Abstract
Aim
A stoma exposes patients to several complications which could impair their quality of life (QoL). In the last decade, the market for stoma therapy in France has evolved, with a significant increase in the activities of home health providers, meeting a need for patient follow‐up and companionship. International studies have demonstrated the impact of the stoma therapist (ST) follow‐up on the improvement of an ostomy patient's QoL. However, the impact of home stoma nurse management has not been analysed. In this context we would like to assess the added value on health‐related QoL from the enhanced follow‐up of ostomy patients by STs.
Methods
This is a randomized, controlled, open, national and multicentre trial (12 centres) which includes patients with an ostomy who benefit from either standard follow‐up or from an enhanced and personalized follow‐up with, in particular, regular consultations with an ST after discharge. The primary end‐point is the 3‐month QoL score obtained from the Stoma‐QoL questionnaire. The secondary end‐points are satisfaction of the care, comparison of QoL scores (Stoma‐QoL and EuroQuol EQ‐5D) and the economic gains by calculating the consumption of resources between the two arms. There will be a modified intention‐to‐treat analysis with 6‐month follow‐up in both study arms.
Discussion
The StomaCare trial will be the first randomized controlled study in France to evaluate the impact on QoL of an enhanced follow‐up at home of ostomy patients by an ST.
Keywords: home health providers, quality of life, randomized, stoma, stoma therapist, study protocol
The content of all this protocol is described according to relevant items of the SPIRIT checklist (Standard Protocol Items: Recommendations for Interventional Trials) and numbers in braces in this protocol refer to SPIRIT checklist item numbers [1].
ADMINISTRATIVE INFORMATION
Trial registration {2a}: ClinicalTrials.gov NCT05076669
World Health Organization Trial Registration Data Set {2b}, Table 1
TABLE 1.
World Health Organization trial registration dataset
| Primary registry and trial identifying number | ClinicalTrials.gov NCT05076669 |
| Date of registration | 13 October 2021 |
| Secondary identifying numbers | FSK‐001 |
| Material support and sponsor | FSK |
| Sponsor's contact | Ingrid Kuhn; +33 7 61 47 98 25; i.kuhn@fsk.fr |
| Public title | Quality of life impact after enhanced follow‐up of ostomy patients |
| Scientific title {1} | Quality of life impact after enhanced follow‐up of ostomy patients by a home healthcare nursing service employing stomal therapy nurse consultants compared with conventional care: study protocol for a multicentre, open, randomized, controlled trial |
| Countries of recruitment | France |
| Health conditions or problems studied | Home healthcare, stomal therapy consultants, ostomy patients, quality of life |
| Intervention |
|
| Key inclusion criteria |
|
| Key exclusion criteria |
|
| Study type |
Interventional Allocation: randomization Intervention model: parallel assignment Open‐label Primary purpose: treatment National and multicentre study Minimal risks and constraints to routine care |
| Date of first enrolment | August 2023 |
| Target sample size | 350 participants |
| Recruitment status | Recruiting |
| Primary outcome | Efficacy (time frame 3 months) based on Stoma‐QoL specific questionnaire |
| Key secondary outcomes |
|
Protocol version {3}: First version 1.0
Funding {4}: Promoter FSK
Authors' contribution {5a}:
All authors contributed to the conception and design of the trial. CdP, MR and JHL drafted the manuscript; all the investigators provided critical revision to the clinical and intellectual content. CdP wrote the study protocol that was reviewed by JHL. DV provided statistical expertise in clinical trial design. Lastly, all authors approved the final manuscript.
Sponsor contact information {5b}
Mrs Ingrid Kuhn
Phone number: +33 7 61 47 98 25
Email: i.kuhn@fsk.fr
Role of study sponsor and funder {5c}
FSK is a home health provider (HHP). It is the sponsor of the study as well as the provider involved in the delivery of the equipment and the follow‐up of the patients through the stoma therapists (STs) and the consultants. As the owner of the data, no use of them can be made without the sponsor's agreement, but the design of the study, the management and analysis of the data are outsourced to an independent company (see below). The sponsor reserves the right to discontinue the trial due to non‐inclusion but no changes to the study can be proposed without a joint decision by the sponsor and the coordinating investigator. The sponsor is also responsible for the conduct of the trial, including insurance coverage and reporting of serious adverse events and material vigilance.
Committees {5d}
The scientific committee is composed of the coordinating investigator (Professor Jérémie H Lefèvre), the scientific manager (Professor Morgan Roupret), a stoma nurse (Liliane Jacob), a statistician (Dewi Vernerey), a patients' association (Association Francois Aupetit) and a clinical data manager (Aurélia Meurisse).
Steering committee: The implementation of the study will be realized by the company Sêmeia who will be responsible for the overall management of the study, operational interaction with the investigator centres, the electronic case report form (e‐CRF), the design and implementation of the data management plan and analysis.
