Abstract
Psychiatric comorbidity is common in patients with chronic pain. In peripheral neuropathic pain, particularly anxiety and mood disorders are frequently present and associated with a high level of catastrophizing. Small fiber neuropathy (SFN) is a peripheral neuropathy dominated by pain. This study aimed to investigate the prevalence of and factors associated with anxiety and depressive symptoms in SFN. All consecutive patients diagnosed with SFN at Maastricht University Medical Center+, between September 2016 and October 2021, were included (n = 1310). Data on demographics, medical history, diagnostic tests, and questionnaires about pain, SFN‐specific symptoms, and mental health were collected once. The Hospital Anxiety and Depression Scale (HADS) was used to measure anxiety and depression and the Pain Catastrophizing Scale (PCS) to measure the degree of catastrophizing. One‐third of the patients had an abnormal HADS score (≥11) on the subscales anxiety and/or depression (26.5% anxiety and 23.0% depression) indicating clinical relevance. Regression analysis showed that higher pain intensity, catastrophizing, and more SFN‐related complaints were significantly associated with an abnormal HADS‐score. In conclusion, the prevalence of reported anxiety or depressive symptoms in SFN is 36.3%. A multidisciplinary approach, not only focusing on pain relief, is therefore essential for the treatment of SFN.
Keywords: anxiety, association, depression, neuropathic pain, small fiber neuropathy
1. INTRODUCTION
Psychiatric comorbidity is common in patients with chronic pain. 1 , 2 A bidirectional relationship is frequently described between chronic pain and mental disorders. 3 , 4 , 5 Especially anxiety and disturbed mood are common with chronic or severe pain conditions. 6 Moreover, in peripheral neuropathic pain, particularly anxiety and mood disorders are frequently present and associated with a high level of catastrophizing. 7 , 8
Small fiber neuropathy (SFN) is a peripheral neuropathy of the small nerve fibers, characterized by chronic neuropathic pain and autonomic dysfunction. 9 An underlying condition is found in 47% of the cases. 10 To establish the diagnosis of SFN, a diagnostic workup is necessary. This consists of a skin biopsy to measure the intraepidermal nerve fiber density (IENFD) and quantitative sensory testing (QST) to determine the temperature thresholds. Skin biopsy is a minimally invasive, objective, and reliable method to quantify the nerve fiber endings in the skin. 11 In QST, patients must indicate when they experience a thermal stimulus. Hence, this method is subjective and demands the cooperation of the patient.
Overall, quality of life (QOL) is severely reduced in SFN patients compared with healthy individuals or patients with various other chronic conditions and might be attributed to both physical (especially pain) and mental factors. 12 In most chronic pain conditions, QOL is reduced 13 and negatively influenced by the presence of anxiety or depressive mood. 14 , 15 , 16 , 17 The worldwide prevalence of an anxiety or depressive disorder is respectively estimate around 4.4%–3.6% (WHO). 18 In chronic neuropathic pain, the prevalence of both disorders is higher, and reported up to 25%–65.6%. 19 , 20 , 21 More specifically, in painful diabetic polyneuropathy a prevalence of 46.7% for depression and 60.7% for anxiety disorders has been described. 22 Prevalence rates for anxiety disorders and depression in fibromyalgia 23 and complex regional pain syndrome type 1 are estimated around 40%, 24 and in psychosomatic disorders, such as irritable bowel syndrome around 30%. 25
Studies about the specific mental health status of SFN patients are scarce. The exact prevalence of anxiety and depression in SFN is not clear. In an American study, consisting of a retrospective chart review and cross‐sectional survey in 100 SFN patients, anxiety or depressive symptoms were described to be present in one‐third of the patients. 26
The risk of developing a mental disorder in (chronic) pain seems to be determined by a longer duration of pain, the daily use of medication, and the severity of pain at baseline. 27 Recognizing the co‐occurrence of mental health conditions and neuropathic pain is important concerning the treatment and management of chronic pain, and a multidisciplinary approach to delivering personalized health care might be more sufficient.
This study aimed to investigate the prevalence of and factors associated with anxiety and depressive symptoms in SFN.
2. MATERIALS AND METHODS
2.1. Study population
The Maastricht University Medical Center+ (Maastricht UMC+) serves as a tertiary referral center for SFN in the Netherlands and the only center in the Netherlands in which the IENFD in skin biopsy can be determined. Patients with possible SFN receive an extensive standardized workup during a daycare setting to establish the diagnosis and to search for an underlying condition associated with SFN. Based on the results, the patients receive a treatment advice. The treatment and follow‐up take place in their own region. In addition, the Maastricht UMC+ has also a regional hospital function. As a result, patients can also be referred directly by a general practitioner.
All consecutive patients being 18 y of age or older who visited the Maastricht UMC+ between September 2016 and October 2021, and who met the diagnostic criteria for SFN, were included in this study. The diagnosis of SFN was confirmed according to the Besta criteria. 28 , 29 The Besta criteria require the combination of at least two clinical signs and symptoms, and abnormal temperature thresholds in QST, and/or reduced IENFD in skin biopsy, with no signs of large fiber involvement. 29 All patients fulfilled several online questionnaires about pain (pain intensity Visual Analog Scale [VAS]), SFN‐related symptoms (SFN‐Symptoms Inventory Questionnaire [SFN‐SIQ]), and mental health (Hospital Anxiety and Depression Scale (HADS), and Pain Catastrophizing Scale (PCS)). All data has been collected prospectively.
The data was used anonymously and patients had the possibility to complain about the use of medical and personal data for research according to the Code of Conduct for the Use of Data in Health Research.
2.2. Measurement tools
The following data were collected: sex, age at visit, age of onset complaints, somatic comorbidity, IENFD, QST, and nerve conduction study results.
Pain intensity was measured by the VAS for the current pain intensity. The VAS ranges from 0 to 100. A score of 0 indicates no pain, and a score of 100 is the worst pain someone can imagine. The pain intensity is divided into three main categories (mild: 0–49, moderate: 50–69 and severe: 70–100) according to the Verbal Rating Scale. 30 In addition to pain intensity, pain locations were indicated to make a distinction between specific peripheral pain locations and diversely distributed pain (see Figure 1). Participants had to indicate the most painful body parts. The sum of each body part and side was calculated (eg, left hand+abdomen+right feet+left feet = 4). The pain intensity, the pain duration and the number of pain sites were divided into four categories. Categorizing data makes it more clinically useful and easier to interpret the results, than using a continuous outcome.
FIGURE 1.
