Abstract
Crimean–Congo haemorrhagic fever (CCHF) is an emerging tick‐borne disease caused by the arbovirus Crimean–Congo haemorrhagic fever virus (CCHFV; family Nairoviridae). Given the public health impact, CCHF is considered a priority disease for the European Union. This study describes the first detection of anti‐CCHFV antibodies in transhumant bovines in Italy. Sera from 794 cattle collected across Basilicata region (Southern Italy) were screened using a commercial ELISA kit. The animal‐level and herd‐level seroprevalences detected were 1.89% [95%CI: 1.12–3.1] and 29.63% [95%CI: 15.68–48.65], respectively. Results of the χ 2 test for trend show that the exposure to CCHFV was significantly associated with increasing age, with the odds 5 times higher in 11–22‐year old cattle than 1–4‐year old cattle. The detection of antibodies against CCHFV in indigenous cattle indicates that the infection occurred in the study area and may warrant further consideration. Additionally, no significant spatial clustering of CCHF infection was detected, supporting the hypothesis that the disease is widespread in the region. Further studies at larger scale are needed to identify the areas at higher risk of zoonotic infection. A One Health approach should be implemented to better understand the disease risk and dynamics in the country, which effectively address the related public health threat.
Keywords: CCHFV, Crimean‐Congo haemorrhagic fever, Italy, Tick‐borne diseases
1. INTRODUCTION
Crimean–Congo haemorrhagic fever (CCHF) is an emerging tick‐borne disease caused by the arbovirus Crimean–Congo haemorrhagic fever virus (CCHFV; family Nairoviridae). The main vector and reservoir of CCHFV is the genus Hyalomma, which is widely distributed across Africa, Southern and Eastern Europe, the Middle East, India and Asia. The virus has been found in other ixodid ticks, despite the lack of a clear evidence of their potential to transmit the virus (Hawman & Feldmann, 2018). In Europe, the most important CCHFV vector is deemed to be Hyalomma marginatum, which is found in Mediterranean‐type landscapes (EFSA Panel on Animal and Welfare [AHAW], 2010), even though H. lusitanicum plays a major role in the Iberian Peninsula (Portillo et al., 2021). The main routes of disease transmission in humans include infected tick bites or exposure to crushed tick tissues and contact with infected animal blood or tissues. Clinical signs of the disease in humans are fever, chills, severe headache, dizziness, photophobia, and back and abdominal pains. In severe cases, haemorrhagic manifestations develop generally 3–6 days after the onset of disease. These non‐specific prodromal symptoms make it difficult to clinically distinguish CCHFV infection from other causes of febrile illness and viral haemorrhagic fevers (Whitehouse, 2004). The case fatality rate ranges from 10% to 40% (WHO, 2020), and there is currently no approved vaccine or specific anti‐viral therapy (Keshtkar‐Jahromi et al., 2011). In contrast to humans, livestock and wild animals do not develop clinical signs and may serve as amplifying hosts both of CCHFV (Hawman & Feldmann, 2018) and ticks (Ruiz‐Fons et al., 2013). Serosurveillance of animals is a valuable tool to provide initial evidence of the virus circulation into new areas (Spengler et al., 2016). Avian species appear to be refractory to CCHFV infection, even though they are able to carry infected ticks, playing an important role in the introduction of the virus into new areas (Whitehouse, 2004).
During the past decades, an increasing trend of CCHF cases have been observed in humans (Nasirian, 2020), along with an expansion of the disease into new areas (Spengler et al., 2019). Accordingly, a significant increase in the number of countries reporting information on the disease in animals has been recorded (Fanelli et al., 2022). Given the wide distribution of its vector, the numerous animals that can serve as hosts, and the suitable climate and ecologic conditions in several European countries, CCHF has been identified as a priority disease for the EU (Maltezou et al., 2010).
