Abstract
Aim
To investigate the safety of skin-to-skin contact initiated immediately after birth on cardiorespiratory parameters in unstable low birth weight infants.
Methods
A randomized clinical trial was conducted in tertiary newborn units in Ghana, India, Malawi, Nigeria and Tanzania in 2017–2020, in infants with birth weight 1.0–1.799 kg. The intervention was Kangaroo mother care initiated immediately after birth and continued until discharge compared to conventional care with Kangaroo mother care initiated after meeting stability criteria. The results of the primary study showed that immediate Kangaroo mother care reduced neonatal mortality by 25% and the results have been published previously. The post-hoc outcomes of this study were mean heart rate, respiratory rate, oxygen saturation during the first four days and the need of respiratory support.
Results
1,602 infants were allocated to control and 1,609 to intervention. Mean birth weight was 1.5 kg (SD 0.2) and mean gestational age was 32.6 weeks (SD 2.9). Infants in the control group had a mean heart rate 1.4 beats per minute higher (95% CI -0.3–3.1, p = 0.097), a mean respiratory rate 0.4 breaths per minute higher (-0.7–1.5, p = 0.48) and a mean oxygen saturation 0.3% higher (95% CI -0.1–0.7, p = 0.14) than infants in the intervention group.
Conclusion
There were no significant differences in cardiorespiratory parameters during the first four postnatal days. Skin-to-skin contact starting immediately after birth is safe in low birth weight infants in limited-resource settings.
Keywords: Kangaroo mother care, Skin-to-skin contact, Low birth weight infant, Randomized clinical trial, Cardiorespiratory parameters
1. Introduction
About 15% of infants worldwide are born with a low birth weight (LBW) and the majority of neonatal deaths occur in this population.1, 2, 3 Kangaroo Mother Care (KMC) involves infant-parent skin-to-skin contact (SSC) and is an effective intervention for preventing neonatal deaths.4,5 SSC and KMC are often used interchangeably. However, KMC is a wider concept beginning with SSC and including breastfeeding support and early discharge with frequent follow-up.6 Traditionally, SSC is initiated when the LBW infant is considered stable as per World Health Organization (WHO) recommendations.7 This may occur after some days from birth. In settings of healthy newborn infants, immediate SSC has been defined as starting within ten minutes and early SSC as starting within 24 h.8 KMC as traditionally described in LBW infants, starting late at around the third postnatal day, has been associated with improved thermal control,4,9 higher breastfeeding rates,4,8 protection against neonatal sepsis4,9 and decreased mortality.4 The WHO immediate Kangaroo mother care (iKMC) Study Group recently reported that SSC initiated already after birth and provided continuously throughout the newborn unit stay in limited resource settings reduced neonatal mortality by 25%.9,10 In terms of physiology, time to clinical stabilization as per predefined criteria was the same in both study allocations. The proportion of infants with hypothermia was lower in the iKMC group.9
Studies investigating cardiorespiratory effects of SSC show conflicting results. Two studies on immediate SSC in low- and middle-resource settings have used a score involving heart rate, fraction of inspired oxygen, oxygen saturation and apnea to describe the composite cardiorespiratory state of the infant, and showed that LBW infants had better cardiorespiratory stability during the first six hours when cared for in SSC compared to conventional care in incubators or cots.11,12 With regards to the fraction of inspired oxygen in very low birth weight infants in SSC, studies have described both lower13 and higher fractions of inspired oxygen.14 One study reported a decrease in bradycardic events during SSC.15 Other studies have reported no differences in heart rate, respiratory rate or oxygen saturation during SSC.16 A recent review described the heterogeneity of studies on SSC and cardiorespiratory outcomes.17 A dose-response relationship has been hypothesized, but one study confirms a positive effect on the physiology of very low birth weight infants even with hour-long sessions of SSC.18 The importance of early tactile stimulation for positive neurodevelopmental experiences is well-described,19,20 as well as mother-infant separation as a source of toxic stress.21
Overall, data on the mechanisms of immediate and early SSC in very preterm and LBW infants is scarce.22 The aim of this report was to investigate the effects of immediate and continuous SSC on cardiorespiratory parameters in the LBW infants of the WHO iKMC study cohort.
