Abstract
Background and Objectives:
Prescription opioid use is associated with substance-related adverse outcomes among adolescents and young adults through a pathway of prescribing, diversion and misuse, and addiction and overdose. Assessing the impact of current prescription drug monitoring programs (PDMPs) on opioid prescribing and overdoses will further inform strategies to reduce opioid-related harms.
Methods:
We performed interrupted time-series analyses to measure the association between state-level implementation of PDMPs with annual opioid prescribing and opioid-related overdoses in adolescents (13–18 years) and young adults (19–25 years) between 2008 and 2019. We focused on PDMPs that included mandatory review by providers. Data were obtained from a commercial insurance company.
Results:
Among 9,344,504 adolescents and young adults, 1,405,382 (15.0%) had a dispensed opioid prescription and 6,262 (0.1%) received treatment for an opioid-related overdose. Mandated PDMP review was associated with a 4.2% (95% CI, 1.9 to 6.4%) reduction in annual opioid dispensations among adolescents and a 7.8% (95% CI, 4.7 to 10.9%) annual reduction among young adults. For opioid-related overdoses, mandated PDMP review was associated with a 16.1% (95% CI, 3.8 to 26.7) and 15.9% (95% CI, 7.6 to 23.4) reduction in annual opioid overdoses for adolescents and young adults, respectively.
Conclusions:
PDMPs were associated with sustained reductions in opioid prescribing and overdoses in adolescents and young adults. While these findings support the value of mandated PDMPs as part of ongoing strategies to reduce opioid overdoses, further study with prospective study designs are needed to fully characterize the impact of these programs.
Introduction
Background
Opioids are commonly prescribed to adolescents and young adults for the treatment of acute and chronic pain. While prescribing rates have been generally decreasing for these populations, nearly half of pediatric opioid prescriptions remain high-risk based on excessive duration or daily dosage.1,2 Adolescents appear to be particularly vulnerable to harmful effects of prescription opioids, with use of prescription opioids prior to the 12th grade associated with a 33% increased risk of future opioid misuse, and nonmedical use of prescription opioids more than doubling the risk of subsequent heroin initiation.3,4 While diversion is a common source of prescription medication misuse, as many as a quarter of adolescents and young adults misusing opioids obtain them directly from one or more healthcare providers.5
Forty-nine states in the US have implemented prescription drug monitoring programs (PDMPs) in an effort to reduce high-risk prescribing practices and opioid-related harm. These programs consist of state-run databases that track information on controlled substances dispensed by pharmacies to individual patients. Clinicians can query the system, identify recently dispensed medications, and prevent prescriptions for controlled substances from multiple providers. As these are state-administered programs, there is some variability in key features of individual PDMPs. In particular, certain states require providers to access the PDMP, referred to as mandatory provider review, prior to prescribing a controlled substance.6 Research has shown that these programs are associated with decreased opioid prescribing by emergency and non-emergency medicine physicians,7–9 although the data on the association between PDMPs and opioid-related deaths are mixed.10–14
Importance
Among adolescents and young adults, PDMPs have been linked to reductions in prescription opioid poisonings reported to poison control centers.15 However, the opioid epidemic has been evolving with synthetic opioids now the most commonly implicated opioid in overdose deaths in adolescents and young adults. Among individuals aged 15 to 24 years, between 2010 and 2019, overdose deaths from synthetic opioids other than methadone (a category that includes heroin and illicitly manufactured fentanyl) increased from 0.5% to 7.1% of all deaths from any cause in this age group.16 Many individuals who overdose on heroin or fentanyl, however, first misused prescription opioids.4,17 Understanding the impact of PDMPs on opioid prescribing practices and opioid overdoses could help elucidate gaps in mitigation strategies for this population and inform further policy development to reduce opioid-related harm.
Goals of This Investigation
Using a national private insurance claims database, we sought to examine the association between PDMPs with a mandated provider review and opioid prescribing among adolescents and young adults. In addition, we assessed the association of PDMPs with adolescent and young adult overdoses related to all opioid products and to heroin, specifically.
Methods
Study Design and Data Sources
We performed interrupted time series analyses to evaluate the association between implementation of PDMPs with mandated provider review and healthcare claims for an opioid prescription or medical care for an opioid-related overdose among adolescents (13–18 years) and young adults (19–25 years) between January 1, 2008 and December 31, 2019. Data were administrative claims from a United States (US) commercial health insurance company that included over 83 million members during the study period. Coverage is offered in all 50 states and the insurance provider has a greater than 10% market share in the US. The name of the company is withheld as per our data use agreement. The database undergoes extensive and repeated quality control procedures to ensure the validity and integrity of the data. Data on race was not included in the database.
Data for the analysis consisted of counts of enrollees, stratified by sex, age, state, year and month. Patients were included in monthly counts during which they were enrolled with both pharmacy and medical coverage. Over 9 million individuals aged 13–25 years met this criterion. To measure prescriptions, patients were included in the monthly count if any day of the given month was encompassed within the period of their prescription claim (i.e., from the fill date to the last prescribed day). To measure overdose diagnoses, patients were included in the monthly count if the date of an encounter with a claim for an opioid-related diagnosis fell within the given month. If enrollment dates or date of birth were missing, patients were excluded from all analyses. If sex or state of residence were missing, patients were excluded from counts in which those variables were of interest.
Dispensed Opioid Prescriptions:
We identified enrollees with dispensed opioid prescriptions using the Cerner Multum™ (https://www.cerner.com/solutions/drug-database) drug categories of “narcotic analgesics” and “narcotic analgesic combinations” (S1 Table).
