Abstract
Cancer cachexia is defined as a multi‐factorial syndrome characterised by an ongoing loss of skeletal muscle mass and progressive functional impairment, estimated to affect 50–80% of patients and responsible for 20% of cancer deaths. Elevations in the morbidity and mortality rates of cachectic cancer patients has been linked to respiratory failure due to atrophy and dysfunction of the ventilatory muscles. Despite this, there is a distinct scarcity of research investigating the structural and functional condition of the respiratory musculature in cancer, with the majority of studies exclusively focusing on limb muscle. Treatment strategies are largely ineffective in mitigating the cachectic state. It is now widely accepted that an efficacious intervention will likely combine elements of pharmacology, nutrition and exercise. However, of these approaches, exercise has received comparatively little attention. Therefore, it is unlikely to be implemented optimally, whether in isolation or combination. In consideration of these limitations, the current review describes the mechanistic basis of cancer cachexia and subsequently explores the available respiratory‐ and exercise‐focused literature within this context. The molecular basis of cachexia is thoroughly reviewed. The pivotal role of inflammatory mediators is described. Unravelling the mechanisms of exercise‐induced support of muscle via antioxidant and anti‐inflammatory effects in addition to promoting efficient energy metabolism via increased mitochondrial biogenesis, mitochondrial function and muscle glucose uptake provide avenues for interventional studies. Currently available pre‐clinical mouse models including novel transgenic animals provide a platform for the development of multi‐modal therapeutic strategies to protect respiratory muscles in people with cancer.
Keywords: cachexia, cancer, exercise, respiratory system
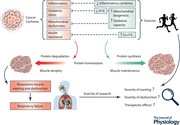
Abstract figure legend Cancer cachexia is characterised by profound loss of skeletal muscle mass. Exercise has the potential to counteract several factors that contribute to muscle wasting. Atrophy and dysfunction of the ventilatory muscles can result in respiratory compromise and ultimately failure. There is a scarcity of research investigating structure and function of the respiratory musculature in cancer. Pre‐clinical and clinical studies focused on mechanisms of exercise‐induced support of muscle are required to drive the development of multi‐modal therapeutic strategies to protect respiratory muscles in people with cancer.

Introduction
Cancer cachexia has been defined as ‘a multi‐factorial syndrome characterised by an ongoing loss of skeletal muscle mass, with or without a loss of fat mass, that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment’ (Fearon et al., 2011). Lack of consensus on an appropriate definition, classification and diagnostic criteria hindered early research (Fox et al., 2009). Therefore, Fearon et al. (2011) developed the above working definition and the internationally recognised framework illustrated in Fig. 1. Incidence rates of the condition have been estimated at 50–80% of cancer patients, with variation observed between cancer types (Gordon et al., 2005). Sufferers of cancer cachexia experience an amalgamation of severe adverse effects. The nature of the induced weight loss causes progressive decline in muscle performance (Weber et al., 2009), exercise capacity (Jones et al., 2012) and activity level (Dodson et al., 2011; Wilcock et al., 2008), resulting in accelerated functional decline (LeBlanc et al., 2015), disability with regard to activities of daily living (Naito et al., 2017), increased fatigue (Stewart et al., 2006) and consequently poorer quality of life (Dewys et al., 1980). Additionally, loss of appetite and anorexia are often present (Laviano et al., 2003). Furthermore, cachexia is considered to contribute significantly toward increased morbidity and mortality in cancer populations (Tisdale, 2002), estimated to be responsible for up to 20% of cancer deaths (Argiles et al., 2014), with death reported to typically occur once weight loss exceeds 30–40% (Arthur et al., 2014). One of the leading hypotheses surrounding the link between muscle atrophy and elevated mortality considers the enhanced susceptibility of cachectic cancer patients to chemotherapy‐induced toxicity (Prado et al., 2007, 2008, 2009), often leading to reductions in treatment, which subsequently impacts patient survival (Andreyev et al., 1998; Martin et al., 2013). What can be stated with conviction is that cachexia imparts a severe burden, with potentially fatal consequences, on populations of cancer patients.
Figure 1. Internationally recognised framework illustrating the continuum of cachexia progression across three clinically relevant stages.

Adapted with permission from Fearon et al. (2011).
In association with critical illness‐related muscle weakness, respiratory muscle dysfunction in cancer has been identified as a distinct and highly relevant issue (Berger et al., 2016). Dysfunction of the inspiratory muscles, primarily the diaphragm, can result in hypoxaemia and hypercapnia. Reduced maximal inspiratory pressure in advanced cancer patients has been correlated with increased prevalence of dyspnoea (Bruera et al., 2000). Additionally, dysfunction of the expiratory muscles can inhibit airway clearance, which can increase risk of pneumonia (Gea et al., 2012; Kravitz, 2009), a major cause of respiratory failure and death in critically ill cancer patients (Azoulay et al., 2004; Windsor & Hill, 1988). Inspiratory muscle weakness is frequently observed among cancer patients (Bruera et al., 2000; Dudgeon & Lertzman, 1998; Dudgeon et al., 2001) and has been reported to contribute significantly to cachexia‐associated morbidity and mortality (Feathers et al., 2003). This contribution is likely related to the observed correlation between reduced inspiratory function and a reduction in the workload necessary to induce respiratory failure (Callahan & Supinski, 2010). Unfortunately, there is a scarcity of observational reporting specific to respiratory muscle in cancer cachexia, as the majority of research in this domain has focused on limb muscle. Ultimately, despite imparting a considerable impact on patient well‐being, relatively little attention has been given to the study of cachexia with the focal point of interest centred on respiratory muscle wasting and function.
A recent systematic review conducted by the American Society of Clinical Oncology aimed to produce evidence‐based recommendations for the clinical management of cancer cachexia (Roeland et al., 2020). The authors concluded that insufficient evidence was available in support of pharmacological intervention. Additionally, although dietary guidance and nutritional supplementation may be of some benefit toward weight gain and improved quality of life, the depth of evidence is poor and findings are equivocal. In consideration of exercise interventions, no eligible trials were identified according to the review criteria. A lack of clinical evidence was also reported by the authors of a recent Cochrane review, who identified only four relevant studies on the effects of exercise in cachexia, hindering their ability to meaningfully interpret the limited data (Grande et al., 2021). The association between severe cancer types and the prevalence of cachexia is likely a major contributing factor to this distinct lack of clinical research. Although there is growing support for the benefits of exercise in cancer patients (Singh et al., 2020), including those with advanced disease (Lazzari et al., 2021), exercise as a therapeutic approach is yet to be recommended in incurable cancer patients (Patel et al., 2019). Additionally, despite encouraging findings, there remain considerable complications associated with the prescription of exercise in cancer populations, with primary barriers to participation reported to be treatment side effects, comorbidities and medical complications (Frikkel et al., 2020). Furthermore, the potential contraindications and complications of considerable muscle wasting cannot be dismissed. As a result, the ability for such results to inform the feasibility and safety of an exercise intervention in cancer cachexia is limited. Inclusion of metrics such as adherence and occurrence of adverse events in future studies of exercise in cancer cachexia populations would help to address such concerns. Preclinical research provides a more bountiful source of information, with murine models of cancer cachexia frequently employed, primarily the Lewis lung carcinoma model (Cai et al., 2004; Zhang et al., 2017) and the C26 colorectal adenocarcinoma model (Bonetto et al., 2016). Results from such research are promising in support of the benefits of exercise in the amelioration of the adverse effects of cancer cachexia (Bowen et al., 2015; Donatto et al., 2013; Hardee et al., 2020). However, the variety of models utilised poses a barrier to synthesis of data across individual studies, and uncertainty remains in the translatability of results from these models to humans (Talbert et al., 2019). Several conclusions can be drawn from the above information. Firstly, there is a need for clinical research examining the therapeutic capacity of exercise in cancer cachexia patients. Secondly, selection of an adequate cancer model capable of accurately representing the cachectic condition as it presents in humans is key to identification of appropriate mechanisms, and thus, therapeutic targets for clinical intervention design.
Considering the uncertainty surrounding the most appropriate therapeutic approaches for sufferers of cachexia, extensive further research toward the development of treatment strategies is needed. Past research indicates that in order to provide impactful benefit, such a strategy would likely need to be multimodal in nature, potentially combining elements of nutrition, exercise and pharmacology. However, due to the complexity of the condition, and the continued work toward complete understanding of the mechanisms and mediating factors, careful consideration must be given to the limits of our current knowledge. Combination of ineffective strategies is unlikely to produce further benefit than said strategies alone. As such, the pursuit of an efficacious multimodal approach must be predicated on comprehensive understanding of the benefits provided by the individual elements. Additionally, the sparsity of cachexia research addressing respiratory muscle wasting and function highlights a particular need for investigations attending to this area. The current review aims to describe the mechanisms implicated in the aetiology of cancer cachexia, and subsequently collate and explore the available respiratory‐ and exercise‐focused literature in consideration of this mechanistic context.
Mechanisms of cachexia
Mechanistically, cachexia is a diverse condition, exhibiting a pro‐inflammatory state and elevations in oxidative stress, resulting in upregulation of protein degradation, inhibition of protein synthesis and reduced insulin sensitivity (Gould et al., 2013; Tisdale, 2009). The underlying mechanisms responsible for mediating the alterations seen in these processes are numerous and far from fully elucidated. Discussion surrounding the causative roots of cachexia has addressed the condition from the sides of both atrophy and hypertrophy, assessing the stimulation and suppression of these processes, respectively. Past investigations have followed logical paths of enquiry, from describing established signalling pathways, to mediating factors of such pathways, to resultant gene expression and translation. Such explorations have greatly expanded our understanding of the mechanisms of the condition. When considered collectively, the multi‐factorial inter‐connecting nature of the contributing mechanisms becomes apparent. Interrelations across pathways and between components complicate the determination of the relative impact of factors which have been studied in isolation. Therefore, although evidence exists indicating the involvement of a range of factors, identifying promising avenues for future research, few conclusive statements can be made in relation to the mechanisms of cancer cachexia. Where possible, the system should be considered holistically. Isolated components which have been evaluated in terms of their influence on the cachectic state need also be considered for their impact on other mediating elements. A thorough integrative understanding of this manner is necessary in order to identify the most valid pursuits aimed at enhancing the mechanistic understanding of cancer cachexia.
Protein degradation/skeletal muscle atrophy
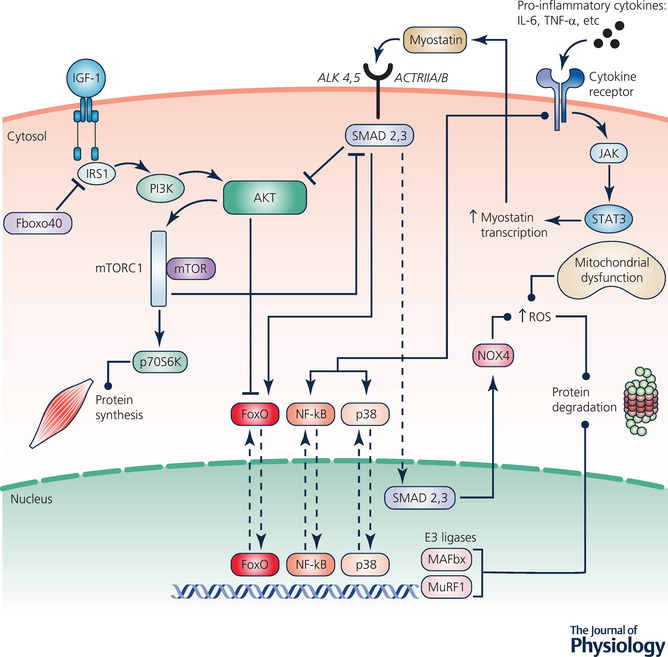
Regarding protein breakdown, the ubiquitin–proteasome system (UPS) is the primary pathway responsible for the removal of unwanted and damaged intracellular proteins (Goldberg, 2003). This is particularly the case with regard to the degradation of myofibrillar proteins in skeletal muscle (Attaix et al., 1998). The ubiquitination of proteins involves the action of the E1 ubiquitin‐activating enzyme, E2 ubiquitin‐conjugating enzymes and E3 ubiquitin‐protein ligases, the latter of which act to closely regulate proteolysis in skeletal muscle (Kitajima et al., 2020). Of particular interest in the context of muscle degradation are two muscle‐specific E3 ligases, namely muscle RING finger 1 (MuRF1) and muscle atrophy F‐Box (MAFbx; also known as Atrogin‐1). Interest in these genes stems from the work of Bodine, Latres et al. (2001) and Gomes et al. (2001) who detected them at low levels in resting skeletal muscle, with significant increases following immobilisation or food deprivation in mice. Considerable evidence has shown similar upregulation of MuRF1 and MAFbx mRNA across many preclinical models, and in response to every condition which induces skeletal muscle atrophy (Bodine & Baehr, 2014), including cachexia (Lecker et al., 2004). Furthermore, suppression of MuRF1 or MAFbx may support maintenance of muscle mass (Bodine, Latres et al., 2001), although this effect is not evident across all atrophy conditions (Baehr et al., 2011). This has led to further investigations into the potentially key regulatory roles of these factors. Some findings suggest MAFbx may be linked to the control of protein synthesis via degradation of MyoD and eIF3‐f (Lagirand‐Cantaloube et al., 2008; Tintignac et al., 2005). However, greater credence is attributed to the role of MuRF1 in the control of protein degradation via the specific targeting of myosin heavy chain and other thick filament proteins for ubiquitination (Cohen et al., 2009). Notably, evidence in clinical instances is lacking, which represents the primary limitation of such hypotheses. In addition, due to the ability of a given E3 ligase to bind to different E2 enzymes dependent on the cellular environment, it remains a possibility that the substrates of MuRF1 and MAFbx may vary as a function of the condition responsible for the induction of the muscle wasting (Bodine & Baehr, 2014). Therefore, whilst evidence indicates MuRF1 and MAFbx are implicated in the early induction and regulation of skeletal muscle atrophy, further in vivo research is necessary to accurately determine the mechanistic role of these factors. Upstream of these factors, expression of ubiquitin ligase is regulated by the Forkhead box O (FoxO) transcription factors (Sandri et al., 2004), whose sub‐cellular location in turn is affected by Akt. Phosphorylation of FoxO transcription factors by Akt results in their translocation from the nucleus to the cytoplasm, thus dampening the transcription and subsequent production of MuRF1 and MAFbx (Kitajima et al., 2020). Such activity illustrates the interconnected nature of the processes responsible for protein degradation and synthesis, as Akt also plays an important role in muscle hypertrophy. Under normal conditions, and when regulated appropriately, proteolysis via the UPS is critical in the prevention of disease and cellular dysfunction (Rock et al., 1994), and is necessary in the facilitation of healthy ageing. However, dysregulation and subsequent increased activity of this system is thought to contribute to the increased proteolysis evident in the cachectic condition (Fig. 2).
Figure 2. Outline of the primary signalling factors and mediators implicated in the pathways of protein synthesis and protein degradation in skeletal muscle via the activation of mTOR and the ubiquitin–proteasome system, respectively.
Protein synthesis/skeletal muscle hypertrophy
Several anabolic pathways facilitate the induction of muscle hypertrophy and an intracellular shift away from muscle protein degradation toward protein synthesis. The primary identified pathways are the Akt–mammalian target of rapamycin (mTOR), mitogen‐activated protein kinase (MAPK) and calcium (Ca2+)‐dependent pathway (Schoenfeld, 2010). Of these, the Akt–mTOR pathway is considered to be of particular importance, with mTOR being widely known as the master regulator of skeletal muscle growth (Bodine, Stitt et al., 2001; Thomas & Hall, 1997). Thus, in addressing the contribution of alterations in protein synthesis toward the development of cachexia, suppression of this pathway is of considerable interest.
Exploring the implicated factors from the top down, insulin‐like growth factor‐1 (IGF‐1) acts as an upstream regulator. Binding of IGF‐1 to its receptor recruits insulin receptor substrate 1 (IRS1) (Bohni et al., 1999), phosphorylation of which is necessary for the activation of many downstream signalling events (Egerman & Glass, 2014), illustrating the regulatory role of IGF‐1 in the modulation of protein synthesis. This is demonstrated in the potential for inactivation of the IGF‐1 pathway via ubiquitin‐mediated degradation of IRS1, which limits the downstream activation of the IRS1–phosphoinositide 3‐kinase (PI3K)–Akt pathway (Shi et al., 2011). Unimpeded phosphorylation of IRS1 initiates a signalling cascade, involving recruitment and phosphorylation of PI3K, which subsequently results in Akt phosphorylation. This is of critical importance in the hypertrophy process (Rommel et al., 2001), with Akt acting both to stimulate anabolism and to inhibit catabolic signalling (Nader, 2005; Toigo & Boutellier, 2006), via FoxO phosphorylation. Delving deeper, the master regulator of muscle growth, mTOR, is a downstream target of Akt (Nave et al., 1999). Briefly, Akt, via intermediary signalling events, activates mTOR complex‐1 (mTORC1), resulting in phosphorylation of p70s6k, which promotes protein synthesis via activation of ribosomal protein S6 (Yoshida & Delafontaine, 2020). This pathway, linking IGF‐1 to Akt to mTOR, has been established as the primary process of mediation in the stimulation of muscle hypertrophy (Glass, 2003).
