Abstract
Introduction
The IMPACT study established the role of controlled esophageal cooling in preventing esophageal thermal injury during radiofrequency (RF) ablation for atrial fibrillation (AF). The effect of esophageal cooling on ablation lesion delivery and procedural and patient outcomes had not been previously studied. The objective was to determine the effect of esophageal cooling on the formation of RF lesions, the ability to achieve procedural endpoints, and clinical outcomes.
Methods
Participants in the IMPACT trial underwent AF ablation guided by Ablation Index (30 W at 350–400 AI posteriorly, 40 W at ≥450 AI anteriorly). A blinded 1:1 randomization assigned patients to the use of the ensoETM® device to keep esophageal temperature at 4°C during ablation or standard practice using a single‐sensor temperature probe. Ablation parameters and clinical outcomes were analyzed.
Results
Procedural data from 188 patients were analyzed. Procedure and fluoroscopy times were similar, and all pulmonary veins were isolated. First‐pass pulmonary vein isolation and reconnection at the end of the waiting period were similar in both randomized groups (51/64 vs. 51/68; p = 0.54 and 5/64 vs. 7/68; p = 0.76, respectively). Posterior wall isolation was also similar: 24/33 versus 27/38; p = 0.88. Ablation effect on tissue, measured in impedance drop, was no different between the two randomized groups: 8.6Ω (IQR: 6–11.8) versus 8.76Ω (IQR: 6–12.2; p = 0.25). Arrhythmia recurrence was similar after 12 months (21.1% vs. 24.1%; 95% CI: 0.38–1.84; HR: 0.83; p = 0.66).
Conclusions
Esophageal cooling has been shown to be effective in reducing ablation‐related thermal injury during RF ablation. This protection does not compromise standard procedural endpoints or clinical success at 12 months.
Keywords: Ablation Index, AF ablation, efficacy, efficiency, esophageal cooling
1. INTRODUCTION
Recent randomized data on controlled esophageal cooling suggests that it can prevent esophageal thermal injury, 1 an important contributor to serious complications of ablation for atrial fibrillation (AF). 2 Esophageal cooling during RF left atrial ablation involves application of thermal energy at both ends of the spectrum, in the esophageal luminal and left atrial endocardial walls, which are in close proximity to one another and may be at juxtaposed sites anatomically. The impact of esophageal cooling on ablation lesion formation is unknown.
Ablation Index (AI) was introduced in 2017 as an improved method to help standardize radiofrequency (RF) energy deliveries to ensure the creation of durable ablation lesions with irrigated, contact‐force sensing catheters. 3 , 4 , 5 AI is a nonlinear weighted formula which incorporates time, contact force, and power to provide the operator with a real‐time measure of ablation lesion formation. The AI system collects data about the characteristics of each RF delivery and the response to that delivery in electrical impedance, where a 5–10Ω drop has been viewed to be a marker of successful lesion formation. 6 , 7 AI has been validated in several studies and has been shown to improve both acute and long‐term outcomes of catheter ablation in AF. 8 , 9 , 10
IMPACT was a randomized study that evaluated the ability of an esophageal temperature control device (ensoETM®, Attune Medical) to reduce thermal injury during AI‐guided AF ablation. 11 The device is a double‐lumen silicone tube; it permits closed‐loop water irrigation 2.4 L/min at a temperature as cold as 4°C, creating a large capacity to extract heat from the vicinity. 12 , 13 The IMPACT study endoscopy results showed significant evidence of protection, with a relative reduction of thermal injury of 83.4%. 11
In this further study of the IMPACT study cohort, we sought to determine if esophageal cooling affected the ability to achieve acute ablation procedural endpoints and clinical success.
2. METHODS
2.1. Trial design
The IMPACT study was an investigator‐initiated single‐center, prospective double‐blind randomized trial of esophageal temperature control in adult patients undergoing AF ablation using AI technology (ClinicalTrials.gov NCT03819946). 11 The study was approved by the London‐Stanmore Research Ethics Committee (IRAS ID 253844, NIHR CPMS ID 40619).
2.2. Study purpose
The primary and secondary endpoints of the IMPACT study have been outlined. 11 In this further analysis, we sought to determine the effect of esophageal cooling on the characteristics of RF lesions associated with esophageal injury.
2.3. Study population
All adult patients attending for RF ablation for AF under general anesthesia by participating electrophysiologists were screened for study eligibility during pre‐assessment. Both first‐time and redo AF ablations were included in the study. Table 1 illustrates the proportion of first‐time and redo patients in each randomized group, with no significant differences between them. Indications for catheter ablation for AF were in keeping with international guidelines. Exclusion criteria were: Age <18 or >88 years; inability to consent for any reason and contra‐indication for upper gastrointestinal endoscopy for any reason.
Table 1.