Opioid overdoses:
Enrollees receiving medical care for an opioid-related overdose were defined as those with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis code of opioid poisoning (S2 Table).18,19 These codes do not reliably distinguish between different opioid types, such as prescription opioids vs illicitly manufactured fentanyl. However, there are specific codes for heroin and we selected these to identify patients with heroin-related poisonings.
Prescription drug monitoring programs (PDMPs):
Information on state-level PDMPs was obtained from the Prescription Drug Monitoring Program Training and Technical Assistance Center at Brandeis University, the Prescription Drug Abuse Policy System (PDAPS) database, and individual state PDMP administrators.20,21 We restricted our analysis to states with PDMPs that included mandatory use requirements as this feature has been found to decrease opioid prescribing and overdoses.11,22–26 However, all states, regardless of PDMP status, were included in the analysis, with non-implementing states serving as controls and contributing data exclusively to the pre-implementation period. We defined the “implementation of a PDMP” as the date a program instituted a mandatory use provision and became electronically available in clinical care.8,15 With the state-year as the unit of analysis, we defined a binary PDMP variable as positive if the given year was subsequent to the year of PDMP implementation (or if month of implementation was prior to July 1 when the given year coincided with the year of implementation), and negative if antecedent.
Statistical Analysis
We calculated mean annual proportions of prescribing and overdose diagnoses as the number of events per 100 and 100,000 enrollees, respectively, stratified by age group, sex, region, and time. States were grouped into regions (Northeast, Midwest, South, and West) according to US Census Bureau classifications.27
To test the association between implementation of PDMPs with mandated review and opioid prescribing and overdoses, we used data at the state-year level to perform interrupted time series analyses, leveraging the variation in timing of PDMP implementation across states. We estimated a set of population-averaged negative binomial models as follows:
where is the count outcome variable measured at each equally spaced time point , is time measured in years relative to the year of PDMP adoption, is the annual state-level binary PDMP indicator, and is the time-by-PDMP interaction term to compare pre- and post-implementation slopes. Each model included the log of the annual total number of enrollees as the offset, a first-order autoregressive within-state correlation structure, and a robust variance estimator.
To measure the immediate change following PDMP implementation, we performed a Wald test comparing the outcome measure as derived from the fitted model at the end of the pre-implementation period to the first month of the post-implementation period.28 All states were included in the analysis of pre-implementation and post-implementation trajectories to account for baseline trends in prescribing and overdose. The primary models examined all opioid-related overdoses and secondary analyses included heroin overdoses only. Adolescent and young adult age groups were combined in the analyses of heroin overdoses due to the small number of these overdoses among adolescents.
To account for other state-level policies that may be associated with changes in opioid prescribing and overdose, each model included as covariates implementation of pain clinic legislation and opioid prescribing guidelines.9,15,29,30 Information on these state policies was obtained from PDAPS, the Prescription Drug Monitoring Program Training and Technical Assistance Center at Brandeis University, and prior published research.20,21,31
The negative binomial models provided parameter estimates as incidence rate ratios. However, the units of analysis were individual enrollees (i.e., not person-time). Thus, to express our results in a format consistent with our data, estimates of annual slopes and comparisons between slopes were presented as mean annual percent changes with corresponding 95% confidence intervals (CIs). Estimates of outcome changes from the initial to the final year of the post-implementation period were presented as proportion differences with 95% CIs. Predicted annual proportions of each outcome, stratified by age group and PDMP status, were graphically presented. These graphs included extrapolated estimates of pre-PDMP outcome proportions (i.e., predicted annual outcome proportions if the pre-PDMP period had extended to the end of the post-PDMP period), as generated by the margins command in STATA.
Analyses were conducted with the software package STATA, version 16.0 (College Station, TX). Figures were created using R version 3.6.1 (R Foundation, Vienna, Austria) and the ggplot2 package. The Institutional Review Board at Boston Children’s Hospital determined that this study was exempt as it utilized only de-identified patient data and met criteria for waiver of informed consent. Data analysis took place between January 1 and March 1, 2022. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline.
Results
Study Cohort
Among 9,344,504 adolescents and young adults in the dataset, 1,405,382 (15.0%) had one or more prescription opioid claims and 6,262 (0.1%) received treatment for an opioid overdose between 2008 and 2019 (S1 Figure). Overall, the mean length of enrollment of patients in the study cohort was 21 months (standard deviation, 22 months; median 12 [IQR 6, 25]).
Dispensed Opioid Prescriptions
There were 524,455 (12.5%) adolescents and 949,326 (15.4%) young adults with at least one dispensed opioid prescription (Table 1). More females (772,593; 16.5%) than males (632,711; 13.6%) received an opioid prescription. The South (590,357; 15.9%) accounted for the largest number of enrollees with an opioid prescription, followed by the West (314,950; 15.1%), Midwest (202,493; 14.1%), and Northeast (300,849; 13.7%). Mean annual proportion peaked in 2010 at 1.1 (95% CI, 1.0 to 1.3) and 2.4 (95% CI, 2.3 to 2.5) dispensed prescriptions per 100 adolescents and young adults, respectively. Proportions subsequently decreased to 0.6 (95% CI, 0.5 to 0.7) and 0.9 (95% CI, 0.8 to 1.0) dispensed prescriptions per 100 adolescents and young adults, respectively, by 2019.
Table 1.