In each investigator centre a senior lead will be identified and responsible for identification, giving information, obtaining consent, recruitment, data collection and completion of the e‐CRF.
In view of the low risks identified above, it was decided not to set up a supervisory committee.
INTRODUCTION
Background and rationale {6a and 6b}
France currently has about 80 000 ostomy patients, 88% of whom have gastrointestinal stomas and 12% urinary stomas [2]. Stoma creation exposes patients to several complications which could impair their quality of life (QoL) [3, 4].
The management of ostomy patients varies between institutions according to how the patient's medical equipment is supplied and whether or not specialized personnel are available. In the last decade, the market for stoma therapy in France has evolved, with a significant increase in the delivery of equipment by HHPs, meeting a need for patient follow‐up and companionship. This increased preference of HHPs to use specialist nurses seems to be explained by the added benefits. Early international studies have demonstrated the impact of ST follow‐up on the improvement of ostomy patients' health‐related QoL [5, 6, 7, 8, 9]. However, none of the studies available on follow‐ups performed by STs included the French population. Furthermore, the studies show methodological gaps, limited time spans and are based on hypotheses. Finally, in most of these studies, follow‐up by an ST consisted mainly of visits while in hospital or during dedicated consultations, but few studies looked at home follow‐up by an ST.
Objectives {7}
The main objective of this trial is to demonstrate the effectiveness on the QoL of an enhanced follow‐up of ostomy patients by involving an HHP with an ST.
The main secondary objectives are as follows:
to evaluate patients' satisfaction;
to analyse the impact of this management on QoL in different targeted subgroups (randomization criteria, temporary or permanent stomas, planned or unplanned surgery);
to compare health‐related QoL longitudinally between the two study arms;
to investigate clinical and/or demographic factors associated with QoL in ostomy patients;
to evaluate the financial cost (equipment, products, hospitalization, medication, consultations etc.) of such enhanced follow‐up;
to assess the rate of rehospitalization for ostomy‐related complications.
Trial design {8}
It is an interventional, randomized, controlled, open‐label, national and multicentre superiority trial with minimal risks and constraints to routine care.
METHODS: ASSIGNMENT OF INTERVENTIONS
Allocation {16}
Randomization (generated by CleanWeb™) will be balanced with a 1:1 ratio between the parallel arms: interventional versus control. It will be realized by minimization and according to (i) the centre, (ii) the type of stoma (ileostomy, colostomy and urostomy/mixed stoma), (iii) the ostomy indication, (iv) gender and (v) the Stoma‐QoL score at inclusion divided into four groups: [0–25], [25–50], [50–75], [75–100].
In order to reinforce the random effect and prevent anticipation of the next allocated group, in 20% of cases the software does not use the minimization algorithm and allocates the treatment completely randomly.
Blinding/masking {17}
Given the nature of the intervention, the study necessarily will be open‐label. The situation in which the evaluation is conducted by telephone and not by email (see explanations below) will be an exception because in this case the third party collecting the information will be blind to the patient's randomization arm.
METHODS: PARTICIPANTS, INTERVENTIONS, OUTCOMES
Study setting {9}
This is a French national multicentre study, with 12 investigating centres throughout the country (Table 2). These centres will have both gastrointestinal surgery and urology departments and create stomas regularly. They are all expert centres, both public and private establishments.
TABLE 2.
Investigator centres listed
| Hospital | Department | Referent investigator | Referent stoma nurse |
|---|---|---|---|
| Saint Antoine Hospital, AP‐HP, Paris | Digestive surgery | Pr Jérémie H Lefèvre | Anne Tripon, Dominique Tincelin, Jeanne Sixdenier |
| Pitié‐Salpêtrière Hospital, AP‐HP, Paris | Urology | Pr Morgan Roupret | Axelle Pierre‐Joseph |
| Bicêtre Hospital, AP‐HP, Kremlin Bicêtre | Digestive surgery | Pr Antoine Brouquet | Corinne Bonneau, Aurélie Courcol |
| Saint Louis Hospital, AP‐HP, Paris | Digestive surgery | Pr Léon Maggiori | Amandine Toutain |
| Georges Pompidou Hospital, AP‐HP, Paris | Urology | Dr François Audenet | Laurence Philibert |
| Foch Hospital, Suresnes | Digestive surgery | Dr Frédéric Kanso | Elsa Loscot, Sandrine Decamps, Liénor Rafii, Sydonie Baba |
| Foch Hospital, Suresnes | Urology | Pr Yann Neuzillet | |
| Lyon‐Sud Hospital, Lyon | Digestive surgery | Pr Eddy Cotte | Arianne Deluga |
| Rangueil Hospital, Toulouse | Urology | Dr Mathieu Roumiguié | Carine Humbert |
| CHU, Nantes | Digestive surgery | Pr Guillaume Meurette | Agnès Deschamps, Magalie Pottier, Christelle Cathy‐Lemoine |
| CHU Tenon, Paris | Urology | Pr Véronique Phé | Laeticia Quenault |
| Clinique Saint‐Augustin, Bordeaux | Urology | Dr Nam‐San Vuong | Katia Cousin |
| Clinique Esquirol St‐Hilaire, Agen | Urology | Dr Xavier Cuvillier | |
| CHU Tours | Digestive Surgery | Pr Mehdi Ouaissi | Valérie Desvilettes Emilie Houssier |
In addition, data on the management of ostomy patients on discharge from hospital by the investigating centres will be collected: the presence of an ST in the department, organization and number of dedicated ST consultations after returning home, referral of patients to an HHP or pharmacy on discharge and type of discharge (home, hospital etc.).
