Abstract
Objectives
To determine safety and feasibility of ex‐situ coronary angiography.
Background
To cater for the perpetually growing demand for heart donors, interest in donation following circulatory death (DCD) has been rekindled. Further pursuit of donor pool expansion has led to eligibility extension to “marginal” donors who are at higher risk of coronary artery disease (CAD). Excluding CAD in potentially eligible DCD donors, for whom ante‐mortem angiography is commonly not permitted, is therefore challenging. Ex‐situ coronary angiography serves as an ethical and feasible diagnostic tool to assess for preclusive CAD.
Methods
We undertook a systematic review of the published literature and institutional retrospective review of case experience with ex‐situ coronary angiography of donor hearts, supported by a portable organ care system.
Results
Combined literature and institutional case review yielded nine total cases of ex‐situ coronary angiography of donor human hearts plus one experimental porcine model. Of the eight cases of ex‐situ coronary angiography performed at our institute, all were conducted without complication or injury to the allograft. Two thirds of reported human cases have proceeded to successful transplantation.
Conclusions
Diagnostic coronary angiography of the ex‐situ beating donor heart is safe, feasible, and demonstrates novel clinical utility in mitigating subsequent transplantation of unsuitable allografts. In the setting of suspected coronary atherosclerosis of the donor heart, which may preclude favorable transplantation outcomes, ex‐situ coronary angiography should be considered at eligible transplant centers.
Keywords: cardiac transplantation, coronary angiography, donation after circulatory death, interventional cardiology
Abbreviations
- DCD
donation following circulatory death
- OCS
organ care system
- RAO
right anterior oblique
- WIT
warm ischemia time
- WLS
withdrawal of life support
1. INTRODUCTION
As the age of the human population increases, so does the morbidity burden of advanced heart failure. As such, the demand for donor hearts continues to rise. As a means of expanding the donor pool to ameliorate the supply–demand mismatch, interest in heart donation following circulatory death (DCD) has been rekindled. This follows a period of temporary abandonment following establishment of brain death criteria, advances in static cold organ preservation, ethical controversy surrounding the definition of circulatory death and concern over warm‐ischemic damage to the heart during withdrawal of life support (WLS). The TransMedics organ care system (OCS) is a portable, normothermic perfusion and monitoring system, designed to keep a donor heart in a near‐human metabolically active state to facilitate transport to the recipient. At our institution, Dhital et al. 1 performed the first successful heart transplantation after circulatory death with the distant procurement of the reanimated donor heart, supported by the OCS. Since this initial report, 74 heart transplants from DCD donors have been performed at our center. Heart transplant programs from DCD donors have now been established in multiple European countries commencing with Papworth in the United Kingdom in 2015. 2 In 2019, the first DCD cardiac transplant was performed in the North America. 3 More than 450 DCD heart transplants have been performed globally.
Further expansion of the donor pool has been facilitated by extending eligibility to “marginal” donors, being of older age with variable atherosclerotic risk profiles. In these donors the probability of undiagnosed atherosclerotic vascular disease is higher and to mitigate the known poor post‐transplant survival outcomes associated with significant multivessel disease, delineation of the coronary anatomy before transplantation is preferable. 4 Following declaration of brain‐death, donor coronary angiography is both feasible and economically favorable (by obviating unnecessary retrieval missions). 5 As such, in Australia, coronary angiography may be performed, following declaration of brain death, at the request of the retrieving transplant physician or surgeon with provisional acceptance of the heart pending an acceptable result. 6 However, it is important to note that due to ethical and legal restrictions surrounding DCD donors, ante‐mortem cardiac catheterization, or CT coronary angiography for the purpose of assessing the suitability of the heart for donation is not permitted in most jurisdictions. In the setting of anticipated coronary atherosclerosis, which may be suspected based on palpable disease at the time of retrieval, ex‐situ coronary angiography serves as an ethical and feasible diagnostic tool to assess for the presence of significant and prohibitive coronary artery disease. To explore the feasibility, safety, and procedural approach for ex‐situ coronary angiography, we performed a systematic review of the literature and retrospective analysis of our institutional experience.
