Abstract
It is important to clarify the associations between modifiable lifestyle factors such as physical activity and breast cancer prognosis to enable the development of evidence‐based survivorship recommendations. We performed a systematic review and meta‐analyses to summarise the evidence on the relationship between postbreast cancer diagnosis physical activity and mortality, recurrence and second primary cancers. We searched PubMed and Embase through 31st October 2021 and included 20 observational studies and three follow‐up observational analyses of patients enrolled in clinical trials. In linear dose‐response meta‐analysis of the observational studies, each 10‐unit increase in metabolic equivalent of task (MET)‐h/week higher recreational physical activity was associated with 15% and 14% lower risk of all‐cause (95% confidence interval [CI]: 8%‐22%, studies = 12, deaths = 3670) and breast cancer‐specific mortality (95% CI: 4%‐23%, studies = 11, deaths = 1632), respectively. Recreational physical activity was not associated with breast cancer recurrence (HR = 0.97, 95% CI: 0.91‐1.05, studies = 6, deaths = 1705). Nonlinear dose‐response meta‐analyses indicated 48% lower all‐cause and 38% lower breast cancer‐specific mortality with increasing recreational physical activity up to 20 MET‐h/week, but little further reduction in risk at higher levels. Predefined subgroup analyses across strata of body mass index, hormone receptors, adjustment for confounders, number of deaths, menopause and physical activity intensities were consistent in direction and magnitude to the main analyses. Considering the methodological limitations of the included studies, the independent Expert Panel concluded ‘limited‐suggestive’ likelihood of causality for an association between recreational physical activity and lower risk of all‐cause and breast cancer‐specific mortality.
Keywords: breast cancer survival, evidence grading, expert panel judgement, physical activity, systematic review
What's new?
While being physically active facilitates improvements in various cancer‐related health outcomes, the role of physical activity in breast cancer prognosis remains unclear. Here, the Global Cancer Update Programme provided a systematic review and meta‐analysis of postdiagnosis recreational physical activity and as‐yet‐understudied prognosis‐related outcomes, namely all‐cause and breast cancer‐specific mortality and recurrence. The independent expert panel concluded that there is limited but suggestive evidence that recreational physical activity is beneficial and can lower all‐cause and breast cancer‐specific mortality rates in women diagnosed with breast cancer. The findings support the development of lifestyle recommendations for breast cancer survivors to be physically active, within the limits of their ability and specific medical advice.
Abbreviations
- AICR
American Institute for Cancer Research
- ACS
American Cancer Society
- AMBER
The Alberta Moving Beyond Breast Cancer
- BMI
body mass index
- Cis
confidence intervals
- CUP
Continuous Update Project
- CUP Global
Global Cancer Update Programme
- HR
hazard ratio
- MET
metabolic equivalent of task
- RCT
randomised controlled trial
- RR
relative risk
- SLR
systematic literature review
- WCRF
World Cancer Research Fund
1. INTRODUCTION
In 2020, approximately 2.3 million women worldwide were diagnosed with incident breast cancer and almost 700 000 died from the disease. 1 Incidence rates have increased in western countries from the 1980s until the late 1990s. Incidence rates continue to increase in many middle‐income countries. 2 , 3 Breast cancer mortality rates have been stable or declining since the 1990s, possibly due to improved early detection and treatment. 2 The number of new breast cancer cases globally is expected to rise from 2.3 million in 2020 to 3.2 million in 2040. 1 It is therefore essential to clarify the relationship between modifiable lifestyle factors (such as physical activity, adiposity and diet) with survival outcomes, in order to develop evidence‐based cancer survivorship recommendations. 4
The role of physical activity in preventing the occurrence of adverse health outcomes and overall mortality is well‐established. 5 In terms of aetiology, being physically active reduces the risk of pre‐ and postmenopausal breast cancer. 4 , 6 The 2019 American College of Sports Medicine (ACSM) guidelines indicated that sufficient evidence supports that being physically active could lead to improvements in various cancer‐related health outcomes such as depression, fatigue and anxiety. 7 , 8 However, questions remain about the role of physical activity in disease‐free survival and mortality. 9 In addition, it is unclear if the benefits of physical activity equally apply to all cancer patients or only for specific subgroups. 10 , 11 A number of reviews and meta‐analyses have been published to date that investigated physical activity and mortality after breast cancer diagnosis. 10 , 12 , 13 , 14 , 15 , 16 , 17 Five of these 10 , 12 , 15 , 16 , 17 published in 2019‐2020, consistently reported that women at the highest levels of physical activity had lower (summary hazard ratio [HR] range = 0.58‐0.74) rate of all‐cause and breast cancer‐specific mortality (summary HR range = 0.59‐0.72) compared to those at the lowest levels. The meta‐analysis by Geidl et al 12 reported that for each 10 metabolic equivalent of task (MET)‐h/week increase in physical activity there was a 22% lower rate of all‐cause mortality and an indication of a nonlinear dose‐response association. This finding was further supported in the meta‐analysis by Friedenreich et al in 2019. 10 Only one 15 of the recently published meta‐analyses investigated physical activity and recurrence, and reported no association (HR = 0.79, 95% confidence interval [CI]: 0.60‐1.05, studies = 5).
As part of its comprehensive assessment of diet, nutrition, adiposity, and physical activity in breast cancer survivorship, the Global Cancer Update Programme (CUP Global) formally known as the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Continuous Update Project (CUP), assessed the available evidence on physical activity and breast cancer prognosis to June 2012 and concluded ‘limited‐suggestive’ evidence for an association between physical activity and lower rate of all‐cause and breast cancer‐specific mortality in breast cancer survivors. 4 , 18 The CUP Global updated this evidence in its 2018 report. 4 This manuscript further updates the systematic review and meta analyses findings and Expert Panel evidence grading on postdiagnosis recreational physical activity and breast cancer prognosis. We sought to evaluate the evidence, identify gaps in the literature and define future research directions. This article presents the evidence on physical activity and breast cancer outcomes, whereas evidence on body fatness, diet and the overall summary are presented in the accompanied papers. 19 , 20 , 21
2. MATERIALS AND METHODS
This work was conducted as part of the ongoing CUP Global. 22 The peer‐reviewed protocol is available online 23 and the PRISMA checklist 24 is provided in Table S1.
2.1. Search strategy, selection criteria and data extraction
We searched in PubMed and Embase through 31st October 2021 for peer‐reviewed observational studies or randomised controlled trials (RCTs) in women diagnosed with primary breast cancer during adulthood. Search terms are listed in Table S2A,B. The search strategy included terms not only related to physical activity but also diet and anthropometry because it was part of the broader CUP Global SLR that also covered other exposures. Study selection included an initial screening of all titles and abstracts, followed by full‐text and reference list screening. A second reviewer checked at least 10% of the study selection and data extraction. Any disagreements on study eligibility were resolved through consensus‐based discussion. For the purposes of this review, we classified physical activity into types, namely ‘total’ and ‘recreational’. Total physical activity describes studies that reported on recreational, occupational, transportation and household physical activities combined. We restricted our review to studies that assessed physical activity postdiagnosis and we therefore excluded studies that reported only on prediagnosis physical activity 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 and studies that likely reflected a combination of pre‐ and postdiagnosis physical activity. 43 , 44 , 45 If a study reported data separately for pre‐ and postdiagnosis physical activity, we included it and used the estimate for postdiagnosis physical activity in analysis. We excluded ecologic, cross‐sectional, and case–control studies, case reports, surveys, conference abstracts, commentaries and any studies on passive physical activity (eg, aided by a physiotherapist). Outcomes of interest were all‐cause and breast cancer‐specific mortality, breast cancer recurrence (as defined in studies) and second primary cancers. We therefore excluded studies that investigated only quality of life (QoL) outcomes. From each included study, we extracted study, participant and disease characteristics, treatment information, exposure/intervention and cancer outcome/s, HRs or relative risks (RRs), 95% CIs and/or P‐values for each exposure category and covariate adjustments. Based on available literature, important potential confounders of the association of physical activity and breast cancer prognosis include age, alcohol intake, smoking, stage, treatment and relevant prediagnostic exposures. The quality of individual studies was not graded using a specific tool. Instead, relevant study characteristics that could be used to explore potential sources of bias were included into the CUP Global database. Information on potential for residual confounding, risk of information and selection bias was retrieved after identifying the most likely influential sources of bias in cancer survival studies. 46 , 47 In the Expert Panel meeting, whether the studies had serious quality issues was discussed when evaluating the evidence for each exposure‐outcome association.
2.2. Data synthesis
We computed summary HRs and 95% CIs for the highest vs the lowest levels of physical activity and for dose‐response analyses using random‐effects models to account for the variation within and between studies. 48 For dose‐response analysis, we summarised HR estimates and 95% CIs for continuous increments as reported in the articles, if provided, and for studies that only reported categorised exposures, dose‐response estimates were derived using the method described by Greenland and Longnecker. 49 , 50 Details of the estimations are provided in Appendix S2. In analyses of ‘highest’ vs ‘lowest’ categories, we included studies that reported results of physical activity either in h/week or metabolic equivalent of task (MET)‐h/week. One MET is defined as the amount of oxygen consumed while sitting at rest that equals to 3.5 mL/O2 per kg of body‐weight*min. 51 Dose‐response analyses are presented using 10 MET‐h/week as the unit increment, that reflects approximately 1.25 hours of vigorous activity per week or 2.5 hours of moderate activity per week. If there were multiple publications from the same study, we selected the one with the largest number of events for analysis if it provided the necessary data. We used results of pooling projects instead of their component studies when available for analysis. We conducted nonlinear dose‐response analyses when we had at least five studies with three or more exposure categories by using restricted cubic splines, with knots at 10th, 50th, and 90th percentiles of the exposure distribution and combined them using multivariate meta‐analysis. 52 , 53 For each study, the results from fully adjusted models were included in analyses.