Patient and procedure characteristics of all participants recruited to the IMPACT study
| Protected (n = 89) | Control (n = 99) | p‐value | |
|---|---|---|---|
| Patient characteristics | |||
| Male | 52 (58%) | 63 (63%) | 0.55 |
| Age (year) | 65.1 ± 10.4 | 65.2 ± 10.3 | 0.95 |
| Body mass index (kg/m2) | 28.7 ± 5.8 | 29.8 ± 6.6 | 0.23 |
| Prior cerebrovascular accident | 1 (1%) | 6 (6%) | 0.2 |
| Renal dysfunction | 5 (6%) | 3 (3%) | 0.48 |
| Diabetes mellitus | 16 (18%) | 6 (6%) | 0.01 |
| LA diameter (cm) | 4.5 ± 3.8 | 4.2 ± 0.6 | 0.44 |
| Left ventricular ejection fraction (%) | 51.4 ± 8.4 | 52.3 ± 7.9 | 0.45 |
| Direct oral anticoagulant | 78 (88%) | 79 (79%) | 0.17 |
| Antiarrhythmic drug therapy | 31 (35%) | 28 (28%) | 0.35 |
| Endoscopy performed | 60 (67%) | 60 (60%) | 0.33 |
| Arrhythmia characteristics | |||
| Paroxysmal AF, first‐time ablation | 35 (39%) | 41 (41%) | 0.88 |
| Persistent AF, first‐time ablation | 29 (33%) | 27 (27%) | 0.52 |
| Repeat LA ablation | 27 (30%) | 29 (29%) | >0.9 |
| Procedure characteristics | |||
| PVI without other LA lesions | 44 (49%) | 46 (46%) | 0.77 |
| PVI and roof without posterior line | 1 (1%) | 5 (5%) | 0.21 |
| Posterior wall isolation attempted | 30 (34%) | 37 (37%) | 0.65 |
| PVI + mitral line | 5 (6%) | 4 (4%) | 0.74 |
| PVI + focal ablation | 3 (3%) | 2 (2%) | 0.67 |
Note: Values are mean ± standard deviation or n (%). Patient and procedure characteristics.
Abbreviations: AF, atrial fibrillation; LA, left atrial; PVI, pulmonary vein isolation.
Enrolled participants were randomized 1:1 (via electronic randomization www.sealedenvelope.com) to either receive thermal protection with the ensoETM® device or standard care consisting of the use of a single sensor temperature probe during ablation. Participants were blinded to the treatment assignment.
2.4. Definitions
Technical success was defined as demonstrable isolation of all pulmonary veins (PVs). This was assessed by standard methods; we determined entrance and exit block, using multipolar mapping catheters either the Lasso or the Pentaray (Biosense Webster). The achievement of enduring first‐pass isolation of the veins was a desirable outcome, defined as isolation that occurred on completion or before completion of the encircling lesion set for each pair of veins and endured for at least 20 min including adenosine provocation when performed. Adenosine provocation testing was not a substitute for the full waiting period but was permitted to be performed in this protocol during the waiting period. This option of either a full waiting period or a waiting period and adenosine testing was in line with standard ablation practice at this center. Proven block across all other lines created was also a desired endpoint. The duration of the procedure and duration of fluoroscopy were documented, as well as ablation delivery parameters including total RF ablation time, power, force, force‐time integral, and AI. All the lesion‐related data stored in the Carto® system (Biosense Webster) were exported into Microsoft Excel for analysis (Figure 1).
Figure 1.
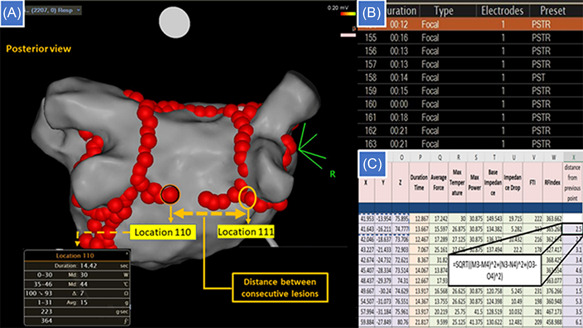
Collection and processing of Ablation Index data. (A) The posterior line is nearing completion. The operator has moved from point 110 at the left side of the line to a point abutting the right pulmonary vein lesion set, giving a distance of 25 mm between consecutive lesions, although adjacent lesions are <6 mm apart. (B) Data are stored in the CARTO system for each lesion, including a timestamp for the start and end of each delivery and three‐dimensional coordinates for its position. (C) Exported data are analyzed, including calculation of the interval between lesions from the time‐stamps, and calculation of the distance between consecutive points by trigonometry.
Clinical success was also measured as freedom from atrial arrhythmia at >3 months after ablation. Atrial arrhythmia recurrence was defined as a record of AF or related atrial tachycardia of >30 s from standard cardiac monitoring devices available at standard care such as 24‐h ECG, implanted loop recorders, and so on. A major adverse cardiovascular or cerebrovascular event (MACCE) was defined as in‐hospital death from any cause, acute myocardial infarction, or acute ischemic stroke and was screened for acutely up to 12‐month follow‐up.
2.5. Method of RF ablation
All procedures were performed under general anesthesia. Patients continued anticoagulation as per local practice at the center. Ultrasound‐guided vascular access was available for all operators. Transseptal punctures were guided by fluoroscopy and transesophageal echocardiography. Ablation was performed using an irrigated, contact force‐sensing catheter (Thermocool SmartTouch Surround Flow or Qdot Micro, Biosense Webster) with a 3D mapping system (Carto® version 6 or 7, Biosense Webster).
All ablation lesions were guided by AI with those in the anterior part of the left atrium created at 40 W with an AI target of ≥450; posterior lesions were at 30 W with an AI target of 350–400. Anterior and posterior segmentation was in keeping with previously recognized definitions. 3 Point‐by‐point ablation was performed with interlesion distance <6 mm. The Visitag SurpointTM was standardized at the recommended settings: minimum force of 5 g, force overtime of 25%, and lesion tag size of 3 mm. Respiratory adjustment was enabled.
2.6. Ablation strategy
Pulmonary vein isolation (PVI) was completed in all patients. At the operator's discretion, additional linear and/or focal ablation lesion sets could be delivered in patients with persistent AF. In cases where posterior wall isolation was attempted, the approach was the same for all participating operators: A roofline and a low posterior wall line were created to construct a “box” lesion set, with the posterior line at the level of the inferior margin of the inferior veins. When possible, operators waited for 20 min after the last RF delivery, then retested isolation of the PVs with a bolus injection of adenosine 15 mg for each side.