2. METHODS
A systematic search of two large databases (SCOPUS and PubMed) was conducted using the following combination of MeSH and keyword terms: coronary angiography, heart transplant, donation after circulatory death, DCD, OCS. The databases were queried from their inception until March 2022. To ensure comprehensive capture, an additional manual reference check of pertinent literature including recent review articles as well as article metric and citation analyses were performed to identify additional studies. Second, a retrospective analysis of all DCD cardiac allografts that underwent ex‐situ coronary angiography at our institution was performed. This study received institutional ethical review board approval (2022/ETH00580) and data collection was performed according to national and institutional guidelines. Pertinent procurement, transport and procedural data were retrieved from our database. Angiographic images were audited by two members of our interventional cardiology team (T. M. and M. H.) to determine prior angiographic views and angulations, which were subsequently reproduced and bench‐tested in an empty catheterization suite with an inactive TransMedics OCS. Data are presented descriptively, including percentages for categorical variables and means and standard deviation for normally distributed continuous variables, or median and interquartile range where nonnormally distributed. Comparisons were not performed owing to the small sample size.
3. RESULTS
Systematic literature review yielded 164 studies and after screening and duplicate removal, four reports were identified comprising three cases of ex‐situ coronary angiography on human donor hearts (two of which were from our institute), 7 , 8 , 9 and one on an experimental pig heart. 10 As of March 2022, our institute has performed 97 DCD retrievals, resulting in 74 successful DCD transplantations. We have performed eight cases of ex‐situ coronary angiography, all of which were conducted without complication or injury to the allograft. Donor and allograft details are outlined in Table 1; the two prior case reports from our center 7 , 9 have been included in the present case series.
Table 1.
Summary of globally reported ex‐situ coronary angiography cases including our institutional experience
| Case | Donor age (years) | Sex | BMI (kg/m2) | Smoker | Diabetes | HTN | HL | Functional WIT (min) | Asystolic WIT (min) | Time on OCS (min) | Fluoro time (s) | Angiographic findings | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case series | |||||||||||||
| 1 | 49 | M | 26.3 | Yes | No | Yes | No | NR | NR | 319 | 438 | Severe multivessel disease | Unsuitable (DBD research run) |
| 2 | 58 | M | 25.7 | No | No | No | Yes | NR | NR | 265 | NR | Mild (<30%) atherosclerosis | Transplanted (DBD) |
| 3 | 54 | M | 32 | No | No | Yes | Yes | 21 | 14 | 276 | 259 | Mild (<30%) atherosclerosis | Transplanted |
| 4 | 51 | M | 29.4 | No | No | Yes | Yes | 26 | 14 | 205 | 535 | Mild (<30%) atherosclerosis | Unsuitable on clinical grounds |
| 5 | 53 | F | 26 | No | No | No | Yes | 23 | 11 | 241 | NR | Minimal (<10%) atherosclerosis; separate origins of the LAD and LCx | Transplanted |
| 6 | 49 | M | 29.4 | Yes | No | Yes | No | 13 | 10 | 296 | 135 | Mild (<30%) atherosclerosis | Transplanted |
| 7 | 42 | M | 29.4 | Yes | No | No | No | 21 | 14 | 160 | 186 | Mild (<30%) atherosclerosis | Transplanted |
| 8 | 53 | F | 46.1 | Yes | Yes | Yes | Yes | 24 | 14 | 285 | 216 | Mild (<30%) atherosclerosis | Transplanted |
| Literature search | |||||||||||||
| Shibilsky et al. 10 | NA | NR | Porcine model—moderate LAD stenosis; IVUS feasible | NA | |||||||||
| Ghodisizad et al. 8 | 46 | M | 24 | NR | 37 | 570 | NR | Minor diffuse atherosclerosis | Unsuitable due to haemodynamic parameters |
Note: aWIT is the time from cessation of circulation in the donor to delivery of preservation flush to the donor heart, >15 min is a predictor for severe primary graft dysfunction and perioperative ECMO. Functional WIT, functional warm ischemic time is the time from systolic of <90 mmHg in the donor to delivery of preservation flush to the donor heart, an upper limit of 30 min is permitted for this timeframe.
Abbreviations: CVD, cardiovascular disease; ECMO, extra corporeal membrane oxygenation; HL, hyperlipidaemia; HTN, hypertension; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCx, left circumflex coronary artery; OCS, organ care system; NR, not reported/recorded.