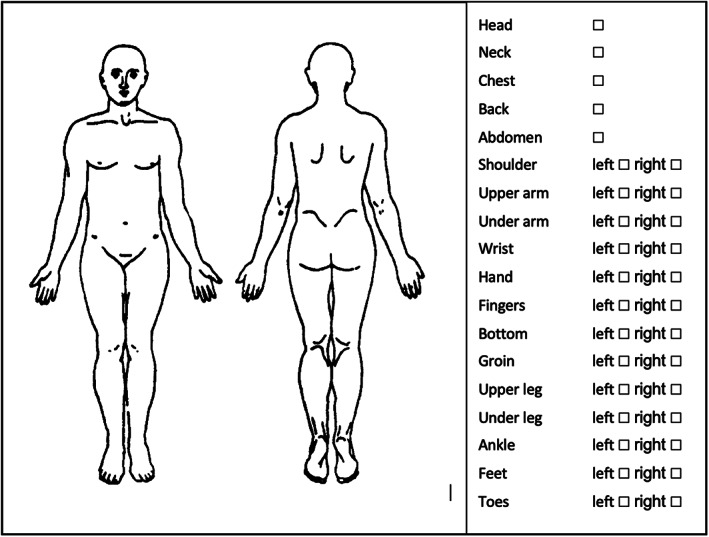
Questionnaire about the pain locations. The figure is available in the online questionnaire for patients who had the diagnostic workup of SFN in the Maastricht UMC+. The following question is followed by this figure: “Could you indicate the locations where you experience the maximum pain intensity?”. It is possible for patients to indicate several pain locations.
The SFN‐SIQ, a Rasch‐based questionnaire, is a valid and reliable tool to measure SFN‐specific complaints. 31 The questionnaire consists of 14 questions (9 about dysautonomia) with four answer options: never, sometimes, most of the time, and always. The centile metric total scores were calculated, which range from 0 to 100. A higher score indicates more complaints.
The Dutch version of the HADS was used to screen for anxiety and depressive symptoms. 32 The questionnaire consists of 14‐items, of which seven are related to depression (HADS‐D) and seven to anxiety (HADS‐A) with a maximal score of 21 on both subscales. A cut‐off point of ≥11, on the subscales, is considered to be abnormal and clinically relevant indicating a probable presence of anxiety or depressive symptoms. A score between 0 and 7 on both subscales is considered to be normal, and scores between 8 and 10 are considered to be borderline. 33
The PCS questionnaire was used to measure the level of catastrophizing and consists of 13 questions, with five answer options (0 not at all; 1 to a slight degree; 2 to a moderate degree; 3 to a great degree; 4 all the time). 34 The PCS ranges from 0 to 52. A higher total score indicates the presence of catastrophic thoughts. 35 The PCS total scores were divided into four categories.
2.3. Statistical analysis
The demographic and clinical information of the total population were calculated with mean and SD for continuous variables. Absolute and relative frequencies were calculated for categorical variables. Pearson coefficient correlation was used for normally distributed variables to evaluate linear correlations. A significance level of 0.05 was set.
Univariate and multivariate logistic regression analysis was used to identify variables independently associated with the subscales of the HADS questionnaire. First, a univariate logistic regression analysis (model 1) was performed to assess the relationship between potential relevant factors and scores on the subscales of the HADS questionnaire (<11 vs ≥11). Second, variables that were associated with the HADS questionnaire score according to a significance level of α ≤ 0.10. A backward stepwise regression selection was performed until the final model was reached including only variables associated with the HADS questionnaire with a statistically significant level of α ≤ 0.05. Odds ratios including 95% confidence intervals and P‐values were presented.
Statistical analyses were performed in SPSS (Version 25.0, SPSS Inc., Chicago, IL, USA).
3. RESULTS
3.1. Patients
A total of 1608 consecutive patients were screened at the Maastricht UMC+ between September 2016 and October 2021, of whom 298 patients were excluded; 288 patients did not meet the diagnostic criteria, and 10 did not have had a complete workup in our hospital. A total of 1310 patients with SFN were included in the analysis (see Figure 2). The demographic and clinical characteristics of all SFN participants are presented in Table 1. Of the included patients, 911 (69.5%) were female. The average age of the total group was 54.71 ± 12.91 years. Thirty‐six percent of the patients had an abnormal score (≥11) on the HADS‐A and/or HADS‐D sub‐scale (26.5% on the HADS‐A, 23.0% on the HADS‐D), considered to be abnormal and indicating the presence of underlying anxiety symptoms or depressive symptoms. Diversely distributed pain (≥11 pain sites) was present in 34.5%.
FIGURE 2.
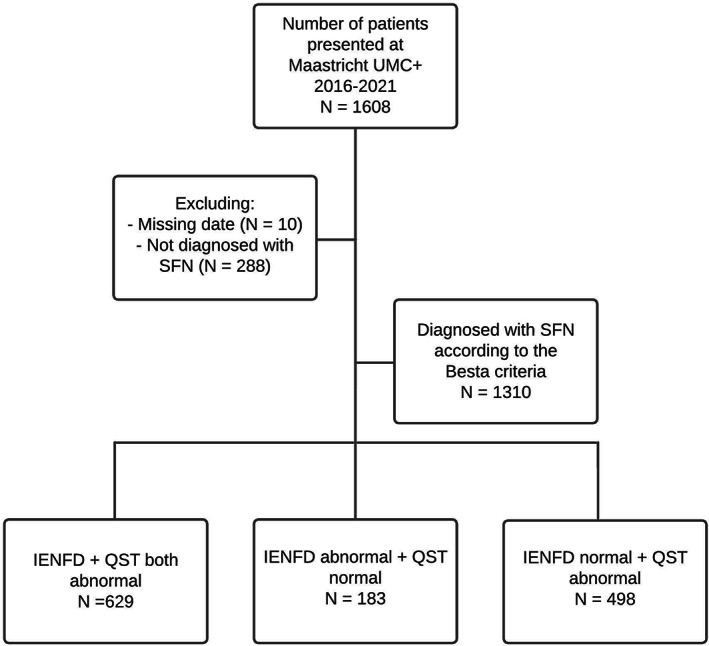
Flowchart. In total 1608 patients were screened. 298 patients were excluded. Reasons for exclusion: not having a complete workup in the Maastricht UMC+ (N = 10), and not meeting the diagnostic criteria of SFN (N = 288). A total of 1310 patients were diagnosed with SFN according to the Besta criteria and included for further analysis.
TABLE 1.
