Abstract
Objective
The Canadian Tofacitinib for Rheumatoid Arthritis Observational (CANTORAL) is the first Canadian prospective, observational study assessing tofacitinib. The objective was to assess effectiveness and safety for moderate to severe rheumatoid arthritis (RA). Coprimary and secondary outcomes are reported from an interim analysis.
Methods
Patients initiating tofacitinib from October 2017 to July 2020 were enrolled from 45 Canadian sites. Coprimary outcomes (month 6) included the Clinical Disease Activity Index (CDAI)–defined low disease activity (LDA) and remission. Secondary outcomes (to month 18) included the CDAI and the 4‐variable Disease Activity Score in 28 joints (DAS28) using the erythrocyte sedimentation rate (ESR)/C‐reactive protein (CRP) level to define LDA and remission; the proportions of patients achieving mild pain (visual analog scale <20 mm), and moderate (≥30%) and substantial (≥50%) pain improvements; and the proportions of patients achieving a Health Assessment Questionnaire disability index (HAQ DI) score greater or equal to normative values (≤0.25) and a HAQ DI score greater or equal to minimum clinically important difference (MCID) (≥0.22). Safety was assessed to month 36.
Results
Of 504 patients initiating tofacitinib, 44.4% received concomitant methotrexate. At month 6, 52.9% and 15.4% of patients were in CDAI‐defined LDA and remission, respectively; a similar proportion of patients achieved outcomes by month 3 (first post‐baseline assessment). By month 3, 27.2% and 41.7% of patients, respectively, were in DAS28‐ESR–defined LDA and DAS28‐CRP <3.2; 14.7% and 25.8% achieved DAS28‐ESR remission and DAS28‐CRP <2.6. By month 3, mild pain and moderate and substantial pain improvements occurred in 29.6%, 55.6%, and 42.9% of patients, respectively; 19.9% and 53.7% of patients achieved a HAQ DI score greater than or equal to normative values and a HAQ DI score greater than or equal to MCID, respectively. Outcomes were generally maintained to month 18. Incidence rates (events per 100 patient‐years) for treatment‐emergent adverse events (AEs), serious AEs, and discontinuations due to AEs were 126.8, 11.9, and 14.5, respectively, and AEs of special interest were infrequent.
Conclusion
Tofacitinib was associated with early and sustained improvement in RA signs and symptoms in real‐world patients. Effectiveness and safety were consistent with the established tofacitinib clinical profile.
INTRODUCTION
A treat‐to‐target approach is recommended in patients with rheumatoid arthritis (RA), with the goal of sustained remission or low disease activity (LDA) (1, 2, 3). The importance of a rapid clinical response post‐treatment is recognized by European, US, and Canadian treatment guidelines, which advocate disease improvement screening by month 3 (1, 2, 3) and achievement by month 6 (1, 2). The positive impact of therapy on disease activity has been established, with active uncontrolled disease resulting in significant disease burden due to physical, psychosocial, and economic factors (2, 4).
SIGNIFICANCE & INNOVATIONS.
This is the first report of the real‐world effectiveness and safety of tofacitinib (JAK inhibitor) in Canadian patients with rheumatoid arthritis (RA).
Tofacitinib provided early and sustained improvement in RA signs and symptoms in a real‐world setting. These data provide insight into the outcomes expected with tofacitinib in a mixed real‐world population of biologic disease‐modifying antirheumatic drug (bDMARD)–naive and –experienced patients, and when administered as monotherapy or with conventional synthetic DMARDs.
These results are consistent with the known efficacy and safety profile of tofacitinib and complement findings from previous Canadian observational studies of bDMARDs in RA.
First‐line RA therapy comprises conventional synthetic disease‐modifying antirheumatic drugs (csDMARDs) as monotherapy or combination therapy. Addition of a biologic DMARD (bDMARD; e.g., tumor necrosis factor inhibitor [TNFi]) (1, 2, 3) or targeted synthetic DMARD (tsDMARD; e.g., JAK inhibitor) (2, 3) is recommended for patients with inadequate response to csDMARDs. Tofacitinib is an oral JAK inhibitor for the treatment of RA. Tofacitinib has been approved for the treatment of RA in Canada since 2014 (5).
Tofacitinib efficacy and safety in RA have been established across 23 phase I, II, III, and IIIb/IV clinical trials and 2 long‐term extension studies, with up to 10.5 years of observation and 23,497 patient‐years of exposure (6, 7, 8). Regulator‐mandated post‐authorization safety studies, including ORAL Surveillance (9) and an analysis of the US‐based CorEvitas (formerly Corrona) registry (10), have been completed.
Real‐world assessment is necessary to inform clinicians of appropriate treatment targets and to provide insights into safety outcomes in populations ineligible for clinical trials. Such benefit/risk profile assessments of tofacitinib, and other JAK inhibitors in patients with RA have been described globally (10, 11, 12, 13, 14, 15, 16, 17, 18, 19). Demographic information and persistence of tofacitinib in Canadian patients with RA have been reported in an analysis of data from a patient‐support program; however, clinical effectiveness or safety was not assessed (20). CANTORAL (Canadian Tofacitinib for Rheumatoid Arthritis Observational) is the first Canadian prospective, observational cohort study assessing tofacitinib effectiveness, safety, and persistence for moderate to severe RA in patients receiving routine clinical care. Here, we report the coprimary and secondary effectiveness measures, as well as safety outcomes.
PATIENTS AND METHODS
Study design and patient population
CANTORAL enrolled adult Canadian patients with moderate to severe active RA per the revised 1987 American College of Rheumatology (ACR) criteria or the 2010 ACR/European Alliance of Associations for Rheumatology criteria (21, 22, 23). Methotrexate inadequate‐responder patients initiated tofacitinib from October 2017 to July 2020 per the Canadian product monograph (5). Additional study design information is included in Supplementary Appendix A, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966.
The fully enrolled and ongoing study involved 45 Canadian sites across 7 provinces. Patients were followed per routine clinical care for up to 36 months, with recommended follow‐up visits at months 3, 6, and 12, and every 6 months thereafter. In this interim analysis, effectiveness to month 18 and safety to month 36 were evaluated in patients with at least 1 post‐baseline visit as of July 16, 2021 (data cut). In this analysis, all patients (except for one with a postponed month 12 visit) who remained in the study (and had not previously discontinued) had completed their month 12 visit.
CANTORAL was conducted in compliance with the Declaration of Helsinki, International Council for Harmonization Guidelines for Good Clinical Practice and local country regulations. The study protocol was approved by Advarra (MOD00903929) and the Institutional Review Board or Independent Ethics Committee at each center, per institutional or clinical requirements. Patients provided written informed consent.
Effectiveness
Coprimary outcomes were the proportion of patients achieving Clinical Disease Activity Index (CDAI)–defined LDA (<10) and remission (<2.8) at month 6; outcomes were also assessed at months 3, 6, 12, and 18. Achievement of CDAI‐defined LDA and remission in bDMARD‐naive and bDMARD‐experienced patients, and in patients initiating tofacitinib with versus without concomitant csDMARDs, were evaluated at months 3 and 6.
