Abstract
IL-12 family members IL-12, IL-23, IL-27 and IL-35 are heterodimeric cytokines that are important players in autoimmunity. Subunits are shared among the IL-12 family and these cytokines, excluding IL-35, are mainly produced by antigen presenting cells. In this review we will initially describe the structure, regulation and functional relationship among IL-12 family members. We will also discuss how these cytokines contributed to a paradigm shift in T cell biology and orchestrate immune mediated inflammation. We further highlight that the cross-regulation between these factors is linked to adaptive immunity. Our discussion will be focused on each family member’s impact on rheumatoid arthritis (RA). We finally discuss how results from the bench can assist us in designing future therapeutic approaches for RA. However, the findings regarding the IL-12 family members are a relatively new area of research and the data is mostly at the preclinical stage therefore, more human data is required to determine whether these cytokines may be valid therapeutic targets in RA.
Keywords: IL-12 family cytokines, IL-23, IL-27, IL-35, Rheumatoid arthritis
INTRODUCTION
IL-23, IL-27 and IL-35, a family of IL-12 related heterodimeric cytokines
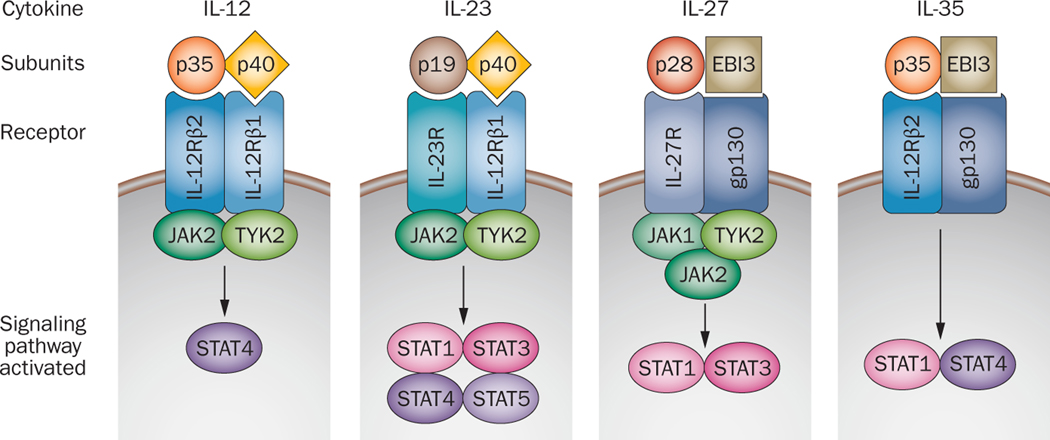
IL-12 family members consisting of IL-12, IL-23, IL-27 and IL-35 are unique type I cytokines that are pleiotropic by nature, share receptor subunits and are influential in determining the T cell fate. IL-12, the family prototype, is composed of IL-12p40 and IL-12p35 subunits. The p40 subunit is shared between IL-12 and IL-23, and heterodimerization of p19 with the p40 domain is required for IL-23 secretion 1. While IL-35 shares its p35 subunit with IL-12 and the EBI3 domain with IL-27, the EBI3 also dimerizes with p28 to form IL-27 2 (Figure 1). Because of the structural relationship between IL-12 and IL-23, IL-12Rβ1 is used by both cytokines. IL-12Rβ1 combines with IL-12Rβ2 to form the receptor for IL-12 and with IL-23R to bind to IL-23 3. The IL-27 receptor is comprised of the IL-27R and gp130 (Figure 1), while the IL-35 receptor consists of IL-12Rβ2 and gp130 4. In addition to it role in binding to IL-27 and IL-35, the gp130 receptor is shared with a variety of other cytokines including IL-6 2, 4 (Figure 1).
Figure 1. The IL-12 cytokine family; their structure and activated signaling pathways.
