Abstract
Australian wildlife rehabilitators (AWR) are at increased risk of developing Q fever, a serious zoonotic disease caused by the intracellular bacterium Coxiella burnetii. Previous studies have suggested that Australian wildlife may be a potential C. burnetii infection source for humans. However, a recent serological survey of AWR found no association between C. burnetii exposure and direct contact with any wildlife species. To further explore the potential risk that wildlife may pose, this study aimed to identify associations between self‐reported Q fever in AWR and risk factors for exposure to C. burnetii. An online cross‐sectional survey was implemented in 2018 targeting AWR nationwide. Risk factors for self‐reported Q fever were determined using multivariable logistic regression. Medically diagnosed Q fever was self‐reported in 4.5% (13/287) of unvaccinated respondents. Rehabilitators who self‐reported medically diagnosed Q fever were significantly more likely to: primarily rehabilitate wildlife at a veterinary clinic (OR 17.87, 95% CI: 3.09–110.92), have domestic ruminants residing on the property where they rehabilitate wildlife (OR 11.75, 95% CI: 2.91–57.42), have been educated at a High School/Technical and Further Education level (OR 10.29, 95% CI: 2.13–84.03) and be aged >50 years (OR 6.61, 95% CI: 1.60–38.35). No association was found between self‐reported Q fever and direct contact with wildlife. These findings support previous work suggesting that AWR are at increased risk of C. burnetii infection and may develop Q fever potentially via exposure to traditional infection sources including livestock, other domestic animals, or contaminated environments, in association with their rehabilitation practices and lifestyle. Although Q fever vaccination is recommended for AWR, vaccine uptake is low in this population. Future studies should aim to determine the level of Q fever awareness and identify barriers to Q fever vaccination in this at‐risk group. The difficulty in accessing the AWR population also highlights the need for a national centralized AWR database.
Keywords: Australia, Coxiella burnetii, Q fever, wildlife rehabilitators
Impacts.
Australian wildlife rehabilitators (AWR) are at risk of exposure to Coxiella burnetii and developing Q fever.
Rehabilitating wildlife primarily at a veterinary clinic, or on a property housing domestic ruminants, were identified as risk factors for Q fever in AWR, however, direct contact with macropods or other wildlife species was not.
Despite an increased risk of exposure to C. burnetii and Q fever vaccination being recommended for AWR, uptake of the vaccine in this cohort is low, suggesting interventions are needed to promote vaccination to this population.
1. INTRODUCTION
Q fever is a zoonotic disease initially described in 1935 among abattoir workers in Queensland, Australia (Derrick, 1937), but has since been found worldwide, except for New Zealand (Hilbink et al., 1993). The Q fever agent C. burnetii is an obligate intracellular bacterium that may cause acute and chronic human infections (Angelakis & Raoult, 2010; Marrie, 1990). C. burnetii also exists as a highly infectious extracellular spore‐like form, which can persist in the environment for at least 12 months (Kersh et al., 2013) and can be easily dispersed by the wind over long distances (Hawker et al., 1998). Domestic ruminants are regarded as the major reservoirs of human infection (Marrie et al., 1996). Infected ruminants contaminate the environment by shedding C. burnetii in their milk, urine, faeces and, to a greater extent, products of conception (Marrie, 1990). Infection is mostly acquired following inhalation of contaminated aerosols.
In humans, the clinical manifestations of C. burnetii infection are broad, ranging from asymptomatic seroconversion in approximately in 20–80% of cases, to acute disease, which typically presents as a self‐limiting “influenza‐like” illness, characterized by high fevers, headaches, chills, and fatigue, with hepatitis and pneumonia as potential complications (Million & Raoult, 2015). Post‐Q fever fatigue syndrome and persistent focal infection (previously “chronic Q fever”) are well recognized sequelae of C. burnetii infection, which may manifest years after primary infection, regardless of the initial clinical presentation (Eldin et al., 2017; Maurin & Raoult, 1999). Due to non‐specific clinical symptoms, Q fever cases may go undiagnosed or result in delayed diagnosis (Million & Raoult, 2015). In Australia, Q fever has been nationally notifiable since 1977 (Garner et al., 1997), with approximately 500 human cases notified annually (National Notifiable Diseases Surveillance System, 2021). Australia is the only country where an effective licensed human Q fever vaccine (Q‐Vax®; Seqirus, Parkville, Vic.) is available. Q fever vaccination is recommended for those engaged in high‐risk occupations, including abattoir workers, veterinarians and zoo and wildlife workers (Australian Technical Advisory Group on Immunisation, 2021).
In addition to traditional domestic animal sources, Australian wildlife have been suggested as potential sources of C. burnetii infection for humans. Evidence of C. burnetii exposure or infection has been observed in many wildlife species including bandicoots, possums, koalas, flying foxes (Bennett et al., 2011; Tozer et al., 2014) and macropods (Banazis et al., 2010; Pope et al., 1960; Potter et al., 2011; Shapiro et al., 2020). Seroprevalence rates of between 21 and 33% have been reported in kangaroos in Western Australia (WA) and Queensland (QLD) (Banazis et al., 2010; Cooper et al., 2012; Potter et al., 2011). The detection of C. burnetii DNA in macropod faeces (Banazis et al., 2010; Potter et al., 2011) and in raw meat containing kangaroo intended for pet consumption (Shapiro et al., 2020) suggests that macropods exposed to C. burnetii may become infected and subsequently amplify and shed the bacterium. Studies examining Q fever notification data have identified macropod exposure as a possible risk factor for C. burnetii infection in people with limited or no known exposure to the traditional high risk animals (Chong et al., 2003; Clutterbuck et al., 2018; Gale et al., 2007; Islam et al., 2011; Parker et al., 2010). Additionally, human Q fever cases in which patients were exposed to kangaroo and wallaby carcasses (Stevenson et al., 2015), kangaroo faeces and joeys, and worked in outdoor environments inhabited by kangaroos (Flint et al., 2016; Pickard, 2016), in the absence of exposure to traditional reservoir species such as livestock, have been reported. However, the link between Q fever and macropods remains circumstantial, and the role of macropods in C. burnetii transmission to humans remains poorly understood.
A recent serological survey investigating the link between wildlife exposure and Q fever identified Australian wildlife rehabilitators (AWR) as an at‐risk population for C. burnetii infection (Mathews, Toribio, et al., 2021) with the 6.1% C. burnetii seropositivity among the cohort being 70% greater than that reported in a study of healthy Australian blood donors (3.6%) (Gidding et al., 2019). Furthermore, 2% of the unvaccinated AWR participants self‐reported having had medically diagnosed Q fever. However, an association between direct wildlife exposure and C. burnetii seropositivity was not identified in the study, and risk factors for self‐reported Q fever were unable to be evaluated due to the limited number of medically diagnosed Q fever cases (Mathews, Toribio, et al., 2021).
This study aimed to build on the findings of Mathews, Toribio, et al. (2021) by using an online survey directed at AWR to (i) determine the prevalence of Q fever in AWR and (ii) identify the association between self‐reported medically diagnosed Q fever and risk factors for exposure to C. burnetii.