The study design was defined to meet the outcomes as objectively as possible while ensuring benefit for the patients. This is based on the experience of each of the authors of this study, and the scientific committee whose objective was to consider the complaints and questions of patients in their clinical practice.
Eligibility criteria {10}
The study population will consist of ostomy patients. The study will be proposed by the investigator to patients in the month before the stoma is created and up to 10 days after an operation so as to include emergencies. Patients must provide written, informed consent (see Appendix A).
Inclusion criteria are (i) aged ≥18 years, (ii) patients with a temporary or permanent ostomy made less than 10 days before or (iii) during the current hospitalization (iv) performed as part of emergency or planned surgery, and (v) patients informed orally and in writing via the information sheet and having signed the informed consent.
Exclusion criteria are (i) palliative care patients, (ii) patients participating in another clinical study concerned with ostomy care, (iii) patients not affiliated to a social security regime or to the French universal health insurance (CMU), (iv) patients under guardianship or curatorship, (v) patients deprived of their liberty (prison or psychiatric care without consent) and (vi) patients with difficulty in understanding or reading French.
Interventions: procedure for patients in the interventional group {11a}, Figure 1
FIGURE 1.
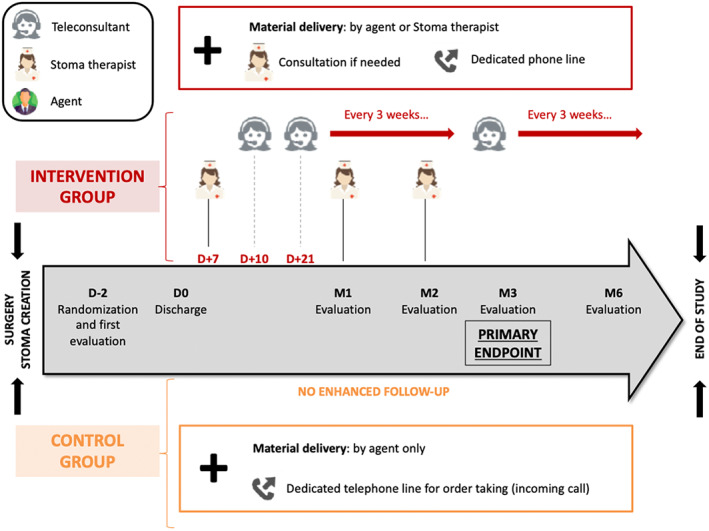
Flow chart of the protocol
The HHP FSK personalized support system mobilizes three types of personnel:
The agent makes the first delivery of equipment to the hospital or patient's home or another place chosen by the patient. They will be in contact with the prescribing surgeon, to whom they will hand over, in an envelope, the reports produced by the ST following a consultation with the patient.
The teleconsultants provide telephone support to patients and are of two types: first, the teleconsultants in the central office who only make outgoing calls to check on patients regularly, help with the ordering and delivery of equipment and request STs if necessary; second, the regional teleconsultants who handle incoming calls from patients to ensure the coordination of the patients' or the central office's requests with the various regional activities of the ST.
The FSK ST advisors carry out initial deliveries of equipment, advise the patient on the use of medical equipment and the various treatments, provide liaison with the nursing staff and in particular the home nurses, and carry out physical or virtual consultations proactively or at the request of the patient.
First phase: Delivery of the equipment that will be carried out by an agent or by an ST and will be accompanied by a check of the equipment, an explanation of the services and the provision of a guide to good practice.
Second phase: Continuous and personalized patient support, based on regular telephone follow‐up by central patient relations teleconsultants, physical or virtual consultations (via EasyConsult™) and a patient service for handling incoming calls. The telephone follow‐up by the teleconsultants will be done on days 10 and 21 and then every 3 weeks, to review the equipment and answer any questions. The ST will carry out physical or virtual consultations on day 7 and at 1 and 2 months. Each consultation (including additional consultations requested by the patient) will result in a report being written and sent to the prescribing doctor. Videos will not be recorded in order to maintain privacy.