Within our institutional case series, six have proceeded to successful transplantation; Case 1 was conducted in 2014 for research purposes only and was not destined for clinical use; transplantation for Case 4 was abandoned owing to palpable coronary disease, however, subsequent research angiography demonstrated mild nonobstructive coronary disease. Seventy‐five percent (n = 6) of donors were male, the mean age was 51 ± 4.1 years. Mean BMI was 30.5 ± 6.6 kg/m2, four (50%) were smokers, five (63%) had a history of hypertension, and five (63%) hyperlipidaemia. Only one donor was a diabetic (13%). The mean duration on the OCS was 4 h 15 min (±52 min, n = 8). The mean fluoroscopy time was 4 min 54 s (±156 s, n = 6).
To date, the only other reported case of human ex‐situ angiography was performed by Ghodisizad et al. 8 although angiography was successful, transplantation was precluded due to an adverse haemodynamic profile, perhaps due to prolonged support on the OCS.
3.1. The OCS and anatomical considerations
Donation after circulatory death donors, by definition, do not meet the criteria for declaration of brain death, and therefore require a unique procurement procedure involving WLS, and a subsequent variable progression to cessation of circulation before retrieval of donor organs. This process exposes the cardiac allograft to an unavoidable period of warm ischemia time (WIT) and concerns about the impact of this time period previously precluded utilization of donor hearts via this pathway. However, preclinical research demonstrated that the combination of pharmacological post‐conditioning 11 and normothermic ex‐situ perfusion of the donor allograft 12 permitted full recovery of DCD hearts exposed to a period of warm ischemia up to 30 minutes.
Although there are several methods for the retrieval of DCD allografts, the most commonly used is that of direct procurement with ex‐situ perfusion (DPP). Due to the significant insult WLS and the unavoidable WIT places on the DCD donor allograft, surgical technique in DPP retrieval focuses on the rapid delivery of the preservation flush to halt the detrimental effects of ongoing warm ischemia, followed by resuscitation and assessment on the OCS device. During organ procurement, 1.2–1.5 L of donor blood is collected, which is passed through a leukocyte depleting filter, and then added to the pump reservoir with 500 ml of a proprietary priming solution containing metabolic nutrients. Once the preservation flush, a modified St Thomas' cardioplegic solution containing pharmacological conditioning agents, is delivered to the donor heart, it is explanted and prepared for resuscitation on the OCS platform. The donor aorta as well as pulmonary artery are cannulated, and both the superior and inferior vena cavae are closed, to establish a “closed circuit” or right heart and coronary perfusion. A vent is introduced into the left ventricle (LV), via the open left atrium and secured. The heart is then connected to the OCS device and perfusion commenced. The heart is placed onto the OCS device in a “face down” position, with the base and posterior walls of the LV and RV visible on the left and right side of the OCS module, respectively (Figure 1).
Figure 1.
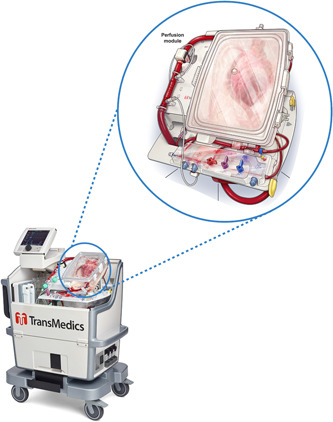
TransMedics organ care system. Zoomed window illustrates a beating donor heart mounted in the perfusion module. [Color figure can be viewed at wileyonlinelibrary.com]
A pulsatile pump delivers oxygenated blood from the reservoir to the ascending aorta in a retrograde fashion. In the setting of a competent, closed aortic valve the perfusate then flows into the coronary arteries in an anterograde fashion, before returning to the right atrium via the coronary sinus. Deoxygenated blood is then ejected by the right ventricle via the return pulmonary artery cannula (where a flow sensor monitors this volume and reports it onto the console as coronary flow). The blood is then passed through a low‐resistance membrane oxygenator and returned to the reservoir. 11 While on the OCS device, haemodynamic and metabolic parameters (mainly production and utilization of lactate) are monitored to assess the organ for transplantation.
3.2. Indications for angiography
Present guidelines recommend the consideration of donor coronary angiography (for brain death donors) in select cases, 4 including:
-
1.
Donor history of coronary artery disease (e.g., prior myocardial infarction).
-
2.
LV dysfunction on echocardiography (regional wall motion abnormalities on echocardiography; reduced left ventricular ejection fraction <45%).
-
3.
Known atherosclerotic risk factors (age > 50 years, BMI > 30 kg/m2, hypercholesterolemia, diabetes, smoking, cocaine use, and significant family history).