Also of interest is the myokine myostatin, a member of the transforming growth factor‐β family which is responsible for the regulation of cell growth via hypertrophy inhibition (Lee & McPherron, 1999; McPherron et al., 1997). Myostatin acts via binding to its receptors, activin receptor IIA/activin receptor IIB and activin‐like kinase‐4/5. Subsequent intracellular signalling activates the transcription factors Smad2 and Smad3, initiating their translocation to the nucleus and activation of target genes (McCroskery et al., 2003). Myostatin can reduce protein synthesis by suppressing Akt or increase protein degradation by elevating FoxO transcription. There is evidence to suggest IGF‐1 can maintain Akt phosphorylation when applied concurrently with myostatin to myotubes (Trendelenburg et al., 2009). This suggests that the effect of IGF‐1 on mTORC1 signalling involves a feedback loop, with increased myostatin‐induced Smad2 phosphorylation observed following mTORC1 inhibition. Therefore, within the complex hypertrophy signalling framework, there is apparent myostatin–Akt crosstalk, whereby IGF‐1 and myostatin act as antagonists operating a level above mTOR phosphorylation, influencing the respective stimulation and repression of muscle hypertrophy. The multi‐faceted influence between myostatin signalling and the Akt–mTOR pathway, in addition to its ability to elevate FoxO transcription, present an intriguing narrative for the exploration of myostatin's contribution to the development of the cachectic condition.
The adverse influence of cachexia
Inflammation
Elevated systemic inflammation is a hallmark of cancer (Argiles et al., 2012; Korniluk et al., 2017). Inflammatory cytokines are released both from the tumour and from immune cells in response to the disease (Argiles et al., 2012), and are heavily associated with the development of cachexia (Paval et al., 2022). Inflammatory cytokines mediate alterations in the UPS by promoting activation of transcription factors related to wasting (Mangner, et al., 2013; Puppa, et al., 2014), and they are the subjects of extensive research in this regard. Proinflammatory cytokines implicated in muscle wasting, such as IL‐6, IL‐1, IL‐8, tumour necrosis factor‐α (TNF‐α), TNF receptor‐associated factor 6, tumour necrosis factor‐like weak inducer of apoptosis (TWEAK), leukaemia inhibitory factor, and γ‐interferon exert their influence via the nuclear factor kappa B (NF‐κB) and the p38 MAP kinase signalling pathways. Activity of these pathways is necessary for the upregulation of MuRF1 and MAFbx (Trendelenburg et al., 2009), and thus can be indicative of UPS dysregulation. Indeed, inhibition of NF‐κB activation was sufficient to prevent muscle wasting due to acute pulmonary and subsequent systemic inflammation in mice, suggesting a requirement for NF‐κB activation in the transition from inflammation to atrophy (Langen et al., 2012). Additionally, inhibition of NF‐κB significantly reduced muscle loss in tumour‐bearing mice in association with downregulation of MuRF1. Initiation of muscle wasting and the subsequent development of cachexia in pre‐cachectic APCmin/+ mice was linked to the overexpression of IL‐6 (Baltgalvis et al., 2008). Additionally, IL‐6 is suggested to contribute to the mitochondrial abnormalities associated with tumour growth, as neutralising IL‐6 antibodies restored altered peroxisome proliferator‐activated receptor‐gamma coactivator (PGC)‐1α levels and mitochondrial content (White et al., 2012). The cytokine TWEAK in particular has been linked to upregulation of MuRF1 and resultant breakdown of the myosin heavy chain contractile component of skeletal muscle (Mittal et al., 2010). Interestingly, inflammatory cytokines also activate the Janus kinase (JAK)–signal transducer and activator of transcription 3 (STAT3) pathway, with an induction of STAT3 phosphorylation observed in cancer (Bonetto et al., 2012). The implication of the JAK/STAT signalling pathway in the development of oxidative stress (Duan & Bai, 2020) highlights a potential interconnecting and synergistic interaction between these mediating states present in cancer cachexia.
Oxidative stress
Oxidative stress is produced by excessive reactive oxygen species (ROS) production, reflected by an imbalance between the manifestation of systemic ROS and ability of the system to detoxify the reactive intermediates or repair the resulting cell damage (Zorov et al., 2006). One potential mechanism responsible for this is mitochondrial dysfunction leading to uncoupling of oxidative phosphorylation, which has been observed in Lewis lung carcinoma (LLC) mice (Tzika et al., 2013). Subsequently, mitochondrial uncoupling contributes to aberrant energy metabolism, which in turn is closely related to ROS emission (Cadenas, 2018). Mitochondrial uncoupling proteins (UCP) act to lower the electrochemical proton gradient across the mitochondrial membrane (Ricquier & Bouillaud, 2000), thus facilitating proton leak. This in turn has been shown to decrease ROS production in isolated mitochondria (Boveris & Chance, 1973). Additionally, increased ROS production was observed in the mitochondria of skeletal muscle from UCP3 knockout mice, suggesting a regulatory role of UCPs in ROS generation (Vidal‐Puig et al., 2000). Notably, UCP3 is almost exclusively expressed in skeletal muscle (Sanchis et al., 1998), and evidence suggests that cancer cachexia leads to the induction of UCP2 and UCP3 via TNF‐α (Collins et al., 2002). Indeed, significant upregulation of UCP3 was observed alongside a downregulation of PGC‐1β and altered energy metabolism in LLC cancer cachectic mice (Constantinou et al., 2011), perhaps suggesting a regulatory antioxidative response of the system to the imposed stress of the condition. However, in parallel to decreased ROS, elevated levels of UCPs result in increases in resting energy expenditure via thermogenesis and the dissipation of energy as heat (Giordano et al., 2003). In this way, the activity of UCPs may be linked to the elevations in basal metabolic rate observed in cachexia sufferers. Additionally, increased expression of UCP3 has also been linked to the activation of the proteolytic systems in cultured myotubes (Busquets et al., 2006), suggesting a potential active role in muscle atrophy. Therefore, although there exists a theoretical argument for the targeted upregulation of UCPs with the intention of combatting conditions associated with oxidative stress, the mechanistic contribution of these factors in cachexia is still unclear.
The development of a pro‐oxidative state imparts an influence on skeletal muscle hypertrophic and atrophic pathways. With regard to atrophy, mitochondrial oxidative stress enhances protein degradation via activation of the UPS and autophagy pathways (Barbieri & Sestili, 2012). In the context of hypertrophy, oxidative stress impairs the differentiation of myoblasts and myotubes, and has been implicated in muscular pathologies defined by imbalances in proliferation, such as sarcopenia and cachexia (Fulle et al., 2004). Furthermore, elevated expression of ROS has been linked to downregulation of IGF‐1 signalling and induction of insulin resistance (Bashan et al., 2009). The damaging effect of elevations in free radical expression also impacts muscle function, inducing unwanted alterations in protein structure, including the sarcoplasmic reticulum Ca2+ release channel/ryanodine receptor (Matecki et al., 2016), cross bridge kinetics or the reduction in myofilament Ca2+ sensitivity (Powers & Jackson, 2008). In assessing a potential pharmacological intervention, Smuder and colleagues (2020) illustrated the impact of mitochondrial dysfunction and excessive ROS production in a C26 cancer cachexia model. Tumour‐bearing control animals experienced significant loss of diaphragm muscle mass and subsequent ventilatory dysfunction associated with increased ROS and inflammatory marker expression. The contributions of oxidative stress and inflammation toward muscle wasting in cancer cachexia may work in parallel, or indeed promote the action of one another, as cytokines, particularly TNF‐α, are associated with oxidative stress in skeletal muscle during cancer (Powrozek et al., 2018). Additionally, the JAK–STAT3 signalling pathway is strongly linked to IL‐6 and was found to be activated in the diaphragm of a cancer cachexia mouse model, inducing oxidative stress and diaphragm atrophy and dysfunction (Bonetto et al., 2012). Activation of STAT3 also directly leads to increased expression of myostatin, caspase‐3, atrogin and MuRF, further contributing to protein degradation via ubiquitination (Bonetto et al., 2012; Demaria et al., 2010; Tang et al., 2015). The myostatin‐regulated transcription factor Smad3 has been implicated in the excessive production of ROS via induction of mitochondrial NADPH oxidase 4 (NOX4) (Goodman et al., 2013). Thus, increased STAT3 activation contributes to a pro‐oxidative state via increases in myostatin and subsequent expression of Smad3.
Respiratory muscle function and wasting
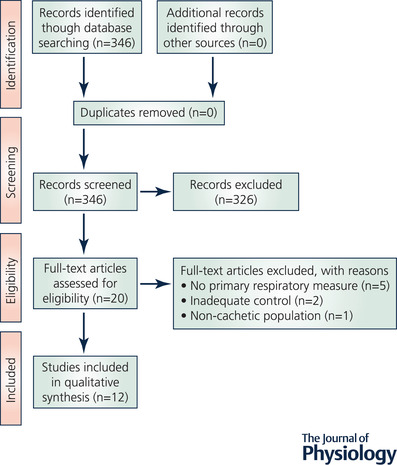
To collate and interpret the relevant existing studies concerning the respiratory system in cancer cachexia a systematic search of the literature was conducted. This process involved the definition of appropriate inclusion and exclusion criteria (Appendix A) from which a search strategy was developed according to the PICO tool, as recommended by the Cochrane Collaboration (Higgins et al., 2019). Indexed headings, identified via the Medical Subject Headings (MeSH) browser (https://meshb.nlm.nih.gov/search), and appropriate keywords were inputted into a table (Appendix B) and used to guide the development of a systematic search strategy. A database search using this strategy was then conducted in PubMed (Appendix C). The resultant screening of papers is illustrated in the PRISMA flow diagram (Moher et al., 2009) in Fig. D1 (Appendix D). Descriptive information including study characteristics, aims and relevant findings are presented in Table 1.
Table 1.
Qualitative data obtained from the studies (n = 12) isolated during a systematic search of the PubMed database
| Respiratory relevant findings | ||||||
|---|---|---|---|---|---|---|
| Author (Year) | Aim | Design | Primary comparison | Alternative comparisons | ||
| Bachmann et al. (2009) | Investigate the influence of cachexia on fat, muscle and lung function in patients with pancreatic cancer |
Non‐cachectic PDA patients Cachectic PDA patients |
CC vs. non‐CC ↓ relative VC ↔ absolute VC ↔ absolute FEV1 ↔ relative FEV1 |
|||
| Chacon‐Cabrera et al. (2014) | Assess the effects of treatment with NF‐κB, MAPK or proteasome inhibitors on respiratory and limb muscle in cancer cachexia |
Control LP07 LC LP07 LC + proteasome inhibitor LP07 LC + NF‐κB inhibitor LP07 LC + MAPK inhibitor |
CC vs. control Diaphragm ↓ muscle mass ↑ protein degradation ↑ myostatin ↓ myogenin ↓ MyHC |
CC + proteasome vs. CC ↔ muscle mass ↓ protein degradation ↓ myostatin ↔ myogenin ↔ MyHC |
CC + NF‐κB vs. CC ↑ muscle mass ↓ protein degradation ↓ myostatin ↑ myogenin ↑ MyHC |
CC + MAPK vs. CC ↑ muscle mass ↓ protein degradation ↓ myostatin ↑ myogenin ↑ MyHC |
| Choi et al. (2013) | Assess the validity of the Lewis lung carcinoma model and examine its effects on skeletal muscle |
Control LLC |
CC vs. control Diaphragm ↓ specific force |
|||
| Fermoselle et al. (2013) | Explore whether cancer cachexia alters MRC complexes and oxygen uptake in respiratory and limb muscles |
Control LP07 LC LP07 LC + NAC LP07 LC + NF‐κB inhibitor LP07 LC + MAPK inhibitor |
CC vs. control Diaphragm ↓ muscle mass ↔ citrate synthase ↓ MRC complex I, II, IV ↓ oxygen consumption |
CC + NAC vs. CC ↔ muscle mass ↔ citrate synthase ↔ MRC complex I, II, IV ↑oxygen consumption |
CC + NF‐κB vs. CC ↑ muscle mass ↑ citrate synthase ↑ MRC complex I, II, IV ↑ oxygen consumption |
CC + MAPK vs. CC ↑ muscle mass ↔ citrate synthase ↔ MRC complex I, II ↑ MRC complex IV ↑ oxygen consumption |
| Fields et al. (2019) | Examine neural involvement in cachexia‐linked respiratory insufficiency |
Control C26 |
Normoxia ↔ V T ↑ breathing frequency ↑ |
Hypoxia ↓ V T ↔ breathing frequency ↓ ↓ inspiratory burst amplitude ↔ phrenic nerve firing frequency |
Hypercapnia ↔ V T ↔ breathing frequency ↔ ↔ inspiratory burst amplitude ↔ phrenic nerve firing frequency |
Maximal Chemoreflex ↔ V T ↔ breathing frequency ↔ ↔ inspiratory burst amplitude ↔ phrenic nerve firing frequency |
| Murphy et al. (2012) | Characterise functional impairments in mild and severe cachexia to inform the suitability of the C26 model |
Control C26‐mild C26‐severe |
CC‐mild vs. control Diaphragm ↔ specific force ↔ twitch characteristics ↓ force (fatigued) |
CC‐severe vs. control ↓ specific force ↔ twitch characteristics ↓ force (fatigued) |
||
| Murphy et al. (2013) | Assess whether treatment with perindopril enhances whole body and skeletal muscle function in cancer cachexia |
Control C26‐mild C26‐mild + treatment C26‐severe C26‐severe + treatment |
CC‐mild vs. control Diaphragm ↓ specific force ↔ twitch characteristics ↔ specific force–freq. relationship |
CC‐mild vs. perindopril ↔ specific force ↔ twitch characteristics ↔ specific force‐freq. relationship |
CC‐severe vs. control ↓ specific force ↔ twitch characteristics ↓ force across force–freq. relationship |
CC‐severe vs. perindopril ↔ specific force ↔ twitch characteristics ↓ force across force–freq. relationship |
| Nosacka et al. (2020) | Assess pathophysiological differences between limb and diaphragm muscle in cancer cachexia |
Control PDAC‐PDX |
Diaphragm ↓ fibre CSA ↑ extracellular space ↑ irregular shaped fibres ↑ no. mononuclear cells ↑ no. necrotic fibres |
Tibialis anterior ↓ fibre CSA ↔ extracellular space ↔ fibre shape ↔ no. mononuclear cells ↔ no. necrotic fibres |
Transcriptome No. genes upregulated TA vs. DIA = 30 overlap No. genes downregulated TA vs. DIA = 39 overlap |
|
| Rosa‐Caldwell et al. (2020) | Investigate signalling related to mitochondrial function, ROS production and protein synthesis during cancer cachexia development |
Control – week 0 LLC – week 1 LLC – week 2 LLC – week 3 LLC – week 4 |
Week 1 vs. week 0 ↔ mitochondrial RCR ↔ mitochondrial content ↔ PGC‐1α ↔ ROS production ↔ SOD1, SOD2 or SOD3 ↔ FSR |
Week 2 vs. week 0 ↓ mitochondrial RCR ↔ mitochondrial content ↔ PGC‐1α ↑ ROS production ↔ SOD1, SOD2 or SOD3 ↔ FSR |
Week 3 vs. week 0 ↔ mitochondrial RCR ↔ mitochondrial content ↔ PGC‐1α ↔ ROS production ↔ SOD1, SOD2 or SOD3 ↔ FSR |
Week 4 vs. week 0 ↓ mitochondrial RCR ↔ mitochondrial content ↔ PGC‐1α ↔ ROS production ↔ SOD1, SOD2 or SOD3 ↔ FSR |
| Salazar‐Degracia et al. (2018) | Assess the effects of treatment with β2 agonist formoterol on atrophy signalling pathways and muscle metabolism of limb and respiratory muscle in cancer cachexia |
Control Control + formoterol CC CC + formoterol |
CC vs. control Diaphragm ↓ muscle mass ↓ muscle fibre CSA ↑ muscle structure abnormalities ↑ NF‐κB activity ↑ FoxO activity ↑ myostatin levels ↓ mTOR activity levels ↓ PGC‐1α |
CC + formoterol vs. control ↓ muscle mass ↔ muscle fibre CSA ↔ muscle structure abnormalities ↔ NF‐κB activity levels ↑ FoxO activity ↔ myostatin levels ↓ mTOR activity levels ↓ PGC‐1α |
CC + formoterol vs. CC ↔ muscle mass ↑ muscle fibre CSA ↓ muscle structure abnormalities ↓ NF‐κB activity levels ↔ FoxO activity ↓ myostatin levels ↔ mTOR activity levels ↔ PGC‐1α |
|
| Smith et al. (2016) | Determine whether the JAK1/3 signalling pathway contributes to cancer cachexia‐mediated diaphragm muscle weakness |
Control Control + JAK inhibitor C26 C26 + JAK inhibitor |
CC vs. control Diaphragm ↓ specific force |
CC + JAK vs. control ↔ specific force |
||
| Smuder et al. (2020) | Assess the impact of pharmacological treatment of mitochondrial dysfunction and ROS production in cancer cachexia |
Control + saline Control + SS‐31 C26 + saline C26 + SS‐31 |
CC vs. control Diaphragm ↓ specific force ↓ fibre CSA ↓ normoxic V T ↓ normoxic ↓ hypoxic VT ↓ hypoxic ↑ ROS emission ↓ mitochondrial RCR |
CC + SS‐31 vs. control ↔ specific force ↔ fibre CSA ↔ normoxic VT ↔ normoxic ↔ hypoxic VT ↔ hypoxic ↔ ROS emission ↔ mitochondrial RCR |
||
↑, significant increase; ↓, significant decrease; ↔, no significant difference; C26, colon 26 adenocarcinoma; CC, cancer cachexia; CSA, cross‐sectional area; DIA, diaphragm; FEV1, forced expiratory volume in 1 s; FSR, fractional synthesis rate; LC, lung cancer; LLC, Lewis lung carcinoma; MRC, mitochondrial respiratory chain; NAC, N‐acetyl cysteine; PDA, pancreatic ductal adenocarcinoma; PDAC‐PDX, pancreatic ductal adenocarcinoma patient derived xenograft; RCR, respiratory control ratio; ROS, reactive oxygen species; SOD, superoxide dismutase; TA, tibialis anterior; VC, vital capacity; V T, tidal volume; , minute ventilation.