Cochran's Q and the I squared statistic (I 2) were used to examine between‐study heterogeneity. A P‐value <.10 for Q test was considered significant and an I 2 statistic greater or equal to 25%, 50% and 75% indicated a low, moderate and high proportion of variation attributable to heterogeneity. 54 , 55 95% CIs of I 2 were calculated to aid interpretation. 56 We further investigated potential sources of heterogeneity by conducting predefined subgroup analyses across strata of BMI, menopausal and hormone receptor status, recreational physical activity intensity (ie, moderate, moderate‐to‐vigorous, vigorous), adjustment for confounders (stage, treatments, smoking and alcohol), number of deaths and analyses restricted to studies that assessed physical activity after completion of initial treatment. This analysis assumes that physical activity after initial treatment is a less variable measure and amount of physical activity may differ from during‐to‐after treatment. It is therefore important to clarify these associations with outcomes, especially for women who survive long term to allow more targeted recommendations and/or interventions. We performed sensitivity analyses by sequential omission of one study at a time (leave‐one‐out analyses) 57 to explore the potential influence of single studies on results. Egger's regression asymmetry test and visual inspection of funnel plots were used to assess presence of small‐study effects such as publication bias, when there were 10 or more studies in analyses. A P‐value less than .10 indicated evidence of small‐study effects, as the test has limited power in the presence of few studies. 58 We used fixed‐effect model as sensitivity analysis, to compare with the random‐effects models 59 , 60 , 61 and results are presented in tables.
We used R 62 version 4.0.5. packages: ‘meta’, 63 ‘metafor’, 64 ‘dosresmeta’ 65 and ‘tidyverse’. 66
2.3. Evidence grading criteria
The independent Expert Panel (ELG, MJG, AAJ, EK, VL, SKC, AMT) graded the quality of the evidence as strong (subgrades evaluating likelihood of causality: substantial effect on risk unlikely, probable or convincing) or limited (subgrades evaluating likelihood of causality: limited—no conclusion or limited—suggestive) according to predefined criteria listed in Table S3 which evaluate the quantity, consistency, magnitude and precision of the summary estimates, existence of a dose‐response, risk of bias, generalisability and mechanistic plausibility of the results.
3. RESULTS
Study selection is shown in Figure 1. Of 3212 full texts assessed for inclusion, 20 cohort studies 11 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 and three follow‐up analyses of patients enrolled in clinical trials 91 , 92 met our inclusion criteria. Among these, two publications were from the After Breast Cancer Pooling Project (ABCPP). Beasley et al 75 comprised four individual cohort studies, three from the United States that is, The Women's Healthy Eating and Living Study (WHEL), Life After Cancer Epidemiology study (LACE), Nurses' Health Study (NHS) and one from China that is, the Shanghai Breast Cancer Survival Study (SBCSS). Nechuta et al 70 comprised the three US cohorts (WHEL, LACE, NHS). When possible, we used Beasley et al in analyses otherwise we used Nechuta et al.
FIGURE 1.
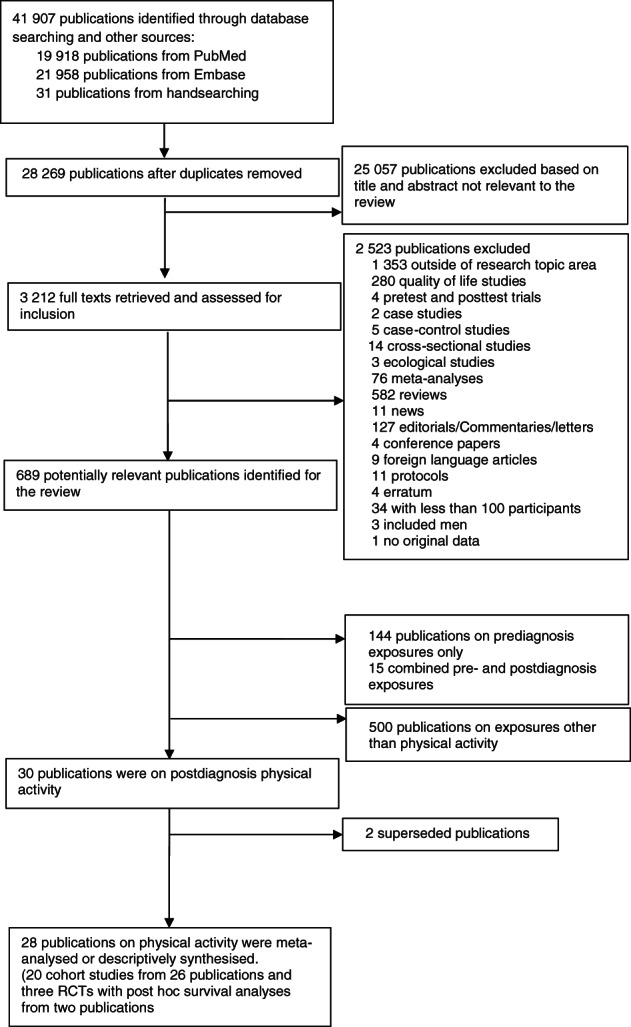
Flow chart of study selection process
The sample size of the identified studies ranged from 103 to 13 302 women. Most (n = 17, 65%) originated from the United States, 11 , 67 , 68 , 70 , 73 , 76 , 77 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 88 , 93 one (4%) from Mexico, 87 two (8%) from China, 72 , 78 one (4%) from Germany, 89 one (4%) from Denmark, 69 one (4%) from Norway, 71 one (4%) from Turkey 90 and one (4%) 74 included women from various countries. Most studies reported on recreational physical activity. 11 , 67 , 68 , 69 , 70 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 81 , 82 , 83 , 84 , 85 , 86 One reported on total physical activity only, 71 one reported total and recreational physical activities, 79 one reported household and recreational physical activities 69 and another one reported results on household physical activity. 87 One study 93 reported on sedentary time (television watching). Our review concentrated on recreational physical activities (eg, aerobics, walking, running) since most of the studies published data for this domain and we were unable to perform analyses across other domains of activity. Details of the physical activity definitions are presented in Table S4. Characteristics of the studies included in the meta‐analyses are presented in Table S5A,B. The two publications 71 , 79 that investigated total physical activity (ie, combined: occupational, household, recreational and transportation) reported null associations for all‐cause mortality, breast cancer‐specific mortality and breast cancer recurrence (Figure S1). Seven publications 69 , 71 , 76 , 77 , 82 , 89 , 90 examined the association between physical activity changes from before to after diagnosis and breast cancer prognosis but could not be meta‐analysed; therefore, we provide a descriptive synthesis of these results (Appendix S3).
Two publications reported follow‐up observational analyses of patients enrolled in three RCTs. The Supervised Trial of Aerobic vs Resistance Training (START) trial 92 evaluated the effect of participation in a 17 week highly‐supervised, moderate‐intensity resistance‐only or aerobic‐only intervention during chemotherapy. The impact of physical activity on survival was reported, although the trial was not originally designed to address these outcomes. The two Exercise for Health trials (EfH) 91 involved a mixed‐type (aerobic‐ and resistance‐based), unsupervised moderate‐intensity intervention, monitored by frequent face‐to‐face or telephone‐based contact with an exercise physiologist after surgery. These follow‐up analyses of patients enrolled RCTs 91 , 92 suggest a beneficial impact of physical activity on outcomes after breast cancer. The interpretation of these results was limited by the post hoc analysis, small number of events and wide CIs (Figure S2 and Table S6).
3.1. Recreational physical activity and all‐cause mortality
Recreational physical activity was associated with lower risk of all‐cause mortality. Twelve observational studies were included in the dose‐response meta‐analysis comprising 31 563 women, of whom 3670 (~12%) died. 67 , 69 , 74 , 75 , 76 , 81 , 82 , 84 , 89 Most of these studies had less than 10 years median follow‐up (Table S5B). The summary HR per 10 MET‐h/week was 0.85 (95% CI: 0.78‐0.92) with evidence of substantial heterogeneity (I 2 = 87%, P‐heterogeneity ≤ .01), and an indication of small‐study effects (P‐Egger's = .01; Figures 2A, S3 and Table 1). Highest vs lowest levels of physical activity were associated with a 44% lower risk of all‐cause mortality (HR = 0.56, 95% CI: 0.49‐0.64; I 2 = 34%, P‐heterogeneity = .08, P‐Egger's = .34, 15 studies) 67 , 68 , 69 , 73 , 74 , 75 , 76 , 81 , 82 , 85 , 88 , 89 (Figures 2B, S4 and Table 1). Sequential omission of studies did not change the direction or magnitude of the summary estimates (Figure S5). Analysis of 10 studies 69 , 74 , 75 , 76 , 81 , 82 , 89 showed a dose‐dependent 47% reduction in risk of all‐cause mortality at 20 MET‐h/week, but little or no further reduction in risk at higher levels. The data were sparse at higher physical activity levels (P‐nonlinearity < .001) (Figure 3A and Table S7).
FIGURE 2.
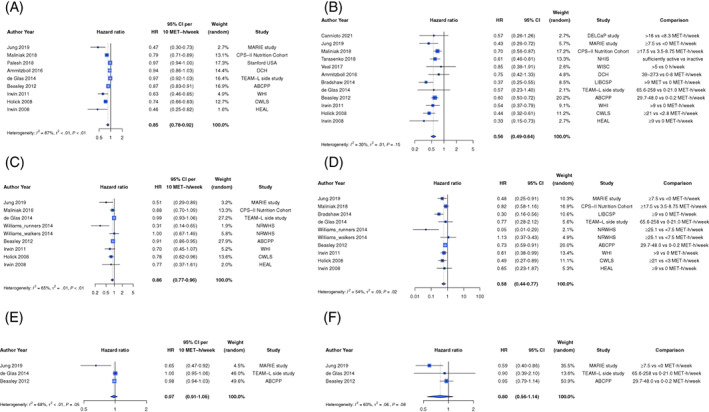
Summary hazard ratio estimate (95% CI) of (A) all‐cause mortality for every 10 MET‐h/week of recreational physical activity after diagnosis, (B) all‐cause mortality for the highest compared to the lowest level of recreational physical activity after diagnosis, (C) breast cancer‐specific mortality for every 10 MET‐h/week of recreational physical activity after diagnosis, (D) breast cancer‐specific mortality for the highest compared to the lowest level of recreational physical activity after diagnosis, (E) recurrence for every 10 MET‐h/week of recreational physical activity after diagnosis and (F) recurrence for the highest compared to the lowest level of recreational physical activity after diagnosis. Forest plot shows results from the random effects model. Diamond represents the summary hazard ratio. Each square represents the hazard ratio estimate of each study and the horizontal line across each square represents the 95% confidence interval (CI) of the hazard ratio estimate. ABCPP (Beasley 2012) included data from three US cohorts that is, LACE, NHS, WHEL and one Chinese cohort SBCSS. For the CPS‐II Nutrition Cohort (Maliniak 2018), the HR estimates for the two age groups reported were combined using fixed effects models before inclusion in the meta‐analysis
TABLE 1.
