Abstract
Aims
Cognitive and motivational processes are thought to underlie cannabis use disorder (CUD), but research assessing how cognitive processes [e.g. interference control (IC)] interact with implicit [e.g. attentional bias (AB)] and explicit motivation (i.e. craving) is lacking. We assessed the presence of AB in cannabis users with varying use severity and tested models of moderation, mediation and moderated mediation to assess how AB, craving and IC interact in their association with measures of cannabis use.
Design
A cross‐sectional study design was used.
Setting and participants
Eight studies performed by our laboratory in the Netherlands including never‐sporadic, occasional (≤ 1/month) and regular cannabis users (≥ 2/week), and individuals in treatment for CUD were combined (n = 560; 71% male).
Measurements
Studies included a classic Stroop task (IC), a cannabis Stroop task (AB) and measures of session‐induced and average session craving. Both heaviness of cannabis use (grams/week) and severity of use related problems were included.
Findings
Only those in treatment for CUD showed an AB to cannabis (P = 0.019) and group differences were only observed when comparing CUD with never‐sporadic users (P = 0.007). In occasional and regular users, IC was negatively associated with heaviness (β = 0.015, P < 0.001), but not severity of use. Average session craving (exploratory), but not session‐induced craving (confirmatory), mediated this association between AB and heaviness (β = 0.050, P = 0.011) as well as severity of use (β = 0.083, P = 0.009); higher AB was associated with heavier use and more severe problems through increased craving.
Conclusions
Attentional bias only appears to be present in cannabis users with the most severe problems and craving appears to mediate the association between attentional bias and both heaviness and severity of use in occasional and regular users. The association of interference control with heaviness but not severity of use may point to subacute intoxication effects of cannabis use on interference control.
Keywords: Attentional bias, cannabis use, cognition, craving, interference control, motivation
INTRODUCTION
Excessive cannabis use and cannabis use disorder (CUD) are considered major health problems. Trends in cannabis legalization, increasing potency and decreasing harm perceptions [1] highlight the urgency of research into the mechanisms underlying CUD. Traditional theories of addiction propose central roles for both cognitive and motivational processes [2], but research assessing both cognitive and motivational processes and their interactions in cannabis users is lacking.
The increased salience of substance‐related cues in substance users is thought to bias behaviour towards substance use, which can present itself as a cue‐induced attentional bias (AB) and craving [3]. These drug‐orientated motivational processes may more easily result in actual substance use in individuals with relatively limited cognitive control [4, 5]. The classical Stroop task has been used to measure cognitive control [6], in which slower responses on incongruent trials, controlled for congruent trials, are an indication of lower IC. Modified drug Stroop tasks have been developed (e.g. Ataya et al. [7]), and the extent to which substance‐related (e.g. weed or blunt) relative to matched neutral words (e.g. floor or table) slow down colour‐naming is taken as an index of AB, which is expected to relate to substance use [8].
Several studies have investigated the role of IC, AB and craving in cannabis use and CUD. One study, using the classical Stroop to measure IC, found poorer IC and altered brain activity in weekly to daily users relative to non‐sporadic using controls when responding to incongruent trials [9]. However, others found no performance differences when comparing near‐daily users and controls (e.g. Takagi et al. [10]) or only found differences in task‐related brain activity in at‐risk and treatment samples (e.g. previous works [11, 12, 13]). Similarly, AB has been identified in cannabis users ranging from life‐time users to those in treatment for CUD [14, 15, 16], while others do not observe AB using a cannabis Stroop even in near‐daily users and those in treatment for CUD [17, 18, 19]. Craving, however, has consistently been associated with heavier use [20] and has been shown to be predictive of cannabis use and related problems 6 months later [16]. Also, craving has been association with both AB (e.g. Field [21]) and IC (e.g. Cousijn et al. [15]) in studies using the cannabis Stroop and the classical Stroop.
These mixed findings could in part be explained by the differential role that AB, craving and IC play across trajectories of cannabis use towards CUD. IC may be lower and AB and craving may be higher in heavier and dependent users [15, 19, 20, 22, 23, 24]. Unfortunately, most studies look at these constructs separately and have a limited range of cannabis use severity included in the sample. It remains unclear which cannabis users have an AB and how this relates to craving and IC (e.g. Cousijn et al. [16]). Furthermore, a meta‐analysis [21] found a small but significant association between AB and craving in substance users, indicating that previous studies might lack power to detect such small effects. To overcome these problems and systematically assess the potential interactions between cognitive and motivational processes in a large sample of cannabis users with variable use frequency, this study combines eight studies conducted in our laboratory that included a pencil‐and‐paper version of the classical Stroop and the cannabis Stroop, and similar assessments of craving.
First, focusing on AB, we will assess whether groups of never‐sporadic users, occasional users, regular users and those in treatment for CUD show an AB towards cannabis cues and whether AB differs between these groups. We expect an AB in regular users and those in treatment for CUD only, that differs from the never‐sporadic users [20]. In occasional and regular users, excluding the CUD group to avoid effects of recent cessation on the outcomes, we will assess whether AB, craving, IC, heaviness of current use and severity of cannabis use‐related problems are indeed associated with each other in this broad range of users.