Demographic and clinical information of patients with small fiber neuropathy
| Characteristics | N = 1310 | Male | Female |
|---|---|---|---|
| N = 399 | N = 911 | ||
| Age in years [Mean, SD, range] | 54.71 (12.91), 19–88 | 56.47 (11.71) | 53.94 (13.34) |
| Pain duration in years [mean, SD, n (%), range] | 8.53 (6.05), 1–45 | 8.22 (5.51) | 8.66 (6.27) |
| 1–5 yr | 464 (35.4) | 143 (35.8) | 321 (35.2) |
| 6–10 yr | 503 (38.4) | 154 (38.6) | 349 (38.3) |
| 11–15 yr | 192 (14.7) | 64 (16.0) | 128 (14.1) |
| >15 yr | 151 (11.5) | 38 (9.5) | 113 (12.4) |
| Number of pain sites [mean, SD, n (%), range] | 9.09 (6.41) | 8.64 (6.34) | 9.29 (6.44) |
| 1–5 locations | 480 (36.6) | 164 (41.1) | 316 (34.7) |
| 6–10 locations | 378 (28.9) | 114 (28.6) | 264 (29.0) |
| 11–15 locations | 233 (17.8) | 59 (14.8) | 174 (19.1) |
| >15 locations | 219 (16.7) | 62 (15.5) | 157 (17.2) |
| VAS (current pain intensity) [mean, SD, range] | 52.11 (27.08), 0–100 | 52.44 (27.76) | 51.97 (26.80) |
| SFN diagnosis based on [n (%)] | |||
| Reduced IENFD in the skin biopsy | 183 (14.0) | 37 (9.3) | 146 (16.0) |
| Abnormal QST | 498 (38.0) | 144 (36.1) | 354 (38.9) |
| Reduced IENFD in the skin biopsy and abnormal QST | 629 (48.0) | 218 (54.6) | 411 (45.1) |
| Idiopathic SFN [n (%)] | 802 (61.2) | 248 (62.2) | 554 (60.8) |
| HADS‐score [mean, SD] | 15.24 (8.00) | 16.04 (8.43) | 14.89 (7.76) |
| Total score ≥ 11 | 475 (36.3) | 163 (40.9) | 312 (34.2) |
| HADS anxiety, total score [mean, SD, n (%)] | 7.97 (4.41) | 8.14 (4.47) | 7.90 (4.38) |
| Normal (0–7) | 651 (49.7) | 188 (47.1) | 463 (50.8) |
| Borderline (8–10) | 312 (23.8) | 105 (26.3) | 207 (22.7) |
| Abnormal (11–21) | 347 (26.5) | 106 (26.6) | 241 (26.5) |
| Only total score ≥ 11 | 174 (13.3) | 89 (22.3) | 85 (9.3) |
| HADS depression, total score [mean, SD, n (%)] | 7.27 (4.41) | 7.91 (4.77) | 6.99 (4.22) |
| Normal (0–7) | 737 (56.3) | 201 (50.4) | 536 (58.8) |
| Borderline (8–10) | 272 (20.8) | 85 (21.3) | 187 (20.5) |
| Abnormal (11–21) | 301 (23.0) | 113 (28.3) | 188 (20.6) |
| Only total score ≥ 11 | 128 (9.8) | 44 (11.0) | 84 (9.2) |
| PCS score [mean, SD] | 20.82 (12.00) | 23.31 (12.45) | 19.70 (11.59) |
| 0–19 | 670 (51.1) | 168 (42.1) | 502 (55.1) |
| 20–34 | 447 (34.1) | 149 (37.3) | 298 (32.7) |
| ≥35 | 187 (14.3) | 80 (20.1) | 107 (11.7) |
| SFN‐SIQ score (centile metric) [mean, SD] | 45.88 (9.20) | 42.36 (9.50) | 47.42 (8.62) |
Abbreviations: HADS, Hospital Anxiety and Depression Scale; IENFD, intra‐epidermal nerve fiber density; PCS, Pain Catastrophizing Scale; QST, quantitative sensory testing; SD, standard deviation; SFN, Small Fiber Neuropathy; SFN‐SIQ, SFN‐Symptoms Inventory Questionnaire; VAS, Visual Analogue Scale; yr, year.
3.2. Associations with anxiety
The analysis of factors associated with the HADS‐A scores is presented in Table 2. In the univariate regression analyses, an increasing number of pain sites (0–5 sites vs >15 sites) (OR = 0.65 [95% CI: 0.46–0.94]; P‐value = 0.022), a higher current pain intensity (Mild vs Moderate: OR = 0.47 [95% CI: 0.35–0.64]; P‐value <0.001, Moderate vs Severe: OR = 0.70 [95% CI: 0.52–0.94]; P‐value = 0.017), a high score on the catastrophizing scale ([0–19] vs [>35]: OR = 0.08 per [95% CI: 0.05–0.12]; P‐value <0.001, [20–34] vs [>35]: OR = 0.30 [95% CI: 0.21–0.42]; P‐value <0.001), and a high score on the SFN‐SIQ questionnaire (OR = 1.05 per 1‐point increase [95% CI:1.03–1.06]; P‐value <0.001) were associated with an increased likelihood of having a high HADS‐A sub‐score (≥11). Mean age, the duration of pain, a reduced IENFD in skin biopsy, abnormal QST, and idiopathic SFN were not statistically significantly associated with a high HADS‐A.
TABLE 2.
Analysis of factors associated with high scores and low scores on HADS questionnaire subscale anxiety (HADS‐A) in small fiber neuropathy (N = 1310)
| Variables | High score on HADS‐A (≥11) (N = 347) | Low score on HADS‐A (<11) (N = 963) | Model 1 a | Model 2 b | ||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Mean (SD) | N (%) | Mean (SD) | OR [95%CI] | P‐value | OR [95% CI] | P‐value | |
| Sex | ||||||||
| Female | 241 (69.5) | 670 (69.6) | 0.99 [0.76–1.30] | 0.966 | ||||
| Male | 106 (30.5) | 293 (30.4) | Reference | |||||
| Mean age in years | 54.65 (12.88) | 54.73 (12.93) | 1.00 [0.99–1.00] | 0.921 | ||||
| Pain duration in years | 8.64 (5.99) | 8.49 (6.07) | ||||||
| 1–5 yr | 109 (31.4) | 355 (36.9) | 0.98 [0.63–1.51] | 0.930 | ||||
| 6–10 yr | 144 (41.5) | 359 (37.3) | 1.28 [0.84–1.95] | 0.250 | ||||
| 11–15 yr | 58 (16.7) | 134 (13.9) | 1.38 [0.85–2.24] | 0.190 | ||||
| >15 yr | 36 (10.4) | 115 (11.9) | Reference | |||||
| Number of pain sites | 9.99 (6.78) | 8.77 (6.25) | ||||||
| 0–5 | 106 (30.5) | 374 (38.8) | 0.65 [0.46–0.94] | 0.022 | ||||
| 6–10 | 108 (31.1) | 270 (28.0) | 0.92 [0.64–1.33] | 0.685 | ||||
| 11–15 | 67 (19.3) | 166 (17.2) | 0.93 [0.62–1.40] | 0.747 | ||||
| >15 | 66 (19.0) | 153 (15.9) | Reference | |||||
| VAS (current pain intensity) | 59.21 (27.58) | 49.55 (26.45) | ||||||
| Mild | 87 (25.4) | 357 (37.6) | 0.47 [0.35–0.64] | <0.001 | 1.45 [1.01–2.18] | 0.043 | ||
| Moderate | 115 (33.6) | 320 (33.7) | 0.70 [0.52–0.94] | 0.017 | 1.30 [0.93–1.83] | 0.127 | ||
| Severe | 140 (40.9) | 272 (28.7) | Reference | Reference | ||||
| Reduced IENFD in the skin biopsy | ||||||||
| Yes | 213 (61.4) | 599 (62.2) | Reference | |||||
| No | 134 (38.6) | 364 (37.8) | 1.03 [0.80–1.33] | 0.788 | ||||
| Abnormal QST | ||||||||
| Yes | 290 (83.6) | 837 (86.9) | Reference | |||||
| No | 57 (16.4) | 126 (13.1) | 1.30 [0.93–1.83] | 0.124 | ||||
| Idiopathic SFN | ||||||||
| Yes | 210 (60.5) | 592 (61.5) | Reference | |||||
| No | 137 (39.5) | 371 (38.5) | 1.04 [0.81–1.34] | 0.754 | ||||
| PCS score | 29.29 (11.99) | 17.73 (10.38) | ||||||
| 0–19 | 80 (23.1) | 590 (61.6) | 0.08 [0.05–0.12] | <0.001 | 0.07 [0.05–0.11] | <0.001 | ||
| 20–34 | 149 (43.1) | 298 (31.1) | 0.30 [0.21–0.42] | <0.001 | 0.29 [0.20–0.43] | <0.001 | ||
| ≥35 | 117 (33.8) | 70 (7.3) | Reference | Reference | ||||
| SFN‐SIQ centile metric score | 48.84 (8.54) | 44.81 (9.19) | 1.05 [1.03–1.06] | <0.001 | 1.05 [1.04–1.07] | <0.001 | ||
Abbreviations: CI, Confidence Interval; HADS, Hospital Anxiety and Depression Scale; HADS‐A, Hospital Anxiety and Depression Scale subscale Anxiety; IENFD, intra‐epidermal nerve fiber density; OR, Odds Ratio; PCS, Pain Catastrophizing Scale; QST, quantitative sensory testing; SD, standard deviation; SFN, Small Fiber Neuropathy; SFN‐SIQ, SFN‐Symptoms Inventory Questionnaire; VAS, Visual Analogue Scale; yr, years.