2. Methods
The methods of the iKMC study have been published9,10 and are briefly described as follows: The two-armed, multi-country, non-blinded randomized clinical trial was registered in Australian-New Zealand Clinical Trials Registry Number ACTRN12618001880235 and India Clinical Trials Registry Number CTRI/2018/08/015,369. The study protocol was approved by the WHO Ethics Review Committee with reference number: EC0002901 and by local institutional review boards of the sites. A detailed description of the ethical approvals, sample size calculation, recruitment procedure and intervention was described in the protocol10 and the publication of the primary outcome.9 Cardiac and respiratory parameters were not defined as outcomes in the study protocol and were hence analyzed post-hoc.
2.1. Selection and description of participants
3211 newborns, 1609 in intervention and 1602 in control, with birth weight 1.000–1.799 kg regardless of gestational age, mode of birth or single or twin status were enrolled at tertiary-level newborn units in Ghana, India, Malawi, Nigeria and Tanzania between December 2017 and January 2020. Exclusion criteria were a mother younger than 15 years of age, triplet pregnancies or more, if the mother was considered too sick to be likely to provide KMC within three days after birth, if the mother resided outside the study uptake area, if the child did not breathe spontaneously within the first postnatal hour, if the infant was unlikely to be enrolled within two hours after birth, or had major congenital malformations. Randomization was done in strata per site and by birth weight < 1.500 and ≥ 1.500 kg.
2.2. Essential newborn care
To ensure harmonized standard of good newborn care across study sites, consultants from the Karolinska Institutet provided training in and monitoring of Essential newborn care23 and Helping Babies Breathe,24 including thermal control, early and frequent breast milk feeding, infection prevention and control, respiratory support for respiratory distress syndrome and assessment of the newborn. The sites were provided with continuous positive airway pressure (CPAP) machines: bubble CPAPs (Diamedica, UK) and pulse oximeters (Masimo Radical, V 5.0; Masimo Corp., Irvine, CA, USA). Before the study, CPAP machines had been variably available at the sites.
2.3. Definition of infant stability
The protocol definition of clinical stability was respiratory rate of 40 to 60 breaths per minute, oxygen saturation of above 90%, no respiratory support including supplementary oxygen, no apneas, heart rate of 80 to 179 per minute, an axillary temperature of 36.0 to 37.4 °C and no need for intravenous fluids during the last 24 h.
This article presents the results of the post-hoc analyses of newborn cardiorespiratory and support parameters during the first four days for the study population.
2.4. Definition of immediate kangaroo mother care
The definition of iKMC was newborn care in non-separation from the mother, meaning the mother or surrogate caregiver and infant being admitted to the newborn unit together for SSC initiated as soon as possible and provided continuously, aiming at 20 h per day, integrated in the conventional medical and nursing care.10 The iKMC study reported a median SSC initiation time with interquartile range (IQR) of 1.3 h (IQR 0.8–2.7) after birth in the intervention group and 53.6 h (IQR 34–101) in the control group.9 The daily SSC duration was 16.9 h (IQR 13.0–19.7) in the intervention group and 1.5 h (IQR 0.3–3.3) in the control group during the first three postnatal days.9
2.5. Data collection
A first observation of cardiorespiratory parameters was performed at enrolment, after which an outcome measurement team performed observations. Observations were done at pre-specified hours of the day, six-hourly, until the infant had met stability criteria for 24 h and was transferred to the KMC unit. Whether the infant was in SSC or in another place was not taken into account, nor was the state of the infant such as if the infant was sleeping or active at the time of data collection. As an individual infant could have been born anytime during the day, in this report we included data from the first four calendar days after birth in order to ensure data from the first 72 postnatal hours for all infants. The parameters collected were heart rate, respiratory rate, oxygen saturation, any CPAP including positive end-expiratory pressure, supplementary oxygen including flow, axillary temperature and duration of SSC during the last 12-hour period. Data was later entered into an electronic case report form (RedCap, Vanderbilt, USA25). Mean heart rate, mean respiratory rate, mean oxygen saturation and any respiratory support during days zero to three were analyzed in this study.