2. MATERIALS AND METHODS
2.1. Study design and recruitment
This cross‐sectional online survey targeted AWR over 18 years of age from all Australian states and territories. Study data were collected and managed using REDCap electronic data capture tools hosted at The University of Sydney (Harris et al., 2009, 2019). REDCap (Research Electronic Data Capture) is a secure, web‐based application designed to support data capture for research studies, providing: (i) an intuitive interface for validated data entry; (ii) audit trails for tracking data manipulation and export procedures; (iii) automated export procedures for seamless data downloads to common statistical packages and (iv) procedures for importing data from external sources. Wildlife rehabilitators were recruited from June through to August 2019, with survey distribution aided by the following organizations: Wildlife Health Australia, For Australian Wildlife Needing Aid (FAWNA), Western Australian Wildlife Rehabilitation Council Inc., Tasmanian Wildlife Rehabilitation Council, Wildlife Victoria, Australian Wildlife Carer's Network Inc., and New South Wales (NSW) Wildlife Council, who advertised the survey to their members via email, newsletters and postings to social media groups and on websites. Reminders were sent after approximately 12 weeks. To maximize the response rate, the opportunity to win an electronic tablet was used as an incentive to motivate participation in the survey.
2.2. Sample size calculation
An estimated prevalence of 8% of Q fever in AWR was used to calculate the sample size required for this study. This was based on the prevalence of self‐reported medically diagnosed Q fever in other Australian cohorts, including AWR (2%) (Mathews, Toribio, et al., 2021), veterinary personnel (2%) (Sellens et al., 2016) and cat breeders (6%) (Shapiro et al., 2017). The estimate was also based on the assumption that Q fever prevalence generally is underestimated, given many cases are undiagnosed or misdiagnosed (Kermode et al., 2003), and on the assumption that approximately 8% of AWR would be vaccinated (Mathews, Toribio, et al., 2021), and therefore unavailable for prevalence determination. This estimated vaccinated proportion was therefore added to the sample size calculation. Using these assumptions and a 0.1–1% prevalence of medically diagnosed Q fever in the general Australian population (National Notifiable Diseases Surveillance System, 2021), this study required a sample size of between 246 and 350 (i.e., 228 + ~18 [8%] vaccinated AWR and 324 + ~26 [8%] vaccinated AWR) to achieve a power of 80% for detecting a difference in proportions of 7–8% between exposed and unexposed groups with a two‐sided p‐value of .05 (Dhand & Khatkar, 2014).
2.3. Questionnaire design and implementation
The questionnaire (Appendix S2) was developed with reference to previous studies (Guy & Banks, 2012; Sánchez & Baker, 2016; Sellens et al., 2016; Shapiro et al., 2017), and in consultation with key stakeholders, including wildlife public health researchers, wildlife veterinarians and wildlife rehabilitators. Pretesting of the questionnaire via a pilot testing group (consisting of members of Wildlife Heath Australia, practicing AWR and one wildlife veterinarian) allowed questions to be modified for clarity. The questionnaire consisted of 12 open, 23 closed, 16 checklist, nine Likert scale and four multiple choice questions which were divided across six sections. Questions focused on (i) the rehabilitator and the geographical and physical location used to rehabilitate wildlife, (ii) the type of wildlife rehabilitated and other animals residing nearby, (iii) rehabilitation and husbandry practices, (iv) knowledge and attitudes regarding Q fever and its causative agent C. burnetii, (v) Q fever vaccination status and (vi) Q fever disease and exposure to the agent. Participants were required to answer all questions and branching logic was employed to direct them through the questionnaire. Participants accessed the questionnaire via a hyperlink distributed via email, web page or social media. A participant information statement was provided explaining the purpose and expected outcomes of the research, and consent was obtained before questionnaire commencement. The study was approved by the Human Research Ethics Committee of the University of Sydney (project number 2018/270).
2.4. Data management and analysis
Upon survey closure, the data were exported from REDCap (Harris et al., 2009, 2019) into Microsoft® Excel® (Microsoft Corporation, Washington, USA) for preliminary exploration and processing and statistical analysis was performed using R statistical program® (R Core Team, 2022).
2.4.1. Explanatory variables
Descriptive statistics, including mean (± standard error; SE), median (interquartile range; IQR) and range for continuous variables, and contingency tables for categorical variables, were generated to obtain information regarding their distribution. Continuous variables were transformed into categorical variables and where necessary, were re‐categorized based on their distribution, biological plausibility and previous studies (Mathews, Toribio, et al., 2021).
2.4.2. Outcome variable Q fever
The primary outcome variable was Q fever status. Participants were classified as having had Q fever if they had self‐reported, medically diagnosed Q fever. Participants who had not heard of Q fever before survey participation were also classified as not having had Q fever, based on the assumption that they would have remembered being medically diagnosed given the uncommon diagnosis. Similarly, participants who were unvaccinated for Q fever or were unsure of their vaccination status were classified as non‐vaccinates, given that Q fever vaccination is a multi‐step process and therefore more likely to be “memorable” (Sellens et al., 2018). Vaccinated participants were excluded from the logistic regression analysis as no vaccinated respondents self‐reported having had Q fever.
2.4.3. Univariable analysis
Univariable logistic regression was conducted to evaluate the associations between potential risk factors and the outcome variable Q fever. All potential risk factors were screened and unadjusted odds ratios were calculated. Variables significantly associated with Q fever (p < .2) were included in multivariable analyses. Highly correlated variables were identified if Cramer's V statistic was > .7. Only the variable deemed more biologically plausible was included in subsequent multivariable analysis.
2.4.4. Multivariable analysis
Multivariable logistic regression was undertaken to examine relationships between screened risk factors and the outcome variable Q fever. Biologically or practically relevant two‐way interactions between explanatory variables were evaluated. Each interaction term was added to the base model and removed if the likelihood ratio statistic was insignificant (p > .01). A backward elimination approach was used to build the final model. All relevant risk factor variables were placed in the multivariable model and evaluated for confounding. Each variable was removed sequentially (starting with the variable with the highest p‐value) and was considered to be a confounder, and therefore retained in the model, if it was significant (p < .05) or if its removal resulted in >10% change in parameter estimates of explanatory variables, irrespective of its significance level. Multicollinearity between variables in the final multivariable model was identified when the variance inflation factor (VIF) was > 5.
3. RESULTS
3.1. Response rate and descriptive analysis
In total, 405 participants accessed the questionnaire via the hyperlink and the final data set consisted of 338 (338/398; 84.9%) questionnaire responses (Figure 1). Given the total number of people who received the survey via electronic means (email lists, websites, social media and newsletters) was unknown, a true response rate could not be calculated. However, assuming a denominator of between approximately 4000–17,000 active AWR (Englefield, Candy, et al., 2019; Mathews, Toribio, et al., 2021), the estimated response rate of this study may have been as low as 2% (405/17,000) to 10% (405/4000).
FIGURE 1.
