Abstract
Introduction
Radiomics in uro-oncology is a rapidly evolving science proving to be a novel approach for optimizing the analysis of massive data from medical images to provide auxiliary guidance in clinical issues. This scoping review aimed to identify key aspects wherein radiomics can potentially improve the accuracy of diagnosis, staging, and grading of renal and bladder cancer.
Material and methods
A literature search was performed in June 2022 using PubMed, Embase, and Cochrane Central Controlled Register of Trials. Studies were included if radiomics were compared with radiological reports only.
Results
Twenty-two papers were included, 4 were pertinent to bladder cancer, and 18 to renal cancer. Radiomics outperforms the visual assessment by radiologists in contrast-enhanced computed tomography (CECT) to predict muscle invasion but are equivalent to CT reporting by radiologists in predicting lymph node metastasis. Magnetic resonance imaging (MRI) radiomics outperforms radiological reporting for lymph node metastasis. Radiomics perform better than radiologists reporting the probability of renal cell carcinoma, improving interreader concordance and performance. Radiomics also helps to determine differences in types of renal pathology and between malignant lesions from their benign counterparts. Radiomics can be helpful to establish a model for differentiating low-grade from high-grade clear cell renal cancer with high accuracy just from contrast-enhanced CT scans.
Conclusions
Our review shows that radiomic models outperform individual reports by radiologists by their ability to incorporate many more complex radiological features.
Keywords: diagnosis, computer-assisted, neoplasm staging, urinary bladder neoplasms, renal neoplasms, radiomics
INTRODUCTION
Prostate, bladder, and kidney cancers are the most frequent tumors faced by urologists.
Renal cell carcinoma (RCC) is the 14th cancer in the world in the number of new cases (431000/year) and the 15th in the number of deaths (179000/year) [1].
Bladder cancer is the 10th most common cancer, with 573278 estimated new cases and 212536 deaths worldwide in 2020 [1].
Urologists commonly rely on radiological features of either contrast-enhanced computed tomography (CECT) scan or magnetic resonance imaging (MRI) for both diagnosis and staging in urological cancers. Yet, these imaging modalities may miss or sometimes under/overstage tumours as was noted by Kim et al. [2]. With a rise in incidentally detected small renal masses, this problem has recently increased. Additionally, renal tumour patients are often subjected to further interventions and 20% of surgically removed renal tumours eventually turn out benign on histopathology despite increased sensitivity using both CECT and MRI [3]. Likewise, in bladder cancers as well, urological decisions on the initial modality of intervention and prognostic significance fundamentally rely on a radiologist’s interpretation of the Vesical imaging reporting and data system (VI-RADS) score in MRI which superseded the limitations of assessing muscle invasion [4]. Yet, this too is not without its limitations.
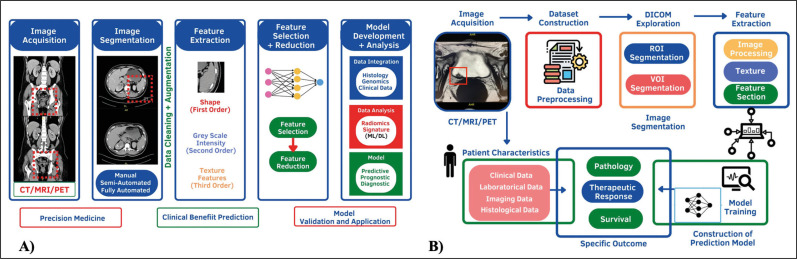
Radiomics in uro-oncology is a rapidly evolving science proving to be a novel method for enhancing the analysis of large data from medical images to offer auxiliary guidance in clinical issues. These techniques, in fact, being able to directly process images, have allowed a series of research sub-lines: detection of malignant and benign tumors, segmentation, detection of tumor grading, prediction of the most appropriate therapy, understanding of the evolution of tumors, and efficacy of given therapies (Figure 1A) [5].
Figure 1.
A) Radiomics image processing. B) Inherent ability of radiomics to integrate key data.signatures from large image bases.
CT – computed tomography; MRI – magnetic resonance imaging; PET – positron emission tomography; ML – machine learning, DL – deep learning
Our scoping review aimed to identify key oncological aspects wherein radiomics can potentially improve the accuracy of either diagnosis, staging, or grading of kidney, bladder, and prostate cancer. Often urologists rely on investigative reports from radiologists and need to deploy multiple investigations before embarking on a definitive treatment for these tumours. We hypothetically believe that radiomics, by its inherent ability to integrate key data signatures from large image bases pertinent to a pathology, may be able to bridge the gap of missing parameters that preclude radiologists from being unable to conclusively provide a diagnosis (Figure 1B). This limitation is potentiated by the inability to make direct comparisons between two different imaging modalities.