2.6. Statistical analysis
Data was analyzed by intention to treat using Stata/IC 15.0 (StataCorp LLC, Texas, USA). Descriptive statistics were used to present baseline variables. Mean heart rate, respiratory rate and oxygen saturation during days zero to three were compared with linear regression and also analyzed with multilevel mixed-effects linear regression with random effects and an independent structure. The time factor in this model was the infant's individual postnatal age in hours, whereas in the graphical presentations mean parameter per order of observation was calculated, translating to different exact postnatal ages. Subgroup analyses were done by birth weight 1.0 to 1.499 and 1.5 to 1.799 kg, gestational age below 32 and above 31.9 weeks and for the infants who later died. All tests were two-sided and controlled at a significance level of 0.05. As in the publication of the primary outcome, adjustments were done for family income, mother's age, mother's years of schooling, mode of birth, multiple births, birth weight, sex and Apgar score at five minutes and for clustering of sites.
3. Results
3.1. Infant characteristics
There were no statistically significant differences between groups regarding gestational age and birth weight. Overall mean gestational age was 32.6 gestational weeks (SD 2.9) and mean birth weight was 1.5 kg (SD 0.2). Median time to clinical stabilization was 73.8 h (IQR 26.8–138.5) in the intervention group and 74.8 h (IQR 25.3–140.6) in the control group. Moreover, median stay in the newborn unit before transfer to the KMC unit was 6.3 days (IQR 4.0–8.9) for the iKMC group and 6.0 days (IQR 3.5–9.9) for the control group, a difference with 95% confidence interval (CI) of 6 h (95% CI −4–15, p = 0.24) after adjustments in favor of the control group. More infant characteristics are presented in Table 1.
Table 1.
Infant characteristics.
| Control, n = 1602 | iKMC, n = 1609 | |
|---|---|---|
| BW, kg, mean (SD) | 1.5 (0.2) | 1.5 (0.2) |
| GA, weeks, mean (SD) | 32.6 (2.8) | 32.6 (3.1) |
| Twin, n (%) | 430 (27) | 430 (27) |
| CS, n (%) | 614 (38.3) | 559 (34.7) |
| Male, n (%) | 748 (47) | 752 (47) |
| Ever CPAP, n (%) | 960 (60) | 998 (62) |
| Ever oxygen, n (%) | 1029 (64) | 1006 (63) |
| Time to transfer NICU-KMC unit, days, median (IQR) | 6.0 (3.5–9.9) | 6.3 (4–8.9) |
| Died, n (%) | 249 (15.7) | 191 (12.0) |
BW=birth weight, CPAP=continuous positive airway pressure, CS=Caesarean section, GA=gestational age, iKMC=immediate Kangaroo mother care, IQR=interquartile range, NICU=neonatal intensive care unit, SD=standard deviation.
3.2. Cardiorespiratory parameters