Although the extent of research investigating cachexia in the context of its impact on respiratory muscle atrophy and function is scarce, limited results have been published, and these findings support the need for further consideration in this domain. Early results were equivocal, with a number of the original studies addressing this concept reporting no atrophy of the diaphragm in mouse models of cachexia (Murphy et al., 2011; Tessitore et al., 1993). However, a follow‐up study observed slight reductions in maximum specific force and absolute force in diaphragm strips obtained from C26 mice that lost 22% tumour‐free body mass (Murphy et al., 2012). This study represents the earliest evidence of diaphragm muscle atrophy and contractile dysfunction in a mouse model of cancer cachexia. Supportive of this negative impact of cachexia, Roberts, Ahn and colleagues (2013) presented evidence of reduced respiratory muscle mass and function in C26 mice. The severe cachectic condition of these animals was associated with reductions in diaphragm muscle cross‐sectional area of 24%, 22% and 29% in type I, type IIa and type IIb/x fibres, respectively, illustrating uniform wasting across fibre types. Additionally, mRNA expression of the atrophy‐related biomarkers MuRF1 and MAFbx was elevated 2.8‐ and 2.2‐fold in the diaphragm, respectively. Beyond muscle wasting, dysfunction of the diaphragm was evident in reduced submaximal and maximal diaphragm specific force production. In further elucidating the cause of such dysfunction, calcium‐stimulated contractile characteristics were assessed in permeabilised single fibres. Findings were indicative of sarcomeric dysfunction and reduced calcium sensitivity, and this was accompanied by a 27% reduction in myosin heavy chain protein levels. Collectively, these results illustrate not only muscle wasting, but intrinsic dysfunction of the diaphragm beyond the observed decrease in muscle cross‐sectional area. More recent investigations expand our understanding of the nature of the respiratory dysfunction present in cancer cachexia. Examining the neural drive of the phrenic nerve in C26 mice whilst manipulating inspired air, Fields et al. (2019) reported reduced inspiratory burst amplitude in response to hypoxia, but not hypercapnia or maximal hypercapnic/hypoxic response. This is suggestive of selective impairment of the neural mechanisms responsible for the hypoxic respiratory response, most likely at the level of hypoxic sensing. Similar to Roberts, Ahn et al. (2013) and Roberts, Frye et al., 2013), these findings highlight the complexity of the respiratory dysfunction beyond simple atrophy of the associated musculature.
Studies have highlighted an intuitive commonality in the mechanisms responsible for limb muscle wasting in cachexia, and those implicated in the generation of respiratory muscle weakness, atrophy and dysfunction. As is evident in studies concerned with cachexia and limb muscle, elevations in inflammatory factors have also been identified as a contributing mechanism of muscle atrophy and contractile dysfunction in ventilatory muscles (Janssen et al., 2005; Li et al., 2000). Additionally, the NF‐κB pathway was reported to be requisite for the transition of systemic inflammation to muscle atrophy in COPD‐associated cachexia (Mittal et al., 2010). Pharmacological inhibition of either the NF‐κB or the MAPK pathway led to a restoration of both limb and diaphragm muscle mass and force in a lung cancer mouse model (Chacon‐Cabrera et al., 2014). Mitochondrial oxidative stress is also suggested to be implicated in the multifactorial mechanisms of diaphragm atrophy and dysfunction (Duan & Bai, 2020). For example, oxidative stress induced by overexpression of Smad3 was associated with protein degradation in a ventilation‐induced diaphragm dysfunction rat model. Inhibition of Smad3 prevented the protein degradation and reduced diaphragm contractility (Tang et al., 2017). Furthermore, there appears to exist an interconnected signalling network involving STAT3 signalling, Smad3 and FoxOs which contributes to diaphragm muscle atrophy and associated dysfunction, either directly or via mitochondrial dysfunction and oxidative stress (Duan & Bai, 2020). Interestingly, impaired mitochondrial function has been shown to present prior to the development of cancer cachexia in LLC mice (Brown et al., 2017), suggesting potential involvement in the development of the condition. These factors and signalling pathways that are implicated in limb muscle atrophy and altered by inflammatory and pro‐oxidative states are of potential interest as therapeutic targets with the aim of minimising respiratory muscle atrophy and maintaining or restoring respiratory function. However, available information in cachexia‐specific circumstances is limited and there is a distinct need for definitive profiling of the respiratory musculature under the imposed stresses of cachexia.
Further to the issue of limited respiratory focused studies in the context of cachexia, Nosacka and colleagues (2020) provided preliminary evidence to be considered in the generalisation of results obtained in the examination of limb muscle atrophy, which forms the focus of the majority of cachexia research. Transcriptome analysis suggested differential gene expression in tibialis anterior muscle compared to diaphragm muscle in response to the cancer‐induced cachectic state. Additionally, while no apparent evidence of morphological damage was observed in limb muscle, consistent with past literature (Langen et al., 2012), histological analysis of diaphragm cryosections revealed disrupted muscle architecture, including increased extracellular space, irregularity of muscle fibre shape and necrotic fibres. Therefore, although commonalities are evident such as inflammation and oxidative stress, the manifestation of wasting and the response of the musculature to the stresses imposed may differ to a degree between limb and respiratory muscles. Such findings further emphasise the need for additional research aiming to characterise the profile of diaphragmatic muscle atrophy and dysfunction in cachexia.
Benefits of exercise
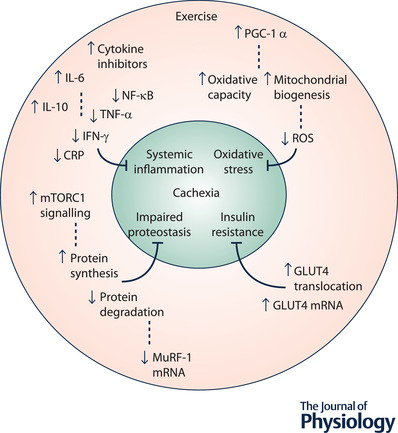
The potential utility of exercise in reducing the severity of cachexia is promising. Exercise may provide both anti‐inflammatory (Petersen & Pedersen, 2005) and antioxidative stimuli (Ji, 1999). Additionally, exercise type may impart a specific influence. For example, resistance exercise provides a potent hypertrophic stimulus in elevating levels of protein synthesis (Chesley et al., 1992) via activation of the mTOR pathway (Song et al., 2017). Alternatively, endurance exercise provides a potent metabolic stimulus by increasing mitochondrial biogenesis (Holloszy & Booth, 1976) via PGC‐1α signalling (Pilegaard et al., 2006) (Fig. 3). The influence of such mechanistic stimuli can present in increased skeletal muscle strength and function, exercise capacity, and decreased fatigue, ultimately resulting in improved patient quality of life and handling of the condition (Knols et al., 2005; Schneider et al., 2007). Theoretically, these effects would act antagonistically to the fundamental mechanistic basis of the condition. Whereas cachexia is associated with an increase in protein degradation and concurrent decrease in protein synthesis, exercise may work to combat the adverse effects of the condition, whilst simultaneously increasing lean tissue mass. Contradictory to this, a recent study demonstrated that voluntary exercise was ineffective in improving the muscle phenotype of cachectic C26 mice (Hiroux et al., 2021). Additionally, it has been hypothesised that increased energy demand due to tumour burden elevates the risk of over‐reaching (Allan et al., 2022), an acute instance of insufficient recovery following exercise which if not recognised and accommodated for can progress to overtraining syndrome (Urhausen & Kindermann, 2002). In this way inappropriate implementation of exercise may exacerbate the cachectic state. These findings illustrate the need for careful implementation of a structured intervention defined by appropriate parameters should the benefits of exercise in cancer cachexia be optimally realised. Further research in this area is needed to elucidate such parameters and these investigations should be conducted in consideration of the knowledge transfer to clinical populations. However, prior to assessment of the specifics of intervention, additional foundational investigations into the mechanisms of effect of exercise in cachexia are needed.
Figure 3. The potential benefits of exercise in cachexia as reported in the literature.
Tumour burden
The influence of tumour burden on the cachectic state is considerable (De Lerma Barbaro et al., 2015). Reasonable preclinical evidence suggests exercise training may inhibit the progression of tumour growth and subsequently mitigate the impact of tumour burden on muscle tissue. For example, aerobic exercise training slowed tumour growth and attenuated cancer‐induced muscle wasting in C26 mice (Khamoui et al., 2016). Similar observations have also been reported in Walker 256 breast tumour‐bearing rats (Lira et al., 2008) and LLC tumour‐bearing mice (Penna et al., 2011). The study of Pedersen et al. (2016) emphasised this beneficial effect of exercise, reporting a 60% reduction in tumour incidence and growth across multiple cancer models following voluntary wheel running. This effect was attributed to the acute immune response following exercise. Additionally, 16 days of high‐intensity interval training has been shown to reduce tumour mass by 52% in LLC mice (Alves et al., 2018). This was accompanied by increases in mRNA levels of genes associated with inflammation and immunomodulation. These findings are particularly impressive considering the aggressive tumour progression and atrophy development of such cancer cachexia models. In addressing a considerable limitation of these studies, further research exploring the degree to which effects of exercise are maintained in orthotopic models is required. Such evidence would be informative as to the benefit of exercise in combatting tumour burden alongside the adverse impact of the associated tumour microenvironment.
Hypertrophy/protein synthesis
An intuitive potential benefit of an exercise intervention in combatting cancer cachexia is the stimulation of muscle hypertrophy, whereby activation of the associated pathways would act to create a shift in favour of protein synthesis and to disrupt and attenuate atrophic signalling. In this manner, regardless as to whether or not the stimulus is sufficient to produce considerable muscle growth, the mechanism of hypertrophic adaptation may reduce the severity of the cachectic condition by acting antagonistically to the mechanisms responsible for muscle atrophy and protein degradation. Resistance exercise training (RET) provides a potent stimulus toward such adaptation. Recent studies focusing on eccentric exercise training have provided evidence of its capability to activate mediating pathways of muscle growth, in addition to inhibiting mediators of muscle atrophy in preclinical cancer cachexia models. A maximal eccentric exercise intervention of 4 weeks resulted in elevated phosphorylation of p70S6K and rpS6, implying increased mTORC1 signalling in C26 mice. This was accompanied by a reduction in MuRF‐1 mRNA and increased gastrocnemius muscle mass (Tatebayashi et al., 2018). An intervention consisting of eight sessions of repeated eccentric contractions in male APCmin/+ mice reflected similar results, with reduced muscle wasting, increased mTORC1 signalling and improved oxidative metabolism (Hardee et al., 2020). These results support the capability of exercise to reverse the imbalance of increased protein degradation and decreased protein synthesis as evident in the cachectic condition, helping to establish a state of proteostasis more compatible with the maintenance or recovery of muscle mass.
It is well established that eccentric contractions consume less oxygen and energy for a given muscle force than concentric contractions (Abbott et al., 1952). As a result eccentric RET is less energy demanding than both concentric RET (Lastayo et al., 1999) and endurance training (Bloomer, 2005), and hence, may be of particular benefit to those suffering from cachexia. Interest in the potential for imposing high loads on muscle tissue whilst reducing energy requirements led to the development of the Resistance Exercise via Eccentric Work (RENEW) modality, which advocates for moderate load eccentric exercise (LaStayo et al., 2014). Based on previous research, general protocol design would involve a frequency of 3 times per week, at an initial intensity of 50–75 W, for initial durations of 5–10 min. Progressive ramping of loads can then be introduced over 2–3 weeks without undue muscle soreness (LaStayo et al., 2017). Results support the use of this approach in producing similar gains in muscle strength and volume as traditional training, and it has been recommended in many clinical populations including cancer, sarcopenia and cachexia (Hoppeler, 2016; Julian et al., 2018; LaStayo et al., 2014). A range of techniques and equipment have been utilised to facilitate this form of eccentric training, including upper and lower limb ergometers (LaStayo et al., 2017), adapted treadmills to simulate downhill walking, or ergometers which mimic stair descending (Isner‐Horobeti et al., 2013; Theodorou et al., 2013).
Notably, it is well established that eccentric contractions result in greater degrees of muscle damage in comparison to other contraction types (Fridén & Lieber, 1992). It is possible that excessive muscle damage may be detrimental to subsequent training efforts via decreased capability to produce force and increased levels of delayed‐onset muscle soreness (Tee et al., 2007). Despite this, a reasonable body of evidence recommends eccentric training over concentric or isometric RET for muscle hypertrophy (Julian et al., 2018). However, the evidence in this regard is not conclusive, as a systematic review found similar changes in hypertrophy between eccentric and concentric training modalities (Franchi et al. 2017). Additionally, the implication of greater muscle damage in potentially enhancing the hypertrophic stimulus has been challenged, considering muscle damage is not essential to induce skeletal muscle hypertrophy and muscle becomes increasingly less susceptible to damage with repeated exercise (Nosacka et al., 2003). Ultimately, the optimal degree of exercise‐induced muscle damage has not been determined, although it is hypothesised from past literature that a moderate degree should be sufficient in order to induce muscle hypertrophy (Schoenfeld, 2012). Should there be potential contraindications surrounding the implementation of eccentric training, submaximal contractions of progressive intensity, such as those employed in the RENEW modality, are recommended to minimise the likelihood of experiencing negative side effects (Hody et al., 2019).
Anti‐inflammatory effect
Exercise is associated with the considerable production of anti‐inflammatory cytokines (Ostrowski et al., 1999). First to appear in the circulation, IL‐6 potentially increases 100‐fold in response to exercise (Pedersen & Hoffman‐Goetz, 2000; Steensberg et al., 2002), exhibiting a contrasting influence to that of its chronic presence in systemic inflammation. The anti‐inflammatory influence of IL‐6 is demonstrated in its antagonism of pro‐inflammatory cytokine production, including TNF‐α and IL‐1 (Gould et al., 2013; Schindler et al., 1990). Additionally, IL‐6 is implicated in the mediation of muscle growth (Serrano et al., 2008). As opposed to being correlated with reduced muscle cross‐sectional area when chronically elevated, muscle growth induced by eccentric RET was positively correlated with IL‐6 levels in APCmin/+ mice (Hardee et al., 2020). Exercise also results in increased levels of the anti‐inflammatory cytokine IL‐10, which inhibits the production of IL‐1α, IL‐1β and TNF‐α, and cytokine inhibitors, which act to block the signalling capability of pro‐inflammatory factors (Helmark et al., 2010; Petersen & Pedersen, 2005). Considered to be the most practical, cost‐effective and robust biomarker of cancer cachexia, C‐reactive protein (CRP) is used to assess prognosis and predict quality of life in cancer cachexia patients (Fearon et al., 2011). A meta‐analysis of cancer‐focused research found that combined resistance and endurance exercise training lead to reductions in CRP in cancer survivors (Khosravi et al., 2019). Additionally, 3 months of lower body RET was effective in reducing TNF‐α levels in a frail elderly population (Greiwe et al., 2001). Similarly, RET reduced IFN‐γ levels after 12 weeks in elderly women (Roh et al., 2020), and after 8 weeks in prostate cancer patients (Papadopoulos et al., 2021). Finally, although not in a cachectic model, loaded ladder climbing, representing concentric RET, decreased NF‐κB expression in the inflammatory condition of Parkinson's disease in mice (Kim et al., 2021). However, it should be noted that many of these results were observed in non‐cachectic cohorts and not in consideration of the subsequent impact on muscle atrophy.