A recent risk assessment evaluated the risk of CCHFV introduction and spread in CCHF‐free countries in Southern and Western Europe concluding that the likelihood of occurrence is low for all the EU free‐countries, with the exception of France, Germany and Italy where it is estimated to be medium. In particular, the risk of introduction through infected vectors attached to ground feeding migratory birds flying from endemic areas is higher in Italy than other countries. Additionally, Italy is the only CCHF‐free country with a widely distributed H. marginatum–resident population. Thus, the establishment and spread of the virus is likely to occur once introduced (Fanelli & Buonavoglia, 2021).
Considering the previously mentioned issues, this study aims to assess the circulation of CCHFV in Italy, targeting an at‐risk livestock population.
2. MATERIALS AND METHODS
A serological survey was conducted on livestock in Basilicata region (Southern Italy). This region is characterized by large forested areas, a Mediterranean climate, high biodiversity and low anthropic pressure (Cillis et al., 2021). All these features create an ideal environment for ticks to thrive (Rinaldi et al., 2014). The target population is represented by transhumant bovines, which are raised using a traditional and sustainable system involving the seasonal movement of herds between summer and winter pastures. These animals are kept outdoors all the year round, and they have been found heavily infested by ticks (Figure 1), with H. marginatum, the most common species (Fanelli unpublished data). Data on the study population was retrieved from the National Database (BDN) for livestock registration, defining a population of 12,081 heads raised in 132 herds (average herd size = 91). To substantiate freedom from CCHF, the minimum sample size required was calculated according to Cameron and Baldock (1998). The parameters used were as follows: a design prevalence (minimal prevalence that is to be expected if the disease is present in the population) of 1%, a significance level α = 0.05, 98.9% sensitivity and 100% specificity of the ELISA employed (ID Screen CCHF Double Antigen Multi‐species, IDvet, Grabels, France) (Sas et al., 2018). Given these assumptions, the minimal sample size was estimated to be 299. A simple random sampling was applied to select the sera among those of cattle aged over 1 year collected during the official brucellosis control activities (Spring 2021), which consists in a comprehensive testing of all the herds in the region twice a year, and all adult animals (≥12 months) within the herd. ELISA‐positive sera were sent to the Virology Laboratory of the National Reference Centre for the Study of Exotic Diseases (The Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise ‘Giuseppe Caporale’ [IZSAM]), which confirmed the results repeating the ELISA test (ID Screen CCHF Double Antigen Multi‐species, IDvet, Grabels, France) (Sas et al., 2018). Epidemiological characteristics, including animal‐level and herd‐level seroprevalences, were computed along with their 95% confidence intervals using the method recommended by Agresti and Coull (1998). The association between CCHFV exposure and sex was tested using the χ 2 test. The variable age was transformed into a categorical variable using four equally spaced quantile groups, and the χ 2 test for trend was implemented to test for a linear trend over the ordered categories (1–4 years old, 5–8 years old, 9–11 years old and 12–22 years old). Statistical calculations were performed in Epi Info 7.0 (Dean et al., 2011). An odds ratio (OR) was used to measure the association of risk factors with CCHFV exposure. Eventually, the D function (difference in estimated K functions for a set of cases and controls) with a Monte Carlo test (simulated random labellings = 999) was computed to investigate the spatial pattern of the positive and negative herds (Diggle & Chetwynd, 1991). Spatial analysis was done in R software version 4.1.1 (R Core Team, 2021).
FIGURE 1.