IL-12, IL-23, IL-27 and IL-35 are heterodimeric cytokines that belong to IL-12 family. These IL-12 family siblings comprise of both common as well as unique subunits and binding receptors. In addition to their structural relationship, the IL-12 family members activate overlapping JAK/STAT signaling pathways which explains some of their mutual characteristics. IL-12, IL-23 and IL-27 structure, expression and mechanism of action is well studied and our knowledge about IL-35 is rapidly growing.
In addition to the structural relationship between the IL-12 family members they share some biological characteristics 5. Except for IL-35, all IL-12 family cytokines are produced from monocytes, macrophages and dendritic cells in response to microbial or host immune stimuli such as toll like receptors (TLRs) and interferons (IFNs) 5. Further, IL-12p35, IL-23p19 and IL-27p28 alone are poorly secreted in the absence of heterodimerization, with p40 (for p35 and p19) or with EBI3 for p28, since tight regulation of these factors avoids severe inflammation 5. Another common feature of this group is that they signal through the JAK/STAT pathway, hence, a mechanism contributing to their overlapping effects on T cells (Figure 1) 6. Despite these similarities, each family member has distinct characteristics. In following sections, we discuss details of each IL-12 family member and also its link to RA pathogenesis reported in preclinical as well as in clinical studies.
Role of IL-12 in RA and experimental arthritis
IL-12R is expressed on T cells, NK cells and dendritic cells. IL-12 has a key role in promoting polarization of naïve CD4+ T cells to mature TH-1 effector cells that produce IFN-γ. IL-12 is also capable of inducing production of IFN-γ from NK cells and CD8+ T cells. IL-18 is another cytokine that promotes TH-1 differentiation and IFN-γ production 7. Further, IL-18 synergizes with IL-12 in modulating secretion of IFN-γ from a variety of different cell types which include CD4+ and CD8+ T cells, NK cells and mouse bone marrow derived macrophages7. Polarization of TH-1 cells is initially mediated through ligation of IL-12 to its receptor leading to activation of STAT1 and T-bet. However after the initiation of TH-1 differentiation, the transcription factor T-bet which is the TH-1 master regulator is mainly induced by IFN-γ and this process is potentiated by IL-12 3. Additionally positive feed back regulation exists between T-bet transcription and increased IFN-γ which promotes further IL-12R expression enhancing the secretion of IFN-γ 6. The stimulatory effect of IFN-γ and the inhibitory effect of IL-4 on IL-12Rβ2 expression, is an important regulatory mechanism for TH-1 differentiation. Another shared effect of IL-12 and IFN-γ is their ability to antagonize TH-2 polarization and the production of IL-4 and IL-13 3.
Initial observations indicated that TH-1 cells may be associated with RA pathogenesis as IL-12 is elevated in RA serum and synovial fluid and its levels correlate with disease activity score 8. Also TH-1 cells were shown to be the most frequent T cells despite low levels of IFN-γ detected in the RA joint 9. However recent studies demonstrate that the ratio of TH-17 to TH-1 cells is greatly increased in RA synovial fluid compared to RA peripheral blood in early naïve RA patients (>3 months disease onset) 10, consistent with the observation of higher levels of IL-17 compared to IFN-γ in RA joint.
Hence to further examine role of TH-1 and TH-17 cells in disease pathogenesis, collagen induced arthritis (CIA), a murine model of RA that has many similarities to human disease including symmetrical synovitis and presence of rheumatoid factor was employed 11. Earlier studies demonstrate that co-administration of collagen type II with IL-12 exacerbated CIA 12. In contrast, IL-12 treatment in established CIA mice alleviated the disease suggesting that IL-12 therapy may have a pro- or anti-inflammatory effect depending on when the treatment is initiated 13. Results from gene targeted mice deficient in IFN-γ or IFN-γ receptor demonstrated that loss of IFN-γ unexpectedly exacerbated CIA which was shown to be due to activation of the TH-17/IL-17 pathway and reduction of TH-1 cells, suggesting that IFN-γ plays a protective role in CIA by inhibiting TH-17 differentiation 14, 15 (Table 1, supplemental material). Because of mechanistic differences, animal models only partially reflect the human disease therefore further clinical studies are required to determine effective targets for RA treatment.