There were 49,190 observations during days zero to three for the 3209 infants included in the post-hoc analyses. Two infants allocated to iKMC died before any observations were done. Missing data was equally distributed between allocations at 5% respectively for heart rate, respiratory rate and oxygen saturation. For respiratory support, CPAP or supplementary oxygen, missing data was 5% in the control and 4% in the intervention group. Missing data was mainly due to research staff not being able to collect data when the infant was not available due to medical procedures. The multilevel mixed-effects linear regression model handled missing data by method and marginal mean imputation was performed for missing data in the other analyses.
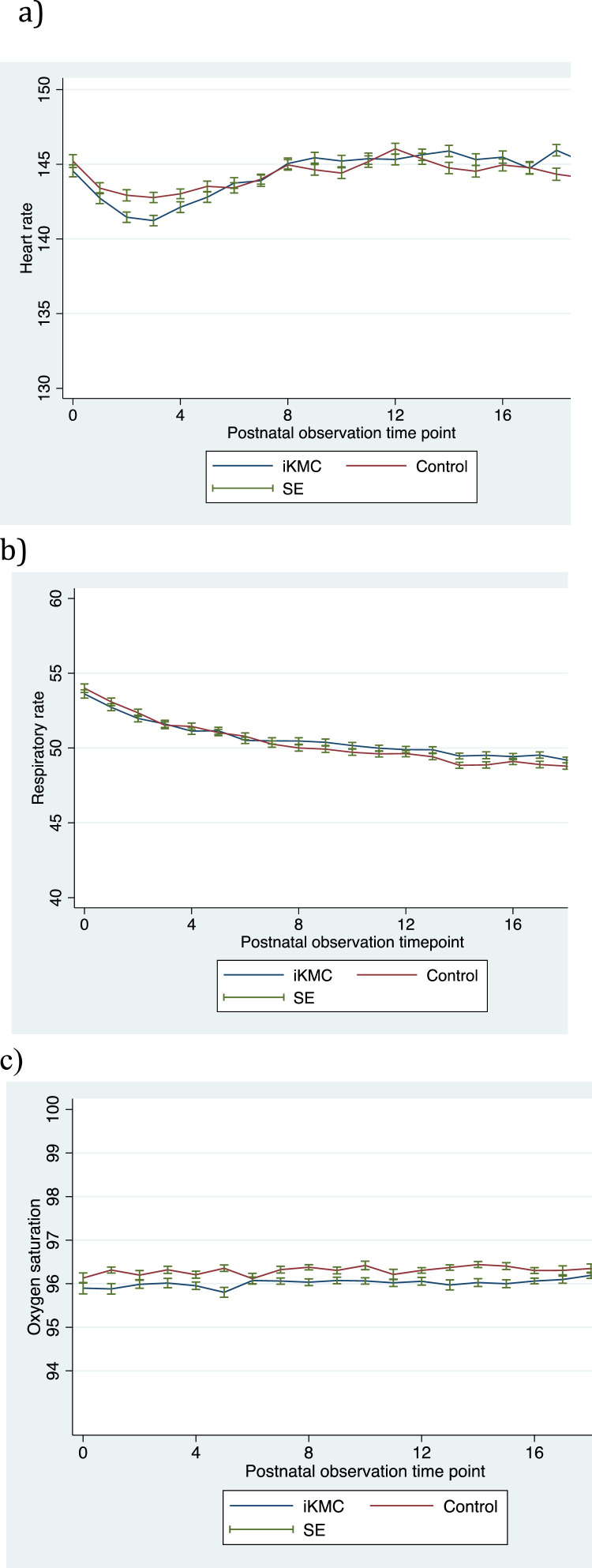
During days zero to three, infants of the control group had a mean heart rate 1.4 beats per minute higher (95% CI −0.3–3.1), p = 0.097, than infants of the iKMC group and an interaction effect of allocation and time on heart rate of −0.0 (95% CI −0.1- −0.0), p = 0.004 (Fig. 1a). Infants in the control group had a mean respiratory rate 0.4 breaths per minute higher (−0.7–1.5), p = 0.48 and an interaction effect on respiratory rate of allocation and time of −0.0 (95% CI −0.0- −0.0), p = 0.01 (Fig. 1b). Infants of the control group had an oxygen saturation 0.3% higher (95% CI −0.1–0.7), p = 0.14 with an interaction effect on oxygen saturation of allocation and time of 0.0 (95% CI −0.0- 0.0), p = 0.93 (Fig. 1c). During the first day, infants in the control group had a heart rate 1.3 beats per minute higher (95% CI 0.7–1.9), p<0.001 than infants in the iKMC group.
Fig. 1.
Mean heart rate, respiratory rate and oxygen saturation.
Mean heart rate in beats per minute (a), respiratory rate in breaths per minute (b) and oxygen saturation in percent (c) with standard errors for infants allocated to iKMC or control. Range of heart rate according to stability criteria: 120–160 beats per minute. Range of respiratory rate according to stability criteria: 40–60 breaths per minute. Range of oxygen saturation according to stability criteria: >90%. The number on the X-axis refers to the order of six-hourly observation time-points for each child, four observations per 24 h. iKMC=immediate Kangaroo mother care, SE=standard error.
Pooled parameters collected for days zero to three showed that the mean heart rate was 144 beats per minute (SD 14) in both allocations and 1.3% of observations in both groups had heart rates outside the normal range of 80 to 179 beats per minute. Mean respiratory rate was 51 breaths per minute (SD 9) in both groups and 14% of observations in both groups were outside the normal range of 40 to 60 breaths per minute. Mean oxygen saturation was 96% (SD 3) in both allocations, regardless of whether the infant was on supplementary oxygen, CPAP or no respiratory support. Oxygen saturation below 90% was observed in 1.1% of the control group and 1.3% of the iKMC group. In infants with no respiratory support, oxygen saturation was under 90% in 0.3% of the observations in the control group and 0.5% in the iKMC group. Heart rate, respiratory rate and oxygen saturation by gestational age group, by birth weight groups and in those who later died are presented in Table 2.
Table 2.
