Abstract
Background:
Although viral infection is known to be associated with asthma exacerbations, prior research has not identified reliable predictors of acute symptom severity in virus-related asthma exacerbations (VRAEs).
Objective:
To determine the effect of asthma control and viral infection on the severity of current illness and evaluate biomarkers related to acute symptoms during asthma exacerbations.
Methods:
We prospectively enrolled 120 children with physician-diagnosed asthma and current wheezing who presented to Arkansas Children’s Hospital emergency department. The asthma control test (ACT) stratified controlled (ACT > 19) and uncontrolled (ACT ≤ 19) asthma, whereas pediatric respiratory symptom scores evaluated symptoms. Nasopharyngeal swabs were obtained for viral analysis, and inflammatory mediators were evaluated by nasal filter paper and Luminex assays.
Results:
There were 33 children with controlled asthma and 87 children with uncontrolled asthma. In those with uncontrolled asthma, 77% were infected with viruses during VRAE compared with 58% of those with controlled asthma. Uncontrolled subjects with VRAE had more acute symptoms compared with the controlled subjects with VRAE or uncontrolled subjects without a virus. The uncontrolled subjects with VRAE and allergy had the highest acute symptom scores (3.363 point pediatric respiratory symptom; P = .04). Children with asthma with higher symptom scores had more periostin (P = .02).
Conclusion:
Detection of respiratory viruses is frequent in those with uncontrolled asthma. Uncontrolled subjects with viruses have more acute symptoms during exacerbations, especially in those with allergy. Periostin was highest in subjects with the most acute symptoms, regardless of control status. Taken together, these data imply synergy between viral infection and allergy in subjects with uncontrolled asthma when considering acute asthma symptoms and nasal inflammation during an exacerbation of asthma.
Introduction
Asthma exacerbations are most often associated with viral infections, specifically rhinovirus (RV), which accounts for 60% to 80% of these events.1–3 Other viruses have also been implicated in exacerbations of asthma, including respiratory syncytial virus (RSV), coronaviruses, human metapneumovirus, human parainfluenza viruses, and enteroviruses.4–6
In an effort to understand mechanisms of virus-related asthma exacerbations (VRAEs), subjects with controlled asthma have undergone experimental inoculation with RV.7–10 Although this approach has provided information regarding immune responses and symptoms during VRAE, it has become clear that more research is needed in those with uncontrolled asthma, as clinical outcomes are often worse within this population. A 2015 letter to the editor by Jackson et al11 considers the effect of asthma control over the prior month during viral infection by evaluating asthma symptoms during an experimental inoculation with RV16. They observed more severe exacerbations after RV infection in those with uncontrolled asthma, irrespective of asthma severity or treatment status. However, little is known regarding the effects of asthma control on acute symptoms during exacerbations of asthma due to other viruses. Furthermore, these data have not been replicated in cross-sectional, case-control studies of asthma exacerbations secondary to viral infection in children seen in the emergency department (ED). Finally, there have been few studies that consider the effects of atopy and viral infections on acute symptoms experienced by patients during VRAE.
In this study, we prospectively enrolled subjects with acute exacerbations of asthma seen in the ED at Arkansas Children’s Hospital (ACH). We hypothesized that uncontrolled asthma in the prior month as determined by the asthma control test (ACT) and the presence of allergy would account for more severe respiratory symptoms during VRAE. Furthermore, we investigated the correlation of these symptoms, the presence of allergy, and viral infections to the presence of nasal inflammatory mediators.
Methods
Study Population
We prospectively enrolled 120 subjects aged 4 to 18 years from the ED at ACH in Little Rock, Arkansas. Patients were enrolled from the fall of 2014 to the fall of 2017. Inclusion criteria required that subjects have a physician diagnosis of asthma and active wheezing requiring a beta-agonist during the ED visit. Children with chronic lung disease, congenital heart disease, immunodeficiency, or oncologic disorders were excluded. Informed consent was obtained from parents, and informed assent was obtained from children 7 years of age or older. This study was approved by the Institutional Review Board at the University of Arkansas for Medical Sciences.
Survey Instruments and Clinical Data
Surveys were obtained from the caregivers during the visit, including questionnaires detailing past and present medical history, the ACT,12 and the pediatric respiratory symptom (PRS) score.13 The ACT was used to determine relative asthma control over the prior 1 month. Subjects were classified as controlled (ACT > 19) or uncontrolled (ACT ≤ 19).14 PRS score was used to compare current asthma symptoms during exacerbations. As previously described, we defined symptoms as mild (PRS of 1 to 5) or moderate and severe (PRS of 6 to 15).13 In addition, we evaluated the impact of allergy based on caregiver-reported allergy, identified from surveys performed at the time of enrollment.
Rhinovirus Loads by Quantitative Polymerase Chain Reaction and Viral Genome Sequencing
A nasopharyngeal swab was obtained at the time of enrollment and placed in viral transport media, and a small aliquot was removed 24 hours after collection. The aliquot and sample were stored at −80° C. The aliquots were tested for rhinovirus (RV) by quantitative polymerase chain reaction (qPCR), whereas the samples were batch-shipped to the University of New Mexico Health Sciences Center (UNM HSC) on dry ice for virus detection and genome sequencing for a large panel of respiratory viruses (eTable 1).
Rhinovirus Quantitative Polymerase Chain Reaction
Total cellular RNA was harvested from 200 μL of viral transport media using TriReagent (Qiagen, Venlo, Netherlands), and complementary DNA (cDNA) was generated using TaqMan Reverse Transcription kits with primers for RV, as previously described.15,16 Briefly, cDNA was amplified using primers specific for conserved regions of RV and detected by qPCR (forward—5′-CCTCCGGCCCCTGAA; reverse—5′-AAACACGGACACCCAAAGTAGT). Subject samples were evaluated in duplicate, and the values were compared with a standard curve to determine the copies of RV virions.15
Viral Genome Sequencing
Respiratory virus detection and genome sequencing were also performed, as previously described.17,18 RNA extraction used TriReagent (Qiagen, Venlo, Netherlands) (1:3 ratio) with processing using the Zymo DirectZol isolation kit thereafter (per manufacturer’s instructions, Zymo Research, Freiburg, Germany).
RNA was isolated from all samples and analyzed for quality, and sequencing libraries were created following a slightly modified version of the RNA TruSeq Access (Illumina, San Diego, California) library preparation protocol. Briefly, 1 to 20 ng of isolated total RNA was reverse transcribed using random hexamers in the presence of actinomycin D to maintain strand information to produce cDNA. Dual index, Illumina-barcoded adapters were ligated to the ends of the cDNA to create Illumina sequencing libraries. Samples were pooled together in equal molar concentrations, and hybridization-based enrichment was performed using the UNM ResVir (respiratory viral) panel probe set containing 5683 hybridization probes of 80 nucleotides in length designed to be complementary to coding sequence regions of 24 human respiratory viruses (eTable 1). The sequencing library pool was mixed with the viral probe set, and blocking oligonucleotides and the mixture were heated at 95°C to denature the libraries, followed by hybridization on step down lowering of the temperature to an annealing temperature of 65°C. The virus-specific, biotin-labeled probe, Illumina sequence library hybrids were isolated using magnetic streptavidin-coated beads. Next, a minimal polymerase chain reaction amplification step was performed on the enriched pool of sequencing libraries, followed by quality assessment. Prepped and enriched libraries were pooled and seeded at a cluster density of 1200 to 1400 k/mm2 on an Illumina MiSeq. Next-generation sequencing was performed using v3 chemistry and paired 75 bp reads.
