Abstract
Ureteral stents are useful devices in urological surgery. The main objective of a ureteric stent is to allow passage of urine and reduce early or late complications related to obstruction in the urinary tract.
Despite their widespread use, there is a general lack of knowledge and awareness in stent composition and indication of application.
We represented a synthesis of our extensive research over materials, coatings and shapes available on the market and then analyzed the main characteristics and peculiarities of ureteral stents. We have also focused our attention over the side effects and complication that must be considered when placing a ureteral stent. Encrustation, microbial colonization, stent-related symptoms and patient’s history must always be evaluated when there is the need for a ureteral stent.
The perfect stent should have many characteristics including easy insertion and removal, easy manipulation, resistance to encrustation and migration, lack of complications, biocompatibility, radio-opacity, biodurability, affordability (cost-effectiveness), tolerability and optimal flow characteristics.
Nevertheless, further research and studies need to be done to provide more information about stent composition and efficacy in vivo.
In this narrative review, we covered the basic information and main characteristics of ureteral stents, in order to help clinicians choose the appropriate device needed for a given situation.
Keywords: ureteral stent, urolithiasis, ureteral obstruction, kidney calculi, ureteroscopy, RIRS
INTRODUCTION
Ureteral stents are useful devices in urological surgery. Their placement is useful to facilitate passage of stone fragments after treatment and to prevent ureteral obstruction or delayed formation of ureteral stricture [1]. Stents may also be placed in an emergency to drain obstructed infected kidneys or before surgical procedures to passively dilate the ureter in preparation for subsequent surgery. The main objective of a ureteric stent is therefore to allow passage of urine, and to reduce early or late complications related to obstruction in the urinary tract [2, 3].
The main matter of concern about stents is their tolerability. Stents may cause encrustation and microbial colonization and may therefore lead to symptomatic urinary tract infections. Other stent-related symptoms (SRS) may be caused by the presence of the stent itself or by its misplacement or migration.
The perfect stent should have many characteristics including easy insertion and removal, easy manipulation, resistance to encrustation and migration, lack of complications, biocompatibility, radio-opacity, biodurability, affordability (cost-effectiveness), tolerability and optimal flow characteristics [1, 2].
MATERIAL AND METHODS
Materials
A fundamental characteristic of the urinary system is its poor stability with an environment of many chemical changes. The biodurability and biocompatibility of the stents are attacked by the changes in the grade of saturation of the surface protector proteins between its outer layer and the urine. Hence these stent characteristics need to be considered whenever there is the need to deploy a ureteral stent [4]. The key features to create the ideal stent that needs be taken into consideration are:
Elasticity and memory: this feature determines the ability of the stent to maintain its position within the ureter. Polymeric elastomers have a memory that allows the construction of elastic stents that reform the coils when the insertion guidewire is removed. The strength of this memory can vary within the same material.
Tensile strength and elongation capacity: crystallization and crosslinking of biomaterials cause variations in tensile strength. When a stent has a high tensile strength, internal and external diameters can be wider, and they can have more side-holes that improves urine passage. Elongation capacity is the degree of elongation at stent rupture, a feature that is high in thermoplastic elastomers [4].
Biodurability: this is the stent’s ability to remain within the body without being degraded in structure and function. The urinary tract is a hostile environment and unpredictable variations may occur within the interface between the body and the stent, determining different levels of durability [4].
Biocompatibility: measures the stent tolerance and the problems caused by the interface between the stent and the body, issues such as encrustation and infection. Hydrophilic polymers make a stent more biocompatible because they cause lower protein absorption and bacterial adherence [4]. Ideally, the presence of the stent should not affect the patients’ quality of life, thus tolerance is a key issue that must be considered [3].
Coefficient of friction: determines how easily a stent passes or is exchanged. Hydrophilic coatings are useful to reduce the coefficient of friction [4]. Insertion and removal processes should therefore be easy and should not lead to any discomfort in patients [3].
Radio-opacity: this is needed to see the stents radiologically, which are usually placed under fluoroscopic visualization. Some stents may have fillers that enhance their radio-opacity [4].
The incidence of stent-related symptoms is influenced by the stent composition. Soft ureteral stents might reduce SRS when compared to hard ones, even if the quality of evidence is low [5]. SRS are also determined by the encrustation rate and the bacterial adhesion incidence [5].