As this topic is far-reaching, we limited our scope to answer the following questions that are considered rate limiting before choosing a modality of intervention in a two-part series: part 1 is focused on urinary bladder and renal cancers, whilst part 2 is on prostate cancer.
MATERIAL AND METHODS
Evidence acquisition
The literature search was performed on 9th June 2022 using PubMed, Embase, and Cochrane Central Controlled Register of Trials (CENTRAL). The following terms and Boolean operators were used: (Machine learning OR radiomics) AND (computed tomography OR magnetic resonance imaging) AND (bladder cancer OR bladder neoplasm); (Machine learning OR radiomics) AND (computed tomography OR magnetic resonance imaging) AND (prostate cancer OR prostate neoplasms); (Machine learning OR radiomics) AND (computed tomography OR magnetic resonance imaging) AND (renal cancer OR renal neoplasms). No date limits were imposed.
Selection criteria
Studies were included if radiomics were compared with radiological reports only. Only English language papers were accepted. Pediatric and animal studies were excluded. Meeting abstracts, letters to the editor, and editorials were also excluded.
Study screening and selection
Two independent authors screened all retrieved records through Covidence Systematic Review Management® (Veritas Health Innovation, Melbourne, Australia). Discrepancies were solved by a discussion. Meeting abstracts, reviews, case reports, letters to the editor, and editorials were excluded. The full text of the screened papers was selected if found relevant to the present review.
Evidence synthesis
Literature screening
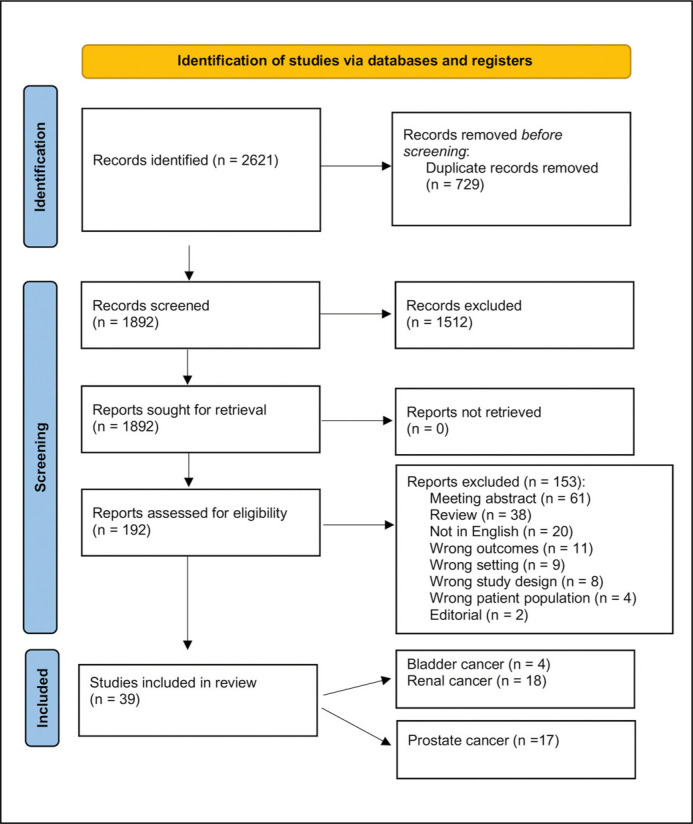
The literature search retrieved 2621 papers. A total of 729 duplicate studies were automatically excluded. After title and abstract screening of the remaining 1892 unique references, 1512 records were excluded because they were irrelevant to this study's aim. The full texts of the remaining 380 studies were assessed for eligibility. Finally, 39 studies were accepted and included. Four papers were pertinent to bladder cancer [6–9], and 18 to renal cancer [10, 11, 20–27, 12–19]. Figure 2 shows the flow chart of the literature search. In part 1 we discuss the role and utility of radiomics and compare and contrast this vis a-vis radiological investigations pertinent only to aspects of bladder and renal cancer. The following aspects are discussed:
Figure 2.