Bioinformatics
To identify viruses, sequences were compared with the KRAKEN metagenomics database.19 Further identification and virus characterization were conducted using a custom-designed workflow in CLC Genomics Workbench v9 (Qiagen, Germantown, Maryland). Briefly, fastq sequences were aligned to a genome reference database of human respiratory viruses in the UNM ResVir panel. Coverage statistics for each genome were calculated, and a reference-guided consensus sequence was generated. Using a conservative threshold to eliminate false-positive results, we defined a sample as positive for a virus if it had more than or equal to 500 reads align to reference genome and more than or equal to 10% of the genome covered.
Serum Allergy Testing by ImmunoCAP
A blood sample (5–7 mL) was obtained by venipuncture on a subset of subjects (n = 40), and sera were analyzed for total serum immunoglobulin E (IgE) and specific IgE (IU/mL) to several relevant allergens (Dermatophagoides farinae, Dermatophagoides pteronyssinus, cat, dog, Alternaria, Aspergillus, Bermuda, Timothy, oak, birch, tree mix, and ragweed) using the Phadia ImmunoCap Assay (Phadia/Thermo. Fisher, Hercules, California). Sera with greater than or equal to 0.35 IU/mL of IgE Ab to any of the allergens tested were considered positive for sensitization. Evaluations between total IgE and acute asthma symptom (PRS score) and any positive-specific IgE and PRS score were performed.
Inflammatory Mediator Testing by Luminex
Grade 42 Whatman paper cut into similar-sized strips was placed into each nostril of the children for 2 minutes on each side. Samples were stored at −80°C until batched analysis could be performed. Filter papers were eluted using previously described methods.20 Luminex assays (MilliporeSigma, Burlington, Massachusetts) for periostin (detection limit = 0.12 ng/mL), eotaxin (detection limit = 4 pg/mL), interleukin (IL)-8 (detection limit = 0.4 pg/mL), and IL-13 (detection limit = 1.3 pg/mL) were used to determine levels of these cytokines. Comparisons of nasal inflammatory mediators and acute symptoms in VRAE and no virus subjects were performed.
Statistics
Values were expressed as percentages for discrete variables or as mean and SD or median and interquartile range for continuous variables. We used Pearson’s χ2 and descriptive statistics, such as median and quartiles, for categorical variables. Mann-Whitney was used to compare viral loads. PRS scores were analyzed by a 2 × 2 × 2 factorial analysis of variance design using asthma control, VRAE, and allergy rhinitis as factors in a full interaction model. Multiple comparisons were done at the full interaction level using a Holm correction for P values. Associations between cytokines and acute symptoms were tested using Spearman rank correlation. All tests were 2-sided, assuming a significance level of 5%. Plots and analyses were performed using GraphPad Prism, version 8.3.1 (GraphPad Software, Inc, La Jolla, California) and R, version 4.2.0.21
Results
Baseline Characteristics
A total of 120 children with acute asthma exacerbations were enrolled prospectively into the study. There were 33 children with controlled asthma (ACT > 19) and 87 children with uncontrolled asthma (ACT ≤ 19). The mean ACT score for those with controlled asthma was 21.5 (SD, 1.73) and 14.07 (SD, 3.52) for those with uncontrolled asthma. Children with uncontrolled asthma were found to have similar overall PRS scores when compared with those with controlled asthma (controlled, mean [SD], 4.839 [3.184]; uncontrolled, 6.205 [3.432]; P = .06).
Of the subjects, 73% had a history of allergy by caregiver report. There were no differences in the frequency of reported allergy in either the controlled or uncontrolled asthma cohorts (controlled, 67%; uncontrolled, 78%; P = .19). To confirm the validity of the subjective responses of caregivers as to who had allergy, we evaluated sensitization by serum-specific IgE in a subgroup of our patients. Of those with reported allergy who had a blood sample drawn (n = 40), 67% were found be sensitized to more than or equal to 1 allergen, and 11% were not sensitized. Of those without reported allergic rhinitis (AR), 22% were sensitized to more than or equal to 1 allergen. No other substantial differences were found in the demographics or environmental exposures between the cohorts. Table 1 summarizes these findings.
Table 1.
Demographics
| n = 120 | Controlled (n = 33) | Uncontrolled (n = 87) | P |
|---|---|---|---|
| Age (y), median (Q1, Q3) | 11.05 (6.1, 15.0) | 11.15 (7.6, 15.0) | .92 |
| Male sex, n (%) | 20 (60.6%) | 52 (59.8%) | >.99 |
| Race and ethnicity, n (%) | .21 | ||
| White | 5 (15.1%) | 18 (20.7%) | |
| Black | 27 (81.8%) | 71 (81.6%) | |
| Hispanic | 1 (3.0%) | 1 (1.1%) | |
| Native American | 0 (0%) | 2 (2.3%) | |
| Residence type, n (%) | .90 | ||
| Apartment | 7 (21.2%) | 18 (20.7%) | |
| House | 23 (68%) | 23 (70%) | |
| Townhouse or duplex | 1 (3.0%) | 4 (4.6%) | |
| Trailer house | 1 (3.0%) | 5 (5.7%) | |
| No answer | 1 (3.0%) | 1 (1.1%) | |
| Carpet in house, n (%) | 19 (57.6%) | 61 (70.1%) | .19 |
| Pets, n (%) | 10 (30.3%) | 35 (40.2%) | .31 |
| Heating and air, n (%) | .31 | ||
| Central | 21 (63.6%) | 69 (79.3%) | |
| Window units | 0 (0%) | 2 (2.3%) | |
| Electric base board | 6 (18.2%) | 7 (8.0%) | |
| Heat pump | 2 (6.1%) | 3 (3.4%) | |
| Other | 4 (12.1%) | 5 (5.7%) | |
| No answer | 0 (0%) | 1 (1.1%) | |
| Inhaled corticosteroids, n (%) | 18 (54.5%) | 57 (65.5%) | .26 |
| ACT, mean (SD) | 21.54 (1.75) | 14.42 (3.37) | |
| FEV1, mean (SD) (n = 27) (5 Controlled) (22 Uncontrolled) |
2.54 (0.33) | 1.91 (0.73) | .01 |
| Positive viral tests, n (%) | 18 (58.1%) | 65 (75.6%) | .06 |
| Positive RV tests, n (%) | 10 (32.3%) | 36 (45.0%) | .22 |
| RV co-infections, n (%) | 4 (12.9%) | 14 (17.9%) | .72 |
Abbreviations: ACT, asthma control test; FEV1, forced expiratory volume in 1 second; RV, rhinovirus.
Seasonal Differences in Viral Infections, Asthma Control, and Asthma Exacerbation Symptoms
As expected, we found an increased number of positive viral test results during the winter, spring, and fall with more positive test results for RV infection during these same seasons (eFig 1A). There were no differences in asthma control (eFig 1B) or PRS scores based on season of enrollment (eFig 1C).
Frequency of Positive Viral Infections in Those With Controlled and Uncontrolled Asthma With Virus-Related Asthma Exacerbation
We found that 72% of the 120 children with asthma seen in the ED for exacerbation of symptoms had a virus at the time of presentation (VRAE). RV was the most common virus associated with VRAE, accounting for 48% of those with a virus, followed by RSV (18%), and influenza (15%) (Fig 1). Other viruses within both cohorts included the following: coronavirus, human metapneumovirus, parainfluenza virus, adenovirus, WU polyomavirus, bocavirus, and enterovirus.
Figure 1.