We propose that ex‐situ coronary angiography of the ex‐situ heart should be considered where there is significant concern for atherosclerotic coronary disease as examined by the retrieving surgeon, and in the presence of haemodynamic features demonstrated on the OCS consistent with obstructive coronary artery disease, such as persistently elevated mean arterial pressure. 13
3.3. Staffing and equipment
Angiography should be performed in a dedicated cardiac catheterization laboratory at the recipient hospital, with standard haemodynamic monitoring and radiographic capabilities. In addition to the interventional cardiologist, it is vital to have scrub and scout nurses, a radiographer as well as a cardiothoracic surgeon and perfusionist, who will accompany the OCS. Table 2 summarizes minimum recommended procedural equipment, usually included in standard angiography packs, illustrated as well in Figure 2. Exact equipment may vary depending on vendors and geographical location.
Table 2.
List of minimum recommended equipment
|
|
Figure 2.
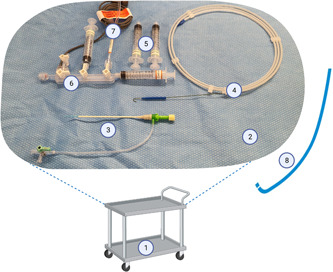
Example table setup. Equipment labels correspond to numbering in Table 1. Items 9–12 not pictured. Not to scale. [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Radiographic considerations
The OCS should be positioned centrally within the catheterization laboratory, usually necessitating removal or rotation of the patient table. The C‐arm should be positioned horizontally, equivalent to left anterior oblique 90° (Figures 3 and 4). We recommend positioning the OCS such that the posterior surface of the OCS rig (anterior surface of the ex‐situ heart) faces the detector (Figures 3 and 4), simulating the approximate spatial orientation achieved with a patient supine on the table—that is, the detector “in‐front” of the heart. The OCS should be positioned approximately 75 cm from the X‐ray tube, and as proximate to the detector as possible to reduce beam scatter. The ex‐situ heart must then be aligned with the beam iso‐center. We have found that the necessary floor elevation of the C‐arm when imaging an ex‐situ heart on the TransMedics OCS is approximately 85 cm, depending on the ex‐situ heart size. We recommend utilizing laser alignment, where available, to mitigate screening the heart into the correct position. A lead shield should be positioned between the OCS and the operator, who stands adjacent.
Figure 3.
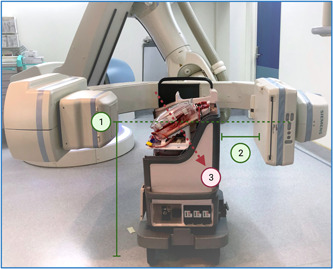
Spatial relationship of the OCS to the C‐arm. 1—The c‐arm should be angulated horizontally and elevated such that the ex‐situ heart sits within the iso‐center; 2—The distance between the detector and the posterior surface of the OCS should be minimized; 3—The ex‐situ heart sits prone in the OCS. OCS, organ care system. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4.
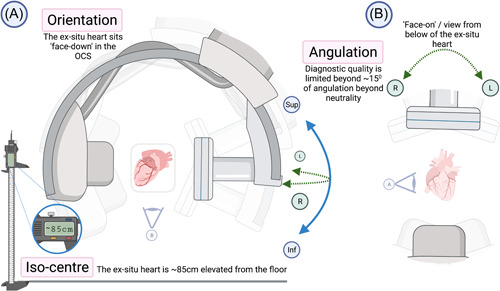
Positioning of the OCS in the angiographic suite. (A) The unscaled spatial relationship of the OCS to the C‐arm from the right‐hand side of the ex‐situ heart. (B) The lateral detector angulations at a view from below. OCS, organ care system. [Color figure can be viewed at wileyonlinelibrary.com]
3.5. Procedural method
Following appropriate positioning, the OCS Tuohy‐Borst valve should be prepared with sterile cleaning solution, before the application of a small fenestrated drape—we use our standard 1 m2 radial drape. The Tuohy‐Borst valve will not permit introduction of a standard 0.035″ J‐tipped guidewire alone, and so introduction of a vascular sheath into the valve is necessary first. It is preferable to use a 5‐Fr vascular sheath owing to the internal diameter of the OCS Tuohy‐Borst valve, however, use of a 6‐Fr sheath is possible. The sheath with its dilator is introduced directly, with great care, into the valve and this should be done with a bung or saline flush attached to the back end of the dilator to mitigate loss of perfusate (Figure 2, Label 3) through the dilator lumen. Following prompt removal of the dilator, the sheath is aspirated and flushed through the side‐port (Figure 5).