Flow diagram of the literature screening.
n – number
– Bladder cancer: prediction of muscle invasion and lymph node metastasis
– Renal cancer: differentiating benign from malignant lesions, nuclear grade in renal cell carcinoma (RCC), identification of histological variants of RCC and lymph node, and distant metastasis.
DISCUSSION
Bladder cancer
Prediction of muscle invasion
Discrimination between muscle-invasive and non-muscle-invasive disease is of paramount importance in the management of bladder cancer patients. CECT has many weaknesses for local tumor staging, mainly a lack of visualization of the bladder wall and specificity for early detection of extravesical invasion of the tumor. Findings suggestive of extravesical invasion of the tumor are unspecific, including perivesical fat stranding and adjacent soft tissue nodularity [28]. A study on 265 patients by Tritschler et al. reported that the inability of CECT to accurately evaluate the deepness of tumor invasion (low accuracy rate at 49%), leads to over-staging in 23.4% and under-staging in 24.7% of cases, as confirmed by cystectomy [29]. The use of MRI and the introduction of the VI-RADS score overcame the limitation of CECT scan in assessing muscle invasion [4].
Radiomics has the potential to better assess bladder wall invasion as compared with CT and MRI.
Cui et al. retrospectively assessed 188 patients with histopathologically confirmed bladder cancer who underwent CECT before transurethral resection [6]. Patients were divided into the training (120 patients) and validation group (68 patients). Two radiologists evaluated each CT study for the preoperative prediction of muscle-invasion. Radiomics analysis was also performed including 102 radiomics feature extraction and model development (data normalization, feature redundancy reduction, feature selection, and classifier). The radiomics model outperformed the visual assessment of both radiologists in the training [area under the curve (AUC) 0.979 vs 0.865 vs 0.894] and validation dataset (AUC 0.894 vs 0.766 vs 0.826). The specificity of the radiomics model was better than the radiologists (85.3–96.7% vs 47.1–58.3%,), but sensitivity did not significantly differ (79.4–90% versus 91.2–96.7%).
Xu et al. assessed 54 patients with pathologically proven non-muscle-invasive (24 patients) and muscle-invasive bladder cancer (30 patients) who underwent preoperative 3-T MRI with T2-weighted and multi-b-value diffusion-weighted sequences [9]. A total of 1104 radiomics features were extracted from bladder tumors and a support vector machine with recursive feature abolition and synthetic minority oversampling method was applied to build the model. The performance of the radiomics model was matched with that of an expert radiologist. The radiomics model showed a sensitivity of 92.6%, specificity of 100%, and accuracy of 96.3% (AUC 0.9857) which outperformed the radiologist’s visual assessment (sensitivity, specificity, and accuracy of 91.11%, 88.89%, and 90.12%, respectively).
Prediction of lymph node metastasis
Accurately predicting the presence of lymph node metastasis in bladder cancer is integral for TNM classification upon which the entire treatment and prognosis rest. From the days of using deoxyribonucleic acid flow cytometry techniques [30] to more advanced methods like gene transcription signatures [31] and nomograms [32], urologists rely on radiological evidence in imaging to determine this. Of these, CECT and MRI are the standard and positron emission tomography scans are the newer modalities of choice [33]. Yet there is significant variability in the accuracy that current imaging modalities achieve across different studies because CT and MRI criteria for lymph node metastasis rely mainly on lymph node size. However, up to 25% of muscle-invasive bladder cancer patients who are clinically node-negative have occult metastases at radical cystectomy and pelvic lymph node dissection [34].
In a recent meta-analysis of 860 patients, Kozikow-ski et al. concluded that radiomics shows high diagnostic performance in predicting MIBC, is relatively homogeneous in its diagnostic accuracy and has the potential to become a useful adjunct in the clinical management of bladder cancer [35]. Here, we analyzed if the ability of radiomics to apply rapidly evolving imaging analysis methods using artificial intelligence algorithms could prove equally useful in lymph node metastasis too.
Starmans et al. extracted 564 radiomics features after manual segmentation of lymph nodes in 209 patients with clinically T2-T4aN0-N1M0 muscle-invasive bladder cancer, of whom preoperative CT scans and pathology reports were available [7]. Among the included patients, 50 patients were pathologically proven node-negative at radical cystectomy. Seven radiomics models were developed but none performed better than radiologists and random guessing, showing no statistically significant differences in patients with negative or positive lymph node disease.