Recreational physical activity after diagnosis and all‐cause mortality, summary of results
| Heterogeneity | Small‐study effects | ||||||
|---|---|---|---|---|---|---|---|
| Analysis | Number of studies (number of publications) | Number of events a | Summary HR (95% CI) random‐effects | Summary HR (95% CI) fixed‐effect | I 2% (95% CI) | Q value, df, Q P‐value | Egger's P‐value |
| Dose‐response meta‐analyses (HR per 10 MET‐h/week) | |||||||
| Main analysis (all‐studies) | 12 (9) | 3670 | 0.85 (0.78‐0.92) | 0.93 (0.91‐0.94) | 87 (78‐93) | 62, 8, <.01 | .01 |
| Stratified by hormone receptor status | |||||||
| Negative b | – | – | – | – | – | – | |
| Positive c , d | 4 (2) | 1267 | 0.91 (0.80‐1.04) | 0.93 (0.89‐0.97) | 89 (58‐97) | 9.0, 1, <.01 | |
| Stratified by menopausal status | |||||||
| Premenopausal | 1 (1) | 186 | 0.89 (0.65‐1.22) | 0.89 (0.65‐1.22) | – | – | – |
| Postmenopausal | 5 (5) | 1773 | 0.72 (0.58‐0.89) | 0.91 (0.87‐0.95) | 89 (76‐95) | 35.0, 4, <.01 | – |
| Stratified by adjustment variables | |||||||
| Adjusted for stage: Yes | 10 (7) | 3484 | 0.86 (0.80‐0.93) | 0.93 (0.91‐0.95) | 86 (78‐93) | 56, 7, <.01 | – |
| Adjusted for stage: No | 1 (1) | 186 | 0.63 (0.46‐0.85) | – | – | – | – |
| Adjusted for treatments: Yes | 9 (6) | 2205 | 0.89 (0.83‐0.96) | 0.93 (0.91‐0.95) | 87 (74‐94) | 39, 5, <.01 | – |
| Adjusted for treatments: No | 3 (3) | 1465 | 0.65 (0.49‐0.87) | 0.75 (0.68‐0.83) | 71 (0‐91) | 6.8, 2, .03 | – |
| Adjusted for smoking: Yes | 7 (4) | 2906 | 0.85 (0.77‐0.93) | 0.87 (0.84‐0.90) | 70 (14‐90) | 9.9, 3, .02 | – |
| Adjusted for smoking: No | 5 (5) | 764 | 0.84 (0.74‐0.95) | 0.95 (0.93‐0.98) | 89 (77‐95) | 35.9, 4, <.01 | – |
| Adjusted for alcohol: Yes | 3 (3) | 1438 | 0.81 (0.68‐0.97) | 0.86 (0.81‐0.92) | 80 (36‐94) | 9.9, 2, .01 | – |
| Adjusted for alcohol: No | 9 (6) | 2232 | 0.86 (0.78‐0.94) | 0.93 (0.91‐0.95) | 90 (80‐95) | 48, 5, <.01 | – |
| Stratified by median deaths | |||||||
| Less than median deaths | 5 (5) | 511 | 0.93 (0.86‐1.00) | 0.96 (0.94‐0.99) | 72 (30‐89) | 14.4, 4, .01 | – |
| More than median deaths | 7 (4) | 3159 | 0.77 (0.68‐0.88) | 0.84 (0.81‐0.88) | 80 (48‐93) | 15.2, 3, .00 | – |
| Stratified by timing of physical activity assessment in relation to cancer treatment | |||||||
| After the end of primary cancer treatment | 5 (4) | 1815 | 0.91 (0.83‐0.98) | 0.97 (0.96‐0.99) | 87 (70‐95) | 23.5, 3, <.01 | – |
| Highest vs lowest meta‐analyses | |||||||
| Main analysis (all‐studies) | 15 (12) | 4919 | 0.56 (0.49‐0.64) | 0.58 (0.53 to 0.64) | 34 (0‐67) | 17.0, 11, .12 | .34 |
| Stratified by BMI | |||||||
| BMI < 25 (kg/m2) e | 5 (5) | 359 | 0.44 (0.30‐0.64) | 0.49 (0.38‐0.61) | 51 (0‐82) | 8.2, 4, .09 | – |
| BMI 25–29.9 (kg/m2) f | 2 (2) | 55 | 0.62 (0.29‐1.35) | 0.59 (0.38‐0.92) | 65 (0‐92) | 2.9, 1, .09 | – |
| BMI ≥ 25 kg/mg | 3 (3) | 194 | 0.50 (0.32‐0.78) | 0.53 (0.40‐0.72) | 49 (0‐85) | 3.9, 2, .14 | – |
| BMI ≥ 30 kg/mh | 2 (2) | 63 | 0.83 (0.51‐1.34) | 0.83 (0.51‐1.34) | 0 (n/a) | 0.05, 1, .82 | – |
| Stratified by hormone receptor status | |||||||
| Negative b , e | 5 (5) | 219 | 0.50 (0.38‐0.66) | 0.48 (0.38‐0.62) | 14 (0‐82) | 4.6, 4, .33 | – |
| Positive c , d | 8 (6) | 1583 | 0.52 (0.33‐0.81) | 0.65 (0.58‐0.74) | 84 (67‐92) | 31.6, 5, <.01 | – |
| Stratified by menopausal status | |||||||
| Premenopausal | 1 (1) | 186 | 0.86 (0.58‐1.27) | – | – | – | – |
| Postmenopausal | 5 (5) | 1773 | 0.60 (0.51‐0.71) | 0.61 (0.53‐0.71) | 11 (0‐82) | 4.5, 4, .34 | – |
| Stratified by adjustment variables | |||||||
| Adjusted for stage: Yes | 10 (7) | 3414 | 0.57 (0.47‐0.68) | 0.59 (0.52‐0.66) | 37 (0‐74) | 9.6, 6, .14 | – |
| Adjusted for stage: No | 5 (5) | 1505 | 0.54 (0.42‐0.68) | 0.54 (0.45‐0.65) | 26 (0‐71) | 5.4, 4, .25 | – |
| Adjusted for treatments: Yes | 10 (7) | 2642 | 0.51 (0.41‐0.64) | 0.54 (0.47‐0.62) | 41 (0‐75) | 10.2, 6, .11 | – |
| Adjusted for treatments: No | 5 (5) | 2277 | 0.62 (0.53‐0.72) | 0.62 (0.53‐0.72) | 0 (0‐77) | 3.6, 4, .46 | – |
| Adjusted for smoking: Yes | 9 (6) | 3737 | 0.63 (0.56‐0.71) | 0.63 (0.56‐0.71) | 0 (0‐53) | 2.7, 5, .75 | – |
| Adjusted for smoking: No | 6 (6) | 1182 | 0.42 (0.35‐0.52) | 0.42 (0.35‐0.52) | 0 (0‐31) | 1.8, 5, .87 | – |
| Adjusted for alcohol: Yes | 4 (4) | 1548 | 0.67 (0.56‐0.80) | 0.67 (0.56‐0.80) | 0 (0‐75) | 1.9, 3, .60 | – |
| Adjusted for alcohol: No | 10 (8) | 3371 | 0.51 (0.44‐0.60) | 0.53 (0.47‐0.60) | 24 (0‐65) | 9.2, 7, .24 | – |
| Stratified by median deaths | |||||||
| Less than median deaths | 6 (6) | 581 | 0.55 (0.41‐0.73) | 0.55 (0.41‐0.73) | 0 (0‐73) | 4.7, 5, .45 | – |
| More than median deaths | 9 (6) | 4338 | 0.55 (0.47‐0.66) | 0.58 (0.52‐0.64) | 54 (0‐82) | 10.8, 5, .06 | – |
| Stratified by timing of physical activity assessment after in relation to cancer treatment | |||||||
| After the end of primary cancer treatment | 6 (6) | 2093 | 0.70 (0.59‐0.84) | 0.70 (0.59‐0.84) | 25 (0‐69) | 6.7, 5, .25 | – |
| Stratified by physical activity intensity | |||||||
| Moderate | 2 (2) | 599 | 0.54 (0.39‐0.74) | 0.53 (0.41‐0.69) | 31 (n/a) | 1.5, 1, .23 | – |
| Moderate‐vigorous | 4 (4) | 1629 | 0.62 (0.50‐0.78) | 0.65 (0.56‐0.76) | 34 (0‐77) | 4.5, 3, .21 | – |
| Vigorous | 2 (2) | 599 | 0.93 (0.72‐1.20) | 0.93 (0.72‐1.20) | 0 (n/a) | 0.5, 1, .49 | – |
Note: Where the number of studies differs from the number of publications is because we have used the pooling project ABCPP in analyses.
Abbreviations: BMI, body mass index; CI, confidence interval; df, degrees of freedom; ER−, oestrogen receptor negative; ER+, oestrogen receptor positive; HR, hazard ratio; MET, metabolic equivalent of task; PR−, progesterone receptor negative; PR+, progesterone receptor positive.
Not all studies that are included in the analyses reported the number of events. Bradshaw 2014 (LIBCSP) and Sternfeld 2009 (LACE) do not provide the number of events for each receptor or BMI subgroup.
Hormone receptor negative analysis includes ER− or PR−, ER − PR−, ER−.
Hormone receptor positive analysis includes ER + PR+, ER+.
Bradshaw 2014 (LIBCSP) is used in analysis.
Both Sternfeld and Bradshaw are in the analysis.
Sternfeld 2009 (LACE) is used in analysis.
FIGURE 3.
