Abstract
Early studies reported a 4‐ to 6‐fold risk of breast cancer between women with extremely dense and fatty breasts. As most early studies were case‐control studies, we took advantage of a population‐based screening program to study density and breast cancer incidence in a cohort design. In the Capital Region, Denmark, women aged 50 to 69 are invited to screening biennially. Women screened November 2012 to December 2017 were included, and classified by BI‐RADS density code, version 4, at first screen after recruitment. Women were followed up for incident breast cancer, including ductal carcinoma in situ (DCIS), to 2020 in nationwide pathology data. Rate ratios (RRs) and 95% confidence intervals (CI) were compared across density groups using Poisson‐regression. We included 189 609 women; 1 067 282 person‐years; and 4110 incident breast cancers/DCIS. Thirty‐three percent of women had BI‐RADS density code 1; 38% code 2; 24% code 3; 4.7% code 4; and missing 0.3%. Using women with BI‐RADS density code 1 as baseline; women with code 2 had RR 1.69 (95% CI 1.56‐1.84); women with code 3, RR 2.06 (95% CI 1.89‐2.25); and women with code 4, RR 2.37 (95% CI 1.05‐2.74). Results differed between observations accumulated during screening and above screening age. Our results indicated less difference in breast cancer risk across level of breast density than normally stated. Translated into absolute risk of breast cancer after age 50, we found a 6.2% risk for the one‐third of women with lowest density, and 14.7% for the 5% of women with highest density.
Keywords: breast cancer, breast density, cohort study, screening
What's new?
Breast density is a known risk factor for breast cancer, with previous studies suggesting a 4‐ to 6‐fold increase in risk for women with highly dense breast tissue. In our study, the authors leveraged a population‐based screening program in Denmark to more closely investigate the relationship between breast density and breast cancer risk. Using a cohort design, analyses show a 6.2% cumulative risk for women with lowest breast density and a 14.7% cumulative risk for women with highest breast density. The findings indicate that the difference in risk across different breast densities is smaller than earlier estimates.
Abbreviations
- BI‐RADS
Breast Imaging Reporting and Data System
- CI
confidence interval
- DCIS
ductal carcinoma in situ
- MRI
magnetic resonance imaging
- OR
odds ratio
- PMD
percent mammographic density
- RR
rate ratio
- SNOMED
Systemized Nomenclature of Medicine
1. INTRODUCTION
In the 2000s, breast density was identified as a risk factor for breast cancer. A frequently quoted size of this association was a 4‐ to 6‐fold increased risk in women with a percent mammographic density (PMD) at or higher than 75% compared to women with a density below 5% or 10%. 1 , 2 This made density one of the strongest known breast cancer risk factors. 3
Breast density can be categorized in different ways. Besides PMD, the Breast Imaging Reporting and Data System (BI‐RADS) density coding version 4 has been used widely; code 1 refers to <25% glandular tissue; code 2 to 25% to 50%; code 3 to 51% to 75%; and code 4 to >75%. 4 In the Capital Region of Denmark, encompassing a population of 1.8 million persons, BI‐RADS version 4 has been used for density coding in the population‐based screening program since November 2012. Based on early data from the Capital Region, we analyzed breast cancer risk by BI‐RADS density for women followed up for 2‐years 5 ; 5% had BI‐RADS density code 4; 28% had code 1; and there was a 2‐fold difference in breast cancer risk between the groups; relative risk 2.0 (95% confidence interval [CI] 1.3‐2.8). In the ground‐breaking case‐control study by Boyd et al, 6 an odds ratio (OR) of 4.7 (95% CI 3.0‐7.4) was reported for high vs low density; with 4% of control women in the highest category and 33% in the lowest; approximately the same distribution as seen in our material.
On this background, we extended the analysis of the Capital Region data from Denmark to include both a longer recruitment period and a longer follow‐up period for incident breast cancers. As a novelty, we analyzed density‐specific breast cancer incidence by time of data accumulation after start of screening age, during repeated rounds of screening, and after end of screening age.