Conversely, Wu et al. demonstrated that T2-weighted MRI-based radiomics performed better than radiologists in assessing lymph node metastasis in 103 patients [8]. Radiologists’ subjective MRI status of lymph nodes showed that 55.2% (16/29) of the pN1-3 patients were understaged (reported to be cN0), while 6.8% (5/74) of the pN0 patients were overstaged (reported to be cN1-3). In the radiomics feature, only 18.8% (16/85) of cN0 patients were understaged. The radiomics model also showed good discrimination with an optimism-corrected AUC of 0.8872 (95% CI, 0.7827–0.9496).
Kidney cancer
Differentiating benign from malignant tumors
Xu et al. built a MRI-based deep learning (DL) model for the distinction of malignant and benign kidney tumors and to evaluate its discrimination performance with that of radiomics models and by radiologists [24]. The authors found that the AUC of the DL model based on T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and their combination was 0.906, 0.846, and 0.925 in the testing cohorts, respectively. The AUC of the radiomics models based on T2WI, DWI, and the combination was 0.824, 0.742, and 0.826 in the testing cohorts, which was better than the AUC of two radiologists (0.724 and 0.667) in the testing cohorts. Therefore, the MRI-based DL model was valuable for discriminating benign from malignant renal tumors, demostrating that an association of signatures that can be assessed in radiomic models was superior to individual radiological features.
Said et al. assessed 104 RCCs [29 papillary (pRCC), 51 clear cell RCC (ccRCC), and 24 subtypes] and 21 benign tumors in 125 patients [18]. Significant qualitative and quantitative radiomics features were included for analysis. Models with the best diagnostic performance on validation sets showed an AUC of 0.73 [confidence interval (CI) 0.5–0.96] for differentiating RCC from benign tumors: AUC of 0.77 (CI 0.62–0.92) for diagnosing ccRCC (using radiomics features), and AUC of 0.74 (CI 0.53–0.95) for diagnosing pRCC (using qualitative features).
In the study by Sun et al. comparing outcomes of radiomics models vis a vis the CECT reports of experts radiologists, radiomics performed better in differentiating benign from malignant lesions or ccRCC from papillary RCC and chromophobe RCC (chrRCC) as well as in distinguishing ccRCC from angiomyolipoma (AML) and oncocytomas [20]. After 10-fold cross-validation, the radiologic-radiomic machine learning (ML) model yielded the following performance values for differentiating ccRCCs from pRCCs and chrRCCs, ccRCCs from fat-poor angioleiomyolipomas (fp-AML) and oncocytomas, and pRCCs and chrRCCs from fp-AML and oncocytomas: a sensitivity of 90.0%, 86.3%, and 73.4% and a specificity of 89.1%, 83.3%, and 91.7%, respectively. Conversely, expert-level radiologists had great variation in performance for differentiating benign from malignant solid kidney masses. Therefore, radiologic-radiomics can be a potential tool to improve interreader performance and concordance.
Renal oncocytoma accounts for 18% of all benign renal tumors and is often diagnosed post-surgery due to radiological misdiagnosis for absence of CT specificity [36]. This misdiagnosis is generally owing to overlapping imaging features. Li et al. described the building of a radiomics nomogram based on clinical data and radiomics signature for the preoperative differentiation of renal oncocytoma from ccRCC on tri-phasic contrast-enhanced CT [13]. Central stellate area and perirenal fascia thickening were selected to build the clinical factors model. Eleven radiomics features were combined to construct the radiomics signature. The AUCs of the radiomics nomogram, which was based on the selected clinical factors and Rad-score, were 0.960 and 0.898 in the training and validation sets, respectively. The decision curves of the radiomics nomogram and radiomics signature in the validation set indicated an overall net benefit over the clinical factors model. In a further study, the same group evaluated pre-operative differentiation between renal oncocytoma and chRCC [14]. This study aimed to develop and validate a CT-based radiomics nomogram for the pre-operative differentiation of renal oncocytoma from chRCC. Twelve features from CT images were selected to develop the radiomics signature. The radiomics nomogram combining a clinical factor (segmental enhancement inversion) and radiomics signature showed an AUC value of 0.988 in the validation set. Decision curve analysis revealed that the diagnostic performance of the radiomics nomogram was better than the clinical model and the radiomics signature.
Another study aimed to discriminate fp-AML from ccRCC by constructing radiomics-based logistic classifiers in comparison with conventional CT analysis at three CT phases [15]. The authors found that whole-tumor radiomics CT analysis showed superior characteristics to conventional CT in the differentiation of fp-AML from ccRCC. Cyst degeneration, pseudocapsule, and sum rad-score were the most notable aspects.