Interventions: procedure for patients in the control group {11a}, Figure 1
First phase: Delivery of the equipment to the patient's home, hospital or another place chosen by the patient. This delivery will be carried out only by an agent and will be accompanied by a check of the equipment and the provision of a phone guide regarding renewing equipment orders.
Second phase: Absent. However, there is a phone line that the patient can use to contact the HHP to reorder or change the location of the equipment delivery. Any advice given by telephone will be limited by the competence of the online contact person. In the case of need or problems with equipment and devices, the patient will be asked to contact their healthcare facility again or call for a visit by a nurse at home.
Modifications {11b and 25}
The sponsor is authorized to modify the protocol, in consultation with the coordinating investigator. If necessary, a request for substantial amendments is sent to the Comité de protection des personnes (CPP) (ethics committee) for approval. On receipt of a favourable assessment, the amended version of the protocol will then be sent by the sponsor to all the investigators.
Substantial amendment is defined as an amendment that has a significant impact on any aspect of the research (protection of individuals, conditions of validity of the research, quality and safety of the products tested, interpretation of the scientific documents, procedures for conducting the research), whereas a non‐substantial amendment is a minor amendment or clarification with no impact on the conduct of the trial, and will not be submitted to the competent authorities. This will be agreed between the sponsor and the investigator and clearly documented.
The criterion for premature cessation of the trial is early achievement of the recruitment target. The study may also be terminated by the sponsor due to poor recruitment or by a joint decision of the competent authority, the sponsor and the coordinating investigator.
Adherence {11c}
First, we expect to have good adherence by the participants given the low compliance burden and the low risk to the patients in this study. Indeed, the objective of this study is to evaluate an additional service delivered by an HHP as a complement to current practice and not as a substitute for standard care. Nevertheless, to ensure optimal adherence, several elements are proposed:
Comprehensive information about the research protocol at the time of inclusion with all relevant documents.
Patients in both groups benefit from an ostomy equipment delivery service and a dedicated hotline provided by FSK in addition to what is usually available to them on their return home (home nurse, consultation with general practitioner, consultation with an ST).
In addition, in the interventional group, continuous and comprehensive support will be provided both for the supply of equipment and for the provision of nurse/ST advisors.
To facilitate the fluidity of responses to the questionnaires, they will be sent by email directly to the patient. This avoids the inconvenience of attending consultations or using the postal service. In addition, phone follow‐ups will be carried out by a third party who has no knowledge of the assignments to the different arms in order to follow up patients who have not responded to the questionnaires or who are reluctant to use digital tools.
Concomitant care {11d}
Given the low‐constraint design of the study, the management of both arms by the same sponsor FSK, and the consideration of major bias in the randomization time, few constraints will be given to the patients. The only clearly identified constraint will be the inability to use any HHP other than FSK for the whole study.
Outcomes {12}
The primary outcome is the 3‐month QoL score obtained from the Stoma‐QoL health‐specific QoL questionnaire [10]. A score on a scale of 20–80 will be obtained after adding the answers to the 20 questions asked with four response modalities: all the time (1 point), sometimes (2 points), rarely (3 points), never (4 points). This score will then be converted to a scale of 0 (worst possible score) to 100 (best possible score).
The secondary outcomes are as follows:
the satisfaction of care evaluated by a continuous score (between 1 and 10), at 1, 2, 3 and 6 months;
QoL scores from the Stoma‐QoL questionnaire at inclusion, 1, 2, 3 and 6 months and from the EuroQuol EQ‐5D‐5L questionnaire at 1, 2 and 3 months [10, 11];
the consumption of resources (equipment, products, rehospitalization, drugs, consultations etc.) and comparing overall and specific costs for each expenditure category between the two arms based on standardized and published price references [12];
the rate of rehospitalization for complications related to the stoma.
All the proposed outcomes were agreed collectively, taking into account relevant and validated criteria for the patients and the paramedical staff. These criteria are in line with a desire to respond to the complaints and wishes expressed by patients having an ostomy. They were validated by a scientific committee including a stoma nurse and a patients' association (Association Francois Aupetit, AFA) represented by Eric Balez.
Participant timeline {13}, Table 3
TABLE 3.
Study period of the protocol
| Screening | Randomization | Post‐allocation | |||||
|---|---|---|---|---|---|---|---|
| Time point | D‐10 a | Postoperative time | M1 | M2 | M3 | M6 | In case of SC b |
| Enrolment | |||||||
| Eligibility screen | × | ||||||
| Informed consent | × | ||||||
| Administrative and baseline data | × | ||||||
| Allocation | × | ||||||
| Interventions | |||||||
| Intervention group |

|
||||||
| Control group |

|
||||||
| Assessments | |||||||
| Stoma‐QoL questionnaire | × | × | × | × | × | × | |
| EQ‐5D‐5L questionnaire | × | × | × | × c | |||
| Rate of rehospitalization d | × | × | × | ||||
| Patients' satisfaction | × | × | × | × | × | ||
| Cost evaluation | × | × | × | ||||
| Location of patient (hospital, home etc.) | × | × | × | × | × | × | |
| How to recover the medical material | × | × | × | × | × | ||
| Possible assistance in providing care | × | × | × | × | × | ||
| Relationship with the health provider | × | × | × | × | × | ||
| Relationship with the ST | × | × | × | × | × | ||
| Control of the material by the patient | × | × | × | × | × | ||
Abbreviations: D‐10, 10 days before surgery; QoL, quality of life; SC, stoma closure; ST, stoma therapist.