Univariable adjusted logistic regression.
Backward stepwise logistic regression, including variables with a P‐value<0.05.
The higher current pain intensity [Mild vs Severe: OR = 0.47 (95% CI 0.35–0.64); P‐value <0.001], a high score on the catastrophizing scale [(0–19) vs (>35): OR = 0.08 (95% CI: 0.05–0.12); P‐value <0.001, (20–34) vs (>35): OR = 0.30 (95% CI: 0.21–0.44); P‐value <0.001] and SFN‐SIQ questionnaire [OR = 1.05 per 1‐point increase (95% CI: 1.03–1.06); P‐value <0.001] showed a significant association with a high HADS‐A sub‐score in the multivariable adjusted model.
The HADS‐A was correlated with several outcomes: number of pain sites (r = 0.13, P‐ value <0.001), higher current pain intensity (r = 0.21, P‐value <0.001), catastrophic thoughts measured with PCS (r = 0.55, P‐value <0.001), and SFN‐SIQ questionnaire (r = 0.22, P‐value <0.001). The HADS‐A was not correlated with age (r = −0.02, P‐value = 0.398), and the duration of pain (r = −0.02, P‐value = 0.424), see Figure 3 (1–6).
FIGURE 3.
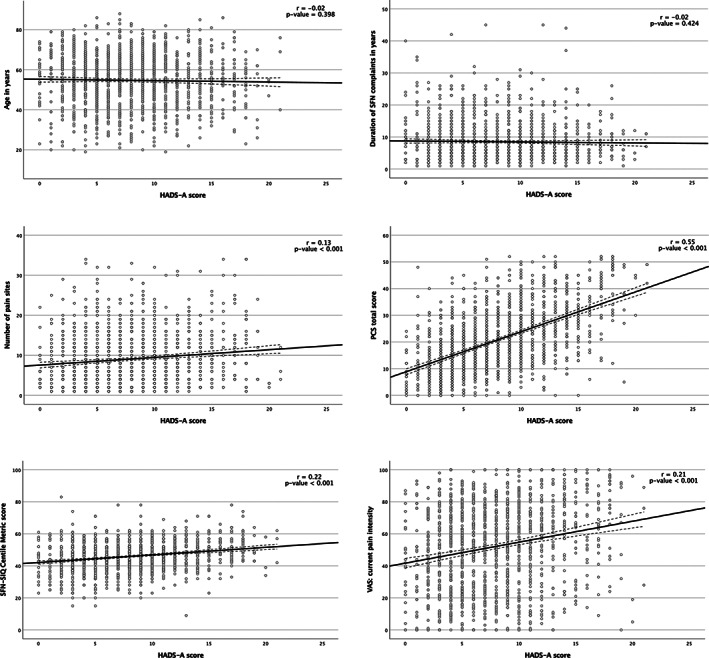
Pearson correlations of HADS subscale anxiety with several outcomes. The outcome of HADS subscale anxiety (HADS‐A) was correlated with the following outcomes: age, the number of pain locations, the current pain intensity, the duration of SFN complaints, the PCS total score and the SFN‐SIQ centile metric score. Abbreviations: HADS‐A, HADS questionnaire subscale anxiety; PCS, pain catastrophizing scale; r, Pearson r coefficient; SFN, small fiber neuropathy; SFN‐SIQ, SFN related symptom inventory questionnaire.
3.3. Associations with depression
In the univariate analyses, female gender (OR = 0.63 [95% CI: 0.45–0.87]; P‐value = 0.006) were less likely to have a high HADS‐D score (≥11), compared to males. Furthermore, an increasing number of pain sites ([0–5] vs [>15]: OR = 0.47 [95% CI: 0.33–0.68]; P‐value <0.001, [6–10] vs [>15]: OR = 0.65 [95% CI: 0‐45‐0.94]; P‐value = 0.023), higher current pain intensity (Mild vs Severe: OR = 0.24 [95% CI: 0.16‐0.34]; P‐value <0.001, Moderate vs Severe: OR = 0.50 [95% CI: 0.37–0.67]; P‐value <0.001), a high score on the catastrophizing scale ([0–19] vs [>35]: OR = 0.06 [95% CI: 0.04–0.10]; P‐value <0.001, [20–34] vs [>35]: OR = 0.32 [95% CI: 0.22–0.45]; P‐value <0.001), and a higher score on the SFN‐SIQ questionnaire (OR = 1.05 per 1‐point increase [95% CI: 1.04–1.07]; P‐value <0.001) were associated with an increased likelihood of having a high HADS‐D score (see Table 3).
TABLE 3.