Secondary outcomes included the proportion of patients achieving the 4‐variable Disease Activity Score in 28 joints (DAS28) using the erythrocyte sedimentation rate (DAS28‐ESR)–defined LDA (<3.2) and remission (<2.6); the proportion of patients reaching DAS28 using the C‐reactive protein (DAS28‐CRP) <3.2 and <2.6; and the proportion of patients meeting ACR criteria for 20%/50%/70% improvement in disease activity (ACR20/50/70) at months 3, 6, 12, and 18.
Additional outcomes were the proportion of patients achieving a patient‐reported pain (visual analog scale [VAS]) score <20 mm (mild pain), and pain improvements ≥30% (moderate) and ≥50% (substantial) from baseline; the proportion of patients achieving Health Assessment Questionnaire disability index (HAQ DI) score greater or equal to normative values (≤0.25), remission (≤0.50), and improvements from baseline greater or equal to the minimum clinically important difference (MCID; ≥0.22); and the proportion of patients achieving Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT‐F) score greater or equal to normative levels (≥40.1) and improvements from baseline greater or equal to the MCID (≥3.56).
Safety
Safety events were investigator‐reported per protocol and included treatment‐emergent adverse events (AEs), serious AEs (SAEs), deaths, and treatment‐emergent AEs of special interest (serious infections, herpes zoster [serious and nonserious], major adverse cardiovascular events, malignancies [excluding nonmelanoma skin cancer], nonmelanoma skin cancer, hepatic events, thromboembolism, and gastrointestinal perforation) (see Supplementary Appendix A, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966). All‐cause study discontinuations and discontinuations due to AEs (including intolerance) and lack/loss of efficacy were assessed 1) in all patients, 2) in bDMARD‐naive versus bDMARD‐experienced patients, and 3) in patients initiating tofacitinib with versus without concomitant csDMARDs.
Statistical analysis
Given the observational nature of this study, no predefined hypotheses were tested. Therefore, multiplicity correction was not applied across outcomes. Effectiveness measures were assessed in the full analysis set, comprising all enrolled and consenting patients. Last observation carried forward (LOCF)/baseline observation carried forward (BOCF) methodology was used to account for missing values in effectiveness outcomes; further details are included in Supplementary Appendix A, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966. Safety was assessed in the safety analysis set, comprising all full analysis set patients with ≥1 post‐baseline assessment. Sample size calculations are described in Supplementary Appendix A.
Descriptive statistics are presented for study variables and treatment‐response outcomes, including the mean (95% confidence interval [95% CI]) for continuous variables, and numbers/proportions of patients for categorical variables. For continuous disease parameters, least squares mean change from baseline to each study visit was assessed using mixed models with repeated measures, adjusted for baseline values of the outcome.
Achievement of CDAI‐defined LDA and remission at months 3 and 6 was stratified by prior bDMARD exposure and by concomitant csDMARD use at treatment initiation; between‐group differences were assessed using the chi‐square test (P < 0.05). Mean/median (95% CI) times to study discontinuation were determined for all‐cause discontinuations, and discontinuations due to AEs and lack/loss of efficacy, using the Kaplan‐Meier estimator of the survival function. AEs were coded per the Medical Dictionary for Regulatory Activities, version 23.0. Safety outcomes are reported as the number/proportion of patients with an event and by incidence rate (events per 100 patient‐years of exposure).
RESULTS
Baseline demographic and disease characteristics
A total of 504 patients participated. Of these, 49.8% (n = 251) discontinued from the study before month 36 of tofacitinib treatment/follow‐up. Lack/loss of efficacy was the most common reason for discontinuation (discontinued patients: 29.5%, n = 74; lack of efficacy: 18.7%, n = 47; loss of efficacy: 10.8%, n = 27), followed by no longer willing to participate (25.1%, n = 63), AEs (20.3%, n = 51), and other (17.1%; n = 43; most commonly “patient and doctor decision to stop medication,” “patient moved to another clinic,” and “screen failure”).
Baseline demographic characteristics are shown in Table 1. The mean age was 59.3 years (95% CI 58.2–60.4), mean duration since RA diagnosis was 10.2 years (95% CI 9.3–11.1), and most patients were female (77.8%), White (82.9%), and bDMARD‐naive (66.3%). Concomitant csDMARDs were reported in 62.7% of patients, most commonly methotrexate (44.4%), and 19.6% of patients received concomitant glucocorticoids at baseline. Comorbidities included hypertension (23.8%), chronic pulmonary disease/asthma (15.1%), diabetes mellitus (12.7%), and malignancy (6.5%). Mean CDAI, DAS28‐ESR, and DAS28‐CRP scores were 29.4 (95% CI 28.3–30.5), 5.0 (95% CI 4.8–5.1), and 4.8 (95% CI 4.6–4.9), respectively, while the mean HAQ DI score was 1.3 (95% CI 1.3–1.4) (Table 1). Mean patient global assessment, physician global assessment, and patient‐reported pain (VAS) scores were 58.1 (95% CI 55.9–60.3), 59.4 (95% CI 57.6–61.1), and 59.1 (95% CI 56.7–61.4), respectively.
Table 1.
Baseline patient demographic and disease characteristics*
| Characteristic | Full analysis set (n = 504) |
|---|---|
| Demographic information | |
| Age, years | 59.3 (58.2–60.4) |
| Age ≥65 years, no. (%) | 194 (38.5) |
| Duration since RA diagnosis, years (n = 489)† | 10.2 (9.3–11.1) |
| Body mass index, kg/m2 (n = 363)‡ | 29.7 (29.0–30.5) |
| Female, no. (%) | 392 (77.8) |
| Race, no. (%) | |
| White | 418 (82.9) |
| Black/African American | 10 (2.0) |
| Asian | 34 (6.7) |
| American Indian or Alaska Native | 11 (2.2) |
| Native Hawaiian or Other Pacific Islander | 1 (0.2) |
| Other | 30 (6.0) |
| Herpes zoster vaccination, no. (%) (n = 424)§ | 224 (52.8) |
| Prior and concomitant RA medications, no. (%) | |
| bDMARD‐naive | 334 (66.3) |
| Number of prior bDMARDs | |
| 1 | 126 (25.0) |
| 2 | 30 (6.0) |
| 3 | 9 (1.8) |
| ≥4 | 5 (1.0) |
| Concomitant csDMARD¶ | 316 (62.7) |
| Azathioprine | 1 (0.2) |
| Sulfasalazine | 2 (0.4) |
| Hydroxychloroquine | 51 (10.1) |
| Leflunomide | 65 (12.9) |
| Methotrexate | 224 (44.4) |
| Concomitant glucocorticoid use | 99 (19.6) |
| Comorbidity status, no. (%)# | |
| Chronic pulmonary disease/asthma | 76 (15.1) |
| Coronary artery disease | 5 (1.0) |
| Diabetes mellitus | 64 (12.7) |
| Hypertension | 120 (23.8) |
| Malignancy** | 33 (6.5) |
| Myocardial infarction | 23 (4.6) |
| Active tuberculosis | 3 (0.6) |
| AIDS | 4 (0.8) |
| Disease characteristics†† | |
| Rheumatoid factor positive, no. (%) (n = 434) | 274 (63.1) |
| Anti‐CCP positive, no. (%) (n = 316) | 179 (56.6) |
| Erythrocyte sedimentation rate, mm/hour (n = 299) | 24.9 (22.4–27.4) |
| C‐reactive protein, mg/dl (n = 357) | 1.2 (1.0–1.4) |
| Tender joint count based on 28 joints (n = 499) | 9.8 (9.2–10.3) |
| Swollen joint count based on 28 joints (n = 499) | 7.8 (7.4–8.3) |
| Patient global assessment (100‐mm VAS) (n = 489) | 58.1 (55.9–60.3) |
| Physician global assessment (100‐mm VAS) (n = 486) | 59.4 (57.6–61.1) |
| Patient‐reported pain (100‐mm VAS) (n = 488) | 59.1 (56.7–61.4) |
| DAS28‐ESR (n = 289) | 5.0 (4.8–5.1) |
| DAS28‐CRP (n = 341) | 4.8 (4.6–4.9) |
| Clinical Disease Activity Index (n = 472) | 29.4 (28.3–30.5) |
| Health Assessment Questionnaire disability index (n = 478) | 1.3 (1.3–1.4) |
| FACIT‐F (n = 474) | 27.2 (26.2–28.3) |
Values are the mean (95% confidence interval) unless indicated otherwise. The full analysis set includes all patients providing consent to participate in the study. anti‐CCP = anti–cyclic citrullinated peptide; bDMARD = biologic disease‐modifying antirheumatic drug; csDMARD = conventional synthetic DMARD; DAS28‐CRP = 4‐variable Disease Activity Score in 28 joints using C‐reactive protein; DAS28‐ESR = DAS28 using erythrocyte sedimentation rate; FACIT‐F = Functional Assessment of Chronic Illness Therapy–Fatigue; RA = rheumatoid arthritis; VAS = visual analog scale.