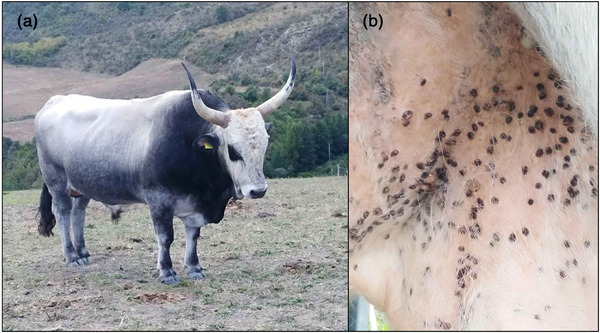
Podolian cattle (traditional cattle breed in Southern Italy) (a) highly infested by hard ticks in the inguinal region (b)
3. RESULTS
A total of 794 bovines (female = 766, male = 28) from 27 herds were screened for CCHFV antibodies. The number of animals tested per herd ranged from 21 to 30 (on average 29 animals per herd). The sample size was roughly three times larger than the one required to demonstrate a seroprevalence of 1%. The sample was composed by different breeds, namely Charolais (0.38%), Chianina (3.78%), Limousine (4.41%), Marchigiana, (4.41%) Maremmana (0.38%), Mixed breed (30.35%), Podolian (53.53%), Italian Red Pied (0.38%), Romagnola (1%) and Simmental (1.38%). The average age of the animals sampled was 8 years old (sd = 4). The animal‐level and herd‐level seroprevalences were 1.89% (95%CI: 1.12–3.1) and 29.63 (95%CI: 15.68–48.65), respectively. The within‐herd prevalence ranged from 0.03% to 13.33%. Out of 15 positive animals, 6 were home‐born animals (born in the tested herds). The remaining positive animals were purchased by farmers from herds in the same study area. No significant difference was found between males and females (χ 2 = 0, p‐value = 1). Conversely, a statistically significant higher odds of exposure was identified with increasing age (OR = 5 [12–22 years olds], OR = 1.23 [9–11 years old], OR = 1.26 [5–8 years old], OR = 1 [1–4 years old], χ 2 test for trend = 6.07, p‐value = .01). Figure 2 shows the spatial distribution of the positive and negative herds. The test for clustering using the test statistic D gave a p‐value of 0.86, indicating the spatial randomness of CCHFV exposure across the region (Figure S1).
FIGURE 2.

The geographical locations of Crimean‐Congo haemorrhagic fever virus (CCHFV) seropositive (red dots) and seronegative herds (blue dots) in Basilicata region (Southern Italy). Dot size is proportional to the within‐herd prevalence.
4. DISCUSSION
This study reports for the first time the presence of anti‐CCHFV antibodies in transhumant bovines in Italy. To the best of our knowledge, only one previous study investigated the circulation of the CCHFV in the country, in coastal provinces of Central Italy, by a serosurvey conducted on ovine animals, all testing negative (De Liberato et al., 2018). Nevertheless, the authors confirmed the introduction of CCHFV potential vectors transported by migratory birds arriving from endemic areas, highlighting the need of a continuous serological monitoring of susceptible domestic animals for an early detection of CCHFV circulation (De Liberato et al., 2018). Similarly, another study detected the virus in a nymph collected from an African migrating bird arrived in Ventotene Island (Central Italy) (Mancuso et al., 2019).
In this study, the serosurvey was carried out on a target population considered at high risk of infection, and in a sample area at high risk of CCHFV occurrence, which increased the probability of detecting positive animals. Indeed, the Basilicata region is characterized by a Mediterranean climate favourable to the biology and ecology of ticks, with a risk for Hyalomma spp. infestation higher in cattle than in small ruminants (Rinaldi et al., 2014). The ELISA used is characterized by a high sensitivity and specificity, and it is more reliable than a neutralization test considering the weak neutralising antibody response to this virus (Arteaga et al., 2020). The purpose of this report was to demonstrate the exposure of livestock to the virus rather than estimate the true seroprevalence. Therefore, the low animal‐level seroprevalence detected may not reflect the strength of CCHFV circulation in the transhumant bovine population. However, this result may suggest either a relatively recent introduction of the virus, as higher seroprevalences have been reported in cattle in endemic countries (Spengler et al., 2016), or that the virus has not found yet an appropriate environment to become prevalent. Indeed Italy, similar to Spain, is one of the main migratory routes of birds between Africa and Asia, and it is closer to the Balkans than Spain, where genotypes V and VII are enzootic (Fanelli & Buonavoglia, 2021). Therefore, the hypothesis that the virus has been introduced a long time ago cannot be ruled out. It is essential to consider that no human cases have been officially reported in Italy so far. This can be due to the fact that human and cattle infection by CCHFV are difficult to compare (Papa et al., 2016), with the latter considered the most sensitive indicator of low‐level CCHFV circulation (Spengler et al., 2016).