Role of IL-23 in RA and murine models of RA
IL-23 mediates chronic inflammation through survival and maintenance of TH-17 cells, production of IL-17 from non-T cells (neutrophils) or induction of IL-1, TNF-α and IL-6 production from myeloid cells and/or RA synovial fibroblasts as summarized in Figure 216, 17. Nevertheless, the TH-17 cells play a key role in IL-23 induced inflammation since neutralization of IL-23 significantly reduces the in vivo induction of IL-17, and TH-17 cells are absent in IL-23 deficient p19−/− or p40−/− mice 18.
Although IL-17 and IL-23p19 protein levels were elevated in RA synovial tissue, fluid and peripheral blood compared to osteoarthritis (OA) controls 17, in the same RA specimens IL-23p19 protein levels were significantly higher than the p40 expression19, 20. However, biologically active IL-23 requires the production and heterodimerization of both the p40 and p19 subunits within the same cell, which was either undetectable or found at very low levels in synovial fluid, tissue and sera of RA patients 19, 20. These observations question the role of IL-23 in promoting TH-17 differentiation in RA, suggesting other mechanisms such as IL-1β and IL-6 might be more important in this disease. Nonetheless, IL-23p19 is elevated in IL-17 positive compared to IL-17 negative synovial tissues 21 suggesting that there may be a positive feed back loop between low levels of biologically active IL-23 and TH-17 cells in the RA joint. Further, RA synovial fluid macrophages as well as normal monocyte derived dendritic cells, are capable of producing IL-23p19p40 in response to TLR ligation, while IL-23p19 expression is upregulated by IL-1β, TNF-α and IL-17 in RA synovial tissue fibroblasts 19, 20, 22, 23. Also supporting the importance of IL-23p19 in the pathogenesis of RA, serum IL-23p19 levels correlate with serum concentrations of IL-1β, TNF-α and IL-17 as well as bony erosion 24. Given these varied observations, it may take a therapeutic trial to neutralize IL-23 to determine its role in RA.
Earlier studies have shown that IL-23 mediates osteoclastogenesis directly as well as indirectly via IL-17. IL-23 directly activates osteoclast differentiation through induction of RANK and cathepsin K in RAW cells in the absence of IL-17 25. It was also shown that IL-23 can induce production of RANKL in the conditioned media of RA fibroblasts cocultured with osteoclast precursors resulting in osteoclast differentiation 26. Further, employing RA synovial tissue, a positive correlation has been observed between IL-23p19 and RANKL mRNA suggesting that IL-23 activated RANKL may contribute to bone destruction 27. However, IL-23 also contributes to osteoclastogenesis through maintainence of TH-17 cells and production of IL-17, as it has been shown that IL-17 can promote bone destruction through activation of prostaglandin E2 (PGE2) as well as RANKL 28.
Previous findings show that IL-17 deficient mice 29 are not as resistant as IL-23 p19−/− mice 18 in developing CIA, further supporting the role of IL-23 in mediating inflammation and bone erosion independent of IL-17 16. Other IL-17 independent pathways associated with IL-23 induced pathology include production of TNF-α and IL-1β from antigen presenting cells as well as IL-6 and IL-8 from synovial tissue fibroblasts. Supporting this notion, levels of TNF-α and IL-1β were elevated in IL-23p19 transgenic mice which exhibit systemic inflammation, impaired cell growth and premature death, however there was no definitive evidence to conclude that these observations were independent of TH-17 and/or IL-17 pathway 30. In a recent study peptide-based vaccine against IL-23p19 ameliorated CIA without affecting joint, spleen and lymph node IL-17 mRNA levels further suggesting that the effects of IL-23 may not be restricted to IL-17/TH-17 cells 31. In summary, although experimental arthritis models document an important role for IL-23 in disease pathology, the fact that p40 is weakly expressed in RA synovial tissue, fluid and serum, and bioactive IL-23 is not increased in RA synovial fluid and peripheral blood raises the question whether or not therapeutic approaches directed against IL-23 will be effective in RA, as they have been in experimental models.