Calculated among patients with known duration since RA diagnosis.
Calculated among patients with available BMI.
Calculated among patients with available herpes zoster vaccination status. Proportion of patients receiving the live attenuated varicella‐zoster vaccine versus the adjuvant recombinant subunit varicella‐zoster virus vaccine is not known.
Patients may have reported >1 concomitant csDMARD.
Any prior history of chronic pulmonary disease/asthma, malignancy, myocardial infarction, and AIDS is reported.
Includes preferred terms: basal cell carcinoma, breast cancer, cholangiocarcinoma, chondrosarcoma, colon cancer, endometrial adenocarcinoma, invasive ductal breast carcinoma, lip squamous cell carcinoma, lung neoplasm malignant, malignant melanoma, osteosarcoma, prostate cancer, renal cancer, skin cancer, squamous cell carcinoma of skin, and uterine leiomyoma.
Calculated among patients with available data per routine care.
Coprimary outcomes
CDAI‐defined LDA and remission at month 6 were achieved by 52.9% (95% CI 47.8–58.0) and 15.4% (95% CI 12.1–19.5) of patients, respectively, with similar proportions reported by month 3 (first post‐baseline assessment) (Figure 1A). CDAI‐defined LDA and remission rates were generally maintained to month 12. From months 12 to 18, the proportion of patients attaining CDAI‐defined LDA and remission was numerically reduced, with overlapping 95% CIs (Figure 1A); however, when implementing LOCF/BOCF methodology, response rates remained consistent or increased numerically (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966). Mean CDAI score improved over time, ranging from 12.8 (95% CI 11.8–13.9) by month 3 to 8.4 (95% CI 7.1–9.6) at month 18 (see Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966).
Figure 1.
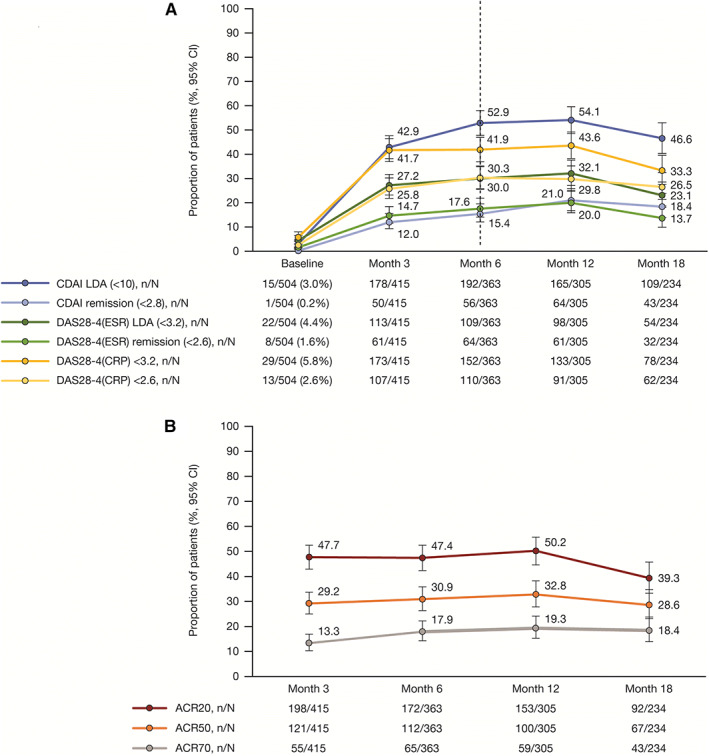
Achievement of disease activity thresholds over time for patients receiving tofacitinib. A, CDAI‐, DAS28‐4(ESR)–, and DAS28‐4(CRP)–defined LDA and remission; B, ACR20/50/70 response rates. For A, the dotted line at month 6 indicates the time point for assessment of the coprimary end points (achievement of CDAI‐defined LDA and remission). Error bars correspond to the 95% confidence interval (95% CI) around the response rates. Remission and LDA values for DAS28‐4(CRP) (<2.6 and <3.2, respectively) have not been validated (ref. 24), but are commonly used in rheumatology (ref. 25). ACR20/50/70 = American College of Rheumatology criteria for 20%, 50%, or 70% improvement in disease activity; CDAI = Clinical Disease Activity Index; DAS28‐4(CRP) = 4‐variable Disease Activity Score in 28 joints using the C‐reactive protein level; DAS28‐4(ESR) = DAS28 using the erythrocyte sedimentation rate; LDA = low disease activity; n = number of patients meeting response criteria; N = number of patients with available data at each visit.
A significantly greater proportion of bDMARD‐naive versus bDMARD‐experienced patients achieved CDAI‐defined LDA (P = 0.014) and remission (P = 0.021) at month 6 (Table 2), with similar results observed by month 3 (see Supplementary Table 3, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966). CDAI‐defined LDA and remission rates at month 6 were generally comparable in patients receiving tofacitinib with csDMARDs versus monotherapy, with nonsignificant differences observed (P = 0.127 and P = 0.370, respectively) (Table 2). At month 3, similar proportions of patients receiving tofacitinib with versus without concomitant csDMARDs achieved CDAI‐defined LDA (43.8% versus 41.3%; P = 0.997) and remission (12.5% versus 11.2%; P = 0.852) (see Supplementary Table 3, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966).
Table 2.