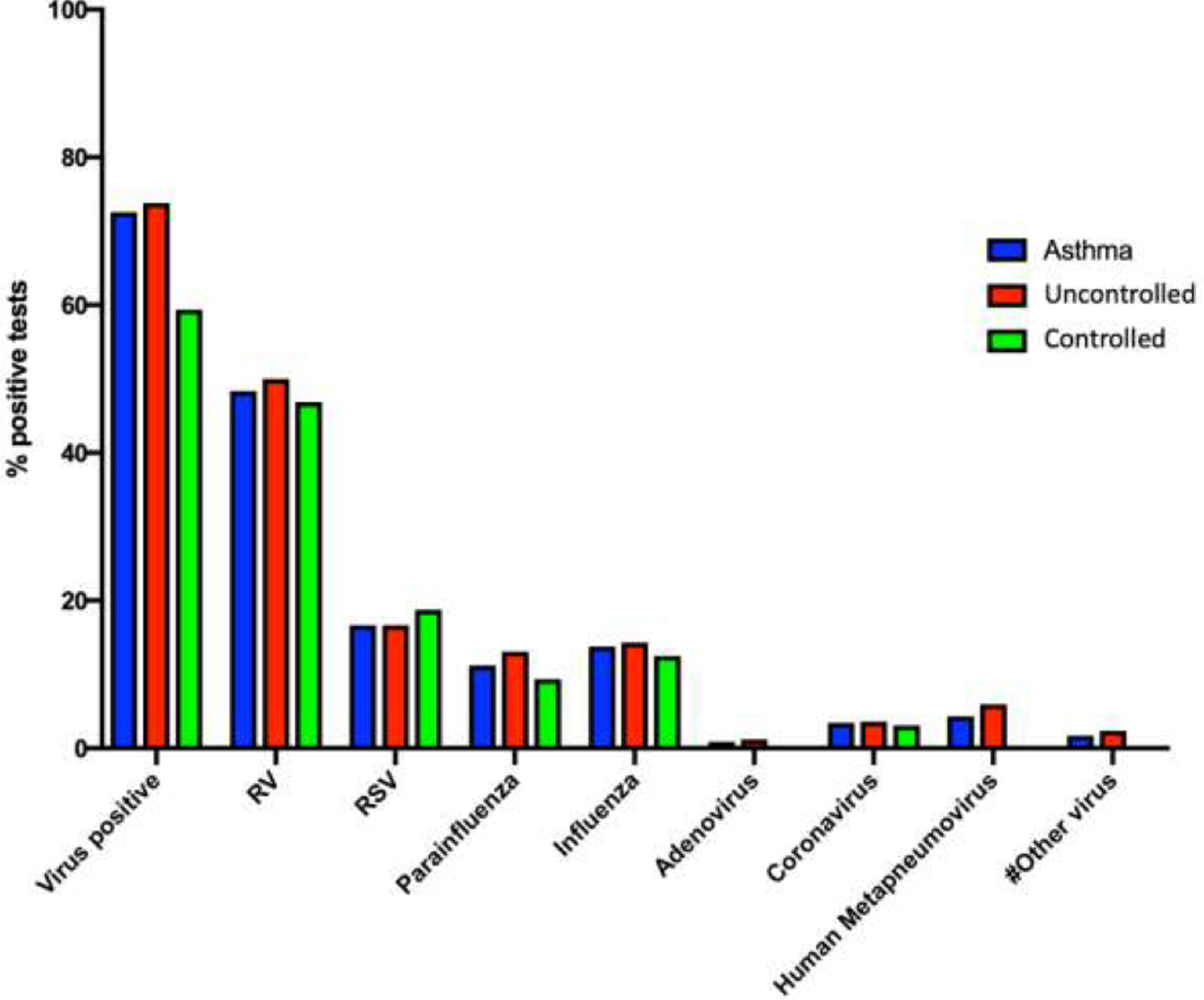
Frequency of viral infections in subjects with asthma at the time of presentation to the ED. Subjects with uncontrolled asthma had more positive viral test results than those who were controlled at the time of presentation to the ED (uncontrolled 77%; controlled 58%; P = .05). #Other virus includes WU polyomavirus, bocavirus, and enterovirus. ED, emergency department.
Of those with uncontrolled asthma, 77% had VRAE at presentation, compared with 58% of those with controlled asthma (P = .05). The most common virus for those with uncontrolled and controlled asthma was also rhinovirus (45% and 32%, respectively; P = .20). Those with uncontrolled asthma had a co-infection with RV and another virus 17% of the time. The most common co-infection with RV in either group was RSV (Fig 1).
Acute Symptoms in Those With Uncontrolled and Controlled Asthma With Virus-Related Asthma Exacerbation
Unadjusted comparisons between uncontrolled and controlled asthma with VRAE and PRS score revealed that subjects with uncontrolled asthma with VRAE had more acute symptoms at the time of evaluation (controlled with VRAE, mean [SD], 4.41 [3.39]; uncontrolled with VRAE, mean [SD] 6.26 [3.19]). When only considering uncontrolled asthma, those with VRAE had more symptoms by PRS than those without virus (uncontrolled with VRAE, mean [SD], 6.26 [3.19]; uncontrolled, no virus, mean [SD], 3.43 [3.80]), suggesting viral infection considerably enhanced acute symptoms within this population (Fig 2A). Those with uncontrolled asthma with RV-induced exacerbations also had higher PRS scores at the time of presentation compared with those with controlled asthma with RV-induced exacerbations (uncontrolled with RV, mean [SD] 6.74 [3.66]; controlled with RV, mean [SD], 4.40 [3.46]) (Fig 2B).
Figure 2.
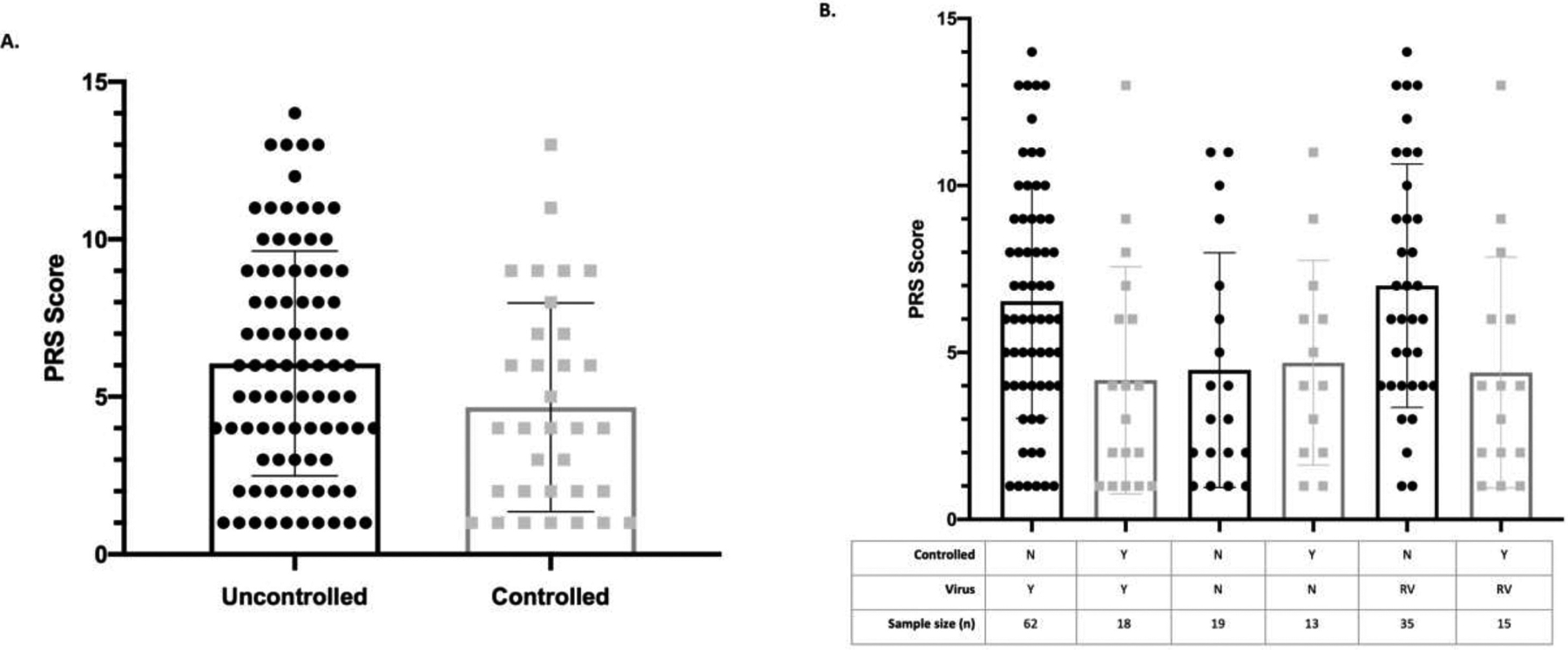
Acute symptoms according to asthma control and viral infection. In unadjusted analysis, (A) those with uncontrolled asthma do not have significantly more symptoms than those with controlled asthma during acute exacerbations. (B) However, uncontrolled VRAE and uncontrolled with RV had significantly more acute symptoms than controlled VRAE or uncontrolled without virus. RV, rhinovirus; VRAE, virus-related asthma exacerbation.