Nie et al. developed a radiomics nomogram for preoperative differentiating renal angiomyolipoma without visible fat from homogeneous ccRCC (hm-ccRCC) [17]. Fourteen features were used to construct the radiomics signature. The radiomics signature demonstrated good discrimination in the training (AUC, 0.879; 95%; [CI], 0.793–0.966) and the validation set (AUC, 0.846; 95% CI, 0.643–1.000). The radiomics nomogram showed good calibration and discrimination in the training set (AUC, 0.896; 95% CI, 0.810–0.983) and the validation set (AUC, 0.949; 95% CI, 0.856–1.000) and showed better discrimination proficiency compared with the clinical factor model (AUC, 0.788; 95% CI, 0.683–0.893) in the training set. Decision curve analysis proved that the nomogram outperformed the clinical factors model and radiomics signature in terms of clinical utility.
Predicting nuclear grade in renal cell carcinoma
Tumor grade is one of the well-known prognostic factors of ccRCC and is regarded as an predictor of cancer-specific survival [37]. Radiomics analysis is a potentially useful method that could be used to assess the pathological grade for guiding personalized cancer treatment.
Sun et al. demonstrated that radiomics combined with CT images were useful to establish a model for differentiating low-grade [2016 International Society of Urological Pathology (ISUP)/ World Health Organization (WHO) grade 1–2] from high-grade (2016 ISUP/WHO grade 3–4) ccRCC [19]. Three-phase CECT images of 227 patients with ISUP-grade ccRCC (155 cases in the low-grade group and 72 cases in the high-grade group) were analyzed retrospectively and a model was built using the optimal features. The support vector machine (SVM) model constructed using the screening features for the 2-stage joint samples effectively discriminated high- and low-grade ccRCC, and obtained the highest prediction accuracy (AUC value in the training and validation group of 0.88 and 0.91, respectively).
In a 2020 study, Han et al. compared the prediction models for the ISUP/WHO grade of ccRCC based on CT radiomics and conventional CECT [11]. The corticomedullary phase images were gathered from 119 cases of high-grade (3 and 4) and low-grade (1 and 2) ccRCC. In the training set, the C-statistics of the radiomics prediction model was statistically higher than that of the CECT. In addition, validation set decision curve analysis showed net benefit increase of CT radiomics prediction model in the range of 3–81% over CECT.
Yi et al. developed a ML radiomic model achieving a good performance in discriminating low-grade from the high-grade ccRCC [25]. A total of 264 patients with ccRCC (206 patients in the low-grade group and 58 in the high-grade group) were included in this study. The model built with traditional radiological characteristics (baseline and post-enhancing CT density, and tumor size) reached an AUC of 0.9175 (95% CI: 0.8765–0.9585) and 0.8088 (95% CI: 0.7064–0.9113) in differentiating the low-grade from the high-grade ccRCC for the training cohort and the validation cohort, respectively. The radiomics model built with textural features yielded an AUC value of 0.8170 (95% CI: 0.7353–0.8987) and 0.8017 (95% CI: 0.6878–0.9157) for the training cohort and the validation cohort, respectively. The combined model incorporating the traditional radiological characteristics with the radiomic textural features achieved the highest accuracy, with an AUC of 0.9235 (95% CI: 0.8646–0.9824) and an AUC of 0.9099 (95% CI: 0.8324–0.9873) for the training cohort and validation cohort, respectively.
Li et al. developed an MRI-based radiomic model for preoperative prediction WHO/ISUP nuclear grade in ccRCC [12]. A total of 379 patients with histologically confirmed ccRCC were included. The radiomic signature demonstrated a good performance in discriminating high-grade (grades 3 and 4) from low-grade (grades 1 and 2) ccRCC, with sensitivity, specificity, and AUC of 77.3%, 80.0%, and 0.842, respectively. The radiomic model, combining radiomic signature and clinico-radiologic features, demonstrated good ability to predict high-grade tumors (sensitivity of 63.6%, specificity of 93.3%, and accuracy of 88.2%).
Wang et al. proved that a radiological model constructed from CT radiomic features effectively predicted the WHO/ISUP pathological grade of ccRCC in 197 ccRCC patients. The prediction model constructed with seven radiomic features showed the best performance in identification for WHO/ISUP pathological grades, with AUC, sensitivity, and specificity of 0.89, 0.85, and 0.84 respectively.