Unique roles of IL-12 and IL-23 in CIA
Distinctive roles of IL-12 and IL-23 were demonstrated in CIA disease development since mice deficient in p19 (IL-23) and p40 (IL-23 and IL-12) were resistant to CIA while p35 (IL-12) knockout mice were highly susceptible 18 (Table 1, supplemental material). Later, it was shown that the suppressive effect of TH-1 cells and/or their defining cytokine IFN-γ in CIA may be due in part to its suppressive effect on the IL-23/TH-17 axis 15. These findings suggest that IL-23 is directly linked to CIA progression and that the inflammatory process may be dependent upon the balance between TH-1 and TH-17 cells.
Multiple functions of IL-27 in RA and other autoimmune disease
IL-27 is expressed in RA synovial tissue and is also induced by ligation of RA synovial fluid macrophages by TLR ligands 20, 32. Expression of IL-27 is also modulated by IFN-γ and IL-27 promotes the initial step of TH-1 differentiation via STAT1 activation and IFN-γ production 33. These findings were confirmed in IL-27R deficient mice where antigen stimulation demonstrated impaired TH-1 development and reduction in IFN-γ production which resulted in shift from a TH-1 to a TH-2 response 34. In proteoglycan-induced arthritis, a TH-1 mediated arthritis model, IL-27R−/− mice developed less severe arthritis due to reduced IFN-γ producing T cells 35.
Besides IL-27’s effect on TH-1 and TH-2 differentiation, recent evidence demonstrates that IL-6 and TGF-β induced TH-17 polarization are suppressed by IL-27. It was further documented that IL-27R deficient mice develop more severe experimental autoimmune encephalomyelitis (EAE) by enhancing TH-17 cell differentiation and EAE disease severity which was ameliorated employing a neutralizing antibody to IL-17 36, 37. The suppressive effect of IL-27 on TH-17 cells was shown to be independent of the TH-1/IFN-γ pathway since double knockouts of IFN-γ and IL-27R demonstrated a more severe EAE compared to either knockout alone 36, 37. Others have shown that IL-27 is a strong suppressor of TH-17 cell differentiation through an IL-10 and a STAT1/STAT3 dependent mechanism 38. More recently it was demonstrated that IL-27 promotes the development of CD4+/Foxp3‾ regulatory T cells, referred as Tr-1 cells, which produce IL-10 in response to IL-27, while inhibiting the development of IL-2 expressing CD4+/Foxp3+ Tregs 39.
Recent studies support the anti-inflammatory role of IL-27 in myeloid cells. IL-27’s anti-inflammatory effect is mediated by IL-10 production from murine bone marrow derived macrophages 40 as well as suppressing macrophage responses to TNF-α and IL-1β by downregulating their corresponding receptors 41. IL-27 can also directly restrain pre-osteoclast differentiation to mature osteoclasts by suppressing RANK expression on myeloid cells 42.
The anti-inflammatory effects of IL-27 treatment early in the course of CIA include the reduction of joint IL-6 and IL-1β, cytokines responsible for TH-17 polarization, as well as suppression of IL-17 mediated monocyte migration and vascularization 43 (Figure 2). In contrast IL-27 treatment during established disease does not suppress CIA progression 32. For IL-27 to effectively inhibit TH-17 cell polarization higher levels of this cytokine need to be present in the early stage of experimental arthritis, prior to IL-17 production from mature TH-17 cells. Perhaps like in experimental arthritis models, RA patients may differentially respond to IL-27 therapy depending on whether their disease is in early onset or established stage.