Acute Symptoms in Those With Uncontrolled and Controlled Asthma With Allergy and Virus-Related Asthma Exacerbation
Using a factorial analysis of variance statistical approach, those with uncontrolled asthma and VRAE with reported allergy had higher PRS scores than those with well-controlled asthma, allergy, and virus (3.363 point increase in PRS; P = .04) (Fig 3). In these analyses, there were no other comparisons that were significantly different (P ≥ .35).
Figure 3.
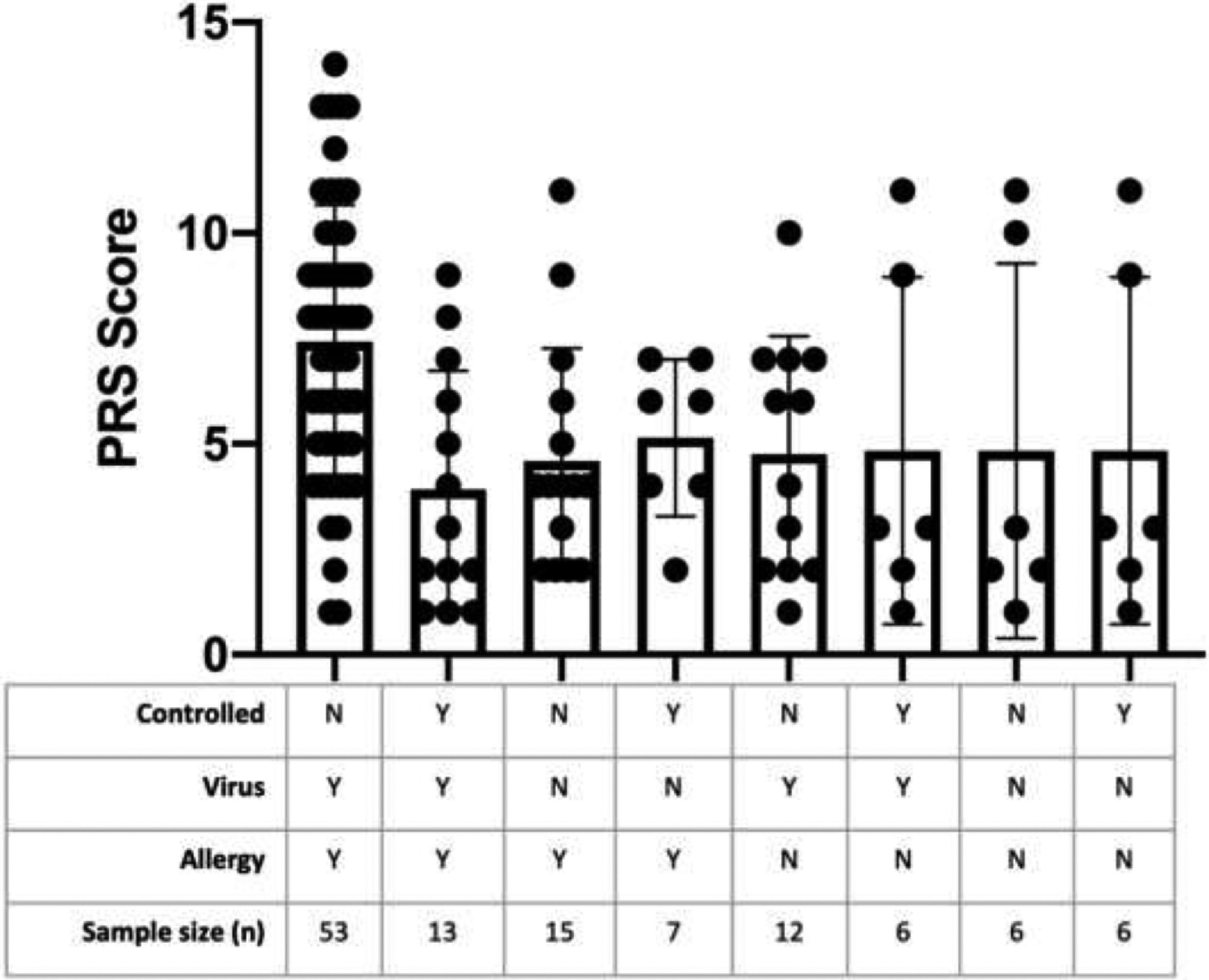
Acute symptoms according to asthma control, caregiver-reported allergy, and viral infection. There was a 3.363 point increase in PRS scores for those with uncontrolled asthma with VRAE and allergy compared with those with controlled asthma (P = .04). PRS, pediatric respiratory symptom; VRAE, virus-related asthma exacerbation.
In unadjusted analyses, subjects with a total IgE more than 371 IU/dL7 had modestly increased acute symptoms in those with uncontrolled asthma with VRAE compared with those with uncontrolled asthma with VRAE with low total IgE (<371 IU/dL) (uncontrolled with VRAE, high total IgE, mean [SD], 7.75 [3.32]; uncontrolled, virus (−), low IgE, mean [SD], 4.12 [5.07]) (eFig 2A). If we considered those subjects with any positive specific IgE test result, there was an increase in acute asthma symptoms between those with uncontrolled asthma with VRAE who had a positive specific IgE test result compared with those with uncontrolled asthma with VRAE and no positive IgE test result (uncontrolled with VRAE, allergy (+), mean [SD], 7.23 [3.14]; uncontrolled, virus (−), allergy (−), mean [SD], 3.24 [1.86]) (eFig 2B).
Rhinovirus Load and Association With Asthma Control
Subjects with uncontrolled asthma had similar viral loads compared with those with controlled asthma (uncontrolled, geometric mean [GM] [95% confidence interval (CI)], 21,826 virions/2 μL cDNA [11,629–40,968]; controlled, GM [95% CI], 45,031 virions/2 μL cDNA [14,883–136,247]; P = .03) (Fig 4A). RV load in either group did not correlate with acute asthma symptoms or ACT at the time of presentation (Fig 4B).
Figure 4.