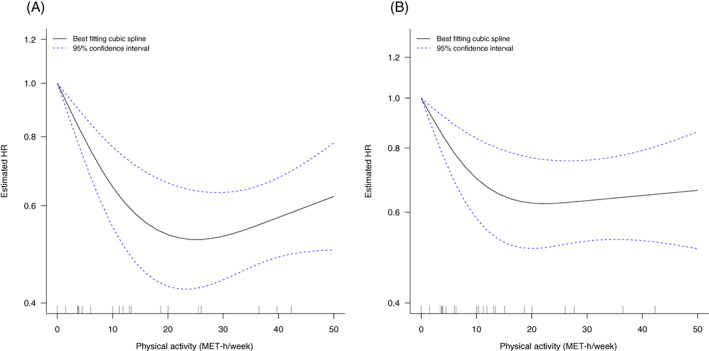
Nonlinear association between recreational physical activity after diagnosis and (A) all‐cause mortality and (B) breast cancer‐specific mortality. The curves show the nonlinear dose–response associations between recreational physical activity after diagnosis and (A) all‐cause mortality and (B) breast cancer‐specific mortality, estimated using restricted cubic spline regression with three knots at 10th, 50th and 90th percentiles of distribution of the exposure and pooled with random‐effects meta‐analysis. Recreational physical activity at 0 metabolic equivalent of task (MET) was chosen as reference.
We conducted predefined subgroup meta‐analyses to explore potential sources of heterogeneity (Table 1). Among women with hormone positive tumours, a marginal inverse association was observed (HR per 10 MET‐h/week = 0.91, 95% CI: 0.80‐1.04; I 2 = 89%, P‐heterogeneity < .01, four studies, deaths = 1267, Figure S6). 70 , 74 Highest vs lowest levels of recreational physical activity were associated with a 48% (HR = 0.52, 95% CI: 0.33‐0.81; I 2 = 84%, P‐heterogeneity < .01, deaths = 1583) and 50% (HR = 0.50, 95% CI: 0.38‐0.66; I 2 = 14%, P‐heterogeneity = .33, deaths = 219) lower risk of all‐cause mortality among women with hormone receptor positive 70 , 73 , 74 , 76 , 78 , 82 and hormone receptor negative tumours, 73 , 76 , 78 , 79 , 82 respectively (Figure S7 and Table 1).
In linear dose‐response analysis restricted to postmenopausal women, a 10 MET‐h/week higher recreational physical activity was associated with a 28% lower risk of all‐cause mortality (HR = 0.72, 95% CI: 0.58‐0.89; I 2 = 89%, P‐heterogeneity < .01, five studies, 67 , 74 , 76 , 78 , 89 deaths = 1773) (Figure S8). Highest vs lowest levels of recreational physical activity were associated with a 40% (HR = 0.60, 95% CI: 0.51‐0.71; I 2 = 11%, P‐heterogeneity = .34, deaths = 1773) lower risk of all‐cause mortality among postmenopausal women. 67 , 74 , 76 , 78 , 89 (Figure S9 and Table 1). One study 78 restricted to premenopausal women and was indicative of an inverse association between recreational physical activity and all‐cause mortality; however, the CI was wide and included the null both in dose‐response and categorical analysis (Table 1).
We could not conduct dose‐response analyses within strata of BMI due to insufficient data. Highest vs lowest levels of recreational activity were associated with a 56% lower risk of all‐cause mortality in women with BMI < 25 kg/m2 (HR = 0.44, 95% CI: 0.30‐0.64; I 2 = 51%, P‐heterogeneity = .09, five studies 73 , 76 , 78 , 79 , 82 ) and 50% lower risk for those with BMI ≥ 25 kg/m2 (HR = 0.50, 95% CI: 0.32‐0.78; I 2 = 51%, P‐heterogeneity = .14, three studies 73 , 78 , 82 ). The summary HR was 0.62 (95% CI: 0.29‐1.35; I 2 = 65%, P‐heterogeneity = .09) among women with BMI 25‐29.9 kg/m2 and 0.83 (95% CI: 0.51‐1.34; I 2 = 0%, P‐heterogeneity = .82) among women with BMI ≥ 30 kg/m2; however, these analyses were based on only two studies (Figure S10).
We explored the association between different recreational physical activity intensities and breast cancer prognosis, but dose‐response meta‐analysis was not possible due to limited data. With few available studies, categorical meta‐analyses were indicative of an inverse association between moderate (HR = 0.54, 95% CI: 0.39‐0.74; I 2 = 31%, P‐heterogeneity = .23, two studies, 79 , 81 deaths = 599) and moderate‐to‐vigorous intensity recreational physical activity (HR = 0.62, 95% CI: 0.50‐0.78; I 2 = 34%; P‐heterogeneity = .21, four studies 67 , 76 , 77 , 79 deaths = 1629) and all‐cause mortality (Table 1). There was little evidence of an association for vigorous recreational physical activity (HR = 0.93, 95% CI: 0.72‐1.20, I 2 = 0%; P‐heterogeneity = .49; deaths = 599) (Figure S11) but this analysis was based on two studies. 79 , 81 No studies reported on light‐intensity recreational physical activity.
To avoid the influence of possible change of physical activity habits due to active treatment, we conducted meta‐analysis restricted to studies that assessed physical activity after completion of initial treatment, excluding hormone therapy. Recreational physical activity was associated with a 9% lower risk of all‐cause mortality in dose‐response analysis (HR per 10 MET‐h/week = 0.91 95% CI: 0.83‐0.98; I 2 = 87%, P‐heterogeneity < .01, four studies, 67 , 74 , 77 , 83 deaths = 1815), and a 30% lower risk in categorical meta‐analysis (HR = 0.70 95% CI: 0.59‐0.84; I 2 = 25%, P‐heterogeneity = .25, six studies, 67 , 74 , 77 , 79 , 83 , 88 deaths = 2093; Figure S12).
Subgroup analyses by adjustment factors and by median number of deaths gave similar results to the main analyses (Table 1).
3.2. Recreational physical activity and breast cancer‐specific mortality
The findings for breast cancer‐specific mortality were similar, in general, to those for all‐cause mortality (Table 2). A 14% lower risk was observed between recreational physical activity and breast cancer‐specific mortality (HR per 10 MET‐h/week = 0.86, 95% CI: 0.77‐0.96; I 2 = 65%, P‐heterogeneity = .01, 11 studies 67 , 74 , 75 , 76 , 81 , 82 , 86 , 89 comprising 31 242 women, 1632 [5%] of whom died; Figure 2C and Table 2). Results were consistent in the categorical analysis (HR = 0.60, 95% CI: 0.47‐0.77; I 2 = 42%, P‐heterogeneity = .08, 12 studies 67 , 73 , 74 , 75 , 76 , 81 , 82 , 86 , 89 ; Figures 2D, S13 and Table 2). The summary estimates did not materially change in the leave‐one‐out analysis (Figure S14). We found evidence of nonlinearity in dose‐response meta‐analysis with 10 studies. 74 , 75 , 76 , 81 , 82 , 86 , 89 There was a dose‐dependent, 38% reduction in risk of breast cancer‐specific mortality with increasing physical activity levels observed at 20 MET‐h/week, but no further reduction in risk was observed at higher levels (P‐nonlinearity < .001; Figure 3B and Table S7). We could not perform meta‐analysis stratified by hormone receptor status in three publications 11 , 73 , 83 due to high heterogeneity across these subgroups. Results by menopausal status and across BMI strata were consistent with the main analysis (Figures S15‐S17 and Table 2).
TABLE 2.
Recreational physical activity after diagnosis and breast cancer‐specific mortality, summary of results
| Heterogeneity | Small‐study effects | ||||||
|---|---|---|---|---|---|---|---|
| Analysis | Number of studies (number of publications) | Number of events a | Summary HR (95% CI) random‐effects | Summary HR (95% CI) fixed‐effect | I 2% (95% CI) | Q value, df, Q P‐value | Egger's P‐value |
| Dose–response meta‐analyses (HR per 10 MET‐h/week) | |||||||
| Main analysis (all‐studies) | 11 (8) | 1632 | 0.86 (0.77‐0.96) | 0.93 (0.89‐0.96) | 65 (28‐83) | 22.7, 8, .01 | .05 |
| Stratified by menopausal status | |||||||
| Postmenopausal | 4 (4) | 476 | 0.84 (0.68‐1.04) | 0.97 (0.91‐1.03) | 65 (0‐88) | 8.6, 3, .04 | |
| Stratified by timing of physical activity assessment in relation to cancer treatment | |||||||
| After the end of primary cancer treatment | 3 (3) | 585 | 0.91 (0.80‐1.04) | 0.95 (0.90‐1.01) | 69 (0‐91) | 6.53, 2, .04 | |
| Stratified by adjustment variables | |||||||
| Adjusted for stage: Yes | 9 (6) | 1500 | 0.90 (0.82‐0.98) | 0.93 (0.89‐0.97) | 61 (3‐84) | 12.7, 5, .03 | – |
| Adjusted for stage: No | 2 (2) | 132 | 0.65 (0.37‐1.14) | 0.74 (0.56‐0.98) | 73 (10‐92) | 7.5, 2, .02 | – |
| Adjusted for treatments: Yes | 7 (4) | 1149 | 0.90 (0.85‐0.95) | 0.90 (0.85‐0.95) | 1 (0‐90) | 2, 2, .37 | – |
| Adjusted for treatments: No | 4 (4) | 483 | 0.70 (0.50‐0.96) | 0.79 (0.67‐0.93) | 63 (3‐86) | 11, 4, .03 | – |
| Adjusted for smoking: Yes | 6 (3) | 1323 | 0.90 (0.85‐0.95) | 0.90 (0.85‐0.95) | 0 (0‐86) | 1.4, 2, .49 | – |
| Adjusted for smoking: No | 5 (5) | 309 | 0.77 (0.59‐0.99) | 0.96 (0.90‐1.02) | 73 (39‐88) | 18.8, 5, .002 | – |
| Adjusted for alcohol: Yes | 2 (2) | 352 | 0.84 (0.69‐1.02) | 0.84 (0.69‐1.02) | 0 (NA) | 0.84, 1, .36 | – |
| Adjusted for alcohol: No | 9 (7) | 1280 | 0.87 (0.76‐0.98) | 0.93 (0.89‐0.97) | 71 (37‐87) | 20.8, 6, .002 | – |
| Stratified by median deaths | |||||||
| Less than median deaths | 4 (4) | 200 | 0.72 (0.50‐1.05) | 0.98 (0.92‐1.04) | 73 (32‐89) | 14.8, 4, .01 | – |
| More than median deaths | 8 (4) | 1432 | 0.89 (0.83‐0.94) | 0.89 (0.85‐0.94) | 5 (0‐85) | 3.2, 3, .37 | – |
| Highest vs lowest meta‐analyses | |||||||
| Main analysis (all‐studies) | 12 (9) | 1827 | 0.60 (0.47‐0.77) | 0.66 (0.57‐0.77) | 42 (0‐72) | 16, 9, .08 | 0.11 |
| Stratified by BMI | |||||||
| BMI < 25 (kg/m2) b | 3 (3) | 185 | 0.47 (0.20‐1.10) | 0.51 (0.35‐0.75) | 76 (21‐93) | 8.36, 2, .02 | – |
| BMI ≥ 25 (kg/m2) b | 3 (3) | 196 | 0.51 (0.35‐0.74) | 0.51 (0.36‐0.73) | 10 (0‐91) | 2.23, 2, .33 | – |
| Stratified by menopausal status | |||||||
| Premenopausal | 1 (1) | 58 | 0.58 (0.32‐1.05) | 0.58 (0.32‐1.05) | – | – | |
| Postmenopausal | 5 (5) | 682 | 0.71 (0.59‐0.87) | 0.71 (0.59‐0.87) | 0 (0‐67) | 2.5, 4, .65 | – |
| Stratified by adjustment variables | |||||||
| Adjusted for stage: Yes | 9 (6) | 1500 | 0.71 (0.60‐0.83) | 0.71 (0.60‐0.83) | 0 (0‐66) | 3.7, 5, .59 | – |
| Adjusted for stage: No | 3 (3) | 327 | 0.40 (0.17‐0.91) | 0.47 (0.33‐0.67) | 74 (28‐91) | 11, 3, .01 | – |
| Adjusted for treatments: Yes | 9 (5) | 1344 | 0.56 (0.38‐0.82) | 0.64 (0.53‐0.78) | 50 (0‐82) | 7.9, 4, .10 | – |
| Adjusted for treatments: No | 3 (4) | 483 | 0.58 (0.35‐0.97) | 0.68 (0.53‐0.87) | 66 (10‐87) | 12, 4, .02 | – |
| Adjusted for smoking: Yes | 6 (3) | 1323 | 0.74 (0.62‐0.87) | 0.74 (0.62‐0.87) | 0 (0‐79) | 0.98, 2, .61 | – |
| Adjusted for smoking: No | 6 (6) | 504 | 0.47 (0.30‐0.74) | 0.46 (0.34‐0.63) | 49 (0‐79) | 11.8, 6, .07 | – |
| Adjusted for alcohol: Yes | 2 (2) | 352 | 0.74 (0.56‐0.99) | 0.74 (0.56‐0.99) | 0 (NA) | 0.97, 1, .32 | – |
| Adjusted for alcohol: No | 10 (8) | 1475 | 0.52 (0.35‐0.77) | 0.62 (0.52‐0.75) | 60 (13‐82) | 17, 7, .01 | – |
| Stratified by median deaths | |||||||
| Less than median deaths | 5 (5) | 286 | 0.56 (0.34‐0.91) | 0.57 (0.42‐0.79) | 47 (0‐79) | 9.4, 5, .09 | – |
| More than median deaths | 7 (4) | 1541 | 0.60 (0.42‐0.85) | 0.68 (0.57‐0.81) | 68 (7‐89) | 9.33, 3, .03 | – |
| Stratified by timing of physical activity assessment after in relation to cancer treatment | |||||||