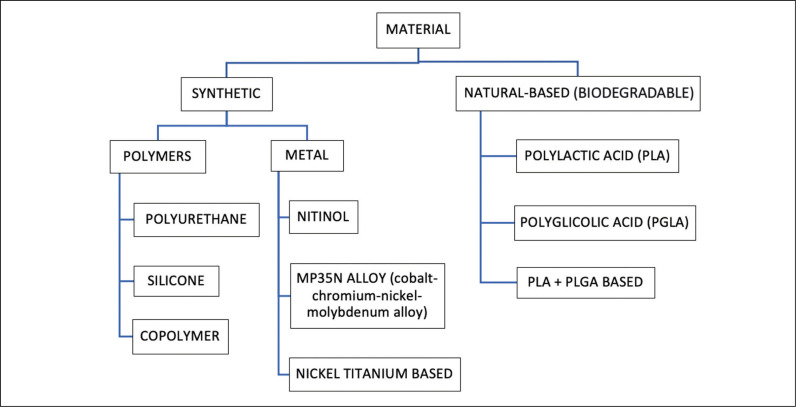
Figure 1 shows the most common materials for ureteral stents.
Figure 1.
Overview of the most common materials for ureteral stents.
Polyurethane (PU) is biocompatible, has good mechanical properties and a high drainage capacity, but it is prone to encrustation, especially by calcium oxalate, struvite, hydroxylapatite and cystine [1, 6].
Silicone, owing to its softness and durability, and because it is inert, non-toxic, more flexible, elastic and softer compared to other polymers (e.g. polyurethane), seems to be the most suitable material for stents [7]. It has the lowest encrustation rate [1] and seems to be the best choice for stenting for stone disease after ureteroscopy (URS) [7].
Copolymers like Cflex®, Silitek®, Percuflex®, Tecoflex®, Hydrothane®, ChronoFlex®, Aquavene® and Soft-Flex® have been studied by different companies to overcome the disadvantages of the other polymers and to provide a better biocompatibility and tolerance, although this has yet to be proven [2].
Metallic stents can resist high compression forces and are useful in long-term drainage [2]. Many of them are self-expandable, balloon-expandable, or thermo-expandable with shape memory [2]. Metals make stents ductile, malleable, easy-to-mold and resistant to compression [2]. Despite the ideal replacement time of six months, the majority of them have been designed to remain in place for up to 1–3 years [2, 8, 9]. Scientific evidence suggests that these stents when compared to other double J (DJ) stents provide less morbidity, a longer indwelling time, a greater patency rate and a better management of the strictures [2]. However, they cause epithelial hyperplasia and ingrowth of this hyperplastic tissue [2], and stent exchange may be challenging [9]. Biodegradable stents may cause reduced morbidity with less SRS and less hassle for removal [10]. Resistance of biodegradable stents, if compared to conventional ones, is high in the first three weeks after positioning and then gradually decreases [11]. They do not necessarily need to be removed [2] but the degradation can determine the formation of stent fragments that favor bacterial adhesion and encrustation [12]. On the other hand, urine flow ameliorates this and modification of the urothelium in terms of hyperplasia or inflammation should be reduced [12].
Shape
Many shapes have been developed over the years with the aim of creating a perfect stent with the least number of complications [13].