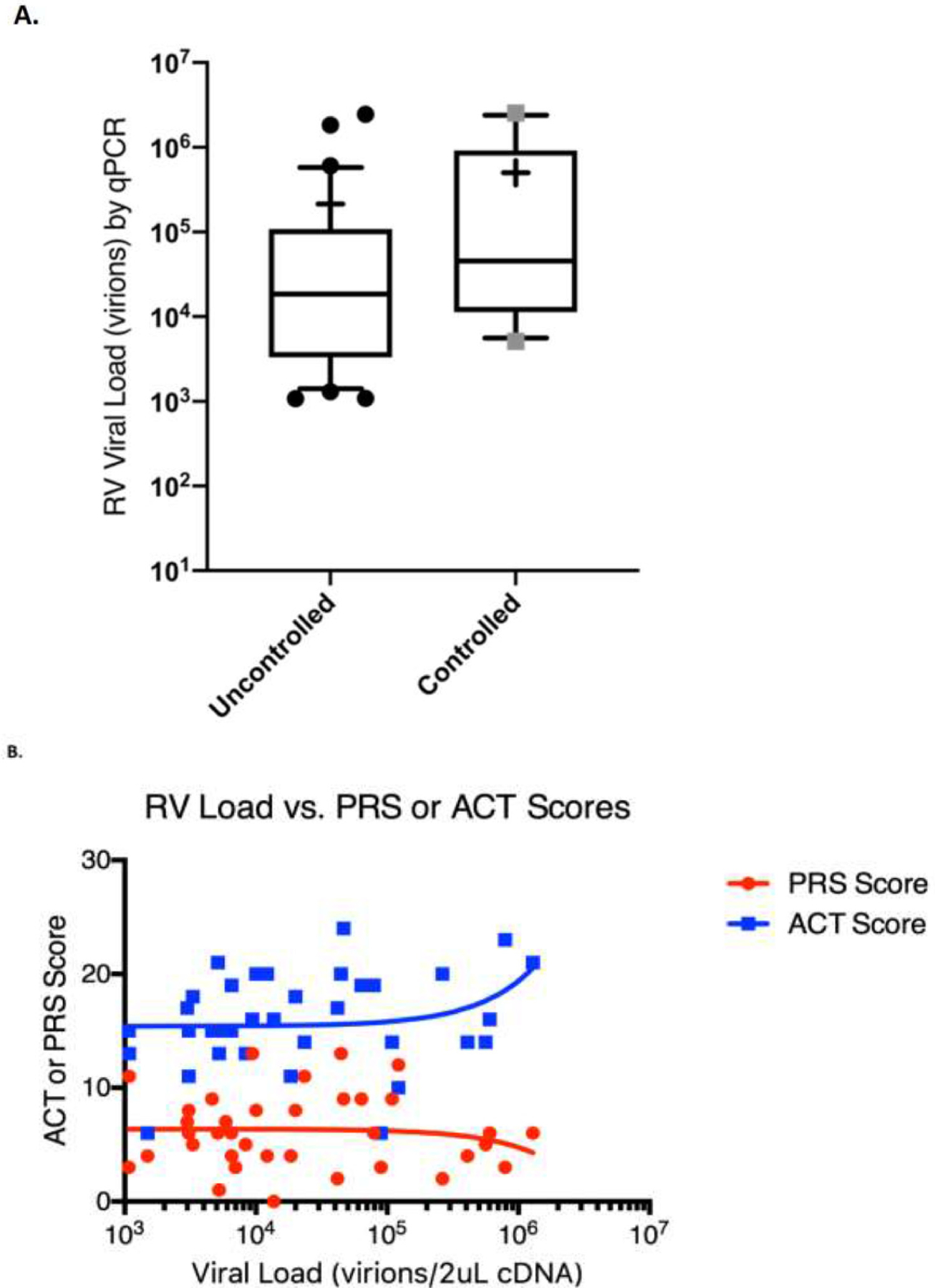
Rhinovirus load comparisons between uncontrolled and controlled asthma. (A) Rhinovirus loads are similar between uncontrolled and controlled and (B) do not correlate with asthma control or acute symptoms.
Nasal Inflammatory Markers During Virus-Related Asthma Exacerbation
When considering all subjects, levels of IL-8 (Rs = 0.17, P = .04) and eotaxin (Rs = 0.13, P = .04) had minimal correlation with acute symptoms (Fig 5A and B, respectively). When considering only uncontrolled with VRAE, only eotaxin remained significantly correlated with acute symptoms (Rs = 0.24, P = .03) (Fig 5C).
Figure 5.
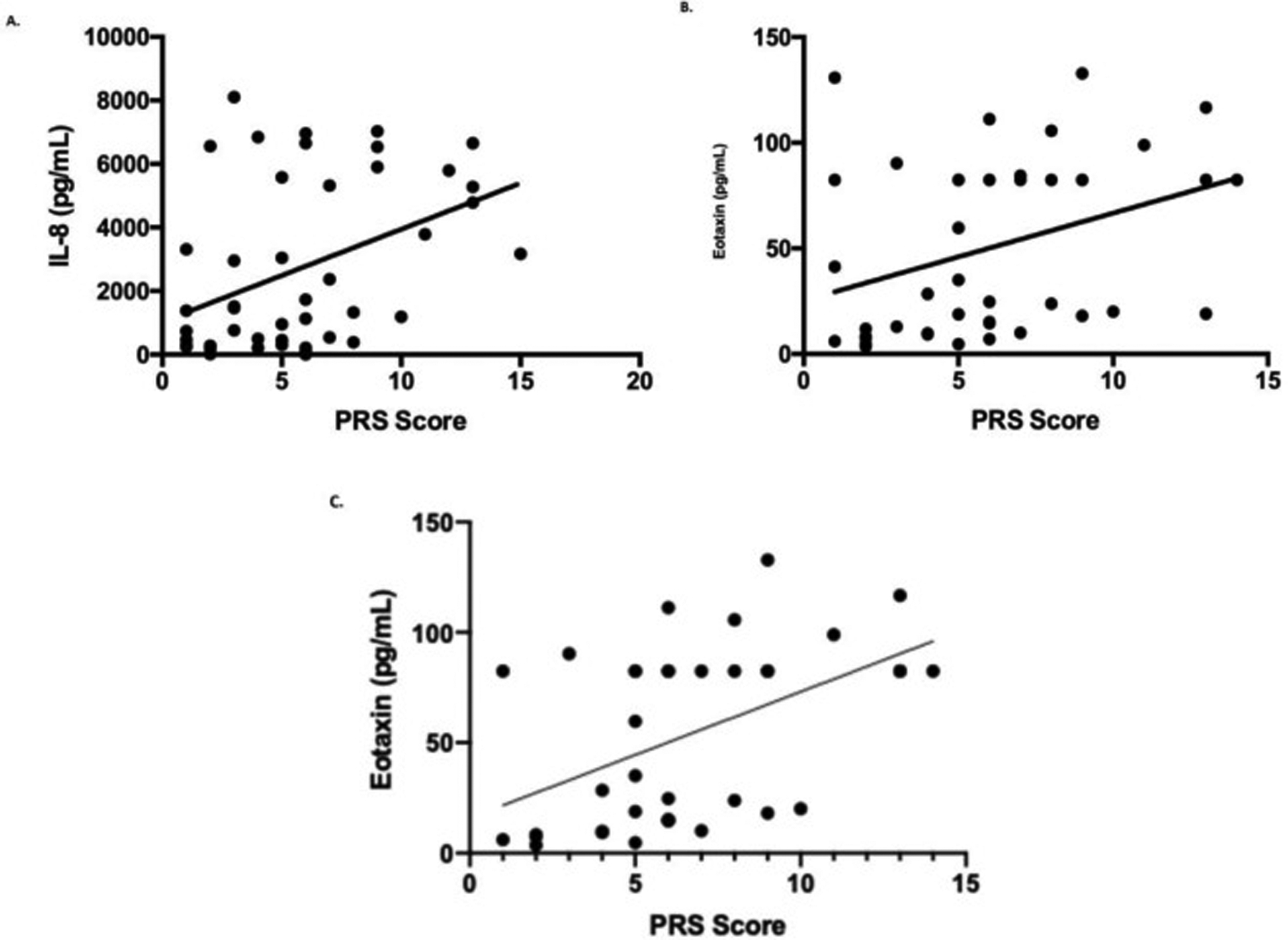
Correlating nasal inflammatory markers and acute symptoms. (A) IL-8 and (B) eotaxin mildly correlate with acute symptoms in those with uncontrolled asthma during exacerbations (all-comers) (R2 = 0.17, 0.13, respectively; P = .05). When considering uncontrolled with VRAE, (C) only eotaxin remains modestly correlated (R2 = 0.24; P < .05). IL-8, interleukin 8; VRAE, virus-related asthma exacerbation.