Secondly, we will assess how the cannabis AB, craving and IC interact in their association with heaviness and severity of cannabis use. We will test different theory‐informed models; we will assess whether AB, craving and/or IC are predictive of heaviness of cannabis use and/or severity of cannabis use‐related problems (Figure 1a; e.g. Kroon et al. [25]). We will then assess the proposed moderating role of cognitive processes in overcoming motivational urges [4, 5] (Figure 1b,c). AB could increase craving or vice versa, subsequently leading to increased cannabis use or use‐related problems (e.g. Field et al. [3, 26]). Therefore, we will also separately assess whether AB or craving act as a mediator in the association between the other variable with heaviness of use and severity of cannabis‐use‐related problems (Figure 1d,e). Then, to combine these moderation and mediation models, we will assess whether IC moderates the association of craving and/or AB with heaviness/severity cannabis use in the proposed mediation models (Figure 1f,g).
FIGURE 1.
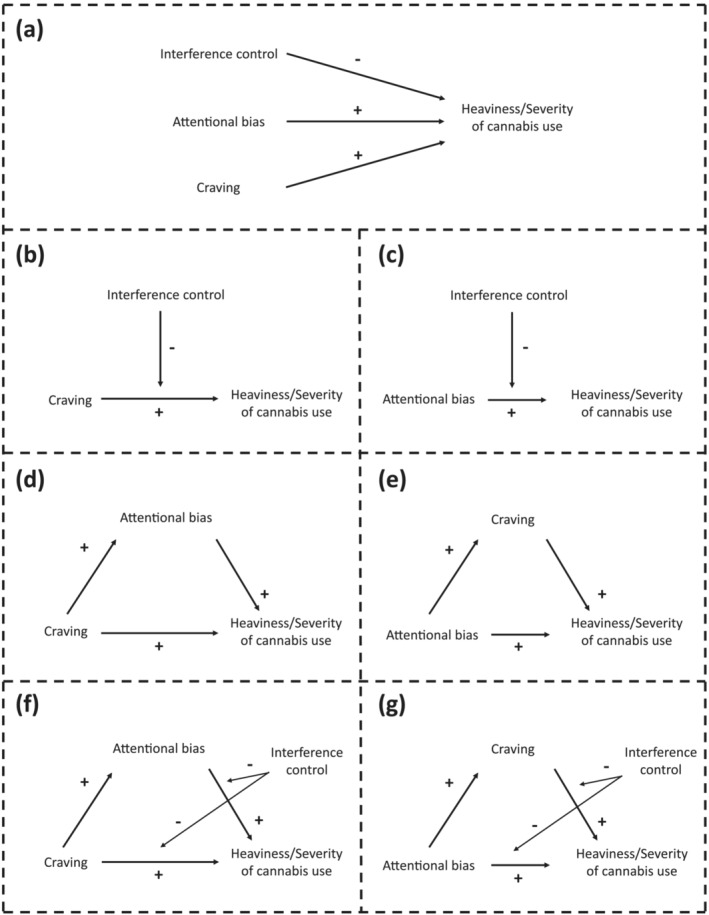
Visual representation of hypotheses
METHODS
We combined data from eight studies (see Supporting information, Figure S1 for study descriptions [15, 16, 19, 27, 28]) conducted by our laboratory that included the same measure of AB, IC and similar measures of craving, resulting in a total of 569 participants. The analysis plan was pre‐registered (https://aspredicted.org/7JT_TN7; 10 November 2021). Deviations from the pre‐registration are reported as exploratory throughout the paper and an overview of the deviations can be found in Supporting information, Figure S2. In all studies, procedures were approved by the ethics committee of the corresponding department and all participants were fully informed and provided informed consent before the start of the experiment.
Materials
Assessments
Participants reported age, gender, weekly cannabis use in grams (heaviness of use) and completed the cannabis use disorder identification test—revised (CUDIT‐R [29]) to assess severity of cannabis use‐related problems. Smoking (yes/no), the Fagerström Test for Nicotine Dependence (FTND [30]) and the Alcohol Use Disorder Identification Test (AUDIT [31]) were included to assess the severity of drug use other than cannabis.
Craving
Craving was assessed using a visual analogue scale (craving VAS) or the marijuana craving questionnaire (MCQ [32]; Supporting information, Figure S1) at the start and the end of the session. To account for differences in measures across studies, session‐induced (SI) craving (start–end score) and an exploratory measure of average session (AS) craving were calculated before the scores were standardized within each scale and combined into single measures of AS and SI craving. Comparability of the MCQ and VAS craving scores was assessed in a subsample (n = 40) in which both were collected during the same session, showing a moderate to high within‐person correlation between the AS craving scores (r = 0.806, P < 0.001) as well as SI craving scores (r = 0.500, P = 0.001) as calculated from the VAS and MCQ. Furthermore, VAS and MCQ were similarly associated with the measures of cannabis use included in this study (Supporting information, Table S2).
Classical Stroop: interference control
The classical Stroop task included three different cards that were presented in a fixed order [6, 33]. All cards included 10 rows of 10 words/blocks which participants were instructed to read over row‐by‐row, as fast as possible, according to the card instructions. First, participants were instructed to read the words red, green, blue and yellow as printed in black (word card). Secondly, participants were instructed to name the colour of the colour blocks (colour card). Thirdly, participants were instructed to name the incongruent colour in which the words red, green, blue and yellow were printed (colour‐word card). Reaction‐times were recorded using a stopwatch and IC scores were calculated using this formula: reaction‐time colour‐word card/[(reaction‐time word card + reaction‐time colour card)/2] [34]. Higher scores indicated lower IC.
