2023;58:35‐45. 10.1002/ppul.26153
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are may be available on request from the study PI. The study is still ongoing, and data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Haland G, Carlsen KC, Sandvik L, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355(16):1682‐1689. [DOI] [PubMed] [Google Scholar]
- 2. den Dekker HT, Sonnenschein‐van der Voort A, de Jongste JC, et al. Early growth characteristics and the risk of reduced lung function and asthma: a meta‐analysis of 25,000 children. J Allergy Clin Immunol. 2016;137(4):1026‐1035. [DOI] [PubMed] [Google Scholar]
- 3. Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non‐selective longitudinal cohort study. Lancet. 2007;370(9589):758‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turner S, Fielding S, Mullane D, et al. A longitudinal study of lung function from 1 month to 18 years of age. Thorax. 2014;69(11):1015‐1020. [DOI] [PubMed] [Google Scholar]
- 5. Dezateux C, Stocks J, Dundas I, Fletcher ME. Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med. 1999;159(2):403‐410. [DOI] [PubMed] [Google Scholar]
- 6. Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319(17):1112‐1117. [DOI] [PubMed] [Google Scholar]
- 7. Guerra S, Lombardi E, Stern DA, et al. Fetal origins of asthma: A longitudinal study from birth to age 36 years. Am J Respir Crit Care Med. 2020;202(12):1646‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1(9):728‐742. [DOI] [PubMed] [Google Scholar]
- 9. Boezen HM, Jansen DF, Postma DS. Sex and gender differences in lung development and their clinical significance. Clin Chest Med. 2004;25(2):237‐245. [DOI] [PubMed] [Google Scholar]
- 10. Schittny JC. Development of the lung. Cell Tissue Res. 2017;367(3):427‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morris MJ, Lane DJ. Tidal expiratory flow patterns in airflow obstruction. Thorax. 1981;36(2):135‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hevroni A, Goldman A, Blank‐Brachfeld M, Abu Ahmad W, Ben‐Dov L, Springer C. Use of tidal breathing curves for evaluating expiratory airway obstruction in infants. J Asthma. 2018;55(12):1331‐1337. [DOI] [PubMed] [Google Scholar]
- 13. Stick SM, Burton PR, Gurrin L, Sly PD, LeSouëf PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet. 1996;348(9034):1060‐1064. [DOI] [PubMed] [Google Scholar]
- 14. Lødrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J. 1997;10(8):1774‐1779. [DOI] [PubMed] [Google Scholar]
- 15. de Gouveia Belinelo P, Collison AM, Murphy VE, et al. Maternal asthma is associated with reduced lung function in male infants in a combined analysis of the BLT and BILD cohorts. Thorax. 2021;76(10):996‐1001. [DOI] [PubMed] [Google Scholar]
- 16. Young S, Arnott J, Le Souef PN, Landau LI. Flow limitation during tidal expiration in symptom‐free infants and the subsequent development of asthma. J Pediatr. 1994;124(5 Pt 1):681‐688. [DOI] [PubMed] [Google Scholar]
- 17. Gray D, Willemse L, Visagie A, et al. Determinants of early‐life lung function in African infants. Thorax. 2017;72(5):445‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beretta F, Lavizzari A, Pesenti N, et al. Effect of human milk and other neonatal variables on lung function at three months corrected age. Pediatr Pulmonol. 2021;56(12):3832‐3838. [DOI] [PubMed] [Google Scholar]
- 19. Mank A, Carrasco Carrasco C, Thio M, et al. Tidal volumes at birth as predictor for adverse outcome in congenital diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. 2020;105(3):248‐252. [DOI] [PubMed] [Google Scholar]
- 20. Anık A, Öztürk S, Erge D, Akcan AB, Türkmen MK, Uysal P. Tidal breath in healthy term newborns: an analysis from the 2nd to the 30th days of life. Pediatr Pulmonol. 2021;56(1):274‐282. [DOI] [PubMed] [Google Scholar]
- 21. Pike KC, Crozier SR, Lucas JSA, et al. Patterns of fetal and infant growth are related to atopy and wheezing disorders at age 3 years. Thorax. 2010;65(12):1099‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turner S, Prabhu N, Danielan P, et al. First‐ and second‐trimester fetal size and asthma outcomes at age 10 years. Am J Respir Crit Care Med. 2011;184(4):407‐413. [DOI] [PubMed] [Google Scholar]
