Abstract
Relugolix, the first orally active, nonpeptide gonadotropin‐releasing hormone receptor antagonist, is approved in the United States and the European Union for the treatment of adult patients with advanced prostate cancer. The recommended dosing regimen is a 360‐mg loading dose followed by a 120‐mg daily dose. Relugolix and testosterone concentration data and clinical information from two phase I studies, two phase II studies, and the phase III safety and efficacy study (HERO) were used to develop a population pharmacokinetic (PopPK) model and a semimechanistic population pharmacokinetic/pharmacodynamic (PopPK/PD) model that characterized relugolix exposure and its relationship to testosterone concentrations. Age, body weight, and Black/African American race had at most minimal effects on relugolix exposure or testosterone concentrations with no clinical relevance. Simulations using the PopPK/PD model confirmed the recommended dosing regimen of relugolix, with the median simulated testosterone concentrations predicted to achieve castration levels (< 50 ng/dL) and profound castration levels (< 20 ng/dL) by day 2 and day 9, respectively, and demonstrated that 97.3% and 85.5% of the patients remained at castration levels (< 50 ng/dL) upon temporary interruption of treatment for 7 days and 14 days, respectively. Collectively, simulations based on the PopPK and PopPK/PD models were consistent with actual data from clinical studies, reflecting the high predictiveness of the models and supporting the reliability of model‐based simulations. These models can be used to provide guidance regarding dosing recommendations under various circumstances (e.g., temporary interruption of treatment, if needed) for relugolix.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Relugolix, the first orally active, nonpeptide gonadotropin‐releasing hormone receptor antagonist, is approved in the United States and the European Union for the treatment of adults with advanced prostate cancer.
WHAT QUESTION DID THIS STUDY ADDRESS?
A population pharmacokinetic (PopPK) model and a semimechanistic pharmacokinetic/pharmacodynamic (PopPK/PD) model for testosterone suppression by relugolix were developed from phases I–III data, to identify covariates with clinical relevance, assess impact of temporary treatment interruption, and demonstrate testosterone recovery after treatment discontinuation.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The PopPK and PopPK/PD models characterized relugolix exposure and its relationship to testosterone concentrations. Age, body weight, race, and health status demonstrated limited/no clinically meaningful impact. Model‐based simulated testosterone profiles were consistent with clinical data for the approved relugolix dosing regimen (a 360‐mg loading dose, followed by a 120‐mg daily dose) and support temporary treatment interruption of ≤ 14 days, if necessary.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Quantitative pharmacology based on clinical data can be implemented to provide dosing recommendations for relugolix.
Prostate cancer is the second most common cancer in men and the fifth leading cause of cancer death in men, with over 1.2 million cases diagnosed and 358,000 deaths reported annually worldwide. 1 Prostate cancer is defined as advanced when it has metastasized beyond the prostate 2 ; the term “advanced” is also used for the disease with prostate‐specific antigen biochemical relapse following primary surgical or radiation therapy of curative intent, or for localized disease unlikely to be cured by local surgical or radiation therapy of curative intent. 3 , 4 Although the overall survival rate of patients with localized disease is high, it decreases dramatically with advanced disease, with a 5‐year survival rate ranging from 26% to 30%. 5 , 6
Testosterone (the primary androgen in men) stimulates prostate cancer cell growth, 7 and surgical or medical castration can induce prostate cancer cell death. 6 , 8 Hence, androgen deprivation therapy (ADT) is recommended by European and US clinical practice guidelines as the foundational treatment for patients with advanced prostate cancer. 9 , 10 Medical castration with testosterone concentrations < 50 ng/dL has been associated with decreased metastases of the primary tumor 11 and has been accepted by regulatory authorities to establish efficacy for prostate cancer treatments. 12 , 13 Profound castration with testosterone concentrations < 20 ng/dL has been associated with improved clinical outcomes compared with testosterone concentrations < 50 ng/dL. 14 , 15 , 16 Upon disease progression to castration‐resistant prostate cancer, where extra‐testicular production of testosterone from the adrenal gland and prostate carcinoma cells develops, tumor growth remains androgen dependent, 17 and therefore treatment with ADT is continued and other treatments with complementary mechanisms of action are co‐prescribed. 18
Gonadotropin‐releasing hormone (GnRH) receptor agonists and antagonists decrease testicular production of testosterone and are therefore used as ADT, 19 with the injectable GnRH receptor agonist (peptide analog) leuprolide acetate being the current mainstay of prostate cancer treatment. 20 , 21 GnRH receptor agonists initially stimulate testicular testosterone production, which leads to an increase in systemic testosterone concentrations, but ultimately causes desensitization and downregulation of GnRH receptors in the anterior pituitary. Consequently, testicular production of testosterone is reduced, with castration levels of testosterone being reached within 4 weeks following treatment initiation. 21 However, the initial testosterone surge can be associated with adverse clinical effects in some patients (e.g., spinal cord compression, pathologic bone fracture, bladder outlet obstruction, and, consequently, death). 22 First‐generation androgen receptor inhibitors (e.g., bicalutamide) are often co‐prescribed to avoid the initial testosterone surge. 23 In addition, until recently, ADT products were only available as injectable depot formulations, which are not only associated with injection‐site reactions, 24 but therapeutic or adverse effects may persist for months following discontinuation of treatment. 25
Relugolix (ORGOVYX), the first orally active, nonpeptide GnRH receptor antagonist, is currently approved in the United States and the European Union for the treatment of adult patients with advanced prostate cancer, with a recommended dosing regimen of a 360‐mg loading dose followed by a 120‐mg daily dose. 26 , 27 By directly blocking GnRH signals, relugolix lowers systemic testosterone concentrations immediately at the start of treatment without an initial testosterone surge. 28 Differences in mechanism of action, formulation, and route of administration result in rapid onset and offset of relugolix action that may provide therapeutic benefits compared with leuprolide acetate and other ADT products. Population pharmacokinetic (PopPK) and semimechanistic PopPK/pharmacodynamic (PopPK/PD) models were developed to characterize the relugolix exposure and its relationship to systemic testosterone concentrations. Relugolix and testosterone concentration data, demographic and clinical information from two phase I studies, two phase II studies, and the phase III safety and efficacy study (HERO) were combined to create a pooled dataset for model development. Simulations using the PopPK and PopPK/PD models were conducted to confirm the recommended relugolix dosing regimen, identify covariates that impact relugolix or testosterone concentrations, assess the impact of temporary treatment interruption on testosterone concentrations, and describe the time‐course of testosterone concentrations following treatment discontinuation.
METHODS
Study designs
Five clinical studies (two phase I studies (C27001, TB‐AK160108), two phase II studies (C27002 and C27003), and the phase III study (HERO)) were included in the PopPK and PopPK/PD analyses. Protocols for all studies were approved by an independent review board prior to study initiation, and all participants provided written informed consent prior to screening. All studies were conducted in accordance with ethical principles originating in the Declaration of Helsinki and in compliance with Good Clinical Practice guidelines and applicable regulations. An overview of the designs of these five clinical studies is provided as Supplementary Material .