Dispensed opioid prescriptions and opioid overdoses among adolescents and young adults, 2008–2019
| Opioid Prescriptions | Opioid Overdoses | |||
|---|---|---|---|---|
| No. of enrollees | Enrollees with dispensed prescription per 100 enrollees | No. of enrollees | Enrollees with overdose per 100,000 enrollees | |
| Age Group | ||||
| 13–18 years (n = 4,195,455) |
524,445 | 12.5 | 1,361 | 32.4 |
| 19–25 years (n = 6,146,070) |
949,326 | 15.4 | 4,941 | 80.4 |
| Sex | ||||
| Female (n = 4,674,101) |
772,593 | 16.5 | 2,758 | 59.0 |
| Male (n = 4,669,413) |
632,711 | 13.6 | 3,504 | 75.0 |
| Region | ||||
| Northeast (n = 2,196,100) |
300,849 | 13.7 | 1,865 | 84.9 |
| Midwest (n = 1,433,120) |
202,493 | 14.1 | 948 | 66.2 |
| South (n = 3,704,149) |
590,357 | 15.9 | 2,145 | 57.9 |
| West (n = 2,085,417) |
314,950 | 15.1 | 1,313 | 63.0 |
The most commonly dispensed opioids among adolescents were acetaminophen-hydrocodone (7.8%), acetaminophen-codeine (2.8%), and acetaminophen-oxycodone (2.4%). Among young adults, acetaminophen-hydrocodone was also most commonly dispensed (9.8%), followed by acetaminophen-oxycodone (4.0%) and acetaminophen-codeine (2.3%).
Opioid Overdoses
Opioid overdoses occurred among 1,361 (32.4 per 100,000 enrollees) adolescents and 4,941 (80.4 per 100,000 enrollees) young adults. Males (3,504; 75.0 per 100,000 enrollees) were more likely to have an overdose compared to females (2,758; 59.0 per 100,000 enrollees). The largest proportion of overdoses was observed in the Northeast (84.9 per 100,000 enrollees), followed by the Midwest (66.2 per 100,000 enrollees), West (63.0 per 100,000 enrollees), and South (57.9 per 100,000 enrollees). For both age groups, the mean annual proportion of opioid overdoses peaked in 2016, with 2.8 (95% CI, 2.5 to 3.2) and 12.1 (95% CI, 11.1 to 13.1) per 100,000 adolescents and young adults, respectively, treated for an opioid overdose.
Association of PDMPs with Opioid Prescribing and Overdose
PDMPs with mandatory use provisions increased from 0 states in 2008 to 39 states by 2019. The largest increase occurred in 2017 with 11 states implementing a PDMP with mandatory use requirement. For the pre-implementation period, there were 249 state-year observations across 50 states with observation periods of up to 5 years. The post-implementation period included 131 state-year observations across 39 states with up to 8 years of follow up.
For both adolescents and young adults, there were significant decreases in the mean annual percentage of enrollees with dispensed opioid prescriptions prior to PDMP implementation, followed by significant decreases after implementation (Table 2, Figure 1 Panel A). Comparing the changes in mean annual percentage of enrollees with dispensed opioid prescriptions in the pre- and post-implementation periods, PDMPs were associated with a 4.2% (95% CI, 1.9 to 6.4%) reduction among adolescents and a 7.8% (95% CI, 4.7 to 10.9%) reduction among young adults. There was also an immediate change in the percentage of enrollees with a dispensed opioid prescription with a 10.2% (95% CI, 6.3 to 14.1%) reduction among adolescents and a 13.6% (95% CI, 10.5 to 16.6%) reduction among young adults. This corresponded to an absolute difference (comparing the first to last year of PDMP implementation) of −0.4 (95% CI, −0.5 to −0.3) prescriptions per 100 adolescents and −0.9 (95% CI, −1.0 to −0.8) prescriptions per 100 young adults (S4 Table).
Table 2.
Adjusted interrupted time series analyses assessing association of PDMPs with mandatory use requirements on dispensed opioid prescriptions and opioid overdoses
| Model Estimates | Opioid Prescriptions % (95% CI) |
Opioid Overdoses % (95% CI) |
||
|---|---|---|---|---|
| Ages 13–18 | Ages 19–25 | Ages 13–18 | Ages 19–25 | |
| Pre-PDMP Mean Annual Change in Percent | −5.4 (−6.8, −4.1) |
−7.1 −9.3, −4.9) |
12.0 (1.9, 23.0) |
21.5 (14.0, 29.5) |
| Post-PDMP Mean Annual Change in Percent | −9.4 (−11.2, −7.5) |
−14.4 (−16.6, −12.1) |
−6.0 (−14.4, 3.2) |
2.2 (−4.8, 9.7) |
| Ratio of Pre-PDMP and Post-PDMP Percent | −4.2 (−6.4, −1.9) |
−7.8 −10.9, −4.7) |
−16.1 (−26.7, −3.8) |
−15.9 (−23.4, −7.6) |
| Immediate Change1 | −10.2 (−14.1, −6.3) |
−13.6 (−16.6, −10.5) |
18.9 (−15.5, 53.3) |
11.9 (−11.5, 35.3) |
Immediate change represents the difference between the outcome percentage derived from the fitted model at the end of the pre-implementation period and the actual percentage at 1 month after implementation.
Figure 1.