Achievement of CDAI‐defined LDA and remission at month 6 in bDMARD‐naive versus bDMARD‐experienced patients receiving tofacitinib, and in patients receiving tofacitinib with versus without concomitant csDMARDs*
| CDAI‐defined LDA | CDAI remission | |||||
|---|---|---|---|---|---|---|
| No./total no. | % (95% CI) | P | No./total no. | % (95% CI) | P | |
| bDMARD‐naive | 139/246 | 56.5 (50.3–62.6) | – | 45/246 | 18.3 (14.0–23.6) | – |
| bDMARD‐experienced | 53/117 | 45.3 (36.6–54.3) | 0.014 | 11/117 | 9.4 (5.3–16.1) | 0.021 |
| Tofacitinib + concomitant csDMARDs | 137/242 | 56.6 (50.3–62.7) | – | 41/242 | 16.9 (12.7–22.2) | – |
| Tofacitinib monotherapy | 55/121 | 45.5 (36.9–54.3) | 0.127 | 15/121 | 12.4 (7.7–19.4) | 0.370 |
P values are for bDMARD‐naive versus bDMARD‐experienced patients and concomitant csDMARD versus monotherapy patients. 95% CI = 95% confidence interval; bDMARD = biologic disease‐modifying antirheumatic drug; CDAI = Clinical Disease Activity Index; csDMARD = conventional synthetic DMARD; LDA = low disease activity; no. = number of patients meeting disease activity threshold; total no. = number of patients with available data at visit.
Secondary outcomes
DAS28‐ESR–defined LDA and DAS28‐CRP <3.2 were achieved by 27.2% (95% CI 23.2–31.7) and 41.7% (95% CI 37.0–46.5) of patients by month 3, respectively, and were generally maintained until month 12 (Figure 1A). By month 3, DAS28‐ESR–defined remission and DAS28‐CRP <2.6 were achieved by 14.7% (95% CI 11.6–18.4) and 25.8% (95% CI 21.8–30.2) of patients, respectively, with responses maintained to month 12. From months 12 to 18, numeric reductions with overlapping 95% CIs were observed in DAS28‐ESR/DAS28‐CRP disease state outcomes, but when LOCF/BOCF methodology was used, outcomes remained consistent or increased numerically (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966). Following initial improvements from baseline to month 3, mean DAS28‐ESR and DAS28‐CRP scores were generally consistent to month 18 (see Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966).
ACR20/50/70 response rates were achieved by 47.7% (95% CI 42.9–52.5), 29.2% (95% CI 25.0–33.7), and 13.3% (95% CI 10.3–16.9) of patients, respectively, by month 3, and remained mostly consistent through month 12 (Figure 1B). Similar to CDAI and DAS28‐ESR/DAS28‐CRP, a numeric decrease in ACR20 was observed from months 12 to 18, but 95% CIs overlapped (Figure 1B). When considering more stringent ACR criteria (ACR50/ACR70), response rates were maintained throughout the study. From months 12 to 18, ACR20/50/70 response rates were numerically increased when LOCF/BOCF was implemented (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966).
Concomitant csDMARDs increased from 62.7% (316 of 504) at tofacitinib initiation to 79.5% (330 of 415) by month 3, then decreased to 71.4% (167 of 234) at month 18 (see Supplementary Table 4, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966). Glucocorticoid usage increased from 19.6% (99 of 504) at treatment initiation to 27.7% (115 of 415) by month 3, then decreased to 24.4% (57 of 234) at month 18 (see Supplementary Table 4). Of note, data only include patients remaining in the study at each time point.
Patients who added csDMARDs to their treatment regimen from baseline to month 3 had a shorter duration since RA diagnosis and were less likely to be bDMARD‐experienced and have comorbidities versus patients who did not add csDMARDs during this time period (see Supplementary Table 5, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966). Patients who added glucocorticoids to their treatment regimen from baseline to month 3 had a longer duration since RA diagnosis, were more likely to be current smokers or bDMARD‐experienced, and generally had more comorbidities versus patients who did not add glucocorticoids during this time period (see Supplementary Table 5). Mean (95% CI) and least squares mean change from baseline in components of the ACR score are shown in Supplementary Table 6, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966.
Pain (VAS), HAQ DI, and FACIT‐F
By month 3, the proportion of patients reporting mild pain (<20 mm) was 29.6% (95% CI 25.4–34.2), while moderate (≥30%) and substantial (≥50%) improvements were observed in 55.6% (95% CI 50.6–60.4) and 42.9% (95% CI 38.1–47.9) of patients, respectively (Figure 2A). The proportions of patients attaining pain thresholds were maintained to month 18.
Figure 2.
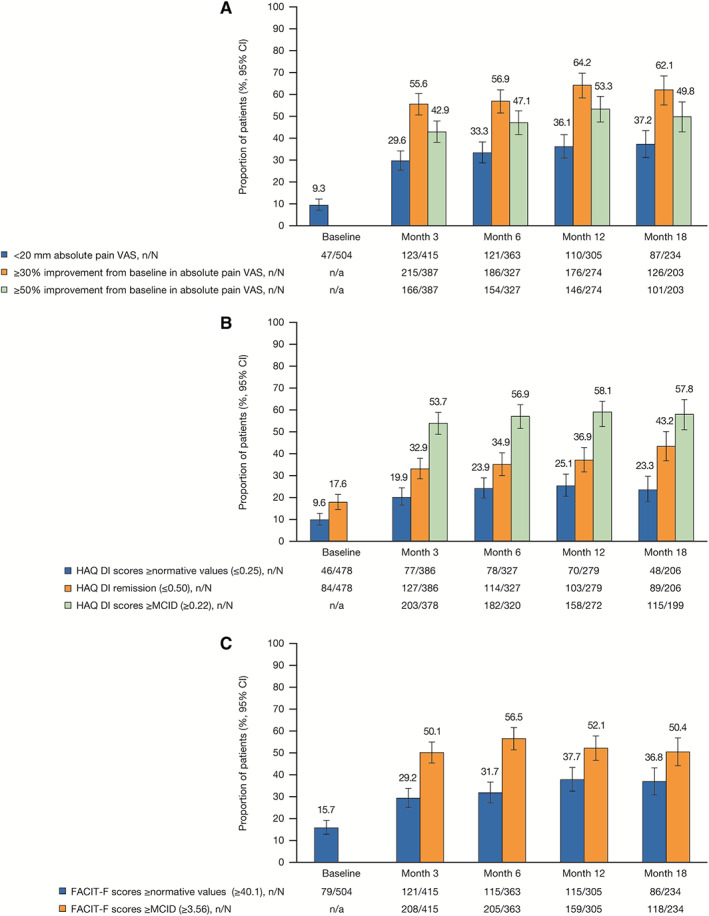
Achievement of patient‐reported response thresholds over time in patients receiving tofacitinib. A, Patient‐reported pain (visual analog scale [VAS]); B, Health Assessment Questionnaire disability index (HAQ‐DI); C, Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT‐F). MCID = minimum clinically important difference; n = number of patients meeting response criteria; N = number patients with available data at each visit; n/a = not applicable. Error bars indicate the 95% confidence interval (95% CI).
HAQ DI score greater or equal to normative values, remission, and improvements from baseline greater or equal to the MCID by month 3 were achieved by 19.9% (95% CI 16.3–24.2), 32.9% (95% CI 28.4–37.7), and 53.7% (95% CI 48.7–58.7) of patients, respectively, with rates stable to month 18 (Figure 2B). In addition, 29.2% (95% CI 25.0–33.7) of patients attained FACIT‐F score greater or equal to normative values and 50.1% (95% CI 45.3–54.9) achieved improvements from baseline greater or equal to the MCID by month 3, with both maintained throughout the study (Figure 2C).