Participants
Healthy adult men were enrolled in one phase I study (C27001) to evaluate the safety, tolerability, PKs, and PDs of relugolix. Adult men with prostate cancer were enrolled in the other four studies (TB‐AK160108, C27002, C27003, and HERO). Inclusion and exclusion criteria are listed in the Supplementary Material section.
Pharmacokinetics and pharmacodynamics
Relugolix plasma concentrations and testosterone serum concentrations were determined using validated high‐performance liquid chromatography with tandem mass spectrometry methods, with isotopically labeled compounds used as internal standards. The lower limit of quantitation (LLOQ) for relugolix in plasma was 0.01 ng/mL for the assays used in the 2 phase I studies and 0.05 ng/mL for assays used in the other 3 studies. The LLOQ of testosterone in serum was 0.5 ng/dL for all 5 clinical studies.
PopPK and PopPK/PD model development
In the development of the PopPK model for relugolix, the structural model was established using relugolix concentration data from the four phase I and II studies. One‐, two‐, and three‐compartment distribution models with linear absorption, linear elimination, and absorption lag‐time were assessed as potential structural models. Parameters related to relugolix absorption and distribution into peripheral compartments were subsequently fixed upon addition of sparsely sampled relugolix concentration data from the HERO study. A PopPK/PD model based on the individual post hoc estimates from the PopPK model and the testosterone concentration data from the five studies was subsequently developed. The PopPK/PD model was based on a previously published semimechanistic model by Romero et al., 29 which included the primary components of the hypothalamic–pituitary‐gonadal (HPG) axis (endogenous GnRH, GnRH receptors, and testosterone), and adapted to incorporate the mechanism of action for relugolix (GnRH receptor antagonist) and physiological feedback mechanisms to optimize characterization of testosterone concentrations over time during treatment.
The PopPK and PopPK/PD analyses were performed using a nonlinear mixed‐effects modeling approach using NONMEM software (version 7.3; ICON Development Solutions, Ellicott City, MD, USA). Model selection was based on the log‐likelihood criterion, goodness‐of‐fit (GoF) plots, and biological plausibility. 30 Interindividual variability (IIV) in the parameters of the PopPK and PopPK/PD models was investigated, and the influence of the potential covariates (age, weight, body mass index, and race) on IIV was assessed graphically and tested by stepwise covariate modeling. 31 A stepwise forward addition with reduction in the objective function value (OFV) ≥ 6.63 (P < 0.01, degrees of freedom = 1) and backward deletion with increase in the OFV > 10.83 (P ≤ 0.001, degrees of freedom = 1) was used to identify covariates to be retained in the model. The final model was evaluated using GoF plots and prediction‐corrected visual predictive checks (pcVPC), 32 and parameter uncertainty was assessed by sampling importance resampling (SIR). 33 Health status (healthy men vs. patients with prostate cancer) and cancer stage based on tumor‐node‐metastasis staging 34 were assessed as potential covariates in the final PopPK and PopPK/PD models, with P < 0.001 as the critical significance level. External validation for the PopPK model was performed by evaluating the GoF plots, pcVPC, and normalized prediction distribution error (NPDE) for the relugolix data from an additional 87 men enrolled from the HERO amendments.
PopPK and PopPK/PD simulations
Simulations using the PopPK model to assess the impact of covariates on relugolix exposure, defined as relugolix trough concentration at steady‐state (Ctrough,ss) and area under the concentration‐time curve (AUC) over the dosing interval at steady‐state (AUCss), were performed using two approaches. For the univariate approach, covariate effects on relugolix exposure were assessed in isolation by keeping all other covariates fixed to their reference values in the phase III study population (non‐Black/African American race; median body weight 80 kg; median age 72 years); vectors of covariates were sampled with replacement from the phase III population and each simulation included 1,000 individuals. For the multivariate approach, covariate effects on relugolix exposure were assessed collectively as the combination of observed covariate distributions associated with each subgroup of the phase III study population, to illustrate the exposure variation within and between subgroups. A virtual population of 10,000 patients was created using sampling with replacement from the phase III study population, to achieve a larger sample size that was anticipated to improve precision on the predicted impact of covariate effects and to better represent the general advanced prostate cancer population. Ctrough,ss and AUCss of relugolix for the virtual population were simulated using the Monte‐Carlo method and were summarized above and below the median value of continuous covariates and by categories of categorical covariates.
Simulations using the PopPK/PD model were performed to confirm the recommended dosing regimen of relugolix, and included: (i) the impact of covariates on testosterone suppression; (ii) the time‐course of testosterone concentrations with different dosing regimens; (iii) the impact of various durations of temporary treatment interruption; and (iv) the time‐course of testosterone concentrations after discontinuation of treatment. For each scenario, a virtual population of 1,000 patients was created by sampling with replacement from the phase III study population. Individual relugolix plasma concentrations simulated by the PopPK model were used in the PopPK/PD model to simulate the associated individual testosterone concentrations. Complete (100%) adherence with daily dosing was assumed for all scenarios, unless otherwise indicated.
RESULTS
Participant demographics
Demographics for participants enrolled in the five clinical studies for the PopPK and PopPK/PD analyses are summarized in Table 1 .
Table 1.