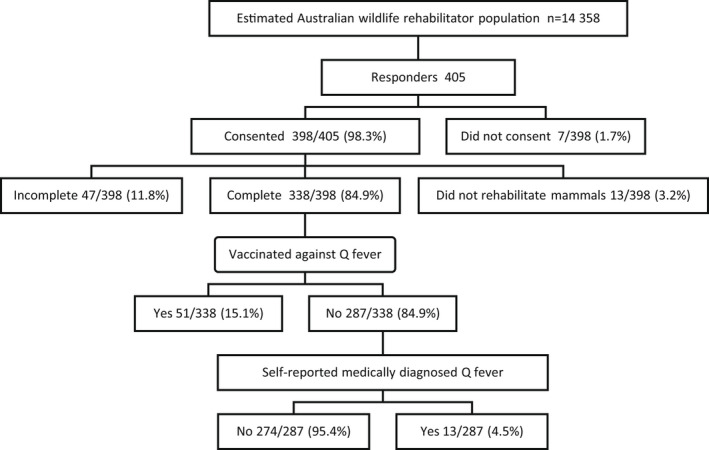
Breakdown of responses from Australian wildlife rehabilitators participating in a nationwide online survey regarding Q fever conducted in 2018. Estimation of the nationwide Australian wildlife rehabilitator population (n = 14,358) is described in Mathews, Toribio, et al. (2021)
Characteristics of the survey respondents are presented in Table 1. Participants were primarily female (282/338; 83.4%) and the median age of respondents was 52 years (19–80; IQR 42–62) with 54.7% (185/338) > 50 years of age. Although all Australian states and territories were represented, compared to the available total national population estimates, the proportion of respondents residing in NSW and Tasmania (TAS) was higher (24% and 8% respectively), the proportions in Victoria (VIC), South Australia (SA) and WA were lower (20%, 6% and 5% respectively), and the proportions within QLD, Northern Territory (NT) and Australian Capital Territory (ACT) (combined 23%) were comparable to the Australian population distribution (Australian Bureau of Statistics, 2018). The proportion of the cohort living in major cities (99/338; 29.3%) was lower, and the proportion living in inner regional Australia (167/338; 49.4%) was higher compared to the distribution in the general Australian population (70% and 18% respectively). Twenty one percent (72/338) of respondents resided in outer regional/remote areas, which is approximately double the population distribution (11%) for these remoteness categories. (National Rural Health Alliance, 2011).
TABLE 1.
Characteristics of Australian wildlife rehabilitators participating in a nationwide online survey conducted in 2018
| Variable | Category | Number | Proportion (%) |
|---|---|---|---|
| Gender | Female | 282 | 83.4 |
| Male | 51 | 15.1 | |
| Prefer not to say | 5 | 1.5 | |
| Age | > 50 | 185 | 54.7 |
| ≤ 50 | 153 | 45.3 | |
| Level of education | University/Postgraduate | 153 | 45.3 |
| High School Level/TAFE or private college | 185 | 54.7 | |
| State of residence a | NSW | 189 | 55.9 |
| Queensland | 71 | 21.0 | |
| Tasmania | 31 | 9.2 | |
| Victoria | 21 | 6.2 | |
| Western Australia | 17 | 5.0 | |
| South Australia | 3 | 0.9 | |
| Northern Territory | 5 | 1.5 | |
| Australian Capital Territory | 1 | 0.3 | |
| Remoteness classification | Major cities of Australia | 99 | 29.3 |
| Inner regional Australia | 167 | 49.4 | |
| Outer regional Australia/remote Australia/very remote Australia | 72 | 21.3 | |
| Active Rehabilitator | No | 15 | 4.4 |
| Yes | 323 | 95.6 | |
| Years rehabilitating Australian mammals | 1–10 | 182 | 53.8 |
| >10 | 156 | 46.2 | |
| Number of animals cared for per year b | 1–50 | 260 | 77.4 |
| More than 50 | 76 | 22.6 | |
| Associated with wildlife groups | No | 28 | 8.3 |
| Yes | 310 | 91.7 | |
| Primary location of rehabilitating wildlife | Wildlife rescue/rehabilitation facility closed to the public | 44 | 13.0 |
| Animal facility open to public | 14 | 4.1 | |
| Veterinary clinic | 30 | 8.9 | |
| Private residence | 296 | 87.6 | |
| Care for wildlife on own property | No | 18 | 5.3 |
| Yes | 320 | 94.7 | |
| Number of people in household | <3 | 123 | 36.4 |
| ≥3 | 95 | 28.3 | |
| Occupational animal contact | No occupational contact | 213 | 63.0 |
| Cattle sheep goats (ruminants) | 50 | 14.8 | |
| Non‐ruminant occupational contact | 75 | 35.2 | |
| Present at non‐human birth | No | 158 | 46.7 |
| Yes | 180 | 53.3 | |
| Hand reared joeys | No | 50 | 14.8 |
| Yes | 288 | 85.2 | |
| Tick bite | No | 185 | 55.1 |
| Yes | 151 | 44.9 |
As determined by Australian postal code.
Missing data n = 2.
Abbreviation: TAFE, Technical and Further Education.
Most participants (91.7%; 310/338) were associated with a wildlife group. Approximately 102 individual wildlife groups associations were reported across the cohort with the greatest number of representatives associated with NSW Wildlife Information, Rescue and Education Inc. (WIRES; 76/310; 24.5%), followed by Wildcare Australia (50/310; 16.1%), FAWNA (30/310; 9.7%), Sydney Metropolitan Wildlife Services (SMWS; 27/310; 8.7%), Wildlife Victoria (13/310; 4.2%) and Northern Rivers Wildlife Carers (NRWLC; 12/310; 3.9%). Most participants (260/336; 77.4%) rehabilitated <50 animals per year. Possums and gliders were the most commonly rehabilitated animals being cared for by 80.5% (272/338) of respondents, followed by macropods (255/338; 75.4%), monotremes (272/338; 35.5%), flying foxes and microbats/bats (111/338; 32.8%), bandicoots (93/338; 27.5%), wombats (83/338; 24.6%), koalas (69/338; 20.4%), dasyurids (e.g. quolls and antechinus) and small marsupials (41/338;12.1%), and birds and reptiles (21/338; 6.2%). Just over half (53.3%; 180/338) reported having been present at, or assisting with, a non‐human birth. Of these 46.7% (84/180) of births attended were ruminant, 48.9% (88/180) cat and dog, 16.1% (29/180) horses and 32.8% (59/180) other species including alpacas, cheetahs, giraffes and rodents.
3.2. Self‐reported Q fever diagnosis
Overall, 51 (51/338; 15.1%) participants reported having been vaccinated against Q fever and were excluded from modelling for the outcome variable Q fever, leaving 287 unvaccinated participants in this dataset. Of the 287 (287/338; 84.9%) unvaccinated participants, 13 (13/287) self‐reported having been medically diagnosed with Q fever (using laboratory testing), corresponding to a Q fever prevalence of 4.5% (95% CI 2.4% – 7.6%). A further seven (7/287; 2.4%) reported being ineligible to receive the vaccine due to a positive pre‐vaccination screening test. Self‐reported Q fever diagnosis occurred over 18 years (from 2000 to 2018), and the age at which the patients were diagnosed ranged from 20–64 years (median 52 years; IQR 12 years). Over half (8/13; 61.5%) were from NSW and most had been educated to a High School/TAFE level (11/13; 84.6%). Just under half (6/13; 46.2%) of the medically diagnosed respondents were hospitalized due to their illness, with the duration of hospitalization ranging from 2–21 days (mean 6.2 ± 3.0 days). The most frequently reported symptoms were chills (13/13; 100%), joint pain (13/13; 100%), fatigue (13/13; 100%), and sweat (12/13; 92.3%). Five participants (5/13; 38.5%) developed pneumonia, two (2/13; 23.1%) hepatitis and one (1/13; 15.4%) endocarditis. No pregnancy associated complications were reported.
Eight (8/13; 61.5%) participants self‐reporting a Q fever diagnosis reported being present at, or assisting with, a non‐human birth, of which six (6/8; 75%) were ruminant births. Of the respondents that reported handling joeys (10/13; 76.9%), nine (9/10; 90%) had handled macropod joeys and one (1/13; 7.7%) had handled possum and koala joeys. Eleven participants (11/13; 84.6%) had rehabilitated kangaroos or wallabies, and of the six (6/13; 46.2%) participants reporting occupational contact with animals, five (5/6; 83.3%) had ruminant contact and two (2/6 33.3%) had contact with kangaroos or wallabies.
3.3. Univariable analysis
Of the 27 risk factors investigated for association with Q fever among the 287 unvaccinated AWR, eight (Appendix S1) progressed to multivariable analysis and no collinearity between any variable was identified (Cramers V < 0.7).