Nasal periostin levels were higher in patients with uncontrolled asthma as compared with those with controlled asthma during an exacerbation, without consideration for the presence of a virus (uncontrolled, mean [SD], 13.98 pg/mL [18.39]; controlled, mean [SD], 4.39 pg/mL [4.09]; P = .02) (Fig 6A). There were no differences when considering uncontrolled asthma with VRAE and uncontrolled asthma without virus (uncontrolled with VRAE, mean [SD], 15.57 ng/mL [20.93]; uncontrolled without virus, 10.58 pg/mL [12.12]; P = .41) (Fig 6A).
Figure 6.
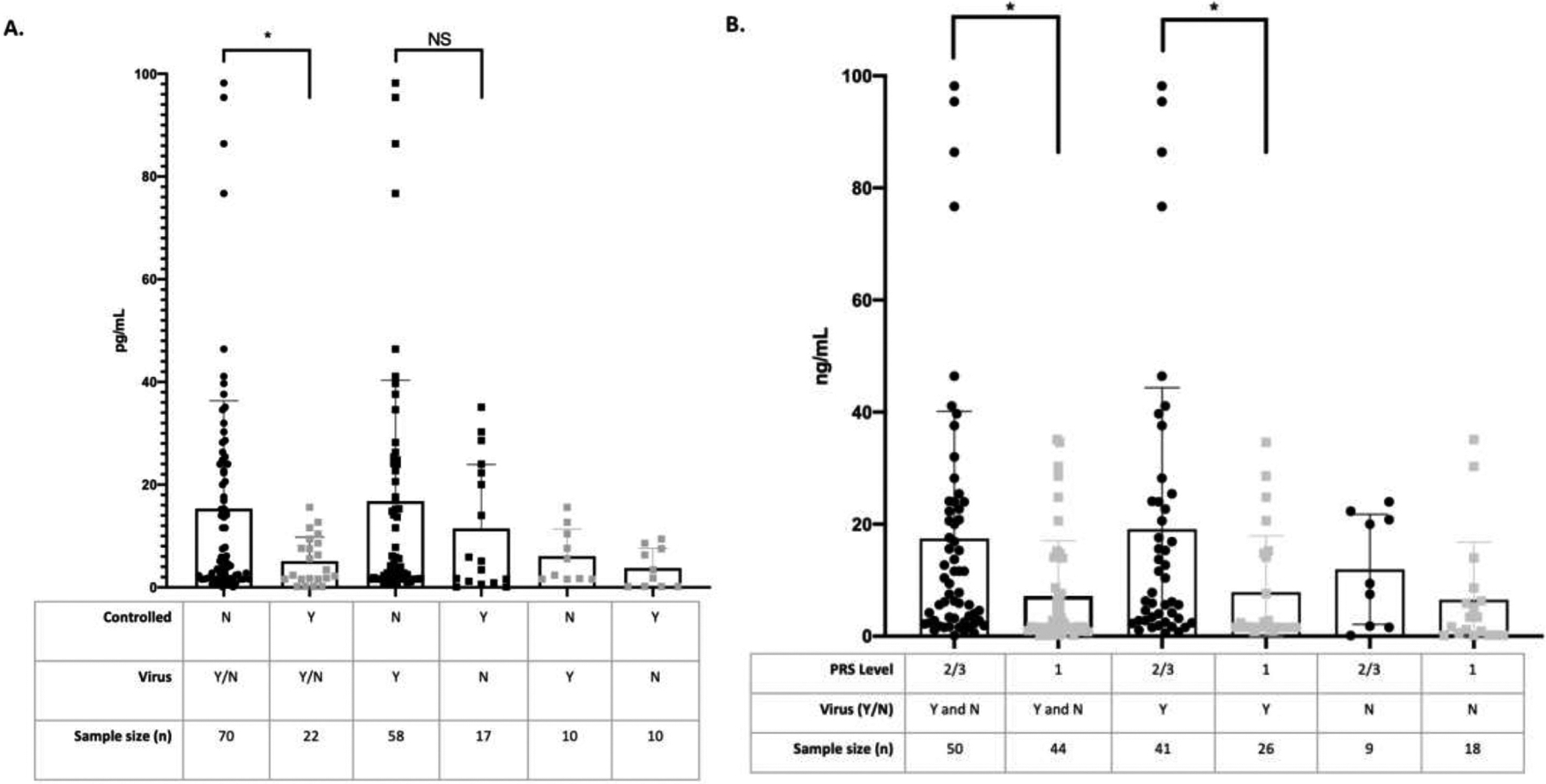
Nasal periostin levels during exacerbations of asthma according to asthma control. (A) Those with uncontrolled asthma had higher periostin levels than those with controlled asthma during exacerbations (P = .02); however, this was not related to VRAE (P = .41). (B) More severe asthma exacerbations according to PRS severity scores had higher levels of periostin during VRAE (P = .01). PRS, pediatric respiratory symptom; VRAE, virus-related asthma exacerbation.
Periostin levels were higher in those patients with more severe PRS scores. Those subjects with moderate/severe (level 2 or 3) symptoms by PRS had higher periostin levels compared with those with mild (level 1) symptoms (asthma and level 2 or 3 PRS, mean [SD], 17.42 pg/mL [9.74]; asthma and level 1 PRS, mean [SD], 7.2 pg/mL [9.83]; P = .01) (Fig 6B). Similarly, subjects with asthma exacerbations and viral infections having moderate/severe symptoms by PRS had increased periostin levels compared with those with mild symptoms and viral infection (asthma with virus and level 2 or 3 PRS, mean [SD], 19.09 pg/mL [25.26]; asthma with virus and level 1 PRS, 7.81 pg/mL [10.1]; P = .01) (Fig 6B). As expected, though not previously found in nasal filter paper studies, periostin levels did correlate to nasal IL-13 levels (Rs = 0.35, P = .02) (eFig 3).22–24
Discussion
We performed a prospective cross-sectional study of children with asthma during exacerbations to determine the effect asthma control over the prior month and atopic status had on current symptoms and nasal inflammation when children were seen in the ED with asthma exacerbations. In our study, there was no difference between those with uncontrolled asthma and those with controlled asthma regarding the presence of a viral infection. As in other studies, RV was the most prominent virus present during asthma exacerbations within both cohorts,1–3,15,25–27 but other viruses were also present, albeit in much lower numbers. There was no difference in the frequency of RV infections between those with uncontrolled asthma and those with controlled asthma at the time seen in the ED.