Demographic and clinical information for participants enrolled in the five clinical studies included in the PopPK and PopPK/PD analyses
| Study phase | Phase I | Phase II | Phase III | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| Study number | TB‐AK160108 | C27001 | C27002 | C27003 | HERO | |||
| (N = 43) | (N = 74) | (N = 110) | (N = 65) | For the development of the PopPK and PopPK/PD models (N = 598) | For the validation of the PopPK model (N = 87) | Participants with updated PK observations (N = 22) | (N = 999) | |
| Age, years | ||||||||
| Mean (SD) | 74.0 (5.00) | 55.6 (7.72) | 72.2 (8.64) | 70.2 (5.65) | 71.2 (7.74) | 70.5 (8.18) | 71.7 (7.96) | 70.2 (8.72) |
| Median (min, max) | 74.0 (65.0, 82.0) | 53.5 (45.0, 74.0) | 73.0 (49.0, 91.0) | 71.0 (52.0, 86.0) | 72.0 (48.0, 91.0) | 70.0 (52.0, 88.0) | 70.5 (57.0, 85.0) | 71.0 (45.0, 91.0) |
| Race | ||||||||
| White | 0 | 69 (93.2) | 88 (80.0) | 58 (89.2) | 419 (70.1) | 58 (66.7) | 13 (59.1) | 705 (70.6) |
| Asian | 43 (100) | 2 (2.7) | 3 (2.7) | 0 (0) | 120 (20.1) | 22 (25.3) | 7 (31.8) | 197 (19.7) |
| Black/African American | 0 | 1 (1.4) | 19 (17.3) | 7 (10.8) | 29 (4.8) | 4 (4.6) | 1 (4.5) | 61 (6.1) |
| Other (including multiple) | 0 | 2 (2.7) | 0 | 0 | 19 (3.2) | 2 (2.3) | 0 | 23 (2.3) |
| Not reported | 0 | 0 | 0 | 0 | 11 (1.8) | 1 (1.1) | 1 (4.5) | 13 (1.3) |
| Ethnicity | ||||||||
| Not Hispanic or Latino | 0 | 74 (100) | 101 (91.8) | 62 (95.4) | 537 (89.8) | 74 (85.1) | 19 (86.4) | 867 (86.8) |
| Hispanic or Latino | 0 | 0 | 8 (7.3) | 2 (3.1) | 51 (8.5) | 12 (13.8) | 1 (4.5) | 74 (7.4) |
| Not reported | 43 (100) | 0 | 1 (0.9) | 1 (1.5) | 10 (1.7) | 1 (1.1) | 2 (9.1) | 58 (5.8) |
| Weight, kg | ||||||||
| Mean (SD) | 61.6 (7.02) | 80.5 (9.24) | 91.3 (19.7) | 93.2 (17.9) | 81.5 (16.3) | 79.4 (18.7) | 80.2 (18.9) | 82.2 (17.5) |
| Median (min, max) | 61.0 (51.2, 87.7) | 79.0 (64.8, 107) | 88.7 (56.4, 193) | 90.9 (58.2, 146) | 79.8 (40.5, 146) | 77.9 (44.3, 142) | 81.3 (50.0, 119) | 80.4 (40.5, 193) |
| Body mass index, kg/m2 | ||||||||
| Mean (SD) | 22.9 (2.69) | 26.2 (2.74) | 28.9 (5.37) | 29.6 (4.95) | 27.3 (4.59) | 26.5 (5.42) | 27.4 (4.83) | 27.3 (4.78) |
| Median (min, max) | 23.4 (18.6, 33.0) | 25.9 (20.7, 32.0) | 27.9 (18.5, 54.7) | 28.9 (19.3, 42.6) | 26.9 (16.3, 47.6) | 25.7 (16.0, 45.3) | 27.6 (17.8, 39.2) | 26.7 (16.0, 54.7) |
| Missing | 0 | 0 | 0 | 0 | 2 (0.3) | 0 | 0 | 2 (0.2) |
| Stage of cancer | ||||||||
| No disease | 0 (0) | 74 (100) | 0 (0) | 0 (0) | 0 (0) | 74 (7.4) | ||
| Localized | 25 (58.1) | 0 (0) | 24 (21.8) | 50 (76.9) | 177 (25.0) | 276 (27.6) | ||
| Locally advanced | 5 (11.6) | 0 (0) | 12 (10.9) | 1 (1.5) | 189 (26.7) | 207 (20.7) | ||
| Metastatic | 0 (0) | 0 (0) | 14 (12.7) | 0 (0) | 284 (40.2) | 298 (29.8) | ||
| Not classifiable | 13 (30.2) | 0 (0) | 0 (0) | 3 (4.6) | 57 (8.1) | 73 (7.3) | ||
| Missing | 0 (0) | 0 (0) | 60 (54.5) | 11 (16.9) | 0 (0) | 71 (7.1) | ||
| Estimated glomerular filtration rate, mL/min/1.73 m 2 | ||||||||
| Mean (SD) | 96.8 (19.2) | 94.6 (14.2) | 77.7 (22.4) | 76.1 (18.1) | 84.5 (19.3) | 86.8 (25.5) | 85.8 (20.0) | 84.7 (20.4) |
| Median (min, max) | 95.0 (58.0, 152) | 93.8 (70.6, 135) | 79.5 (38.7, 158) | 74.7 (30.8, 122) | 84.9 (34.3, 154) | 78.7 (44.5, 232) | 82.7 (42.1, 126) | 84.9 (30.8, 232) |
Data are n (%) of participants, unless otherwise indicated.
Min, minimum; Max, maximum; PD, pharmacodynamic; PK, pharmacokinetic; PopPK, population pharmacokinetic; SD, standard deviation.
PopPK modeling and simulations
The dataset used to develop the PopPK model consisted of 12,174 relugolix plasma concentration‐time datapoints (11,205 (92%) datapoints with quantifiable concentrations; 888 (7.3%) predose and 81 (0.7%) postdose datapoints below the LLOQ) from 912 participants. Relugolix datapoints below the LLOQ were not included in the development of the PopPK model: predose relugolix concentrations below the LLOQ were expected; postdose relugolix concentrations below the LLOQ were < 1% of the relugolix data and, therefore, exclusion was considered appropriate. 35
The PKs of relugolix were characterized by a three‐compartment disposition model with first‐order absorption, a short (0.27 hours (16 minutes)) absorption lag time, and first‐order elimination (Figure S1 ). The slightly greater than proportional increase in exposure with dose can be explained by saturation of intestinal permeability glycoprotein (P‐gp) 26 and was characterized by an effect of dose on relative oral bioavailability. IIV on absorption rate constant (K a), apparent systemic clearance after oral administration (CL/F), and apparent volume of distribution of the central compartment (Vc/F) were included. Age, body weight, and Black/African American race were identified independently as covariates with statistical significance on CL/F; Black/African American race was also identified as a covariate with statistical significance on Vc/F. No apparent effects of other races (Asian, White, or other), health status, or cancer stage on model parameters were identified. A trend between creatinine clearance (CrCL; estimated by the Cockcroft‐Gault equation 36 ) and CL/F was observed visually, but this visual trend was reduced upon inclusion of age, body weight, and race in the final PopPK model. Because CrCL was highly correlated with body size and age, it was not tested in the stepwise covariate analysis. Because the estimated exponent for body weight on CL/F was small (0.322), a sensitivity analysis was performed by applying fixed allometric scaling with body weight using the exponents of 0.75 and 1 on clearance and volume parameters, respectively. This approach resulted in a reduced GoF of the model, as indicated by an increase in OFV of 78.3.
The parameter estimates of the final PopPK model, along with those from the sensitivity analysis, are summarized in Table S1 . Parameters in the final PopPK model were estimated with good precision (relative standard error (RSE) based on SIR ≤ 22.9%). The IIV on CL/F and Vc/F in the final PopPK model were only 2% less than the IIV in the base model, indicating that the included covariates (age, body weight, and Black/African American race) explained only a small proportion of the variability of the PKs of relugolix. The IIV was relatively high for Vc/F (122.9%) and K a (159.9%). The IIV on K a was also associated with a high shrinkage 37 (70.4%), which reflected the relatively low number of participants in the PopPK dataset providing relugolix concentration‐time datapoints during the absorption phase (the majority of the datapoints were from sparse (trough) sampling in the HERO study). The results from the sensitivity analysis showed similar parameter estimates with those of the final PopPK model. The PopPK model developed from the original dataset captured the central tendency and variability of the external validation dataset from the additional 87 patients from the HERO amendments, as shown by GoF plots (Figure S2 ), pcVPC, and NPDE (Figure S3 ).