3.4. Multivariable analysis
None of the interaction terms were significant at the 1% level and therefore were not considered in the final model. Multivariable modelling identified four variables significantly associated with Q fever (Table 2). After controlling for the other variables in the model, AWR with medically diagnosed Q fever were more likely to: primarily rehabilitate wildlife at a veterinary clinic (p < .002), rehabilitate wildlife on a property in which domestic ruminants also resided (p < .001), have secondary or Technical and Further Education (TAFE) level education rather than tertiary level education (p = .010) and be aged >50 years (p = .017). Occupational contact with ruminants was non‐significant (p = .074) but was included in the final model as it confounded the relationship between other variables. Multicollinearity was not observed between the variables in the final model.
TABLE 2.
Results of final multivariable analysis for risk factors associated with self‐reported Q fever among 287 unvaccinated Australian wildlife rehabilitators participating in a nationwide online survey conducted in 2018
| Description | β | SE (β) | Adjusted odds ratio | 95% CI | p‐value |
|---|---|---|---|---|---|
| Intercept | −7.81 | 1.41 | <.001 | ||
| Domestic ruminants living on the same property | |||||
| No | 1 | .001 | |||
| Yes | 2.46 | 0.74 | 11.75 | 2.91–57.42 | |
| Primary rehabilitated Australian wildlife at a veterinary clinic | |||||
| No | 1 | .002 | |||
| Yes | 2.88 | 0.89 | 17.87 | 3.09–110.92 | |
| Education level | |||||
| University/Postgraduate | 1 | .010 | |||
| High School Level/TAFE or private college | 2.33 | 0.91 | 10.29 | 2.13–84.03 | |
| Age | |||||
| ≤50 | 1 | .017 | |||
| >50 | 1.88 | 0.79 | 6.61 | 1.60–38.35 | |
| Occupational exposure to ruminants | |||||
| No | 1 | .074 | |||
| Yes | 1.30 | 0.73 | 3.67 | 0.85–15.53 | |
Abbreviations: CI, confidence intervals; TAFE, Technical and Further Education.
4. DISCUSSION
The 4.5% (13/287) prevalence of medically diagnosed Q fever observed in this study was higher than but similar to the 2.1% (n = 3/147) self‐reported Q fever prevalence found in a cohort of AWR attending a nationwide conference (Mathews, Toribio, et al., 2021). This 4.5% prevalence also extrapolated to approximately 4530 cases of Q fever per 100,000 in AWRs over the 18 years (2000–2018) in which AWR in this study reported having been medically diagnosed with Q fever. This number was approximately 100 fold greater than the cumulative Australian Q fever notifications over the same 18 year period (2000–2018; 43 notifications per 100,000 of population) (National Notifiable Diseases Surveillance System, 2021). Together these studies provide further evidence that AWR are at increased risk of C. burnetii infection and developing Q fever than the Australian general population. In addition, the 4.5% self‐reported Q fever prevalence in this cohort of AWR was comparable to that reported in other high‐risk groups in Australia, including unvaccinated veterinary personnel (2%) (Sellens et al., 2016), cat breeders (6%) (Shapiro et al., 2017) and goat producers (6%) (Gunther et al., 2019). Given the possibility that some AWR failed to recall having had Q fever, and that many cases of Q fever are undiagnosed (e.g., when cases do not seek medical care) or misdiagnosed due to non‐specific symptoms or lack of availability of diagnostic testing (Kermode et al., 2003), the 4.5% Q fever prevalence observed in this study may be an underestimation of the true Q fever prevalence within this cohort. Additionally, if the seven AWR who had positive pre‐vaccination screening tests, indicating prior exposure and possibly undiagnosed Q fever, were included in the numerator for calculation of prevalence (i.e., 13 + 7/287), then the prevalence of Q fever in this study may have been as high as 7%.
However, it is possible that respondents who were aware of, had experience with, or were interested in Q fever may have been more likely to respond to the survey (Tripepi et al., 2010), resulting in a potential overrepresentation of people who had experienced Q fever. Given that this low magnitude of sampling bias may be offset by the possible effect of underdiagnosis, and that around 95% of respondents in this study did not report Q fever, the Q fever prevalence determined in the current study still indicates that AWR are at increased risk of contracting Q fever. In addition, while participants were asked to self‐identify as having been medically diagnosed with Q fever, the questionnaire did not ask them to specify the diagnostic test used for diagnosis. Therefore, there was potential for some degree of measurement bias, given the variation in sensitivity and specificity of the various serological assays and the likelihood of false negative results (Fournier et al., 1998). It is reasonable to assume, however, that those with significant clinical disease were accurately represented and diagnosed in this study.
The results of this study demonstrated that AWR who self‐reported medically diagnosed Q fever were approximately 18 times more likely to have primarily rehabilitated wildlife at a veterinary clinic. This finding was probably due to factors associated with veterinary clinics which may have increased the likelihood of AWR being directly or indirectly exposed to C. burnetii. Small, large, and mixed animal veterinary clinics treat a variety of animal species known to be potential reservoirs of C. burnetii, including livestock species (Marrie, 1990) and companion animals such as cats and dogs (Kopecny et al., 2013; Shapiro et al., 2016). In addition, animals visiting veterinary clinics for reproductive and obstetric procedures, particularly those that were periparturient, may have presented a greater risk due to the organism's predilection for the products of conception. Q fever outbreaks among veterinary personnel have been associated with indirect or direct contact with birth products following dog and cat caesarean sections in small animal veterinary clinics (Gibbons & White, 2014; Kopecny et al., 2013). Furthermore, C. burnetii DNA has been detected in air and soil one year following parturition in livestock (Kersh et al., 2013); therefore, infection may be possible in people without direct exposure to infected animals or their products.
Another possible explanation for why rehabilitating wildlife in a veterinary clinic setting may have resulted in an increased risk for C. burnetii infection was the low levels of QFV in AWR. Although excluded from analysis, only 15.1% of the study cohort reported having been vaccinated, which while higher, was similar to the 8% vaccination rate reported by Mathews, Toribio, et al. (2021). These low vaccination rates are a significant concern for a population for whom vaccination is recommended by the Australian government (Australian Technical Advisory Group on Immunisation, 2021). Other Australian studies have observed similarly low vaccination rates in high‐risk groups for whom QFV is recommended, such as abattoir workers (38%) (Gidding et al., 2019), farmers (28%) (Lower et al., 2017) veterinary nurses (29%) (Sellens et al., 2016) and cat breeders (2%) (Shapiro et al., 2017). The occupation of participants in the current study was not reported; however, it is possible that some of those rehabilitating in a veterinary clinic setting were ancillary veterinary workers (e.g., veterinary nurses or reception staff). Future work could aim to determine the level of Q fever awareness and identify barriers to QFV in AWR, to help formulate strategies for enhancing vaccine uptake in this group, which may help to increase uptake in other at‐risk groups.
In this study, rehabilitators reporting having been diagnosed with Q fever were 10.29 times as likely to have reported achieving a lower level of education (high school, TAFE or private college). Although, as mentioned above, occupation was unmeasured in this study, a potential explanation for this association could be that many AWR are employed as para‐veterinary staff who, unlike those who have been enrolled in veterinary and animal science degrees (Sellens et al., 2016), are not required to be vaccinated as part of their training. These findings are supported by those of Sellens et al. (2016) and emphasize the need to better educate all veterinary clinic employees, and AWR associated with veterinary clinics, about the potential risk of exposure to C. burnetii and the importance of Q fever vaccination.