Cannabis Stroop: attentional bias
The cannabis Stroop task included two different cards presented in counterbalanced order [16]. Each card included eight rows of seven words that were all printed in red, green, blue or yellow. The words on both cards were matched on word length and number of syllables, but on one card the words were neutral (e.g. poster), while the words on the other card were cannabis‐related (e.g. stoned). Participants were instructed to name the colour in which each word was printed, row by row, from left to right, as fast as they could. A stopwatch was used to record the time needed to complete each card. AB scores were calculated using the following formula: reaction‐time cannabis card – reaction‐time neutral card, with higher scores being indicative of a relatively higher bias for cannabis words.
Procedures
While there were variations in the full study protocol and session length between studies (Supporting information, Figure S1), the overlapping measures were identical across studies. Also, the cannabis Stroop was always completed before the classical Stroop. Craving measures were conducted at both the start and the end of the session in all studies. Furthermore, cannabis‐related questionnaires, aside from the pre‐session craving, were always completed after the Stroop tasks.
Data analysis
Grouping and exclusion
Participants were classified as never‐sporadic user (no life‐time or no use in the last year), occasional users (maximum of once per month during the last year), regular users (minimum of twice per week during the last year) or CUD (in treatment at the moment of testing; Table 1) using the first question of the CUDIT‐R [29] (NB: in study 8, grouping was based on self‐reported last year use) and treatment status. Individuals who did not fit any of these groups (n = 8) were excluded (Supporting information, Table S1). IC, AB, craving, grams/week of use and CUDIT‐R scores that were more than 3 standard deviations from the mean were excluded to reduce effects of measurement error (e.g. implausibly high levels of cannabis use or IC scores indicative of potential methodological problems).
TABLE 1.
Sample characteristics
| Variables | Groups | Occasional and regular (n = 358) | |||||
|---|---|---|---|---|---|---|---|
| Never–sporadic (n = 97) | Occasional (n = 35) | Regular (n = 323) | CUD (n = 97) | Group difference | Pairwise difference a | ||
| Gender, % male | 57.7 | 45.7 | 75.9 | 77.7 | χ2 (3, n = 549) = 24.9, P < 0.001 | 2, 3, 4, 5 | 72.9 |
| Age, median (MAD) | 22.0 (2.5) | 22.0 (2.0) | 21.0 (2.0) | 20.0 (2.0) |
F (3,539) = 7.2, P < 0.001, η 2 = 0.04 |
3, 6 | 23.2 (5.8) |
| CUDIT‐R, median (MAD) | _ | 1.0 (0.0) | 15.0 (4.0) | 23.0 (4.0) |
F (2,441) = 169.0, P < 0.001, η 2 = 0.43 |
4, 5, 6 | 14.1 (6.4) |
| Gram/week, median (MAD) | _ |
F (2,415) = 46.0, P < 0.001, η 2 = 0.18 |
4, 5, 6 | 4.2 (4.0) | |||
| Age of onset, median (MAD) | _ | 17.0 (2.0) | 16.0 (1.0) | 16.0 (1.5) |
F (2,429) = 3.7, P < 0.001, η 2 = 0.02 |
4, 5 | 15.8 (2.4) |
| Smoking, % smokers | 19.6 | 40.0 | 64.1 | 85.3 | χ2 (3, n = 550) = 97.5, P < 0.001 | 2, 3, 4, 5, 6 | 61.7 |
| FTND, median (MAD) | 0.0 (0.0) | 2.0 (1.0) | 3.0 (2.0) | 4.0 (2.0) |
F (3,344) = 13.6, P < 0.001, η 2 = 0.11 |
2, 3, 5, 6 | 2.7 (2.3) |
| AUDIT, median (MAD) | 5.0 (2.0) | 6.0 (2.0) | 8.0 (4.0) | 8.0 (4.0) |
F (3,481) = 6.5, P < 0.001, η 2 = 0.06 |
2, 3 | 8.6 (5.5) |
| Session induced craving, median (MAD) | −0.22 (0.1) | −0.22 (0.1) | 0.02 (0.6) | −0.23 (0.4) |
F (3,528) = 8.7, P < 0.001, η 2 = 0.05 |
2, 6 | 0.58 (2.3) |
| Average session craving, median (MAD) | −0.85 (0.0) | −0.85 (0.1) | 0.41 (0.7) | −0.39 (0.6) |
F (3,528) = 45.1, P < 0.001, η 2 = 0.20 |
2, 3, 4, 5, 6 | 0.21 (1.0) |
| Interference control, median (MAD) | 25.5 (7.5) | 23.0 (4.6) | 31.3 (8.9) | 33.2 (8.4) |
F (3,540) = 7.3, P < 0.001, η 2 = 0.04 |
2, 3, 5 | 31.6 (12.5) |
| Attentional bias, median (MAD) | −0.5 (1.9) | 0.0 (2.0) | 0.3 (2.1) | 1.0 (2.3) | F (3,541) = 3.1, P = 0.026, η 2 = 0.02 | 0.28(3.4) | |
| Never‐sporadic versus occasional | t (130) = 0.42, P = 0.673, d = 0.08 | ||||||
| Never‐sporadic versus regular | t (415) = 1.96, P = 0.050, d = 0.23 | ||||||
| Never‐sporadic versus CUD | t (188) = 2.71, P = 0.007, d = 0.39 | ||||||
| Occasional versus regular | t (353) = 0.85, P = 0.398, d = 0.15 | ||||||
| Occasional versus CUD | t (126) = 1.59, P = 0.114, d = 0.32 | ||||||
| Regular versus CUD | t (411) = 1.74, P = 0.084, d = 0.20 | ||||||
AUDIT, alcohol use disorder identification test; CUD, cannabis use disorder; CUDIT‐R, cannabis use disorder identification test; FTND, Fagerström test for nicotine dependence; MAD, median absolute deviation.
pairwise differences (P < 0.05) after Bonferroni correction; pairwise comparisons: 1 = never‐sporadic versus occasional, 2 = never‐sporadic versus regular, 3 = never‐sporadic versus CUD, 4 = occasional versus regular, 5 = occasional versus CUD, 6 = regular versus CUD.