Acute symptoms at the time of the exacerbations were worse for those subjects who were uncontrolled with VRAE compared with those who were controlled with VRAE. In fact, acute symptoms remained higher even when comparing uncontrolled with VRAE and uncontrolled without an identified virus. Taken together, these findings suggest that, within our population, viral infection accounts for enhanced acute symptoms. Similar to the study of Jackson et al,11 those with uncontrolled asthma with VRAE secondary to RV had more acute symptoms than those with controlled asthma with VRAE secondary to RV.
Those subjects with uncontrolled asthma and caregiver-reported allergy had more symptoms than those individuals with uncontrolled asthma without caregiver-reported allergy. The difference in PRS score between those with uncontrolled asthma and those with controlled asthma with allergy and VRAE was 3.363, which is enough to cause a clinically meaningful difference and is statistically significant (P = .04). Subjects with any positive specific IgE test result had more acute asthma symptoms when considering those with uncontrolled asthma with VRAE compared with those with uncontrolled asthma with VRAE and no positive IgE test result. In those with blood tests for total IgE, those with uncontrolled asthma with VRAE trended toward more acute symptoms when considering high total IgE (>371 IU/mL). These findings suggest that there is a synergistic effect between viral infection and allergic responses within our population. Others report similar findings. In a study by Zambrano et al,7 only those subjects with asthma with high total IgE levels (>343 IU/mL) developed hyper-responsiveness to methacholine by day 4 of an experimental RV16 infection. In a study of Costa Rican children, the probability of acute wheezing was significantly associated with increasing IgE titers to dust mites, and this probability substantially increased if the subject was rhinovirus positive during the exacerbation (odds ratio of 30, P < .001).3
Rhinoviral loads during exacerbations of asthma have been studied extensively in the literature with some arguing higher loads in individuals with asthmas secondary to an innate immune deficiency,28–30 whereas others find similar viral loads between those with asthma and controls.15,31 It is important to remember that this study does not have a control group without asthma; however, we found that the RV load in those with controlled asthma was similar to the uncontrolled cohort. This finding is interesting because those with uncontrolled asthma with RV had more acute symptoms than those with controlled asthma with RV. In fact, we reveal that the viral load for RV does not correlate with either acute symptoms or control over the prior month. These findings suggest that viral loads do not influence overall symptoms during an exacerbation of asthma in our population. Ex vivo models of RVC infection in precision-cut lung slices document similar findings. Parikh et al32 revealed that RVC causes airway hyper-responsiveness in precision-cut lung slices by calcium mobilization in airway smooth muscle; however, this finding was uncoupled from viral load.32
This study identifies 2 nasal inflammatory markers that slightly correlate with acute symptoms, IL-8 and eotaxin. One of these markers, IL-8, has been previously correlated with acute symptoms in other studies.33 Eotaxin is a CC chemokine that acts as a chemoattractant for eosinophils, and it is highly associated with allergic responses.34 Eotaxin is the only one of the 3 markers that remained correlated to acute symptoms when considering individuals with asthma with viral infections. The observation that eotaxin levels from nasal filter paper correlate with acute symptoms during an asthma exacerbation also lends credence to the idea that viral infections and allergy are synergistic. To further evaluate this synergistic effect, we evaluated the role of periostin in VRAE. Although periostin levels were higher in uncontrolled asthma, there were no differences in periostin protein levels between those with uncontrolled asthma with VRAE compared with those with uncontrolled asthma without virus. If all asthma was considered without discrimination for control, those with asthma who had worse symptoms by PRS during the exacerbation had the highest levels of periostin, which also held true for those with VRAE. These findings suggest that periostin may be a good indicator of asthma control before exacerbations and asthma symptoms during exacerbations.
This study begins to define the connections that exist between allergy, viral infections, and asthma exacerbations. However, there are limitations that exist. We found increased frequency of viral infections in those patients with uncontrolled asthma at the time of their presentation to the ED. Although this finding could represent susceptibility to viral infection in this population, it is also plausible that this represents a selection bias, given that those with poor control may be more likely to seek care during exacerbations of asthma with or without viral infections. More work is necessary to determine the relevance of this finding. Our study had few children with controlled asthma, which is expected in a population that is being evaluated for asthma exacerbations in the ED. It could be argued that our classification of these subjects as controlled, despite their ACT score, is inaccurate as they were being seen emergently for asthma symptoms. We believe that these children with controlled asthma seen in the ED likely had some acute event that caused their current exacerbation—be that an allergic trigger or viral infection. In our study, we only evaluated for specific respiratory viruses in our population. It is possible that some of the patients without viruses were infected with either respiratory viruses that we did not test or bacteria, especially Mycoplasma species or Chlamydia pneumoniae.35,36 Furthermore, environmental risks besides viral infections and allergy are important. Our study was not powered sufficiently to include every variable related to the environment. We have provided descriptive statistics for some of these variables in Table 1. Finally, we are unable to determine when each of these patients became infected with the virus. This fact could affect the symptom profiles of those with virus and the spectrum of acute symptoms we found in this study.
In conclusion, the results of this investigation reveal that uncontrolled asthma is associated with higher frequency of viral infections at the time of their presentation to the ED, and those with asthma who are uncontrolled with VRAE have more acute symptoms than either controlled VRAE or uncontrolled asthma without virus. Nasal periostin levels are higher in those with uncontrolled asthma, and these levels were associated with the most symptoms in patients with asthma exacerbations. In the future, nasal periostin may serve as a marker for lack of control and provide important information regarding risk for exacerbations in individuals with asthma, including those related to viral infection.
Supplementary Material
eFigure 1. Seasonal variation of viruses detected, asthma control, and acute symptoms. (A) As expected, most of the viruses detected were found in the spring, fall, and winter. (B) There were no differences in asthma control between seasons. (C) Similarly, there were no differences in acute symptoms between seasons.
eTable 1 Respiratory Viral Panel for Viral Genome Sequencing Abbreviations: dsDNA, double-stranded DNA; ssRNA, single-stranded RNA.
eFigure 3. Nasal periostin levels correlate with nasal IL-13 levels (R2 = 0.35; P < .05). IL-13, interleukin 13.
eFigure 2. IgE and acute symptoms during VRAE. (A) Acute symptoms of subjects with uncontrolled VRAE trended higher in those with high total IgE (>371) compared with those with low total IgE (<371) (P = .08). (B) Uncontrolled VRAE with at least one positive specific IgE test had higher PRS than those with uncontrolled VRAE and no positive allergy test result. IgE, immunoglobulin E; PRS, pediatric respiratory symptom; VRAE, virus-related asthma exacerbation.
Funding:
Dr Kennedy discloses receiving funding from the National Institutes of Health (NIH) (1K08AI121345-01A1, R21AI156446, KL2TR000063, UL1TR000039, R21AI154127, and P20GM103625), the American Academy of Allergy Asthma Immunology ARTrust pilot award, Marion B. Lyon New Scientist Career Development Award, the Clinical and Translational Science Award (CTSA) Western Consortium Grant, the University of Arkansas for Medical Sciences Clinician Scientist Program, and the Arkansas Biosciences Institute. Dr Dinwiddie reports receiving support from HHS|NIH| National Center for Advancing Translational Sciences (UL1TR000041 and KL2TR000089), NIH National Institute of Allergy and Infectious Diseases (R21AI156446, R21AI154127), and the CTSA Western Consortium Grant. Dr Perry discloses receiving funding from the National Institutes of Health National Institute of Nursing Research (R01NR015988), National Heart, Lung, and Blood Institute (1R01HL153052, R01HL142691), and the IDeA States Pediatric Clinical Trials Network.