Results of the χ 2 test for trend show that the exposure to CCHFV was significantly associated with increasing age, with the odds five times higher in 11–22‐year‐old cattle than 1–4‐year‐old cattle. This mirrors what it has been observed in other recent studies, suggesting a cumulative exposure of older cattle raised outdoors to tick infestation (Blanco‐Penedo et al., 2021; Zouaghi et al., 2021). Additionally, CCHFV IgG antibodies persist for a long time in both humans and animals (Papa et al., 2016; Spengler et al., 2016), supporting the association between seropositivity and age. This positive association may support the hypothesis that the virus introduction has not been very recent, because it appears that older animals have had more opportunity for exposure than younger animals. A very recent exposure would likely result in a equal seroprevalence in all age categories.
With regard to the positive animals, the detection of antibodies against CCHFV in indigenous cattle warrants further consideration as it indicates that the infection occurred in the study area. Moreover, the lack of spatial clustering suggests that the disease is randomly distributed in the region. This finding, along with the herd‐level seroprevalence detected, provides evidence that CCHFV may circulate across the whole region. It is worth mentioning that, given the small scale of the study area and the low variability among the herd sites, we did not assess the environmental and climatic features associated with the risk of infection. In the context of animal disease surveillance, the application of spatial modelling has increasingly been employed to identify areas at high risk of pathogen circulation, providing valuable insights on the understanding of diseases epidemiology (Fanelli & Tizzani, 2020; Fanelli et al., 2020). Specifically, hotspot areas for CCHFV infection in humans have been detected in several countries, using spatial techniques, and incorporating abiotic and biotic risk factors which affect the tick density (Cuadrado‐Matías et al., 2021; Estrada‐Peña et al., 2008; Papa et al., 2013). Considering this, further studies on a larger scale are needed to identify the areas at higher risk of human infection. Ideally, a One Health approach considering humans, animals, ticks and environmental data should be implemented to better understand the disease risk and dynamics in the country and effectively address the related public health threat.
In conclusion, results of this study highlight that livestock may be successfully used as sentinels for CCHFV circulation in new areas. Given the public health impact of CCHF, it is crucial to perform serological surveys on animals in the areas considered at higher risk of occurrence. The serosurveillance implemented in this study was conducted on samples of cattle raised outdoors and aged over 1 year. The strategy of concentrating efforts on a target population considered at high risk of infection has been found to be a valid method to detect CCHFV circulation in a non‐endemic area. Based on our findings, at‐risk human groups, including farmers, veterinarians and abattoir workers, should be educated on the risk posed by CCHFV. This study also paves the way for further investigations aimed to detect CCHFV genome in indigenous Hyalomma spp., which would provide direct evidence of the virus circulation in Italy.
AUTHOR CONTRIBUTIONS
Angela Fanelli investigated the study, contributed to methodology, curated the data, involved in formal analysis and wrote – original draft preparation. Domenico Buonavoglia supervised the study, contributed to methodology and wrote – review and editing. Gianvito Lanave, Federica Monaco, Vincenzo Quaranta, Roberta Catanzariti and Francisco Ruiz‐Fons wrote – review and editing and validated the study. Canio Buonavoglia conceptualized and supervised the study, contributed to methodology and wrote – review and editing.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICS STATEMENT
All applicable international, national and institutional guidelines for the care and use of animals were followed. Ethics approval was obtained from the Animal Ethics Committee of the department of Veterinary Medicine, University of Bari (Italy).
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank Dr. Paola Serio from ASM Basilicata, Dr. Michele Garaguso from A.R.A. Basilicata, Maria Gabriella Piccirillo, Rocco Masiello, Vito Montagnaro and Giovanni Uricchio from Istituto Zooprofilattico Sperimentale della Puglia e della Basilicata for their assistance during the samples collection.
Open Access Funding provided by Universita degli Studi di Bari Aldo Moro within the CRUI‐CARE Agreement.