Safety outcomes
In total, 495 patients were included, amounting to 791 patient‐years of follow‐up. Treatment‐emergent AEs were reported in 332 patients (67.1%; incidence rate 126.8/100 patient‐years) (Table 3). SAEs were reported in 58 patients (11.7%; 11.9/100 patient‐years), and 88 patients (17.8%) discontinued due to AEs (14.5/100 patient‐years). Four deaths (0.8%; 0.8/100 patient‐years) were reported, including fatal myocardial infarction (n = 1; investigator‐reported as nontreatment‐related), Salmonella bacteremia and sepsis (n = 1; possibly treatment‐related), renal failure due to sepsis (n = 1; possibly treatment‐related), and acute myeloid leukemia and pneumonia (n = 1; possibly treatment‐related). The most common treatment‐emergent AEs were upper respiratory tract infection (5.7%; 3.8/100 patient‐years), urinary tract infection (4.4%; 3.7/100 patient‐years), and headache (4.2%; 2.8/100 patient‐years).
Table 3.
Safety outcomes to month 36*
| Patients with event | Safety analysis set (n = 495; PY = 791) |
|---|---|
| Treatment‐emergent adverse events† | 332 (67.1) [126.8] |
| Serious adverse events‡ | 58 (11.7) [11.9] |
| Study discontinuations due to adverse events | 88 (17.8) [14.5] |
| Deaths§ | 4 (0.8) [0.8] |
| Most frequent treatment‐emergent adverse events (≥3%) | |
| Upper respiratory tract infection | 28 (5.7) [3.8] |
| Urinary tract infection | 22 (4.4) [3.7] |
| Headache | 21 (4.2) [2.8] |
| Hypertension | 19 (3.8) [2.4] |
| Diarrhea | 18 (3.6) [2.3] |
| Nausea | 15 (3.0) [2.0] |
| Treatment‐emergent adverse events of special interest | |
| Serious infection | 22 (4.4) [3.8] |
| Herpes zoster (nonserious/serious)¶ | 10 (2.0) [1.3] |
| Vaccinated | 3 (1.4) [0.8] |
| Unvaccinated | 5 (2.5) [1.7] |
| Major adverse cardiovascular events# | 5 (1.0) [0.9] |
| Malignancies (excluding nonmelanoma skin cancer)** | 8 (1.6) [1.3] |
| Nonmelanoma skin cancer | 4 (0.8) [0.6] |
| Hepatic events†† | 6 (1.2) [0.8] |
| Venous thomboembolism | 2 (0.4) [0.3] |
| Deep vein thrombosis‡‡ | 1 (0.2) [0.1] |
| Pulmonary embolism§§ | 1 (0.2) [0.1] |
| Gastrointestinal perforation | 0 |
| Other scenarios involving tofacitinib exposure¶¶ | 2 (0.4) [0.3] |
Values are the number of patients with events (%) [incidence rate (events/100 patient‐years [PYs])], and include all enrolled patients providing consent to participate in the study and with at least 1 post‐baseline visit. Mean ± SD duration of follow‐up 16.3 ± 10.4 months; cumulative PYs.
Reporting period was from the patient's first dose of tofacitinib (or the time of the patient's informed consent if they were being treated with tofacitinib at study start) until the end of the observation period of the study (≥28 calendar days following the last administration of a drug under study).
Any untoward medical occurrence that resulted in death, was life‐threatening, required in‐patient hospitalization or prolongation of hospitalization, resulted in persistent or significant disability/incapacity, or resulted in congenital anomaly/birth defect.
Renal failure (possibly related to study drug) and sepsis (possibly related to study drug), n = 1; Salmonella bacteremia (possibly related to study drug) and sepsis (possibly related to study drug), n = 1; myocardial infarction (not related to study drug), n = 1; acute myeloid leukemia and pneumonia (possibly related to study drug), n = 1.
Vaccinated refers to patients receiving the herpes zoster (HZ) vaccine: 1 case of serious HZ was reported in an unvaccinated individual (serious adverse event [SAE], related to study drug). Patient vaccination status was unknown in 2 nonserious HZ cases. Frequency and incidence rate of HZ in vaccinated and unvaccinated patients calculated in those patients with corresponding known vaccination status at baseline.
Cardiac failure (2 events across 1 patient; both nonfatal SAEs, both not related to study drug), n = 1; myocardial infarction (1 fatal SAE, 1 nonfatal SAE; neither related to study drug), n = 2; cerebrovascular accident (3 SAEs across 2 patients; 2 events not related to study drug; 1 event causality unknown), n = 2.
Bladder cancer, malignant pleural effusion, myeloproliferative neoplasm, neuroendocrine tumor, polycythemia vera, prostate cancer, and small‐cell lung cancer metastatic.
Hepatic fibrosis, n = 1 (nonserious AE, not related to study drug); hepatic steatosis, n = 4 (nonserious AE; 3 unlikely related and 1 not related to study drug), hepatomegaly, n = 1 (nonserious AE; relationship to study drug unknown).
Nonocclusive thrombus in right posterior tibial vein, n = 1 (SAE, possibly related to study drug).
Pulmonary embolism in patient with acute respiratory failure secondary to COVID‐19 infection, n = 1 (SAE, possibly related to study drug).
Other scenarios involving tofacitinib exposure: maternal exposure during pregnancy, n = 1 (outcome: no fetal/gestational abnormality); product communication issue, n = 1 (patient continued to take study drug after treatment discontinued).
Serious infections occurred in 22 patients (4.4%; 3.8/100 patient‐years) (Table 3). The most common serious infection was pneumonia (n = 6; 1.2%); serious COVID‐19 infection occurred in 2 patients (0.4%). The herpes zoster vaccination rate, among patients with known vaccination status, was 52.8%. Ten cases of herpes zoster (1.3/100 patient‐years) were reported. Three cases occurred in patients with a prior vaccination history (0.8/100 patient‐years) who received concomitant methotrexate (n = 1), hydroxychloroquine (n = 1), and methotrexate and hydroxychloroquine (n = 1); none were reported with concomitant glucocorticoids. Five cases occurred in unvaccinated patients (1.7/100 patient‐years; 2 cases in patients with unknown vaccination status). Nine of the 10 infections were considered to be nonserious. The serious case of herpes zoster occurred in a 59‐year‐old unvaccinated male; tofacitinib treatment was temporarily interrupted and the patient was successfully treated with valacyclovir. Five patients (1.0%) experienced major adverse cardiovascular events (0.9/100 patient‐years); major adverse cardiovascular events were reported as 1 cardiac failure (2 events in 1 patient; both nonfatal), 1 nonfatal myocardial infarction, 1 fatal myocardial infarction, and 2 cerebrovascular accidents (3 events in 2 patients). Malignancy (excluding nonmelanoma skin cancer) was reported in 8 patients (1.6%; 1.3/100 patient‐years), of which 7 cases were reported as serious and none resulted in death; nonmelanoma skin cancer occurred in 4 patients (0.8%; 0.6/100 patient‐years); and hepatic events occurred in 6 patients (1.2%, 0.76/100 patient‐years). Thromboembolism occurred in 2 patients (0.4%, 0.3/100 patient‐years); deep vein thrombosis (nonocclusive thrombus in tibial vein) occurred in a 71‐year‐old male with a history of coronary artery disease, and pulmonary embolism occurred in a 62‐year‐old male with acute respiratory failure secondary to COVID‐19 infection. No arterial thrombotic events or gastrointestinal perforations were reported.