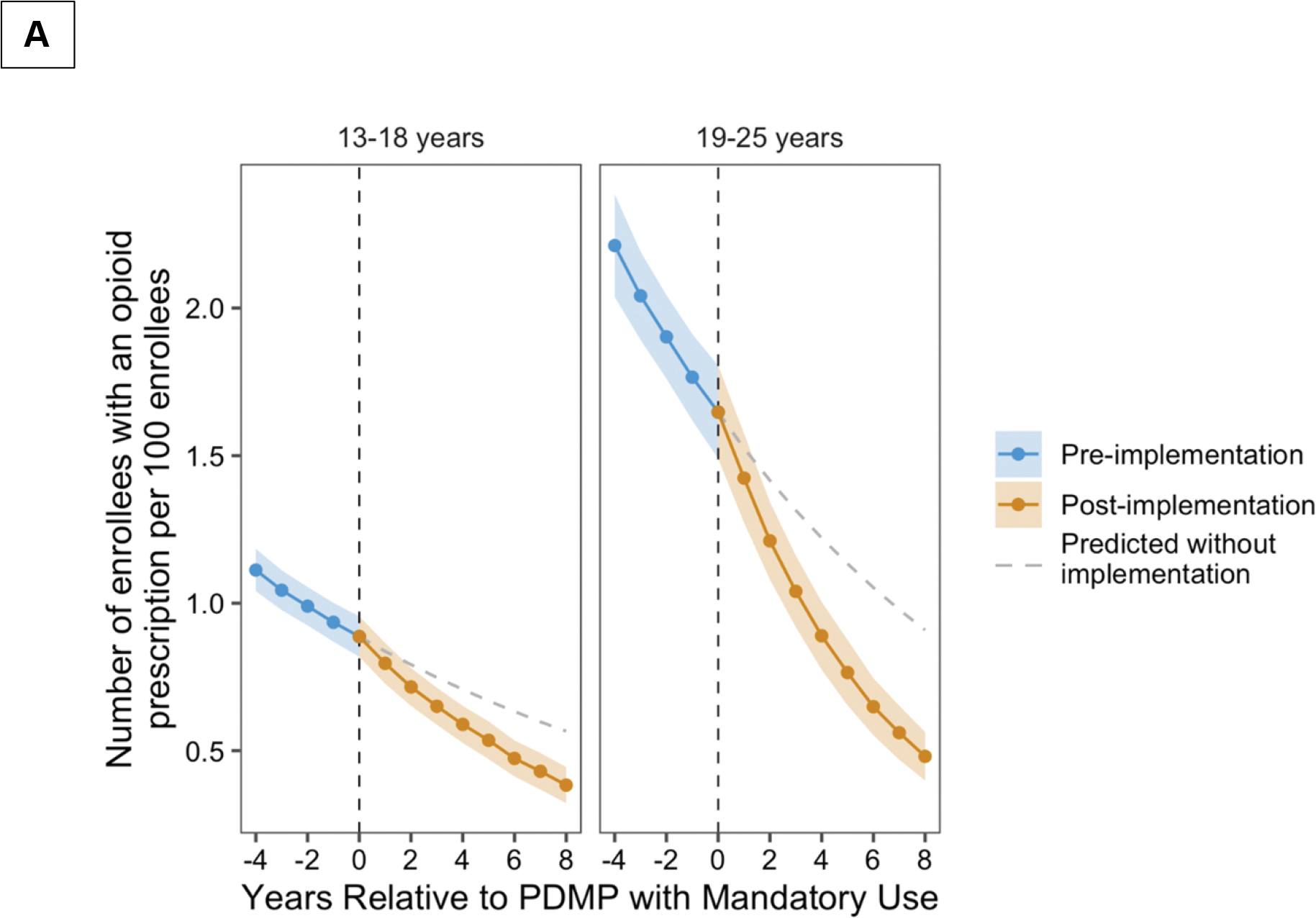
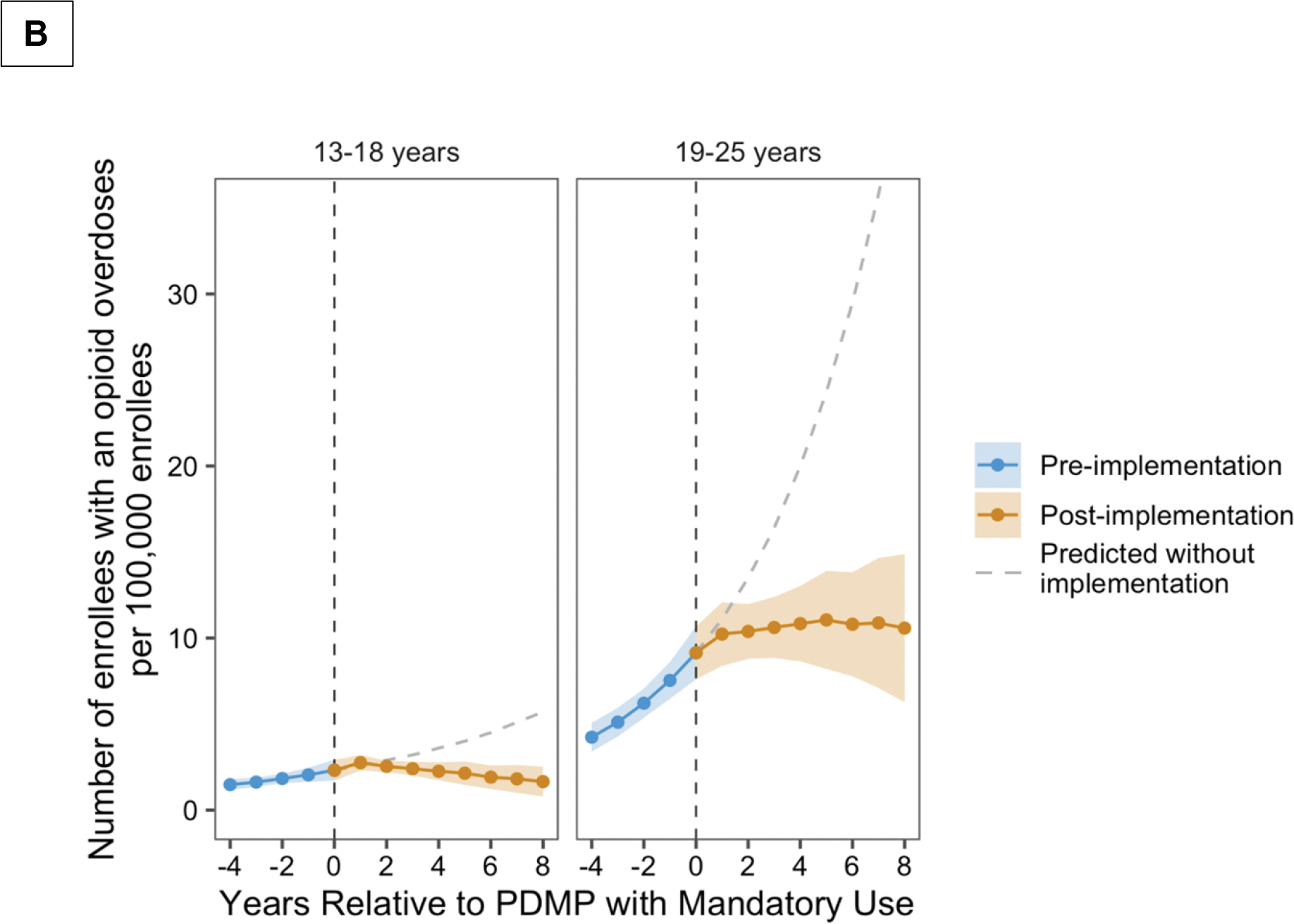
Annual percentage of dispensed opioid prescriptions (Panel A) and opioid-related overdoses (Panel B) in relation to PDMP implementation, stratified by age group and adjusted for opioid prescribing guidelines and pain clinic legislation. The predicted values were derived based on extension of trends in the pre-PDMD period to the post-PDMP period.
The mean annual percentage of enrollees with an opioid overdose increased significantly prior to PDMP implementation among both adolescents and young adults (Figure 1 Panel B). Comparison of the changes in mean annual percentage of enrollees with opioid overdoses in the pre- and post-implementation periods showed significant reductions in opioid overdoses for adolescents (16.1%, [95% CI, 3.8 to 26.7%]) and young adults (15.9%, [95% CI, 7.6 to 23.4%]) following PDMP implementation. No statistically significant immediate change in opioid overdoses was observed in either age group. The absolute difference in opioid-related overdoses between the first and last year of PDMP implementation was −1.1 (95% CI, −2.2 to 0.1) and 0.3 (95% CI, −4.7 to 5.4) overdoses per 100,000 adolescents and young adolescents, respectively.
Heroin Overdoses and Association with PDMPs
Overdoses related to heroin were relatively uncommon among adolescents (4.0 per 100,000 enrollees) compared to young adults (36.4 per 100,000 enrollees) and occurred almost twice as often among males (33.7 per 100,000 enrollees) compared to females (17.3 per 100,000 enrollees). The geographic distribution was similar to that of overall opioid overdoses, with the highest proportion in the Northeast (43.3 per 100,000 enrollees), followed by the Midwest (26.3 per 100,000 enrollees), West (19.1 per 100,000 enrollees) and South (17.8 per 100,000 enrollees). Comparison of changes in mean annual percentage of adolescents and young adults with heroin overdoses in the pre- and post-implementation periods, showed that PDMPs were associated with an annual reduction of 16.7% (95% CI, 5.9 to 26.2%) in heroin overdoses (S3 Table).
Limitations