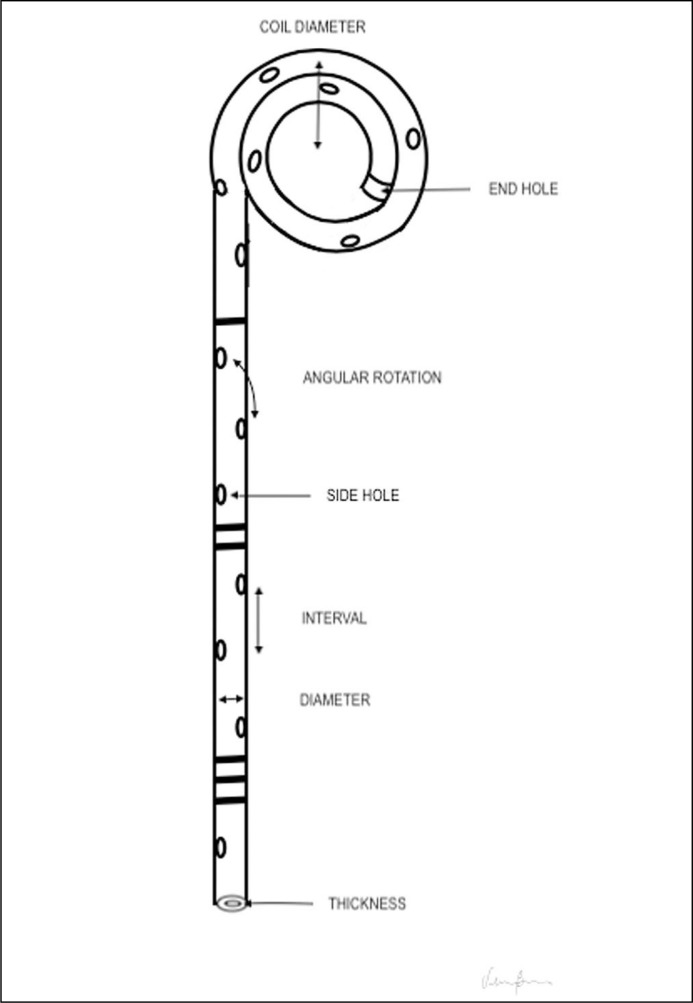
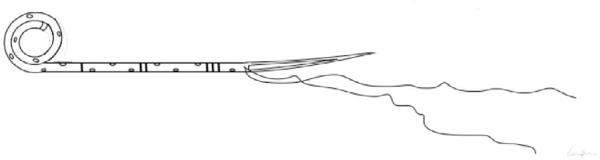
Figure 2 represents the usual structure of a ureteral stent. Urine passes not only through the holes but also alongside the stent, between it and the ureteral internal wall. Side holes, which are useful to allow urine flow [2], may be present only at the extremities of the stent.
Figure 2.
Ureteral stent anatomv. Some stents do not have side holes.
Owing to the fact that the distal ureter has the highest nerve density, modifications of the distal part of the stent have been studied to reduce SRS [10]. Examples of different shapes are displayed and described in Table 1.
Table 1.
Examples of different stent shapes
| SINGLE J (SJ) The proximal end has a J shape, while the distal end goes directly outside the urethra. The distal end can be connected to a separate bag in order to monitor the different diuresis between the two kidneys. |
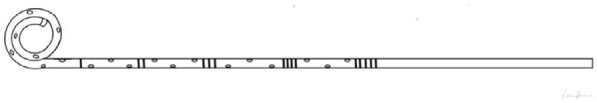
|
| DOUBLE J (DJ) Their name comes from the peculiar J shape of the proximal and distal end, that serves to avoid stent displacement [14]. Each coil usually has one end hole and four side holes, while the shaft may have multiple holes or no holes at all. |
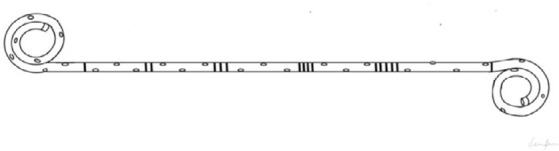
|
| STRING STENT Strings are made of suture material; they run through the urethra and exit from the external urethral meatus [16]. The position of the strings makes the removal process easier [2,15, 16], reducing complication rates and stent-related symptoms (SRS) when removed in a short time. |
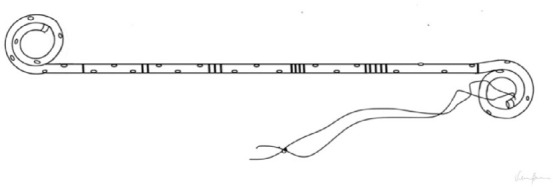
|
| TAIL STENT The loops of polymer on the distal end have been designed to reduce bladder irritation and obstructive urinary symptoms [14]. It is worth noting that these stents are not superior to DJ stents in reducing SRS [17]. |
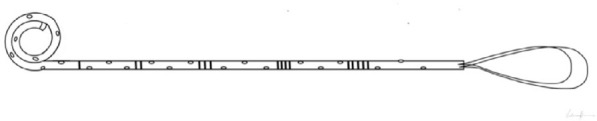
|
| PIGTAIL SUTURE STENT (PSS) 7 Fr x 16 cm stent with a distal 0.3 Fr double suture that reaches the bladder, reducing SRS. It is indicated in proximal stone-related obstruction, after stone treatment (URS - ureterorenoscopy/ureteroscopy), in case of ureteropelvic junction, stones and in strictures [18]. |
|
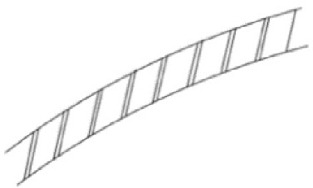