Figure 5.
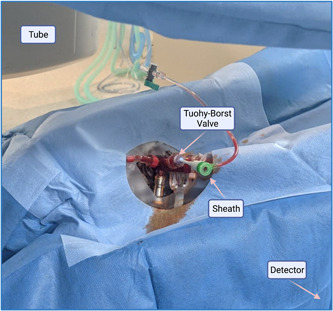
OCS covered in a sterile radial drape. Exposed is the Tuohy‐Borst valve, into which a vascular sheath has been inserted. OCS, organ care system. [Color figure can be viewed at wileyonlinelibrary.com]
Next, we recommend use of a 5‐Fr Judkins right (JR4, Cordis) coronary catheter, preloaded with a 0.035″ J‐tip guidewire, which are inserted a short distance into the sheath in the standard fashion, and the guidewire is advanced to the aortic root. Once the wire position is fluoroscopically confirmed, the catheter can be advanced, and the wire removed. Care should be taken to avoid crossing the aortic valve, which remains closed throughout the cardiac cycle. The catheter is then connected to the manifold, before aspiration and flushing. Although continuous intrinsic flow is provided by the OCS module, we recommend standard pressure transduction via a manifold, which also offers a practical and conventional method to aspirate, flush and administer contrast. The catheter may then be clocked, advanced, or retracted as needed to intubate the coronary ostia sequentially.
3.6. Angiography
It is very important to first consider how the geometry and fixation of the ex‐situ heart will influence image acquisition, lest time, radiation, and contrast be squandered with a trial‐and‐error approach. The ex‐situ heart sits “face‐down” on the OCS in an unloaded state, with the apex pointing directly inferiorly, such that a neutral detector position (Figure 5) equates to a right anterior oblique (RAO) cranial view. As such, to achieve the equivalent standard posterior‐anterior view of a supine “in‐situ” patient, compensation for this angulation (i.e., caudal and left‐anterior‐oblique angulation) is necessary. Table 3 summarizes our recommended angiographic views and their correspondence to standard coronary angiographic angulations.
Table 3.
Recommended angiographic views
| Detector angulation | Conventional view equivalent | Visualization adequacy | |||
|---|---|---|---|---|---|
| LMCA | LAD | LCx | RCA | ||
| Superior 15° | Steep RAO cranial (inverse spider) | ++ | + | + | + |
| Neutral 0° | RAO cranial | + | ++ | ‐ | + |
| Left 15° | PA cranial | + | ++ | + | ++ |
| Inferior 15° | PA | ++ | ++ | + | ++ |
Note: ++, optimal; +, reasonable; ‐, suboptimal.
Abbreviations: LAD, left anterior descending; LAO, left anterior oblique; LCx, left circumflex; LMCA, left main coronary artery; PA, posteroanterior; RAO, right anterior oblique; RCA, right coronary artery.
Additionally, much of the OCS componentry attenuates X‐ray to varying degrees, limiting the available acquisition windows. Specifically, we have found that angulation beyond 15° of neutrality in all planes results in progressively diminishing diagnostic quality and traditional caudal views are seldom possible. Therefore, we recommended commencing angiography in an anatomically favorable engagement position, then angulating the detector systematically to ensure comprehensive assessment of the major epicardial vessels.
A favorable engagement position is achieved with superior (cranial) angulation of the detector by approximately 15°, which yields the equivalent of a conventional steep RAO cranial view, in essence an “inverse spider” view, illustrated in Figure 6. This view nicely differentiates the coronary ostia to simplify catheter rotation, and despite aortic depth not being well appreciated in this axial view, this is obviated by starting the catheter low in the relevant cusp before retracting slowly until engagement is achieved. This view also provides good visualization of the proximal epicardial vessels.
Figure 6.