[Correction added on 25 November 2022, after first online publication: CRUI‐CARE funding statement has been added.]
Fanelli, A. , Buonavoglia, D. , Lanave, G. , Monaco, F. , Quaranta, V. , Catanzariti, R. , Ruiz‐Fons, F. , & Buonavoglia, C. (2022). First serological evidence of Crimean–Congo haemorrhagic fever virus in transhumant bovines in Italy. Transboundary and Emerging Diseases, 69, 4022–4027. 10.1111/tbed.14710
Contributor Information
Angela Fanelli, Email: angela.fanelli@uniba.it.
Gianvito Lanave, Email: gianvito.lanave@uniba.it.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- Agresti, A. , & Coull, B. A. (1998). Approximate is better than “exact” for interval estimation of binomial proportions. The American Statistician, 52, 119–126. [Google Scholar]
- Arteaga, L. M. , Bellido, J. L. M. , Lista, M. C. V. , Santiago, M. V. B. , Soto, P. F. , Bas, I. , Leralta, N. , de Ory Manchon, F. , Negredo, A. I. , Sanchez Seco, M. P. , Sardon, M. A. , Perez Gonzalez, S. , del Bianco, A. J. , Blanco Peris, L. , Alamo‐Sanz, R. , Hewson, R. , Belhassen‐Garcia, M. , & Muro, A. (2020). Crimean‐Congo haemorrhagic fever (CCHF) virus‐specific antibody detection in blood donors, Castile‐León, Spain, summer 2017 and 2018. Eurosurveillance, 25, 1900507. 10.2807/1560-7917.ES.2020.25.10.1900507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco‐Penedo, I. , Obanda, V. , Kingori, E. , Agwanda, B. , Ahlm, C. , & Lwande, O. W. (2021). Seroepidemiology of Crimean‐Congo hemorrhagic fever virus (CCHFV) in cattle across three livestock pastoral regions in Kenya. Dairy, 2, 425–434. 10.3390/dairy2030034 [DOI] [Google Scholar]
- Cameron, A. R. , & Baldock, F. C. (1998). A new probability formula for surveys to substantiate freedom from disease. Preventive Veterinary Medicine, 34, 1–17. [DOI] [PubMed] [Google Scholar]
- Cillis, G. , Statuto, D. , & Picuno, P. (2021). Historical GIS as a tool for monitoring, preserving and planning forest landscape: A case study in a Mediterranean region. Land (Basel), 10, 851. 10.3390/land10080851 [DOI] [Google Scholar]
- Cuadrado‐Matías, R. , Cardoso, B. , Sas, M. A. , García‐Bocanegra, I. , Schuster, I. , González‐Barrio, D. , Reiche, S. , Mertens, M. , Cano‐Terriza, D. , Casades‐Martí, L. , Jiménez‐Ruiz, S. , Martínez‐Guijosa, J. , Fierro, Y. , Gómez‐Guillamón, F. , Gortázar, C. , Acevedo, P. , Groschup, M. H. , & Ruiz‐Fons, F. (2021). Red deer reveal spatial risks of Crimean‐Congo haemorrhagic fever virus infection. Transboundary and Emerging Diseases, 69, e630–e645. 10.1111/tbed.14385 [DOI] [PubMed] [Google Scholar]
- De Liberato, C. , Frontoso, R. , Magliano, A. , Montemaggiori, A. , Autorino, G. L. , Sala, M. , Bosworth, A. , & Scicluna, M. T. (2018). Monitoring for the possible introduction of Crimean‐Congo haemorrhagic fever virus in Italy based on tick sampling on migratory birds and serological survey of sheep flocks. Preventive Veterinary Medicine, 149, 47–52. 10.1016/j.prevetmed.2017.10.014 [DOI] [PubMed] [Google Scholar]
- Dean, A. , Arner, T. , Sunki, G. , Friedman, R. , Lantinga, M. , Sangam, S. , Zubieta, J. , Sullivan, K. , Brendel, K. , Gao, Z. , Fontaine, N. , Shu, M. , Fuller, G. , Smith, D. , Nitschke, D. , & Fagan, R. (2011). Epi InfoTM, a database and statistics program for public health professionals. CDC. [Google Scholar]