Anti‐oxidative effect
The mitochondrial oxidative pathway, which is of critical importance to cell metabolism and growth, is another physiological function impaired in cancer cachexia (VanderVeen et al., 2017), and is hypothesised to be implicated in the development of the condition. Exercise enhances the activity of antioxidant enzymes which play an important role in protecting against cell damage from ROS, with markers of damage being reduced following exercise (Khanna et al., 1998; Neuzil & Stocker 1993; Ohkuwa et al., 1997). Moderate intensity continuous exercise is known to stimulate metabolic alterations which increase antioxidant capacity in both humans and rodents (Gomez‐Cabrera et al., 2008). Predicated on this knowledge, Ballaro et al. (2019) demonstrated the maintained antioxidative capabilities of exercise in the C26 mouse model. Here, moderate intensity treadmill running provided protection against muscle wasting and prevented loss of muscle function in association with reduced levels of ROS and improved oxidative capacity. Additionally, exercise may indirectly decrease ROS production by attenuating systemic inflammation (Bowen et al., 2015), as inflammatory factors such as TNF‐α and IL‐6 can promote a pro‐oxidative state via the JAK–STAT3 signalling pathway (Bonetto et al., 2012; Powrozek et al., 2018). Furthermore, the activity of the NF‐κB and STAT3 pathways have been linked to mitochondrial dysfunction in muscle and thus are relevant therapeutic targets (VanderVeen et al., 2017).
Endurance training was recommended over RET for the reduction of muscle wasting by Aquila et al. (2020) in the context of oxidative stress in cancer cachexia. Indeed, endurance training is known to improve the oxidative capacity of muscle in healthy individuals by enhancing mitochondrial biogenesis and increasing glycogen content (Burgomaster et al., 2007, 2008). Considered in the context of cachexia, Mavropalias and colleagues (2022) suggested that the benefits of endurance exercise may be intensity dependent, with shorter duration, higher intensity training providing the most promise. Conversely, low‐intensity continuous training is hypothesised to be a less than optimal approach due to its higher energy demands (Bloomer, 2005), potentially exacerbating the cachectic condition. In support of this concept, 8 weeks of high‐intensity endurance training proved to be more effective in reducing NF‐κB levels than low‐intensity endurance training in rats (Leite et al., 2021). However, a lack of primary studies assessing the effects of exercise training on cachexia limits our understanding of the impact that altering such exercise parameters can have on the condition. In contradiction of findings which advocate endurance training over RET for the reduction of oxidative stress (Aquila et al., 2020), STAT3 activation was reduced in the muscle of tumour‐bearing cachectic mice following eight eccentric RET sessions across 2 weeks (Hardee et al., 2020) in addition to increasing muscle oxidative capacity and mitochondrial content (Hardee et al., 2020, 2016). Furthermore, basal protein synthesis was correlated with mitochondrial content (Hardee et al., 2020), suggesting a restorative effect on protein synthesis in association with maintenance of mitochondrial content and function.
Attempts to alleviate oxidative stress have also focused on the elevation of the transcription coactivator PGC‐1α, which primarily acts to increase mitochondrial content. However, findings in relation to the inhibition of muscle atrophy are mixed. For example, a large decrease in PGC‐1α mRNA was observed in a mouse model of cancer cachexia, and transgenic overexpression of PGC‐1α exhibited resistance to atrophy through the suppression of FoxO3 action (Sandri et al., 2006). Additionally, the same research group reported that the overexpression of an isoform, PGC‐1α4, facilitated the prevention of cancer cachexia in mice through the activation of IGF‐1 and the repression of myostatin. However, employment of a similar transgenic model failed to illustrate a protective effect of PGC‐1α overexpression in LLC mice (Wang et al., 2012), suggesting that targeting of increased mitochondrial biogenesis may be insufficient in the treatment of cancer cachexia.
Noting the distinct scarcity of research specific to a cachexia context, evidence from healthy and alternative disease populations suggest that RET and high‐intensity endurance training are of potential benefit in combatting the adverse effects of cancer cachexia on mitochondrial function and the associated state of oxidative stress.
Insulin resistance
Peripheral insulin resistance is common in cancer cachexia due to decreased expression of glucose transporter type 4 (GLUT4) and muscle glucose uptake (Puppa et al., 2014). This altered response to insulin likely contributes in some degree to reductions in protein synthesis (Baracos, 2000). TNF‐α appears to bear a mediating influence in the development of insulin resistance in cancer (Noguchi et al., 1998), and so the anti‐inflammatory effect of exercise may be of some benefit. However, likely to be a much more potent stimulus is exercise's ability through muscle contraction to increase expression and translocation of GLUT4 to the muscle membrane, facilitating uptake of glucose from the circulation independent of insulin (Flores‐Opazo et al., 2020; Ren et al., 1994; Richter & Hargreaves, 2013). In cancer cachectic mice with reduced basal levels of GLUT4, a single 30‐min session of electrically stimulated concentric contractions facilitated the increase of GLUT4 mRNA levels by 4.7‐fold (Puppa et al., 2014). This offers evidence that this effect of exercise is maintained in the cachectic state. Insulin sensitivity has been increased in elderly men following a single session of eccentric RET per‐week for 12 weeks, whereas concentric RET did not produce equivalent results (Chen et al., 2017). Further research conducted in alternative populations, including diabetic and healthy adults, implies that exercise reliant on high muscle glucose metabolism may be more efficacious in elevating GLUT4 expression, supporting the implementation of both high‐intensity interval (Burgomaster et al., 2007) and low‐intensity continuous (Bowman et al., 2021) endurance exercise, in addition to concentric RET (Dehghan et al., 2016). The obvious issue in implementing an intervention aimed at maximising glucose metabolism is a higher energy yield is undesirable in the cachectic individual. Nonetheless, muscle contraction‐induced mobilisation of GLUT4 would likely be a beneficial concomitant effect of an exercise intervention aimed at combatting cachexia. Additionally, exercise is known to stimulate angiogenesis (Andersen & Henriksson, 1977), largely via the upregulation of vascular endothelial growth factor (Breen et al., 1996). This is typically observed in response to endurance exercise (Hudlicka et al., 1992). Both chemical and mechanical mechanisms are potentially implicated, including increases in stretch and compression, shear stress and transmural pressure of the vessels (Kissane & Egginton, 2019), in addition to elevations in hypoxia‐inducible factor in response to low partial pressure of oxygen in the blood (Shweiki et al., 1992). Significant increases in capillary density can be observed following only 4 weeks of cycle ergometer training at ∼ 60% maximal oxygen uptake (Hoier et al., 2012), and this timeline is reflected in rodents following voluntary wheel running (Waters et al., 2004). Such an adaptation may further enhance insulin sensitivity through improved blood–tissue exchange properties. A greater capillary network increases the surface area for diffusion, shortens the average diffusion pathway length, and increases the length of time for diffusive exchange between the blood and the muscle (Bloor, 2005). Of note, enhancement of blood exchange properties is dependent on the relative ratio between capillary surface area and muscle fibre surface area (Hepple et al., 2000). In this regard RET has been suggested to be less beneficial or perhaps even detrimental towards this adaptation as hypertrophy of muscle fibres can decrease this ratio (Tesch et al., 1984). However, considering the population in question, any considerable skeletal muscle hypertrophy would far outweigh the benefits which enhanced blood–tissue glucose exchange may have on insulin resistance.
From this brief overview of the literature, we conclude that exercise training has the potential to exert many beneficial effects which may culminate to counteract the adverse effects of cachexia. However, many of these effects are yet to be comprehensively investigated in cachexia specifically, with much of the above information originating from alternative populations. Therefore, although as much information as possible should be gleaned from research into conditions which elicit similar wasting profiles, it is important to acknowledge that all of the effects of exercise may not hold true in cachexia. As a result, further attempts to contribute to the knowledge base surrounding the mechanistic alterations of the cachectic state resulting from exercise would be of considerable value in advancing this area. In order to ensure valid and practically meaningful results are obtained from such investigations an appropriate model of cancer cachexia must be utilised.
Preclinical models of cancer cachexia
There are a number of preclinical methodological options available designed to mimic the human cancer cachectic condition in mice. These include subcutaneous, orthotopic, chemically induced and genetically engineered variations. The classically employed models in the study of cancer cachexia are the subcutaneous C26 and LLC models. An insightful systematic review highlighted the popularity of these models. Compared to orthotopic and genetically engineered mouse models (GEMM), subcutaneous models were found to be the most abundant in the early research, with the C26 variation continuing to be predominant in 2017 (Tomasin et al., 2019). Results from the extensive use of subcutaneous models have facilitated the advancement of our understanding of the mechanisms responsible for muscle atrophy in the condition. However, the clinical translatability of these models has been questioned (Johns et al., 2013). It should be acknowledged that no single model fully recapitulates cancer cachexia in humans (Mueller et al., 2016). However, continued model development with the aim of addressing certain limitations has led to the production of viable alternatives, particularly with regard to the representation of pancreatic cancer cachexia (Delitto et al., 2017; Henderson et al., 2018). Consideration concerning which model to employ should primarily focus on the nature of the resultant cachexia phenotype to ensure that this is in line with the focal point of the research to be conducted and optimises the translation of findings to the clinical setting. Additionally, the associated benefits and drawbacks of each model should also be considered.
Subcutaneous models are an ectopic model, involving the injection of cultured tumour‐derived cells into the flank of the animal which have been reported to be representative of the cancer cachectic condition (Aulino et al., 2010). Supporting the use of the C26 model in juvenile mice, comparable tumour development and proteolytic signalling was observed between 8‐week‐old and 12‐month‐old animals (Talbert et al., 2014). Notably, 12‐month‐old mice were used in this study as this age is before the onset of sarcopenia (Sayer et al., 2013), accounting for this potential confounding variable. Slight differences were reported with young mice experiencing ∼10% greater limb muscle mass loss, which was attributed to a greater relative tumour burden as evident from the tumour mass to body mass ratio (Talbert et al., 2014). Highlighted as an issue of the C26 and LLC models is the typically large resultant tumours, which often represent >10% of whole‐body mass (Talbert et al., 2017; Zhang et al., 2017). This reflects one of the primary criticisms of these models, in that they may represent an inhibition of growth in young animals, as opposed to the active atrophy of established muscle mass seen in cachexia (Talbert et al., 2019). This is likely due to not only the use of younger animals, but also the aggressive nature of the models whereby the induction and development of cachexia occurs over a time scale of only a few weeks (Roberts, Ahn et al., 2013; Talbert et al., 2014). This rapid onset of wasting may be a reflection of the reliance of subcutaneous models on IL‐6 and the induction of an inflammatory phenotype (Bonetto et al., 2011). Although systematic inflammation is associated with and contributes to cachexia progression, the levels observed in subcutaneous models far exceed those of cancer patients (Lerner et al., 2015; Strassmann et al., 1992; Talbert et al., 2018). Furthermore, this narrow window from induction to death shortens the experimental period, limiting the analysis of potential treatments. Another acknowledged limitation of the subcutaneous options, as ectopic models, is the development of the tumour in a non‐physiological growth environment (Bonetto et al., 2016), and hence, the absence of a representative tumour microenvironment, which constitutes a proportion of the disparity between these preclinical models and human cancer patients. This represents one of the primary advantages for which one might consider employing an orthotopic cancer cachexia model.
Orthotopic models are frequently employed and recommended for the study of pancreatic cancer cachexia (Qiu & Su, 2013). Such models involve implantation of human or mouse cancer cell lines or tumour tissue into a syngeneic site. This facilitates the establishment of an organ‐specific tumour microenvironment, allowing the development of site‐specific pathology (Go et al., 2017). In this way, orthotopic models are suggested to be more clinically relevant than subcutaneous models (Delitto et al., 2017; Gengenbacher et al., 2017). Variations of orthotopically induced pancreatic cancer cachexia models have reported results supportive of their ability to reflect numerous aspects of the condition (Henderson et al., 2018), including decreased body mass and limb muscle CSA, upregulated cachexia biomarkers, including FoxO proteins and E3 ubiquitin ligases, and elevated levels of pro‐inflammatory cytokines (Greco et al., 2015; Jones‐Bolin & Ruggeri, 2007; Shukla et al., 2015). However, orthotopic models are also associated with certain limitations, primarily the required use of immunodeficient animals to prevent rejection of the transplanted tissue (Henderson et al., 2018), which likely influences the pathophysiological response of the animal to the condition.
Recently, Talbert and colleagues (2019) designed a novel GEMM of pancreatic ductal adenocarcinoma (PDA), with the intention of accurately recapitulating the human cancer cachectic phenotype, in consideration of the limitations of the C26 and LLC models. The resultant model, named KPP, is reported to express a number of improvements in this regard. Modification of the Kras–p53–Cre model (Parajuli et al., 2018) allows induction of PDA via tamoxifen administration. Characterisation of the model at 60, 75 and 90 days post‐induction illustrated a progressive nature of muscle wasting, with decreases in body weight matching observed decreases in skeletal muscle, heart and adipose mass relative to controls. Interestingly, the tibia lengths of KPP mice were comparable to control animals, suggesting that the observed muscle atrophy was not due to a stunting of development, which is a hypothesised limitation of subcutaneous models. Notably, a comparable phenotype was observed in 1‐year‐old animals, whereby tamoxifen administration induced progressive PDA development over a median post‐injection survival period of 158 days. Importantly, un‐injected KPP mice showed no apparent pancreas pathology. In comparison to the rapid onset of muscle wasting induced in many other preclinical models, the progressive development of the cachectic condition in the KPP model more accurately represents the nature of muscle atrophy observed in cancer patients (Johns et al., 2013). In this way the KPP model could potentially facilitate research aimed at exploring the initial mechanistic alterations that occur in the developmental stages of cancer cachexia, an area of study recommended for the improvement of diagnosis and prevention of the condition (Rosa‐Caldwell et al., 2020). Additionally, these features would allow extensive assessment of therapeutic interventions. For example, the potential benefit of chronic exercise training or training performed prior to the onset of wasting could be explored. In consideration of the variation in cachexia severity dependent on tumour type and site (Dewys et al., 1980), Talbert and colleagues acknowledged the potential for features of the KPP model to be selective to PDA. Nevertheless, the characteristics of this model present it as a promising tool in the future study of cachexia, particularly in the context of muscle atrophy associated with pancreatic cancer, which considering the prevalence reported in this population (Fearon et al., 2011) is an area of utmost importance.
Conclusion
Cachexia is a severe condition associated with the progression of chronic illness, bearing a notable impact in cancer considering its prevalence in this population. Use of the classification of cachexia as a prognostic marker in cancer patients highlights the severe adverse effect imposed during the development of this condition. Elevations in the morbidity and mortality rates of cancer patients suffering from cachexia has been linked to respiratory failure subsequent to atrophy and dysfunction of the ventilatory muscles. Despite this, there is a distinct scarcity of research investigating the structural and functional condition of the respiratory musculature in response to the stresses of cachexia, with the vast majority of studies exclusively focusing on limb muscle. Considering some of the findings of the small minority that have addressed this issue with a respiratory focus, this area of research warrants much further attention. Although a considerable number of reports have been published investigating mechanisms of action of cachexia in limb muscles, research into the presentation of the condition in the respiratory musculature would be valuable due to the importance of this system in disease outcomes and potential differences in response between limb and diaphragm muscle. Therefore, profiling of cancer cachexia in the respiratory musculature would be a valuable pursuit. Furthermore, such results would aid the identification of therapeutic targets, facilitating subsequent investigation into efficacious intervention design for the treatment of the condition. It is accepted that such an intervention would be multi‐factorial, combining elements of pharmacology, nutrition and exercise. Further research is required into each of these components. However, exercise has received comparatively little attention. Considering the presentation of cachexia and the known effects of exercise in healthy individuals in addition to disease populations, it is probable that further research into its bearing on the cachectic state will yield valuable results with potential application to disease management. In time, such research will facilitate exploration into the influence of varying exercise parameters on patient response, allowing optimisation of intervention protocols. Additionally, further understanding of the response of the cachectic individual to exercise in isolation would benefit future studies aiming to design, implement and assess multifactorial therapeutic approaches.
Additional information
Competing interests
None.
Author contributions
B.M. and K.O'H. were responsible for the concept of the article. B.M. wrote the initial draft of the text. All authors contributed to the critical evaluation and revision of the text for important intellectual content. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
None.
Supporting information
Peer Review History
Acknowledgements
Open access funding provided by IReL.
Biography
Ben Murphy graduated with a BSc in Sport and Exercise Sciences from the University of Limerick in 2018 and an MSc in Exercise Physiology from Loughborough University in 2020. He is currently undertaking his PhD studies at University College Cork in collaboration with Dr John Mackrill and Professor Ken O'Halloran. He has a particular interest in the mechanisms which underlie skeletal muscle adaptation to exercise, and how understanding of these mechanisms can inform therapeutic implementation of exercise to combat disease. His studies will focus on exercise as an intervention in novel mouse models of cancer cachexia with a view to the adoption of strategies for implementation in people with cancer.

Appendix A. Inclusion and exclusion criteria
Inclusion
Studies were considered for inclusion provided they fulfilled the following criteria.
Participants/subjects included either patients suffering from cancer cachexia or preclinical models of cancer cachexia.
There was assessment of the impact of cancer cachexia on the respiratory system using primary measures, including, but not limited to, measures of respiratory muscle wasting and/or function.
There was inclusion of an appropriate control group for comparison.
The study was published in English in a peer‐reviewed journal.
Exclusion
Studies were excluded if they fulfilled the following criteria.
They including other wasting conditions, where isolation of cachexia data is not possible.