2. METHODS
2.1. Study population
In the Capital Region, breast cancer screening is offered every second year to all women aged 50 to 69 years. Invitation is personal, originally by letter and now‐a‐days via the e‐box for communication between citizens and public authorities. Women are invited with a fixed, changeable, appointment to visit one of five screening clinics. Participation, assessment of abnormal findings, and eventual treatment are all free of charge for the women. Women not wanting to participate can opt out of the invitation scheme. Use of opportunistic screening is rare, and screening coverage is 74%. 7 Women with a known BRCA1, BRCA2 or other high risk genmutations are offered special surveillance, 8 and data from this surveillance are not included in the present study. All other women are included in the screening program, even women genetically considered to be at high risk but without the listed genmutations. Women previously treated for breast cancer are also invited to the screening program, and for this group the upper age limit was by July 1, 2018 extended to age 79.
Siemens Inspiration digital mammography equipment was used during the data collection period. At screening, the radiographer took a craniocaudal and a mediolateral oblique view, and if needed supplementary views. All mammograms were read and coded independently by two trained radiologists. If they disagreed on malignancy code, a consensus code was made in dialog, and if necessary, a third reader was brought in. All women with screening mammograms suspicious of malignancy were offered assessment. Density was assessed for research purpose only. If the two readers disagreed on the BI‐RADS density code, the highest code was used. The five screening clinics were headed by the same radiologist (author IV) throughout the period. We included all women screened in the program from November 1, 2012 to December 31, 2017. A woman was included at the date of her first registered screen in the period, and she was categorized by the BI‐RADS density code given at that screen.
2.2. Follow‐up
The women were followed up until April 20, 2020 for death and emigration in the Central Population Register, and incident breast cancer cases in the nationwide Danish Pathology Register. 9 We included both screen‐detected cancers, interval cancers, and cancers diagnosed within the follow‐up period but more than 2 years after last screen, and both invasive breast cancer and ductal carcinoma in situ (DCIS); topography T04*** and morphology M8***3 or M85**2 in the Danish version of the Systemized Nomenclature of Medicine (SNOMED) codes. 9 Use of unique identification numbers allowed linkage between screening, vital status and pathology data.
2.3. Dynamic of breast cancer incidence in relation to screening
As the breast density data derived from screening data, the dynamic of breast cancer incidence during screening needs to be considered in the analysis. With “dynamic” we mean the changes in breast cancer incidence caused by the fact that screen‐detected cancer are diagnosed earlier in time than they would have been in absence of screening. In the absence of screening, the incidence of breast cancer in postmenopausal women increases with increasing age. However, at start of screening age, a prevalence peak is expected in breast cancer incidence. During subsequent screens, artificial aging is expected because the new incident breast cancer cases are diagnosed on average at an earlier age than they would have been in absence of screening. After end of screening age, a compensatory dip is expected before the breast cancer incidence gradually returns to the level expected in absence of screening. 10
The sensitivity of screening mammography is higher in women with low density than in women with high density. 11 It is reasonable therefore to expect that unscreened women with fatty breasts will detect abnormalities earlier than unscreened women with dense breasts. 12 If this is true, an excess number of abnormalities in dense breasts are left to be detected at first screen. Following the similar line of thoughts, after end of screening age where abnormalities are detected only by the women themselves, a deficit in diagnosed breast cancers would be expected in women with dense breasts. In the present study, we will investigate therefore the pattern of breast cancer incidence by age at recruitment before summarizing data across ages.
2.4. Statistics
A woman contributed person‐years at risk from date of first screen in the recruitment period until date of death, emigration from Denmark, first incident breast cancer, or April 20, 2020, which ever came first. Only the first incident breast cancer/DCIS case was included. Risk of breast cancer was compared across BI‐RADS density groups using women with code 1 as baseline. To take common practice in the United States and Canada into consideration, a calculation was made also where women with BI‐RADS density codes 3 + 4 were compared to those with code 1. Rate ratios (RRs), crude and adjusted for running age, were calculated with Poisson regression, including 95% CI. To investigate the possible impact of length of follow‐up since recruitment, data were also analyzed divided into three follow‐up periods; 0 to <2 years; 2 to <5 years, and 5+ years.