In the univariate covariate setting, simulations of Ctrough,ss and AUCss of relugolix demonstrated that the magnitude of predicted relative change in the Ctrough,ss and AUCss at the extremes (2.5th and 97.5th percentiles) of the overall range of age and weight in the phase III study population were up to 18.2% compared with the Ctrough,ss and AUCss at the median age and weight, and Black/African American race was associated with 32.8% and 39.3% higher Ctrough,ss and AUCss, respectively, compared with non‐Black/African American races (Figures 1 and 2 ). However, based on the multivariate covariate simulations with a virtual population of 10,000 individuals, Ctrough,ss and AUCss for Black/African American men were 26% and 16% higher, respectively, compared with non‐Black/African American men. The impact of age and weight on the Ctrough,ss and AUCss of relugolix was small, with median differences up to 17% between patients above and below the median age of 72 years and up to 18% between patients above and below the median body weight of 80 kg.
Figure 1.
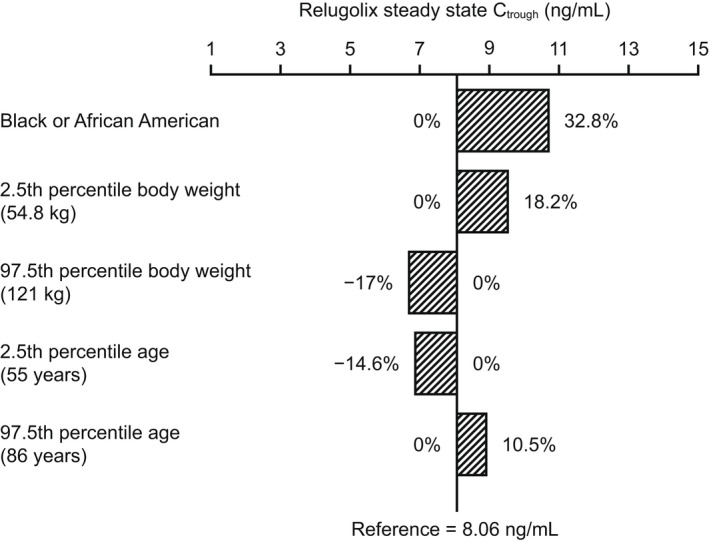
Univariate impact of covariates on relugolix Ctrough,ss upon the recommended dosing regimen (a 360‐mg loading dose, followed by a 120‐mg daily dose). Numbers represent percentage change from the Ctrough,ss value in a reference man in the phase III study population: non‐Black/African American race, median body weight (80 kg), and median age (72 years). Bars represent the relative change (%) from the reference value. Ctrough,ss, trough relugolix concentration at steady‐state.
Figure 2.
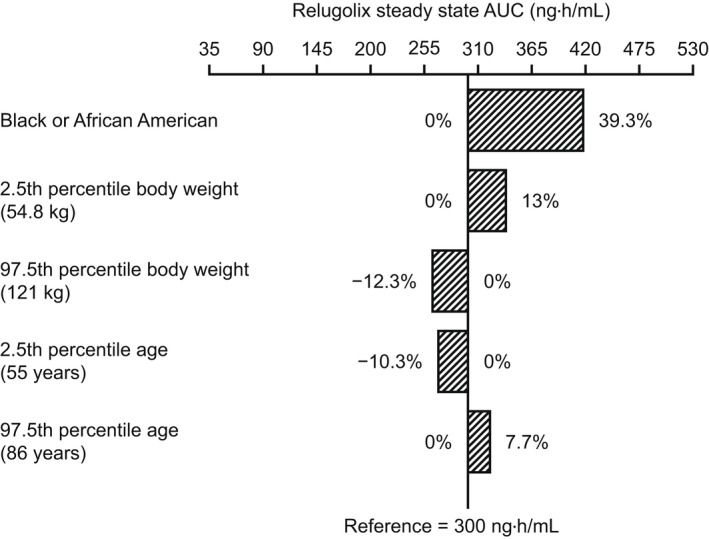
Univariate impact of covariates on relugolix AUCss upon the recommended dosing regimen (a 360‐mg loading dose, followed by a 120‐mg daily dose). Numbers represent percentage change from the AUCss value in a reference man in the phase III study population: non‐Black/African American race, median body weight (80 kg), and median age (72 years). Bars represent the relative change (%) from the reference value. AUCss, area under the relugolix concentration‐time curve over a dosing interval at steady‐state.
PopPK/PD modeling and simulations
The dataset used to develop the PopPK/PD model consisted of 14,491 testosterone serum concentration‐time datapoints (13,983 (96.5%) datapoints with quantifiable concentrations; 131 (0.9%) datapoints below LLOQ and 377 (2.6%) above upper limit of quantification (ULOQ)) from 899 of the 912 participants included in the PopPK dataset. Only participants with both relugolix and testosterone data available were kept for the PopPK/PD analysis. Testosterone concentrations below the LLOQ and above the ULOQ were < 5% of the testosterone data; therefore, exclusion of these observations from the analysis was considered appropriate. 35
The final PopPK/PD model included production and degradation processes for endogenous GnRH, GnRH receptors, and testosterone, with competitive and reversible inhibition of endogenous GnRH binding to GnRH receptors by relugolix, downregulation of GnRH receptor production driven by activation of the GnRH receptor, and a turnover model representing the dynamics of the latent unobserved endogenous GnRH with a modulation of its production by testosterone concentrations (Figure 3 ). IIV on testosterone production rate constant (KinT) was included, for which age was identified as a covariate with statistical significance. Body weight, race, health status, or cancer stage did not have a statistically significant effect on KinT. IIV on activation of the GnRH receptor (DR50; defined as level of GnRH receptor activation at which receptor downregulation is at 50% of maximum) was also assessed; however, inclusion of IIV on DR50 led to instability and non‐normally distributed random effects and was therefore not retained in the final PopPK/PD model. The parameter estimates of the final PopPK/PD model are summarized in Table S2 . Parameters in the PopPK/PD model were estimated with good precision (RSE based on SIR ≤ 17.6%). The IIV on KinT was 45.7% with a low shrinkage 37 (2.8%), reflecting the remaining variability of KinT after incorporation of age effect and the large amount of data available across participants. Standard GoF plots showed no overall bias (Figure S4 ), indicating an appropriate characterization of testosterone concentrations during treatment with relugolix by the PopPK/PD model. The testosterone elimination half‐life derived from the estimate of testosterone degradation rate (KouT) as ln (2)/KouT was 7.6 hours, which is consistent with values reported in the literature. 29 , 38 , 39 , 40
Figure 3.