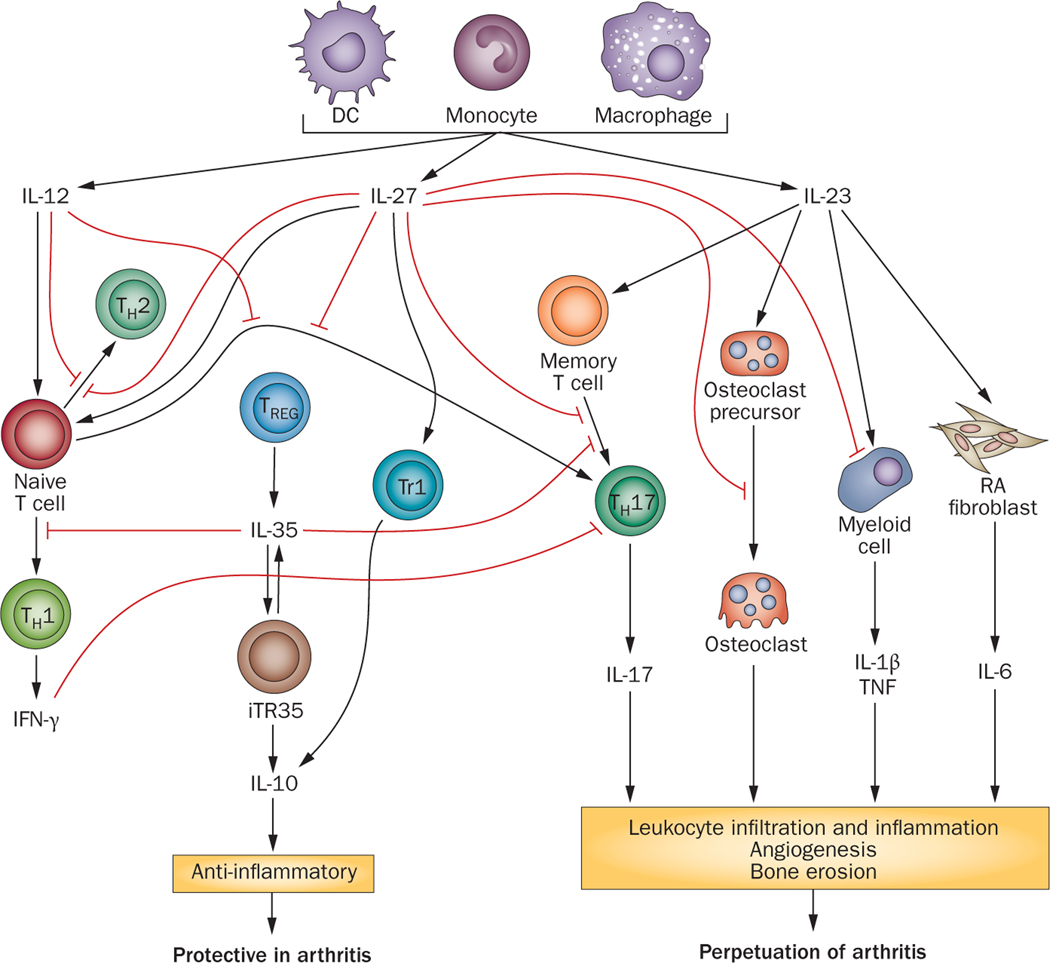
Figure 2. Cross-regulation of IL-12 family members and their effect on RA pathogenesis.
While IL-12, IL-23 and IL-27 are produced from antigen presenting cells, IL-35 is mostly secreted from Tregs. IL-12 and IL-27 drive differentiation of TH-1 cells and both family members suppress TH-2 and TH-17 polarization. The anti-inflammatory effects of IL-27 is also associated with production of IL-10 from Tr1 and myeloid cells as well as reducing the expression of receptors for TNF-α and IL-1β on myeloid cell and maturation of osteoclast precursors to mature osteoclasts. Presence of IL-27 prior to TH-17 development in experimental arthritis inhibits IL-17 mediated leukocyte migration, angiogenesis, bone erosion and ameliorates disease pathogenesis. Consistently, TH-17 cell differentiation is inhibited by IL-35; however, unlike IL-12 and IL-27, IL-35 is also capable of suppressing TH-1 development. The role of IL-23 on disease pathogenesis is due in part to its effect on TH-17 cell maintenance as well as its ability to activate production of IL-1β and TNF-α from myeloid cells and IL-6 from RA fibroblasts. We conclude that while IL-12, IL-27 and IL-35 display a protective role in arthritis in contrast IL-23 perpetuates arthritis pathogenesis.