They presented unpublished data or data only available in abstract form.
They were non‐primary studies such as reviews and meta‐analyses.
Appendix B. Search strategy table
Table B1.
MeSH headings and text words which were combined to generate search strategies
| Population | Intervention | Comparison | Outcome | ||
|---|---|---|---|---|---|
| Medical subject headings (MeSH) | |||||
| Cachexia | Mice | Human | Exercise | Diaphragm | |
| Adult | Respiratory muscles | ||||
| Clinical study | Respiratory system | ||||
| Clinical trial | |||||
| Text words | |||||
| Cancer | Mouse model | Exercise | Respiratory | ||
| Cancer cachexia | Murine | Exercise intervention | Respiratory function | ||
| Colon adenocarcinoma 26 | Training | Respiratory control | |||
| Lewis lung carcinoma | Aerobic training | Diaphragm function | |||
| Aerobic exercise | Diaphragm control | ||||
| Pancreatic ductal adenocarcinoma | Resistance training | Muscle atrophy | |||
| LLC | Resistance exercise | Muscle wasting | |||
| C26 | |||||
| PDA | |||||
Appendix C. PubMed search strategy
((Cachexia[mh]) OR (cancer[tiab] AND cachexia[tiab]) OR cachexia[tiab])
(Mice[mh] OR ‘mouse model’[tiab] OR murine[tiab] OR ‘Colon Adenocarcinoma 26’[tiab] OR C26[tiab] OR ‘Lewis Lung Carcinoma’[tiab] OR LLC[tiab] OR ‘Pancreatic Ductal Adenocarcinoma’[tiab] OR PDA[tiab])
(Human[mh] OR Adult[mh] OR ‘Clinical Study’[mh] OR ‘Clinical Trial’[mh] OR ‘clinical trial’[tiab])
(Diaphragm[mh] OR ‘Respiratory muscles’[mh] OR ‘Respiratory system’[mh] OR Respiratory[tiab] OR ‘Respiratory function’[tiab] OR ‘Respiratory control’[tiab] OR ‘Diaphragm control’[tiab] OR ‘Diaphragm function’[tiab] OR ‘respiratory muscle atrophy’[tiab] OR ‘respiratory muscle wasting’[tiab])
(1) AND (2)
(1) AND (3)
(1) AND (2) AND (4)
(1) AND (3) AND (4)
(7) OR (8)
Appendix D. PRISMA flow diagram
Figure D1.
Figure D1. PRISMA flow diagram illustrating the number of papers excluded at each stage of the screening process (Moher et al., 2009).
Handling Editors: Laura Bennet & Scott Powers
The peer review history is available in the Supporting Information section of this article (https://doi.org/10.1113/JP283569#support‐information‐section).
References
- Abbott, B. C. , Bigland, B. , & Ritchie, J. M. (1952). The physiological cost of negative work. Journal of Physiology, 117(3), 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan, J. , Buss, L. A. , Draper, N. , & Currie, M. J. (2022). Exercise in people with cancer: A spotlight on energy regulation and cachexia. Frontiers in Physiology, 13, 836804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, C. R. R. , das Neves, W. , Tobias, G. C. , de Almeida, N. R. , Barreto, R. F. , Melo, C. M. , de Carneiro C, G. , Garcez, A. T. , de Faria D, P. , Chammas, R. , & Brum, P. C. (2018). High‐intensity interval training slows down tumor progression in mice bearing Lewis lung carcinoma. JCSM Rapid Communications, 1(2), 1–10. [Google Scholar]
- Andersen, P. , & Henriksson, J. (1977). Capillary supply of the quadriceps femoris muscle of man: Adaptive response to exercise. Journal of Physiology, 270(3), 677–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreyev, H. J. N. Norman, A. R. , Oates, J. , & Cunningham, D. (1998). Why do patients with weight loss have a worse outcome when undergoing chemotherapy. European Journal of Cancer, 34(4), 503–509. [DOI] [PubMed] [Google Scholar]
- Aquila, G. , Re Cecconi, A. D. , Brault, J. J. , Corli, O. , & Piccirillo, R. (2020). Nutraceuticals and exercise against muscle wasting during cancer cachexia. Cells, 9(12), 2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiles, J. M. , Busquets, S. , Stemmler, B. , & Lopez‐Soriano, F. J. (2014). Cancer cachexia: Understanding the molecular basis. Nature Reviews. Cancer, 14(11), 754–762. [DOI] [PubMed] [Google Scholar]
- Argiles, J. M. , Lopez‐Soriano, F. J. , & Busquets, S. (2012). Counteracting inflammation: A promising therapy in cachexia. Critical Reviews in Oncogenesis, 17(3), 253–262. [DOI] [PubMed] [Google Scholar]
- Arthur, S. T. , Noone, J. M. , Van Doren, B. A. , Roy, D. , & Blanchette, C. M. (2014). One‐year prevalence, comorbidities and cost of cachexia‐related inpatient admissions in the USA. Drugs Context, 3, 212265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaix, D. , Aurousseau, E. , Combaret, L. , Kee, A. , Larbaud, D. , Ralliere, C. , Souweine, B. , Taillandier, D. , & Tilignac, T. (1998). Ubiquitin‐proteasome‐dependent proteolysis in skeletal muscle. Reproduction Nutrition Development, 38(2), 153–165. [DOI] [PubMed] [Google Scholar]
- Aulino, P. , Berardi, E. , Cardillo, V. M. , Rizzuto, E. , Perniconi, B. , Ramina, C. , Padula, F. , Spugnini, E. P. , Baldi, A. , Faiola, F. , Adamo, S. , & Coletti, D. (2010). Molecular, cellular and physiological characterization of the cancer cachexia‐inducing C26 colon carcinoma in mouse. BMC Cancer, 10(1), 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay, E. , Thiery, G. , Chevret, S. , Moreau, D. , Darmon, M. , Bergeron, A. , Yang, K. , Meignin, V. , Ciroldi, M. , Le Gall, J. R. , Tazi, A. , & Schlemmer, B. (2004). The prognosis of acute respiratory failure in critically ill cancer patients. Medicine, 83(6), 360–370. [DOI] [PubMed] [Google Scholar]
- Bachmann, J. , Ketterer, K. , Marsch, C. , Fechtner, K. , Krakowski‐Roosen, H. , Büchler, M. W. , Friess, H. , & Martignoni, M. E. (2009). Pancreatic cancerrelated cachexia: Influence on metabolism and correlation to weight loss and pulmonary function. BMC Cancer, 9(1), 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr, L. M. , Furlow, J. D. , & Bodine, S. C. (2011). Muscle sparing in muscle RING finger 1 null mice: Response to synthetic glucocorticoids: Response of MuRF1 KO mice to glucocorticoids. Journal of Physiology, 589(19), 4759–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaro, R. , Penna, F. , Pin, F. , Gomez‐Cabrera, M. C. , Vina, J. , & Costelli, P. (2019). Moderate exercise improves experimental cancer cachexia by modulating the redox homeostasis. Cancers, 11(3), 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltgalvis, K. A. , Berger, F. G. , Pena, M. M. , Davis, J. M. , Muga, S. J. , & Carson, J. A. (2008). Interleukin‐6 and cachexia in ApcMin/+ mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 294(2), R393‐R401. [DOI] [PubMed] [Google Scholar]
- Baracos, V. E. (2000). Regulation of skeletal‐muscle–protein turnover in cancer‐associated cachexia. Nutrition (Burbank, Los Angeles County, Calif), 16(10), 1015–1018. [DOI] [PubMed] [Google Scholar]
- Barbieri, E. , & Sestili, P. (2012). Reactive oxygen species in skeletal muscle signaling. Journal of Signal Transduction, 2012, 982794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan, N. , Kovsan, J. , Kachko, I. , Ovadia, H. , & Rudich, A. (2009). Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiological Reviews, 89(1), 27–71. [DOI] [PubMed] [Google Scholar]
- Berger, D. , Bloechlinger, S. , von Haehling, S. , Doehner, W. , Takala, J. , Z'Graggen, W. J. , & Schefold, J. C. (2016). Dysfunction of respiratory muscles in critically ill patients on the intensive care unit. Journal of Cachexia, Sarcopenia and Muscle, 7(4), 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer, R. J. (2005). Energy cost of moderate‐duration resistance and aerobic exercise. Journal of Strength and Conditioning Research, 19, 878–882. [DOI] [PubMed] [Google Scholar]
- Bloor, C. M. (2005). Angiogenesis during exercise and training. Angiogenesis, 8(3), 263–271. [DOI] [PubMed] [Google Scholar]
- Bodine, S. C. , & Baehr, L. M. (2014). Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin‐1. American Journal of Physiology. Endocrinology and Metabolism, 307(6), E469–E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine, S. C. , Latres, E. , Baumhueter, S. , Lai, V. K. M. , Nunez, L. , Clarke, B. A. , Poueymirou, W. T. , Panaro, F. J. , Na, E. , Dharmarajan, K. , & Pan, Z. Q. (2001). Identification of ubiquitin ligases required for skeletal muscle atrophy. Science, 294(5547), 1704–1708. [DOI] [PubMed] [Google Scholar]
- Bodine, S. C. , Stitt, T. N. , Gonzalez, M. , Kline, W. O. , Stover, G. L. , Bauerlein, R. , Zlotchenko, E. , Scrimgeour, A. , Lawrence, J. C. , Glass, D. J. , & Yancopoulos, G. D. (2001b). Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in‐vivo. Nature Cell Biology, 3(11), 1014–1019. [DOI] [PubMed] [Google Scholar]
- Bohni, R. , Riesgo‐Escovar, J. , Oldham, S. , Brogiolo, W. , Stocker, H. , Andruss, B. F. , Beckingham, K. , & Hafen, E. (1999). Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1‐4. Cell, 97(7), 865–875. [DOI] [PubMed] [Google Scholar]
- Bonetto, A. , Aydogdu, T. , Jin, X. , Zhang, Z. , Zhan, R. , Puzis, L. , Koniaris, L. G. , & Zimmers, T. A. (2012). JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL‐6 and in experimental cancer cachexia. American Journal of Physiology. Endocrinology and Metabolism, 303(3), E410‐E421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetto, A. , Aydogdu, T. , Kunzevitzky, N. , Guttridge, D. C. , Khuri, S. , Koniaris, L. G. , & Zimmers, T. A. (2011). STAT3 Activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia ed. Parise G. PLoS One, 6(7), e22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetto, A. , Rupert, J. E. , Barreto, R. , & Zimmers, T. A. (2016). The colon‐26 carcinoma tumor‐bearing mouse as a model for the study of cancer cachexia. Journal of Visualized Experiments, (117), 54893. 10.3791/54893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris, A. , & Chance, B. (1973). The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochemical Journal, 134(3), 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen, T. S. , Schuler, G. , & Adams, V. (2015). Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. Journal of Cachexia, Sarcopenia and Muscle, 6(3), 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, P. R. T. , Smith, G. L. , & Gould, G. W. (2021). Run for your life: Can exercise be used to effectively target GLUT4 in diabetic cardiac disease? PeerJ, 9, e11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen, E. C. , Johnson, E. C. , Wagner, H. , Tseng, H. M. , Sung, L. A. , & Wagner, P. D. (1996). Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. Journal of Applied Physiology, 81(1), 355–361. [DOI] [PubMed] [Google Scholar]
- Brown, J. L. , Rosa‐Caldwell, M. E. , Lee, D. E. , Blackwell, T. A. , Brown, L. A. , Perry, R. A. , Haynie, W. S. , Hardee, J. P. , Carson, J. A. , Wiggs, M. P. , Washington, T. A. , & Greene, N. P. (2017). Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour‐bearing mice. Journal of Cachexia, Sarcopenia and Muscle, 8(6), 926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruera, E. , Schmitz, B. , Pither, J. , Neumann, C. M. , & Hanson, J. (2000). The frequency and correlates of dyspnea in patients with advanced cancer. Journal of Pain and Symptom Management, 19(5), 357–362. [DOI] [PubMed] [Google Scholar]
- Burgomaster, K. A. , Cermak, N. M. , Phillips, S. M. , Benton, C. R. , Bonen, A. , & Gibala, M. J. (2007). Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 292(5), R1970‐R1976. [DOI] [PubMed] [Google Scholar]
- Burgomaster, K. A. , Howarth, K. R. , Phillips, S. M. , Rakobowchuk, M. , MacDonald, M. J. , Mcgee, S. L. , & Gibala, M. J. (2008). Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. Journal of Physiology, 586(1), 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets, S. , Garcia‐Martínez, C. , Olivan, M. , Barreiro, E. , López‐Soriano, F. J. , & Argilés, J. M. (2006). Overexpression of UCP3 in both murine and human myotubes is linked with the activation of proteolytic systems: A role in muscle wasting? Biochimica et Biophysica Acta – General Subjects, 1760(2), 253–258. [DOI] [PubMed] [Google Scholar]
- Cadenas, S. (2018). Mitochondrial uncoupling, ROS generation and cardioprotection. Biochimica et Biophysica Acta –Bioenergetics, 1859(9), 940–950. [DOI] [PubMed] [Google Scholar]
- Cai, D. , Frantz, J. D. , Tawa, N. E. Jr , Melendez, P. A. , Oh, B. C. , Lidov, H. G. , Hasselgren, P. O. , Frontera, W. R. , Lee, J. , Glass, D. J. , & Shoelson, S. E. (2004). IKKß/NF‐kB activation causes severe muscle wasting in mice. Cell, 119(2), 285–298. [DOI] [PubMed] [Google Scholar]
- Callahan, L. A. , & Supinski, G. S. (2010). Diaphragm weakness and mechanical ventilation–what's the critical issue? Critical Care, 14(4), 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon‐Cabrera, A. , Fermoselle, C. , Urtreger, A. J. , Mateu‐Jimenez, M. , Diament, M. J. , de Kier Joffe, E. D. , Sandri, M. , & Barreiro, E. (2014). Pharmacological strategies in lung cancer‐induced cachexia: Effects on muscle proteolysis, autophagy, structure, and weakness. Journal of Cellular Physiology, 229(11), 1660–1672. [DOI] [PubMed] [Google Scholar]
- Chen, T. C. , Tseng, W. C. , Huang, G. L. , Chen, H. L. , Tseng, K. W. , & Nosaka, K. (2017). Superior effects of eccentric to concentric knee extensor resistance training on physical fitness, insulin sensitivity and lipid profiles of elderly men. Frontiers in Physiology, 8, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesley, A. , MacDougall, J. D. , Tarnopolsky, M. A. , Atkinson, S. A. , & Smith, K. (1992). Changes in human muscle protein synthesis after resistance exercise. Journal of Applied Physiology, 73(4), 1383–1388. [DOI] [PubMed] [Google Scholar]
- Choi, E. , Carruthers, K. , Zhang, L. , Thomas, N. , Battaglino, R. A. , Morse, L. R. , & Widrick, J. J. (2013). Concurrent muscle and bone deterioration in a murine model of cancer cachexia. Physiological Reports, 1(6), e00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S. , Brault, J. J. , Gygi, S. P. , Glass, D. J. , Valenzuela, D. M. , Gartner, C. , Latres, E. , & Goldberg, A. L. (2009). During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1‐dependent ubiquitylation. Journal of Cell Biology, 185(6), 1083–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, P. , Bing, C. , McCulloch, P. , & Williams, G. (2002). Muscle UCP‐3 mRNA levels are elevated in weight loss associated with gastrointestinal adenocarcinoma in humans. British Journal of Cancer, 86(3), 372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou, C. , Fontes de Oliveira, C. C. , Mintzopoulos, D. , Busquets, S. , He, J. , Kesarwani, M. , Mindrinos, M. , Rahme, L. G. , Argiles, J. M. , & Tzika, A. A. (2011). Nuclear magnetic resonance in conjunction with functional genomics suggests mitochondrial dysfunction in a murine model of cancer cachexia. International Journal of Molecular Medicine, 27, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan, F. , Hajiaghaalipour, F. , Yusof, A. , Muniandy, S. , Hosseini, S. A. , Heydari, S. , Salim, L. Z. A. , & Azarbayjani, M. A. (2016). Saffron with resistance exercise improves diabetic parameters through the GLUT4/AMPK pathway in‐vitro and in‐vivo. Scientific Reports, 6(1), 25139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lazzari, N. , Niels, T. , Tewes, M. , & Götte, M. (2021). A systematic review of the safety, feasibility and benefits of exercise for patients with advanced cancer. Cancers, 13(17), 4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lerma Barbaro, A. (2015). The complex liaison between cachexia and tumor burden (Review). Oncology Reports, 34(4), 1635–1649. [DOI] [PubMed] [Google Scholar]