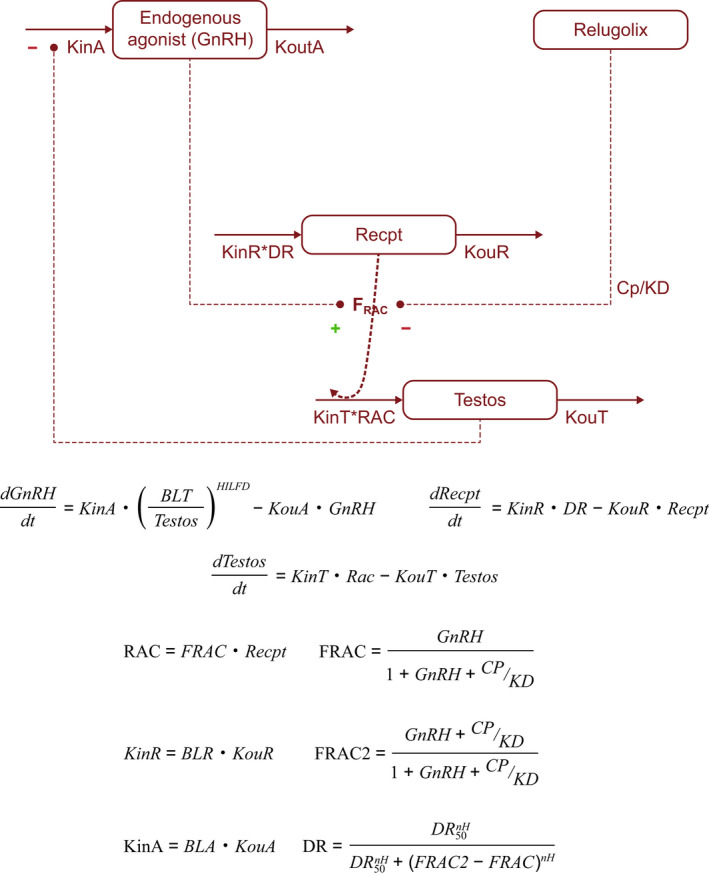
Schematic representation of the PopPK/PD model. BLA, baseline value for the endogenous agonist (GnRH); BLR, baseline value for the GnRH receptor; BLT, baseline value for testosterone; Cp, relugolix plasma concentration; DR, receptor downregulation; DR50, level of receptor activation at which receptor downregulation is at 50% of maximum; FRAC, fraction of activated GnRH receptors (by agonist); FRAC2, fraction of activated GnRH receptors (by agonist and antagonist); GnRH, gonadotropin‐releasing hormone (endogenous agonist); HILFD, Hill coefficient of the testosterone‐feedback on GnRH production; KD, receptor equilibrium dissociation constant; KinA, endogenous GnRH production rate constant; KinR, GnRH receptor production rate constant; KinT, testosterone production rate constant; KouR, GnRH receptor degradation rate constant; KouT, testosterone degradation rate constant; KoutA, endogenous GnRH degradation rate constant; nH, Hill coefficient of the downregulation of GnRH receptor production; PD, pharmacodynamic; PopPK, population pharmacokinetic; RAC, amount of activated receptors; Recpt, receptor; Testos, testosterone.
Simulations of testosterone profile to assess the impact of covariates
The covariate effects on testosterone suppression were assessed by comparing simulated testosterone concentrations by age (a covariate in both final PopPK and PopPK/PD models), Black/African American race, and body weight (covariates in the final PopPK model) for patients in the HERO study. Testosterone concentrations were simulated following the administration of the recommended dosing regimen for 45 days with 100% adherence. Minor differences in testosterone suppression were demonstrated between patients younger and older than the median of 72 years, between patients with a body weight above and below the median of 80 kg, and between Black/African American and non‐Black/African American men, with the 95th percentile of simulated testosterone concentrations remaining at ~ 20 ng/dL (Figure 4 ), indicating a lack of clinically relevant impact of these covariates on the suppression of testosterone by relugolix.
Figure 4.
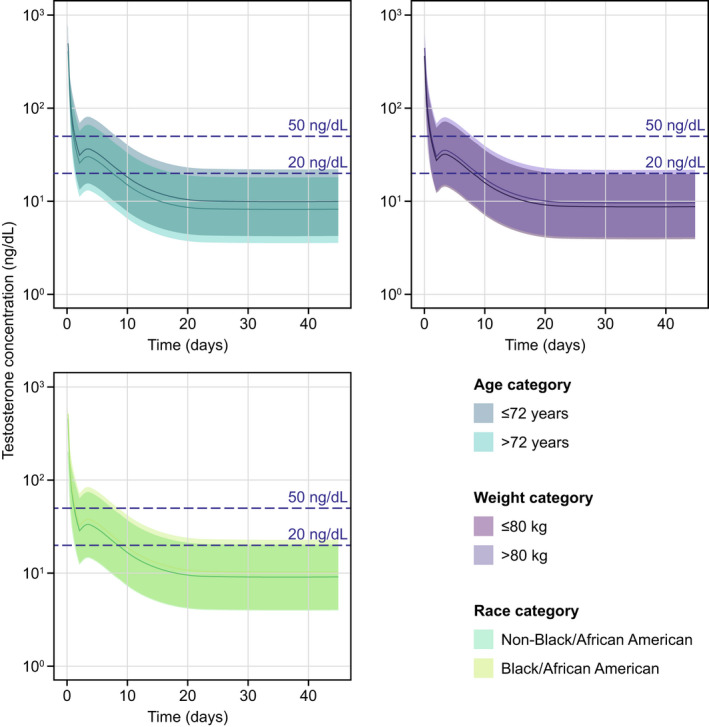
Simulation of testosterone profiles by covariate subgroups according to age, weight, and race. The shaded areas represent the 90% prediction interval (5th to 95th percentile) of the simulations. The solid lines represent the median of the simulations. Relugolix was simulated to be administered for 45 days with 100% adherence. Testosterone concentrations of < 50 ng/dL and < 20 ng/dL represent the castration levels and profound castration levels, respectively.
Simulations of testosterone profile with different dosing regimens
Testosterone response associated with various dosing regimens was simulated to explore the dose–response relationship. The 360‐mg loading dose was associated with an initial rapid decrease in simulated testosterone concentrations within 24 hours postdose, and the 120‐mg daily dose is the minimal daily dose associated with the 95th percentile of simulated individual testosterone concentrations at 20 ng/dL. After administration of the recommended dosing regimen, simulated median testosterone concentrations were predicted to achieve castration levels of testosterone (< 50 ng/dL) by day 2 and profound castration levels of testosterone (< 20 ng/dL) by day 9, and to maintain concentrations below profound castration levels thereafter (Figure S5 ). The 95th percentile of simulated testosterone concentrations was ~ 20 ng/dL from day 25 onward.
Simulations of testosterone profile following temporary treatment interruption
Temporary treatment interruption on testosterone concentrations was investigated by simulating testosterone concentrations with various durations of treatment interruption (up to 28 days). For each scenario, 100% adherence to the recommended dosing regimen for the initial 40 days of treatment was assumed, followed by temporary treatment interruptions for 1, 2, 3, 7, 14, 21, or 28 days prior to resuming treatment with a 120‐mg dose once daily.
Following a consecutive 7‐day temporary treatment interruption, 97.3% of the patients were predicted to remain at castration levels of testosterone (concentrations < 50 ng/dL) and 54.4% at profound castration levels of testosterone (concentrations < 20 ng/dL; Table 2 ); simulated testosterone concentrations were predicted to return to steady‐state after resumption of the 120‐mg daily dose after ~ 13 days (Figure S5 ). Following a 14‐day temporary treatment interruption, 85.5% of the patients were predicted to remain at castration levels of testosterone (concentrations < 50 ng/dL) and 28.2% at profound castration levels of testosterone (concentrations < 20 ng/dL; Table 2 ).
Table 2.