Attentional bias
One‐sample t‐tests were run to assess whether there was an AB to cannabis words (whether the AB was different from zero) per group. An analysis of variance (ANOVA) was performed to assess group differences in AB, with post‐hoc independent‐sample t‐tests to explore the differences. Then, in all occasional and regular users, correlation analyses were conducted to assess how heaviness of cannabis use (grams/week), severity of cannabis use‐related problems (CUDIT‐R score), AB, IC and SI craving were associated with each other. The attentional bias analyses as described above were conducted in JASP (version 0.14.1.0 [35]).
Attentional bias, interference control and craving: their association with cannabis use
Only current occasional and regular users were included in the following analyses (Table 1; excluding the CUD group due to potential effects of recent cessation). Simple and multiple regression analyses were conducted to assess whether AB, IC and/or SI craving were predictive of heaviness of cannabis use and severity of cannabis use‐related problems (Figure 1a). Moderation analyses were conducted to assess whether IC moderates the association between SI craving (Figure 1b) or AB (Figure 1c) and heaviness of cannabis use and severity of cannabis use‐related problems. Then, to assess the proposed relation between AB and SI craving in their association with cannabis use outcomes, we ran a mediation analysis to determine whether AB mediates the association between SI craving and heaviness of cannabis use or severity of cannabis use‐related problems (Figure 1d) or the reverse (Figure 1e, Supporting information, Figure S5A). Combining this, moderated‐mediation analyses were run to assess whether IC moderates the association between SI craving and AB with heaviness of cannabis use or cannabis use related problems in the proposed mediation models (Figure 1f,g and Supporting information, Figure S5B). All included variables were mean‐centred. Additional exploratory analyses were conducted replacing SI craving with AS craving. The models as described above were run in R (version 4.1.2 [36]) creating the models (Figure 1b–g) using the processR (version 0.2.6 [37]) package and running them in lavaan (version 0.6‐9 [38]) using maximum likelihood estimation. Bonferroni‐corrected P‐values (P bonf ) were provided for analysis requiring multiple comparison correction.
RESULTS
Sample characteristics
Individuals with known colour‐blindness (n = 2) and those who tested positive on drugs other than cannabis during the test session (n = 7) were excluded from the analyses, resulting in a total sample of 560 participants (71% male). Outlier exclusion resulted in the omission of six participants’ data regarding grams/week of use, four participants’ craving scores, seven participants’ AB scores and seven participants’ IC scores.
Groups significantly differed on all variables (see Table 1). Exploratory independent‐sample t‐tests showed varying patterns of differences for all variables, with a general tendency of more severe alcohol, cigarette use and more limited IC in more severe cannabis users and no differences between never‐sporadic users and occasional users. Notably, SI craving was only positive in regular users.
Group differences in attentional bias and correlations between variables
Only the CUD group showed an AB to cannabis (t (92) = 2.39, P = 0.019, d = 0.25). However, no significant AB to cannabis was observed in the never‐sporadic (t (96) = 1.31, P = 0.192, d = 0.13), occasional (t (34) = 0.38, P = 0.704, d = 0.07) and regular users (t (319) = 1.72, P = 0.087, d = 0.10). AB differed between groups (F (3,541) = 3.1, P = 0.026, η 2 = 0.017; Table 1), with post‐hoc analyses revealing a higher bias in CUD (and regular users at P = 0.050) versus never‐sporadic users (Figure 2). Exploratory sensitivity analyses, adding the variables that differed between groups (Table 1) as covariates in an analysis of covariance (ANCOVA), showed that the effect was independent of age and IC but no longer significant after correction for AUDIT and FTND.
FIGURE 2.
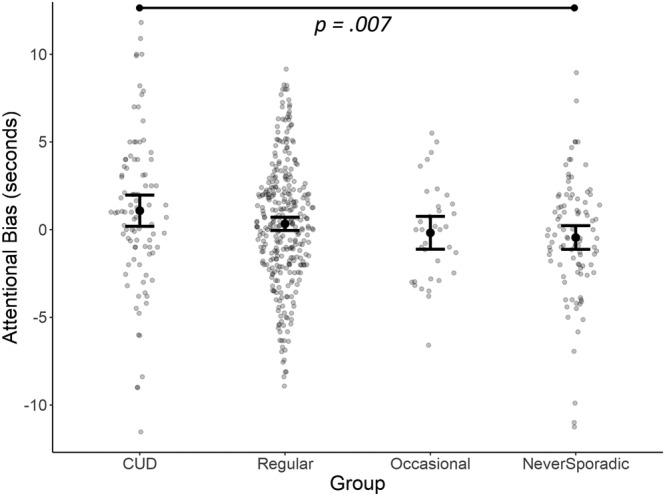
Group differences in attentional bias (AB). Error bars presenting standard error (SE) of the mean. CUD, cannabis use disorder.