2.7. Protected group: Utilizing the ensoETM® device
After transseptal puncture, the transesophageal echocardiography probe was withdrawn and an ensoETM® probe was introduced in its place by the attending anesthetist and connected to a mobile console (Blanketrol III, Gentherm Medical). Before commencing ablation on the posterior part of the left atrium, the probe was set to cooling mode at 4°C for at least 10 min. Cooling continued until posterior wall ablation was complete. Body temperature was recorded throughout with a temperature probe placed in the axilla or nasopharynx.
2.8. Control group
A single‐sensor temperature probe (Level 1® Esophageal Temperature Probe, Smiths Medical) was placed in the esophagus by the attending anesthetist and adjusted approximately to the site of ablation. This followed standard practice for AF ablations at this center. Adjustment of the position of the probe during ablation was performed by the anesthetist at the direction of the electrophysiologist to keep it close to the site of ablation. RF deliveries were interrupted if the local temperature in the esophagus rose above 38°C, and ablation recommenced only once esophageal temperatures fell back to less than 37°C.
2.9. Postprocedural management
All participants were prescribed proton pump inhibitors, postprocedure for a duration of 6–8 weeks (Lansoprazole 30 mg od or an alternative to an equivalent dose).
2.10. Endoscopy
Patients in both groups were invited to attend for esophageal endoscopy at 7 days after ablation by one of two senior endoscopists. The patient and the endoscopist were blinded to the treatment assignment of the patient and following a standardized protocol. The site of any physical or thermal injury to the esophagus was documented, and any abnormality of gastric or esophageal motility. These results have already been reported. 11
2.11. Focus on ablation lesion data
Ablation data were analyzed from all IMPACT study participants, including those who did not have endoscopy performed. Ablation data was extracted from the Carto workstations, which included all the lesions applied in all of the cases included in this study. The additional information yielded includes RF time (sec), force (g), power (W), base impedance (ohms), impedance drop (ohms), FTI, and AI achieved. Due to X, Y, and Z coordinates for each lesion, consecutive interlesion distance could be measured. The maximum ablation catheter temperature tip (degrees) was also recorded per lesion.
2.12. Statistical analysis
Categorical data were compared using the χ² test, post hoc test was used to detect the difference between individual groups. Student's t‐test was used to compare normally distributed data and Mann–Whitney U‐test to compare nonparametric data. Spearman rank correlation coefficient (ρ) was used to measure the ordinal association between nonparametric variables. Kaplan Meier curve was used to measure the fraction of patients with AF recurrence. Analysis was performed with IBM SPSS statistical software (Version 26.0, IBM SPSS Statistics).
3. RESULTS—AI IN IMPACT
One Hundred eighty‐eight patients were recruited from February 2019 to January 2020 and all underwent successful catheter ablation (Supporting Information: Figure 1). Because 36% of participants were unable or unwilling to return for endoscopy after ablation, recruitment was expanded to obtain the 120 with endoscopy required by our study design. Procedure characteristics and outcomes were recorded for all 188 patients; AI data were analyzed for 181 participants, with seven cases (four in the protected group, three control) not retrieved due to file corruption with missing data. A total of 22 829 RF lesions were analyzed, 11 876 in the anterior left atrium, 8422 in the posterior aspect of the PVs, 1399 in the posterior left atrial wall line, 982 in the cavotricuspid isthmus, and 150 in the coronary sinus.
3.1. Patient characteristics
Demographic data of the study groups were well‐matched (Table 1). The mean age in the randomized protected and control groups was 65.1 and 65.2 years respectively and 58% and 63% were males.
3.2. Procedure characteristics
PVI was achieved in all cases. The rates of success in creating block in the left atrial roof, the mitral isthmus, and the cavotricuspid isthmus were similar, and in those in whom isolation of the left atrial posterior wall was attempted, the rate of its success was similar in both groups (Table 2). The total procedure, fluoroscopy, and RF time were similar in both randomized groups (p > 0.05; Table 2). The most common ablation strategies were PVI alone, or PVI combined with isolation of the left atrial posterior wall. 14
Table 2.
Lesion sets, lesion characteristics, and acute clinical effects
| Acute procedural parameters and clinical outcomes | Protected (n = 89) | Control (n = 99) | p‐value |
|---|---|---|---|
| Fluoroscopy duration (min) | 10.4 ± 6.5 | 12.2 ± 8.8 | 0.12 |
| Procedure duration (min) | 181.8 ± 44.7 | 188.9 ± 51 | 0.31 |
| Total RF time (s) | 2066 ± 1062 | 2315 ± 1053 | 0.20 |
| Impedance drop (Ω) | 8.6 (6–11.8) | 8.76 (6–12.2) | 0.25 |
| Measured axillary body temperature <35° | 0 | 0 | |
| Maximum ablation catheter tip temperature (degrees) | 25.5 (24–27.4) | 25.5 (24.1–27.1) | 0.003a |
| Achievement of isolation of all veins | 100% | 100% | |
| First‐pass pulmonary vein isolation achieved (first‐time cases only) | 51/64 (80%) | 51/68 (75%) | 0.54 |
| Reconnection with waiting or adenosine (first‐time cases) | 5/64 (8%) | 7/68 (10%) | 0.76 |
| Enduring first‐pass isolation (first‐time cases only) | 46/64 (72%) | 44/68 (65%) | 0.38 |
| Posterior wall isolation achieved (when attempted) | 24/33 (73%) | 27/38 (71%) | 0.88 |
| Mitral line block achieved (when attempted) | 17/20 (85%) | 21/24 (88%) | 0.81 |
| Roofline block achieved (when performed without posterior line) | 2/2 (100%) | 5/5 (100%) | 1 |
| Cavotricuspid isthmus block achieved (when attempted) | 32/32 (100%) | 39/39 (100%) | 1 |
| Acute complications | 1 | 4 | 0.37 |
| MACCE—within 3 months | 0 | 0 | |
| MACCE—within 6 months | 1 | 0 | 0.46 |
| Arrhythmia recurrence within 6 months | 7 (7.9%) | 9 (9.1%) | 0.80 |
| Re‐ablation since trial‐related procedure | 0 | 1 | >0.9 |
Note: Values are mean ± standard deviation, n (%), or median (interquartile range).