Analysis of factors associated with high scores and low scores on HADS questionnaire subscale depression (HADS‐D) in small fiber neuropathy (N = 1310)
| Variables | High score on HADS‐D (≥11) (N = 301) | Low score on HADS‐D (<11) (N = 1009) | Model 1 a | Model 2 b | ||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Mean (SD) | N (%) | Mean (SD) | OR [95%CI] | P‐value | OR [95% CI] | P‐value | |
| Sex | ||||||||
| Female | 188 (62.5) | 723 (71.7) | 0.66 [0.50–0.86] | 0.002 | 0.63 [0.45–0.87] | 0.006 | ||
| Male | 113 (37.5) | 286 (28.3) | Reference | |||||
| Mean age in years | 54.36 (12.19) | 54.82 (13.12) | 1.00 [0.98–1.00] | 0.584 | ||||
| Pain duration in years | 8.39 (5.43) | 8.57 (6.22) | ||||||
| 1–5 yr | 100 (33.2) | 364 (36.1) | 0.98 [0.63–1.53] | 0.937 | ||||
| 6–10 yr | 120 (39.9) | 383 (38.0) | 1.12 [0.72–1.73] | 0.610 | ||||
| 11–15 yr | 48 (15.9) | 144 (14.3) | 1.20 [0.72–1.97] | 0.500 | ||||
| >15 yr | 33 (11.0) | 118 (11.7) | Reference | |||||
| Number of pain sites | 10.63 (7.14) | 8.63 (6.11) | ||||||
| 0–5 | 86 (28.6) | 394 (39.0) | 0.47 [0.33–0.68] | <0.001 | ||||
| 6–10 | 87 (28.9) | 291 (28.8) | 0.65 [0.45–0.94] | 0.023 | ||||
| 11–15 | 59 (19.6) | 174 (17.2) | 0.73 [0.49–1.11] | 0.145 | ||||
| >15 | 69 (22.9) | 150 (14.9) | Reference | |||||
| VAS (current pain intensity) | 64.78 (24.91) | 48.36 (26.57) | ||||||
| Mild | 52 (17.6) | 392 (39.4) | 0.24 [0.16–0.34] | <0.001 | 0.64 [0.42–0.97] | 0.038 | ||
| Moderate | 95 (32.2) | 340 (34.1) | 0.50 [0.37–0.67] | <0.001 | 0.86 [0.61–1.22] | 0.399 | ||
| Severe | 148 (50.2) | 264 (26.5) | Reference | Reference | ||||
| Reduced IENFD in the skin biopsy | ||||||||
| Yes | 190 (63.1) | 622 (61.6) | Reference | |||||
| No | 111 (36.9) | 387 (38.4) | 0.94 [0.72–1.22] | 0.643 | ||||
| Abnormal QST | ||||||||
| Yes | 259 (86.0) | 868 (86.0) | Reference | |||||
| No | 42 (14.0) | 141 (14.0) | 1.00 [0.69–1.44] | 1.000 | ||||
| Idiopathic SFN | ||||||||
| Yes | 178 (59.1) | 624 (61.8) | Reference | |||||
| No | 123 (40.9) | 385 (38.2) | 1.12 [0.86–1.45] | 0.400 | ||||
| PCS score | 30.29 (11.13) | 17.96 (10.68) | ||||||
| 0–19 | 80 (23.1) | 590 (61.6) | 0.08 [0.05–0.12] | <0.001 | 0.08 [0.05–0.13] | <0.001 | ||
| 20–34 | 149 (43.1) | 298 (31.1) | 0.30 [0.21–0.42] | <0.001 | 0.36 [0.25–0.53] | <0.001 | ||
| ≥35 | 117 (33.8) | 70 (7.3) | Reference | Reference | ||||
| SFN‐SIQ centile metric score | 49.29 (8.88) | 44.86 (9.04) | 1.05 [1.04–1.07] | <0.001 | 1.05 [1.03–1.07] | <0.001 | ||
Abbreviations: CI, Confidence Interval; HADS, Hospital Anxiety and Depression Scale; HADS‐A, Hospital Anxiety and Depression Scale subscale Anxiety; IENFD, intra‐epidermal nerve fiber density; OR, Odds Ratio; PCS, Pain Catastrophizing Scale; QST, quantitative sensory testing; SD, standard deviation; SFN, Small Fiber Neuropathy; SFN‐SIQ, SFN‐Symptoms Inventory Questionnaire; VAS, Visual Analogue Scale; yr, year.
Univariable adjusted logistic regression.
Backward stepwise logistic regression, including variables with a P‐value<0.05.
Gender (OR = 0.63 [95% CI: 0.45–0.87]; P‐value = 0.006), a higher current pain intensity (Mild vs Severe: OR = 0.64 [95% CI: 0.42–0.97]; P‐value = 0.038), a high score on the catastrophizing scale ([0–19] vs [>35]: OR = 0.08 [95% CI: 0.05–0.13]; P‐value <0.001, [20–34] vs [>35]: OR = 0.36 [95% CI: 0.25–0.53]; P‐value <0.001), and a high score on the SFN‐SIQ questionnaire (OR = 1.05 per 1‐point increase [95% CI: 1.03–1.07]; P‐value <0.001) showed a significant association with a high HADS‐D sub‐score in the multivariable adjusted model.
The HADS‐D was correlated with several outcomes: number of pain sites (r = 0.15, P‐ value <0.001), higher current pain intensity (r = 0.31, P‐value <0.001), catastrophic thoughts measured with PCS (r = 0.53, P‐value <0.001), and SFN‐SIQ questionnaire (r = 0.26, P‐value <0.001). The HADS‐D was not correlated with age (r = −0.01, P‐value = 0.773), and the duration of pain (r = −0.03, P‐value = 0.278), see Figure 4 (1–6).
FIGURE 4.
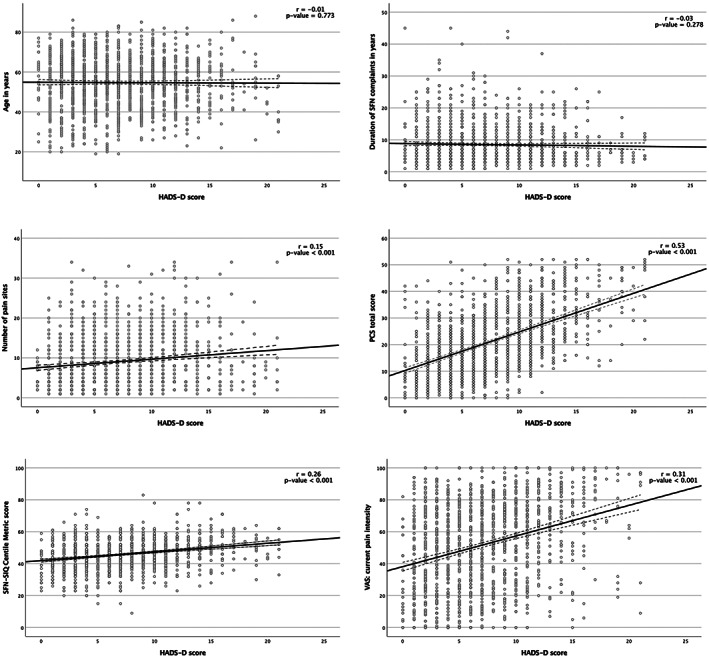
Pearson correlations of HADS subscale depression with several outcomes. The outcome of HADS subscale depression (HADS‐D) was correlated with the following outcomes: age, the number of pain locations, the current pain intensity, the duration of SFN complaints, the PCS total score and the SFN‐SIQ centile metric score. Abbreviations: HADS‐A, HADS questionnaire subscale anxiety; PCS, pain catastrophizing scale; r, Pearson r coefficient; SFN, small fiber neuropathy; SFN‐SIQ, SFN related symptom inventory questionnaire.
4. DISCUSSION
To our knowledge, this study is the first one to screen the presence of anxiety and depressive symptoms in SFN. It indicates that 36.3% of our patients may suffer from underlying anxiety and/or depressive disorder. A higher pain intensity, a higher level of catastrophizing and more SFN‐related (autonomic) complaints were independently associated and strongly correlated with a high HADS score (≥11) on the anxiety and depression subscales.