Stoma made less than 10 days ago or during the current hospitalization.
Between day − 2 and day + 2 from stoma closure.
Only before 3 months.
Rehospitalization due to a stoma problem.
Sample size {14}
Randomization of 178 evaluable patients is required (89 in each arm) to demonstrate a mean difference of 5 points on the Stoma‐QoL [10] score between the two arms, using a two‐sided alpha risk of 5% with a statistical power of 90% and considering a standard deviation of the Stoma‐QoL score of 10.2 [10]. An intermediate analysis using the alpha risk expenditure function with the Lan–Demets method (O'Brien–Fleming limits) is planned at 50% of the information fraction (89 randomized patients) [13].
From the 2019 FSK data (not published), the expected dropout rate at 3 months (death, reinstatement, lost to follow‐up) is a weighted average of 43.26%. In order to compensate for these premature discontinuations as well as patient‐requested study exits, a margin of 49.11% will be applied to the number of patients needed. To this number, we will also take into account the number of incomplete questionnaires returned. So, the number needed to treat is estimated at 350 patients.
Data collection {18}
The patients will be recruited from the investigating centres after obtaining their consent. After the surgery, the investigators will collect the following information: patient characteristics, demographic information, stoma history and Stoma‐QoL questionnaire. These elements will be necessary for randomization. The Stoma‐QoL will be filled by the patient after stoma creation and just before discharge.
Subsequently, during follow‐up, the QoL questionnaire EQ‐5D‐5L will be asked at 1, 2, 3 months and between 2 days before and 2 days after stoma closure in the event that this will be achieved within 3 months. All other data will be collected at 1, 2, 3, 6 months and between 2 days before and 2 days after stoma closure (Table 3).
Patients in both arms will receive the questionnaire via a web link that will be sent to them by email. It is hoped that an automatic reminder will maximize the response rate. In addition, follow‐ups by phone will be carried out by a third party who will be blinded to the assignments in order to follow up patients who have not responded to the questionnaires or who are resistant to the digital tools.
A generalized linear mixed model that can include observations with missing data will be included in the analysis. For tests of significance of differences at 1, 2 and 3 months, only the data available for these dates will be used, so incomplete data will be excluded from these tests.
Data management {19, 21, 23 and 29}
An e‐CRF will be used for this study and will only be accessible to authorized persons via a secure internet connection with a login and password. An e‐CRF will be completed for each patient included in the study. Data will be collected via the CleanWeb™ application—electronic clinical trial management solution from ‘Telemedicine Technologie’ society. This solution meets the requirements of the various Best Practices in Clinics (BPC), International Conference on Harmonisation (ICH), 21 Case Report Form (CFR) part 11 (FDA) regulations in terms of identification, authentication, traceability, data flow encryption and data hosting.
Data entry on the e‐CRF will be in accordance with the directions provided in the instructions. It is the investigator's responsibility to ensure that the e‐CRF is completed, reviewed and approved. Once the pages have been entered and monitored, the investigator will sign them and be responsible for all data entered. All changes to the case report form will be recorded in an audit trail file. Patients will complete the questionnaires via the Cleanweb ePRO system. They will receive a link to enter their answers by email at the different measurement times. The patients' email addresses will be stored in an ‘administrative’ database which will be destroyed when the study is deemed complete by the data manager. This ‘administrative’ database will be hosted on servers different from the data collected via the e‐CRF. Once entered, the data will be reviewed by the clinical research officer mandated by the sponsor and/or the data manager.
Data verification and validation will be carried out according to the data validation plan established for the study. The database freeze will be decided by mutual agreement between the study statistician, the principal investigator and the project leader after a review of the data.
The investigator undertakes to accept checks by the sponsor (monitor and/or auditor) or by the inspector of the competent administrative authority. He guarantees access to the source data (medical records, computer files, study documents etc.).
Statistical analysis {20}
Statistical analysis will be performed according to the modified intention‐to‐treat principle, that is, an intention‐to‐treat population with at least one baseline Stoma‐QoL questionnaire. All statistical analyses will use two‐tailed tests and P ≤ 0.05 will be considered statistically significant.