Abbreviations: MACCE, major adverse cardiovascular or cerebrovascular events; PV, pulmonary vein; RF, radiofrequency.
Common language effect size U1/(n1n2) is small (=0.51). Observed standard effect size, Z/√(n1 + n2) is small (=0.019).
In all but six cases, the Thermocool SmartTouch Surround Flow (STSF) ablation catheter was used. The Qdot Micro was used in 4 protected and 2 control group patients, accounting for 2.8% of the ablation lesions analyzed. The QDot Micro was used in QMODE only, in all cases with the same AI technology and guidance as for STSF, therefore this data was included in the AI analysis, as both the practical use and the data extraction and analysis were the same.
3.3. Enduring first‐pass isolation
The achievement of first‐pass isolation and the recurrence of conduction during the waiting period or in response to adenosine was similar in both groups (51/64 vs. 51/68; p = 0.54 and 5/64 vs. 7/68; p = 0.76). The waiting period of at least 20 min was respected in all cases, with additional adenosine provocation used in this period recorded in 34 cases (18.1%). The adenosine test was not used as a substitute for the waiting period. Points that required additional ablation after completion of the first pass isolation and at points of reconnection were all located at the carina or posterior sites (Figure 2). Additional ablation was performed at these sites, giving re‐isolation in all cases.
Figure 2.
Sites of pulmonary vein reconnection. There were 13 reconnections in 11 patients, with one patient in the control group having 2 points of reconnection, one in each circumferential lesion set. Reconnections were all either posterior or carinal.
Within each of the randomized groups, there were lower median AI values in cases where first‐pass isolation was achieved (control group: 437.3 [IQR: 370.6–493.2] vs. 465.3 [IQR: 383.7–505.8], p < 0.001; protected group: 436.7 [IQR: 371.2–485.6] vs. 439.3 [IQR: 373.4–498.1], p < 0.001) but greater impedance drop (control group: 9.1 [IQR: 6.1–12.7] vs. 8.7 [IQR: 6–11.9], p < 0.001; protected group: 8.8 [IQR: 6.2–11.8] vs. 8.2 [IQR: 6.1–11.2], p = 0.007). Total RF duration was shorter in first‐pass isolation cases (Supporting Information: Table 1).
3.4. Lesion parameters and stability
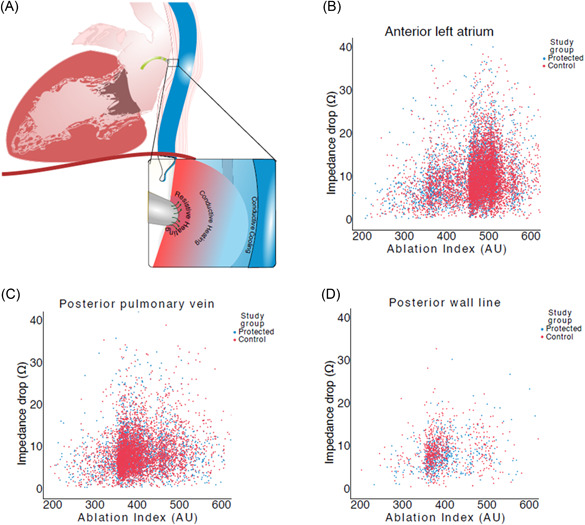
The overall values for impedance drop for all ablation points were similar in the protected group versus the control group (p = 0.25, Table 2, Figure 3).
Figure 3.
Scatter plots showing the relationship between AI and impedance drop in the protected and control groups in each location. (A) In the anterior left atrium, the AI values cluster just above the target of 450 for that region. (B) For the posterior PV encirclement lesions, the clustering of AI values is just above 350. (C) In the posterior wall line, the AI values are similar to the posterior PV set. AI, Ablation Index; PV, pulmonary vein.
RF lesions placed in the posterior PV region were more often in the intended range in the protected group (1801/3603, 50%) than in the control group (2137/4819, 44.3%, p < 0.001). A similar proportion of RF lesions in both groups were in the intended AI target range in the anterior left atrium and in the low posterior wall line (Supporting Information: Table 2a–d).
Lower than intended AI lesions were used as a surrogate for stability. There was similar number of AI points <350 at the low posterior wall line in both randomized groups (p = 0.20). There were significantly more AI lesions <350 at the posterior PVs in the control group (16.9% vs. 14.7%, p = 0.006).
3.5. Impedance drop
AI was found to have a highly significant but weak correlation with impedance drop that was similar in the protected group and the control group (ρ = 0.16–0.25, p < 0.0001; Figure 4).
Figure 4.