Focusing upon occasional and regular users, correlational analysis revealed a positive association between heaviness of cannabis use (gram/week) and severity of cannabis use (CUDIT‐R scores; r s(347) = 0.49, P bonf < 0.001). CUDIT‐R score was not associated with any of the other variables (highest r s = 0.10, with uncorrected P = 0.06), but gram/week was positively associated with classical Stroop scores (r s(343) = 0.20, P bonf < 0.001), indicating worse IC in more severe users. No other correlations between IC, craving and AB were observed (highest r s = 0.08, with uncorrected P = 0.16).
Attentional bias, interference control and craving: their association with cannabis use
In line with the correlational results, simple regression analyses (Figure 1a) showed an association between poorer IC and gram/week (R 2 = 0.037, F = 14.23, β = 0.015, β SE = 0.004, t = 3.772, P bonf < 0.001), but not CUDIT‐R score (R 2 = −0.003, F (1, 350) = 0.023, β = 0.004, β SE = 0.027, t = 0.150, P bonf = 1.0). AB and craving did not directly predict gram/week or CUDIT‐R score (Supporting information, Table S3, Figure S4).
Moderation (Figure 1b,c, Supporting information, Table S4), mediation (Figure 1d,e, Supporting information, Table S5) and moderated‐mediation (Figure 1f,g, Supporting information, Table S6) models revealed no other associations than the consistently present direct association between IC and gram/week (Supporting information, Figure S4).
Exploratory analyses: the role of average session craving
As SI changes in craving do not necessarily reflect absolute feelings of craving, but rather to what extent the session affected craving in the individual, we re‐ran the correlations, simple regressions, moderation, mediation and moderated‐mediation models with AS craving instead of SI craving (Figure 3).
FIGURE 3.
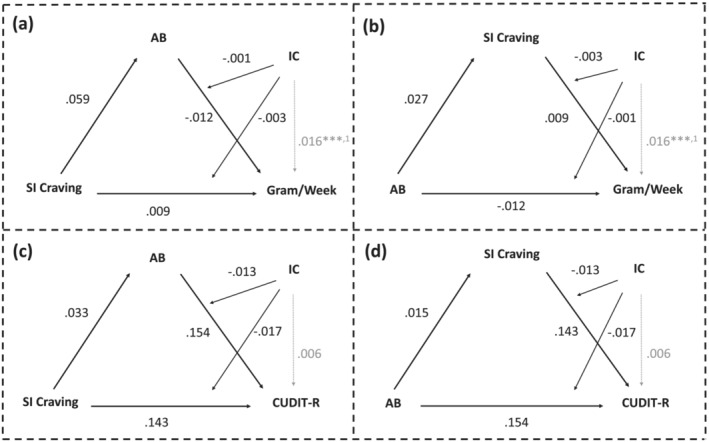
Moderated‐mediation analysis results. Analyses assessing the conditional indirect effects of session induced (SI) craving/attentional bias (AB) on heaviness or severity of use through AB/SI craving, at different levels of interference control (IC). Estimates for all paths reported with indicators of significance: ***p < 0.001, 1 p < 0.001.
Correlational and simple regression analyses showed that AS craving was positively associated with gram/week (r s(330) = 0.30, P bonf < 0.001; R 2 = 0.057, F (1, 330) = 20.93, β = 0.977, β SE = 0.214, t = 4.575, P bonf < 0.001) and CUDIT‐R (r s(338) = 0.26, P bonf < 0.001; R 2 = 0.074, F (1, 338) = 28.19, β = 1.75, β SE = 0.331, t = 5.309, P bonf < 0.001; Supporting information, Table S7) of use. Furthermore, higher AS craving during the session was associated with higher AB (r s(336) = 0.15, P bonf = 0.024) and lower IC (i.e. higher Stroop score; r s(333) = 0.18, P bonf = 0.004).
Moderation analyses revealed similar associations, also including the association between IC and heaviness of use (Supporting information, Table S8). However, mediation analyses revealed that AS craving mediated the association between AB and both gram/week (indirect effect: β = 0.050, β SE = 0.020, Z = 2.556, P bonf = 0.021) and CUDIT‐R score (indirect effect: β = 0.083, β SE = 0.032, Z = 2.602, P bonf = 0.019; Supporting information, Table S8). These mediations were stable across the moderated‐mediation models (CUDIT‐R—indirect effect: β = 0.089, β SE = 0.033, Z = 2.655, P bonf = 0.016; gram/week—indirect effect: β = 0.049, β SE = 0.019, Z = 2.552, P bonf = 0.021), but IC did not act as a moderator: rather, it was directly associated with gram/week only (Supporting information, Table S9; Figure 4).
FIGURE 4.