The finding that AWR self‐reporting Q fever were 11.75 times more likely to rehabilitate wildlife on a property that housed domestic ruminants was not surprising, given that contact with domestic ruminants is an important and well‐known risk factor for human C. burnetii infection (Angelakis & Raoult, 2010). Infected ruminants contaminate the environment by shedding C. burnetii in high numbers in their birth products and to a lesser extent in their milk, urine and faeces (Maurin & Raoult, 1999). C. burnetii transmission to AWR potentially occurred via inhalation of aerosolised organisms through direct contact with ruminants, and/or indirectly through contact with environments contaminated by livestock species.
Finally, the results of this analysis showed that AWR who self‐reported Q fever were more likely (OR; 6.61) to be aged >50 years at the time of the survey. Increasing age is commonly reported in Q fever notification data (Clutterbuck et al., 2018; Sloan‐Gardner et al., 2017), and is thought to be due to the cumulative increased risk of exposure over time and/or the concomitant decline in cellular immunity during the aging process (Weiskopf et al., 2009). While eleven of thirteen AWR self‐reporting Q fever were female, gender was not a risk factor for Q fever. A consistent observation across AWR study cohorts (including the current study cohort) is that most AWR are female (Englefield, Candy, et al., 2019; Haering et al., 2020; Mathews, Toribio, et al., 2021; Tribe & Brown, 2000). Q fever has traditionally been associated with males, most likely as a consequence of the occupations (e.g., abattoir workers, farmers, etc) in which men predominate and where the risk of exposure is high (Chiu et al., 2010; Sloan‐Gardner et al., 2017). However, given the results of this study, and the elevated C. burnetii seroprevalence observed in female AWR (Mathews, Toribio, et al., 2021), medical practitioners should not discount Q fever in their differential diagnosis in female AWR presenting with an acute flu‐like illness.
Consistent with a recent serosurvey in AWR where direct contact with wildlife species was not identified as a risk factor for C. burnetii seropositivity (Mathews, Toribio, et al., 2021), this study did not identify contact with kangaroos or other wildlife species as a risk factor for C. burnetii infection. While there is a body of evidence implicating macropods as a source of C. burnetii infection for humans (Banazis et al., 2010; Clutterbuck et al., 2018; Cooper et al., 2012; Flint et al., 2016; Pope et al., 1960; Potter et al., 2011; Shapiro et al., 2020; Stevenson et al., 2015), the evidence is still largely circumstantial. Additionally, the mechanism by which C. burnetii is amplified and shed into the environment by macropods or other wildlife species remains poorly understood and is an area at which future research could be directed.
Accessing the AWR population for this study proved difficult because, currently, members of the Australian wildlife and rehabilitation sector are registered with different state or territory authorities governed by different licensing arrangements (Englefield et al., 2018; Englefield, Blackman, et al., 2019; Haering et al., 2020). Also, there is no unifying national governing body through which AWR can be contacted either via email or by phone. In addition, the questionnaire used was disseminated electronically via social media platforms, which is a common, cost effective, and convenient way of managing surveys (Wright, 2017). However, the inability to know how wide this online reach was across all wildlife rehabilitation sectors rendered estimating the number of people the survey truly reached impossible. Furthermore, a low number and proportion of AWR were ineligible to (13/398; 3.2%), or could not (47/398; 11.8%), participate in the study (see Figure 1), representing a 15% loss of those AWR who did participate in the study. This loss was considered low and not a significant sampling bias. Finally, the total number of Australians involved in rehabilitating wildlife nationwide was unknown, although numbers have been estimated at between 4150 (involved in marsupial care; Englefield, Candy, et al., 2019) to 14,358 (Mathews, Toribio, et al., 2021) and 17,000 (Englefield, Candy, et al., 2019).
Although 405 survey respondents represented a low response rate, there were several reasons why this sample of AWR obtained in this study was a reasonable and broad representation of the AWR population in Australia. Firstly, the participants of this study reported being associated with a diverse range of wildlife species, and approximately 102 different individual wildlife rehabilitator groups. Secondly, the age and sex distribution among the current cohort aligned with other Australian studies on AWR (Englefield, Candy, et al., 2019; Haering et al., 2020; Mathews, Toribio, et al., 2021; Tribe & Brown, 2000). For example, Englefield, Candy, et al. (2019) found that 70% of AWR were > 46 years and 86% were female. Similarly, Haering et al. (2020) reported that more than 50% of the AWR study cohort were > 50 years and 79% were female. Finally, the number of correctly completed survey responses (n = 338) was higher than that reported in another national online survey of AWR (n = 270) investigating the mental, physical and financial challenges faced by this population (Englefield, Candy, et al., 2019). This latter study was also considered to be broadly representative of AWR in Australia.
The sample size obtained in this study (n = 338 with 287 unvaccinated AWR), while within the range required for an estimated prevalence of 8% (see sample size calculation) was lower than the sample size that would have been required for a prevalence of 4.5% (n = ~430–1118, accounting for the addition of ~15% of AWR that were vaccinated in this study), which was the actual prevalence of Q fever in the current study cohort. Although this resulted in ~5–15% decreased statistical power, reducing the chance of detecting statistically significant differences, this may have been offset by the finding that Q fever prevalence in this study could have been as high as 7% (as discussed previously). This higher prevalence, combined with the general underdiagnosis of Q fever (Kermode et al., 2003), may more accurately reflect Q fever prevalence in AWR, and therefore align well with the estimated 8% disease prevalence and the sample size range (n = 246–350) determined for this study. In addition, despite some loss of statistical power, the results of this study still demonstrated several important large and highly significant associations.
Given the limited information on the distribution of AWR across Australia at the commencement of this study (e.g., between and within States), a simple random sampling strategy was used. This strategy assumed that AWR were independent (where one survey responding AWR had no influence on the chance of another responding), and that every AWR in the population had a reasonably even chance of being included in the sample. Under these assumptions, a sample size calculation for simple random sampling was performed and multivariable logistic regression assuming no levels or hierarchy of data was deemed appropriate. However, the possibility that AWR with Q fever may have been clustered between states and within at least NSW should be noted, given that this state had the greatest number of survey respondents and the highest number of medically diagnosed Q fever cases (8/13). In addition, the geographical distribution of survey respondents according to Australian jurisdiction differed from the national general population distribution, with overrepresentation in NSW and TAS, underrepresentation in VIC, SA and WA and even representation in QLD, NT and ACT. Recent evidence has also confirmed the occurrence of spatiotemporal clustering of Q fever notifications within QLD (Proboste et al., 2022). Although determining this form of outcome clustering of AWR within the state level was beyond the sampling strategy and scope of this study, it is noteworthy that the proportion of AWR with Q fever in each state was approximately the same (e.g., NSW, 4.2%; QLD, 4.2%; VIC, 4.8%; WA, 5.9%), which may have offset some of the loss of power and precision of associations consequent to clustering of AWR with Q fever within NSW or between state levels. Future research in this at‐risk cohort of AWR should therefore carefully consider these important study design issues.