The variables measured at inclusion will be described, for all patients and in each group, by percentages for the qualitative variables and by the minimum, maximum, means, standard deviations and medians for the quantitative variables. To compare the two groups, a χ 2 test (or Fisher's exact test depending on sample size) for categorical variables will be used and a Student's t test for continuous variables. We will use their non‐parametric equivalent (Wilcoxon or Kruskal–Wallis test) when the conditions of application are not respected.
Statistical analyses of the data will be carried out using R version 3.6.1 and SAS version 9.4.
Harms and adverse events {20}
The present protocol does not influence the prescription of stoma equipment which will have been freely prescribed by the investigating physician at the time of the patient's inclusion in the study. Thus, the collection of adverse events and any new information that could influence the assessment of the benefit/risk balance follows the European regulations related to material vigilance.
During the study, the consulting STs and client relationship teleconsultants will complete the material vigilance data file with the patient and send it to the investigator and to FSK (materiovigilance@fsk.fr). The investigator will be responsible for declaring a vigilance incident on the official website (www.signalement‐sante.gouv.fr) and for reporting the incident to the medical device producer. In the event that the internet website is out of order, an official CERFA form no. 10246 will be completed and sent to the relevant regional administrative authority. A copy of this form will be archived and kept for at least 5 years. Finally, a back‐up of all material will be ensured which will itself be quarantined.
Any unexpected death or serious incident or risk of incident related to stoma equipment will be notified to the relevant ethical committees (CPP) by FSK in a timely manner.
ETHICS AND DISSEMINATION
Research ethics approval {24}
Authorizations from the ANSM (Agence Nationale de Sécurité du Médicament et des produits de santé) and CPP were obtained, approval number ID‐RCB: 2021‐A00616‐35.
Authorization from the CNIL (Commission nationale de l'informatique et des libertés) was also obtained, registration number 2221807.
Consent to publication {26a}
Patients provide written, informed consent signed after receiving clear and informed advice.
Confidentiality {27}
All study information will be stored securely in restricted areas. All data will be coded with a unique identification number to maintain participant confidentiality (i.e., data management). Participants' study information will not be released outside of the study without the written permission of the participant.
Conflict of interest {28}: None to declare.
Dissemination policy {31}
FSK will own the data and no use or transmission to a third party will be made without its prior agreement. The scientific integrity of the project requires that the data from all centres be analysed study‐wide and reported as such. The results will be reported in a publication and submitted to a refereed journal with an editorial board. The rank of authors is defined in advance: JHL, DV, PI, MR according to the number of included patients.
DISCUSSION
This paper describes a protocol for a pilot randomized controlled trial that aims to evaluate the impact of enhanced monitoring of ostomy patients by an HHP, involving an ST, on the improvement of patients' health‐related QoL at 3 months compared with conventional follow‐up.
Every year in France 16 000 new stomas are performed. The main indications are malignancy (50% of cases), inflammatory and infectious (inflammatory bowel diseases, diverticulitis, colitis etc), traumatic, congenital (Hirschprung disease) and genetic (familial adenomatous polyposis). The frequency of complications in ostomy patients is important and varies from 10% to 80% [14, 15, 16, 17]. There are early complications (necrosis, retraction, stenosis with obstruction, bleeding and haematoma, abscesses etc.) [18]. Late complications mainly include peristomal hernia (0–48%) [19], stomal prolapse (10%–20% of colostomy) [20], high output (notably with ileostomy) and peristomal skin complications [21, 22] which affect up to a third of colostomies and two‐thirds of ileostomies and urostomies [23] and represent, along with stoma leakage, the earliest complaints by patients. Finally, nearly 30% of ostomy complications will require a surgical intervention. Ostomy management and care are therefore essential elements in the prevention of complications and in improving QoL [24].
Several studies have shown a relationship between the presence of a stoma and a reduced QoL [25, 26, 27]. For example, a study of 391 ostomy patients found that 80% of patients experienced some change in lifestyle, 40% had an alteration to their sex lives, between 35% and 45% of patients had significant anxiety about their stoma and 25% reported being ashamed [28, 29]. Neil et al. showed that each peristomal complication avoided yielded, on average, eight additional quality‐adjusted life days over 1 year [30].
Many international studies have demonstrated the impact of follow‐up by an ST on the improvement of the QoL of an ostomy patient. In fact, STs play a fundamental role in the education and empowerment of the ostomy patient, in better management and prevention of complications, and in providing psychological support, as demonstrated by Becker's study where 89.3% of ostomates consider that STs are crucial and 70.3% claim to live better with their ostomy thanks to them [6]. The Dialogue Study is a multicentre, open and non‐comparative study conducted in North America on 743 patients. QoL was assessed using the Stoma‐QoL scale and the condition of the peristomal skin was assessed with the ostomy skin tool. Erwin‐Toth et al. observed a 2.1‐point increase in the QoL score for patients in regular contact with an ST (P < 0.001) and using a double‐layer adhesive appliance (over a period of 6–8 weeks) [7]. Marquis et al. in 2003 suggested that the QoL of patients with a stoma is particularly correlated with access to ST care, especially within 3–6 months following surgery [9]. Danielsen and Rosenberg conducted a case–control study of 50 patients and demonstrated an improvement in QoL at 6 months after an ostomy with patients benefitting from educational follow‐up (P < 0.001) [8].