| After the end of primary cancer treatment | 4 (4) | 687 | 0.83 (0.61‐1.12) | 0.94 (0.90‐0.98) | 0 | 0.41, 3, .94 | – |
| Stratified by physical activity intensity | |||||||
| Moderate | 2 (2) | 211 | 0.59 (0.38‐0.90) | 0.59 (0.38‐0.89) | 4 | 1.04, 1, .31 | – |
| Moderate to vigorous | 3 (3) | 528 | 0.77 (0.60‐0.99) | 0.77 (0.60‐0.99) | 0 (0‐83) | 1.20, 2, .52 | – |
| Vigorous | 2 (2) | 211 | 1.10 (0.74‐1.63) | 1.10 (0.74‐1.63) | 0 (n/a) | 0.00, 1, 1.00 | – |
Note: Where the number of studies differs from the number of publications is because we have used the pooling project ABCPP in analyses.
Abbreviations: BMI, body mass index; CI, confidence interval; df, degrees of freedom; HR, hazard ratio; MET, metabolic equivalent of task.
Not all studies that are included in the analyses reported the number of events. Bradshaw 2014 (LIBCSP) did not provide the number of events for each BMI subgroup.
Bradshaw 2014 (LIBCSP) is used in analysis.
Women who participated in moderate intensity recreational physical activity had 41% lower risk of breast cancer‐specific mortality (HR = 0.59, 95% CI: 0.38‐0.90; I 2 = 4%; P‐heterogeneity = .31, two studies, 79 , 81 deaths = 211; Figure S18 and Table 2). There was a marginal inverse association for moderate‐to‐vigorous recreational activities (HR = 0.77, 95% CI: 0.60‐0.99; I 2 = 0%; P‐heterogeneity = .52, three studies, 67 , 76 , 79 deaths = 528). There was no association between vigorous physical activity and breast cancer‐specific mortality (HR = 1.10, 95% CI: 0.74‐1.63; I 2 = 0%; two studies, 79 , 81 deaths = 211; Figure S18 and Table 2).
In linear dose‐response meta‐analysis that investigated the association between recreational physical activity and breast cancer‐specific mortality after primary cancer treatment, a marginal inverse association was observed (HR per 10 MET‐h/week = 0.91, 95% CI: 0.80‐1.04; I 2 = 69%, P‐heterogeneity = .04, three studies, 67 , 74 , 83 deaths = 585). Findings were similar in categorical meta‐analysis (HR = 0.83, 95% CI: 0.61‐1.12, I 2 = 0.0%, P‐heterogeneity = .99, four studies 67 , 74 , 79 , 83 deaths = 687; Figure S19 and Table 2).
Subgroup analyses by adjustment factors and number of deaths gave similar results to the main analyses (Table 2).
3.3. Recreational physical activity and breast cancer recurrence
Recreational physical activity was not associated with the risk of breast cancer recurrence. Six studies 74 , 75 , 89 were included in categorical and dose‐response analyses. The 95% CI included the null; both in linear dose‐response (HR per 10 MET‐h/week = 0.97, 95% CI: 0.91‐1.05; I 2 = 68%, P‐heterogeneity = .05, events = 1705) and categorical analyses (HR = 0.80, 95% CI: 0.56‐1.14; I 2 = 60%, P‐heterogeneity = .08, events = 1705) (Figure 2E,F). Data were insufficient to conduct further analyses.
3.4. Recreational activity and second primary cancers
No data for meta‐analysis or descriptive synthesis.
3.5. Evidence grading
The judgement of the independent Expert Panel is presented in Table S8. The Expert Panel concluded that the observed dose‐response relationship for all‐cause and breast cancer‐specific mortality was limited by the methodological quality of the studies. The diversity of breast cancer subtypes and patient characteristics could not be appropriately assessed in the available studies, and control of treatment completion was considered inadequate. The Expert Panel judged the evidence for the likely causality for the association between recreational physical activity and lower risk of all‐cause and breast cancer‐specific mortality as ‘limited‐suggestive’.
4. DISCUSSION
Our systematic review and meta‐analysis showed that higher levels (up to 20 MET‐h/week) of postdiagnosis recreational physical activity were associated with a 48% lower all‐cause and a 38% lower breast cancer‐specific mortality (10 studies). Twenty MET‐h/week is equivalent to about 5 hours of moderate‐intensity physical activity/week (assuming 4 METS for moderate activity). 94 There was little further reduction in risk at higher levels of activity. There was no evidence of an association with risk of breast cancer recurrence in linear dose‐response meta‐analysis, but the number of included studies was smaller. Subgroup analyses (by BMI, hormone receptor, menopausal status, restricted to assessing physical activity after primary cancer treatment, adjustment variables and number of deaths) were generally consistent in direction and magnitude to the main analyses.
Strengths of our review include the comprehensive search strategy covering literature up to 31st October 2021, the predefined subgroup analyses and the rigorous independent process of evaluating the evidence by the independent Expert Panel. Our results are consistent with previously published reviews and meta‐analyses with similar inclusion criteria and methods. 10 , 12 , 13 , 14 , 15 , 16 , 95 , 96 Five recent meta‐analyses 10 , 12 , 15 , 16 , 17 published between 2019 and 2020 consistently reported that women at the highest levels of physical activity had lower (summary HR range = 0.58‐0.74) risk of all‐cause and breast‐cancer specific mortality (summary HR range = 0.59‐0.72) compared to those at the lowest physical activity levels. Three 15 , 16 , 17 of these five meta‐analyses only conducted categorical analyses. Geidl et al 12 reported that for every 10 MET‐h/week increase of physical activity there was a 22% lower risk of all‐cause mortality. These results are in line with our observed 15% lower risk of all‐cause mortality. Our review is the first one to conduct both categorical and linear dose–response meta‐analyses for the three core outcomes that is, all‐cause, breast cancer‐specific mortality and recurrence. The accumulated data allowed the performance of linear and nonlinear dose–response meta‐analyses that were not possible in the 2012 SLR. 4
The studies identified and evaluated have limitations. All the included studies assessed physical activity via self‐reported or interview‐based validated questionnaires. This is a potential source of measurement error due to recall and social desirability bias. 97 Self‐reporting is usually associated with overreporting exercise 98 when compared to objective, device‐based, measurements. 99 Furthermore, variability in physical activity assessment methods across different studies could have created misclassification of the physical activity definition and introduced heterogeneity in the evidence synthesis. Physical activity assessment was implemented once, at the beginning of the studies with no subsequent measurements that could have informed on possible changes during follow‐up. Physical activity encompasses a heterogenous and complex set of behaviours including everyday activities (eg, housework), transportation and occupational activities, which may be performed at different patterns and multiple times throughout a day. 98 Overall patterns of activity are difficult to assess and it is clear that the current literature is concentrated on recreational physical activity while studies on other physical activity domains are limited. Studies on sedentary lifestyle are also limited. Only one study was found examining the effect of sedentary time on breast cancer survival. 93 While our study found no association between television watching time and all‐cause mortality, additional research in larger cohorts of breast cancer survivors is warranted to clarify the prognostic effect of sedentary behaviour. It is important to study the effect of both physical activity and sedentary behaviour on breast cancer survivorship. Various ongoing prospective cohort studies are assessing physical activity, sedentary lifestyle, and cardiorespiratory fitness in relation to prognosis in breast cancer survivors. 100 , 101 , 102 The Alberta Moving Beyond Breast Cancer (AMBER) is the first large ongoing prospective cohort study that aims to address limitations of existing observational studies conducted to date by including objective measurements for physical activity, sedentary behaviour and health‐related fitness at multiple time points postdiagnosis in relation to breast cancer survival. 100
Our work highlighted that among women with breast cancer, few studies investigate associations by varying physical activity intensities and mortality. The inverse association between physical activity with all‐cause and breast cancer‐specific mortality appeared to be stronger (but CIs overlapped) with moderate‐intensity physical activity compared to vigorous activity. While this is an important finding, we acknowledge that these analyses were based on a limited number of studies and more research is warranted in this area. In terms of methodology, this observation could be attributable to measurement error in different exercise intensities. It should be noted that null results in some meta‐analyses with few studies and wide CIs may not imply absence of an association and more studies are needed to draw definitive conclusions. It is likely that women with more advanced cancer were unable to participate or died before an opportunity to participate in the study and therefore we cannot exclude survival bias. Also, some studies reported differences between eligible study participants and nonparticipants (eg, higher proportion of older women among participants 79 ; nonparticipants more likely to have stage I breast cancer 71 ). Selection bias may have an unpredictable impact on study results. Moreover, standardised, cancer‐specific recurrence definitions are required to consistently assess this body of epidemiologic evidence. 103 We performed a subgroup analysis restricted to studies that assessed physical activity after treatment completion and while we acknowledge the importance of assessing the role of physical activity across the cancer continuum, the level of physical activity during cancer treatment may not be entirely representative of posttreatment physical activity levels. 104 It is also possible that the recommended physical activity levels during treatment may differ from the recommended levels postcancer treatment. 105 Moreover, physical activity after treatment is considered a less variable measure and therefore associations with outcomes (especially for women with breast cancer who survive long term) are important to clarify and would allow more targeted interventions and recommendations.