This study has several limitations. First, we analyzed healthcare claims from a single, private insurance provider and are unable to independently verify the quality of the data. Further, results may not be generalizable to individuals with other types of insurance plans. Inherent to any interrupted time series analysis is the possibility of residual confounders. We attempted to address this by controlling for several of the most widely used state policies (e.g., opioid prescribing guidelines and pain clinic legislation) that may be associated with reductions in opioid prescribing and overdose. Additionally, one of the strengths of the current analysis is the staggered implementation of PDMPs with mandatory use requirements, which helps control for temporal trends in opioid prescribing practices.10 However, causality cannot be inferred from our study and it is possible that secular trends contributed to the findings. Further, we measured dispensed prescriptions, which may not necessarily equate to medication use. Finally, healthcare claims data likely underestimate the true number of opioid overdoses as diagnostic codes may be specific, but not sensitive for opioid overdoses, and claims data do not capture fatal and nonfatal overdoses that do not present to healthcare facilities.32 The available data also do not allow further analysis to distinguish between overdoses related to prescription opioids versus synthetic opioids.
Discussion
In this national cohort study of 9.3 million commercially insured US adolescents and young adults, PDMPs with mandatory use requirements were associated with sustained annual reductions in opioid prescribing of 4.2% among adolescents and 7.8% among young adults. Additionally, these PDMPs were associated with 16.1% and 15.9% annual reductions in opioid overdoses, respectively. However, given the retrospective nature of these data, causality cannot be inferred. Further, the small overall reductions, while statistically significant, may have limited clinical implications.
Opioid prescriptions among adolescents and young adults have been linked to subsequent opioid misuse and overdose.3,33 These adverse outcomes appear to be increased irrespective of the acuity of the treatment indication or the practice setting, and extend into later adulthood and to misuse of other illicit substances.34,35 In adult populations, studies have shown a positive impact of PDMPs on reducing initiation and number of opioid prescriptions.7–9,11,36 Our findings show that PDMPs with mandatory provider review are associated with decreased opioid prescribing among adolescents and young adults. However, it is important to note that we cannot exclude the impact of other factors on opioid prescribing patterns, and that the observed reductions may be influenced by secular changes.