Percentage of patients with simulated testosterone concentrations at castration levels (<50 ng/dL) and at profound castration levels (<20 ng/dL) after temporary treatment interruptions of different durations (N = 1,000 for each scenario)
| Testosterone concentration (ng/dL) | Full adherence (%) | Duration of temporary treatment interruption | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 day (%) | 2 days (%) | 3 days (%) | 7 days (%) | 14 days (%) | 21 days (%) | 28 days (%) | ||
| < 50 | 100.0 | 99.9 | 99.4 | 99.3 | 97.3 | 85.5 | 61.2 | 36.5 |
| < 20 | 93.8 | 87.9 | 82.3 | 76.2 | 54.4 | 28.2 | 12.4 | 5.6 |
Simulations of testosterone profile after treatment discontinuation
Testosterone concentrations associated with 100% adherence to the recommended dosing regimen for 40 days followed by discontinuation of treatment were simulated (Figure 5 ). Following discontinuation of treatment, around 50% of patients were predicted to have testosterone concentrations above castration levels by day 25, and to have concentrations ≥ 280 ng/dL (lower limit of the normal range) by day 52.
Figure 5.
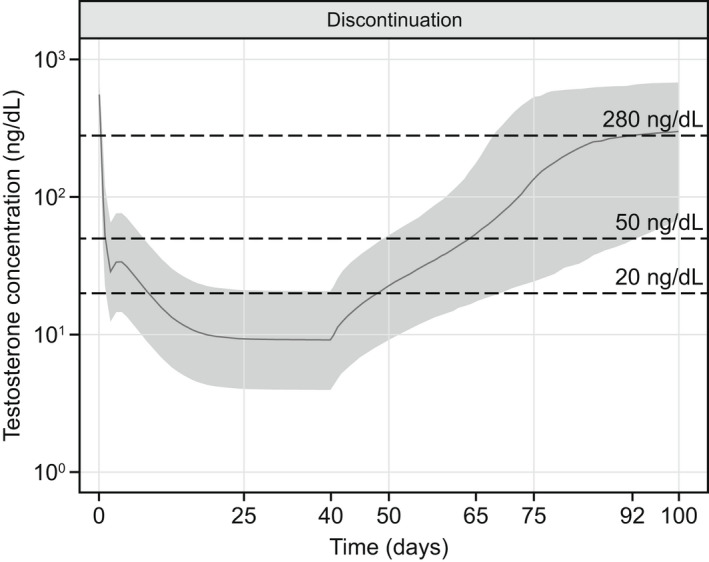
Simulation of testosterone profile after treatment discontinuation. The shaded area represents the 90% prediction interval (5th to 95th percentile) of the simulations. The solid line represents the median of the simulations. Testosterone concentrations of ≥ 280 ng/dL, < 50 ng/dL, and < 20 ng/dL represent the normal range, castration levels, and profound castration levels, respectively. Relugolix was simulated to be administered for 40 days with 100% adherence followed by treatment discontinuation.
DISCUSSION
We describe a PopPK model for relugolix exposure and a semimechanistic PopPK/PD model characterizing the relationship between relugolix exposure and testosterone concentrations. The models were developed based on data from five clinical studies in healthy adult men and men with advanced prostate cancer, including the phase III safety and efficacy study (HERO). The PKs of relugolix were described by a three‐compartment model with first‐order absorption, short (16 minutes) absorption lag time, and first‐order elimination. Age, body weight, and Black/African American race were independently identified as statistically significant covariates in the final PopPK model. The impact of age and body weight on relugolix exposure at steady‐state were relatively small (relative change < 20%). There is no clear explanation for the higher exposure to relugolix in Black/African American race, considering that race was identified as a covariate independent of age or body weight. Additionally, relugolix is a substrate of intestinal P‐gp, 26 in which polymorphisms that could significantly impact drug exposure have not been reported. 41 , 42 Relugolix also undergoes multiple routes of biotransformation and elimination, 26 and therefore it is unlikely for ethnically based differences of a single biotransformation or elimination pathway to have an impact on the overall systemic clearance of relugolix with clinical relevance. 43 The effect of Black/African American race was partly mitigated by the combined effect of other covariates. Based on the multivariate covariate simulations with 10,000 individuals sampled from the phase III population, relugolix exposure in Black/African American men was 16–26% higher than in non‐Black/African American men, indicating that the overall impact of Black/African American race on the exposure to relugolix is not clinically meaningful. The model‐predicted lack of clinical relevance from age, body weight, and race is consistent with analyses from the observed phase III data. 44 , 45 The final PopPK model was externally validated using the relugolix concentration data from the additional 87 patients enrolled in the HERO amendments, which was not included during model development, and the additional data was also adequately described by the PopPK model.
A semimechanistic PopPK/PD model adapted from a previously published model by Romero et al. 29 that included the primary components of the HPG axis, with addition of competitive and reversible GnRH receptor antagonism of relugolix and feedback mechanisms on production of GnRH receptor and testosterone, was developed to characterize the full serum testosterone time‐course over the entire treatment period of relugolix (the initial testosterone drop after start of treatment, changes in testosterone levels during treatment, and a testosterone recovery following treatment discontinuation). Note that IIV was included on testosterone production rate (KinT) but not on other parameters in the final PopPK/PD model, for which age was the only covariate with statistical significance; such limitation could potentially impact inferences of the model. Specifically, although the age‐dependent decline in KinT is consistent with the literature, 46 , 47 the IIV on KinT was high (45.7%), suggesting that the IIV on KinT remained to some extent unexplained after incorporation of age effect. Additionally, the PopPK/PD model with a single IIV parameter may oversimplify the physiological cause of IIV on testosterone concentrations upon treatment with relugolix. Nevertheless, the GoF plots showed no overall bias, demonstrating an appropriate characterization of testosterone concentrations during treatment of relugolix by the PopPK/PD model. Simulations of testosterone profile demonstrated minor effects of age, body weight, or Black/African American race that were considered not to be clinically meaningful.
Based on simulations with the final PopPK/PD model, after administration of the recommended dosing regimen for relugolix, simulated median testosterone concentrations were predicted to achieve castration levels of testosterone (concentrations < 50 ng/dL) by day 2 and profound castration levels of testosterone (concentrations < 20 ng/dL) by day 9, and maintain below profound castration levels thereafter. Simulated testosterone concentrations were predicted to achieve profound castration levels of testosterone in > 90% of patients by day 25, consistent with observed testosterone concentration data from HERO, where mean testosterone concentrations were below castration levels by day 4, the first sampling time point. 26 , 48 Sustained testosterone suppression below castration levels was also observed from day 29 through week 48 in the HERO study, with sustained castration levels achieved in 96.7% of patients through week 48. 26 , 48
Simulations based on the final PopPK/PD model were conducted to assess the impact of temporary treatment interruption on testosterone concentrations after steady‐state is reached: 85.5% of the patients were predicted to remain at castration levels of testosterone after a 14‐day treatment interruption, which supported the dosing recommendations for temporary interruption of treatment for up to 14 days, if needed (e.g., when concomitant use of relugolix and an oral P‐gp inhibitor cannot be avoided and dosing cannot be separated by at least 6 hours). 26 In addition, following a 7‐day treatment interruption, simulated testosterone concentrations were predicted to return to steady‐state ~ 13 days after the resumption of the 120‐mg daily dose. Therefore, a loading dose of 360 mg, which leads to rapid suppression of testosterone concentrations, is recommended after a temporary interruption of longer than 7 days. 26 Of note, 100% compliance with the recommended dose for the initial 40 days prior to temporary treatment interruption was assumed in all scenarios presented herein. Alternate scenarios, including sporadic nonadherence (e.g., a patient missing 2 doses per week) and treatment interruptions occurring before the steady‐state of testosterone concentration is reached, have not been assessed as part of the analyses.