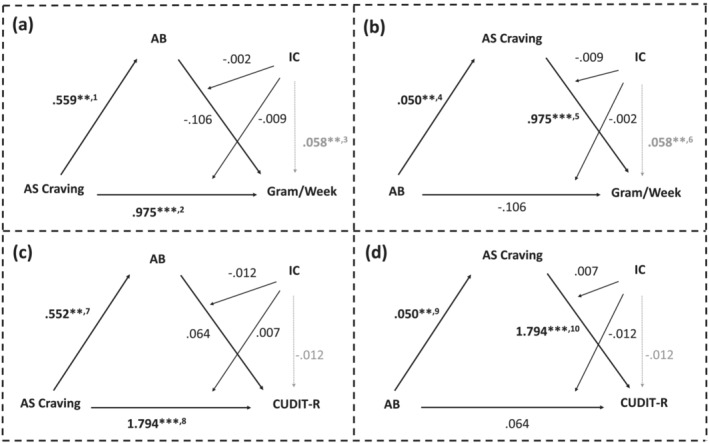
Exploratory moderated‐mediation analysis results including average session (AS) craving instead of session‐induced (SI) craving. Analyses assessing conditional indirect effects of AS craving/attentional bias (AB) on heaviness or severity of use through AB/AS craving, at different levels of interference control (IC). Estimates for all paths reported with indicators of significance: **P < 0.01, ***P < 0.001, 1 P = 0.002, 2 P < 0.001, 3 P = 0.001, 4 P = 0.002, 5 P < 0.001, 6 P = 0.001, 7 P = 0.002, 8 P < 0.001, 9 P = 0.002, 10 P < 0.001.
DISCUSSION
We assessed the presence of AB in cannabis users with different levels of use and how AB interacted with craving and IC in its relationship with heaviness and severity of cannabis use. A clear strength of this study is the inclusion of a large sample with a large range of cannabis use severity (n = 560). Only those users in treatment for CUD showed an AB to cannabis (significantly > 0), which was significantly higher compared to never‐sporadic users, but not compared to occasional and regular users. Poorer IC was consistently associated with heavier cannabis use, but not the severity of use‐related problems. However, in contrast to our hypotheses, IC did not moderate the association between AB and craving in their association with measures of cannabis use. Moreover, SI craving did not mediate the association between AB (or vice versa) and measures of cannabis use, but results changed when using average craving instead; craving mediated the association between AB and heaviness as well as severity of use.
Our results suggest that AB may be a clinical marker of CUD severity, while IC may generally be poorer in heavier users regardless of CUD problem severity. However, the associations between AB, craving IC and cannabis use are complex. Looking at Figure 1, AB appears higher in more frequent users, but AB did not directly relate to our measures of cannabis use (also not when exploratively including the CUD group in the regression analysis). It only related through its positive association with craving; those with higher AB might have higher, potentially more ‘trait‐like’ levels of craving, triggering a higher general likelihood to use. Most studies indicate that the relationship between craving and AB is probably reciprocal [3]; however, our results in which AB affects use through craving are in line with earlier research in alcohol users in which training to increase AB resulted in increased craving and subsequent use [39]. The indirect effects of AB via craving could also explain why some studies did not find direct associations between AB and measures of use (e.g. alcohol Stroop [24]; cocaine Stroop [40]). However, our findings are cross‐sectional and were only significant for average craving, not SI craving. Studies investigating the temporal dynamics between AB and craving are needed to further investigate this.
The specific presence of AB in the treatment (most severe) group could explain some of the null findings of previous studies (e.g. Field et al. [41]) and could indicate its potential value as a clinical marker. However, research evaluating the relevance of assessing AB for other substance use disorders in clinical settings are inconsistent (e.g. Field et al. [26] and Christiansen et al. [42])—while some studies show AB to be associated with worse treatment outcomes or increased relapse rate (heroin [43]; cocaine [44]; alcohol [45]), this is not the case in all studies (cocaine [43]; tobacco [46])—and studies on the value of AB as a marker of CUD severity and treatment outcomes are largely lacking. Hence, further research is required to systematically assess the clinical relevance of AB to cannabis cues in clinical and non‐clinical samples of cannabis users.
It must be noted that the group differences disappeared when controlling for AUDIT and FTND. Polysubstance use is extremely common [47], and the higher use of alcohol and tobacco might arise from the same underlying factors as their heavy cannabis use (e.g. Field [21] and Pennington et al. [48]). Including AUDIT and FTND as covariates is suboptimal for it probably deletes cannabis use‐relevant variance. Furthermore, it seems theoretically unlikely that alcohol and tobacco use directly affect AB for cannabis words, but further research with samples (more closely) matched on these variables are needed to confirm this.
Partially in line with our expectations, we consistently found lower IC to be associated with heavier cannabis use (small–medium effect; r s = 0.20). While it is often argued that this could indicate of a lack of control over use [4, 5], the lack of association with severity of cannabis use‐related problems and the lack of interactions with AB and craving may indicate that this association is a result of the current heaviness of use and associated subacute effects. Some earlier studies also failed to find a moderating role if IC (e.g. Cousijn et al. [15] and van Kampen et al. [19]). Cannabis intoxication has been found to negatively affect Stroop performance (e.g. Hooker & Jones [49]) and there is evidence that several cognitive functions recover with increased abstinence (e.g. Crean et al. [50]). In line with this, an exploratory check in the CUD group, of which the majority have been abstinent for multiple days (53% at least 7 days of abstinence), showed that there is no association between IC and heaviness of use in the CUD group (Supporting information, Table S10). Further research is needed to assess (sub)acute effects and the potential for recovery.
A few limitations of this study should be noted. While combining different studies increases the sample size and allows for more complex models to be tested, it potentially introduces differences in experimenter effects and methodology between studies. However, the classical and cannabis Stroop methodology was the same throughout studies and it is likely that experimenter variability was as large within some studies as between them [in line with low (≤ 0.126) ICC]. Differences between sessions might particularly have affected the results of SI craving, as they differed in length and content aside from the measures included in our analysis. It must be noted that all standardized craving scores were based on two different measures of craving. While subsample analyses showed that within‐person associations between the measures were moderate to high and displayed similar associations with cannabis use outcomes, it is unclear how this approach could have affected the results. Also, the difference in the results between SI and average craving highlight the potential influence of the chosen outcome, even when calculated from the same measures, and the potential incomparability of the results of studies using different outcome measures. Replication of our results using a single measure of craving but using both average session craving and SI craving as outcomes is warranted. Furthermore, it must be investigated whether our results generalize to other measures of cognitive functioning and AB and whether these effects generalize to more ecologically valid situations in which AB could affect craving and cannabis use.