In Denmark, women entering the screening program at age 50 years have on average a 9.8% cumulative risk of breast cancer in their remaining life time. We estimated the density‐specific cumulative risk. First, by using data for women aged 50 to 54 as a proxy for age at start of screening, second by using data for women by actual age at recruitment to the study. The cumulative risk x d for women with density code d (d = 1, 2, 3, 4) can be computed as x d = 0.098 R d/R total, where R d is the breast cancer incidence rate for women with density d, and R total is the rate for all screened women.
Data analysis took place in Statistics Denmark using SAS 9.4 copyright © 2016 by SAS Institute Inc, Cary, North Carolina.
3. RESULTS
We included 189 609 women; of whom approximately 39% were recruited at age 50 to 54; 21% at age 55 to 59; 20% at age 60 to 64; and 21% at age 65 to 69. The BI‐RADS density code at first screen in the recruitment period was 1 for 33% of women; 2 for 38%; 3 for 24%; 4 for 4.7%; and missing for 0.3%. As expected, the density distribution changed by increasing age at recruitment. Among those recruited at age 50 to 54, 25% had BI‐RADS density code 1 and 7.6% code 4. Among those recruited at age 65 to 69, percentages were 41% and 2.4%, respectively, Table 1.
TABLE 1.
Screened women by age and BI‐RADS density code at first screen in recruitment period 2012 to 2017, and number of incident breast cancer cases in follow‐up period 2012 to 2020, Capital Region, Denmark
| Number (%) of women/person‐years/incident breast cancers cases | Total | Age at first screen in recruitment period | |||
|---|---|---|---|---|---|
| 50‐54 y | 55‐59 y | 60‐64 y | 65‐69 y | ||
| Women, % horizontal | 189 609 (100) | 72 916 (38.5) | 39 589 (20.9) | 36 973 (19.5) | 40 131 (21.2) |
| BI‐RADS 1, % vertical | 62 105 (32.8) | 18 235 (25.0) | 13 389 (33.8) | 13 995 (37.8) | 16 486 (41.1) |
| BI‐RADS 2, % vertical | 72 235 (38.1) | 26 489 (36.3) | 15 648 (39.5) | 14 493 (39.2) | 15 605 (38.9) |
| BI‐RADS 3, % vertical | 45 751 (24.1) | 22 385 (30.7) | 9022 (22.8) | 7388 (20.0) | 6956 (17.3) |
| BI‐RADS 4, % vertical | 8960 (4.7) | 5557 (7.6) | 1415 (3.6) | 1015 (2.8) | 973 (2.4) |
| Missing, % vertical | 558 (0.3) | 250 (0.3) | 115 (0.3) | 82 (0.2) | 111 (0.3) |
| Person‐years, % horizontal | 1 067 293 (100) | 375 711 (35) | 235 771 (22) | 219 002 (21) | 236 809 (22) |
| BI‐RADS 1 | 351 823 | 94 494 | 78 646 | 81 920 | 96 763 |
| BI‐RADS 2 | 407 704 | 136 355 | 93 382 | 85 988 | 91 980 |
| BI‐RADS 3 | 256 681 | 115 747 | 54 600 | 44 659 | 41 676 |
| BI‐RADS 4 | 47 954 | 27 806 | 8449 | 5944 | 5755 |
| Missing | 3131 | 1309 | 695 | 493 | 635 |
| Breast cancer cases, % horizontal | 4110 (100) | 1134 (28) | 844 (21) | 1028 (25) | 1104 (27) |