Additional simulations based on the final PopPK/PD model were conducted to demonstrate testosterone recovery after treatment discontinuation: — ~ 50% of patients were predicted to achieve testosterone concentrations above the castration levels on day 25 and above 280 ng/dL on day 52. Simulated testosterone following treatment discontinuation was faster than that observed in the HERO study, which is consistent with other studies demonstrating a correlation between the duration of ADT and the time required for testosterone concentrations to normalize. 49 , 50 Specifically, in a subset of patients in the HERO study who did not receive subsequent ADT for 90 days following discontinuation of treatment with relugolix, the cumulative incidence rate of testosterone concentrations to > 280 ng/dL (lower limit of normal range) or to baseline was 55%. 26 , 48
In summary, the PopPK and semimechanistic PopPK/PD models adequately characterized the systemic relugolix exposure and its relationship to testosterone concentrations. Age, body weight, and Black/African American race demonstrated at most minimal effects with no clinical relevance; other races, health status (healthy men vs. patients with prostate cancer), or stage of cancer demonstrated no effects on relugolix exposure or testosterone concentrations. Model‐based simulations of testosterone profiles were consistent with clinical data, reflecting the high predictive value of the model and supporting the reliability of the model‐based simulations. Simulation using the PopPK/PD model demonstrated that 97.3% and 85.5% of the patients remained at castration levels upon temporary interruption for 7 days and 14 days, respectively. The PopPK and PopPK/PD models can be used to provide guidance regarding dosing recommendations under various circumstances (e.g., temporary interruption of treatment, if needed) for relugolix.
FUNDING
All research and modeling were funded by Myovant Sciences, Inc.
CONFLICTS OF INTEREST
T.‐Y.L. and E.M. are current employees of Myovant Sciences, Inc. and own stocks in the company. P.B.P., Y.‐W.L., R.d.G., A.S.Z., and E.S. are current or former employees of Certara with financial support from Myovant Sciences, Inc.
AUTHOR CONTRIBUTIONS
T.‐Y.L. wrote the manuscript. T.‐Y.L., P.B.P., Y.‐W.L., R.d.G., A.S.Z., and E.M. designed the research. P.B.P., Y.‐W.L., and E.S. performed the research. T.‐Y.L., P.B.P., Y.‐W.L., R.d.G., A.S.Z., and E.M. analyzed the data.
Supporting information
Data S1
ACKNOWLEDGMENTS
Editing support was provided by Ify Sargeant of Twist Medical and was funded by Myovant Sciences and Pfizer Inc. in compliance with Good Publication Practice 3 ethical guidelines. The authors would like to thank Dr. Sinziana Cristea for her contribution to this manuscript.
References
- 1. Bray, F. , Ferlay, J. , Soerjomataram, I. , Siegel, R.L. , Torre, L.A. & Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. A Cancer. J. Clin. 68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 2. Crawford, E.D. Changing concepts in the management of advanced prostate cancer. Urology 44, 67–74 (1994). [Google Scholar]
- 3. Klotz, L. et al. Maximal testosterone suppression in the management of recurrent and metastatic prostate cancer. CUAJ 11, 1–2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moul, J.W. et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J. Urol. 171, 1141–1147 (2004). [DOI] [PubMed] [Google Scholar]
- 5. Steele, C.B. , Li, J. , Huang, B. & Weir, H.K. Prostate cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD‐2 study: US PCa survival by race and stage. Cancer 123, 5160–5177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ritch, C. & Cookson, M. Recent trends in the management of advanced prostate cancer. F1000Res 7, 1513 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huggins, C. , Stevens, R. & Hodges, C.V. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch. Surg. 43, 209–223 (1941). [Google Scholar]
- 8. Bosland, M.C. Testosterone treatment is a potent tumor promoter for the rat prostate. Endocrinology 155, 4629–4633 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parker, C. , Gillessen, S. , Heidenreich, A. & Horwich, A. & ESMO guidelines committee. Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann. Oncol. 26(Suppl 5), v69–v77 (2015). [DOI] [PubMed] [Google Scholar]
- 10. Attard, G. et al. Prostate cancer. Lancet 387, 70–82 (2016). [DOI] [PubMed] [Google Scholar]
- 11. Huggins, C. & Hodges, C.V. Studies on prostatic cancer. I. the effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 22, 232–240 (1972). [DOI] [PubMed] [Google Scholar]
- 12. European Medicines Agency . Assessment report for FIRMAGON (EMEA/H/C/000986) <https://www.ema.europa.eu/en/documents/assessment‐report/firmagon‐epar‐public‐assessment‐report_en.pdf> (2008).
- 13. FDA . Draft Guidance for Industry: Advanced Prostate Cancer: Developing Gonadotropin‐Releasing Hormone Analogues Guidance for Industry <https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/advanced‐prostate‐cancer‐developing‐gonadotropin‐releasing‐hormone‐analogues‐guidance‐industry> (2019).