- Diggle, P. J. , & Chetwynd, A. G. (1991). Second‐order analysis of spatial clustering for inhomogeneous populations. Biometrics, 47, 1155–1163. [PubMed] [Google Scholar]
- EFSA Panel on Animal and Welfare (AHAW) . (2010). Scientific opinion on the role of tick vectors in the epidemiology of Crimean Congo hemorrhagic fever and African swine fever in Eurasia. EFSA Journal, 8, 1703. 10.2903/j.efsa.2010.1703 [DOI] [Google Scholar]
- Estrada‐Peña, A. , Zatansever, Z. , Gargili, A. , Aktas, M. , Uzun, R. , Ergonul, O. , & Jongejan, F. (2008). Modeling the spatial distribution of Crimean‐Congo hemorrhagic fever outbreaks in turkey. Vector‐Borne and Zoonotic Diseases, 7, 667–678. 10.1089/VBZ.2007.0134 [DOI] [PubMed] [Google Scholar]
- Fanelli, A. , & Buonavoglia, D. (2021). Risk of Crimean Congo haemorrhagic fever virus (CCHFV) introduction and spread in CCHF‐free countries in southern and Western Europe: A semi‐quantitative risk assessment. One Health, 13, 100290. 10.1016/j.onehlt.2021.100290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli, A. , & Tizzani, P. (2020). Spatial and temporal analysis of varroosis from 2005 to 2018. Research in Veterinary Science, 131, 215–221. 10.1016/j.rvsc.2020.04.017 [DOI] [PubMed] [Google Scholar]
- Fanelli, A. , Tizzani, P. , & Buonavoglia, D. (2022). Crimean‐Congo hemorrhagic fever (CCHF) in animals: Global characterization and evolution from 2006 to 2019. Transboundary and Emerging Diseases, 69, 1556–1567. 10.1111/tbed.14120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli, A. , Tizzani, P. , Ferroglio, E. , & Belleau, E. (2020). Cheilospirura hamulosa in the rock partridge (Alectoris graeca saxatilis): Epidemiological patterns and prediction of parasite distribution in France. Diversity (Basel), 12, 484. 10.3390/d12120484 [DOI] [Google Scholar]
- Hawman, D. W. , & Feldmann, H. (2018). Recent advances in understanding Crimean–Congo hemorrhagic fever virus. F1000Research, 7, 1715. DOI: 10.12688/f1000research.16189.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshtkar‐Jahromi, M. , Kuhn, J. H. , Christova, I. , Bradfute, S. B. , Jahrling, P. B. , & Bavari, S. (2011). Crimean‐Congo hemorrhagic fever: Current and future prospects of vaccines and therapies. Antiviral Research, 90, 85–92. 10.1016/j.antiviral.2011.02.010. Elsevier [DOI] [PubMed] [Google Scholar]
- Maltezou, H. C. , Andonova, L. , Andraghetti, R. , Bouloy, M. , Ergonul, O. , Jongejan, F. , Kalvatchev, N. , Nichol, S. , Niedrig, M. , Platonov, A. , Thomson, G. , Leitmeyer, K. , & Zeller, H. (2010). Crimean‐Congo hemorrhagic fever in Europe: Current situation calls for preparedness. Eurosurveillance, 15, 48–51. 10.2807/ese.15.10.19504-en [DOI] [PubMed] [Google Scholar]
- Mancuso, E. , Toma, L. , Polci, A. , d'Alessio, S. G. , Di Luca, M. , Orsini, M. , Di Domenico, M. , Marcacci, M. , Mancini, G. , Spina, F. , Goffredo, M. , & Monaco, F. (2019). Crimean‐Congo hemorrhagic fever virus genome in tick from migratory bird, Italy. Emerging Infectious Diseases, 25, 1420. 10.3201/EID2507.181345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasirian, H. (2020). New aspects about Crimean‐Congo hemorrhagic fever (CCHF) cases and associated fatality trends: A global systematic review and meta‐analysis. Comparative Immunology, Microbiology and Infectious Diseases, 69, 101429. 10.1016/j.cimid.2020.101429 [DOI] [PubMed] [Google Scholar]