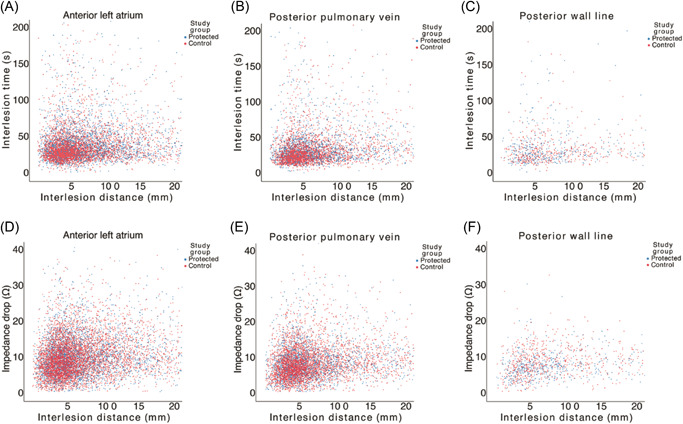
Scatter plots showing the relationships between interlesion distance and interlesion time (A–C), and interlesion distance and impedance drop (D–F) in the protected and control groups in each location.
3.6. Lesion‐to‐lesion progression
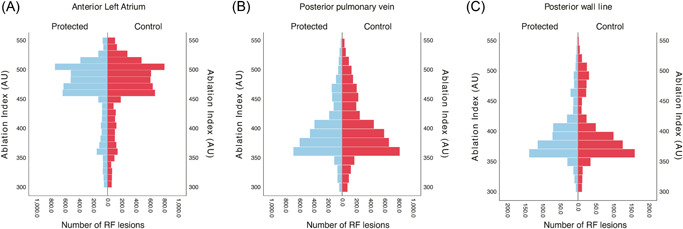
There was a closer spatial relationship between consecutive lesions (not necessarily adjacent lesions) in the protected group in the posterior part of the PV encircling lesion set but with no statistically significant difference (5.4 [IQR = 3.6–9] mm vs. 5.5 [IQR = 3.7–9.3] mm, p = 0.051). There was also no difference between the randomized groups at the anterior left atrium (p = 0.85) or in the posterior wall line in the same analysis of lesion‐to‐lesion progression (Figure 5).
Figure 5.
Population pyramid frequency plot of the Ablation Index values in the protected and control groups. RF, radiofrequency.
Interlesion distance was found to have a highly significant but weak correlation to interlesion time in both randomized groups (ρ = 0.14–0.25; p < 0.0001). A similar correlation was found between interlesion distance and impedance drop in the protected group and in the control group (ρ = 0.15–0.28, p < 0.0001).
3.7. Esophageal injury
There was no case of esophageal perforation or atrioesophageal fistula. Endoscopic findings have been reported 9 : Mucosal injury was less common in the protected patients than in the control group (2/60 vs. 12/60; p = 0.008) with a trend toward lower incidence of gastroparesis in the protected group (2/60 vs. 6/60; p = 0.27).
The occurrence of mucosal injury was not associated with difference in RF duration, maximum power, or impedance drop (p = 0.08–0.69, Supporting Information: Table 3). Patients who developed mucosal injury had higher contact force (17.8 g [IQR: 10.9–26.4] vs. 15.9 g [IQR: 10.5–24.4], p < 0.001) with lower baseline impedance (121.8Ω [IQR: 111.9–132.2] vs. 125.8Ω [IQR: 116.8–137.1], p < 0.001); although overall, there was a greater percentage of ablation points with AI > 500 in cases with thermal injury (24.8% vs. 20%; p < 0.001), only a small proportion of these AI > 500 values were located at posterior sites (32.3%). In those who sustained gastroparesis injury only, there was no difference in the proportion of AI points >500 (19.6% vs. 20%, p = 0.8, Supporting Information: Table 4).
In cases in which thermal injury occurred, there was no significant difference in the number of ablation lesions with AI values >500 (p = 0.46), nor in the proportion of these deployed at the posterior left atrial wall. The impedance drop observed was similar in both groups (impedance drop = 8.7Ω [IQR: 6–12] vs. 8.8Ω [IQR: 6.2–12], p = 0.63).
3.8. Complications
In the protected group there was one acute complication: A pericardial effusion that was conservatively managed without sequelae but was associated with a hospital stay of two nights. In the control group, there were two incidences of vascular access‐related trauma requiring thrombin injection, one of pulmonary edema and one of type 2 respiratory failure requiring additional overnight hospital stay: all recovered well after the acute event. There was 1 MACCE recorded between 3 and 6 months in the protected group that was unrelated to the procedure: Death from sepsis of unknown origin on a background of chronic heart failure; thorough investigation at the time ruled out any other differential diagnoses and atrioesophageal fistula was excluded.
3.9. 12‐month arrhythmia recurrence
The study patients had outpatient arrhythmia recurrence measured by standard of care methods, which involved intermittent, noninvasive monitoring in the form of a Holter for at least 24 h. Only those that had pre‐existing implanted devices such as pacemakers or loop recorders had these devices interrogated to screen for arrhythmia recurrence. Short‐term (>3–6 months) arrhythmia recurrence in the IMPACT study was interrupted by the covid‐19 pandemic in that telephone clinics replaced face‐to‐face clinic appointments but with severe delay in objective assessment of arrhythmia recurrence. Therefore, short‐term results were not calculated. By March 2021, 12‐month outcomes were reliably measured, using these described methods in a standard care setting, either at the same hospital or in linked community hospitals. Kaplan Meier curve was used to measure the fraction of patients with AF recurrence: 12‐month outcomes for arrhythmia recurrence showed no difference between both randomized groups. 21.1% versus 24.1%; 95% CI: 0.38–1.84; HR: 0.83; p = 0.66 (Figure 6).
Figure 6.
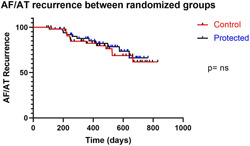
Kaplan–Meier graph of AF or atrial arrhythmia recurrence between the two randomized groups, showing no difference (21.1% vs. 24.1%; 95% CI: 0.38–1.84; HR: 0.83; p = 0.66). AF, atrial fibrillation.