- 14. Morote, J. , Planas, J. , Salvador, C. , Raventós, C.X. , Catalán, R. & Reventós, J. Individual variations of serum testosterone in patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 103, 332–335 (2009). [DOI] [PubMed] [Google Scholar]
- 15. Pickles, T. , Hamm, J. , Morris, W.J. , Schreiber, W.E. & Tyldesley, S. Incomplete testosterone suppression with luteinizing hormone‐releasing hormone agonists: does it happen and does it matter? BJU. Int 110(11 Pt B), E500–E507 (2012). [DOI] [PubMed] [Google Scholar]
- 16. Klotz, L. et al. Nadir testosterone within first year of androgen‐deprivation therapy (ADT) predicts for time to castration‐resistant progression: a secondary analysis of the PR‐7 trial of intermittent versus continuous ADT. J. Clin. Oncol. 33, 1151–1156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chandrasekar, T. , Yang, J.C. , Gao, A.C. & Evans, C.P. Mechanisms of resistance in castration‐resistant prostate cancer (CRPC). Transl. Androl. Urol. 4, 365–380 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohler, P.J.L. & Antonarakis, E.S. NCCN guidelines updates: management of prostate cancer. J. Natl. Compr. Canc. Netw. 17, 583–586 (2019). [DOI] [PubMed] [Google Scholar]
- 19. Plant, T.M. 60 years of neuroendocrinology: the hypothalamo‐pituitary‐gonadal axis. J. Endocrinol. 226, T41–T54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mcleod, D.G. Hormonal therapy: historical perspective to future directions. Urology 61(Suppl 2A), 3–7 (2003). [DOI] [PubMed] [Google Scholar]
- 21. Sharifi, N. , Gulley, J.L. & Dahut, W.L. Androgen deprivation therapy for prostate cancer. JAMA 204, 238–244 (2005). [DOI] [PubMed] [Google Scholar]
- 22. Oh, W.K. , Landrum, M.B. , Lamont, E.B. , McNeil, B.J. & Keating, N.L. Does oral antiandrogen use before leuteinizing hormone‐releasing hormone therapy in patients with metastatic prostate cancer prevent clinical consequences of a testosterone flare? Urology 75, 642–647 (2010). [DOI] [PubMed] [Google Scholar]
- 23. Thompson, I.M. Flare associated with LHRH‐agonist therapy. Rev. Urol. 3(Suppl 3), S10–S14 (2001). [PMC free article] [PubMed] [Google Scholar]
- 24. Sciarra, A. et al. A meta‐analysis and systematic review of randomized controlled trials with degarelix versus gonadotropin‐releasing hormone agonists for advanced prostate cancer. Medicine 95, e3845 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nascimento, B. , Miranda, E.P. , Jenkins, L.C. , Benfante, N. , Schofield, E.A. & Mulhall, J.P. Testosterone recovery profiles after cessation of androgen deprivation therapy for prostate cancer. J. Sex. Med. 16, 872–879 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. FDA . ORGOVYX (relugolix) Prescribing Information <https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214621s000lbl.pdf> (2020).
- 27. European Medicines Agency . ORGOVYX (relugolix) Summary of Product Characteristics <https://www.ema.europa.eu/en/documents/product‐information/orgovyx‐epar‐product‐information_en.pdf> (2022).
- 28. Shore, N.D. & Sutton, J. Plain language summary of the HERO study comparing relugolix with leuprolide for men with advanced prostate cancer. Future Oncol. 18, 2575–2584 (2022). [DOI] [PubMed] [Google Scholar]
- 29. Romero, E. et al. Pharmacokinetic/pharmacodynamic model of the testosterone effects of triptorelin administered in sustained release formulations in patients with prostate cancer. J. Pharmacol. Exp. Ther. 342, 788–798 (2012). [DOI] [PubMed] [Google Scholar]
- 30. Byon, W. et al. Establishing best practices and guidance in population modeling: an experience with an internal population pharmacokinetic analysis guidance. CPT Pharmacometrics Syst. Pharmacol. 2, e51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keizer, R.J. , Karlsson, M.O. & Hooker, A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst. Pharmacol. 2, e50 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bergstrand, M. , Hooker, A.C. , Wallin, J.E. & Karlsson, M.O. Prediction‐corrected visual predictive checks for diagnosing nonlinear mixed‐effects models. AAPS J. 13, 143–151 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dosne, A.G. , Bergstrand, M. & Karlsson, M.O. An automated sampling importance resampling procedure for estimating parameter uncertainty. J. Pharmacokinet. Pharmacodyn. 44, 509–520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosen, R.D. & Sapra, A. TNM classification. In StatPearls [Internet] (StatPearls Publishing, Treasure Island (FL), 2021). http://www.ncbi.nlm.nih.gov/books/NBK553187/. [Google Scholar]
- 35. Bergstrand, M. & Karlsson, M.O. Handling data below the limit of quantification in mixed effect models. AAPS J. 11, 371–380 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cockcroft, D.W. & Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 16, 31–41 (1976). [DOI] [PubMed] [Google Scholar]
- 37. Savic, R.M. & Karlsson, M.O. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J. 11, 558–569 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin, B.J.T. , Chen, K.‐K. , Chen, M.‐T. & Chang, L.S. The time for serum testosterone to reach castrate level after bilateral orchiectomy or oral estrogen in the management of metastatic prostatic cancer. Urology 43, 834–837 (1994). [DOI] [PubMed] [Google Scholar]
- 39. Tornoe, C.W. et al. Population pharmacokinetic/pharmacodynamic (PK/PD) modelling of the hypothalamic‐pituitary‐gonadal axis following treatment with GnRH analogues. Br. J. Clin. Pharmacol. 63, 648–664 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Snelder, N. , Drenth, H.‐J. , Riber Bergmann, K. , Wood, N.D. , Hibberd, M. & Scott, G. Population pharmacokinetic‐pharmacodynamic modelling of the relationship between testosterone and prostate specific antigen in patients with prostate cancer during treatment with leuprorelin. Br. J. Clin. Pharmacol. 85, 1247–1259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leschziner, G.D. , Andrew, T. , Pirmohamed, M. & Johnson, M.R. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J. 7, 154–179 (2007). [DOI] [PubMed] [Google Scholar]
- 42. Wolking, S. , Schaeffeler, E. , Lerche, H. , Schwab, M. & Nies, A.T. Impact of genetic polymorphisms of ABCB1 (MDR1, P‐glycoprotein) on drug disposition and potential clinical implications: update of the literature. Clin. Pharmacokinet. 54, 709–735 (2015). [DOI] [PubMed] [Google Scholar]
- 43. FDA International Conference on harmonisation: guidance on ethnic factors in the acceptability of foreign clinical data. Fed. Regist. 63, 6–7 (1998). [PubMed] [Google Scholar]
- 44. George, D.J. et al. Efficacy and safety of relugolix in black men with advanced prostate cancer: a subgroup analysis from the randomized, phase 3 HERO study versus leuprolide. J. Clin. Oncol. 40(suppl 6), 105 (2021).34652953 [Google Scholar]
- 45. Cookson, M. et al. Impact of age on efficacy and safety of relugolix: a subgroup analysis from the randomized, phase 3 HERO study versus leuprolide in men with advanced prostate cancer. J. Clin. Oncol. 39(suppl 15), 5075 (2021). [Google Scholar]
- 46. Harman, S.M. , Metter, E.J. , Tobin, J.D. , Pearson, J. & Blackman, M.R. Baltimore longitudinal study of aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J. Clin. Endocrinol. Metab. 86, 724–731 (2001). [DOI] [PubMed] [Google Scholar]
- 47. Feldman, H.A. et al. Age trends in the level of serum testosterone and other hormones in middle‐aged men: longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 87, 589–598 (2002). [DOI] [PubMed] [Google Scholar]
- 48. Shore, N.D. et al. Oral relugolix for androgen‐deprivation therapy in advanced prostate cancer. N. Engl. J. Med. 382, 2187–2196 (2020). [DOI] [PubMed] [Google Scholar]
- 49. Kaku, H. et al. Time course of serum testosterone and luteinizing hormone levels after cessation of long‐term luteinizing hormone‐releasing hormone agonist treatment in patients with prostate cancer. Prostate 66, 439–444 (2006). [DOI] [PubMed] [Google Scholar]
- 50. Murthy, V. et al. Recovery of serum testosterone after neoadjuvant androgen deprivation therapy and radical radiotherapy in localized prostate cancer. BJU Int. 97, 476–479 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