Previously, several research groups have examined the prevalence of anxiety and/or depressive disorders in the general population, various chronic pain conditions, and psychosomatic disorders. Our results are in line with the prevalence of anxiety and depression in other chronic (neuropathic) pain conditions and psychosomatic disorders (ie, irritable bowel syndrome [IBS], fibromyalgia, etc.). 19 , 20 , 21 , 22 , 23 , 24 , 25 Schaefer et al. described comparable prevalence rates for anxiety (33%) and depressive symptoms in a retrospective study in SFN (34%). 26
Sometimes it is suggested that patients with an affective disorder may develop a chronic pain disorder, such as fibromyalgia or complex regional pain syndrome. The opposite is also claimed, that chronic pain patients may develop an affective disorder. 36 Chronic pain disorders are heterogenous disorders, 23 , 37 , 38 with overlapping symptoms with affective disorders. 39 The hypothesis that both should appertain to one diagnosis could be considered. However, the co‐occurrence of chronic pain and depressive disorder do not always have the same beneficial effect of antidepressant drugs as the occurrence of only depressive disorder. 40 , 41 These findings plead that chronic pain disorder and affective disorder are separate diagnoses rather than one overarching disorder. On the other hand, it is questionable why certain individuals with chronic pain develop an affective disorder. Predisposing, precipitating and perpetuating factors seem to have a great influence on the development of affective disorders. 23 , 42 , 43 , 44 , 45 Probably, patients with SFN and other chronic pain syndromes tend to have a higher risk to develop affective symptoms and eventually a mental disorder. 46 , 47 However, uncertainty about the risk of mental disorders to developing chronic pain syndrome remains.
In literature, a higher prevalence of psychiatric comorbidities and chronic pain among women has been described. 48 , 49 Similar results were observed in this study. More women were prevalent in both subscales of the HADS questionnaire, and more women are diagnosed with SFN. Men are more likely to have a high HADS‐D score, compared to women. In IBS, research revealed that pain intensity decreases if the affective experience normalizes. 50 Given that, a possible explanation for this finding concerning gender differences might be, that intensive depressive complaints are less present in women due to their openness regarding affective experiences, such as anxiety or depressed mood, and their consecutive treatment in mental health care. 51
Furthermore, the research described the correlation between catastrophizing, chronic pain, and psychiatric disorders. 8 Catastrophizing can be defined as an exaggerated negative mental state experienced during actual or anticipated pain. 52 The results of this study are comparable with previous research on neuropathic pain conditions. 2 A higher level of catastrophizing was found to be strongly correlated with higher scores on the affective subscales in SFN. The results indicate that catastrophizing is a determinant of psychological comorbidity in chronic neuropathic pain, as in SFN.
In general, higher levels of pain intensity are associated with the presence of depression. 7 , 53 In SFN, the levels of pain intensity were significantly higher in patients with a high HADS score on subscale depression and anxiety with a strong correlation, indicating an association between anxiety and depressive symptoms. In painful peripheral diabetic neuropathy, the same findings have been observed. 2 , 47 , 54 A possible explanation could be the change in pain threshold due to affective symptoms. 55 , 56
The IENFD determined by skin biopsy is the most reliable and objective measurement to establish the diagnosis of SFN. Some research groups state that an abnormal IENFD is necessary for the definite diagnosis of SFN. 57 In this study, we have used the Besta criteria to establish the diagnosis of SFN. Using the Besta criteria, a reduced IENFD in skin biopsy is not mandatory to establish SFN. Clinical signs and symptoms, in combination with an abnormal QST, are sufficient for a probable SFN. Even though the outcome of skin biopsy is important, earlier research did not find a correlation between channelopathies in SFN and a reduced IENFD in skin biopsy. 58 In our study, we could not find an association between the outcome of the IENFD and a high score on the HADS subscales, nor a correlation could be found between an abnormal QST and a high score on the HADS subscales, which indicates that neither IENFD nor QST takes the interaction between pain and affective symptoms into account. The reason we hypothesized a probable relationship between QST and affective symptoms, was that QST is a subjective measurement tool. Affective symptoms can have a negative contribution to symptom arousal. 59 , 60 Alteration of temperature thresholds and pain intensity have been observed in affective disorders 61 , 62 and the temperature thresholds seem to be increased in depressive disorders 26 , 55 , 63 , 64 , 65 and anxiety. 65
A strength of our study is the prospective collection of data. All patients underwent the same diagnostic work‐up in the SFN center of expertise at the Maastricht UMC+ and all patients had to complete the same questionnaires. All questionnaires were only available online. Therefore, there were no missing data, making the data reliable.
However, there are also some limitations. First, the HADS‐questionnaire is not developed to establish psychiatric comorbidity. The questionnaire screens for the existence of anxiety and depressive symptoms, thus, further clinical information is important to establish psychiatric disorders. 32 Second, the medical psychiatric history of patients could not be taken into account due to possible underestimation of a psychiatric disorder in the medical history. 66 Patients and physicians do not always mention or ask in depth about the probable psychiatric history due to stigmatization. 67 , 68 , 69 At last, the insecurity of patients at the moment of diagnostic evaluation could have influenced the results of the HADS questionnaire. However, the prevalence of the affective symptoms is in line with the prevalence rates in other chronic pain disorders.
5. CONCLUSION
In this study, the presence of reported anxiety and/or depressive symptoms in SFN is 36.3%. The findings are similar to other chronic pain disorders. A high score on the HADS questionnaire is associated and strongly correlated with a higher intensity of pain, and more catastrophic thoughts. The probable co‐occurrence of mental disorders and/or symptoms and neuropathic pain is important to recognize concerning the treatment and management of chronic pain. A multidisciplinary approach to delivering personalized care may be more effective than mono‐disciplinary trajectories.
CONFLICT OF INTEREST
Dr. Damci, Dr. Schruers, and Dr. Leue declared no conflicts of interest. Dr. Faber reports grants from European Union's Horizon 2020 research and innovation programme Marie Sklodowska‐Curie grant for PAIN‐Net, Molecule‐to‐man pain network (grant no. 721841), grants from Grifols and Lamepro for a trial on IVIg in small fiber neuropathy, grants from Prinses Beatrix Spierfonds, Steering committees/advisory board for studies in small fiber neuropathy of Biogen/Convergence, Vertex, Lilly and OliPass, outside the submitted work. Dr. Hoeijmakers reports a grant from the Prinses Beatrix Spierfonds (W.OK17‐09). This work is generated within the European Reference Network for Neuromuscular Diseases.
Damci A, Schruers KRJ, Leue C, Faber CG, Hoeijmakers JGJ. Anxiety and depression in small fiber neuropathy. J Peripher Nerv Syst. 2022;27(4):291‐301. doi: 10.1111/jns.12514
The present study was funded by the Prinses Beatrix Spierfonds (W.OK17‐09).
Funding information Prinses Beatrix Spierfonds, Grant/Award Number: W.OK17‐09
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, [AD], upon reasonable request.