4. DISCUSSION
4.1. Main findings
The IMPACT study was the first double‐blind randomized controlled trial to identify an effective method for protecting the esophagus from thermal injury during ablation. 11 In this current study, we demonstrate that this effect was achieved without any evident disruption of our ability to create individual RF lesions with similar procedural endpoints reached and no discernible difference in AF recurrence rates at 12‐month analysis. The study results, therefore, support the impression that controlled active cooling of the esophagus did not lead to a paradoxical effect on RF delivery and its effect on myocardial tissue.
4.2. RF ablation guided by AI
Like previous studies, 3 , 4 , 5 , 9 our data show that AI methodology produces a high rate of enduring first‐pass isolation of all PVs. As in previous studies, most points that require additional ablation after completing the first pass were located posteriorly, 9 mostly at the carina (Figure 2). Our data shows that esophageal cooling does not make this difficult area more challenging to ablate.
4.3. Energy delivered and lesion formed
Impedance drop is a surrogate marker of lesion depth, well known from impedance‐guided RF ablation. 15 , 16 With effective application of RF energy, thermal energy disrupts the integrity of local tissue with irreversible cellular dysfunction and necrosis, which lowers the local electrical impedance. While AI integrates the characteristics that go into an effective RF delivery, impedance drop measures its outcome, providing a cross‐check of effectiveness. Our data confirm this with an association between interlesion distance and impedance drop that is independent of AI or its components (Table 2, Figures 4 and 5).
Similar AI values occurred in both study and control groups and the impedance drop achieved in response to that RF delivery was similar. The fact that esophageal protection does not reduce the impedance drop produced by a lesion with a given AI value is strong evidence that lesion formation is not impeded (Figure 5).
4.4. Catheter tip temperature
Catheter tip temperature sensing and its measurements during a procedure are recorded in the ablation data set under “maximum catheter tip temperature.” The median of this temperature parameter did not differ between the two randomized groups in the IMPACT study ablations. This result amongst the rest of the similar ablation lesion application parameters is supportive of the fact that esophageal temperature lowering did not extend significantly to the left atrial tissue myocardium to negatively attenuate or weaken the RF applications. Although further temperature information at the catheter tip level would have been more useful, this provided reassurance alongside other procedural evidence. The biophysics of RF ablations is well described in literature. 17 Tissue in direct contact with the RF catheter undergoes resistive or ohmic heating, whereas there is conductive heating of deeper tissue. Direct resistive heating only affects a shallow layer of tissue as the current dissipates and reduces at a square of the distance from the electrode. Limitation of passive heating of the deeper tissue occurs naturally from surrounding blood vessels; the circulating blood flow creating a convective cooling effect. 17 , 18 This explains why the posterior mitral isthmus line is a challenging area to ablate, as where it is usually drawn, it is close to the coronary sinus, which is often described as a ‘heat sink’. In order for irreversible tissue injury to occur, tissue temperatures need to reach around 50°. Catheter tip temperature is influenced by several factors including degree of convective cooling and direct tissue contact. This understanding of the biophysics of RF energy delivery enables us to draw conclusions that the ensoETM, when used in a protocol like the IMPACT study, did not create a significant convective cooling effect at the site of left atrial myocardial tissue. This is evidenced by the ease of first‐pass isolation and similar rates of PV reconnection requiring additional ablation compared to controls. In addition, RF lesion duration was not significantly prolonged, which takes false or impeded catheter temperature recording out of the equation, as this would have significantly increased the number of steam pops, clot, and char formation and therefore higher incidences of stroke or cardiac tamponade, which was not observed in this study.
4.5. Enduring first‐pass isolation
The achievement of first‐pass isolation and the persistence of that isolation to the end of the waiting period and through adenosine challenge predict greater long‐term success. 19 From this endpoint, it appears that esophageal protection has not hindered the success of ablation.
4.6. Lesion‐to‐lesion progress
The data on the distance between consecutive lesions suggest that the presence of esophageal protection permitted the operators to operate more comfortably in constructing lesion sets in the posterior left atrium. They appear to show a steady progression from one site to the next, adhering in most cases to the desired 4–6 mm between consecutive lesions compared to the control group in which juddering progress was evident with more instances of movement across a distance of more than 15 mm within the posterior region. We interpret this as evidence of hesitancy arising from the occurrence of temperature rises in the esophagus, or from fear of their occurrence. This may be purposeful, as the so‐called “skip” strategy is used by some operators to avoid the heat stacking phenomenon. Juddering movement from one lesion to the next may also reflect intracardiac or intra‐thoracic movement which leads to catheter tip instability. The impact of contact force variation, spatial movement per lesion application as well as the sequential lesion placement on arrhythmia recurrence were explored in the study by Jankelson et al. 20 Here, the study implies that nonsequential ablation lesions were associated with increased risk of arrhythmia recurrence. This was thought to be due to catheter instability leading to contact force variation and spatial movement of the catheter causing shorter ablation applications and therefore leading to nonsequential lesion placement as a result. It is not clear if purposeful nonsequential lesion applications would produce a similar effect. However, this implies that if some of the ablations with longer interlesion distance were not due to purposeful “skip” lesions, this must be related to catheter instability issues, leading to increased risk of AF recurrence. In the clinical follow‐up of the IMPACT study group, both randomized groups had similar levels of AF recurrence, which validates the similar findings on procedural parameters.
4.7. Physical effects of the ensoETM
There was a physical effect of the ensoETM probe on the construction of lesions across the posterior wall. The device made an appreciable physical indentation in the left atrium (Supporting Information: Video 1).