Given that wildlife are acknowledged as major reservoirs for transmitting emerging and zoonotic agents to humans and domestic animals (Kruse et al., 2004), the difficulty accessing the AWR population in a coordinated way is of concern. Rehabilitators at the forefront of the human‐wildlife interface are at increased risk of directly and/or indirectly contracting Q fever and other zoonoses (through vectors and contaminated environments) including rickettsioses (Mathews, Phalen, et al., 2021), Australian bat lyssavirus (Wildlife Health Australia, 2019), salmonellosis (Wildlife Health Australia, 2018), tularaemia (Wildlife Health Australia, 2020) and psittacosis (Wildlife Health Australia, 2017). A centralized national database operating through an organization such as Wildlife Health Australia (https://www.wildlifehealthaustralia.com.au/) may facilitate a channel of coordinated contact with the majority of AWR, providing a means to efficiently relay critical information on wildlife biosecurity, and about the risks, prevention, and management of zoonoses specific to Australia. A centralized database would also serve as a surveillance tool, which could be shared across other sectors such as human and veterinary public health, environmental protection, worksafe, and other wildlife‐livestock‐periurban interfaces and organizations, to help identify new and emerging diseases, and assist with the effective management of any new disease outbreaks.
5. CONCLUSION
The 4.5% prevalence of self‐reported medically diagnosed Q fever observed in this AWR population was consistent with the findings of a recent serosurvey in AWR (Mathews, Toribio, et al., 2021), providing further evidence to support the recommendation of Q fever vaccination for rehabilitators of Australian wildlife. Rehabilitating wildlife on a property that housed domestic ruminants and associations with veterinary clinics were risk factors for Q fever in this study (Table 2). However, associations between Q fever and direct contact with specific wildlife species including macropods were not identified. These findings suggested that AWR may be exposed to C. burnetii and develop Q fever via associations with traditionally recognized animal and environmental sources of infection such as livestock, and potentially through the environment via their wildlife rehabilitation‐associated activities (e.g., collecting feed sources such as browse and recovering or releasing animals), but not necessarily through direct contact with the wildlife themselves. However, given the established evidence that wildlife can become infected with C. burnetii, future studies should aim to determine the presence and location of C. burnetii in wildlife tissues and excretions to enable a better understanding of the infection cycle in wildlife and the risk they pose for human transmission. This study also highlighted that Q fever vaccination rates in AWR are low (at only 15%) despite their recognition as an at‐risk population and therefore future studies are needed to identify barriers to vaccination in this group. The difficulty in accessing the AWR population encountered in this study also highlights the need for a national centralized AWR database.
AUTHOR CONTRIBUTIONS
Karen O. Mathews: Conceptualisation, Writing ‐ original draft, Formal analysis, Investigation, Methodology. Cathie Savage: Conceptualisation, Writing ‐ review & editing, Methodology. Jacqueline M. Norris: Conceptualisation, Writing ‐ review & editing, Investigation, Supervision, Methodology. David Phalen Conceptualisation, Writing ‐ review & editing, Methodology, Investigation, Supervision. Nicholas Malikides: Writing ‐ review & editing, Formal analysis. Paul A. Sheehy: Conceptualisation Writing ‐ review & editing, Methodology, Supervision. Katrina L. Bosward: Conceptualisation, Funding acquisition, Investigation, Writing ‐ review & editing, Supervision, Methodology, Project administration.
FUNDING INFORMATION
We would like to offer our sincere thanks and appreciation to Dr Ramune “Rami” Cobb and Pfizer International Inc. for their support of our research into understanding Q fever.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGEMENTS
The authors would like to thank the wildlife rehabilitators who participated in this study and acknowledge those involved in the pilot testing of the questionnaire and the dissemination of the survey. Open access publishing facilitated by The University of Sydney, as part of the Wiley – The University of Sydney agreement via the Council of Australian University Librarians.
Mathews, K. O. , Savage, C. , Norris, J. M. , Phalen, D. , Malikides, N. , Sheehy, P. A. , & Bosward, K. L. (2023). Risk factors associated with self‐reported Q fever in Australian wildlife rehabilitators: Findings from an online survey. Zoonoses and Public Health, 70, 69–80. 10.1111/zph.13002
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Angelakis, E. , & Raoult, D. (2010). Q fever. Veterinary Microbiology, 140(3), 297–309. 10.1016/j.vetmic.2009.07.016 [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics . (2018). Australian Demographic Statistics, Jun 2018 . https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Jun%202018?OpenDocument
- Australian Technical Advisory Group on Immunisation . (2021). The Australian Immunisation Handbook, (March 4, 2022) . https://immunisationhandbook.health.gov.au/vaccine‐preventable‐diseases/q‐fever
- Banazis, M. J. , Bestall, A. S. , Reid, S. A. , & Fenwick, S. G. (2010). A survey of Western Australian sheep, cattle and kangaroos to determine the prevalence of Coxiella burnetii . Veterinary Microbiology, 143(2), 337–345. 10.1016/j.vetmic.2009.12.002 [DOI] [PubMed] [Google Scholar]
- Bennett, M. D. , Woolford, L. , Banazis, M. J. , O'Hara, A. J. , Warren, K. S. , Nicholls, P. K. , Sims, C. , & Fenwick, S. G. (2011). Coxiella burnetii in western barred bandicoots (Perameles bougainville) from Bernier and Dorre Islands in Western Australia. EcoHealth, 8(4), 519–524. 10.1007/s10393-011-0729-3 [DOI] [PubMed] [Google Scholar]
- Chiu, C. , Dey, A. , Wang, H. , Menzies, R. , Deeks, S. , Mahajan, D. , Macartney, K. , Brotherton, J. , Jardine, A. , Quinn, H. , Jelfs, J. , Booy, R. , Lawrence, G. , Jayasinghe, S. , Roberts‐Witteveen, A. , Senanayake, S. , Wood, N. , & McIntyre, P. (2010). Vaccine preventable diseases in Australia, 2005 to 2007. Communicable Diseases Intelligence Quarterly Report, 34 Supp, S1–S167. [DOI] [PubMed] [Google Scholar]
- Chong, A. K. H. , La Brooy, J. , Norton, R. , & Masson, J. (2003). Q fever: A recent ‘outbreak’ in Townsville. Internal Medicine Journal, 33(4), 208–210. 10.1046/j.1445-5994.2003.00335.x [DOI] [PubMed] [Google Scholar]
- Clutterbuck, H. C. , Eastwood, K. , Massey, P. D. , Hope, K. , & Mor, S. M. (2018). Surveillance system enhancements for Q fever in NSW, 2005–2015. Communicable Diseases Intelligence, 42, S2209‐6051(2218)00012‐00010. https://pubmed.ncbi.nlm.nih.gov/30626297/ [DOI] [PubMed] [Google Scholar]
- Cooper, A. , Barnes, T. , Potter, A. , Ketheesan, N. , & Govan, B. (2012). Determination of Coxiella burnetii seroprevalence in macropods in Australia. Veterinary Microbiology, 155(2), 317–323. 10.1016/j.vetmic.2011.08.023 [DOI] [PubMed] [Google Scholar]
- Derrick, E. H. (1937). “Q” fever, a new fever entity: Clinical features, diagnosis and laboratory investigation. Medical Journal of Australia, 2(8), 281–299. 10.5694/j.1326-5377.1937.tb43743.x [DOI] [PubMed] [Google Scholar]
- Dhand, N. K. , & Khatkar, M. S. (2014). Statulator: An online statistical calculator. Sample size calculator for estimating a single proportion . http://statulator.com/SampleSize/ss1P.html
- Eldin, C. , Melenotte, C. , Mediannikov, O. , Ghigo, E. , Million, M. , Edouard, S. , Mege, J.‐L. , & Raoult, D. (2017). From Q fever to Coxiella burnetii infection: A paradigm change. Clinical Microbiology Reviews, 30(1), 115–190. 10.1128/cmr.00045-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englefield, B. , Blackman, S. A. , Starling, M. , & McGreevy, P. D. (2019). A review of Australian animal welfare legislation, regulation, codes of practice, and policy, and their influence on stakeholders caring for wildlife and the animals for whom they care. Animals, 9(6), 335. 10.3390/ani9060335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englefield, B. , Candy, S. , Starling, M. , & McGreevy, P. (2019). The demography and practice of Australians caring for native wildlife and the psychological, physical and financial effects of rescue, rehabilitation and release of wildlife on the welfare of carers. Animals, 9(12), 1127. 10.3390/ani9121127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englefield, B. , Starling, M. , & McGreevy, P. (2018). A review of roadkill rescue: Who cares for the mental, physical and financial welfare of Australian wildlife carers? Wildlife Research, 45(2), 103. 10.1071/WR17099 [DOI] [Google Scholar]
- Flint, J. , Dalton, C. B. , Merritt, T. D. , Graves, S. , Ferguson, J. K. , Osbourn, M. , Eastwood, K. , & Durrheim, D. N. (2016). Q fever and contact with kangaroos in New South Wales. Communicable Diseases Intelligence, 40(2), 202–203. [DOI] [PubMed] [Google Scholar]
- Fournier, P.‐E. , Marrie, T. J. , & Raoult, D. (1998). Diagnosis of Q fever. Journal of Clinical Microbiology, 36(7), 1823–1834. 10.1128/JCM.36.7.1823-1834.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale, M. , Ketheesan, N. , Govan, B. , Kennedy, R. L. , & Norton, R. (2007). Q fever cases at a North Queensland Centre during 1994–2006. Internal Medicine Journal, 37(9), 644–646. 10.1111/j.1445-5994.2007.01441.x [DOI] [PubMed] [Google Scholar]
- Garner, M. G. , Longbottom, H. M. , Cannon, R. M. , & Plant, A. J. (1997). A review of Q fever in Australia 1991‐1994. Australian and New Zealand Journal of Public Health, 21(7), 722–773. [DOI] [PubMed] [Google Scholar]
- Gibbons, G. C. , & White, P. J. (2014). Q fever in a veterinary hospital; An unusual epidemiology . Paper presented at the Zoonoses Brisbane, Australia.