IL-35 a unique IL-12 family member
The functional mechanism by which IL-35 exerts its effect is a new area of research in humans and thus its importance in human autoimmunity remains to be characterized. Therefore the majority of IL-35 studies have been performed in mice and experimental arthritis models. Unlike other IL-12 family members, IL-35 is not produced from myeloid cells and Tregs are its main source 44. IL-35 is predominantly expressed from CD4+Foxp3+ Tregs and IL-35 can further induce generation of T regulatory cells that produce IL-35 (iTR35) in mice 45 (Figure 2). Consist with this finding, Tregs from mice deficient in EBI3 or p35 demonstrate decreased regulatory activity both in in vitro and in vivo studies suggesting that expression of IL-35 is required for optimal Treg suppressive activity 44
The role of IL-35 has been studied in two CIA studies. Both studies demonstrate that IL-35 ameliorates the severity of CIA. While the earlier investigation shows that the efficacy of treatment was due to reduction of TH-17/IL-17 response and higher levels of IFN-γ 46, the more recent study documents that the IL-35’s effect was associated with induction of Tregs and IL-10 production as well as suppression of TH-17 and TH-1 cell development 47. These data suggest that like IL-12 and IL-27, IL-35 displays anti-inflammatory properties in the pathogenesis of CIA.
Clinical implication of IL-12 family cytokines, IL-23 and IL-27, in rheumatic disease specifically RA
Based on the anti-inflammatory effect of IL-12 family members in CIA (Table 1; supplemental material), it might be expected that IFN-γ treatment in RA might be beneficial. However earlier studies with recombinant IFN-γ demonstrated either a very modest effect on RA disease activity 48 or disease exacerbation 49. These results may be due to the complex effects of IFNγ, which are capable of suppressing TH-17 differentiation, as well as promoting macrophage activation.
More recent, studies have focused on targeting the p40 subunit shared by IL-23 and IL-12. In clinical trial.gov a randomized, double-blind, placebo-controlled clinical study has been registered to determine the safety, tolerability, pharmacokinetic and synovial tissue involvement in RA patients using the IL-12/23 inhibitor, STA-5326 Mesylate. The outcome of this study has not yet been published. However, as reviewed earlier, p40 is not increased in the joints of patients with RA, which means that even though p19 is highly expressed, biologically active IL-23 appears limited 19, 20. Neutralization of p40 will also neutralize IL-12 and based on the early studies with IFN-γ treatment 48, 49, it is not clear what the effect of neutralizing IL-12 might be in RA. Although there are no published studies in RA, employing Ustekinumab, another monoclonal antibody to IL-23/IL-12 p40, in patients with psoriasis 50 and psoriatic arthritis 51, was well tolerated and demonstrated significant improvement in Psoriasis Area and Severity Index (PASI), dermatology life quality index (DLQI) score and quality of life compared to the control group.
In RA other alternative approaches were employed to target IL-17, down stream of IL-23. Addition of a humanized monoclonal antibody to IL-17A (LY2439821) to disease modifying anti-rheumatic drugs (DMARDs) modestly improved symptoms of RA compared to DMARDs alone 52. Further, Amgen’s AMG-827 or Brodalumab, a monoclonal antibody to IL-17R, although registered for RA treatment in clinical trial.gov, there are no published results pertaining to these studies. However, Brodalumab was recently shown to be effective in plaque psoriasis 53.
Based on the data generated in experimental arthritis models, IL-27 and IL-35 may hold a promise in suppressing inflammation due to their inhibitory effect on TH-17 and/or myeloid cells. However, to date use of cytokines has not been proven to be effective in RA. Therefore, while these pathways appear to have potential relevance in the treatment of RA and other forms of inflammatory arthritis, it may be useful to develop strategies that will enhance their endogenous expression in vivo.