Heart rate, respiratory rate and oxygen saturation per gestational age and birth weight strata and in those who later died.
| Strata | Heart rate (SD) | Respiratory rate (SD) | Oxygen saturation (SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| iKMC | Control | Diff (95% CI, p) | iKMC | Control | Diff (95% CI, p) | iKMC | Control | Diff (95% CI, p) | |
| GA <32 v | 146 (15) | 146 (14) | 0.2 (−0.2–0.7, p = 0.31) | 52 (10) | 51 (9) | −0.6 (−0.9- −0.3, p<0.001) | 96 (4) | 96 (4) | 0.2 (0.1–0.3, p = 0.001) |
| GA >32 v | 143 (14) | 143 (14) | 0.1 (−0.2–0.5, p = 0.39) | 50 (8) | 50 (8) | 0.1 (−0.1–0.03, p = 0.26) | 96 (3) | 96 (3) | 0.3 (0.3–0.4, p<0.001) |
| BW <1.5 kg | 145 (15) | 145 (15) | 0.1 (−0.3–0.4, p = 0.25) | 52 (9) | 51 (9) | −0.4 (−0.6- −0.1, p = 0.003) | 96 (4) | 96 (3) | 0.2 (0.05–0.3, p = 0.003) |
| BW >1.5 kg | 143 (14) | 143 (13) | 0.2 (−0.1–0.5, p = 0.27) | 50 (8) | 50 (9) | 0.1 (−0.2–0.3, p = 0.65) | 96 (4) | 96 (3) | 0.4 (0.3–0.5, p<0.001) |
| Died later | 150 (17) | 148 (18) | −1.7 (−2.7 - 0.7, p = 0.002) | 53 (10) | 52 (9) | −1.2 (−1.8- −0.7, p<0.001) | 95 (7) | 95 (6) | 0.5 (0.2–0.9, p = 0.003) |
Mean heart rate in beats per minute, respiratory rate in breaths per minute and oxygen saturation in percent, with standard deviations and the difference between allocations with 95% confidence intervals and p-values per GA and BW strata and for the infants who later died. BW=birth weight, CI=confidence interval, GA=gestational age, SD=standard deviation.
The proportions of infants with any supplementary oxygen in the control and iKMC groups were 64% and 63% respectively, an odds ratio of 1.1 (95% CI 0.9–1.3), p = 0.29 for the control group. The proportion of infants with any CPAP in the control and iKMC groups were 60% and 62%, respectively, corresponding to an odds ratio for the control group of 0.9 (95% CI 0.80–1.1), p = 0.25. There was a difference between sites in overall proportions of infants with any CPAP. The proportions of infants with any CPAP in Ghana, India, Malawi, Nigeria and Tanzania were 71%, 66%, 55%, 78% and 48%, respectively. Of those who did have CPAP, median time with CPAP was 32 h (IQR 12–66) in the iKMC group and 28 h (IQR 11–59) in control group. The difference in duration was 6.6 h after adjustments, (95% CI 1.6–11.6, p = 0.009), in favor of the control group.
4. Discussion
Our post-hoc analyses of the cardiorespiratory parameters collected during the first four postnatal days in the iKMC study demonstrated that there were no significant differences in heart rate, respiratory rate and oxygen saturation between allocations. Hence, previous recommendations that unstable LBW infants should not be provided KMC due to safety concerns are not supported by our report. Previous publications have shown a better cardiorespiratory stabilization in LBW infants in SSC early after birth.11,12,26 However, there were two major differences between our current report and earlier research. The contexts in terms of resources are different between the cited studies and the present. Moreover, data in this report were collected at six-hourly observations during the first four postnatal days whereas in the cited studies data were collected every 15 to 30 min during the first six postnatal hours. This may reflect the period of transition to extra-uterine life taking place in the first few hours, prior to data collection in this study. The frequency of observations may also contribute to the differences. Furthermore, the slightly lower heart rate in the iKMC group was statistically significant during the first day, which might indicate an early impact of SSC. This further highlights that more studies on infant physiology during the first hours are warranted to learn more about the physiological risks and benefits of immediate SSC in the LBW infant.
There was a statistically significant interaction effect of allocation and time on heart rate and respiratory rates, meaning that infants of the control group decreased in heart and respiratory rate quicker than infants in the iKMC group, but there was unlikely a clinical significance as the absolute effect sizes were very small and mean parameters well within normal ranges. Infants in the control group had their CPAP for a quarter of a day shorter than those in the iKMC group, which may be of clinical significance but it may also be explained by confounding by indication. Our study indicates that SSC integrated in the early medical and nursing newborn care for LBW infants in low- and middle-income settings is safe relative to conventional care and with regards to the cardiorespiratory parameters presented here.