- Delitto, D. , Judge, S. M. , Delitto, A. E. , Nosacka, R. L. , Rocha, F. G. , DiVita, B. B. , Gerber, M. H. , George, T. J. , Behrns, K. E. , Hughes, S. J. , Wallet, S. M. , Judge, A. R. , & Trevino, J. G. (2017). Human pancreatic cancer xenografts recapitulate key aspects of cancer cachexia. Oncotarget, 8(1), 1177–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria, M. , Giorgi, C. , Lebiedzinska, M. , Esposito, G. , D'Angeli, L. , Bartoli, A. , Gough, D. J. , Turkson, J. , Levy, D. E. , Watson, C. J. , Wieckowski, M. R. , Provero, P. , Pinton, P. , & Poli, V. (2010). A STAT3‐mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging, 2(11), 823–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewys, W. D. , Begg, C. , Lavin, P. T. , Band, P. R. , Bennett, J. M. , Bertino, J. R. , Cohen, M. H. , Douglass, H. O. , Engstrom, P. F. , Ezdinli, E. Z. , Horton, J. , Johnson, G. J. , Moertel, C. G. , Oken, M. M. , Perlia, C. , Rosenbaum, C. , Silverstein, M. N. , Skeel, R. T. , Sponzo, R. W. , & Tormey, D. C. (1980). Prognostic effect of weight loss prior to chemotherapy in cancer patients. American Journal of Medicine, 69(4), 491–497. [DOI] [PubMed] [Google Scholar]
- Dodson, S. , Baracos, V. E. , Jatoi, A. , Evans, W. J. , Cella, D. , Dalton, J. T. , & Steiner, M. S. (2011). Muscle wasting in cancer cachexia: Clinical implications, diagnosis, and emerging treatment strategies. Annual Review of Medicine, 62(1), 265–279. [DOI] [PubMed] [Google Scholar]
- Donatto, F. F. , Neves, R. X. , Rosa, F. O. , Camargo, R. G. , Ribeiro, H. , Matos‐Neto, E. M. , & Seelaender, M. (2013). Resistance exercise modulates lipid plasma profile and cytokine content in the adipose tissue of tumour‐bearing rats. Cytokine, 61(2), 426–432. [DOI] [PubMed] [Google Scholar]
- Duan, H. , & Bai, H. (2020). Is mitochondrial oxidative stress the key contributor to diaphragm atrophy and dysfunction in critically ill patients? Critical Care Research and Practice, 2020, 8672939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudgeon, D. J. , & Lertzman, M. (1998). Dyspnea in the advanced cancer patient. Journal of Pain and Symptom Management, 16(4), 212–219. [DOI] [PubMed] [Google Scholar]
- Dudgeon, D. J. , Lertzman, M. , & Askew, G. R. (2001). Physiological changes and clinical correlations of dyspnea in cancer outpatients. Journal of Pain and Symptom Management, 21(5), 373–379. [DOI] [PubMed] [Google Scholar]
- Egerman, M. A. , & Glass, D. J. (2014). Signaling pathways controlling skeletal muscle mass. Critical Reviews in Biochemistry and Molecular Biology, 49(1), 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon, K. , Strasser, F. , Anker, S. D. , Bosaeus, I. , Bruera, E. , Fainsinger, R. L. , Jatoi, A. , Loprinzi, C. , MacDonald, N. , Mantovani, G. , Davis, M. , Muscaritoli, M. , Ottery, F. , Radbruch, L. , Ravasco, P. , Walsh, D. , Wilcock, A. , Kaasa, S. , & Baracos, V. E. (2011). Definition and classification of cancer cachexia: an international consensus. The Lancet Oncology, 12(5), 489–495. [DOI] [PubMed] [Google Scholar]
- Feathers, L. S. B. , Wilcock, A. , Manderson, C. , Weller, R. , & Tattersfield, A. E. (2003). Measuring inspiratory muscle weakness in patients with cancer and breathlessness. Journal of Pain and Symptom Management, 25(4), 305–306. [DOI] [PubMed] [Google Scholar]
- Fermoselle, C. , García‐Arumí, E. , Puig‐Vilanova, E. , Andreu, A. L. , Urtreger, A. J. , de Kier Joffé, E. D. B. , Tejedor, A. , Puente‐Maestu, L. , & Barreiro, E. (2013). Mitochondrial dysfunction and therapeutic approaches in respiratory and limb muscles of cancer cachectic mice. Experimental Physiology, 98(9), 1349–1365. [DOI] [PubMed] [Google Scholar]
- Fields, D. P. , Roberts, B. M. , Simon, A. K. , Judge, A. R. , Fuller, D. D. , & Mitchell, G. S. (2019). Cancer cachexia impairs neural respiratory drive in hypoxia but not hypercapnia. Journal of Cachexia, Sarcopenia and Muscle, 10(1), 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Opazo, M. , McGee, S. L. , & Hargreaves, M. (2020). Exercise and GLUT4. Exercise and Sport Sciences Reviews, 48(3), 110–118. [DOI] [PubMed] [Google Scholar]
- Fox, K. M. , Brooks, J. M. , Gandra, S. R. , Markus, R. , & Chiou, C. F. (2009). Estimation of cachexia among cancer patients based on four definitions. Journal of oncology, 2009, 693458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi, M. V. , Reeves, N. D. , & Narici, M. V. (2017). Skeletal muscle remodeling in response to eccentric vs. concentric loading: Morphological, molecular, and metabolic adaptations. Frontiers in Physiology, 8, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden, J. , & Lieber, R. L. (1992). Structural and mechanical basis of exercise‐induced muscle injury: Medicine & Science in Sports & Exercise, 24, 521–530. [PubMed] [Google Scholar]
- Frikkel, J. , Götte, M. , Beckmann, M. , Kasper, S. , Hense, J. , Teufel, M. , Schuler, M. , & Tewes, M. (2020). Fatigue, barriers to physical activity and predictors for motivation to exercise in advanced Cancer patients. BMC Palliat Care, 19(1), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulle, S. , Protasi, F. , Di Tano, G. , Pietrangelo, T. , Beltramin, A. , Boncompagni, S. , Vecchiet, L. , & Fanò, G. (2004). The contribution of reactive oxygen species to sarcopenia and muscle ageing. Experimental Gerontology, 39(1), 17–24. [DOI] [PubMed] [Google Scholar]
- Gea, J. Casadevall, C. , Pascual, S. , Orozco‐Levi, M. , & Barreiro, E. (2012). Respiratory diseases and muscle dysfunction. Expert Review of Respiratory Medicine, 6(1), 75–90. [DOI] [PubMed] [Google Scholar]
- Gengenbacher, N. , Singhal, M. , & Augustin, H. G. (2017). Preclinical mouse solid tumour models: Status quo, challenges and perspectives. Nature Reviews. Cancer, 17(12), 751–765. [DOI] [PubMed] [Google Scholar]
- Giordano, A. , Calvani, M. , Petillo, O. , Carteni’, M. , Melone, M. , & Peluso, G. (2003). Skeletal muscle metabolism in physiology and in cancer disease. Journal of Cellular Biochemistry, 90(1), 170–186. [DOI] [PubMed] [Google Scholar]
- Glass, D. J. (2003). Molecular mechanisms modulating muscle mass. Trends in Molecular Medicine, 9(8), 344–350. [DOI] [PubMed] [Google Scholar]
- Go, K. L. , Delitto, D. , Judge, S. M. , Gerber, M. H. , George, T. J. , Behrns, K. E. , Hughes, S. J. , Judge, A. R. , & Trevino, J. G. (2017). Orthotopic patient‐derived pancreatic cancer xenografts engraft into the pancreatic parenchyma, metastasize, and induce muscle wasting to recapitulate the human disease. Pancreas, 46(6), 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, A. L. (2003). Protein degradation and protection against misfolded or damaged proteins. Nature, 426(6968), 895–899. [DOI] [PubMed] [Google Scholar]
- Gomes, M. D. , Lecker, S. H. , Jagoe, R. T. , Navon, A. , & Goldberg, A. L. (2001). Atrogin‐1, a muscle‐specific F‐box protein highly expressed during muscle atrophy. Proceedings National Academy of Sciences, USA, 98(25), 14440–14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Cabrera, M. C. , Domenech, E. , & Vina, J. (2008). Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radical Biology & Medicine, 44(2), 126–131. [DOI] [PubMed] [Google Scholar]
- Goodman, C. A. , McNally, R. M. , Hoffmann, F. M. , & Hornberger, T. A. (2013). Smad3 induces atrogin‐1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Molecular Endocrinology, 27(11), 1946–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, J. N. , Green, S. R. , & Goggin, P. M. (2005). Cancer cachexia. QJM, 98(11), 779–788. [DOI] [PubMed] [Google Scholar]
- Gould, D. W. , Lahart, I. , Carmichael, A. R. , Koutedakis, Y. , & Metsios, G. S. (2013). Cancer cachexia prevention via physical exercise: Molecular mechanisms. Journal of Cachexia, Sarcopenia and Muscle, 4(2), 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande, A. J. , Silva, V. , Sawaris Neto, L. , Teixeira Basmage, J. P. , Peccin, M. S. , & Maddocks, M. (2021). Exercise for cancer cachexia in adults. Cochrane Database of Systematic Reviews (Online), 3, CD010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco, S. H. , Tomkötter, L. , Vahle, A.‐K. , Rokosh, R. , Avanzi, A. , Mahmood, S. K. , Deutsch, M. , Alothman, S. , Alqunaibit, D. , Ochi, A. , Zambirinis, C. , Mohaimin, T. , Rendon, M. , Levie, E. , Pansari, M. , Torres‐Hernandez, A. , Daley, D. , Barilla, R. , Pachter, H. L. , … Miller, G. (2015). TGF‐β blockade reduces mortality and metabolic changes in a validated murine model of pancreatic cancer cachexia ed. Singh AP. PLoS One, 10(7), e0132786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiwe, J. S. , Cheng, B. , Rubin, D. C. , Yarasheski, K. E. , & Semenkovich, C. F. (2001). Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB Journal, 15(2), 475–482. [DOI] [PubMed] [Google Scholar]
- Hardee, J. P. , Fix, D. K. , Koh, H. J. , Wang, X. , Goldsmith, E. C. , & Carson, J. A. (2020). Repeated eccentric contractions positively regulate muscle oxidative metabolism and protein synthesis during cancer cachexia in mice. Journal of Applied Physiology, 128(6), 1666–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee, J. P. , Mangum, J. E. , Gao, S. , Sato, S. , Hetzler, K. L. , Puppa, M. J. , Fix, D. K. , & Carson, J. A. (2016). Eccentric contraction‐induced myofiber growth in tumor‐bearing mice. Journal of Applied Physiology, 120(1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmark, I. C. , Mikkelsen, U. R. , Borglum, J. , Rothe, A. , Petersen, M. C. , Andersen, O. , Langberg, H. , & Kjaer, M. (2010). Exercise increases interleukin‐10 levels in patients with knee osteoarthritis. Arthritis Research and Therapy, 12(4), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, S. E. , Makhijani, N. , & Mace, T. A. (2018). Pancreatic cancer–induced cachexia and relevant mouse models. Pancreas, 47(8), 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple, R. T. , Hogan, M. C. , Stary, C. , Bebout, D. E. , Mathieu‐Costello, O. , & Wagner, P. D. (2000). Structural basis of muscle O2 diffusing capacity: Evidence from muscle function in situ. Journal of Applied Physiology, 88(2), 560–566. [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. , Thomas, J. , Chandler, J. , Cumpston, M. , Li, T. , Page, M. J. , & Welch, V. A. (2019). Cochrane handbook for systematic reviews of interventions. John Wiley & Sons. [Google Scholar]
- Hiroux, C. , Dalle, S. , Koppo, K. , & Hespel, P. (2021). Voluntary exercise does not improve muscular properties or functional capacity during C26‐induced cancer cachexia in mice. Journal of Muscle Research and Cell Motility, 42(2), 169–181. [DOI] [PubMed] [Google Scholar]
- Hody, S. , Croisier, J.‐L. , Bury, T. , Rogister, B. , & Leprince, P. (2019). Eccentric muscle contractions: Risks and benefits. Frontiers in Physiology, 10, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoier, B. , Nordsborg, N. , Andersen, S. , Jensen, L. , Nybo, L. , Bangsbo, J. , & Hellsten, Y. (2012). Pro‐ and anti‐angiogenic factors in human skeletal muscle in response to acute exercise and training: Angiogenic factors in human muscle. Journal of Physiology, 590(3), 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy, J. O. , & Booth, F. W. (1976). Biochemical adaptations to endurance exercise in muscle. Annual Review of Physiology, 38(1), 273–291. [DOI] [PubMed] [Google Scholar]
- Hoppeler, H. (2016). Moderate load eccentric exercise; a distinct novel training modality. Frontiers in Physiology, 7, 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudlicka, O. , Brown, M. , & Egginton, S. (1992). Angiogenesis in skeletal and cardiac muscle. Physiological Reviews, 72(2), 369–417. [DOI] [PubMed] [Google Scholar]
- Isner‐Horobeti, M.‐E. , Dufour, S. P. , Vautravers, P. , Geny, B. , Coudeyre, E. , & Richard, R. (2013). Eccentric exercise training: Modalities, applications and perspectives. Sports Medicine, 43(6), 483–512. [DOI] [PubMed] [Google Scholar]
- Janssen, S. P. , Gayan‐Ramirez, G. , Van den Bergh, A. , Herijgers, P. , Maes, K. , Verbeken, E. , & Decramer, M. (2005). Interleukin‐6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation, 111(8), 996–1005. [DOI] [PubMed] [Google Scholar]
- Ji, L. L. (1999). Antioxidants and oxidative stress in exercise. PSEBM, 222(3), 283–292. [DOI] [PubMed] [Google Scholar]
- Johns, N. , Stephens, N. A. , & Fearon, K. C. H. (2013). Muscle wasting in cancer. International Journal of Biochemistry & Cell Biology, 45, 2215–2229. [DOI] [PubMed] [Google Scholar]
- Jones, L. W. , Hornsby, W. E. , Goetzinger, A. , Forbes, L. M. , Sherrard, E. L. , Quist, M. , Lane, A. T. , West, M. , Eves, N. D. , Gradison, M. , Coan, A. , Herndon, J. E. , & Abernethy, A. P. (2012). Prognostic significance of functional capacity and exercise behavior in patients with metastatic non‐small cell lung cancer. Lung Cancer, 76(2), 248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones‐Bolin, S. , & Ruggeri, B. (2007). Orthotopic model of human pancreatic ductal adenocarcinoma and cancer cachexia in nude mice. Current Protocols in Pharmacology, 37(1), 14. [DOI] [PubMed] [Google Scholar]
- Julian, V. , Thivel, D. , Costes, F. , Touron, J. , Boirie, Y. , Pereira, B. , Perrault, H. , Duclos, M. , & Richard, R. (2018). Eccentric training improves body composition by inducing mechanical and metabolic adaptations: A promising approach for overweight and obese individuals. Frontiers in Physiology, 9, 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamoui, A. V. , Park, B.‐S. , Kim, D.‐H. , Yeh, M.‐C. , Oh, S.‐L. , Elam, M. L. , Jo, E. , Arjmandi, B. H. , Salazar, G. , Grant, S. C. , Contreras, R. J. , Lee, W. J. , & Kim, J.‐S. (2016). Aerobic and resistance training dependent skeletal muscle plasticity in the colon‐26 murine model of cancer cachexia. Metabolism, 65(5), 685–698. [DOI] [PubMed] [Google Scholar]
- Khanna, S. , Atalay, M. , Lodge, J. K. , Laaksonen, D. E. , Roy, S. , Hanninen, O. , Packer, L. , & Sen, C. K. (1998). Skeletal muscle and liver lipoyllysine content in response to exercise and dietary a‐lipoic acid. IUBMB Life, 46(2), 297–306. [DOI] [PubMed] [Google Scholar]
- Khosravi, N. , Stoner, L. , Farajivafa, V. , & Hanson, E. D. (2019). Exercise training, circulating cytokine levels and immune function in cancer survivors: A meta‐analysis. Brain, Behavior, and Immunity, 81, 92–104. [DOI] [PubMed] [Google Scholar]
- Kim, S. H. , Ko, Y. J. , & Baek, S. S. (2021). Resistance exercise improves short‐term memory through inactivation of NF‐kappaB pathway in mice with Parkinson disease. Journal of Exercise Rehabilitation, 17(2), 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissane, R. W. , & Egginton, S. (2019). Exercise‐mediated angiogenesis. Current Opinion in Physiology, 10, 193–201. [Google Scholar]
- Kitajima, Y. , Yoshioka, K. , & Suzuki, N. (2020). The ubiquitin‐proteasome system in regulation of the skeletal muscle homeostasis and atrophy: From basic science to disorders. Journal of Physiological Sciences, 70(1), 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knols, R. , Aaronson, N. K. , Uebelhart, D. , Fransen, J. , & Aufdemkampe, G. (2005). Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. Journal of Clinical Oncology, 23(16), 3830–3842. [DOI] [PubMed] [Google Scholar]
- Korniluk, A. , Koper, O. , Kemona, H. , & Dymicka‐Piekarska, V. (2017). From inflammation to cancer. Irish Journal of Medical Science, 186(1), 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz, R. M. (2009). Airway clearance in Duchenne muscular dystrophy. Pediatrics, 123(Supplement_4), S231‐S235. [DOI] [PubMed] [Google Scholar]