Studies examining the association between PDMPs and opioid overdoses have generated inconsistent findings, with results varying based on the types of opioids examined and timing of the study. Several analyses have found that PDMPs, particularly those with more robust features such as mandatory review, were associated with reductions in prescription opioid overdoses.11,12,37,38 Others have reported no association between PDMPs and emergency department visits for prescription opioid overdoses or prescription opioid-related deaths.39,40 When examining overdoses related to all opioids (i.e. encompassing prescription opioids, heroin, and synthetic products) an early analysis examining overdose deaths between 1999 and 2005 found no association between PDMPs and overall opioid mortality.41 A subsequent study extending to 2013 reported a reduction of 1.12 opioid-related deaths per 100,000 individuals in the year following PDMP implementation.10 These differences may be attributable to the strengthening of PDMPs with the adoption of additional features between 2005 and 2010, such as mandatory provider review and inter-state data sharing.42 For adolescents and young adults, there have been few studies examining the impact of PDMPs on opioid overdoses. In a prior analysis, we found an association between PDMP implementation and reduction in poisonings from prescription opioids reported to poison control centers.15 Another study examined injection drug use among adolescents and reported a decrease following implementation of PDMPs with mandatory use requirements.25 However, the impact of PDMPs on overall opioid overdoses among adolescents and young adults has not previously been assessed.
PDMPs are expected to reduce opioid-related overdoses by increasing the information available to prescribers to allow more informed and judicious prescribing of opioids. However, it is important to monitor the impact of these programs in the context of the current opioid epidemic in the US, which has evolved from misuse of prescription opioids to use of heroin and subsequently synthetic opioids, such as illicitly produced fentanyl.16 The reasons for this shift are multifactorial: the overall reductions in opioid prescribing that began in 2010, as well as the reformulation of Oxycontin (one of the most widely misused prescription opioids) to a new product that could no longer be crushed to yield a dissolvable powder and forced opioid-dependent individuals to seek alternative opioids, such as heroin, which became more available and cheaper than prescription opioids.43–46 The transition from heroin to fentanyl and fentanyl-analogs arose as illicit fentanyl manufacturers, largely located overseas, took advantage of the potency and powdered nature of fentanyl, flooding markets and adulterating the US heroin supply.47,48 Examining the association of PDMPs on overall opioid-related overdoses provides insight on the impact of these programs during the current state of the opioid epidemic. We found that PDMPs with mandated provider review were associated with sustained reductions in opioid-related overdoses among adolescents and young adults, supporting their value as a component in multi-pronged efforts to reduce opioid overdoses. It is important to stress that PDMPs should be combined with other strategies, such as expanded access to medication for opioid-use disorder and naloxone, to further decrease opioid overdoses in adolescents and young adults.49
A concern that has been raised with the implementation of PDMPs, is that by restricting access to prescription opioids, those with opioid-use disorder or dependence may transition to heroin use.11,50 Among adults, reports have indicated that PDMPs may be associated with increases in heroin-related mortality.50,51 Conversely, misuse of prescription opioids in adolescence has been linked to subsequent initiation of heroin use in later adolescence and early adulthood.17 Our analysis found a reduction of 16.7% in the annual percentage of heroin overdoses after PDMP implementing, suggesting that mandated use of PDMPs does not contribute to an increase in heroin overdoses in this population.
In summary, PDMPs with mandatory use requirements were associated with sustained reductions in dispensed opioid prescriptions to adolescents and young adults, as well as decreases in opioid-related overdoses. These findings support the implementation of mandated PDMPs as part of ongoing strategies to reduce opioid overdoses. However, further study with prospective study designs is needed to fully characterize the impact of these programs.
Supplementary Material
Funding/Support:
Dr. Michael S. Toce received a research award from the Medical Toxicology Foundation (https://www.acmt.net/mtf/mtf-innovative-research-and-teachinggrants.html) in support of this study. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of Interest Disclosures: The authors have no conflicts of interest relevant to this article to disclose.
Meetings: Portions of this work were presented at the ACMT Annual Scientific Meeting, 2020 (virtual).