Finally, many studies agree that follow‐up by an ST improves the QoL of patients with a stoma. A recent randomized controlled study demonstrated that telemedicine follow‐up by an ST decreased the readmission rate due to complications [31]. Unfortunately, in France, no well‐conducted study has evaluated this assumption within the French population. Moreover, access to an ST in France is rather limited and restricted to hospital‐based activity.
Through this study, we hope scientifically to demonstrate an improvement in the QoL of ostomy patients by improving access to care through an ST and by providing continuous follow‐up after discharge from hospital. At the same time, we expect to see a reduction in stoma‐related complications and rehospitalizations in the same population through closer follow‐up and earlier management. In the long term, it will be necessary to discuss the implementation of a protocolized follow‐up of patients with a stoma within the French healthcare system, with more important and direct access to stoma therapy.
This trial has minimal risks and constraints. The risks to the patient are minimal because they are recruited in hospital and the procedures and functional tests required for the study are performed as part of their usual therapeutic management. On discharge from hospital and throughout the study, care will be administered in the usual way.
ACKNOWLEDGEMENTS
Hôpital Saint‐Antoine, Unité de Recherche Clinique de l'Est Parisien (URC‐Est), APHP, Paris, France.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and design of the trial. CdP, MR and JHL drafted the manuscript, all the investigators provided critical revision to the clinical and intellectual content. CdP wrote the study protocol that was reviewed by JHL. DV provided statistical expertise in clinical trial design. Lastly, all authors approved the final manuscript.
ETHICAL APPROVAL
Authorizations from the ANSM (Agence Nationale de Sécurité du Médicament et des produits de santé) and CPP (Comité de protection des personnes) were obtained, approval number ID‐RCB: 2021‐A00616‐35. Authorization from the CNIL (Commission nationale de l’informatique et des libertés) was also obtained, registration number: 2221807.
APPENDIX A.
Informed consent materials {32}: sample consent form given to the patient for inclusion.






Biological specimens {33}: Not applicable in this study.
de Ponthaud C, Roupret M, Vernerey D, Audenet F, Brouquet A, Cotte E, et al. StomaCare: quality of life impact after enhanced follow‐up of ostomy patients by a home healthcare nursing service—a multicentre, randomized, controlled trial. Colorectal Dis. 2023;25:128–143. 10.1111/codi.16343
Trial registration NCT05076669.
Funding information
This study was funded by the Promoter FSK.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ripoche J, Basurko C, Fabbro‐Perray P, Prudhomme M. Parastomal hernia. A study of the French Federation of Ostomy Patients. J Visc Surg. 2011;148(6):e435–41. [DOI] [PubMed] [Google Scholar]
- 3. O'Flynn SK. Care of the stoma: complications and treatments. Br J Community Nurs. 2018;23(8):382–7. [DOI] [PubMed] [Google Scholar]
- 4. Vonk‐Klaassen SM, de Vocht HM, den Ouden MEM, Eddes EH, Schuurmans MJ. Ostomy‐related problems and their impact on quality of life of colorectal cancer ostomates: a systematic review. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2016;25(1):125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fingren J, Lindholm E, Petersén C, Hallén AM, Carlsson E. A prospective, explorative study to assess adjustment 1 year after ostomy surgery among Swedish patients. Ostomy Wound Manage. 2018;64(6):12–22. [PubMed] [Google Scholar]
- 6. Becker A, Schulten‐Oberbörsch G, Beck U, Vestweber KH. Stoma care nurses: good value for money? World J Surg. 1999;23(7):638–42. discussion 642–643. [DOI] [PubMed] [Google Scholar]
- 7. Erwin‐Toth P, Thompson SJ, Davis JS. Factors impacting the quality of life of people with an ostomy in North America: results from the Dialogue Study. J Wound Ostomy Cont Nurs Off Publ Wound Ostomy Cont Nurses Soc. 2012;39(4):417–22. quiz 423–4. [DOI] [PubMed] [Google Scholar]
- 8. Danielsen AK, Rosenberg J. Health related quality of life may increase when patients with a stoma attend patient education—a case–control study. PloS One. 2014;9(3):e90354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marquis P, Marrel A, Jambon B. Quality of life in patients with stomas: the Montreux Study. Ostomy Wound Manage. 2003;49(2):48–55. [PubMed] [Google Scholar]