Predicting histological variants in renal cell carcinoma
The prognosis of ccRCC and non-ccRCC is different and the early diagnosis of RCC subtypes is important from a treatment point of view.
Meng et al. built a CT-based radiomics model for the differentiation of sarcomatoid RCC and ccRCC in 128 patients [16]. In total, 1029 radiomics features were gathered from the corticomedullary and nephrographic phase images. A statistically significant difference was found between the radiomics model and the radiologist’s subjective findings with an AUC of 0.966 and 0.792, whilst the combined features showed the highest accuracy (AUC 0.974).
Wang et al. developed a CT-based radiomics model to distinguish ccRCC from non-ccRCC [21]. A total of 190 patients were included, 147 cases with ccRCC and 43 cases with non-ccRCC (24 cases with papillary RCC, 13 chRCC, and 6 collecting duct carcinoma). The sensitivity and specificity of radiologist’s ability to differentiate ccRCC from non-ccRCC were 0.850 and 0.581, respectively (AUC 0.69). The radiomics model augmented radiological diagnosis with a sensitivity of 0.956 and a specificity of 0.538 (AUC 0.909).
Predicting/evaluating lymph node and distant metastasis in renal cancer
RCC presents with metastases in 15–30% of the cases at the time of diagnosis, whilst 30% of localized RCC eventually progresses to metastasis [38].
In our review, only one study analyzed the use of radiomics in predicting distant metastasis. Wen et al. examined the role of contrast-enhanced CT images in predicting synchronous distant metastasis in 172 ccRCC patients [23]. They extracted 2994 quantitative radiomic features and the least absolute shrinkage and selection operator regression was applied for dimension reduction, feature selection, and model construction. Nine radiomic features were used for the construction of the synchronous distant metastasis prediction model. The authors found that the model yielded moderate diagnostic efficacy in both the training (AUC 0.89; 95% CI 0.81–0.97) and the validation cohort (AUC 0.83; 95% confidence interval, 0.69–0.95) in predicting synchronous distant metastasis. This model can be used as a non-invasive, personalized approach for distant metastasis prediction in patients with ccRCC.
Take-home messages
Radiomics in muscle-invasive bladder cancer
Radiomics outperforms the visual assessment by radiologists in CECT to predict muscle invasion
Specificity of the radiomics model was higher than the radiologists
May be equivalent to CECT reporting by radiologists for diagnosis of MIBC and predicting lymph node metastasis.
MRI radiomics outperforms radiological reporting for lymph node metastasis
Radiomics in renal cancer
Radiomics performs better than radiologists reporting the probability of RCC which was validated by deep learning models
Radiologic-radiomic ML can be a potential way to improve interreader concordance and performance often seen even in expert-level radiologists reporting.
Radiomics helps determine differences in types of renal pathology and between malignant lesions from their benign counterparts like fat poor AML or oncocytoma.
Radiomics can be used to establish a model for differentiating low-grade (2016 ISUP/WHO grade 12) from high-grade (2016 ISUP/WHO grade 3–4) ccRCC with high accuracy just from CECT scans.
Radiomics models can be used as a non-invasive, personalized approach for synchronous distant metastasis prediction in patients with ccRCC
Limitations and strengths of adopting radiomics
Conclusive evidence in our study shows that radiomics outshines any individual radiological investigative modality for renal and bladder cancer and yet it has not gained widespread use. A plausible explanation is to examine how a radiomic model is constructed. A typical model involves three aspects: feature selection, modeling methodology, and model validation. Possibly the lack of standardization in how and what parameters must be chosen for each of these fundamentals of model building, also seen in our studies included, makes this science complex and non-reproducible for everyone's interpretation. This was also noted in prior studies [39].
Whilst this science needs a large volume of data to create these signatures its utility in oncology is far-reaching for bladder and renal cancers. What is potentially required is a uniform way of interpretation. Once achieved, radiomics will pave the way forward for radiologists to conclusively interpret any investigation, allowing urologists to have a possible non-invasive diagnosis and staging of tumors.