Footnotes
Supplementary Data
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.anai.2022.06.017.
Disclosures: The authors have no conflicts of interest to report.
References
- 1.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159(3):785–790. [DOI] [PubMed] [Google Scholar]
- 2.Heymann PW, Carper HT, Murphy DD, Platts-Mills TAE, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114(2):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soto-Quiros M, Avila L, Platts-Mills TAE, Hunt JF, Erdman DD, Carper H, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol. 2012;129(6):1499–1505. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudd PA, Thomas BJ, Zaid A, MacDonald M, Kan-o K, Rolph MS, et al. Role of human Metapneumovirus and respiratory syncytial virus in asthma exacerbations: where are we now? Clin Sci (Lond). 2017;131(14):1713–1721. [DOI] [PubMed] [Google Scholar]
- 5.Kistler A, Avila PC, Rouskin S, Wang D, Ward T, Yagi S, et al. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196(6):817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khetsuriani N, Kazerouni NN, Erdman DD, Lu X, Redd SC, Anderson LJ, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119(2):314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zambrano JC, Carper HT, Rakes GP, Patrie J, Murphy DD, Platts-Mills TAE, et al. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003;111(5):1008–1016. [DOI] [PubMed] [Google Scholar]
- 8.Roestenberg M, Hoogerwerf MA, Ferreira DM, Mordmuller B, Yazdanbakhsh M. Experimental infection of human volunteers. Lancet Infect Dis. 2018;18(10):e312–e322. [DOI] [PubMed] [Google Scholar]
- 9.Beale J, Jayaraman A, Jackson DJ, Macintyre JDR, Edwards MR, Walton RP, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6(256):256ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson DJ, Makrinioti H, Rana BMJ, Shamji BWH, Trujillo-Torralbo MB, Footitt J, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190(12):1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson DJ, Trujillo-Torralbo MB, del-Rosario J, Bartlett NW, Edwards MR, Mallia P, et al. The influence of asthma control on the severity of virus-induced asthma exacerbations. J Allergy Clin Immunol. 2015;136(2):497–500. e3. [DOI] [PubMed] [Google Scholar]
- 12.Voorend-van Bergen S, Vaessen-Verberne AA, de Jongste JC, Pijnenburg MW. Asthma control questionnaires in the management of asthma in children: a review. Pediatr Pulmonol. 2015;50(2):202–208. [DOI] [PubMed] [Google Scholar]
- 13.Rutman L, Migita R, Spencer S, Kaplan R, Klein EJ. Standardized asthma admission criteria reduce length of stay in a pediatric emergency department. Acad Emerg Med. 2016;23(3):289–296. [DOI] [PubMed] [Google Scholar]
- 14.Al Moamary MS, Al-Kordi AG, Al Ghobain MO, Tamim HM. Utilization and responsiveness of the asthma control test (ACT) at the initiation of therapy for patients with asthma: a randomized controlled trial. BMC Pulm Med. 2012;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy JL, Shaker M, McMeen V, Gern J, Carper H, Murphy D, et al. Comparison of viral load in individuals with and without asthma during infections with rhinovirus. Am J Respir Crit Care Med. 2014;189(5):532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A. 2015;112 (17):5485–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kothari A, Burgess MJ, Crescencio JCR, Kennedy JL, Denson JL, Schwalm KC, et al. The role of next generation sequencing in infection prevention in human parainfluenza virus 3 infections in immunocompromised patients. J Clin Virol. 2017;92:53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Flaherty BM, Li Y, Tao Y, Paden CR, Queen K, Zhang J, et al. Comprehensive viral enrichment enables sensitive respiratory virus genomic identification and analysis by next generation sequencing. Genome Res. 2018;28(6):869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15(3):R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alam R, Sim TC, Hilsmeier K, Grant JA. Development of a new technique for recovery of cytokines from inflammatory sites in situ. J Immunol Methods. 1992;155 (1):25–29. [DOI] [PubMed] [Google Scholar]
- 21.Core Team R. R: A Language and Environment for Statistical Computing. 4.2.0 ed Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- 22.Ito Y, Al Mubarak R, Roberts N, Correll K, Janssen W, Finigan J, et al. IL-13 induces periostin and eotaxin expression in human primary alveolar epithelial cells: comparison with paired airway epithelial cells. PLOS ONE. 2018;13(4): e0196256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Ma R, Zhang J, Wu X, Yu G, Hu X, et al. Excessive periostin expression and Th2 response in patients with nasal polyps: association with asthma. J Thorac Dis. 2018;10(12):6585–6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ninomiya T, Noguchi E, Haruna T, Hasegawa M, Yoshida T, Yamashita Y, et al. Periostin as a novel biomarker for postoperative recurrence of chronic rhinosinitis with nasal polyps. Sci Rep. 2018;8(1):11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee WM, Lemanske RF Jr, Evans MD, Vang F, Pappas T, Gangnon R, et al. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186(9):886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemanske RF Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571–577. [DOI] [PubMed] [Google Scholar]
- 28.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PAB, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12(9):1023–1026. [DOI] [PubMed] [Google Scholar]
- 29.Laza-Stanca V, Message SD, Edwards MR, Parker HL, Zdrenghea MT, Kebadze T, et al. The role of IL-15 deficiency in the pathogenesis of virus-induced asthma exacerbations. [published correction appears in PLOS Pathog. 2012;8(4)] PLOS Pathog. 2011;7(7): e1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105 (36):13562–13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller EK, Hernandez JZ, Wimmenauer V, Shepherd BE, Hijano D, Libster R, et al. A mechanistic role for type III IFN-λ1 in asthma exacerbations mediated by human rhinoviruses. Am J Respir Crit Care Med. 2012;185(5):508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parikh V, Scala J, Patel R, Corbi C, Lo D, Bochkov YA, et al. Rhinovirus C15 induces airway hyperresponsiveness via calcium mobilization in airway smooth muscle. Am J Respir Cell Mol Biol. 2020;62(3):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner RB, Weingand KW, Yeh CH, Leedy DW. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin Infect Dis. 1998;26(4):840–846. [DOI] [PubMed] [Google Scholar]
- 34.Conroy DM, Williams TJ. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir Res. 2001;2(3):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metz G, Kraft M. Effects of atypical infections with Mycoplasma and Chlamydia on asthma. Immunol Allergy Clin North Am. 2010;30(4):575–585. vii-viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Wang Y, Chen C, Liu K. Mycoplasma pneumoniae infection and risk of childhood asthma: a systematic review and meta-analysis. Microb Pathog. 2021;155: 104893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Seasonal variation of viruses detected, asthma control, and acute symptoms. (A) As expected, most of the viruses detected were found in the spring, fall, and winter. (B) There were no differences in asthma control between seasons. (C) Similarly, there were no differences in acute symptoms between seasons.
eTable 1 Respiratory Viral Panel for Viral Genome Sequencing Abbreviations: dsDNA, double-stranded DNA; ssRNA, single-stranded RNA.
eFigure 3. Nasal periostin levels correlate with nasal IL-13 levels (R2 = 0.35; P < .05). IL-13, interleukin 13.
eFigure 2. IgE and acute symptoms during VRAE. (A) Acute symptoms of subjects with uncontrolled VRAE trended higher in those with high total IgE (>371) compared with those with low total IgE (<371) (P = .08). (B) Uncontrolled VRAE with at least one positive specific IgE test had higher PRS than those with uncontrolled VRAE and no positive allergy test result. IgE, immunoglobulin E; PRS, pediatric respiratory symptom; VRAE, virus-related asthma exacerbation.