| BI‐RADS 1 | 888 | 143 | 185 | 277 | 283 |
| BI‐RADS 2 | 1684 | 366 | 338 | 435 | 545 |
| BI‐RADS 3 | 1265 | 485 | 275 | 267 | 238 |
| BI‐RADS 4 | 256 | 137 | 44 | 45 | 30 |
| Missing | 17 | Not reportable | Not reportable | Not reportable | Not reportable |
| Mean follow‐up period in years | 5.6 | 5.2 | 6.0 | 5.9 | 5.9 |
| Breast cancer incidence per 100 000 | 385 | 302 | 358 | 469 | 466 |
| % change in rate from previous age | Not relevant | +19% | +31% | −1% | |
In total, 1 067 293 person‐years were accumulated. Mean follow‐up time was 5.6 years; 5.9 to 6.0 years in women recruited at age 55 to 69, and 5.2 years in women recruited at age 50 to 54, where new cohorts came in. In total, 4110 women developed breast cancer in the follow‐up period. Among women recruited at age 50 to 54, observations were accumulated during the prevalence peak at entry into screening and during artificial aging. Among women recruited at ages 55 to 59 and 60 to 64 almost all observations were accumulated during artificial aging; and among women recruited at age 65 to 69 most observations were accumulated during the compensatory dip, Figure 1. This pattern was reflected in the breast cancer incidence rate, which was 302 per 100 000 in the 50 to 54 age‐group; 358 in the 55 to 59 age‐group, where women were older but beyond the prevalence peak; and 469 in women recruited at age 60 to 64, Table 1. In women recruited at age 65 to 69 and observed mainly during the compensatory dip, the overall rate was only 466. The numbers were small and the CIs broad when the data were divided simultaneously by age and BI‐RADS density, but the pattern indicated that the compensatory dip affected in particular in women with BI‐RADS density codes 3 and 4, Figure 2.
FIGURE 1.
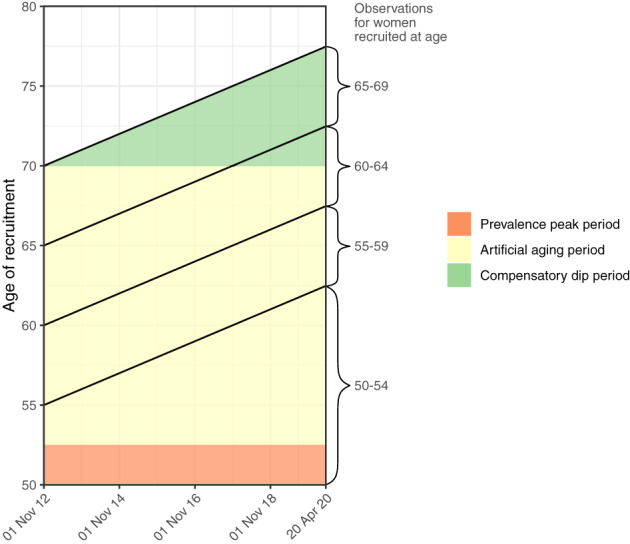
Study design. Women participating in mammography screening in the Capital Region, Denmark, 2012 to 2020
FIGURE 2.
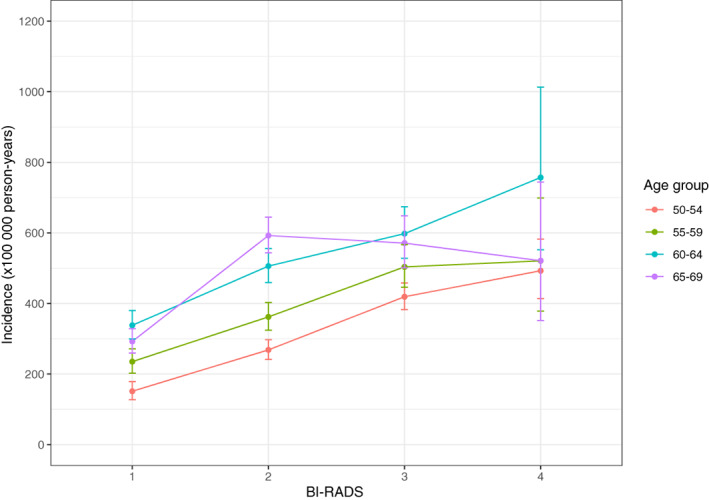
Women participating in mammography screening in the Capital Region, Denmark, 2012 to 2020. Breast cancer incidence by age at recruitment and BI‐RADS version 4 density code
Compared to women with a BI‐RADS density code 1, women with higher codes had statistically significantly increased incidence of breast cancer, Table 2. The gradient was steepest for women aged 50 to 54 years at recruitment with a RR of 3.19 (95% CI 2.52‐4.03) for women with BI‐RADS density code 4. Gradients up to RRs of 2.21 (95% CI 1.59‐3.07) and 2.24 (9%% CI 1.64‐3.07), respectively, were seen for women aged 55 to 59 and 60 to 64 at recruitment. As expected from the pattern in Figure 2, the gradient was smaller for women aged 65 to 69 at recruitment; BI‐RADS 4 vs BI‐RADS 1 gave a RR of 1.76 (95% CI 1.21‐2.57).