- Lagirand‐Cantaloube, J. , Offner, N. , Csibi, A. , Leibovitch, M. P. , Batonnet‐Pichon, S. , Tintignac, L. A. , Segura, C. T. , & Leibovitch, S. A. (2008). The initiation factor eIF3‐f is a major target for Atrogin1/MAFbx function in skeletal muscle atrophy. EMBO Journal, 27(8), 1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen, R. C. , Haegens, A. , Vernooy, J. H. , Wouters, E. F. , de Winther, M. P. , Carlsen, H. , Steele, C. , Shoelson, S. E. , & Schols, A. M. (2012). NF‐kappaB activation is required for the transition of pulmonary inflammation to muscle atrophy. American Journal of Respiratory Cell and Molecular Biology, 47(3), 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaStayo, P. , Marcus, R. , Dibble, L. , Frajacomo, F. , & Lindstedt, S. (2014). Eccentric exercise in rehabilitation: Safety, feasibility, and application. Journal of Applied Physiology, 116(11), 1426–1434. [DOI] [PubMed] [Google Scholar]
- LaStayo, P. , Marcus, R. , Dibble, L. , Wong, B. , & Pepper, G. (2017). Eccentric versus traditional resistance exercise for older adult fallers in the community: A randomized trial within a multi‐component fall reduction program. BMC Geriatrics, 17(1), 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastayo, P. C. , Reich, T. E. , Urquhart, M. , Hoppeler, H. , & Lindstedt, S. L. (1999). Chronic eccentric exercise: Improvements in muscle strength can occur with little demand for oxygen. American Journal of Physiology, 276, R611–615. [DOI] [PubMed] [Google Scholar]
- Laviano, A. , Meguid, M. M. , & Rossi‐Fanelli, F. (2003). Cancer anorexia‐cachexia syndrome. The Lancet Oncology, 4(11), 686–694. [DOI] [PubMed] [Google Scholar]
- LeBlanc, T. W. , Nipp, R. D. , Rushing, C. N. , Samsa, G. P. , Locke, S. C. , Kamal, A. H. , Cella, D. F. , & Abernethy, A. P. (2015). Correlation between the international consensus definition of the Cancer Anorexia‐Cachexia Syndrome (CACS) and patient‐centered outcomes in advanced non‐small cell lung cancer. Journal of Pain and Symptom Management, 49(4), 680–689. [DOI] [PubMed] [Google Scholar]
- Lecker, S. H. , Jagoe, R. T. , Gilbert, A. , Gomes, M. , Baracos, V. , Bailey, J. , Price, S. R. , Mitch, W. E. , & Goldberg, A. L. (2004). Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB Journal, 18(1), 39–51. [DOI] [PubMed] [Google Scholar]
- Lee, S. J. , & McPherron, A. C. (1999). Myostatin and the control of skeletal muscle mass. Current Opinion in Genetics & Development, 9(5), 604–607. [DOI] [PubMed] [Google Scholar]
- Leite, A. B. , Lima, H. N. , Flores, C. O. , Oliveira, C. A. , Cunha, L. E. C. , Neves, J. L. , Correia, T. M. L. , de Melo, F. F. , Oliveira, M. V. , de Magalhaes, A. C. M. , Soares, T. J. , & Amaral, L. S. B. (2021). High‐intensity interval training is more effective than continuous training to reduce inflammation markers in female rats with cisplatin nephrotoxicity. Life Sciences, 266, 118880. [DOI] [PubMed] [Google Scholar]
- Lerner, L. , Hayes, T. G. , Tao, N. , Krieger, B. , Feng, B. , Wu, Z. , Nicoletti, R. , Chiu, M. I. , Gyuris, J. , & Garcia, J. M. (2015). Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. Journal of Cachexia, Sarcopenia and Muscle, 6(4), 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Moody, M. R. , Engel, D. , Walker, S. , Clubb, F. J. Jr , Sivasubramanian, N. , Mann, D. L. , & Reid, M. B. (2000). Cardiac‐specific overexpression of tumor necrosis factor‐alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation, 102(14), 1690–1696. [DOI] [PubMed] [Google Scholar]
- Lira, F. S. , Tavares, F. L. , Yamashita, A. S. , Koyama, C. H. , Alves, M. J. , Caperuto, E. C. , Batista, M. L. , & Seelaender, M. (2008). Effect of endurance training upon lipid metabolism in the liver of cachectic tumour‐bearing rats. Cell Biochemistry and Function, 26(6), 701–708. [DOI] [PubMed] [Google Scholar]
- Mangner, N. , Linke, A. , Oberbach, A. , Kullnick, Y. , Gielen, S. , Sandri, M. , Hoellriegel, R. , Matsumoto, Y. , Schuler, G. , & Adams, V. (2013). Exercise training prevents TNF‐alpha induced loss of force in the diaphragm of mice. PLoS One, 8(1), e52274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, L. , Birdsell, L. , Macdonald, N. , Reiman, T. , Clandinin, M. T. , McCargar, L. J. , Murphy, R. , Ghosh, S. , Sawyer, M. B. , & Baracos, V. E. (2013). Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. Journal of Clinical Oncology, 31(12), 1539–1547. [DOI] [PubMed] [Google Scholar]
- Matecki, S. , Dridi, H. , Jung, B. , Saint, N. , Reiken, S. R. , Scheuermann, V. , Mrozek, S. , Santulli, G. , Umanskaya, A. , Petrof, B. J. , Jaber, S. , Marks, A. R. , & Lacampagne, A. (2016). Leaky ryanodine receptors contribute to diaphragmatic weakness during mechanical ventilation. Proceedings of the National Academy of Sciences, USA, 113(32), 9069–9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavropalias, G. , Sim, M. , Taaffe, D. R. , Galvao, D. A. , Spry, N. , Kraemer, W. J. , Hakkinen, K. , & Newton, R. U. (2022). Exercise medicine for cancer cachexia: Targeted exercise to counteract mechanisms and treatment side effects. Journal of Cancer Research and Clinical Oncology, 148(6), 1389–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery, S. , Thomas, M. , Maxwell, L. , Sharma, M. , & Kambadur, R. (2003). Myostatin negatively regulates satellite cell activation and self‐renewal. Journal of Cell Biology, 162(6), 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron, A. C. , Lawler, A. M. , & Lee, S. J. (1997). Regulation of skeletal muscle mass in mice by a new TGF‐beta superfamily member. Nature, 387(6628), 83–90. [DOI] [PubMed] [Google Scholar]
- Mittal, A. , Bhatnagar, S. , Kumar, A. , Lach‐Trifilieff, E. , Wauters, S. , Li, H. , Makonchuk, D. Y. , Glass, D. J. , & Kumar, A. (2010). The TWEAK‐Fn14 system is a critical regulator of denervation‐induced skeletal muscle atrophy in mice. Journal of Cell Biology, 188(6), 833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. [DOI] [PubMed] [Google Scholar]
- Mueller, T. C. , Bachmann, J. , Prokopchuk, O. , Friess, H. , & Martignoni, M. E. (2016). Molecular pathways leading to loss of skeletal muscle mass in cancer cachexia – can findings from animal models be translated to humans? BMC Cancer, 16(1), 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, K. T. , Chee, A. , Gleeson, B. G. , Naim, T. , Swiderski, K. , Koopman, R. , & Lynch, G. S. (2011). Antibody‐directed myostatin inhibition enhances muscle mass and function in tumor‐bearing mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 301(3), R716‐R726. [DOI] [PubMed] [Google Scholar]
- Murphy, K. T. , Chee, A. , Trieu, J. , Naim, T. , & Lynch, G. S. (2012). Importance of functional and metabolic impairments in the characterization of the C‐26 murine model of cancer cachexia. Disease Models & Mechanisms, 5, 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, K. T. , Chee, A. , Trieu, J. , Naim, T. , & Lynch, G. S. (2013). Inhibition of the renin–angiotensin system improves physiological outcomes in mice with mild or severe cancer cachexia. International Journal of Cancer, 133(5), 1234–1246. [DOI] [PubMed] [Google Scholar]
- Nader, G. A. (2005). Molecular determinants of skeletal muscle mass: Getting the “AKT” together. International Journal of Biochemistry & Cell Biology, 37(10), 1985–1996. [DOI] [PubMed] [Google Scholar]
- Naito, T. , Okayama, T. , Aoyama, T. , Ohashi, T. , Masuda, Y. , Kimura, M. , Shiozaki, H. , Murakami, H. , Kenmotsu, H. , Taira, T. , Ono, A. , Wakuda, K. , Imai, H. , Oyakawa, T. , Ishii, T. , Omori, S. , Nakashima, K. , Endo, M. , Omae, K. , …, & Takahashi, T. (2017). Unfavorable impact of cancer cachexia on activity of daily living and need for inpatient care in elderly patients with advanced non‐small‐cell lung cancer in Japan: A prospective longitudinal observational study. BMC Cancer, 17(1), 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave, B. T. , Ouwens, M. , Withers, D. J. , Alessi, D. R. , & Shepherd, P. R. (1999). Mammalian target of rapamycin is a direct target for protein kinase B: Identification of a convergence point for opposing effects of insulin and amino‐acid deficiency on protein translation. Biochemical Journal, 344(2), 427–431. [PMC free article] [PubMed] [Google Scholar]
- Neuzil, J. , & Stocker, R. (1993). Bilirubin attenuates radical‐mediated damage to serum albumin. FEBS Letters, 331(3), 281–284. [DOI] [PubMed] [Google Scholar]
- Noguchi, Y. Yoshikawa, T. , Marat, D. , Doi, C. , Makino, T. , Fukuzawa, K. , Tsuburaya, A. , Satoh, S. , Ito, T. , & Mitsuse, S. (1998). Insulin resistance in cancer patients is associated with enhanced TNF‐a expression in skeletal muscle. Biochemical and Biophysical Research Communications, 253(3), 887–892. [DOI] [PubMed] [Google Scholar]
- Nosacka, K. , Lavender, A. , Newton, M. , & Sacco, P. (2003). Muscle damage in resistance training ‐ is muscle damage necessary for strength gain and muscle hypertrophy? International Journal of Sport and Health Science, 1(1), 1–8. [Google Scholar]
- Nosacka, R. L. , Delitto, A. E. , Delitto, D. , Patel, R. , Judge, S. M. , Trevino, J. G. , & Judge, A. R. (2020). Distinct cachexia profiles in response to human pancreatic tumours in mouse limb and respiratory muscle. Journal of Cachexia, Sarcopenia and Muscle, 11(3), 820–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuwa, T. , Sato, Y. , & Naoi, M. (1997). Glutathione status and reactive oxygen generation in tissues of young and old exercised rats. Acta Physiologica Scandinavica, 159(3), 237–244. [DOI] [PubMed] [Google Scholar]
- Ostrowski, K. , Rohde, T. , Asp, S. , Schjerling, P. , & Pedersen, B. K. (1999). Pro‐ and anti‐inflammatory cytokine balance in strenuous exercise in humans. Journal of Physiology, 515(1), 287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos, E. , Gillen, J. , Moore, D. , Au, D. , Kurgan, N. , Klentrou, P. , Finelli, A. , Alibhai, S. M. H. , & Santa Mina, D. (2021). High‐intensity interval training or resistance training versus usual care in men with prostate cancer on active surveillance: A 3‐arm feasibility randomized controlled trial. Applied Physiology, Nutrition and Metabolism, 46(12), 1535–1544. [DOI] [PubMed] [Google Scholar]
- Parajuli, P. , Kumar, S. , Loumaye, A. , Singh, P. , Eragamreddy, S. , Nguyen, T. L. , Ozkan, S. , Razzaque, M. S. , Prunier, C. , Thissen, J. P. , & Atfi, A. (2018). Twist1 activation in muscle progenitor cells causes muscle loss akin to cancer cachexia | elsevier enhanced reader. Developmental Cell, 45(6), 712–725.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, A. V. , Friedenreich, C. M. , Moore, S. C. , Hayes, S. C. , Silver, J. K. , Campbell, K. L. , Winters‐Stone, K. , Gerber, L. H. , George, S. M. , Fulton, J. E. , Denlinger, C. , Morris, G. S. , Hue, T. , Schmitz, K. H. , & Matthews, C. E. (2019). American college of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Medicine & Science in Sports & Exercise, 51, 2391–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paval, D. R. , Patton, R. , McDonald, J. , Skipworth, R. J. E. , Gallagher, I. J. , Laird, B. J. , & Caledonian Cachexia, C. (2022). A systematic review examining the relationship between cytokines and cachexia in incurable cancer. Journal of Cachexia, Sarcopenia and Muscle, 13(2), 824–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, B. K. , & Hoffman‐Goetz, L. (2000). Exercise and the immune system: Regulation, integration, and adaption. Physiological Reviews, 80(3), 1055–1081. [DOI] [PubMed] [Google Scholar]
- Pedersen, K. S. , Dethlefsen, C. , & Nielsen, J. (2016). Voluntary running suppresses tumor growth through epinephrine‐and IL‐6‐dependent NK cell mobilization and redistribution. Cell Metabolism, 23(3), 554–562. [DOI] [PubMed] [Google Scholar]
- Penna, F. , Busquets, S. , Pin, F. , Toledo, M. , Baccino, F. M. , López‐Soriano, F. J. , Costelli, P. , & Argilés, J. M. (2011). Combined approach to counteract experimental cancer cachexia: Eicosapentaenoic acid and training exercise. Journal of Cachexia, Sarcopenia and Muscle, 2(2), 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, A. M. , & Pedersen, B. K. (2005). The anti‐inflammatory effect of exercise. Journal of Applied Physiology, 98(4), 1154–1162. [DOI] [PubMed] [Google Scholar]
- Pilegaard, J. A. M. , Leick, L. H. , & Hood, D. A. (2006). Control of gene expression and mitochondrial biogenesis in the muscular adaptation to endurance exercise ed. Wagenmakers AJM. Essays in Biochemistry, 42, 13–29. [DOI] [PubMed] [Google Scholar]
- Powers, S. K. , & Jackson, M. J. (2008). Exercise‐induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiological Reviews, 88(4), 1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrozek, T. , Mlak, R. , Brzozowska, A. , Mazurek, M. , Golebiowski, P. , & Malecka‐Massalska, T. (2018). Relationship between TNF‐alpha ‐1031T/C gene polymorphism, plasma level of TNF‐alpha, and risk of cachexia in head and neck cancer patients. Journal of Cancer Research and Clinical Oncology, 144(8), 1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado, C. M. , Baracos, V. E. , McCargar, L. J. , Mourtzakis, M. , Mulder, K. E. , Reiman, T. , Butts, C. A. , Scarfe, A. G. , & Sawyer, M. B. (2007). Body composition as an independent determinant of 5‐fluorouracil‐based chemotherapy toxicity. Clinical Cancer Research, 13(11), 3264–3268. [DOI] [PubMed] [Google Scholar]
- Prado, C. M. , Baracos, V. E. , McCargar, L. J. , Reiman, T. , Mourtzakis, M. , Tonkin, K. , Mackey, J. R. , Koski, S. , Pituskin, E. , & Sawyer, M. B. (2009). Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clinical Cancer Research, 15(8), 2920–2926. [DOI] [PubMed] [Google Scholar]
- Prado, C. M. M. , Lieffers, J. R. , McCargar, L. J. , Reiman, T. , Sawyer, M. B. , Martin, L. , & Baracos, V. E. (2008). Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population‐based study. The Lancet Oncology, 9(7), 629–635. [DOI] [PubMed] [Google Scholar]
- Puppa, M. J. , Gao, S. , Narsale, A. A. , & Carson, J. A. (2014). Skeletal muscle glycoprotein 130's role in Lewis lung carcinoma‐induced cachexia. FASEB Journal, 28(2), 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, W. , & Su, G. H. (2013). Development of orthotopic pancreatic tumor mouse models. In Su G. H. (ed.), Pancreatic Cancer, Methods in Molecular Biology (pp. 215–223). Humana Press, Totowa, NJ. http://link.springer.com/10.1007/978‐1‐62703‐287‐2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, J. M. , Semenkovich, C. F. , Gulve, E. A. , Gao, J. , & Holloszy, J. O. (1994). Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin‐stimulated glycogen storage in muscle. Journal of Biological Chemistry, 269(20), 14396–14401. [PubMed] [Google Scholar]
- Richter, E. A. , & Hargreaves, M. (2013). Exercise, GLUT4, and skeletal muscle glucose uptake. Physiological Reviews, 93(3), 993–1017. [DOI] [PubMed] [Google Scholar]
- Ricquier, D. , & Bouillaud, F. (2000). The uncoupling protein homologues : UCP1, UCP2, UCP3, StUCP and AtUCP. Biochemical Journal, 345(2), 161–179. [PMC free article] [PubMed] [Google Scholar]
- Roberts, B. M. , Ahn, B. , Smuder, A. J. , Al‐Rajhi, M. , Gill, L. C. , Beharry, A. W. , Powers, S. K. , Fuller, D. D. , Ferreira, L. F. , & Judge, A. R. (2013). Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB Journal, 27(7), 2600–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, B. M. , Frye, G. S. , Ahn, B. , Ferreira, L. F. , & Judge, A. R. (2013). Cancer cachexia decreases specific force and accelerates fatigue in limb muscle. Biochemical and Biophysical Research Communications, 435(3), 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock, K. L. , Gramm, C. , Rothstein, L. , Clark, K. , Stein, R. , Dick, L. , Hwang, D. , & Goldberg, A. L. (1994). Inhibitors of the proteasome block the degradation of most cell proteins. Cell, 78(5), 761–771. [DOI] [PubMed] [Google Scholar]