References
- 1.Renny MH, Yin HS, Jent V, Hadland SE, Cerdá M. Temporal Trends in Opioid Prescribing Practices in Children, Adolescents, and Younger Adults in the US From 2006 to 2018. JAMA Pediatr. 2021;175(10):1043. doi: 10.1001/jamapediatrics.2021.1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua K, Brummett CM, Conti RM, Bohnert AS. Opioid Prescribing to US Children and Young Adults in 2019. Pediatrics. 2021;148(3):e2021051539. doi: 10.1542/peds.2021-051539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miech R, Johnston L, O’Malley PM, Keyes KM, Heard K. Prescription Opioids in Adolescence and Future Opioid Misuse. Pediatrics. 2015;136(5):e1169–77. doi: 10.1542/peds.2015-1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley-Quon LI, Cho J, Strong DR, et al. Association of Nonmedical Prescription Opioid Use With Subsequent Heroin Use Initiation in Adolescents. JAMA Pediatr. 2019;90033:e191750. doi: 10.1001/jamapediatrics.2019.1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudgins JD, Porter JJ, Monuteaux MC, Bourgeois FT. Prescription opioid use and misuse among adolescents and young adults in the United States: A national survey study. Alegria M, ed. PLOS Med. 2019;16(11):e1002922. doi: 10.1371/journal.pmed.1002922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haffajee R, Jena A, Weiner S. Mandatory Use of Prescription Drug Monitoring Programs. Jama. 2015;313(9):891–892. doi: 10.1001/jama.2014.18514.Conflict [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haffajee RL, Mello MM, Zang F, Zaslavsky AM, Larochelle MR, Wharam JF. Four states with robust prescription drug monitoring programs reduced opioid dosages. Health Aff. 2018;37(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao Y, Pan Y, Taylor A, et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Aff. 2016;35(6):1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutkow L, Chang H-Y, Daubresse M, Webster DW, Stuart E a., Alexander GC. Effect of Florida’s Prescription Drug Monitoring Program and Pill Mill Laws on Opioid Prescribing and Use. JAMA Intern Med. 2015;175(10):1642. doi: 10.1001/jamainternmed.2015.3931 [DOI] [PubMed] [Google Scholar]
- 10.Patrick SW, Fry CE, Jones TF, Buntin MB. Implementation Of Prescription Drug Monitoring Programs Associated With Reductions In Opioid-Related Death Rates. Health Aff. 2016;35(7):1324–1332. doi: 10.1377/hlthaff.2015.1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowell D, Zhang K, Noonan RK, Hockenberry JM. Mandatory Provider Review And Pain Clinic Laws Reduce The Amounts Of Opioids Prescribed And Overdose Death Rates. Health Aff (Millwood). 2016;35(10):1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerdá M, Ponicki WR, Smith N, et al. Measuring Relationships Between Proactive Reporting State-level Prescription Drug Monitoring Programs and County-level Fatal Prescription Opioid Overdoses. Epidemiology. 2020;31(1):32–42. doi: 10.1097/EDE.0000000000001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fink DS, Schleimer JP, Sarvet A, et al. Association Between Prescription Drug Monitoring Programs and Nonfatal and Fatal Drug Overdoses. Ann Intern Med. 2018;168(11):783. doi: 10.7326/M17-3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finley EP, Garcia A, Rosen K, McGeary D, Pugh MJ, Potter JS. Evaluating the impact of prescription drug monitoring program implementation: a scoping review. BMC Health Serv Res. 2017;17(1):420. doi: 10.1186/s12913-017-2354-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toce MS, Michelson K, Hudgins J, Burns MM, Monuteaux MC, Bourgeois FT. Association of State-Level Opioid-Reduction Policies With Pediatric Opioid Poisoning. JAMA Pediatr. 2020;02115:1–8. doi: 10.1001/jamapediatrics.2020.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute on Drug Abuse: Overdose Death Rates. https://www.drugabuse.gov/drug-topics/trends-statistics/overdose-death-rates. Accessed October 5, 2021.
- 17.Cerdá M, Santaella J, Marshall BDL, Kim JH, Martins SS. Nonmedical Prescription Opioid Use in Childhood and Early Adolescence Predicts Transitions to Heroin Use in Young Adulthood: A National Study. J Pediatr. 2015;167(3):605–612.e2. doi: 10.1016/j.jpeds.2015.04.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaither JR, Shabanova V, Leventhal JM. US National Trends in Pediatric Deaths From Prescription and Illicit Opioids, 1999–2016. JAMA Netw Open. 2018;1(8):e186558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua K-P, Brummett CM, Conti RM, Bohnert A. Association of Opioid Prescribing Patterns With Prescription Opioid Overdose in Adolescents and Young Adults. JAMA Pediatr. 2020;174(2):141. doi: 10.1001/jamapediatrics.2019.4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prescription Drug Abuse Policy System. http://pdaps.org/. Accessed December 1, 2017.
- 21.Prescription Drug Monitoring Program Training and Technical Assistance Center. http://www.pdmpassist.org/. Accessed June 1, 2020.
- 22.Brown R, Riley MR, Ulrich L, et al. Impact of New York prescription drug monitoring program, I-STOP, on statewide overdose morbidity. Drug Alcohol Depend. 2017;178(February):348–354. doi: 10.1016/j.drugalcdep.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 23.Grecu AM, Dave DM, Saffer H. Mandatory Access Prescription Drug Monitoring Programs and Prescription Drug Abuse. J Policy Anal Manag. 2019;38(1):181–209. doi: 10.1002/pam.22098 [DOI] [PubMed] [Google Scholar]
- 24.Lee B, Zhao W, Yang KC, Ahn YY, Perry BL. Systematic Evaluation of State Policy Interventions Targeting the US Opioid Epidemic, 2007–2018. JAMA Netw Open. 2021;4(2):2007–2018. doi: 10.1001/jamanetworkopen.2020.36687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Earlywine JJ, Hadland SE, Raifman J. State-level prescription drug monitoring program mandates and adolescent injection drug use in the United States, 1995–2017: A difference-in-differences analysis. PLoS Med. 2020;17(9):1–13. doi: 10.1371/journal.pmed.1003272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchmueller TC, Carey C. The effect of prescription drug monitoring programs on opioid utilization in medicare. Am Econ J Econ Policy. 2018;10(1):77–112. doi: 10.1257/pol.20160094 [DOI] [Google Scholar]
- 27.Census Regions and Divisions of the United States. https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf. Accessed January 1, 2019.