TABLE 2.
Rate ratio (RR) and 95% confidence interval (in parentheses) of breast cancer incidence by BI‐RADS density code at first screen in recruitment period, Capital Region, Denmark, 2012 to 2020, by age at recruitment
| BI‐RADS density at time of recruitment | ||||
|---|---|---|---|---|
| Age at recruitment | 2 | 3 | 4 | 3 + 4 |
| 50‐54 y | ||||
| Crude RR | 1.77 (1.46‐2.15) | 2.77 (2.30‐3.34) | 3.26 (2.58‐4.12) | 2.86 (2.39‐3.43) |
| Age‐adjusted a RR | 1.77 (1.46‐2.14) | 2.76 (2.29‐3.33) | 3.19 (2.52‐4.03) | 2.85 (2.37‐3.41) |
| 55‐59 y | ||||
| Crude RR | 1.54 (1.29‐1.84) | 2.14 (1.78‐2.58) | 2.21 (1.59‐3.08) | 2.15 (1.79‐2.58) |
| Age‐adjusted a RR | 1.53 (1.28‐1.83) | 2.13 (1.77‐2.57) | 2.21 (1.59‐3.07) | 2.14 (1.79‐2.57) |
| 60‐64 y | ||||
| Crude RR | 1.50 (1.29‐1.74) | 1.77 (1.49‐2.09) | 2.24 (1.63‐3.07) | 1.82 (1.55‐2.14) |
| Age adjusted a RR | 1.51 (1.30‐1.75) | 1.77 (1.50‐2.10) | 2.24 (1.64‐3.07) | 1.83 (1.56‐2.15) |
| 65‐69 y | ||||
| Crude RR | 2.03 (1.75‐2.34) | 1.95 (1.64‐2.32) | 1.78 (1.22‐2.60) | 1.93 (1.63‐2.28) |
| Age adjusted a RR | 2.03 (1.76‐2.34) | 1.94 (1.64‐2.31) | 1.76 (1.21‐2.57) | 1.92 (1.63‐2.27) |
| Total 50‐69 y | ||||
| Crude RR | 1.64 (1.51‐1.78) | 1.95 (1.79‐2.13) | 2.12 (1.84‐2.43) | 1.97 (1.82‐2.15) |
| Age adjusted a RR | 1.69 (1.56‐1.84) | 2.06 (1.89‐2.25) | 2.37 (2.05‐2.74) | 2.13 (1.96‐2.32) |
| Total 50‐64 y | ||||
| Crude RR | 1.52 (1.38‐1.68) | 2.01 (1.82‐2.23) | 2.26 (1.94‐2.63) | 2.05 (1.86‐2.26) |
| Age adjusted a RR | 1.55 (1.40‐1.71) | 2.11 (1.91‐2.33) | 2.46 (2.11‐2.87) | 2.18 (1.98‐2.40) |
Note: Baseline = BI‐RADS density 1.
Adjusted by running age in 5‐year age groups.