- Roeland, E. J. , Bohlke, K. , Baracos, V. E. , Bruera, E. , Del Fabbro, E. , Dixon, S. , Fallon, M. , Herrstedt, J. , Lau, H. , Platek, M. , Rugo, H. S. , Schnipper, H. H. , Smith, T. J. , Tan, W. , & Loprinzi, C. L. (2020). Management of cancer cachexia: ASCO guideline. Journal of Clinical Oncology, 38(21), 2438–2453. [DOI] [PubMed] [Google Scholar]
- Roh, H. T. , Cho, S. Y. , & So, W. Y. (2020). A cross‐sectional study evaluating the effects of resistance exercise on inflammation and neurotrophic factors in elderly women with obesity. Journal of Clinical Medicine, 9(3), 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel, C. , Bodine, S. C. , Clarke, B. A. , Rossman, R. , Nunez, L. , Stitt, T. N. , Yancopoulos, G. D. , & Glass, D. J. (2001). Mediation of IGF‐1‐induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nature Cell Biology, 3(11), 1009–1013. [DOI] [PubMed] [Google Scholar]
- Rosa‐Caldwell, M. E. , Benson, C. A. , Lee, D. E. , Brown, J. L. , Washington, T. A. , Greene, N. P. , & Wiggs, M. P. (2020). Mitochondrial function and protein turnover in the diaphragm are altered in LLC tumor model of cancer cachexia. International Journal of Molecular Sciences, 21(21), 7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar‐Degracia, A. , Busquets, S. , Argilés, J. M. , Bargalló‐Gispert, N. , López‐Soriano, F. J. , & Barreiro, E. (2018). Effects of the beta2 agonist formoterol on atrophy signaling, autophagy, and muscle phenotype in respiratory and limb muscles of rats with cancer‐induced cachexia. Biochimie, 149, 79–91. [DOI] [PubMed] [Google Scholar]
- Sanchís, D. , Busquets, S. , Alvarez, B. , Ricquier, D. , López‐Soriano, F. J. , & Argilés, J. M. (1998). Skeletal muscle UCP2 and UCP3 gene expression in a rat cancer cachexia model. FEBS Letters, 436(3), 415–418. [DOI] [PubMed] [Google Scholar]
- Sandri, M. , Lin, J. , Handschin, C. , Yang, W. , Arany, Z. P. , Lecker, S. H. , Goldberg, A. L. , & Spiegelman, B. M. (2006). PGC‐1a protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy‐specific gene transcription. Proceedings of the National Academy of Sciences, 103(44), 16260–16265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri, M. , Sandri, C. , Gilbert, A. , Skurk, C. , Calabria, E. , Picard, A. , Walsh, K. , Schiaffino, S. , Lecker, S. H. , & Goldberg, A. L. (2004). Foxo transcription factors induce the atrophy‐related ubiquitin ligase atrogin‐1 and cause skeletal muscle atrophy. Cell, 117(3), 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer, A. A. , Robinson, S. M. , Patel, H. P. , Shavlakadze, T. , Cooper, C. , & Grounds, M. D. (2013). New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age and Ageing, 42(2), 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, R. , Mancilla, J. , Endres, S. , Ghorbani, R. , Clark, S. C. , & Dinarello, C. A. (1990). Correlations and interactions in the production of interleukin‐6 (IL‐ 6), IL‐1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL‐6 suppresses IL‐1 and TNF. Blood, 75(1), 40–47. [PubMed] [Google Scholar]
- Schneider, C. M. , Hsieh, C. C. , Sprod, L. K. , Carter, S. D. , & Hayward, R. (2007). Cancer treatment‐induced alterations in muscular fitness and quality of life: The role of exercise training. Annals of Oncology, 18(12), 1957–1962. [DOI] [PubMed] [Google Scholar]
- Schoenfeld, B. J. (2010). The mechanisms of muscle hypertrophy and their application to resistance training. Journal of Strength and Conditioning Research, 24(10), 2857–2872. [DOI] [PubMed] [Google Scholar]
- Schoenfeld, B. J. (2012). Does exercise‐induced muscle damage play a role in skeletal muscle hypertrophy? Journal of Strength and Conditioning Research, 26(5), 1441–1453. [DOI] [PubMed] [Google Scholar]
- Serrano, A. L. , Baeza‐Raja, B. , Perdiguero, E. , Jardi, M. , & Munoz‐Canoves, P. (2008). Interleukin‐6 is an essential regulator of satellite cell‐mediated skeletal muscle hypertrophy. Cell Metabolism, 7(1), 33–44. [DOI] [PubMed] [Google Scholar]
- Shi, J. , Luo, L. , Eash, J. , Ibebunjo, C. , & Glass, D. J. (2011). The SCF‐Fbxo40 complex induces IRS1 ubiquitination in skeletal muscle, limiting IGF1 signaling. Developmental Cell, 21(5), 835–847. [DOI] [PubMed] [Google Scholar]
- Shukla, S. K. , Dasgupta, A. , Mehla, K. , Gunda, V. , Vernucci, E. , Souchek, J. , Goode, G. , King, R. , Mishra, A. , Rai, I. , Nagarajan, S. , Chaika, N. V. , Yu, F. , & Singh, P. K. (2015). Silibinin‐mediated metabolic reprogramming attenuates pancreatic cancer‐induced cachexia and tumor growth. Oncotarget, 6(38), 41146–41161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shweiki, D. , Itin, A. , Soffer, D. , & Keshet, E. (1992). Vascular endothelial growth factor induced by hypoxia may mediate hypoxia‐initiated angiogenesis. Nature, 359(6398), 843–845. [DOI] [PubMed] [Google Scholar]
- Singh, B. , Hayes, S. C. , Spence, R. R. , Steele, M. L. , Millet, G. Y. , & Gergele, L. (2020). Exercise and colorectal cancer: A systematic review and meta‐analysis of exercise safety, feasibility and effectiveness. International Journal of Behavioral Nutrition and Physical Activity, 17(1), 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, I. J. , Roberts, B. , Beharry, A. , Godinez, G. L. , Payan, D. G. , Kinsella, T. M. , Judge, A. R. , & Ferreira, L. F. (2016). Janus kinase inhibition prevents cancer‐ and myocardial infarction‐mediated diaphragm muscle weakness in mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 310(8), R707–R710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuder, A. J. , Roberts, B. M. , Wiggs, M. P. , Kwon, O. S. , Yoo, J. K. , Christou, D. D. , Fuller, D. D. , Szeto, H. H. , & Judge, A. R. (2020). Pharmacological targeting of mitochondrial function and reactive oxygen species production prevents colon 26 cancer‐induced cardiorespiratory muscle weakness. Oncotarget, 11(38), 3502–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Z. , Moore, D. R. , Hodson, N. , Ward, C. , Dent, J. R. , O'Leary, M. F. , Shaw, A. M. , Hamilton, D. L. , Sarkar, S. , Gangloff, Y.‐G. , Hornberger, T. A. , Spriet, L. L. , Heigenhauser, G. J. , & Philp, A. (2017). Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co‐localisation in human skeletal muscle. Scientific Reports, 7(1), 5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg, A. , Keller, C. , Starkie, R. L. , Osada, T. , Febbraio, M. A. , & Pedersen, B. K. (2002). IL‐6 and TNF‐a expression in, and release from, contracting human skeletal muscle. American Journal of Physiology. Endocrinology and Metabolism, 283(6), E1272‐E1278. [DOI] [PubMed] [Google Scholar]
- Stewart, G. D. , Skipworth, R. J. , & Fearon, K. C. (2006). Cancer cachexia and fatigue. Clinical Medicine, 6(2), 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann, G. , Fong, M. , Kenney, J. S. , & Jacob, C. O. (1992). Evidence for the involvement of interleukin 6 in experimental cancer cachexia. Journal of Clinical Investigation, 89(5), 1681–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert, E. E. , Cuitino, M. C. , Ladner, K. J. , Rajasekerea, P. V. , Siebert, M. , Shakya, R. , Leone, G. W. , Ostrowski, M. C. , Paleo, B. , Weisleder, N. , Reiser, P. J. , Webb, A. , Timmers, C. D. , Eiferman, D. S. , Evans, D. C. , Dillhoff, M. E. , Schmidt, C. R. , & Guttridge, D. C. (2019). Modeling human cancer‐induced cachexia. Cell Reports, 28(6), 1612–1622.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert, E. E. , Lewis, H. L. , Farren, M. R. , Ramsey, M. L. , Chakedis, J. M. , Rajasekera, P. , Haverick, E. , Sarna, A. , Bloomston, M. , Pawlik, T. M. , Zimmers, T. A. , Lesinski, G. B. , Hart, P. A. , Dillhoff, M. E. , Schmidt, C. R. , & Guttridge, D. C. (2018). Circulating monocyte chemoattractant protein‐1 (MCP‐1) is associated with cachexia in treatment‐naive pancreatic cancer patients. Journal of Cachexia, Sarcopenia and Muscle, 9(2), 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert, E. E. , Metzger, G. A. , He, W. A. , & Guttridge, D. C. (2014). Modeling human cancer cachexia in colon 26 tumor‐bearing adult mice. Journal of Cachexia, Sarcopenia and Muscle, 5(4), 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert, E. E. , Yang, J. , Mace, T. A. , Farren, M. R. , Farris, A. B. , Young, G. S. , Elnaggar, O. , Che, Z. , Timmers, C. D. , Rajasekera, P. , Maskarinec, J. M. , Bloomston, M. , Bekaii‐Saab, T. , Guttridge, D. C. , & Lesinski, G. B. (2017). Dual inhibition of MEK and PI3K/Akt rescues cancer cachexia through both tumor‐extrinsic and ‐intrinsic activities. Molecular Cancer Therapeutics, 16(2), 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, H. , LK C., Lee, M. , Gao, Y. , Xia, H. , Olguin, F. , Fraga, D. A. , Ayers, K. , Choi, S. , Kim, M. , Tehrani, A. , Sowb, Y. A. , Rando, T. A. , & Shrager, J. B. (2017). Smad3 initiates oxidative stress and proteolysis that underlies diaphragm dysfunction during mechanical ventilation. Scientific Reports, 7(1), 14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, H. , Smith, I. J. , Hussain, S. N. , Goldberg, P. , Lee, M. , Sugiarto, S. , Godinez, G. L. , Singh, B. K. , Payan, D. G. , Rando, T. A. , Kinsella, T. M. , & Shrager, J. B. (2015). The JAK‐STAT pathway is critical in ventilator‐induced diaphragm dysfunction. Molecular Medicine, 20(1), 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi, D. , Himori, K. , Yamada, R. , Ashida, Y. , Miyazaki, M. , & Yamada, T. (2018). High‐intensity eccentric training ameliorates muscle wasting in colon 26 tumor‐bearing mice. PLoS One, 13(6), e0199050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee, J. C. , Bosch, A. N. , & Lambert, M. I. (2007). Metabolic consequences of exercise‐induced muscle damage: Sports Medicine, 37(10), 827–836. [DOI] [PubMed] [Google Scholar]
- Tesch, P. A. , Thorsson, A. , & Kaiser, P. (1984). Muscle capillary supply and fiber type characteristics in weight and power lifters. Journal of Applied Physiology, 56(1), 35–38. [DOI] [PubMed] [Google Scholar]
- Tessitore, L. , Costelli, P. , Bonetti, G. , & Baccino, F. M. (1993). Cancer cachexia, malnutrition, and tissue protein turnover in experimental animals. Archives of Biochemistry and Biophysics, 306(1), 52–58. [DOI] [PubMed] [Google Scholar]
- Theodorou, A. A. , Panayiotou, G. , Paschalis, V. , Nikolaidis, M. G. , Kyparos, A. , Mademli, L. , Grivas, G. V. , & Vrabas, I. S. (2013). Stair descending exercise increases muscle strength in elderly males with chronic heart failure. BMC Research Notes, 6(1), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, G. , & Hall, M. N. (1997). TOR signalling and control of cell growth. Current Opinion in Cell Biology, 9(6), 782–787. [DOI] [PubMed] [Google Scholar]
- Tintignac, L. A. , Lagirand, J. , Batonnet, S. , Sirri, V. , Leibovitch, M. P. , & Leibovitch, S. A. (2005). Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. Journal of Biological Chemistry, 280(4), 2847–2856. [DOI] [PubMed] [Google Scholar]
- Tisdale, M. J. (2002). Cachexia in cancer patients. Nature Reviews. Cancer, 3, 883–889. [DOI] [PubMed] [Google Scholar]
- Tisdale, M. J. (2009). Mechanisms of cancer cachexia. Physiological Reviews, 89(2), 381–410. [DOI] [PubMed] [Google Scholar]
- Toigo, M. , & Boutellier, U. (2006). New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. European Journal of Applied Physiology, 97(6), 643–663. [DOI] [PubMed] [Google Scholar]
- Tomasin, R. , Martin, A. , & Cominetti, M. R. (2019). Metastasis and cachexia: Alongside in clinics, but not so in animal models. Journal of Cachexia, Sarcopenia and Muscle, 10(6), 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg, A. U. , Meyer, A. , Rohner, D. , Boyle, J. , Hatakeyama, S. , & Glass, D. J. (2009). Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. American Journal of Physiology. Cell Physiology, 296(6), C1258‐C1270. [DOI] [PubMed] [Google Scholar]
- Tzika, A. A. , Fontes‐Oliveira, C. C. , Shestov, A. A. , Constantinou, C. , Psychogios, N. , Righi, V. , Mintzopoulos, D. , Busquets, S. , Lopez‐Soriano, F. J. , Milot, S. , Lepine, F. , Mindrinos, M. N. , Rahme, L. G. , & Argiles, J. M. (2013). Skeletal muscle mitochondrial uncoupling in a murine cancer cachexia model. International Journal of Oncology, 43(3), 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urhausen, A. , & Kindermann, W. (2002). Diagnosis of overtraining: What tools do we have? Sports Medicine, 32(2), 95–102. [DOI] [PubMed] [Google Scholar]
- VanderVeen, B. N. , Fix, D. K. , & Carson, J. A. (2017). Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: A role for inflammation. Oxidative Medicine and Cellular Longevity, 2017, 3292087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal‐Puig, A. J. , Grujic, D. , Zhang, C.‐Y. , Hagen, T. , Boss, O. , Ido, Y. , Szczepanik, A. , Wade, J. , Mootha, V. , Cortright, R. , Muoio, D. M. , & Lowell, B. B. (2000). Energy metabolism in uncoupling protein 3 gene knockout mice. Journal of Biological Chemistry, 275, 16258–16266. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Pickrell, A. M. , Zimmers, T. A. , & Moraes, C. T. (2012). Increase in muscle mitochondrial biogenesis does not prevent muscle loss but increased tumor size in a mouse model of acute cancer‐induced cachexia. PLoS One, 7(3), e33426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, R. E. , Rotevatn, S. , Li, P. , Annex, B. H. , & Yan, Z. (2004). Voluntary running induces fiber type‐specific angiogenesis in mouse skeletal muscle. American Journal of Physiology. Cell Physiology, 287(5), C1342‐C1348. [DOI] [PubMed] [Google Scholar]
- Weber, M. A. , Krakowski‐Roosen, H. , Schroder, L. , Kinscherf, R. , Krix, M. , Kopp‐Schneider, A. , Essig, M. , Bachert, P. , Kauczor, H. U. , & Hildebrandt, W. (2009). Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer‐related cachexia. Acta Oncologica, 48(1), 116–124. [DOI] [PubMed] [Google Scholar]
- White, J. P. , Puppa, M. J. , Sato, S. , Gao, S. , Price, R. L. , Baynes, J. W. , Kostek, M. C. , Matesic, L. E. , & Carson, J. A. (2012). IL‐6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skeletal Muscle, 2(1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock, A. Maddocks, M. , Lewis, M. , England, R. , & Manderson, C. (2008). Symptoms limiting activity in cancer patients with breathlessness on exertion: Ask about muscle fatigue. Thorax, 63(1), 91–92. [DOI] [PubMed] [Google Scholar]
- Windsor, J. A. , & Hill, G. L. (1988). Risk factors for postoperative pneumonia. The importance of protein depletion. Annals of Surgery, 208(2), 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, T. , & Delafontaine, P. (2020). Mechanisms of IGF‐1‐mediated regulation of skeletal muscle hypertrophy and atrophy. Cells, 9(9), 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, G. , Liu, Z. , Ding, H. , Miao, H. , Garcia, J. M. , & Li, Y. P. (2017). Toll‐like receptor 4 mediates Lewis lung carcinoma‐induced muscle wasting via coordinate activation of protein degradation pathways. Scientific Reports, 7(1), 2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov, D. B. , Juhaszova, M. , & Sollott, S. J. (2006). Mitochondrial ROS‐induced ROS release: An update and review. Biochimica et Biophysica Acta, 1757(5–6), 509–517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer Review History