REFERENCES
- 1. Castro M, Kraychete D, Daltro C, Lopes J, Menezes R, Oliveira I. Comorbid anxiety and depression disorders in patients with chronic pain. Arq Neuropsiquiatr. 2009;67:982‐985. [DOI] [PubMed] [Google Scholar]
- 2. Radat F, Margot‐Duclot A, Attal N. Psychiatric co‐morbidities in patients with chronic peripheral neuropathic pain: a multicentre cohort study. Eur J Pain. 2013;17:1547‐1557. [DOI] [PubMed] [Google Scholar]
- 3. Gureje O, Von Korff M, Kola L, et al. The relation between multiple pains and mental disorders: results from the world mental health surveys. Pain. 2008;135:82‐91. [DOI] [PubMed] [Google Scholar]
- 4. Hooten WM. Chronic pain and mental health disorders: shared neural mechanisms, epidemiology, and treatment. Mayo Clin Proc. 2016;91:955‐970. [DOI] [PubMed] [Google Scholar]
- 5. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127‐133. [DOI] [PubMed] [Google Scholar]
- 6. Brouwer B, Waardenburg S, Jacobs C, et al. Biopsychosocial baseline values of 15 000 patients suffering from chronic pain: Dutch DataPain study. Reg Anesth Pain Med. 2020;45:774‐782. [DOI] [PubMed] [Google Scholar]
- 7. Glette M, Stiles TC, Jensen MP, Nilsen TIL, Borchgrevink PC, Landmark T. Impact of pain and catastrophizing on the long‐term course of depression in the general population: the HUNT pain study. Pain. 2021;162:1650‐1658. [DOI] [PubMed] [Google Scholar]
- 8. Linton SJ, Nicholas MK, MacDonald S, et al. The role of depression and catastrophizing in musculoskeletal pain. Eur J Pain. 2011;15:416‐422. [DOI] [PubMed] [Google Scholar]
- 9. Sopacua M, Hoeijmakers JGJ, Merkies ISJ, Lauria G, Waxman SG, Faber CG. Small‐fiber neuropathy: expanding the clinical pain universe. J Peripher Nerv Syst. 2018;24:19‐33. [DOI] [PubMed] [Google Scholar]
- 10. de Greef BTA, Hoeijmakers JGJ, Gorissen‐Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy ‐ a large cohort study and review of the literature. Eur J Neurol. 2018;25:348‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lauria G, Cazzato D, Porretta‐Serapiglia C, et al. Morphometry of dermal nerve fibers in human skin. Neurology. 2011;77:242‐249. [DOI] [PubMed] [Google Scholar]
- 12. Bakkers M, Faber CG, Hoeijmakers JG, Lauria G, Merkies IS. Small fibers, large impact: quality of life in small‐fiber neuropathy. Muscle Nerve. 2014;49:329‐336. [DOI] [PubMed] [Google Scholar]
- 13. Smith B, Torrance N, Bennett M, Lee A. Health and quality of life associated with chronic pain of predominantyl neuropathi origin int he community. Clin J Pain. 2007;23:143‐149. [DOI] [PubMed] [Google Scholar]
- 14. Evans S, Banerjee S, Leese M, Huxley P. The impact of mental illness on quality of life: a comparison of severe mental illness, common mental disorder and healthy population samples. Qual Life Res. 2007;16:17‐29. [DOI] [PubMed] [Google Scholar]
- 15. Inoue S, Taguchi T, Yamashita T, Nakamura M, Ushida T. The prevalence and impact of chronic neuropathic pain on daily and social life: a nationwide study in a Japanese population. Eur J Pain. 2017;21:727‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jensen M, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health‐related quality of life: review and implications. Neurology. 2007;68:1178‐1182. [DOI] [PubMed] [Google Scholar]
- 17. Rapti E, Damigos D, Apostolara P, Roka V, Tzavara C, Lionis C. Patients with chronic pain: evaluating depression and their quality of life in a single center study in Greece. BMC Psychol. 2019;7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Organization WH. Depression and Other Common Mental Disorders: Global Health Estimates. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 19. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433‐2445. [DOI] [PubMed] [Google Scholar]
- 20. Cherif F, Zouari HG, Cherif W, Hadded M, Cheour M, Damak R. Depression prevalence in neuropathic pain and its impact on the quality of life. Pain Res Manag. 2020;2020:7408508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naranjo C, Del Reguero L, Moratalla G, Hercberg M, Valenzuela M, Failde I. Anxiety, depression and sleep disorders in patients with diabetic neuropathic pain: a systematic review. Expert Rev Neurother. 2019;19:1201‐1209. [DOI] [PubMed] [Google Scholar]
- 22. Kec D, Rajdova A, Raputova J, et al. Risk factors for depression and anxiety in painful and painless diabetic polyneuropathy: a multicentre observational cross‐sectional study. Eur J Pain. 2022;26:370‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gracely RH, Ceko M, Bushnell MC. Fibromyalgia and depression. Pain Res Treat. 2012;2012:486590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duong HP, Konzelmann M, Vuistiner P, et al. Psychiatric comorbidity and complex regional pain syndrome through the lens of the biopsychosocial model: a comparative study. J Pain Res. 2020;13:3235‐3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee C, Doo E, Choi JM, et al. The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: systematic review and meta‐analysis. J Neurogastroenterol Motil. 2017;23:349‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schaefer C, Mann R, Sadosky A, et al. Health status, function, productivity, and costs among individuals with idiopathic painful peripheral neuropathy with small fiber involvement in the United States: results from a retrospective chart review and cross‐sectional survey. J Med Econ. 2014;17:394‐407. [DOI] [PubMed] [Google Scholar]
- 27. Gerrits MM, Vogelzangs N, van Oppen P, van Marwijk HW, van der Horst H, Penninx BW. Impact of pain on the course of depressive and anxiety disorders. Pain. 2012;153:429‐436. [DOI] [PubMed] [Google Scholar]
- 28. Devigili G, Cazzato D, Lauria G. Clinical diagnosis and management of small fiber neuropathy: an update on best practice. Expert Rev Neurother. 2020;20:967‐980. [DOI] [PubMed] [Google Scholar]
- 29. Devigili G, Rinaldo S, Lombardi R, et al. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain. 2019;142:3728‐3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101:17‐24. [DOI] [PubMed] [Google Scholar]
- 31. Brouwer BA, Bakkers M, Hoeijmakers JG, Faber CG, Merkies IS. Improving assessment in small fiber neuropathy. J Peripher Nerv Syst. 2015;20:333‐340. [DOI] [PubMed] [Google Scholar]
- 32. Spinhoven P, Ormel J, Sloekers PPA, Kempen GIJM, Speckens AEM, VanHemert AM. A validation study of the hospital anxiety and depression scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27:363‐370. [DOI] [PubMed] [Google Scholar]
- 33. Breeman S, Cotton S, Fielding S, Jones GT. Normative data for the hospital anxiety and depression scale. Qual Life Res. 2015;24:391‐398. [DOI] [PubMed] [Google Scholar]