- Gidding, H. F. , Faddy, H. M. , Durrheim, D. N. , Graves, S. R. , Nguyen, C. , Hutchinson, P. , Massey, P. , & Wood, N. (2019). Seroprevalence of Q fever among metropolitan and non‐metropolitan blood donors in New South Wales and Queensland, 2014–2015. Medical Journal of Australia, 210(7), 309–315. 10.5694/mja2.13004 [DOI] [PubMed] [Google Scholar]
- Gunther, M. J. , Heller, J. , Hayes, L. , & Hernandez‐Jover, M. (2019). Dairy goat producers' understanding, knowledge and attitudes towards biosecurity and Q‐fever in Australia. Preventive Veterinary Medicine, 170, 104742. 10.1016/j.prevetmed.2019.104742 [DOI] [PubMed] [Google Scholar]
- Guy, A. J. , & Banks, P. (2012). A survey of current rehabilitation practices for native mammals in eastern Australia. Australian Mammalogy, 34, 108–118. 10.1071/AM10046 [DOI] [Google Scholar]
- Haering, R. , Wilson, V. , Zhuo, A. , & Stathis, P. (2020). Towards a more effective model of wildlife care and rehabilitation: A survey of volunteers in New South Wales, Australia. Australian Zoologist, 40, 605–627. 10.7882/AZ.2019.018 [DOI] [Google Scholar]
- Harris, P. A. , Taylor, R. , Minor, B. L. , Elliott, V. , Fernandez, M. , O'Neal, L. , McLeod, L. , Delacqua, G. , Delacqua, F. , Kirby, J. , & Duda, S. N. (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, P. A. , Taylor, R. , Thielke, R. , Payne, J. , Gonzalez, N. , & Conde, J. G. (2009). Research electronic data capture (REDCap)—A metadata‐driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker, J. I. , Ayres, J. G. , Blair, I. , Evans, M. R. , Smith, D. L. , Smith, E. G. , Burge, P. S. , Carpenter, M. J. , Caul, E. O. , Coupland, B. , Dessellberger, U. , Farrell, I. D. , Saunders, P. J. , & Wood, M. J. (1998). A large outbreak of Q fever in the west midlands: Windborne spread into a metropolitan area? Communicable Disease and Public Health, 1(3), 180–187. [PubMed] [Google Scholar]
- Hilbink, F. , Penrose, M. , Kovacova, E. , & Kazar, J. (1993). Q fever is absent from New Zealand. International Journal of Epidemiology, 22(5), 945–949. 10.1093/ije/22.5.945 [DOI] [PubMed] [Google Scholar]
- Islam, A. , Ferguson, J. , Givney, R. , & Graves, S. (2011). Seroprevalence to Coxiella burnetii among residents of the hunter New England region of New South Wales, Australia. American Journal of Tropical Medicine and Hygiene, 84(2), 318–320. 10.4269/ajtmh.2011.10-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermode, M. , Yong, K. , Hurley, S. , & Marmion, B. (2003). An economic evaluation of increased uptake in Q fever vaccination among meat and agricultural industry workers following implementation of the National Q Fever Management Program. Australian and New Zealand Journal of Public Health, 27(4), 390–398. 10.1111/j.1467-842X.2003.tb00415.x [DOI] [PubMed] [Google Scholar]
- Kersh, G. J. , Fitzpatrick, K. A. , Self, J. S. , Priestley, R. A. , Kelly, A. J. , Lash, R. R. , Marsden‐Haug, N. , Nett, R. J. , Bjork, A. , Massung, R. F. , & Anderson, A. D. (2013). Presence and persistence of Coxiella burnetii in the environments of goat farms associated with a Q fever outbreak. Applied and Environmental Microbiology, 79(5), 1697–1703. 10.1128/aem.03472-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecny, L. , Bosward, K. L. , Shapiro, A. J. , & Norris, J. M. (2013). Investigating Coxiella burnetii infection in a breeding cattery at the Centre of a Q fever outbreak. Journal of Feline Medicine and Surgery, 15(12), 1037–1045. 10.1177/1098612x13487360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse, H. , Kirkemo, A.‐M. , & Handeland, K. (2004). Wildlife as source of zoonotic infections. Emerging Infectious Diseases, 10(12), 2067–2072. 10.3201/eid1012.040707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lower, T. , Corben, P. , Massey, P. , Depczynski, J. , Brown, T. , Stanley, P. , Osbourn, M. , & Durrheim, D. (2017). Farmers' knowledge of Q fever and prevention approaches in New South Wales. Australian Journal of Rural Health, 25(5), 306–310. 10.1111/ajr.12346 [DOI] [PubMed] [Google Scholar]
- Marrie, T. J. (1990). Q fever ‐a review. The Canadian Veterinary Journal, 31(8), 555–563. [PMC free article] [PubMed] [Google Scholar]
- Marrie, T. J. , Stein, A. , Janigan, D. , & Raoult, D. (1996). Route of infection determines the clinical manifestations of acute Q fever. The Journal of Infectious Diseases, 173(2), 484–487. 10.1093/infdis/173.2.484 [DOI] [PubMed] [Google Scholar]
- Mathews, K. O. , Phalen, D. , Norris, J. M. , Stenos, J. , Toribio, J.‐A. , Wood, N. , Graves, S. R. , Sheehy, P. A. , Nguyen, C. , & Bosward, K. L. (2021). Serological evidence of exposure to spotted fever group and typhus group rickettsiae in Australian wildlife rehabilitators. Pathogens, 10(6), 745. 10.3390/pathogens10060745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews, K. O. , Toribio, J.‐A. , Norris, J. M. , Phalen, D. , Wood, N. , Graves, S. R. , Sheehy, P. A. , & Bosward, K. L. (2021). Coxiella burnetii seroprevalence and Q fever in Australian wildlife rehabilitators. One Health, 12, 100197. 10.1016/j.onehlt.2020.100197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin, M. , & Raoult, D. (1999). Q fever. Clinical Microbiology Reviews, 12(4), 518–553. 10.1128/CMR.12.4.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million, M. , & Raoult, D. (2015). Recent advances in the study of Q fever epidemiology, diagnosis and management. Journal of Infection, 71, S2–S9. 10.1016/j.jinf.2015.04.024 [DOI] [PubMed] [Google Scholar]
- National Notifiable Diseases Surveillance System . (2021). Notifications of selected diseases by state territory and year . http://www9.health.gov.au/cda/source/cda‐index.cfm
- National Rural Health Alliance . (2011). The Little book of rural health numbers . https://www.ruralhealth.org.au/book/demography