Time to study discontinuation
Of the 49.8% of patients (n = 251) in the full analysis set who discontinued from the study, mean ± SE time to discontinuation was 23.7 ± 0.8 months (median 24.2 months [95% CI 19.6–30.8]) (Figure 3). Mean time to discontinuation was 36.6 months (95% CI 35.4–37.9) for AEs and 34.9 months (95% CI 33.6–36.2) for lack/loss of efficacy (Figure 3).
Figure 3.
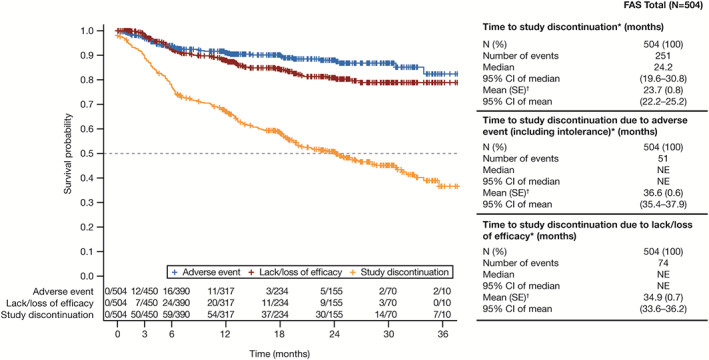
Kaplan‐Meier time to discontinuation in the overall study population (Canadian Tofacitinib for Rheumatoid Arthritis Observational [CANTORAL] study ongoing at data cut, July 16, 2021). Broken line shows the median survival rate. * = time from treatment initiation until premature study discontinuation or last available assessment date. † = mean (SE) survival time may have been underestimated because the estimation was restricted to the largest observation, which was censored. 95% CI = 95% confidence interval; FAS = full analysis set; NE = nonestimable.
Mean time to discontinuation, as well as time to discontinuation due to AEs and lack/loss of efficacy, was longer in bDMARD‐naive versus bDMARD‐experienced patients (see Supplementary Figure 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966). Mean time to discontinuation was longer in patients receiving tofacitinib with versus without concomitant csDMARDs; however, mean time to discontinuation due to AEs or lack/loss of efficacy was similar (see Supplementary Figure 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24966).
DISCUSSION
CANTORAL is the first study reporting the real‐world effectiveness and safety of a JAK inhibitor for moderate to severe RA in Canada and complements previous Canadian observational studies of patients with RA receiving bDMARDs (26, 27, 28, 29). The proportion of patients with CDAI‐defined LDA and remission at month 6 was 52.9% and 15.4%, respectively, demonstrating the effectiveness of tofacitinib for moderate to severe RA. Improvements in disease activity outcomes, achievement of near‐normative functional status, and improvements in patient‐reported outcomes were observed by month 3 (first post‐baseline assessment), with sustained reductions in signs and symptoms observed throughout the study. These data provide insights for clinicians regarding expected tofacitinib responses in a real‐world setting and could assist with determining treatment targets.
The coprimary end points (CDAI‐defined LDA and remission at month 6) in CANTORAL are consistent with expected disease state outcomes in response to tofacitinib treatment for real‐world patients with RA. Analyses of the US CorEvitas and Swiss Clinical Quality Management registries have reported lower CDAI‐defined LDA rates at months 6 and 12, respectively (13, 18), than in the current analysis. However, both studies included higher proportions of treatment‐refractory (i.e., bDMARD‐experienced) patients than CANTORAL. Conversely, a study of the Australian OPAL real‐world data set, with a comparable proportion of bDMARD‐naive patients to CANTORAL, reported higher month 6 CDAI‐defined LDA and remission rates than in the current analysis (11, 12). However, OPAL included a higher proportion of patients in remission at tofacitinib initiation, and patients had a lower median disease duration versus CANTORAL, which may explain these differences.
In this study, CDAI‐ and DAS28‐ESR/DAS28‐CRP–defined LDA and remission rates were slightly reduced from months 12 to 18, although when LOCF/BOCF methodology was implemented, response rates were consistent or numerically increased from months 12 to 18. The attenuation in “as observed” data may be partly attributed to the COVID‐19 global pandemic. Overall, 53 of 113 patients (46.9%) did not attend their month 18 visit in person due to government‐mandated closures, from March to December 2020. After December 2020, patients could continue with remote visits or attend in‐clinic assessments. Patients with well‐controlled disease may have opted to conduct remote assessments, whereas patients with higher disease activity may have presented for a full rheumatology examination.
In this interim analysis of CANTORAL, the median time to study discontinuation was 24.2 months (95% CI 19.6–30.8). The median tofacitinib maintenance in the Swiss Clinical Quality Management registry was 1.9 years (95% CI 1.5–2.3) (13), and the median tofacitinib persistence in the OPAL data set was 34.2 months (95% CI 32.2–not reached) (11). A longer median time to treatment discontinuation for tofacitinib 5 mg twice daily was reported in the long‐term extension studies from the global RA clinical trial program (5.2 years [95% CI 4.9–5.7]) (30) than in the Swiss Clinical Quality Management registry and OPAL data set, reflecting a population known to respond to tofacitinib and meeting eligibility criteria for a randomized controlled trial.
The results of this study further inform the practical utility of tofacitinib in real‐world settings. In CANTORAL, CDAI response and persistence were higher in bDMARD‐naive patients versus bDMARD‐experienced patients. These findings were anticipated based on previously published (17, 18, 20, 31, 32, 33) and common clinical knowledge, that patients’ first advanced therapy is typically the most effective.
Overall, effectiveness and persistence in patients receiving tofacitinib with versus without csDMARDs were similar across real‐world studies (12, 13, 18). In the tofacitinib global clinical trial program, a phase IIIb/IV study showed numeric efficacy differences favoring tofacitinib combination therapy versus monotherapy (34), while persistence with tofacitinib combination and monotherapy were comparable in long‐term extension studies (30). In CANTORAL, comparable effectiveness was observed in patients initiating tofacitinib with and without csDMARDs. Patients initiating tofacitinib with concomitant csDMARDs versus monotherapy had a longer time to all‐cause discontinuation, supporting potential clinical benefits of concomitant csDMARDs. However, time to discontinuation due to AEs or lack/loss of efficacy in patients receiving tofacitinib with and without concomitant csDMARDs was similar.