- 28.Bohnert ASB, Guy GP, Losby JL. Opioid prescribing in the United States before and after the centers for disease control and prevention’s 2016 opioid guideline. Ann Intern Med. 2018;169(6):367–375. doi: 10.7326/M18-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiner SG, Baker O, Poon SJ, et al. The Effect of Opioid Prescribing Guidelines on Prescriptions by Emergency Physicians in Ohio. In: Annals of Emergency Medicine. Vol 70. American College of Emergency Physicians; 2017:799–808.e1. doi: 10.1016/j.annemergmed.2017.03.057 [DOI] [PubMed] [Google Scholar]
- 30.Liang D, Shi Y. The association between pain clinic laws and prescription opioid exposures: New evidence from multi-state comparisons. Drug Alcohol Depend. 2020;206(November 2019):107754. doi: 10.1016/j.drugalcdep.2019.107754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis CS, Lieberman AJ. Laws limiting prescribing and dispensing of opioids in the United States, 1989–2019. Addiction. 2021. doi: 10.1111/add.15359 [DOI] [PubMed] [Google Scholar]
- 32.Rowe C, Vittinghoff E, Santos GM, Behar E, Turner C, Coffin PO. Performance Measures of Diagnostic Codes for Detecting Opioid Overdose in the Emergency Department. Acad Emerg Med. 2017;24(4):475–483. doi: 10.1111/acem.13121 [DOI] [PubMed] [Google Scholar]
- 33.Schroeder AR, Dehghan M, Newman TB, Bentley JP, Park KT. Association of Opioid Prescriptions from Dental Clinicians for US Adolescents and Young Adults with Subsequent Opioid Use and Abuse. JAMA Intern Med. 2019;179(2):145–152. doi: 10.1001/jamainternmed.2018.5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Worsham CM, Woo J, Jena AB, Barnett ML. Adverse events and emergency department opioid prescriptions in adolescents. Health Aff. 2021;40(6):970–978. doi: 10.1377/hlthaff.2020.01762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinn PD, Fine KL, Rickert ME, et al. Association of Opioid Prescription Initiation during Adolescence and Young Adulthood with Subsequent Substance-Related Morbidity. JAMA Pediatr. 2020;174(11):1048–1055. doi: 10.1001/jamapediatrics.2020.2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang HY, Lyapustina T, Rutkow L, et al. Impact of prescription drug monitoring programs and pill mill laws on high-risk opioid prescribers: A comparative interrupted time series analysis. Drug Alcohol Depend. 2016;165:1–8. doi: 10.1016/j.drugalcdep.2016.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardo B Do more robust prescription drug monitoring programs reduce prescription opioid overdose? Addiction. 2017;112(10):1773–1783. doi: 10.1111/add.13741 [DOI] [PubMed] [Google Scholar]
- 38.Pauly NJ, Slavova S, Delcher C, Freeman PR, Talbert J. Features of prescription drug monitoring programs associated with reduced rates of prescription opioid-related poisonings. Drug Alcohol Depend. 2018;184(August 2017):26–32. doi: 10.1016/j.drugalcdep.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maughan BC, Bachhuber MA, Mitra N, Starrels JL. Prescription monitoring programs and emergency department visits involving opioids, 2004–2011. Drug Alcohol Depend. 2015;156(January):282–288. doi: 10.1016/j.drugalcdep.2015.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nam YH, Shea DG, Shi Y, Moran JR. State prescription drug monitoring programs and fatal drug overdoses. Am J Manag Care. 2017;23(5):297–303. [PubMed] [Google Scholar]
- 41.Paulozzi LJ, Kilbourne EM, Desai H a, Sci MM, Church F, Hamilton BA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12(November):747–754. doi: 10.1111/j.1526-4637.2011.01062.x [DOI] [PubMed] [Google Scholar]
- 42.Smith N, Martins SS, Kim J, et al. A typology of prescription drug monitoring programs: a latent transition analysis of the evolution of programs from 1999 to 2016. Addiction. 2019;114(2):248–258. doi: 10.1111/add.14440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cicero TJ, Ellis MS, Surratt HL. Effect of Abuse-Deterrent Formulation of OxyContin. N Engl J Med. 2012;367(2):187–189. doi: 10.1056/NEJMc1204141 [DOI] [PubMed] [Google Scholar]
- 44.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA psychiatry. 2014;71(7):821–826. doi: 10.1001/jamapsychiatry.2014.366 [DOI] [PubMed] [Google Scholar]
- 45.Guy GP, Zhang K, Bohm MK, et al. Vital Signs: Changes in Opioid Prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cicero TJ, Inciardi JA, Muñoz A. Trends in abuse of OxyContin® and other opioid analgesics in the United States: 2002–2004. J Pain. 2005;6(10):662–672. doi: 10.1016/j.jpain.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 47.Ciccarone D Fentanyl in the US heroin supply: A rapidly changing risk environment. Int J Drug Policy. 2017;46(2017):107–111. doi: 10.1016/j.drugpo.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciccarone D The triple wave epidemic: Supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019;71:183–188. doi: 10.1016/j.drugpo.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toce MS, Hadland SE. Commentary on Hill et al. : Breaking down barriers—increasing access to lifesaving opioid use disorder medications to save lives. Addiction. 2021;116(6):1512–1513. doi: 10.1111/add.15375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martins SS, Ponicki W, Smith N, et al. Prescription drug monitoring programs operational characteristics and fatal heroin poisoning. Int J Drug Policy. 2019;74(2019):174–180. doi: 10.1016/j.drugpo.2019.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delcher C, Wang Y, Wagenaar AC, Goldberger BA, Cook RL, Maldonado-Molina MM. Prescription and Illicit Opioid Deaths and the Prescription Drug Monitoring Program in Florida. Am J Public Health. 2016;106(6):e10–e11. doi: 10.2105/AJPH.2016.303104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