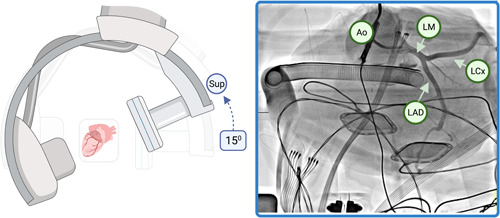
Example of superior detector angulation. Superior (further cranial) angulation of the detector, without lateral angulation, yields an equivalent steep RAO cranial appearance of the left coronary, in essence an “inverse spider.” Ao, aorta; LAD, left anterior descending artery; LCx, left circumflex artery; LMCA, left main coronary artery; RAO, right anterior oblique. [Color figure can be viewed at wileyonlinelibrary.com]
We have found that the JR4 catheter alone is sufficient to image both the right and left systems, as the straightening of the natural aortic curvature due to fixation in the OCS combined with the enhanced effect of gravity mitigates the need for conventional catheters shaped for left coronary engagement. It is not mandatory to always engage the left coronary system first, and the serendipitous engagement of either main coronary ostia should not be misspent. As previously stated, the catheter should not cross the aortic valve and left ventriculography is unnecessary, as the LV is vented and does not eject while on the OCS. Figure 7 demonstrates a comprehensive angiographic acquisition of the right coronary artery with lateral detector angulation yielding the equivalent straight cranial view of a supine patient.
Figure 7.
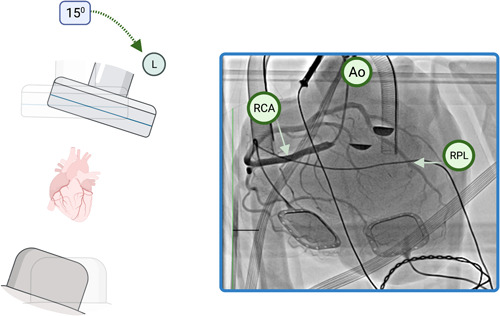
Example of lateral detector angulation. Pure left lateral angulation of the detector (left 15°) during angiography of the right coronary artery, yielding an equivalent straight cranial view. Contrast hang‐up can be seen at the base of the aortic cusps (star). Ao, aorta; RCA, right coronary artery; RPL, right posterolateral branch. [Color figure can be viewed at wileyonlinelibrary.com]
With respect to contrast administration, we use a low‐concentration, nonionic, water‐soluble, iodinated radiographic contrast medium (Omnipaque™; GE Healthcare). The approximate coronary blood flow can be calculated from invasive indices measured by the perfusion module, to then determine the minimum injection volumes and rates. As with standard angiography, we recommend using the lowest possible volume of contrast to opacify the vessels to prevent visible myocardial staining and minimize transient ischemia. Contrast is rapidly cleared through the coronary circulation, however, remains diluted within the perfusate. We have not noted any haemodynamic or metabolic abnormalities following contrast administration, and fortunately the transition to transplantation is usually very rapid once the coronary anatomy is confirmed.
3.7. Transplantation considerations
Following the acquisition of diagnostic images, the findings should be discussed with the transplantation team. The presence of significant multivessel coronary atherosclerosis is generally accepted as a contraindication to transplantation. 6 However, concomitant revascularisation with coronary artery bypass grafting has been demonstrated previously, 14 and may be considered for isolated single vessel disease. Musci et al. report a case of angioplasty and stenting during angiography of a brain‐death donor to facilitate transplantation, but to our knowledge there have been no attempts made at ex‐situ coronary intervention.
3.8. Limitations
Our recommendations herein are based on experience derived from eight live procedures, in addition to bench testing, at a single transplant center. There exists only one other external report of angiography of the ex‐situ human heart. We have attempted to outline broad foundational principles to this unique procedure, recognizing that variability in equipment and resources will exist between transplant centers. We anticipate, and hope, these recommendations will evolve with time as international experience improves.
4. CONCLUSIONS
We have described a safe and feasible method for diagnostic coronary angiography of the ex‐situ beating donor heart, as well as its novel utility in mitigating subsequent transplantation of unsuitable allografts. In the setting of suspected coronary atherosclerosis of the donor heart, which may preclude favorable transplantation outcomes, ex‐situ coronary angiography should be considered at eligible transplant centers.