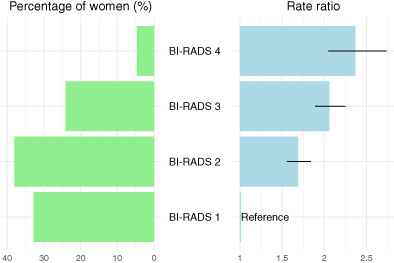
Summarized across age groups, the gradient was steeper in the age‐adjusted RRs than in the crude RRs due to the large size of the high density group in women recruited at age 50 to 54. Using BI‐RADS density code 1 women as baseline, the age‐adjusted RRs for women aged 50 to 64 years were 1.55 (95% CI 1.40‐1.71) for women with code 2; 2.11 (95% CI 1.91‐2.33) for women with code 3; and 2.46 (95% CI 2.11‐2.87) for women with code 4. Across all ages, the age‐adjusted RRs were 1.69 (95% CI 1.56‐1.84) for women with code 2; 2.06 (95% CI 1.89‐2.25) for women with code 3; and 2.37 (95% CI 2.05‐2.74) for women with code 4, Figure 3. The estimate across all ages for women with codes 3 and 4 combined was 2.18 (95% CI 1.98‐2.40). For comparison with all women, see Table S1.
FIGURE 3.
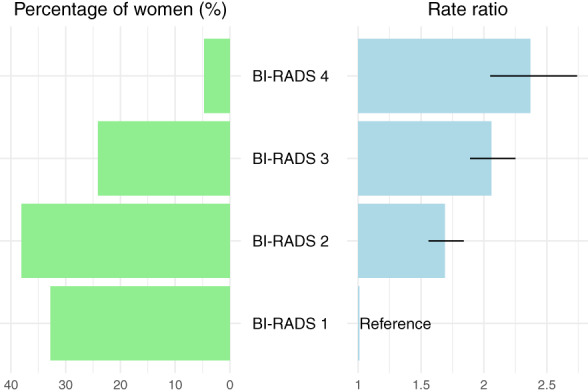
Women participating in mammography screening in the Capital Region, Denmark, 2012 to 2020. Percent distribution of screened women and rate ratio and 95% confidence interval for breast cancer incidence by BI‐RADS version 4 density code
The increasing RR by level of BI‐RADS density code was found within the first 2 years of follow‐up after recruitment, a bit less regular within 2 to <5 years of follow‐up, and again very regular within 5 years or more of follow‐up, Table 3.
TABLE 3.
Rate ratio of breast cancer incidence by BI‐RADS density code at first screen in recruitment period, Capital Region, Denmark, 2012 to 2020
| BI‐RADS | Rate ratio adjusted by running age, 95% confidence interval (in parentheses) | |||||
|---|---|---|---|---|---|---|
| Person‐years a | Breast cancer cases a | Follow‐up time | ||||
| Total | Total | Total BC/PY | 0‐<2 y | 2‐<5 y | 5+ y | |
| Code 1 | 351 823 | 888 |
1 (baseline) 888/351 823 |
1 (baseline) 335/3549 |
1 (baseline) 428/48 453 |
1 (baseline) 125/299 821 |
| Code 2 | 407 704 | 1684 |
1.69 (1.56‐1.84) 1684/407 704 |
1.83 (1.60‐2.09) 676/6131 |
1.53 (1.36‐1.72) b 761/65 809 |
1.69 (1.36‐2.09) 247/335 764 |
| Code 3 | 256 681 | 1265 |
2.06 (1.89‐2.25) 1265/256 681 |
2.02 (1.75‐2.32) 468/4510 |
2.04 (1.80‐2.32) 578/47 309 |
2.36 (1.89‐2.95) 219/204 863 |
| Code 4 | 47 954 | 256 |
2.37 (2.05‐2.74) 256/47 954 |
2.60 (2.07‐3.26) 107/1037 |
1.84 (1.50‐2.26) b 113/11 649 |
2.61 (1.79‐3.82) 36/35 268 |
Note: Total and by length of follow‐up. Adjusted by running age in 5‐years age groups.
Abbreviations: BC, breast cancer/ductal carcinoma in situ; PY, person‐years.
3131 person‐years and 17 breast cancer cases had missing BI‐RADS density code.
Crude rate ratio, as the model did not converge due to collinearity, but this means also that age‐adjustment would not change the estimate.