CONCLUSIONS
Our scoping review conclusively shows that radiomic models outperform individual reports by radiologists by their ability to incorporate many more complex radiological features.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J Clin. 2021;71: 209-249. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Sun HY, Hwang J, et al. Diagnostic accuracy of contrast- enhanced computed tomography and contrast-enhanced magnetic resonance imaging of small renal masses in real practice: sensitivity and specificity according to subjective radiologic interpretation. World J Surg. Oncol. 2016; 14: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol. 2022; 82: 399-410. [DOI] [PubMed] [Google Scholar]
- 4.Del Giudice F, Flammia RS, Pecoraro M, et al. The accuracy of Vesical Imaging-Reporting and Data System (VI-RADS): an updated comprehensive multi-institutional, multi-readers systematic review and meta-analysis from diagnostic evidence into future clinical recommendations. World J Urol. 2022; 40: 1617-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B. Radiomics in medical imaging-"how-to" guide and critical reflection. Insights Imaging. 2020; 11: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui Y, Sun Z, Liu X, Zhang X, Wang X. CT-based radiomics for the preoperative prediction of the muscle-invasive status of bladder cancer and comparison to radiologists’ assessment. Clin Radiol. 2022; 77: e473-82. [DOI] [PubMed] [Google Scholar]
- 7.Starmans MPA, Ho LS, Smits F, Beije N, et al. Optimization of Preoperative Lymph Node Staging in Patients with Muscle-Invasive Bladder Cancer Using Radiomics on Computed Tomography. J Pers Med. 2022; 12: 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, Zheng J, Li Y, et al. Development and Validation of an MRI-Based Radiomics Signature for the Preoperative Prediction of Lymph Node Metastasis in Bladder Cancer. EBioMedicine. 2018; 34: 76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Zhang X, Tian Q, W, et al. Quantitative Identification of Nonmuscle-Invasive and Muscle-Invasive Bladder Carcinomas: A Multiparametric MRI Radiomics Analysis. J. Magn. Reson. Imaging. 2019; 49: 1489-98. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Yin F, Yu Y, Zhang H, Wen G. CT-based multi-phase Radiomic models for differentiating clear cell renal cell carcinoma. Cancer Imaging. 2021; 21: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han D, Yu Y, Yu N, et al. Prediction models for clear cell renal cell carcinoma ISUP/WHO grade: comparison between CT radiomics and conventional contrast-enhanced CT. Br J Radiol. 2020; 93: 20200131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Liu Y-J, Dong D, et al. Multiparametric MRI Radiomic Model for Preoperative Predicting WHO/ISUP Nuclear Grade of Clear Cell Renal Cell Carcinoma. J. Magn. Reson. Imaging 2020; 52: 1557-1566. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Ma Q, Tao C, Liu J, Nie P, Dong C. A CT-based radiomics nomogram for differentiation of small masses (< 4 cm) of renal oncocytoma from clear cell renal cell carcinoma. Abdom Radiol. (NY). 2021; 46: 5240-5249. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Ma Q, Nie P, Zheng Y, Dong C, Xu W. A CT-based radiomics nomogram for differentiation of renal oncocytoma and chromophobe renal cell carcinoma with a central scar-matched study. Br J Radiol. 2022; 95: 20210534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Cao F, Xu X, Ma W. Can whole-tumor radiomics-based CT analysis better differentiate fat-poor angiomyolipoma from clear cell renal cell caricinoma: compared with conventional CT analysis? Abdom Radiol. (NY) 2020; 45: 2500-2507. [DOI] [PubMed] [Google Scholar]
- 16.Meng X, Shu J, Xia Y, Yang R. A CT-Based Radiomics Approach for the Differential Diagnosis of Sarcomatoid and Clear Cell Renal Cell Carcinoma. Biomed Res Int. 2020; 2020: 7103647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nie P, Yang G, Wang Z, et al. A CT-based radiomics nomogram for differentiation of renal angiomyolipoma without visible fat from homogeneous clear cell renal cell carcinoma. Eur Radiol. 2020; 30: 1274-1284. [DOI] [PubMed] [Google Scholar]
- 18.Said D, Hectors SJ, Wilck E, et al. Characterization of solid renal neoplasms using MRI-based quantitative radiomics features. Abdom Radiol. (NY) 2020; 45: 2840-2850. [DOI] [PubMed] [Google Scholar]