Our results indicate that AB as measured by the cannabis Stroop might only be present in those cannabis users with the most severe problems, but that greater AB could be associated with higher craving and, in turn, higher cannabis use and related problems even in less severe cannabis users. While systematic research into the clinical relevance of these associations is crucial, these results highlight the potential importance of AB in both heaviness and severity of cannabis use as well as the mechanisms by which AB through increased craving could affect efforts to reduce or stop using cannabis.
DECLARATION OF INTERESTS
None.
AUTHOR CONTRIBUTIONS
Emese Kroon: Conceptualization; data curation; formal analysis; investigation; methodology; visualization. Lauren Kuhns: Conceptualization; data curation; formal analysis; investigation; methodology. Annette Dunkerbeck: Investigation. Janna Cousijn: Conceptualization; funding acquisition; investigation; methodology; supervision.
CLINICAL TRIAL REGISTRATION
The analysis plans were preregistered (https://aspredicted.org/7JT_TN7; November 10, 2021).
Supporting information
Data S1. Supporting Information
ACKNOWLEDGEMENTS
This research was supported by grant 1R01 DA042490‐01A1 awarded to Janna Cousijn from the National Institute on Drug Abuse/National Institute of Health.
Kroon E, Kuhns L, Dunkerbeck A, Cousijn J. The who and how of attentional bias in cannabis users: associations with use severity, craving and interference control. Addiction. 2023;118(2):307–316. 10.1111/add.16059
Funding information National Institute on Drug Abuse/National Institute of Health, Grant/Award Number: 1R01 DA042490‐01A1
REFERENCES
- 1. United Nations Office on Drugs and Crime (UNODC) . Drug Market Trends: Cannabis Opioids. World Drug Report 2021. Vienna, Austria: UNODC; 2021.
- 2. Bickel WK, Mellis AM, Snider SE, Athamneh LN, Stein JS, Pope DA. 21st century neurobehavioral theories of decision making in addiction: review and evaluation. Pharmacol Biochem Behav. 2018;164:4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. [DOI] [PubMed] [Google Scholar]
- 4. Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Phil Trans Royal Soc B Biol Sci. 2008;363:3137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hester R, Luijten M. Neural correlates of attentional bias in addiction. CNS Spectr. 2014;19:231–8. [DOI] [PubMed] [Google Scholar]
- 6. Stroop RJ. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;121:15–23. [Google Scholar]
- 7. Ataya AF, Adams S, Mullings E, Cooper RM, Attwood AS, Munafò MR. Internal reliability of measures of substance‐related cognitive bias. Drug Alcohol Depend. 2012;121:148–51. [DOI] [PubMed] [Google Scholar]
- 8. Smith DG, Ersche KD. Using a drug‐word Stroop task to differentiate recreational from dependent drug use. CNS Spectr. 2014;19:247–55. [DOI] [PubMed] [Google Scholar]
- 9. Battisti RA, Roodenrys S, Johnstone SJ, Pesa N, Hermens DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology. 2010;212:613–24. [DOI] [PubMed] [Google Scholar]
- 10. Takagi M, Lubman DI, Cotton S, Fornito A, Baliz Y, Tucker A, et al. Executive control among adolescent inhalant and cannabis users. Drug Alcohol Rev. 2011;30:629–37. [DOI] [PubMed] [Google Scholar]
- 11. Banich MT, Crowley TJ, Thompson LL, Jacobson BL, Liu X, Raymond KM, et al. Brain activation during the Stroop task in adolescents with severe substance and conduct problems: a pilot study. Drug Alcohol Depend. 2007;90:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kober H, Devito EE, Deleone CM, Carroll KM, Potenza MN. Cannabis abstinence during treatment and one‐year follow‐up: relationship to neural activity in men. Neuropsychopharmacology. 2014;39:2288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thayer RE, Feldstein Ewing SW, Dodd AB, Hansen NS, Mayer AR, Ling JM, et al. Functional activation during the Stroop is associated with recent alcohol but not marijuana use among high‐risk youth. Psychiatry Res Neuroimag. 2015;234:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cane JE, Sharma D, Albery IP. The addiction Stroop task: examining the fast and slow effects of smoking and marijuana‐related cues. J Psychopharmacol. 2009;23:510–9. [DOI] [PubMed] [Google Scholar]
- 15. Cousijn J, Watson P, Koenders L, Vingerhoets WAM, Goudriaan AE, Wiers RW. Cannabis dependence, cognitive control and attentional bias for cannabis words. Addict Behav. 2013;38:2825–32. [DOI] [PubMed] [Google Scholar]
- 16. Cousijn J, van Benthem P, van der Schee E, Spijkerman R. Motivational and control mechanisms underlying adolescent cannabis use disorders: a prospective study. Dev Cogn Neurosci. 2015;16:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug‐dependent outpatient sample. Addict Behav. 2006;31:174–81. [DOI] [PubMed] [Google Scholar]
- 18. Asmaro D, Carolan PL, Liotti M. Electrophysiological evidence of early attentional bias to drug‐related pictures in chronic cannabis users. Addict Behav. 2014;39:114–21. [DOI] [PubMed] [Google Scholar]
- 19. van Kampen AC, Cousijn J, Engel C, Rinck M, Dijkstra BAG. Attentional bias, craving and cannabis use in an inpatient sample of adolescents and young adults diagnosed with cannabis use disorder: the moderating role of cognitive control. Addict Behav. 2020;100:106126. [DOI] [PubMed] [Google Scholar]