| SPIRAL STENT First made up of a metal wire that maintains a stable opening of the ureteric lumen in case of extrinsic compression or blockage [14], they are now polymeric spirally cut stents that adapt to the ureter's shape [19]. |
|
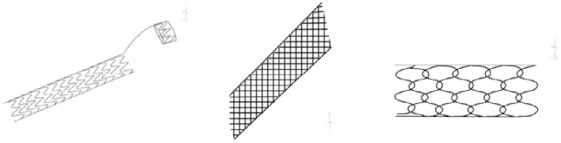
| MESH STENT Self-expanding stents created to reduce the irritation of the urinary tract and to facilitate the flow within the stent (when compared to JJ) [2]. Bioactive compounds can be eluted using mesh structure as a drug reservoir [14]. |
|
| ANTI-REFLUX Anti-reflux membrane valve should reduce reflux and its related symptoms [10]. The valves can be made of two liplike membranes with an inner cavity, that functions as a fluidic channel and a pathway for insertion of guidewires [20]. |
|
| MAGNETIC-TIPPED The magnetic end is composed of a stainless-steel bead that should make the removal procedure easier [2, 3]. The magnet at the distal coil of the ureter should also serve to prevent migration [10]. |
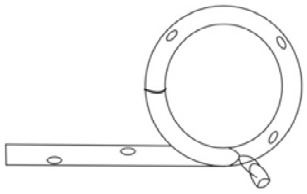
|
Size
The length is usually between 12 and 30 cm (5–12 inches) and the diameter varies from 4.5 to 18 Fr (0.06–0.2 inches) [1]. Multi-length stents have been developed to minimize stent-associated problems caused by the idea that a fixed size should fit every patient [15]. By the way, symptoms do not appear to be reduced [21].
Many studies revealed that the stent diameter is not associated with its function and success; a smaller diameter does not increase stent-associated complications [22, 23, 24]. Two meta-analyses showed that the incidence of ureteral stent-related symptoms is inversely proportional to the stent diameter [25, 26]; on the other hand, the diameter seems not to affect the incidence of stent migration, analgesic use and stone-free rate [26].
Coating
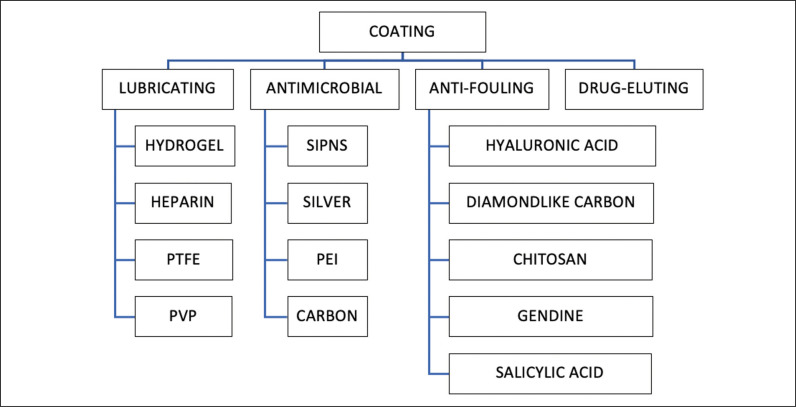
Stent coating could be a solution to reduce stent-associated complications. Coatings can be divided into four categories: lubricating, antimicrobial, anti-fouling and drug-eluting (Figure 3).
Figure 3.
Overview of different coatings for ureteral stents.
PTFE – TeflonTM; SIPNS – sequential interpenetrating polymer networks; PVP – polyvinylpyrrolidone, PEI – polyethyleneimine
Evidence on stent coating is variable [5].