The newborn unit represents an adverse environment for the LBW or preterm infant, with a large amount of unexpected and painful exposures.27 The concept of mother-infant couplet care has been described28 and may provide buffering of stressful events by mother-infant co-regulation, especially by the affectionate caregiver touch that SSC provides.19,20 The long-term neurodevelopmental consequences of iKMC are currently being investigated.29
4.1. Strengths and limitations
A strength of the study is the large number of observations of physiological parameters. However, for the individual infant, the daily observations were quite few and the place or state of the infant at the time of data collection were not taken into account. The six-hourly recording of single values was a convenient compromise and a limitation compared to the above-mentioned studies with a higher frequency of observations. Although physiological parameters were not part of the pre-specified primary or secondary outcomes of the study, the proportion of missing data was low.
5. Conclusion
In addition to the reduction in neonatal mortality, immediate SSC in unstable LBW infants is safe in terms of cardiorespiratory parameters including its practice in settings with limited resources and little previous experience. This supports a change in guidelines to recommend KMC initiation already after birth also for LBW infants in need of medical care and the implementation and scale-up of mother-infant couplet care units. More studies that focus on infant physiology are needed to learn more of the cardiorespiratory effects of SSC immediately after birth in unstable LBW infants.
Declaration of Competing Interest
The authors report no conflicts of interest to declare.
Acknowledgements
We would like to thank all the infants and families who participated in the iKMC study, all research staff who collected the data and all members of the WHO iKMC writing committee. The iKMC study was funded by the Bill and Melinda Gates Foundation, grant number OPP1151718, but the work with the post-hoc analyses presented in this manuscript received no specific funding.
References
- 1.UNICEF, WHO. Low birthweight estimates: levels and trends 2000–2015. 2019. Accessed 20211020, 2021.
- 2.Liu L., Oza S., Hogan D., et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet North Am Ed. 2016;388(10063):3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn J.E., Cousens S., Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 4.Conde-Agudelo A., Diaz-Rossello J. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016;(8) doi: 10.1002/14651858.CD002771.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhutta Z.A., Das J.K., Bahl R., et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet North Am Ed. 2014;384(9940):347–370. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]
- 6.Nyqvist-Hedberg K., Anderson G.C., Bergman N., et al. State of the art and recommendationsKangaroo mother care: application in a high-tech environment. Acta Paediatr. 2010;99(6):812–819. doi: 10.1111/j.1651-2227.2010.01794.x. [DOI] [PubMed] [Google Scholar]
- 7.WHO . 2003. Kangaroo Mother Care - A Practical Guide; p. 2019. [Google Scholar]
- 8.Moore E., Bergman N., Anderson G., Medley N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2016;11 doi: 10.1002/14651858.CD003519.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO iKMC Study Group Immediate “Kangaroo Mother Care” and survival of infants with low birth weight. N Engl J Med. 2021;384(21):2028–2038. doi: 10.1056/NEJMoa2026486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO iKMC Study Group Impact of continuous Kangaroo Mother Care initiated immediately after birth (iKMC) on survival of newborns with birth weight between 1.0 to < 1.8 kg: study protocol for a randomized controlled trial. Trials. 2020;21(1):280. doi: 10.1186/s13063-020-4101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman N.J., Linley L., Fawcus S. Randomized controlled trial of skin-to-skin contact from birth versus conventional incubator for physiological stabilization in 1200- to 2199-gram newborns. Acta Paediatr. 2004;93(6):779–785. doi: 10.1111/j.1651-2227.2004.tb03018.x. [DOI] [PubMed] [Google Scholar]
- 12.Chi Luong K., Long Nguyen T., Huynh Thi D.H., Carrara H., Bergman N.J. Newly born low birthweight infants stabilise better in skin-to-skin contact than when separated from their mothers: a randomised controlled trial. Acta Paediatr. 2016;105(4):381–390. doi: 10.1111/apa.13164. [DOI] [PubMed] [Google Scholar]