Mechanisms that may contribute to reduced cancer progression mediated by physical activity have been proposed. 103 , 106 These include alterations in sex hormones, metabolic dysregulation, chronic low‐grade inflammation, immune function, oxidative stress and gene mutations. It is unclear if physical activity independently influences these pathways or if it exerts its action via reductions in adipose tissue volume. In addition, prolonged sedentariness is hypothesised to be a cancer prognosis risk factor that may act independently of physical inactivity and body fat, by elevating circulating concentrations of numerous protumorigenic growth factors and hormones. 103 , 107 Animal model studies have shown that exercise can normalise abnormal tumour blood vessels leading to greater tumour oxygenation and perfusion. Exercise and drug delivery could act in a synergistic manner to increase treatment efficacy. 17 More than half of women with breast cancer gain weight during and after cancer treatment that is correlated with the treatment duration and type. 82 , 108 , 109 Changes, such as higher fat mass and maintenance or lean tissue atrophy, increase the risk of comorbidities such as cardiovascular disease or type 2 diabetes, which consequently impact recurrence and survival. 108 Promoting exercise during and after cancer treatment could therefore help to prevent critical alterations in body composition and possibly improve breast cancer prognosis. 108
The 2019 ACSM updated recommendations, 7 concluded that sufficient evidence supports that exercise training is safe throughout cancer treatment and could facilitate improvements in cancer‐related health outcomes. The authors have provided the ‘Moving Through Cancer’ guidelines 8 to aid oncologists and practitioners to continue prescribing exercise. Our review supports the 2018 WCRF/AICR Cancer Prevention Recommendations that is, for women with breast cancer diagnosis to be physically active for at least 150 minutes a week and sit less, when they can and as advised by their cancer management team. 4 These recommendations are in line with the guidelines issued by other authoritative organisations, such as the American Cancer Society (ACS), 110 World Health Organisation (WHO) 111 and US Department of Health and Human Services Physical Activity Guidelines Advisory (PAGA) 2018, 112 for those with chronic medical conditions, including cancer. Certain unique aspects of the CUP Global work are that it offers up to date meta‐analyses of physical activity and several prognosis‐related outcomes that have not been well‐studied in the past (all‐cause and breast cancer‐specific mortality, recurrence). Nonlinear meta‐analyses were also performed to better characterise the shape of the associations and important predefined subgroup and sensitivity analyses. The evidence was then reviewed and graded by the independent Expert Panel using a standardised grading system that is evaluating several nutrition‐related factors and survivorship.
Considering the potential methodological limitations of this observational body of evidence, the independent Expert Panel did not reach a unanimous decision on physical activity and breast cancer prognosis and therefore agreed to cautiously grade the evidence as ‘limited‐suggestive’. The Expert Panel could not rule out residual confounding, selection bias and reverse causation as a factor in the observed associations. In general, most of the studies we reviewed controlled for age, stage, and type of treatment, but less studies adjusted for other critical factors such as alcohol consumption and smoking. No study adjusted for cancer treatment dose and completion. Few studies adjusted for prediagnosis physical activity and other relevant important prediagnosis exposures. Moreover, variables such as BMI could be in the causal pathway between physical activity and outcomes after breast cancer diagnosis therefore studies should consider this in analyses. 113 Carefully designed prospective cohort studies with long follow‐up, repeated measures, individual‐level data and objective measures of physical activity are needed to further solidify our results, determine the most beneficial levels of activity, and further investigate these associations across important subgroups. Well‐designed RCTs of physical activity with recurrence and survival outcomes could be useful in addressing limitations of observational studies such as residual confounding and reverse causation. 113 , 114
In conclusion, our findings provide limited but suggestive evidence that higher postdiagnostic levels of recreational physical activity are beneficial and can lower mortality rates in women diagnosed with breast cancer. These results support the development of lifestyle recommendations for breast cancer survivors to be physically active if they can and depending on their specific medical advice.
AUTHOR CONTRIBUTIONS
Konstantinos K. Tsilidis and Doris S. M. Chan are coprincipal investigators of CUP Global at Imperial College London. Konstantinos K. Tsilidis was part of the Expert Panel but was not involved with judging the evidence after becoming a coprincipal investigator of CUP Global. Teresa Norat and Doris S. M. Chan wrote the protocol based on the advice from the Protocol Expertise Group and implemented the study with Konstantinos K. Tsilidis. Doris S. M. Chan and Neesha Nanu did the literature search. Leila Abar, Katia Balducci, Nerea Becerra‐Tomas, Margarita Cariolou, Neesha Nanu and Rita Vieira did the study selections and data extraction. Margarita Cariolou checked, analysed and interpreted the data. Dagfinn Aune, Georgios Markozannes and Nerea Becerra‐Tomás were CUP Global team members who revised the manuscript. Darren C. Greenwood was statistical adviser. Anne McTiernan, Steven K. Clinton, Edward L. Giovannucci, Ellen Kampman, Alan A. Jackson, Konstantinos K. Tsilidis, Marc J. Gunter and Vivien Lund (lay member) were the WCRF/AICR Expert Panel members who provided judgements on the evidence and advised on the interpretation of the review. Elio Riboli and Amanda J. Cross were Expert Panel observers. Kate Allen, Nigel T Brockton, Helen Croker, Daphne Katsikioti, Deirdre McGinley‐Gieser, Panagiota Mitrou and Martin Wiseman were the CUP Global Secretariat members who provided overall coordination for the work and convened and facilitated discussions with the Expert Panel. Margarita Cariolou drafted the original manuscript. All authors reviewed and provided comments on the manuscript. Doris S. M. Chan is the guarantor and has full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. The work reported in the article has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
This work was funded by the World Cancer Research Fund network of charities (American Institute for Cancer Research (AICR); World Cancer Research Fund (WCRF); Wereld Kanker Onderzoek Fonds (WKOF)) (CUP GLOBAL Special Grant 2018). Konstantinos K. Tsilidis, Doris S. M. Chan, Rita Vieira, Dagfinn Aune, Katia Balducci, Margarita Cariolou, Georgios Markozannes and Nerea Becerra‐Tomás are supported by the World Cancer Research Fund network of charities. Leila Abar and Neesha Nanu were previously supported by the World Cancer Research Fund network of charities. Teresa Norat was supported by the World Cancer Research Fund network of charities as principal investigator of the WCRF/AICR Continuous Update Project (CUP) and by WCRF Internationl as the CUP Global scientific advisor. Dr McTiernan was supported by grants from the Breast Cancer Research Foundation (BCRF‐19‐107/BCRF‐20‐107/BCRF‐21‐107). The funders of our study had no role in the decisions about the design and conduct of the study; collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The process used was based on the method developed by WCRF International's Methodology Task Force for the WCRF/AICR Second Expert Report. The CUP Global Secretariat, led by WCRF International, provided overall coordination for the work and convened and facilitated discussions with a WCRF/AICR Expert Panel who provided judgements on the evidence. The views expressed in this review are the opinions of the authors. They may differ from those in future updates of the evidence related to food, nutrition, physical activity, and cancer incidence and survival.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
Supporting information
Appendix S1. Supporting information
ACKNOWLEDGEMENTS
We thank Teresa Norat for leading the WCRF/AICR Continuous Update Project (WCRF/AICR‐CUP) as principal investigator from 2007 to 2020. We thank the Protocol Expertise Group: Annie Anderson (University of Dundee), Steven Clinton (The Ohio State University), Ellen Copson (Southampton University), Wendy Demark‐Wahnefried (UAB Comprehensive Cancer Center, Birminham, AL), John Mathers (Newcastle University), Anne McTiernan (Fred Hutchinson Cancer Research Center), Andrew Renehan (University of Manchester), Lesley Turner (patient representative), Franzel van Duijnhoven (Wageningen University) and Galina Velikova (University of Leeds), for their expert opinion on the review protocol. We thank the CUP Global team members: Sonia Chemlal, Jakub Sobiecki, Britta Talumaa and Victoria White, for their contribution to the literature search and data extraction; and database managers: Yusuf O. Anifowoshe, Christophe Stevens, Lam Teng and Rui Vieira, for implementing and updating the CUP Global database. We also acknowledge the input of Isobel Bandurek and Susannah Brown as past CUP Global Secretariat members.