Lubricating coatings are made of a hydrophilic material that provide a low surface friction [1]; hydrophilicity discourages the adhesion of hydrophobic bacterial surfaces [28].
Hydrogel coated stents are highly biocompatible and have a low coefficient of friction; with the addition of an antimicrobial, they may reduce bacterial growth [5, 27]. Hydrophilic gel alone, however, does not reduce bacterial adhesion [28]. A single study shows that SRS seems not to be modified by the presence or lack of hydrogel [29].
Heparin used as a coating can theoretically resist encrustation and adhesion of cellular organisms [1, 27]. In vitro studies did not demonstrate a reduction of bacterial adhesion [27].
Polyvinylpyrrolidone-coated stents have a soft, smooth and non-adhesive surface, that makes them highly lubricious. Even bacterial adhesion seems to be reduced [1].
PTFE (Teflon™) has a low friction coefficient n [12] and has been studied as slow-release storage for the delivery of liposomal lipid and liposomal drugs [30].
Silver inhibits enzyme activity and destabilizes bacterial membranes thereby reducing biofilm formation [3]. It has a broad-spectrum antimicrobial activity without being toxic as other heavy metals [27]. Actually, this coating has been widely studied for urethral catheters, but information about its use in ureteral stents are still lacking [28].
Anti-fouling coatings create a hydrophilic environment on the stent surface that reduces bacterial and cell adhesion [1, 3]. Irregularities over the stent surface in fact, create a favorable substrate for the adhesion of crystal, biological molecules and microorganisms [28].
Drug-eluting coatings have been developed to avoid microbial adhesion and to overcome stent-related discomfort and complications like cellular hyperplasia. A special stent coating can be made with antibiotics and antimicrobials (Tachyplesin, Triclosan), nonsteroidal anti-inflammatory drugs (ketorolac), Povidone-iodine, chlorhexidine and anti-tumor drugs [1, 2, 10, 27].
DISCUSSION
This review covers the basic information and main characteristics of ureteral stents. Additionally, it is fundamental to know the patients’ history and search for clinical and radiological findings that can help to decide if there is a real need for a ureteral stent. Secondly, to choose the best stent in each situation, it is necessary to know their characteristics. Patient comfort must also be considered in order to reduce hospital readmissions and the need for medications to treat stent-related symptoms.
We have made an attempt to cover all aspects of stents, yet it is difficult to represent every stent shape, composition and feature of all available stents worldwide. It is fundamental to remember that there is not a broad evidence around the best choice for each patient. Particularly for coating, many of them have been studied only in vitro and may have a potential favorable effect in vivo, but demonstrations are still needed.
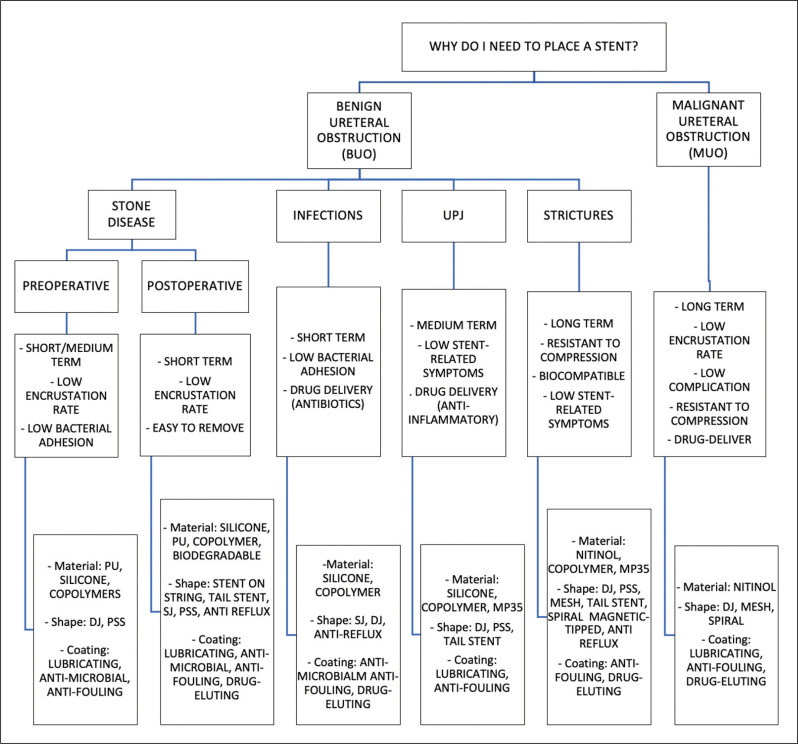
Figure 4 merely represents a suggestion based on the current available literature and on our experience, and not a recommendation or guideline. We hope to implement and correct this vademecum in the future, based on new evidence.