- 23. Turner SW, Campbell D, Smith N, et al. Associations between fetal size, maternal {alpha}‐tocopherol and childhood asthma. Thorax. 2010;65(5):391‐397. [DOI] [PubMed] [Google Scholar]
- 24. Britto IS, Tedesco GD, Herbst SR, et al. New anatomical landmarks to study the relationship between fetal lung area and thoracic circumference by three‐dimensional ultrasonography. J Matern Fetal Neonatal Med. 2012;25(10):1927‐1932. [DOI] [PubMed] [Google Scholar]
- 25. Yoshimura S, Masuzaki H, Gotoh H, Fukuda H, Ishimaru T. Ultrasonographic prediction of lethal pulmonary hypoplasia: comparison of eight different ultrasonographic parameters. Am J Obstet Gynecol. 1996;175(2):477‐483. [DOI] [PubMed] [Google Scholar]
- 26. Laudy JA, Tibboel D, Robben SG, de Krijger RR, de Ridder MA, Wladimiroff JW. Prenatal prediction of pulmonary hypoplasia: clinical, biometric, and Doppler velocity correlates. Pediatrics. 2002;109(2):250‐258. [DOI] [PubMed] [Google Scholar]
- 27. Johnson A, Callan NA, Bhutani VK, Colmorgen GH, Weiner S, Bolognese RJ. Ultrasonic ratio of fetal thoracic to abdominal circumference: an association with fetal pulmonary hypoplasia. Am J Obstet Gynecol. 1987;157(3):764‐769. [DOI] [PubMed] [Google Scholar]
- 28. Carel RS, Greenstein A, Ellender E, Melamed Y, Kerem D. Factors affecting ventilatory lung function in young Navy selectees. Am Rev Respir Dis. 1983;128(2):249‐252. [DOI] [PubMed] [Google Scholar]
- 29. Zeng X, Xu X, Zhang Y, Li W, Huo X. Chest circumference and birth weight are good predictors of lung function in preschool children from an e‐waste recycling area. Environ Sci Pollut Res Int. 2017;24(28):22613‐22621. [DOI] [PubMed] [Google Scholar]
- 30. Lødrup Carlsen KC, Rehbinder EM, Skjerven HO, et al. Preventing Atopic Dermatitis and ALLergies in children—the PreventADALL study. Allergy. 2018;73(10):2063‐2070. [DOI] [PubMed] [Google Scholar]
- 31. Bains KES, Gudmundsdóttir HK, Färdig M, et al. Infant lung function: criteria for selecting tidal flow‐volume loops. ERJ Open Res. 2022:00165‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. [DOI] [PubMed] [Google Scholar]
- 33. Johnsen SL, Wilsgaard T, Rasmussen S, Sollien R, Kiserud T. Longitudinal reference charts for growth of the fetal head, abdomen and femur. Eur J Obstet Gynecol Reprod Biol. 2006;127(2):172‐185. [DOI] [PubMed] [Google Scholar]
- 34. Johnsen SL, Rasmussen S, Sollien R, Kiserud T. Fetal age assessment based on ultrasound head biometry and the effect of maternal and fetal factors. Acta Obstet Gynecol Scand. 2004;83(8):716‐723. [DOI] [PubMed] [Google Scholar]
- 35. Johnsen SL, Rasmussen S, Sollien R, Kiserud T. Fetal age assessment based on femur length at 10‐25 weeks of gestation, and reference ranges for femur length to head circumference ratios. Acta Obstet Gynecol Scand. 2005;84(8):725‐733. [DOI] [PubMed] [Google Scholar]
- 36. Vintzileos AM, Campbell WA, Neckles S, Pike CL, Nochimson DJ. The ultrasound femur length as a predictor of fetal length. Obstet Gynecol. 1984;64(6):779‐782. [PubMed] [Google Scholar]
- 37. Melamed N, Yogev Y, Linder N, et al. Role of fetal length in the prediction of fetal weight. J Ultrasound Med. 2012;31(5):687‐694. [DOI] [PubMed] [Google Scholar]
- 38. den Dekker HT, Jaddoe VWV, Reiss IK, de Jongste JC, Duijts L. Fetal and infant growth patterns and risk of lower lung function and asthma. The generation R study. Am J Respir Crit Care Med. 2018;197(2):183‐192. [DOI] [PubMed] [Google Scholar]
- 39. Wignarajah D, Cock ML, Pinkerton KE, Harding R. Influence of intrauterine growth restriction on airway development in fetal and postnatal sheep. Pediatr Res. 2002;51(6):681‐688. [DOI] [PubMed] [Google Scholar]
- 40. Rao L, Tiller C, Coates C, et al. Lung growth in infants and toddlers assessed by multi‐slice computed tomography. Acad Radiol. 2010;17(9):1128‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turner SW, Devereux G. Fetal ultrasound: shedding light or casting shadows on the fetal origins of airway disease. Am J Respir Crit Care Med. 2012;185(7):694‐695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are may be available on request from the study PI. The study is still ongoing, and data are not publicly available due to privacy or ethical restrictions.