- 19.Sun X, Liu L, Xu K, et al. Prediction of ISUP grading of clear cell renal cell carcinoma using support vector machine model based on CT images. Medicine (Baltimore). 2019; 98: e15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun X-Y, Feng Q-X, Xu X, et al. Radiologic-Radiomic Machine Learning Models for Differentiation of Benign and Malignant Solid Renal Masses: Comparison With Expert-Level Radiologists. AJR Am J Roentgenol. 2020; 214: W44-54. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Pei X, Yin X-P, et al. Radiomics models based on enhanced computed tomography to distinguish clear cell from non-clear cell renal cell carcinomas. Sci Rep. 2021; 11: 13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R, Hu Z, Shen X, et al. Computed Tomography-Based Radiomics Model for Predicting the WHO/ISUP Grade of Clear Cell Renal Cell Carcinoma Preoperatively: A Multicenter Study. Front Oncol. 2021; 11: 543854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen R, Huang J, Gao R-Z, et al. CT Radiomics for the Prediction of Synchronous Distant Metastasis in Clear Cell Renal Cell Carcinoma. J Comput Assist Tomogr. 2021; 45: 696-703. [DOI] [PubMed] [Google Scholar]
- 24.Xu Q, Zhu Q, Liu H, et al. Differentiating Benign from Malignant Renal Tumors Using T2- and Diffusion-Weighted Images: A Comparison of Deep Learning and Radiomics Models Versus Assessment from Radiologists. J Magn Reson imaging 2022; 55: 1251-1259. [DOI] [PubMed] [Google Scholar]
- 25.Yi X, Xiao Q, Zeng F, et al. Computed Tomography Radiomics for Predicting Pathological Grade of Renal Cell Carcinoma. Front. Oncol. 2020; 10: 570396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Yin F, Chen M, et al. A Reliable Prediction Model for Renal Cell Carcinoma Subtype Based on Radiomic Features from 3D Multiphase Enhanced CT Images. J Oncol. 2021; 2021: 6595212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Yin F, Chen M, et al. Development and Validation of a CT-Based Radiomics Nomogram for Predicting Postoperative Progression-Free Survival in Stage I-III Renal Cell Carcinoma. Front Oncol. 2021; 11: 742547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrentschuk N, Lee ST, Scott AM. Current role of PET, CT, MR for invasive bladder cancer. Curr Urol Rep. 2013; 14: 84-89. [DOI] [PubMed] [Google Scholar]
- 29.Tritschler S, Mosler C, Straub J, et al. Staging of muscle-invasive bladder cancer: can computerized tomography help us to decide on local treatment? World J Urol. 2012; 30: 827-831. [DOI] [PubMed] [Google Scholar]
- 30.Shaaban AA, Tribukait B, el-Bedeiwy AF, Ghoneim MA. Prediction of lymph node metastases in bladder carcinoma with deoxyribonucleic acid flow cytometry. J Urol. 1990; 144: 884-887. [DOI] [PubMed] [Google Scholar]
- 31.Seiler R, Lam LL, Erho N, et al. Prediction of Lymph Node Metastasis in Patients with Bladder Cancer Using Whole Transcriptome Gene Expression Signatures. J Urol. 2016; 196: 1036-1041. [DOI] [PubMed] [Google Scholar]
- 32.Tian Z, Meng L, Wang X, , et al. Predictive Nomogram and Risk Factors for Lymph Node Metastasis in Bladder Cancer. Front Oncol. 2021; 11: 690324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crozier J, Papa N, Perera M, et al. Comparative sensitivity and specificity of imaging modalities in staging bladder cancer prior to radical cystectomy: a systematic review and meta-analysis. World J Urol. 2019; 37: 667-690. [DOI] [PubMed] [Google Scholar]
- 34.Perera M, McGrath S, Sengupta S, Crozier J, Bolton D, Lawrentschuk N. Pelvic lymph node dissection during radical cystectomy for muscle-invasive bladder cancer. Nat Rev Urol. 2018; 15: 686-692. [DOI] [PubMed] [Google Scholar]
- 35.Kozikowski M, Suarez-Ibarrola R, Osiecki R, et al. Role of Radiomics in the Prediction of Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur Urol. Focus 2021; 8: 728-738. [DOI] [PubMed] [Google Scholar]
- 36.Trevisani F, Floris M, Minnei R, Cinque A. Renal Oncocytoma: The Diagnostic Challenge to Unmask the Double of Renal Cancer. Int J Mol Sci. 2022; 23: 2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017; 15: 804-834. [DOI] [PubMed] [Google Scholar]
- 38.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009; 373: 1119-1132. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Yi X, Lu C, Qi L, Zhang Y, Li M, et al. Applications of radiomics in genitourinary tumors. Am J Cancer Res. 2020; 10: 2293-2308. [PMC free article] [PubMed] [Google Scholar]