5. PERSPECTIVES
The demand for heart donors continues to grow. Excluding preclusive coronary artery disease in marginal DCD donors is ethically and logistically challenging, but may be facilitated with ex‐situ coronary angiography. There exist only scattered case reports of this procedure, and no published description of procedural technique. We demonstrate safety, feasibility, and novel utility of this procedure together with our institutional technical recommendations (Central Illustration). Further refinement of the procedural technique is expected with increasing global implementation.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors would like to thank the Heart Transplantation Team at St Vincent's Hospital, Sydney, the Cardiac Transplantation Laboratory at the Victor Chang Cardiac Research Institute, Claude Soto for his cardiac perfusion expertise and the provision of a test OCS for bench testing in our cardiac catheterization suite, Matthew Cameron and Sean Hull for their interventional radiographic expertise. Figures created with BioRender.com. Dr. Meredith receives PhD scholarship funding from The Cardiac Society of Australia and New Zealand. Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
Meredith T, Scheuer S, Hoffman M, et al. Coronary angiography of the ex‐situ beating donor heart in a portable organ care system. Catheter Cardiovasc Interv. 2022;100:1252‐1260. 10.1002/ccd.30455
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex‐vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385(9987):2585‐2591. [DOI] [PubMed] [Google Scholar]
- 2. Messer S, Cernic S, Page A, et al. A 5‐year single‐center early experience of heart transplantation from donation after circulatory‐determined death donors. J Hear Lung Transplant. 2020;39(12):1463‐1475. [DOI] [PubMed] [Google Scholar]
- 3. Duke University School of Medicine Newsroom . Doctors at Duke University Hospital Perform First DCD Heart Transplant in U.S. Duke Department of Surgery [Internet]. 2019. Accessed March 29, 2022. https://surgery.duke.edu/news/doctors-duke-university-hospital-perform-first-dcd-heart-transplant-us
- 4. Grauhan O, Siniawski H, Dandel M, et al. Coronary atherosclerosis of the donor heart—impact on early graft failure. Eur J Cardiothorac Surg. 2007;32(4):634‐638. https://academic.oup.com/ejcts/article/32/4/634/493718 [DOI] [PubMed] [Google Scholar]
- 5. Grauhan O, Wesslau C, Hetzer R. Routine screening of donor hearts by coronary angiography is feasible. Transplant Proc. 2006;38(3):666‐667. https://europepmc.org/article/med/16647438 [DOI] [PubMed] [Google Scholar]
- 6.TSANZ. Clinical Guidelines for Organ Transplantation from Deceased Donors The Transplantation Society of Australia and New Zealand Produced in partnership with [Internet]. 2021. Accessed March 2022. https://tsanz.com.au/storage/documents/TSANZ_Clinical_Guidelines_Version-15_29042021.pdf
- 7. Chris A, Jonathan M, Marino C, et al. Ex vivo coronary angiographic evaluation of a beating donor heart. Circulation. 2014;130(25):e341‐e343. https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.114.010289 [DOI] [PubMed] [Google Scholar]
- 8. Ghodsizad A, Bordel V, Ungerer M, Karck M, Bekeredjian R, Ruhparwar A. Ex vivo coronary angiography of a donor heart in the organ care system. Hear Surg Forum. 2012;15(3):161‐163. [DOI] [PubMed] [Google Scholar]
- 9. Nadel J, Scheuer S, Kathir K, Muller D, Jansz P, Macdonald P. Successful transplantation of high‐risk cardiac allografts from DCD donors following ex‐vivo coronary angiography. J Hear Lung Transplant. 2020;39(12):1496‐1499. https://www.jhltonline.org/article/S1053-2498 [DOI] [PubMed] [Google Scholar]
- 10. Schibilsky D, Zhou Q, Wengenmayer T, et al. Coronary angiography and intravascular ultrasound in an ex‐vivo perfused heart using the organ care system (OCS). J Hear Lung Transplant. 2019;38(4):S271. [Google Scholar]
- 11. Iyer A, Gao L, Doyle A, et al. Increasing the tolerance of DCD hearts to warm ischemia by pharmacological postconditioning. Am J Transplant. 2014;14(8):1744‐1752. https://onlinelibrary.wiley.com/doi/10.1111/ajt.12782 [DOI] [PubMed] [Google Scholar]
- 12. Iyer A, Gao L, Doyle A, et al. Normothermic ex vivo perfusion provides superior organ preservation and enables viability assessment of hearts from DCD donors. Am J Transplant. 2015;15(2):371‐380. https://onlinelibrary.wiley.com/doi/10.1111/ajt.12994 [DOI] [PubMed] [Google Scholar]
- 13. Vela MM, Sáez DG, Simon AR. Current approaches in retrieval and heart preservation. Ann Cardiothorac Surg. 2018;7(1):67‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Musci M, Pasic M, Grauhan O, et al. Orthotopic heart transplantation with concurrent coronary artery bypass grafting or previous stent implantation. Z Kardiol. 2004;93(12):971‐974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.