- Parker, N. , Robson, J. , & Bell, M. (2010). A serosurvey of Coxiella burnetii infection in children and young adults in south West Queensland. Australian and New Zealand Journal of Public Health, 34(1), 79–82. 10.1111/j.1753-6405.2010.00478.x [DOI] [PubMed] [Google Scholar]
- Pickard, N. (2016, 29 June). St Georges basin resident urges others to get Q fever vaccination, newspaper article (online). South Coast Register (online). https://www.southcoastregister.com.au/story/3998834/q‐fever‐brings‐st‐georges‐basin‐man‐close‐to‐death/
- Pope, J. H. , Scott, W. , & Dwyer, R. (1960). Coxiella burnetii in kangaroo and kangaroo ticks in Western Queensland. The Australian Journal of Experimental Biology and Medical Science, 38(1), 17–27. [DOI] [PubMed] [Google Scholar]
- Potter, A. S. , Banazis, M. J. , Yang, R. , Reid, S. A. , & Fenwick, S. G. (2011). Prevalence of Coxiella burnetii in Western Grey kangaroos (Macropus fuliginosus) in Western Australia. Journal of Wildlife Diseases, 47(4), 821–828. 10.7589/0090-3558-47.4.821 [DOI] [PubMed] [Google Scholar]
- Proboste, T. , Clark, N. J. , Tozer, S. , Wood, C. , Lambert, S. B. , & Soares Magalhães, R. J. (2022). Profiling risk factors for household and community spatiotemporal clusters of Q fever notifications in Queensland between 2002 and 2017. Pathogens, 11(8), 830. 10.3390/pathogens11080830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing; https://www.R‐project.org/ [Google Scholar]
- Sánchez, C. A. , & Baker, M. L. (2016). Disease risk perception and safety practices: A survey of Australian flying fox rehabilitators. PLoS Neglected Tropical Diseases, 10(2), e0004411. 10.1371/journal.pntd.0004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellens, E. , Norris, J. , Dhand, N. K. , Heller, J. , Hayes, L. , Gidding, H. F. , Willaby, H. , Wood, N. , & Bosward, K. (2018). Willingness of veterinarians in Australia to recommend Q fever vaccination in veterinary personnel: Implications for workplace health and safety compliance. PLoS One, 13(6), e0198421. 10.1371/journal.pone.0198421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellens, E. , Norris, J. M. , Dhand, N. K. , Heller, J. , Hayes, L. , Gidding, H. F. , Willaby, H. , Wood, N. , & Bosward, K. L. (2016). Q fever knowledge, attitudes and vaccination status of Australia's veterinary workforce in 2014. PLoS One, 11(1), e0146819. 10.1371/journal.pone.0146819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, A. J. , Bosward, K. L. , Mathews, K. O. , Vincent, G. , Stenos, J. , Tadepalli, M. , & Norris, J. M. (2020). Molecular detection of Coxiella burnetii in raw meat intended for pet consumption. Zoonoses and Public Health, 67(4), 443–452. 10.1111/zph.12707 [DOI] [PubMed] [Google Scholar]
- Shapiro, A. J. , Norris, J. M. , Bosward, K. L. , & Heller, J. (2017). Q fever (Coxiella burnetii) knowledge and attitudes of Australian cat breeders and their husbandry practices. Zoonoses and Public Health, 64(4), 252–261. 10.1111/zph.12305 [DOI] [PubMed] [Google Scholar]
- Shapiro, A. J. , Norris, J. M. , Heller, J. , Brown, G. , Malik, R. , & Bosward, K. L. (2016). Seroprevalence of Coxiella burnetii in Australian dogs. Zoonoses and Public Health, 63(6), 458–466. 10.1111/zph.12250 [DOI] [PubMed] [Google Scholar]
- Sloan‐Gardner, T. S. , Massey, P. D. , Hutchinson, P. , Knope, K. , & Fearnley, E. (2017). Trends and risk factors for human Q fever in Australia, 1991–2014. Epidemiology and Infection, 145(4), 787–795. 10.1017/S0950268816002843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, S. , Gowardman, J. , Tozer, S. , & Woods, M. (2015). Life‐threatening Q fever infection following exposure to kangaroos and wallabies. BMJ Case Reports, 2015. 10.1136/bcr-2015-210808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozer, S. , Lambert, S. , Strong, C. , Field, H. , Sloots, T. , & Nissen, M. (2014). Potential animal and environmental sources of Q fever infection for humans in Queensland. Zoonoses and Public Health, 61(2), 105–112. 10.1111/zph.12051 [DOI] [PubMed] [Google Scholar]
- Tribe, A. , & Brown, P. R. (2000). The role of wildlife rescue groups in the care and rehabilitation of Australian fauna. Human Dimensions of Wildlife, 5(2), 69–85. 10.1080/10871200009359180 [DOI] [Google Scholar]
- Tripepi, G. , Jager, K. J. , Dekker, F. W. , & Zoccali, C. (2010). Selection bias and information bias in clinical research. Nephron. Clinical Practice, 115(2), c94–c99. 10.1159/000312871 [DOI] [PubMed] [Google Scholar]
- Weiskopf, D. , Weinberger, B. , & Grubeck‐Loebenstein, B. (2009). The aging of the immune system. Transplant International, 22(11), 1041–1050. 10.1111/j.1432-2277.2009.00927.x [DOI] [PubMed] [Google Scholar]
- Wildlife Health Australia . (2017). Chlamydia in Australian wild birds – Fact sheet . https://www.wildlifehealthaustralia.com.au/Portals/0/Documents/FactSheets/Avian/Chlamydia%20in%20Australian%20Wild%20Birds.pdf
- Wildlife Health Australia . (2018). Salmonella infection in Australian reptiles fact sheet . https://wildlifehealthaustralia.com.au/Portals/0/Documents/FactSheets/Reptiles/Salmonella_infection_in_Australian_Reptiles.pdf
- Wildlife Health Australia . (2019). Australian bat lyssavirus factsheet . https://wildlifehealthaustralia.com.au/Portals/0/Documents/FactSheets/mammals/Australian_Bat_Lyssavirus.pdf
- Wildlife Health Australia . (2020). Tullaraemia and Australian wildlife‐fact sheet . https://wildlifehealthaustralia.com.au/Portals/0/Documents/FactSheets/Public%20health/Tularaemia_and_Australian_Wildlife.pdf
- Wright, K. B. (2017). Researching internet‐based populations: Advantages and disadvantages of online survey research, online questionnaire authoring software packages, and web survey services. Journal of Computer‐Mediated Communication, 10(3), JCMC1034. 10.1111/j.1083-6101.2005.tb00259.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