Figure 4.
How to choose the right stent.
BUO – benign ureteral obstruction; MUO – malignant ureteral obstruction; UPJ – ureteropelvic junction; PU – polyurethane; SJ – single J; DJ – double J; PSS – pigtail suture stent
CONCLUSIONS
Ureteral stents constitute a fundamental instrument in urologic surgery. Stent placement is still one of the most commonly practiced procedures in urologists’ everyday work. According to this, it is fundamental for physicians to know every aspect connected with stent design, material, type and positioning. Besides, stent-related symptoms and complications are challenging, and our guide can help clinicians choose the best stent needed for a given situation. We should consider, though, that many studies and research still need to be carried out in order to provide more information about stent composition and efficacy in vivo.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Venkatesan N, Shroff S, Jayachandran K, Doble M. Polymers as ureteral stents. J Endourol. 2010; 24: 191-198. [DOI] [PubMed] [Google Scholar]
- 2.Sali GM, Joshi HB. Ureteric stents: Overview of current clinical applications and economic implications. Int J Urol. 2020; 27: 7-15. [DOI] [PubMed] [Google Scholar]
- 3.Mosayyebi A, Vijayakumar A, Yue QY, et al. Engineering solutions to ureteral stents: Material, coating and design. Cent European J Urol. 2017; 70: 270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee L. Urinary Stone Diseases: The Practical Guide to Medical and Surgical Management. Ann R Coll Surg Engl. 2009; 91: 448. [Google Scholar]
- 5.Boeykens M, Keller EX, Bosio A, et al. Impact of Ureteral Stent Material on Stent-related Symptoms: A Systematic Review of the Literature. Eur Urol Open Sci. 2022; 45: 108-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas A, Cloutier J, Villa L, Letendre J, Ploumidis A, Traxer O. Prospective Analysis of a Complete Retrograde Ureteroscopic Technique with Holmium Laser Stent Cutting for Management of Encrusted Ureteral Stents. J Endourol. 2017; 31: 476-481. [DOI] [PubMed] [Google Scholar]
- 7.Wiseman O, Ventimiglia E, Doizi S, et al. Effects of Silicone Hydrocoated Double Loop Ureteral Stent on Symptoms and Quality of Life in Patients Undergoing Flexible Ureteroscopy for Kidney Stone: A Randomized Multicenter Clinical Study. J Urol. 2020; 204: 769-777. [DOI] [PubMed] [Google Scholar]
- 8.Gu A, Oyo L, Grossmann NC, et al. Tumor Stent for Chronic Ureteral Obstruction: Which Are Predictors of Stent Failure? J Endourol. 2022; 36: 819-826. [DOI] [PubMed] [Google Scholar]
- 9.Sampogna G, Grasso A, Montanari E. Expandable metallic ureteral stent: indications and results. Minerva Urol Nefrol. 2018; 70: 275-285. [DOI] [PubMed] [Google Scholar]
- 10.Forbes C, Scotland KB, Lange D, Chew BH. Innovations in Ureteral Stent Technology. Urol Clin North Am. 2019; 46: 245-255. [DOI] [PubMed] [Google Scholar]
- 11.Yang G, Xie H, Huang Y, et al. Immersed multilayer biodegradable ureteral stent with reformed biodegradation: An in vitro experiment. J Biomater Appl. 2017; 31: 1235-1244. [DOI] [PubMed] [Google Scholar]
- 12.Barros AA, Oliveira C, Ribeiro AJ, et al. In vivo assessment of a novel biodegradable ureteral stent. World J Urol. 2018; 36: 277-283. [DOI] [PubMed] [Google Scholar]
- 13.Finney RP. Experience with new double J ureteral catheter stent. J Urol. 1978; 120: 678-681. [DOI] [PubMed] [Google Scholar]