The physical presence of an indentation in the left atrium cannot be ignored. The authors' impression was that it may actually have enhanced catheter stability in the posterior component of each of the PV encirclement lesion sets: These lines run vertically, typically lying just to either side of the esophagus, so probe‐related indentation may have kept the line straight and the catheter stable. This impression was supported by the fact that significantly fewer ablation points were below the intended AI target range in the protected group, which was an indicator or surrogate of ablation catheter stability.
The indentation produced by the esoETM may have made the posterior line more difficult. It is hard to balance a catheter tip on the apex of a curve that indents the chamber. This effect would account for the apparent slight facilitation of PV isolation in the protected group but an absence of this effect in posterior wall isolation with similar ablation points not reaching the intended AI value. But reassuringly, similar rates of enduring posterior wall isolation achieved suggest that any physical effect at this level was not clinically significant.
4.8. Clinical results versus mathematical modeling
This study provides real‐life confirmation of effects predicted by a mathematical model of heat transfer at the interface between the heart and the esophagus. 21 The model used average values for the thermal and electrical properties for left atrial myocardium, esophagus, and pericardium and a variety of different values for ablation settings, set temperature of the device, and tissue thickness. It predicted that esophageal cooling, even with the device set at body temperature (37°C) would reduce esophageal thermal injury and that cooling to 5° would not impact on temperature or lesion formation in the myocardium during RF application for 20 s at 10–50 W.
4.9. Secondary endpoints: Procedure metrics and complications
Overall procedural workflow was not compromised by esophageal cooling as demonstrated by the similar procedural time and fluoroscopy duration in both groups (Table 2). Acute complication rates were similarly low in both groups, as were MACCE up to 12 months postprocedure.
4.10. Long‐term follow‐up
Introduction of new technology or changes to procedural workflow as part of research necessitates scrutiny on long‐term outcomes in addition to monitoring of the procedural and acute parameters as outlined so far. 12‐month follow‐up from the IMPACT study patients show that there was no difference in arrhythmia recurrence between the two randomized groups. The method in which AF recurrence was measured was using noninvasive, intermittent Holter monitoring as per standard care. The similar rate of recurrence measured at 12 months reassuringly supports the hypothesis that controlled esophageal cooling for esophageal protection is not at the expense of the efficacy of the RF ablation procedure when analyzed by lesion application, procedure workflow, and its clinical or therapeutic effect on patients.
4.11. Limitations
This is a single‐center study and although further experience with this device and technique in esophageal protection has since been reported, 22 , 23 a multicenter study is required to further investigate and confirm these findings. A multicenter randomized study is currently being set up to build on this work.
Both the follow‐up methods and the frequency of Holter monitoring in both randomized groups fell in line with standard care. Although this was favorable from a logistical perspective, this was also a limitation, as the evidence becomes clear that continuous ECG monitoring improves the objective measurement of AF recurrence and so true recurrence for both randomized groups may be higher. Short‐term recurrence rates (>3–6 months) are not presented as this was limited by the first wave of the covid‐19 pandemic, where ECG diagnostics were severely rationalized as part of the pandemic protocol.
5. CONCLUSION
Esophageal cooling has been shown to be effective in reducing ablation‐related thermal injury during RF ablation. This protection does not compromise standard procedural endpoints or clinical success at 12 months.
ETHICS STATEMENT
London‐Stanmore Research Ethics Committee (IRAS ID 253844, NIHR CPMS ID 40619). Patient consent was obtained as part of study NCT03819946.
IMPACT STUDY GROUP
Gurpreet Dhillon MRCP, Banu Evranos MD, Hanney Gonna MRCP, Idris Harding MD, Nawaf Al‐Subaie PhD, John Louis‐Auguste MD, Jamal Hayat MD.
Supporting information
Supplementary Figure: Flowchart of patients randomized to the IMPACT study, whose Ablation Index data were then studied.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supplementary Video: Use of the EnsoETM device during atrial fibrillation ablation. (Voiceover by Dr Mark M Gallagher).
ACKNOWLEDGMENTS
All authors confirm their contribution to this manuscript (data collection, analysis, writing, and editing). Dr. Leung's research fellowship was funded by the charitable organization “Cardiac Risk in the Young” (CRY). Additional funding support was provided by Attune Medical (Chicago, IL).
Leung LWM, Akhtar Z, Elbatran AI, et al. Effect of esophageal cooling on ablation lesion formation in the left atrium: insights from Ablation Index data in the IMPACT trial and clinical outcomes. J Cardiovasc Electrophysiol. 2022;33:2546‐2557. 10.1111/jce.15717
Disclosure: Dr. Leung has received research support from Attune Medical. Dr. Gallagher has received research funding from Attune Medical and has acted as a consultant and a paid speaker for Boston Scientific and Cook Medical. Anthony Li: Other—Travel and accommodation support (modest): Abbott. Abhay Bajpai: Honoraria (modest) Bayer. Other—Educational and Travel Grant (modest): Biosense Webster, Other—Educational Grant (modest): Medtronic. Speaker/Speaker's Bureau (modest) Diachii Sankyo, Pfizer/BMS.
DATA AVAILABILITY STATEMENT
Data availability was available upon request to corresponding author.