- 20. Kroon E, Kuhns L, Hoch E, Cousijn J. Heavy cannabis use, dependence and the brain: a clinical perspective. Addiction. 2020;115:559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Field M. A meta‐analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135:589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marhe R, Waters AJ, Van De Wetering BJM, Franken IHA. Implicit and explicit drug‐related cognitions during detoxification treatment are associated with drug relapse: an ecological momentary assessment study. J Consult Clin Psychol. 2013;81:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Waters AJ, Marhe R, Franken IHA. Attentional bias to drug cues is elevated before and during temptations to use heroin and cocaine. Psychopharmacology. 2015;219:909–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hallgren K, McCrady B. Interference in the alcohol Stroop task with college student binge drinkers. J Behav Health. 2013;2:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kroon E, Kuhns L, Cousijn J. The short‐term and long‐term effects of cannabis on cognition: recent advances in the field. Curr Opin Psychol. 2021;38:49–55. [DOI] [PubMed] [Google Scholar]
- 26. Field M, Marhe R, Franken IHA. The clinical relevance of attentional bias in substance use disorders. CNS Spectr. 2014;19:225–30. [DOI] [PubMed] [Google Scholar]
- 27. Cousijn J, Snoek RWM, Wiers RW. Cannabis intoxication inhibits avoidance action tendencies: a field study in the Amsterdam coffee shops. Psychopharmacology. 2013;229:167–76. [DOI] [PubMed] [Google Scholar]
- 28. Cousijn J, van Duijvenvoorde ACK. Cognitive and mental health predictors of withdrawal severity during an active attempt to cut down cannabis use. Front Psychol. 2018;9:301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adamson SJ, Kay‐Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, et al. An improved brief measure of cannabis misuse: the cannabis use disorders identification test‐revised (CUDIT‐R). Drug Alcohol Depend. 2010;110:137–43. [DOI] [PubMed] [Google Scholar]
- 30. Heatherton T, Kozlowski L, Frecker R, Fagerström K. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–27. [DOI] [PubMed] [Google Scholar]
- 31. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. Addiction. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 32. Heishman SJ, Singleton EG, Liguori A. Marijuana craving questionnaire: development and initial validation of a self‐report instrument. Addiction. 2001;96:1023–34. [DOI] [PubMed] [Google Scholar]
- 33. Hammes JGW. The Stroop Kleur–Woord Test. Handleiding Lisse: Swets and Zeitlinger; 1971. [Google Scholar]
- 34. Scarpina F, Tagini S. The Stroop color and word test. Front Psychol. 2017;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. JASP Team . JASP (version 0.14.1) 2020. https://jasp-stats.org/
- 36. R Core Team. R . A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 37. Moon KW. processR: Implementation of the ‘PROCESS’ Macro 2021. Available at: http://rpubs.com/cardiomoon/468602
- 38. Rosseel Y. Lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48:1–36. [Google Scholar]
- 39. Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology. 2005;183:350–7. [DOI] [PubMed] [Google Scholar]
- 40. Hester R, Dixon V, Garavan H. A consistent attentional bias for drug‐related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006;81:251–7. [DOI] [PubMed] [Google Scholar]
- 41. Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102:579–86. [DOI] [PubMed] [Google Scholar]
- 42. Christiansen P, Schoenmakers TM, Field M. Less than meets the eye: reappraising the clinical relevance of attentional bias in addiction. Addict Behav. 2015;44:43–50. [DOI] [PubMed] [Google Scholar]
- 43. Marissen MAE, Franken IHA, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–12. [DOI] [PubMed] [Google Scholar]
- 44. Carpenter KM, Martinez D, Vadhan NP, Barnes‐Holmes D, Nunes EV. Measures of attentional bias and relational responding are associated with behavioral treatment outcome for cocaine dependence. Am J Drug Alcohol Abuse. 2012;38:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug Alcohol Depend. 2002;68:237–343. [DOI] [PubMed] [Google Scholar]
- 46. Spiegelhalder K, Jähne A, Kyle SD, Beil M, Doll C, Feige B, et al. Is smoking‐related attentional bias a useful marker for treatment effects? Behav Med. 2011;37:26–34. [DOI] [PubMed] [Google Scholar]
- 47. United Nations Office on Drugs and Crime (UNODC) . World Drug Report 2016. Vienna, Austria: UNODC; 2016.
- 48. Pennington CR, Shaw DJ, Adams J, Kavanagh P, Reed H, Robinson M, et al. Where's the wine? Heavy social drinkers show attentional bias towards alcohol in a visual conjunction search task. Addiction. 2020;115:1650–9. [DOI] [PubMed] [Google Scholar]
- 49. Hooker WD, Jones RT. Increased susceptibility to memory intrusions and the Stroop interference effect during acute marijuana intoxication. Psychopharmacology. 1987;91:20–4. [DOI] [PubMed] [Google Scholar]
- 50. Crean RD, Crane NA, Mason BJ. An evidence‐based review of acute and long‐term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