The proportion of patients receiving concomitant csDMARDs and glucocorticoids was numerically increased from tofacitinib initiation to month 3. Compared with patients who did not add concomitant csDMARDs from tofacitinib initiation to month 3, patients who added concomitant csDMARDs had a shorter duration since RA diagnosis and were less likely to be bDMARD‐experienced and to have comorbidities. This finding indicates a population that is earlier in their disease course or less treatment‐experienced and, therefore, more apt to optimize tofacitinib treatment by adding csDMARDs. In contrast, patients who added glucocorticoids versus those who did not add glucocorticoids from tofacitinib initiation to month 3 had longer disease duration and were more likely to be bDMARD‐experienced with more comorbidities, signaling a potentially harder‐to‐treat patient cohort. The subsequent decrease in concomitant csDMARD and glucocorticoid use beyond month 3 may be explained by discontinuation of concomitant treatments due to side effects and/or attainment of clinical response with tofacitinib, aligned with treatment guidelines for patients receiving bDMARDs/tsDMARDs (2, 3). Importantly, this analysis only included patients remaining in the study at each time point; further investigation would be needed to understand the reasons for this trend or whether the observation was founded on a discontinuation bias.
Improvements in pain and HAQ DI score following tofacitinib treatment were reported in CorEvitas (18) and an observational Latin American study (35), respectively, yet data on real‐world changes in clinically important patient‐reported outcomes are limited. Despite originating from a real‐world patient population, with 62.7% receiving concomitant csDMARDs, long disease duration, and varied comorbidity status, patients in CANTORAL experienced improvements in mild, moderate, and substantial pain, and FACIT‐F and HAQ DI normative thresholds aligned with values reported in a post hoc analysis of tofacitinib phase III studies (36).
A post‐authorization safety study within CorEvitas evaluating tofacitinib versus bDMARDs showed comparable AEs of special interest rates, including death, serious infections, major adverse cardiovascular events, thrombosis, malignancies (excluding nonmelanoma skin cancer), and nonmelanoma skin cancer; the exception was herpes zoster, where rates were higher with tofacitinib (10). The safety outcomes reported herein complement those observed in CorEvitas, excluding malignancies (excluding nonmelanoma skin cancer), which cannot be appropriately assessed due to CANTORAL's limited follow‐up period and sample size. These current results also support evaluations of serious infections, venous thromboembolism, and malignancy generated from US claims databases (37, 38, 39), and the findings of an integrated safety analysis of the tofacitinib clinical trial program (40). Notably, in CANTORAL, the incidence of herpes zoster in vaccinated patients was half that of unvaccinated patients, suggesting that herpes zoster vaccination could serve as a risk minimization measure in patients receiving tofacitinib.
A heightened focus has been placed on JAK inhibitor safety following the completion of ORAL Surveillance and subsequent regulatory reviews, wherein tofacitinib failed to demonstrate noninferiority versus TNFi for the coprimary end points of adjudicated major adverse cardiovascular events and malignancy (excluding nonmelanoma skin cancer) (9). The primary analysis included both tofacitinib doses versus TNFi, although tofacitinib 10 mg twice daily is not approved for RA in most countries. Insights gained from clinical trials, such as ORAL Surveillance, together with CANTORAL and other real‐world safety evaluations, complement the growing knowledge supporting the benefit/risk of tofacitinib.
Limitations of this study include the lack of a comparator group, variable follow‐up assessments, missing data, and potential selection bias due to the requirement for patient consent for study participation. CANTORAL was not designed to assess tofacitinib effectiveness and safety versus other treatments. Instead, the focus was on observing patients and collecting data related to the real‐world use of tofacitinib, with the aim of educating health care practitioners and informing treat‐to‐target strategies.
Overall, the results of CANTORAL indicate that tofacitinib provides early and sustained improvement of disease signs and symptoms in Canadian patients with RA, complementing other real‐world data sets. These data further demonstrate that tofacitinib is an option for bDMARD‐naive and bDMARD‐experienced patients and can be administered with or without csDMARDs. The safety profile is consistent with previously published post‐marketing and clinical data. These results further substantiate the established tofacitinib safety profile, provide insight into expected effectiveness outcomes, and inform real‐world patient outcomes, which may serve as waypoints for symptomatic resolution in patients initiating tofacitinib.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Kinch had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Haraoui, Roy, Gruben, Woolcott, Sampalis, Keystone.
Acquisition of data
Haraoui, Khraishi, Lisnevskaia, Teo, Kinch, Galos, Roy, Sampalis, Keystone.
Analysis and interpretation of data
Haraoui, Khraishi, Choquette, Lisnevskaia, Teo, Kinch, Galos, Roy, Gruben, Woolcott, Vaillancourt, Sampalis.
ROLE OF THE STUDY SPONSOR
The study was designed by Pfizer authors in collaboration with the academic authors. Pfizer authors were involved in data acquisition, and analysis and interpretation of data, as well as manuscript drafting, reviewing, and development. Medical writing support was funded by Pfizer Canada ULC. Editorial support was funded by Pfizer Inc. Publication of this article was not contingent upon approval by Pfizer.
Supporting information
Disclosure Form
Appendix S1: Supplementary Information
ACKNOWLEDGMENTS
Medical writing support, under the guidance of the authors, was provided by JSS Medical Research, and funded by Pfizer Canada ULC, Kirkland, Quebec, Canada, in accordance with Good Publication Practice. Editorial support was provided by Karen Irving, PhD, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer Inc, New York, NY, in accordance with Good Publication Practice.
Sponsored by Pfizer.
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr.24966&file=acr24966‐sup‐0001‐Disclosureform.pdf.
Contributor Information
Cassandra Kinch, Email: Cassandra.kinch@pfizer.com.
Canadian Tofacitinib for Rheumatoid Arthritis Observational Investigators:
Karen Pont, Michelle Teo, John Chan, Shahin Jamal, Milton Baker, Timothy McCarthy, Majed Khraishi, Al‐Amin Proton Rahman, Evelyn Sutton, Juris Lazovskis, Viktoria Pavlova, Derek A. Haaland, Tripti Papneja, Sanjay Dixit, Vikas Pandith, Pauline Boulos, Arthur Lau, Manisha Mulgund, Raman Rai, Alaa Dekis, Thanu Ruban, Andrew Chow, Brandusa Florica, Imtiaz Khan, Carter Thorne, Rajwinder Dhillon, Larissa Lisnevskaia, Algis Jovaisas, Suneil Kapur, Angela Montgomery, Saeed Shaikh, Arthur Karasik, Edward Keystone, Stacey Pedvis, Sabeen Anwar, Jude Rodrigues, Sabrina Fallavollita, Boulos Haraoui, Jan Shulz, Alexander Tsoukas, Michel Zummer, Louis Bessette, Paul R. Fortin, Isabelle Fortin, Nabil Attie, Gilles Boire, Ariel Masetto, Clode Lessard, Keltie Anderson, Regan Arendse, and Latha Naik
REFERENCES
- 1. Bykerk VP, Akhavan P, Hazlewood GS, et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease‐modifying antirheumatic drugs. J Rheumatol 2012;39:1559–82. [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Landewé RB, Bijlsma JW, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 3. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2021;73:1108–23. [DOI] [PubMed] [Google Scholar]
- 4. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:1094–108. [DOI] [PubMed] [Google Scholar]
- 5. Pfizer Canada Inc . Xeljanz (product monograph). 2019. URL: https://www.pfizer.ca/sites/default/files/202206/Xeljanz_PM_EN_258173_09‐May‐2022.pdf.