- 13.Jones H., Santamaria N. An observational cohort study examining the effect of the duration of skin-to-skin contact on the physiological parameters of the neonate in a neonatal intensive special care unit. Adv Neonatal Care. 2018;18(3):208–214. doi: 10.1097/ANC.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 14.Bisanalli S., Nesargi S., Govindu R.M., Rao S.P.N. Kangaroo mother care in hospitalized low birth-weight infants on respiratory support. Adv Neonatal Care. 2019;19(6):21–25. doi: 10.1097/ANC.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 15.Kommers D.R., Joshi R., van Pul C., et al. Features of heart rate variability capture regulatory changes during kangaroo care in preterm infants. J Pediatr. 2017;182:92–98. doi: 10.1016/j.jpeds.2016.11.059. e91. [DOI] [PubMed] [Google Scholar]
- 16.Heimann K., Vaeβen P., Peschgens T., Stanzel S., Wenzl T.G., Orlikowsky T. Impact of skin to skin care, prone and supine positioning on cardiorespiratory parameters and thermoregulation in premature infants. Neonatology. 2010;97(4):311–317. doi: 10.1159/000255163. [DOI] [PubMed] [Google Scholar]
- 17.Cristóbal Cañadas D., Bonillo Perales A., Galera Martínez R., Casado-Belmonte MdP, Parrón Carreño T. Effects of kangaroo mother care in the NICU on the physiological stress parameters of premature infants: a meta-analysis of RCTs. Int J Environ Res Public Health. 2022;19(1):583. doi: 10.3390/ijerph19010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boju S.L., Gopi Krishna M., Uppala R., Chodavarapu P., Chodavarapu R. Short spell kangaroo mother care and its differential physiological influence in subgroups of preterm babies. J Trop Pediatr. 2011;58(3):189–193. doi: 10.1093/tropej/fmr072. [DOI] [PubMed] [Google Scholar]
- 19.Mariani Wigley I.L.C., Mascheroni E., Bonichini S., Montirosso R. Epigenetic protection: maternal touch and DNA-methylation in early life. Curr Opin Behav Sci. 2022;43:111–117. [Google Scholar]
- 20.Carozza S., Leong V. The role of affectionate caregiver touch in early neurodevelopment and parent–infant interactional synchrony. Front Neurosci. 2021;14 doi: 10.3389/fnins.2020.613378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergman N.J. Birth practices: maternal-neonate separation as a source of toxic stress. Birth Defects Res. 2019;111(15):1087–1109. doi: 10.1002/bdr2.1530. [DOI] [PubMed] [Google Scholar]
- 22.Gupta N., Deierl A., Hills E., Banerjee J. Systematic review confirmed the benefits of early skin-to-skin contact but highlighted lack of studies on very and extremely preterm infants. Acta Paediatr. 2021;110(8):2310–2315. doi: 10.1111/apa.15913. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Consultation on improving measurement of the quality of maternal, newborn and child care in health facilities. 2014.
- 24.Niermeyer S., Little G.A., Singhal N., Keenan W.J. A short history of helping babies breathe: why and how, then and now. Pediatrics. 2020;146(Suppl 2):S101–S111. doi: 10.1542/peds.2020-016915C. [DOI] [PubMed] [Google Scholar]
- 25.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linnér A., Lode Kolz K., Klemming S., Bergman N., Lilliesköld S., Markhus Pike H., et al. Immediate skin-to-skin contact may have beneficial effects on the cardiorespiratory stabilisation in very preterm infants. Acta Paediatr. 2022;111(8):1507–1514. doi: 10.1111/apa.16371. [DOI] [PubMed] [Google Scholar]
- 27.Provenzi L., Guida E., Montirosso R. Preterm behavioral epigenetics: a systematic review. Neurosci Biobehav Rev. 2018;84:262–271. doi: 10.1016/j.neubiorev.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Klemming S., Lilliesköld S., Westrup B. Mother-newborn couplet care from theory to practice to ensure zero separation for all newborns. Acta Paediatr. 2021;110(11):2951–2957. doi: 10.1111/apa.15997. [DOI] [PubMed] [Google Scholar]
- 29.Adejuyigbe E.A., Agyeman I., Anand P., et al. Evaluation of impact of continuous KMC initiated immediately after birth compared to KMC initiated after stabilization, in newborns with birth weight 1.0 to <1.8kg on neurodevelopmental outcomes: protocol for a follow-up study. Research Square Preprint. 2022 doi: 10.21203/rs.3.rs-1876440/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]