Cariolou M, Abar L, Aune D, et al. Postdiagnosis recreational physical activity and breast cancer prognosis: Global Cancer Update Programme (CUP Global) systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):600‐615. doi: 10.1002/ijc.34324
Konstantinos K. Tsilidis and Doris S. M. Chan contributed equally and share last authorship.
Funding information American Institute for Cancer Research (AICR), Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018; Breast Cancer Research Foundation, Grant/Award Number: BCRF‐19‐107/BCRF‐20‐107/BCRF‐21‐107; Wereld Kanker Onderzoek Fonds, Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018; World Cancer Research Fund, Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018
DATA AVAILABILITY STATEMENT
Only publicly available data were used in our study. Data sources and handling of these data are described in the Materials and Methods section. Further details are available from the corresponding author upon request.
REFERENCES
- 1. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today. Accessed February 15, 2021 [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
- 3. Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000‐2012. JAMA. 2018;319(2):154‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Cancer Research Fund International/American Institute for Cancer Research . Diet, Nutrition, Physical activity and cancer: A Global Perspective. Continuous Update Project Report . 2018. https://www.wcrf.org/dietandcancer. Accessed May 18, 2021
- 5. Bull FC, Al‐Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rezende LFM, Sá TH, Markozannes G, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2018;52(13):826‐833. [DOI] [PubMed] [Google Scholar]
- 7. Campbell KL, Winters‐Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown JC, Winters‐Stone K, Lee A, Schmitz KH. Cancer, physical activity, and exercise. Compr Physiol. 2012;2(4):2775‐2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friedenreich CM, Stone CR, Cheung WY, et al. Physical activity and mortality in cancer survivors: a systematic review and meta‐analysis. JNCI Cancer Spectr. 2019;4(1):pkz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones LW, Kwan ML, Weltzien E, et al. Exercise and prognosis on the basis of clinicopathologic and molecular features in early‐stage breast cancer: the LACE and pathways studies. Cancer Res. 2016;76(18):5415‐5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geidl W, Schlesinger S, Mino E, Miranda L, Pfeifer K. Dose‐response relationship between physical activity and mortality in adults with noncommunicable diseases: a systematic review and meta‐analysis of prospective observational studies. Int J Behav Nutr Phys Act. 2020;17(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta‐analysis of epidemiological studies. Acta Oncol. 2015;54(5):635‐654. [DOI] [PubMed] [Google Scholar]
- 14. Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res. 2016;22(19):4766‐4775. [DOI] [PubMed] [Google Scholar]
- 15. Spei ME, Samoli E, Bravi F, la Vecchia C, Bamia C, Benetou V. Physical activity in breast cancer survivors: a systematic review and meta‐analysis on overall and breast cancer survival. Breast. 2019;44:144‐152. [DOI] [PubMed] [Google Scholar]
- 16. Lee J. A meta‐analysis of the association between physical activity and breast cancer mortality. Cancer Nurs. 2019;42(4):271‐285. [DOI] [PubMed] [Google Scholar]
- 17. Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391‐2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Cancer Research Fund International/American Institute for Cancer Research Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Breast Cancer Survivors . 2014. https://www.wcrf.org/wp-content/uploads/2021/03/Breast-Cancer-Survivors-2014-Report.pdf. Accessed May 18, 2021.
- 19. Tsilidis KK, Cariolou M, Becerra‐Tomás N, et al. Post‐diagnosis body fatness, recreational physical activity, dietary factors and breast cancer prognosis: Global Cancer Update Programme (CUP Global) summary of evidence grading. Int J Cancer. 2023;152(4):635‐644. 10.1002/ijc.34320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Becerra‐Tomás N, Balducci K, Abar L, et al. Post‐diagnosis dietary factors, supplement use and breast cancer prognosis: post‐diagnosis dietary factors, supplement use and breast cancer prognosis: Global Cancer Update Programme (CUP global) systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):616‐634. 10.1002/ijc.34321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan DSM, Vieira R, Abar L, et al. Post‐diagnosis body fatness, weight change and breast cancer prognosis: Global Cancer Update Programme (CUP Global) systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):572‐599. 10.1002/ijc.34322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Global Cancer Update Programme (CUP Global). 2022 . https://www.wcrf.org/diet-activity-and-cancer/global-cancer-update-programme/about-the-global-cancer-update-programme/. Accessed September, 2022.
- 23. Imperial College London CUP Global Team . Cancer Update Programme on Diet and Cancer: Protocol for the Data Collection and Systematic Literature Reviews on the Role of Diet, Nutrition and Physical Activity on Outcomes After Diagnosis of Breast Cancer. Version 3 . 2019. https://www.imperial.ac.uk/school-public-health/epidemiology-and-biostatistics/research/cancer-and-nutritional-epidemiology/global-cancer-update-programme/. Accessed July 27, 2022.
- 24. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borugian MJ, Sheps SB, Kim‐Sing C, et al. Insulin, macronutrient intake, and physical activity: are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev. 2004;13(7):1163‐1172. [PubMed] [Google Scholar]
- 26. Abrahamson PE, Gammon MD, Lund MJ, et al. Recreational physical activity and survival among young women with breast cancer. Cancer. 2006;107(8):1777‐1785. [DOI] [PubMed] [Google Scholar]
- 27. Eng JA, Clough‐Gorr K, Cabral HJ, Silliman RA. Predicting 5‐ and 10‐year survival in older women with early‐stage breast cancer: self‐rated health and walking ability. J Am Geriatr Soc. 2015;63(4):757‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cleveland RJ, Eng SM, Stevens J, et al. Influence of prediagnostic recreational physical activity on survival from breast cancer. Eur J Cancer Prev. 2012;21(1):46‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Emaus A, Veierød MB, Tretli S, et al. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res Treat. 2010;121(3):651‐660. [DOI] [PubMed] [Google Scholar]
- 30. Hellmann SS, Thygesen LC, Tolstrup JS, Grønbæk M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev. 2010;19(5):366‐373. [DOI] [PubMed] [Google Scholar]
- 31. Friedenreich CM, Gregory J, Kopciuk KA, Mackey JR, Courneya KS. Prospective cohort study of lifetime physical activity and breast cancer survival. Int J Cancer. 2009;124(8):1954‐1962. [DOI] [PubMed] [Google Scholar]
- 32. Keegan TH, Milne RL, Andrulis IL, et al. Past recreational physical activity, body size, and all‐cause mortality following breast cancer diagnosis: results from the Breast Cancer Family Registry. Breast Cancer Res Treat. 2010;123(2):531‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keegan TH, Shariff‐Marco S, Sangaramoorthy M, et al. Neighborhood influences on recreational physical activity and survival after breast cancer. Cancer Causes Control. 2014;25(10):1295‐1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu Y, John EM, Sullivan‐Halley J, et al. History of recreational physical activity and survival after breast cancer: the California Breast Cancer Survivorship Consortium. Am J Epidemiol. 2015;181(12):944‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCullough LE, Chen J, Cho YH, et al. Modification of the association between recreational physical activity and survival after breast cancer by promoter methylation in breast cancer‐related genes. Breast Cancer Res. 2017;19(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McLaughlin VH, Trentham‐Dietz A, Hampton JM, et al. Lifestyle factors and the risk of a second breast cancer after ductal carcinoma in situ. Cancer Epidemiol Biomarkers Prev. 2014;23(3):450‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinkston CM, Baumgartner RN, Connor AE, Boone SD, Baumgartner KB. Physical activity and survival among Hispanic and non‐Hispanic white long‐term breast cancer survivors and population‐based controls. J Cancer Surviv. 2015;9(4):650‐659. [DOI] [PubMed] [Google Scholar]
- 38. Rohan TE, Fu W, Hiller JE. Physical activity and survival from breast cancer. Eur J Cancer Prev. 1995;4(5):419‐424. [DOI] [PubMed] [Google Scholar]
- 39. Schmidt ME, Chang‐Claude J, Vrieling A, et al. Association of pre‐diagnosis physical activity with recurrence and mortality among women with breast cancer. Int J Cancer. 2013;133(6):1431‐1440. [DOI] [PubMed] [Google Scholar]
- 40. Tao MH, Hainaut P, Marian C, et al. Association of prediagnostic physical activity with survival following breast cancer diagnosis: influence of TP53 mutation status. Cancer Causes Control. 2013;24(12):2177‐2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. West‐Wright CN, Henderson KD, Sullivan‐Halley J, et al. Long‐term and recent recreational physical activity and survival after breast cancer: the California Teachers Study. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2851‐2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Escala‐Garcia M, Morra A, Canisius S, et al. Breast cancer risk factors and their effects on survival: a Mendelian randomisation study. BMC Med. 2020;18(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buck K, Vrieling A, Zaineddin AK, et al. Serum enterolactone and prognosis of postmenopausal breast cancer. J Clin Oncol. 2011;29(28):3730‐3738. [DOI] [PubMed] [Google Scholar]
- 44. Johnsson A, Broberg P, Krüger U, Johnsson A, Tornberg ÅB, Olsson H. Physical activity and survival following breast cancer. Eur J Cancer Care (Engl). 2019;28(4):e13037. [DOI] [PubMed] [Google Scholar]
- 45. Delrieu L, Jacquet E, Segura‐Ferlay C, et al. Analysis of the StoRM cohort reveals physical activity to be associated with survival in metastatic breast cancer. Sci Rep. 2020;10(1):10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chubak J, Boudreau DM, Wirtz HS, McKnight B, Weiss NS. Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst. 2013;105(19):1456‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Savitz DA, Wellenius GA, Trikalinos TA. The problem with mechanistic risk of bias assessments in evidence synthesis of observational studies and a practical alternative: assessing the impact of specific sources of potential bias. Am J Epidemiol. 2019;188(9):1581‐1585. [DOI] [PubMed] [Google Scholar]
- 48. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 49. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135(11):1301‐1309. [DOI] [PubMed] [Google Scholar]
- 50. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose‐response data. Stata J. 2006;6(1):40‐57. [Google Scholar]
- 51. Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13(8):555‐565. [DOI] [PubMed] [Google Scholar]
- 52. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta‐analyses. Stat Med. 2010;29(12):1282‐1297. [DOI] [PubMed] [Google Scholar]
- 53. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 55. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta‐analyses. BMJ. 2007;335(7626):914‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta‐analysis. Res Synth Methods. 2010;1(2):112‐125. [DOI] [PubMed] [Google Scholar]
- 58. Egger M, Davey Smith G, Schneider M, et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Res Synth Methods. 2010;1(2):97‐111. [DOI] [PubMed] [Google Scholar]
- 60. Haidich A‐BJ. Meta‐analysis in medical research. Hippokratia. 2010;14(Suppl 1):29‐37. [PMC free article] [PubMed] [Google Scholar]
- 61. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820‐826. [DOI] [PubMed] [Google Scholar]
- 62. Team RC . R: A Language and Environment for Statistical Computing . 2020.