- 6. Burmester GR, Nash P, Sands BE, et al. Adverse events of special interest in clinical trials of rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and psoriasis with 37 066 patient‐years of tofacitinib exposure. RMD Open 2021;7:e001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wollenhaupt J, Lee EB, Curtis JR, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open‐label, long‐term extension study. Arthritis Res Ther 2019;21:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamanaka H, Tanaka Y, Takeuchi T, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open‐label, long‐term extension study. Arthritis Res Ther 2016;18:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. [DOI] [PubMed] [Google Scholar]
- 10. Kremer JM, Bingham CO, Cappelli LC, et al. Postapproval comparative safety study of tofacitinib and biological disease‐modifying antirheumatic drugs: 5‐year results from a United States‐based rheumatoid arthritis registry. ACR Open Rheumatol 2021;3:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bird P, Littlejohn G, Butcher B, et al. Real‐world evaluation of effectiveness, persistence, and usage patterns of tofacitinib in treatment of rheumatoid arthritis in Australia. Clin Rheumatol 2020;39:2545–51. [DOI] [PubMed] [Google Scholar]
- 12. Bird P, Littlejohn G, Butcher B, et al. Real‐world evaluation of effectiveness, persistence, and usage patterns of monotherapy and combination therapy tofacitinib in treatment of rheumatoid arthritis in Australia. Clin Rheumatol 2022;41:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finckh A, Tellenbach C, Herzog L, et al. Comparative effectiveness of antitumour necrosis factor agents, biologics with an alternative mode of action and tofacitinib in an observational cohort of patients with rheumatoid arthritis in Switzerland. RMD Open 2020;6:e001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harnett J, Gerber R, Gruben D, et al. Evaluation of real‐world experience with tofacitinib compared with adalimumab, etanercept, and abatacept in RA patients with 1 previous biologic DMARD: data from a U.S. administrative claims database. J Manag Care Spec Pharm 2016;22:1457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iwamoto N, Tsuji S, Takatani A, et al. Efficacy and safety at 24 weeks of daily clinical use of tofacitinib in patients with rheumatoid arthritis. PLoS One 2017;12:e0177057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Machado MA, Moura CS, Guerra SF, et al. Effectiveness and safety of tofacitinib in rheumatoid arthritis: a cohort study. Arthritis Res Ther 2018;20:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mori S, Yoshitama T, Ueki Y. Tofacitinib therapy for rheumatoid arthritis: a direct comparison study between biologic‐naïve and experienced patients. Intern Med 2018;57:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reed GW, Gerber RA, Shan Y, et al. Real‐world comparative effectiveness of tofacitinib and tumor necrosis factor inhibitors as monotherapy and combination therapy for treatment of rheumatoid arthritis. Rheumatol Ther 2019;6:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schneeberger EE, Salas A, Medina LF, et al. Real world use of tofacitinib in rheumatoid arthritis: data from Latin America [abstract]. Ann Rheum Dis 2017;76 Suppl 2:AB0419. [Google Scholar]
- 20. Pope J, Bessette L, Jones N, et al. Experience with tofacitinib in Canada: patient characteristics and treatment patterns in rheumatoid arthritis over 3 years. Rheumatology (Oxford) 2020;59:568–74. [DOI] [PubMed] [Google Scholar]
- 21. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 22. Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford) 2012;51:vi5–9. [DOI] [PubMed] [Google Scholar]
- 23. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 24. Fleischmann R, van der Heijde D, Koenig AS, et al. How much does Disease Activity Score in 28 joints ESR and CRP calculations underestimate disease activity compared with the Simplified Disease Activity Index? Ann Rheum Dis 2015;74:1132–7. [DOI] [PubMed] [Google Scholar]
- 25. Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bessette L, Haraoui B, Chow A, et al. Effectiveness and safety of certolizumab pegol in rheumatoid arthritis patients in Canadian practice: 2‐year results from the observational FαsT‐CAN study. Ther Adv Musculoskelet Dis 2019;11:1759720X19831151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haraoui B, Jamal S, Ahluwalia V, et al. Real‐world tocilizumab use in patients with rheumatoid arthritis in Canada: 12‐month results from an observational, noninterventional study. Rheumatol Ther 2018;5:551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahman P, Baer P, Keystone E, et al. Long‐term effectiveness and safety of infliximab, golimumab and golimumab‐IV in rheumatoid arthritis patients from a Canadian prospective observational registry. BMC Rheumatol 2020;4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thorne C, Bensen WG, Choquette D, et al. Effectiveness and safety of infliximab in rheumatoid arthritis: analysis from a Canadian multicenter prospective observational registry. Arthritis Care Res (Hoboken) 2014;66:1142–51. [DOI] [PubMed] [Google Scholar]
- 30. Pope JE, Keystone E, Jamal S, et al. Persistence of tofacitinib in the treatment of rheumatoid arthritis in open‐label, long‐term extension studies up to 9.5 years. ACR Open Rheumatol 2019;1:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hyrich KL, Lunt M, Watson KD, et al. Outcomes after switching from one anti–tumor necrosis factor α agent to a second anti‐tumor necrosis factor alpha α in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheumatol 2007;56:13–20. [DOI] [PubMed] [Google Scholar]
- 32. Frazier‐Mironer A, Dougados M, Mariette X, et al. Retention rates of adalimumab, etanercept and infliximab as first and second‐line biotherapy in patients with rheumatoid arthritis in daily practice. Joint Bone Spine 2014;81:352–9. [DOI] [PubMed] [Google Scholar]
- 33. Greenberg JD, Reed G, Decktor D, et al. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann Rheum Dis 2012;71:1134–42. [DOI] [PubMed] [Google Scholar]
- 34. Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double‐blind, head‐to‐head, randomised controlled trial. Lancet 2017;390:457–68. [DOI] [PubMed] [Google Scholar]
- 35. Madariaga H, Reyes J, Gutierrez M, et al. Patient‐reported outcomes in rheumatoid arthritis patients treated with tofacitinib or biological DMARDs in real life conditions in two Latin America countries [abstract]. Arthritis Rheumatol 2020;72 Suppl 10:1241. [Google Scholar]
- 36. Strand V, Kremer JM, Gruben D, et al. Tofacitinib in combination with conventional disease‐modifying antirheumatic drugs in patients with active rheumatoid arthritis: patient‐reported outcomes from a Phase III randomized controlled trial. Arthritis Care Res (Hoboken) 2017;69:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mease P, Charles‐Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real‐world data. Ann Rheum Dis 2020;79:1400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Desai RJ, Pawar A, Weinblatt ME, et al. Comparative risk of venous thromboembolism in rheumatoid arthritis patients receiving tofacitinib versus those receiving tumor necrosis factor inhibitors: an observational cohort study. Arthritis Rheumatol 2019;71:892–900. [DOI] [PubMed] [Google Scholar]
- 39. Pawar A, Desai RJ, Gautam N, et al. Risk of admission to hospital for serious infection after initiating tofacitinib versus biologic DMARDs in patients with rheumatoid arthritis: a multidatabase cohort study. Lancet Rheumatol 2020;2:E84–98. [DOI] [PubMed] [Google Scholar]
- 40. Cohen SB, Tanaka Y, Mariette X, et al. Long‐term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open 2020;6:e001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Appendix S1: Supplementary Information