REFERENCES
- 1. Kapur S, Barbhaiya C, Deneke T, Michaud GF. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation. 2017;136:1247‐1255. [DOI] [PubMed] [Google Scholar]
- 2. Leung LW, Gallagher MM, Santangeli P, et al. Esophageal cooling for protection during left atrial ablation: a systematic review and meta‐analysis. J Interv Card Electrophysiol. 2019;59:347‐355. 10.1007/s10840-019-00661-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hussein A, Das M, Riva S, et al. Use of ablation index‐guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients. The PRAISE study results. Circ Arrhythm Electrophysiol. 2018;11:e006576. [DOI] [PubMed] [Google Scholar]
- 4. Solimene F, Lepillier A, Ruvo E, et al. Reproducibility of acute pulmonary vein isolation guided by the ablation index. Pacing Clin Electrophysiol. 2019;42:874‐881. [DOI] [PubMed] [Google Scholar]
- 5. Das M, Loveday JJ, Wynn GJ, et al. Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. Europace. 2017;19:775‐783. [DOI] [PubMed] [Google Scholar]
- 6. Strickberger SA, Ravi S, Daoud E, Niebauer M, Man KC, Morady F. Relation between impedance and temperature during radiofrequency ablation of accessory pathways. Am Heart J. 1995;130(5):1026‐1030. [DOI] [PubMed] [Google Scholar]
- 7. Reithmann C, Remp T, Hoffmann E, Matis T, Wakili R, Steinbeck G. Different patterns of the fall of impedance as the result of heating during ostial pulmonary vein ablation: implications for power titration. Pacing Clin Electrophysiol. 2005;28(12):1282‐1291. [DOI] [PubMed] [Google Scholar]
- 8. Phlips T, Taghji P, El Haddad M, et al. Improving procedural and one‐year outcome after contact force‐guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’‐protocol. Europace. 2018;20:f419‐f427. [DOI] [PubMed] [Google Scholar]
- 9. Dhillon G, Ahsan S, Honarbakhsh S, et al. A multicentered evaluation of ablation at higher power guided by ablation index: establishing ablation targets for pulmonary vein isolation. J Cardiovasc Electrophysiol. 2019;30:357‐365. [DOI] [PubMed] [Google Scholar]
- 10. Stabile G, Lepillier A, De Ruvo E, et al. Reproducibility of pulmonary vein isolation guided by the ablation index: one year outcome of the AIR registry. J Cardiovasc Electrophysiol. 2020;31:1‐8. [DOI] [PubMed] [Google Scholar]
- 11. Leung LWM, Bajpai A, Zuberi Z, et al. Randomized comparison of oesophageal protection with a temperature control device—results of the IMPACT study. Europace. 2021;23(2):205‐215. 10.1093/europace/euaa276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hegazy AF, Lapierre DM, Butler R, Martin J, Althenayan E. The esophageal cooling device: a new temperature control tool in the intensivist's arsenal. Heart & Lung. 2017;46:143‐148. [DOI] [PubMed] [Google Scholar]
- 13. Bhatti F, Naiman M, Tsarev A, Kulstad E. Esophageal temperature management in patients suffering from traumatic brain injury. Ther Hypothermia Temp Manag. 2019;9:238‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elbatran AI, Gallagher MM, Li A, et al. Isolating the entire pulmonary venous component versus isolating the pulmonary veins for persistent atrial fibrillation: a propensity‐matched analysis. Pacing Clin Electrophysiol. 2020;43:68‐77. [DOI] [PubMed] [Google Scholar]
- 15. Ullah W, Hunter RJ, Finlay MC, et al. Ablation index and surround flow catheter irrigation. JACC Clin Electrophysiol. 2017;3:1080‐1088. [DOI] [PubMed] [Google Scholar]
- 16. Martin CA, Martin R, Gajendragadkar PR, et al. First clinical use of novel ablation catheter incorporating local impedance data. J Cardiovasc Electrophysiol. 2018;29:1197‐1206. [DOI] [PubMed] [Google Scholar]
- 17. Nath S, DiMarco JP, Haines DE. Basic aspects of radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 1994;5(10):863‐876. [DOI] [PubMed] [Google Scholar]
- 18. Houmsse M, Daoud EG. Biophysics and clinical utility of irrigated‐tip radiofrequency catheter ablation. Expert Rev Med Devices. 2012;9(1):59‐70. [DOI] [PubMed] [Google Scholar]
- 19. Pranata R, Vania R, Huang I. Ablation‐index guided versus conventional contact‐force guided ablation in pulmonary vein isolation—systematic review and meta‐analysis. Indian Pacing Electrophysiol J. 2019;19(4):155‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jankelson L, Dai M, Aizer A, et al. Lesion sequence and catheter spatial stability affect lesion quality markers in atrial fibrillation ablation. JACC Clin Electrophysiol. 2021;7(3):367‐377. [DOI] [PubMed] [Google Scholar]
- 21. Mercado M, Leung L, Gallagher M, Shah S, Kulstad E. Modeling esophageal protection from radiofrequency ablation via a cooling device: an analysis of the effects of ablation power and heart wall dimensions. Biomed Eng Online. 2020;19(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clark B, Alvi N, Hanks J, Suprenant B. A pilot study of an esophageal cooling device during radiofrequency ablation for atrial fibrillation. medRxiv. 2020. 10.1101/2020.01.27.20019026 [DOI] [PMC free article] [PubMed]
- 23. Tschabrunn CM, Attalla S, Salas J, et al. Active esophageal cooling for the prevention of thermal injury during atrial fibrillation ablation: a randomized controlled pilot study. J Interv Card Electrophysiol. 2021;63:197‐205. 10.1007/s10840-021-00960-w [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure: Flowchart of patients randomized to the IMPACT study, whose Ablation Index data were then studied.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supplementary Video: Use of the EnsoETM device during atrial fibrillation ablation. (Voiceover by Dr Mark M Gallagher).
Data Availability Statement
Data availability was available upon request to corresponding author.