- 34. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:525‐532. [Google Scholar]
- 35. Pare C, Thibault P, Cote P, et al. The relationship between level of catastrophizing and mental health comorbidity in individuals with whiplash injuries. Clin J Pain. 2019;35:880‐886. [DOI] [PubMed] [Google Scholar]
- 36. Hauser W, Fitzcharles MA. Facts and myths pertaining to fibromyalgia. Dialogues Clin Neurosci. 2018;20:53‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brinkers M, Rumpelt P, Lux A, Kretzschmar M, Pfau G. Psychiatric disorders in complex regional pain syndrome (CRPS): the role of the consultation‐liaison psychiatrist. Pain Res Manag. 2018;2018:2894360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosom Med. 2004;66:837‐844. [DOI] [PubMed] [Google Scholar]
- 39. Wilson KG, Mikail SF, D'Eon JL, Minns JE. Alternative diagnostic criteria for major depressive disorder in patients with chronic pain. Pain. 2001;91:227‐234. [DOI] [PubMed] [Google Scholar]
- 40. Onghena P, Van Houdenhove B. Antidepressant‐induced analgesia in chronic non‐malignant pain: a meta‐analysis of 39 placebo‐controlled studies. Pain. 1992;49:205‐219. [DOI] [PubMed] [Google Scholar]
- 41. Roughan WH, Campos AI, Garcia‐Marin LM, et al. Comorbid chronic pain and depression: shared risk factors and differential antidepressant effectiveness. Front Psych. 2021;12:643609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin‐releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5‐HTT gene. Science. 2003;301:386‐389. [DOI] [PubMed] [Google Scholar]
- 44. Galvez‐Sanchez CM, Duschek S, Reyes Del Paso GA. Psychological impact of fibromyalgia: current perspectives. Psychol Res Behav Manag. 2019;12:117‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kato K, Sullivan PF, Evengard B, Pedersen NL. Importance of genetic influences on chronic widespread pain. Arthritis Rheum. 2006;54:1682‐1686. [DOI] [PubMed] [Google Scholar]
- 46. Dworkin RH, Gitlin MJ. Clinical aspects of depression in chronic pain patients. Clin J Pain. 1991;7:79‐94. [DOI] [PubMed] [Google Scholar]
- 47. Rohde C, Finnerup N, Schmitz N, Jensen TS, Thomsen RW, Ostergaard SD. Is diabetic neuropathy associated with increased risk of developing mental disorders? Eur J Endocrinol. 2022;186:K39‐K43. [DOI] [PubMed] [Google Scholar]
- 48. Faravelli C, Alessandra Scarpato M, Castellini G, Lo Sauro C. Gender differences in depression and anxiety: the role of age. Psychiatry Res. 2013;210:1301‐1303. [DOI] [PubMed] [Google Scholar]
- 49. Munce SE, Stewart DE. Gender differences in depression and chronic pain conditions in a national epidemiologic survey. Psychosomatics. 2007;48:394‐399. [DOI] [PubMed] [Google Scholar]
- 50. Kreiter D, Drukker M, Mujagic Z, et al. Symptom‐network dynamics in irritable bowel syndrome with comorbid panic disorder using electronic momentary assessment: a randomized controlled trial of escitalopram vs. placebo. J Psychosom Res. 2021;141:110351. [DOI] [PubMed] [Google Scholar]
- 51. Liddon L, Kingerlee R, Barry JA. Gender differences in preferences for psychological treatment, coping strategies, and triggers to help‐seeking. Br J Clin Psychol. 2018;57:42‐58. [DOI] [PubMed] [Google Scholar]
- 52. Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52‐64. [DOI] [PubMed] [Google Scholar]
- 53. Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain. 2001;17:165‐172. [DOI] [PubMed] [Google Scholar]
- 54. D'Amato C, Morganti R, Greco C, et al. Diabetic peripheral neuropathic pain is a stronger predictor of depression than other diabetic complications and comorbidities. Diab Vasc Dis Res. 2016;13:418‐428. [DOI] [PubMed] [Google Scholar]
- 55. Bar KJ, Greiner W, Letsch A, Kobele R, Sauer H. Influence of gender and hemispheric lateralization on heat pain perception in major depression. J Psychiatr Res. 2003;37:345‐353. [DOI] [PubMed] [Google Scholar]
- 56. Blackburn‐Munro G, Blackburn‐Munro RE. Chronic pain, chronic stress and depression: coincidence or consequence? J Neuroendocrinol. 2001;13:1009‐1023. [DOI] [PubMed] [Google Scholar]
- 57. Freeman R, Gewandter JS, Faber CG, et al. Idiopathic distal sensory polyneuropathy: ACTTION diagnostic criteria. Neurology. 2020;95:1005‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eijkenboom I, Sopacua M, Hoeijmakers JGJ, et al. Yield of peripheral sodium channels gene screening in pure small fibre neuropathy. J Neurol Neurosurg Psychiatry. 2018;90(3):342‐352. [DOI] [PubMed] [Google Scholar]
- 59. Georgopoulos V, Akin‐Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta‐analysis. Pain. 2019;160:1920‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ursin H, Eriksen HR. Sensitization, subjective health complaints, and sustained arousal. Ann N Y Acad Sci. 2001;933:119‐129. [DOI] [PubMed] [Google Scholar]
- 61. Lautenbacher S, Krieg JC. Pain perception in psychiatric disorders: a review of the literature. J Psychiatr Res. 1994;28:109‐122. [DOI] [PubMed] [Google Scholar]
- 62. Lautenbacher S, Spernal J, Schreiber W, Krieg RC. Relationship between clinical pain complaints and pain sensitvy in patients with depression and panic disorder. Psychosom Med. 1999;61:822‐827. [DOI] [PubMed] [Google Scholar]
- 63. Davis GC, Buchsbaum MS, Bunney WE Jr. Analgesia to painful stimuli in affective illness. Am J Psychiatry. 1979;136:1148‐1151. [DOI] [PubMed] [Google Scholar]
- 64. Marazziti D, Castrogiovanni P, Rossi A, et al. Pain threshold is reduced in depression. Int J Neuropsychopharmacol. 1998;1:45‐48. [DOI] [PubMed] [Google Scholar]
- 65. Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84:65‐75. [DOI] [PubMed] [Google Scholar]
- 66. Leue C, Driessen G, Strik JJ, et al. Managing complex patients on a medical psychiatric unit: an observational study of university hospital costs associated with medical service use, length of stay, and psychiatric intervention. J Psychosom Res. 2010;68:295‐302. [DOI] [PubMed] [Google Scholar]
- 67. Clement S, Schauman O, Graham Thomas J, et al. What is the impact of mental health‐related stigma on help‐seeking? A systematic review of quantitative and qualitative studies. Psychol Med. 2015;45:11‐27. [DOI] [PubMed] [Google Scholar]
- 68. Corrigan PW, Druss BG, Perlick DA. The impact of mental illness stigma on seeking and participating in mental health care. Psychol Sci Public Interest. 2014;15:37‐70. [DOI] [PubMed] [Google Scholar]
- 69. Salama R, Tadroos T, Sikander I, Ashraf A, Khan A. Attitudes of healthcare professionals towards mental illness: a survey study in ras al khaimah. Open J Psychiatry. 2021;11:160‐173. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [AD], upon reasonable request.