- 63. Schwarzer G. meta: an R package for meta‐analysis. R News. 2007;7(3):40‐45. [Google Scholar]
- 64. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;36(3):1‐48. [Google Scholar]
- 65. Crippa A, Orsini N. Multivariate dose‐response meta‐analysis: the dosresmeta R package. J Stat Softw. 2016;72(1):1‐15. [Google Scholar]
- 66. Wickham H, Averick M, Bryan J, et al. Welcome to the Tidyverse. Open J. 2019;4(43):1686. [Google Scholar]
- 67. Maliniak ML, Patel AV, McCullough ML, et al. Obesity, physical activity, and breast cancer survival among older breast cancer survivors in the Cancer Prevention Study‐II Nutrition Cohort. Breast Cancer Res Treat. 2018;167(1):133‐145. [DOI] [PubMed] [Google Scholar]
- 68. Veal CT, Hart V, Lakoski SG, et al. Health‐related behaviors and mortality outcomes in women diagnosed with ductal carcinoma in situ. J Cancer Surviv. 2017;11(3):320‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ammitzboll G, Sogaard K, Karlsen RV, et al. Physical activity and survival in breast cancer. Eur J Cancer. 2016;66:67‐74. [DOI] [PubMed] [Google Scholar]
- 70. Nechuta S, Chen WY, Cai H, et al. A pooled analysis of post‐diagnosis lifestyle factors in association with late estrogen‐receptor‐positive breast cancer prognosis. Int J Cancer. 2016;138(9):2088‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Borch KB, Braaten T, Lund E, Weiderpass E. Physical activity before and after breast cancer diagnosis and survival—the Norwegian women and cancer cohort study. BMC Cancer. 2015;15:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bao PP, Zhao GM, Shu XO, et al. Modifiable lifestyle factors and triple‐negative breast cancer survival: a population‐based prospective study. Epidemiology. 2015;26(6):909‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bradshaw PT, Ibrahim JG, Khankari N, et al. Post‐diagnosis physical activity and survival after breast cancer diagnosis: the Long Island Breast Cancer Study. Breast Cancer Res Treat. 2014;145(3):735‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. de Glas NA, Fontein DB, Bastiaannet E, et al. Physical activity and survival of postmenopausal, hormone receptor‐positive breast cancer patients: results of the Tamoxifen Exemestane Adjuvant Multicenter Lifestyle study. Cancer. 2014;120(18):2847‐2854. [DOI] [PubMed] [Google Scholar]
- 75. Beasley JM, Kwan ML, Chen WY, et al. Meeting the physical activity guidelines and survival after breast cancer: findings from the after breast cancer pooling project. Breast Cancer Res Treat. 2012;131(2):637‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Irwin ML, McTiernan A, Manson JE, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the women's health initiative. Cancer Prev Res (Phila). 2011;4(4):522‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bertram LA, Stefanick ML, Saquib N, et al. Physical activity, additional breast cancer events, and mortality among early‐stage breast cancer survivors: findings from the WHEL study. Cancer Causes Control. 2011;22(3):427‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen X, Lu W, Zheng W, et al. Exercise after diagnosis of breast cancer in association with survival. Cancer Prev Res (Phila). 2011;4(9):1409‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sternfeld B, Weltzien E, Quesenberry CP Jr, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):87‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Holmes MD, Chen WY, Hankinson SE, Willett WC. Physical activity's impact on the association of fat and fiber intake with survival after breast cancer. Am J Epidemiol. 2009;170(10):1250‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Holick CN, Newcomb PA, Trentham‐Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379‐386. [DOI] [PubMed] [Google Scholar]
- 82. Irwin ML, Smith AW, McTiernan A, et al. Influence of pre‐ and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958‐3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479‐2486. [DOI] [PubMed] [Google Scholar]
- 84. Palesh O, Kamen C, Sharp S, et al. Physical activity and survival in women with advanced breast cancer. Cancer Nurs. 2018;41(4):E31‐e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tarasenko YN, Linder DF, Miller EA. Muscle‐strengthening and aerobic activities and mortality among 3+ year cancer survivors in the U.S. Cancer Causes Control. 2018;29(4–5):475‐484. [DOI] [PubMed] [Google Scholar]
- 86. Williams PT. Significantly greater reduction in breast cancer mortality from post‐diagnosis running than walking. Int J Cancer. 2014;135(5):1195‐1202. [DOI] [PubMed] [Google Scholar]
- 87. Cao Y, Baumgartner KB, Visvanathan K, Boone SD, Baumgartner RN, Connor AE. Ethnic and biological differences in the association between physical activity and survival after breast cancer. NPJ Breast Cancer. 2020;6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cannioto RA, Hutson A, Dighe S, et al. Physical activity before, during, and after chemotherapy for high‐risk breast cancer: relationships with survival. J Natl Cancer Inst. 2021;113(1):54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jung AY, Behrens S, Schmidt M, et al. Pre‐ to postdiagnosis leisure‐time physical activity and prognosis in postmenopausal breast cancer survivors. Breast Cancer Res. 2019;21(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Akdeniz N, Kaplan MA, Küçüköner M, et al. The effect of exercise on disease‐free survival and overall survival in patients with breast cancer. Ir J Med Sci. 2021;191:1587‐1597. [DOI] [PubMed] [Google Scholar]
- 91. Hayes SC, Steele ML, Spence RR, et al. Exercise following breast cancer: exploratory survival analyses of two randomised, controlled trials. Breast Cancer Res Treat. 2018;167(2):505‐514. [DOI] [PubMed] [Google Scholar]
- 92. Courneya KS, Segal RJ, McKenzie DC, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc. 2014;46(9):1744‐1751. [DOI] [PubMed] [Google Scholar]
- 93. George SM, Smith AW, Alfano CM, et al. The association between television watching time and all‐cause mortality after breast cancer. J Cancer Surviv. 2013;7(2):247‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498‐S504. [DOI] [PubMed] [Google Scholar]
- 95. Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta‐analysis. Ann Oncol. 2014;25(7):1293‐1311. [DOI] [PubMed] [Google Scholar]
- 96. Ibrahim EM, Al‐Homaidh A. Physical activity and survival after breast cancer diagnosis: meta‐analysis of published studies. Med Oncol. 2011;28(3):753‐765. [DOI] [PubMed] [Google Scholar]
- 97. Doherty A, Smith‐Byrne K, Ferreira T, et al. GWAS identifies 14 loci for device‐measured physical activity and sleep duration. Nat Commun. 2018;9(1):5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gorzelitz J, Peppard PE, Malecki K, Gennuso K, Nieto FJ, Cadmus‐Bertram L. Predictors of discordance in self‐report versus device‐measured physical activity measurement. Ann Epidemiol. 2018;28(7):427‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Prince SA, Cardilli L, Reed JL, et al. A comparison of self‐reported and device measured sedentary behaviour in adults: a systematic review and meta‐analysis. Int J Behav Nutr Phys Act. 2020;17(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Friedenreich CM, Vallance JK, McNeely ML, et al. The Alberta moving beyond breast cancer (AMBER) cohort study: baseline description of the full cohort. Cancer Causes Control. 2022;33(3):441‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kwan ML, Ambrosone CB, Lee MM, et al. The pathways study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control. 2008;19(10):1065‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Paskett ED, Caan BJ, Johnson L, et al. The Women's Health Initiative (WHI) Life and Longevity After Cancer (LILAC) study: description and baseline characteristics of participants. Cancer Epidemiol Biomarkers Prev. 2018;27(2):125‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Friedenreich CM, Shaw E, Neilson HK, Brenner DR. Epidemiology and biology of physical activity and cancer recurrence. J Mol Med (Berl). 2017;95(10):1029‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kampshoff CS, van Mechelen W, Schep G, et al. Participation in and adherence to physical exercise after completion of primary cancer treatment. Int J Behav Nutr Phys Act. 2016;13(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Irwin ML, Ainsworth BE. Physical activity interventions following cancer diagnosis: methodologic challenges to delivery and assessment. Cancer Invest. 2004;22(1):30‐50. [DOI] [PubMed] [Google Scholar]
- 106. Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment‐related adverse effects. Epidemiol Rev. 2017;39(1):71‐92. [DOI] [PubMed] [Google Scholar]
- 107. Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise‐dependent regulation of the tumour microenvironment. Nat Rev Cancer. 2017;17(10):620‐632. [DOI] [PubMed] [Google Scholar]
- 108. Schmidt S, Monk JM, Robinson LE, Mourtzakis M. The integrative role of leptin, oestrogen and the insulin family in obesity‐associated breast cancer: potential effects of exercise. Obes Rev. 2015;16(6):473‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. van den Berg MM, Winkels RM, de Kruif JT, et al. Weight change during chemotherapy in breast cancer patients: a meta‐analysis. BMC Cancer. 2017;17(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rock CL, Thomson CA, Sullivan KR, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72(3):230‐262. [DOI] [PubMed] [Google Scholar]
- 111. WHO Guidelines on Physical Activity and Sedentary Behaviour . https://www.who.int/publications/i/item/9789240015128. Accessed August 11, 2021.
- 112. Physical Activity Guidelines for Americans . 2018. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed June 8, 2021.
- 113. Shiroma EJ, Lee IM. Can we proceed with physical activity recommendations if (almost) no clinical trial data exist on mortality? Br J Sports Med. 2018;52(14):888‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. McTiernan A, Friedenreich CM, Katzmarzyk PT, et al. Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc. 2019;51(6):1252‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information
Data Availability Statement
Only publicly available data were used in our study. Data sources and handling of these data are described in the Materials and Methods section. Further details are available from the corresponding author upon request.