CONCLUSIONS
Initially IL-12/TH-1 cells were implicated in the pathogenesis of autoimmune disease and RA. However mutagenesis of IL-12 and/or IL-23 subtype specific animals were required to clarify that IL-23 is essential for initiation and progression of experimental arthritis, while IL-12 has a protective role against inflammation. It was found that IL-23 mediated joint inflammation is mediated in part through differentiation of TH-17 cells. Recently it has come to light that IL-12, IL-27 and IL-35 counteract the effects of the IL-23/TH-17 pathway in order to put a brake on inflammation mediated by adaptive immunity. The new additions to the IL-12 family members have altered the TH-1/TH-2 paradigm and the majority of the work has been performed in experimental models, however these models may partially be reflective of human disease. As demonstrated, despite the significance of IL-23 and IL-17 in animal models, RA patients that received DMARDs plus neutralizing monoclonal antibodies against IL-17 achieved a modest response compared to those receiving DMARDs alone. Therefore, a deeper understanding of IL-12 family members and their interaction with each other in human autoimmunity is required prior to designing more effective therapeutics for RA and other rheumatic diseases.
Supplementary Material
Acknowledgments
This work was supported in part by awards from the National Institutes of Health (AR056099, AR055240), grant from Within Our Reach from The American College of Rheumatology, funding provided by Department of Defense PR093477 and Arthritis Foundation Innovative Research Grant.
Footnotes
Competing Interests
Both authors declare no competing interests.
REFERENCES
- 1.Kastelein RA, Hunter CA & Cua DJ Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol 25, 221–42 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Collison LW & Vignali DA Interleukin-35: odd one out or part of the family? Immunol Rev 226, 248–62 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3, 133–46 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Collison LW et al. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol 13, 290–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter CA New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol 5, 521–31 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Weaver CT, Hatton RD, Mangan PR & Harrington LE IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25, 821–52 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi K, Yoshimoto T, Tsutsui H. & Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine & Growth Factor Reviews. 12, 53–72 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Petrovic-Rackov L. & Pejnovic N. Clinical significance of IL-18, IL-15, IL-12 and TNF-alpha measurement in rheumatoid arthritis. Clin Rheumatol 25, 448–52 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Yin Z. et al. The elevated ratio of interferon gamma-/interleukin-4-positive T cells found in synovial fluid and synovial membrane of rheumatoid arthritis patients can be changed by interleukin-4 but not by interleukin-10 or transforming growth factor beta. Rheumatology (Oxford) 38, 1058–67 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Leipe J. et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum 62, 2876–85 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Holmdahl R. et al. Collagen induced arthritis as an experimental model for rheumatoid arthritis. Immunogenetics, pathogenesis and autoimmunity. Apmis 97, 575–84 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Germann T. et al. Administration of interleukin 12 in combination with type II collagen induces severe arthritis in DBA/1 mice. Proceedings of the National Academy of Sciences of the United States of America 92, 4823–4827 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joosten LA, Lubberts E, Helsen MM & van den Berg WB Dual role of IL-12 in early and late stages of murine collagen type II arthritis. J Immunol 159, 4094–102 (1997). [PubMed] [Google Scholar]
- 14.Paunovic V, Carroll HP, Vandenbroeck K. & Gadina M. Signalling, inflammation and arthritis: crossed signals: the role of interleukin (IL)-12, −17, −23 and −27 in autoimmunity. Rheumatology (Oxford) 47, 771–6 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Sarkar S. & Fox DA Targeting IL-17 and Th17 cells in rheumatoid arthritis. Rheum Dis Clin North Am 36, 345–66 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Iwakura Y. & Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest 116, 1218–22 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paradowska-Gorycka A, Grzybowska-Kowalczyk A, Wojtecka-Lukasik E. & Maslinski S. IL-23 in the pathogenesis of rheumatoid arthritis. Scand J Immunol 71, 134–45 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Murphy CA et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med 198, 1951–7 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brentano F. et al. Abundant expression of the interleukin (IL)23 subunit p19, but low levels of bioactive IL23 in the rheumatoid synovium: differential expression and Toll-like receptor-(TLR) dependent regulation of the IL23 subunits, p19 and p40, in rheumatoid arthritis. Annals of the Rheumatic Diseases. 