- 10. Prieto L, Thorsen H, Juul K. Development and validation of a quality of life questionnaire for patients with colostomy or ileostomy. Health Qual Life Outcomes. 2005;12(3):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balestroni G, Bertolotti G. EuroQol‐5D (EQ‐5D): an instrument for measuring quality of life. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 2012;78(3):155–9. [DOI] [PubMed] [Google Scholar]
- 12. Meisner S, Lehur PA, Brendan M, Martins L, Jemec GBE. Peristomal skin complications are common, expensive, and difficult to manage: a population based cost modeling study. PloS One. 2012;7:e37813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Follmann DA, Proschan MA, Geller NL. Monitoring pairwise comparisons in multi‐armed clinical trials. Biometrics. 1994;50(2):325–36. [PubMed] [Google Scholar]
- 14. Hotouras A, Murphy J, Thaha M, Chan CL. The persistent challenge of parastomal herniation: a review of the literature and future developments. Colorectal Dis. 2013;15(5):e202–14. [DOI] [PubMed] [Google Scholar]
- 15. Shabbir J, Britton DC. Stoma complications: a literature overview. Colorectal Dis. 2010;12(10):958–64. [DOI] [PubMed] [Google Scholar]
- 16. Leong AP, Londono‐Schimmer EE, Phillips RK. Life‐table analysis of stomal complications following ileostomy. Br J Surg. 1994;81(5):727–9. [DOI] [PubMed] [Google Scholar]
- 17. Londono‐Schimmer EE, Leong AP, Phillips RK. Life table analysis of stomal complications following colostomy. Dis Colon Rectum. 1994;37(9):916–20. [DOI] [PubMed] [Google Scholar]
- 18. Duchesne JC, Wang YZ, Weintraub SL, Boyle M, Hunt JP. Stoma complications: a multivariate analysis. Am Surg. 2002;68(11):961–6. discussion 966. [PubMed] [Google Scholar]
- 19. Carne PWG, Robertson GM, Frizelle FA. Parastomal hernia. Br J Surg. 2003;90(7):784–93. [DOI] [PubMed] [Google Scholar]
- 20. McErlain D, Kane M, McGrogan M, Haughey S. Clinical protocols for stoma care: 5. Prolapsed stoma. Nurs Stand R Coll Nurs G B 1987. 2004;18(18):41–2. [PubMed] [Google Scholar]
- 21. Güenaga KF, Lustosa S, Saad SS, Saconato H, Matos D. Ileostomy or colostomy for temporary decompression of colorectal anastomosis. Cochrane Database Syst Rev. 2007;2007(1):CD004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stelton S. CE: stoma and peristomal skin care: a clinical review. Am J Nurs. 2019;119(6):38–45. [DOI] [PubMed] [Google Scholar]
- 23. Persson E, Berndtsson I, Carlsson E, Hallén AM, Lindholm E. Stoma‐related complications and stoma size—a 2‐year follow up. Colorectal Dis. 2010;12(10):971–6. [DOI] [PubMed] [Google Scholar]
- 24. Schiergens TS, Hoffmann V, Schobel TN, Englert GH, Kreis ME, Thasler WE, et al. Long‐term quality of life of patients with permanent end ileostomy: results of a nationwide cross‐sectional survey. Dis Colon Rectum. 2017;60(1):51–60. [DOI] [PubMed] [Google Scholar]
- 25. Silva MA, Ratnayake G, Deen KI. Quality of life of stoma patients: temporary ileostomy versus colostomy. World J Surg. 2003;27(4):421–4. [DOI] [PubMed] [Google Scholar]
- 26. Krouse R, Grant M, Ferrell B, Dean G, Nelson R, Chu D. Quality of life outcomes in 599 cancer and non‐cancer patients with colostomies. J Surg Res. 2007;138(1):79–87. [DOI] [PubMed] [Google Scholar]
- 27. Sprangers MA, Taal BG, Aaronson NK, te Velde A. Quality of life in colorectal cancer. Stoma vs. nonstoma patients. Dis Colon Rectum. 1995;38(4):361–9. [DOI] [PubMed] [Google Scholar]
- 28. Nugent KP, Daniels P, Stewart B, Patankar R, Johnson CD. Quality of life in stoma patients. Dis Colon Rectum. 1999;42(12):1569–74. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y, Xian H, Yang Y, Zhang X, Wang X. Relationship between psychosocial adaptation and health‐related quality of life of patients with stoma: a descriptive, cross‐sectional study. J Clin Nurs. 2019;28(15–16):2880–8. [DOI] [PubMed] [Google Scholar]
- 30. Neil N, Inglese G, Manson A, Townshend A. A cost–utility model of care for peristomal skin complications. J Wound Ostomy Cont Nurs Off Publ Wound Ostomy Cont Nurses Soc. 2016;43(1):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Augestad KM, Sneve AM, Lindsetmo RO. Telemedicine in postoperative follow‐up of STOMa PAtients: a randomized clinical trial (the STOMPA trial). Br J Surg. 2020;107(5):509–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