- 14.Mosayyebi A, Manes C, Carugo D, Somani BK. Advances in Ureteral Stent Design and Materials. Curr Urol Rep. 2018; 19: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandra M, Mosayyebi A, Carugo D, Somani BK. Strategies to improve patient outcomes and qol: Current complications of the design and placements of ureteric stents. Res Reports Urol. 2020; 12: 303-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliver R, Wells H, Traxer O, et al. Ureteric stents on extraction strings: a systematic review of literature. Urolithiasis. 2018; 46: 129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosio A, Alessandria E, Agosti SC, et al. Loop-tail stents fail in reducing stent-related symptoms: results of a prospective randomised controlled trial. BJU Int. 2022; 129: 123-129. [DOI] [PubMed] [Google Scholar]
- 18.Vogt B, Desgrippes A, Desfemmes FN. Changing the double-pigtail stent by a new suture stent to improve patient’s quality of life: a prospective study. World J Urol. 2015: 33: 1061-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chew BH, Rebullar KA, Harriman D, McDougall E, Paterson RF, Lange D. Percuflex Helical Ureteral Stents Significantly Reduce Patient Analgesic Requirements Compared to Control Stents. J Endourol. 2017; 31: 1321-1325. [DOI] [PubMed] [Google Scholar]
- 20.Park CJ, Kim HW, Jeong S, et al. Anti-Reflux Ureteral Stent with Polymeric Flap Valve Using Three-Dimensional Printing: An in Vitro Study. J Endourol. 2015; 29: 933-938. [DOI] [PubMed] [Google Scholar]
- 21.Calvert RC, Wong KY, Chitale SV, et al. Multi-length or 24 cm ureteric stent? A multicentre randomised comparison of stent-related symptoms using a validated questionnaire. BJU Int. 2013; 111: 1099-1104. [DOI] [PubMed] [Google Scholar]
- 22.Sebastian Nestler, Witte B, Schilchegger L, Jones J. Size does matter: ureteral stents with a smaller diameter show advantages regarding urinary symptoms, pain levels and general health. World J Urol. 2020; 38: 1059-1063. [DOI] [PubMed] [Google Scholar]
- 23.Taguchi M, Yoshida K, Sugi M, Kinoshita H, Matsuda T. Effect of ureteral stent diameter on ureteral stent-related symptoms. Low Urin Tract Symptoms. 2019; 11: 195-199. [DOI] [PubMed] [Google Scholar]
- 24.Gang Wu, Fengze Sun, Kai Sun, et al. Impact of differential ureteral stent diameters on clinical outcomes after ureteroscopy intracorporeal lithotripsy: A systematic review and meta-analysis. Int J Urol. 2021; 28: 992-999. [DOI] [PubMed] [Google Scholar]
- 25.Wu G, Sun F, Sun K, et al. Impact of differential ureteral stent diameters on clinical outcomes after ureteroscopy intracorporeal lithotripsy: A systematic review and meta-analysis. Int J Urol. 2021; 28: 992-999. [DOI] [PubMed] [Google Scholar]
- 26.Diatmika AANO, Djojodimedjo T, Kloping YP, Hidayatullah F, Soebadi MA. Comparison of ureteral stent diameters on ureteral stent-related symptoms: A systematic review and meta-analysis. Turk J Urol. 2022; 48: 30-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liaw A, Knudsen B. Urinary tract infections associated with ureteral stents: A Review. Arch Esp Urol. 2016; 69: 479-484. [PubMed] [Google Scholar]
- 28.Yang L, Whiteside S, Cadieux PA, Denstedt JD. Ureteral stent technology: Drug-eluting stents and stent coatings. Asian J Urol. 2015; 2: 194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph V. Candela and Gary C. Bellman Ureteral Stents: Impact of Diameter and Composition on Patient Symptoms. J Endourol. 1997; 11: 45-47. [DOI] [PubMed] [Google Scholar]
- 30.Antimisiaris SG, Siablis D, Liatsikos E, et al. Liposome-coated metal stents: An in vitro evaluation of controlled-release modality in the ureter. J Endourol. 2000; 14: 743-747. [DOI] [PubMed] [Google Scholar]