68, 143–50 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Shahrara S, Huang Q, Mandelin AM 2nd & Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther 10, R93 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamp LK, Easson A, Pettersson L, Highton J. & Hessian PA Monocyte derived interleukin (IL)-23 is an important determinant of synovial IL-17A expression in rheumatoid arthritis. J Rheumatol 36, 2403–8 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Langrish CL et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev 202, 96–105 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Liu FL et al. Interleukin (IL)-23 p19 expression induced by IL-1beta in human fibroblast-like synoviocytes with rheumatoid arthritis via active nuclear factor-kappaB and AP-1 dependent pathway. Rheumatology (Oxford) 46, 1266–73 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Kim HR et al. The clinical role of IL-23p19 in patients with rheumatoid arthritis. Scand J Rheumatol 36, 259–64 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Wei XQ, Evans B, Jiang W. & Aeschlimann D. IL-23 promotes osteoclast formation by up-regulation of receptor activator of NF-kappaB (RANK) expression in myeloid precursor cells. Eur J Immunol 38, 2845–54 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Li X. et al. IL-23 induces receptor activator of NF-kappaB ligand expression in fibroblast-like synoviocytes via STAT3 and NF-kappaB signal pathways. Immunol Lett 127, 100–7 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Sato K. et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203, 2673–82 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotake S. et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103, 1345–52 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakae S, Nambu A, Sudo K. & Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol 171, 6173–7 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Wiekowski MT et al. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol 166, 7563–70 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Ratsimandresy RA et al. Active immunization against IL-23p19 improves experimental arthritis. Vaccine 29, 9329–36 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Niedbala W. et al. Interleukin 27 attenuates collagen-induced arthritis. Annals of the Rheumatic Diseases. 67, 1474–9 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Pflanz S. et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity 16, 779–90 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Diveu C, McGeachy MJ & Cua DJ Cytokines that regulate autoimmunity. Curr Opin Immunol 20, 663–8 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Cao Y, Doodes PD, Glant TT & Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol 180, 922–30 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Batten M. et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol 7, 929–36 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Stumhofer JS et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 7, 937–45 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Stumhofer JS et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 8, 1363–71 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Wojno ED & Hunter CA New directions in the basic and translational biology of interleukin-27. Trends Immunol 33, 91–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iyer SS, Ghaffari AA & Cheng G. Lipopolysaccharide-mediated IL-10 transcriptional regulation requires sequential induction of type I IFNs and IL-27 in macrophages. J Immunol 185, 6599–607 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalliolias GD, Gordon RA & Ivashkiv LB Suppression of TNF-alpha and IL-1 signaling identifies a mechanism of homeostatic regulation of macrophages by IL-27. J Immunol 185, 7047–56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalliolias GD, Zhao B, Triantafyllopoulou A, Park-Min KH & Ivashkiv LB Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheum 62, 402–13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pickens SR et al. Local expression of IL-27 ameliorates collagen induced arthritis. Arthritis Rheum (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collison LW et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450, 566–9 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Collison LW et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol 11, 1093–101 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niedbala W. et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 37, 3021–9 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Kochetkova I, Golden S, Holderness K, Callis G. & Pascual DW IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol 184, 7144–53 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veys EM et al. Interferon gamma in rheumatoid arthritis--a double blind study comparing human recombinant interferon gamma with placebo. J Rheumatol 15, 570–4 (1988). [PubMed] [Google Scholar]
- 49.Seitz M, Franke M. & Kirchner H. Induction of antinuclear antibodies in patients with rheumatoid arthritis receiving treatment with human recombinant interferon gamma. Annals of the Rheumatic Diseases. 47, 642–4 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leonardi CL et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371, 1665–74 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Gottlieb A. et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 373, 633–40 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Genovese MC et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum 62, 929–39 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Papp KA et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 366, 1181–9 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.