- Papa, A. , Sidira, P. , Kallia, S. , Ntouska, M. , Zotos, N. , Doumbali, E. , Maltezou, H. C. , Demiris, N. , & Tsatsaris, A. (2013). Factors associated with IgG positivity to Crimean‐Congo hemorrhagic fever virus in the area with the highest seroprevalence in Greece. Ticks and Tick‐borne Diseases, 4, 417–420. 10.1016/j.ttbdis.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Papa, A. , Sidira, P. , & Tsatsaris, A. (2016). Spatial cluster analysis of Crimean‐Congo hemorrhagic fever virus seroprevalence in humans, Greece. Parasite Epidemiology and Control, 1, 211–218. 10.1016/j.parepi.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo, A. , Palomar, A. M. , Santibáñez, P. , & Oteo, J. A. (2021). Epidemiological aspects of Crimean‐Congo hemorrhagic fever in Western Europe: What about the future? Microorganisms, 9, 1–19. 10.3390/microorganisms9030649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2021). A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Rinaldi, L. , Morgoglione, M. E. , Noviello, E. , Bosco, A. , Prestera, G. , & Cringoli, G. (2014). Ixodidae ticks in sheep and cattle in the Basilicata region (Southern Italy). Parasites & Vectors, 7, 2014. 10.1186/1756-3305-7-s1-p8 [DOI] [Google Scholar]
- Ruiz‐Fons, F. , Acevedo, P. , Sobrino, R. , Vicente, J. , Fierro, Y. , & Fernández‐de‐Mera, I. G. (2013). Sex‐biased differences in the effects of host individual, host population and environmental traits driving tick parasitism in red deer. Frontiers in Cellular and Infection Microbiology, 3, 23. 10.3389/fcimb.2013.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas, M. A. , Comtet, L. , Donnet, F. , Mertens, M. , Vatansever, Z. , Tordo, N. , Pourquier, P. , & Groschup, M. H. (2018). A novel double‐antigen sandwich ELISA for the species‐independent detection of Crimean‐Congo hemorrhagic fever virus‐specific antibodies. Antiviral Research, 151, 24–26. 10.1016/j.antiviral.2018.01.006 [DOI] [PubMed] [Google Scholar]
- Spengler, J. R. , Bergeron, É. , & Rollin, P. E. (2016). Seroepidemiological studies of Crimean‐Congo hemorrhagic fever virus in domestic and wild animals. PLoS Neglected Tropical Diseases, 10, 1–28. 10.1371/journal.pntd.0004210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler, J. R. , Bergeron, É. , & Spiropoulou, C. F. (2019). Crimean‐Congo hemorrhagic fever and expansion from endemic regions. Current Opinion in Virology, 34, 70–78. 10.1016/j.coviro.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse, C. A. (2004). Crimean‐Congo hemorrhagic fever. Antiviral Research, 64, 145–160. 10.1016/j.antiviral.2004.08.001 [DOI] [PubMed] [Google Scholar]
- WHO (2020). Crimean‐Congo haemorrhagic fever (CCHF) [Online]. Available at http://www.emro.who.int/health‐topics/crimean‐congo‐haemorrhagic‐fever/index.html (accessed January 7, 2021)
- Zouaghi, K. , Bouattour, A. , Aounallah, H. , Surtees, R. , Krause, E. , Michel, J. , Mamlouk, A. , Nitsche, A. , & M'ghirbi, Y. (2021). First serological evidence of Crimean‐Congo hemorrhagic fever virus and rift valley fever virus in ruminants in Tunisia. Pathogens, 10, 769. 10.3390/pathogens10060769 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Data available on request from the authors.