4. DISCUSSION
4.1. Main findings
In this large study from a population‐based program, there was overall a 2.37‐fold difference in breast cancer incidence between women with the highest and women with the lowest density level. For women recruited at age 50 to 64 and still within screening age during the follow‐up period, incidence rates increased systematically with increasing density. For women recruited at age 65 to 69 and mostly followed up beyond screening age where only symptomatic breast cancers are diagnosed, women with BI‐RADS density codes 3 and 4 tended to have lower incidence rates than women with BI‐RADS density code 2 though based on small numbers and with overlapping CIs.
4.2. Other studies
Following the early observation of an increased risk of breast cancer in women with dense breasts, numerous studies have been undertaken. Pettersson et al 13 in 2014 summarized results from 19 case‐control studies all providing digitalized prediagnostic film mammograms, density assessed by computer‐assisted technique, and relevant covariable data. For postmenopausal women, they found women in the highest quartile of absolute dense area of the breast to have a multivariate‐adjusted OR of 2.33 (95% CI 2.10‐2.60) for breast cancer compared to women in the lowest quartile. When measured in percentage dense area, the OR was 2.85 (95% CI 2.48‐3.28). For premenopausal women, the results for absolute dense area were similar, while the gradient for percentage dense area was slightly steeper than for postmenopausal women; OR 3.11 (95% CI 2.49‐3.88). It should be noted that when the highest density group is defined by the highest quartile, less contrast is expected than in studies where the highest density group constitutes only a smaller proportion of the study population.
It is therefore interesting to note also the gradients reported from more recent studies using other classification methods. In a case‐control study from Florence, Italy, the 6% of women with BI‐RADS density code 4, had an OR of 2.67 (95% CI 1.08‐6.62) for breast cancer compared to the 43% of women with BI‐RADS density code 1, with no difference between age‐ and multivariate‐adjusted ORs. 14 A fairly similar result was seen in a case‐control study from England where only age was controlled for. The 12% of women with highest Volpara density grade had an OR of 2.6 (95% CI 1.3‐5.7) for breast cancer compared to the 5% of women with lowest grade Volpara density. 15 In a Swedish cohort study, the 40% of women with PMD >25% had an age‐ and body mass index adjusted hazard ratio of 2.54 (95% CI 1.92‐3.37) for breast cancer compared to the 22% of women with PDM <5%. 16 In a young South Korean cohort, the 47% of women with BI‐RADS density code 4, had an age‐adjusted hazard ratio of 2.93 and a multivariate‐adjusted hazard ratio of 2.86 (95% CI 2.04‐4.01) of breast cancer compared to the 10% of women with BI‐RADS density codes 1 or 2. 17 Based on the reported data from a cohort study from the Massachusetts General Hospital, we estimated that the 5% of women with a BI‐RADS version 5 density code D had a crude relative risk of 2.3 (95% CI 1.7‐3.1) for breast cancer as compared to the 10% of women with density code A. 18
Due to differences in study design, results are difficult to compare across recent studies, first of all because the proportion of women falling into the extreme dense category of the density distribution varied considerably across settings; from 6% in the Florence cohort recruited at the age of 35 to 64, 14 to 47% in the South Korean cohort recruited at age less than 35. 17 It was nevertheless the general pattern that the excess risk of breast cancer in women with the highest density as compared to women with the lowest density was in the order of 2.3 to 2.9. These associations were assessed with ORs or hazard ratios, but as breast cancer is a rare event even in women with dense breast, these measures, as well as the RR used in our study, can be interpreted as relative risks. The outcome of these newer observations are therefore in good agreement with our estimate of a 2.37‐fold risk of breast cancer in comparison between women with highest and lowest breast density. In a meta‐analysis of studies published 2005 to 2016, Bond‐Smith and Stone 19 found an OR of 2.00 (95% CI 1.12‐3.42) for women with BI‐RADS version 5 density code D compared to code A. When they used categorial PMD data and included also older studies, they found the gradient to be steeper in older than in newer studies, and they concluded that it would be important to adjust clinical interpretations based on older data.
4.3. Strengths and limitations
It was a strength of our study that it included comprehensive data from a population‐based screening program, and complete follow‐up for death, emigration and incident breast cancer was ensured by linkage to comprehensive, nationwide registers. Furthermore, screening and density coding have remained stable during the recruitment period. In the analysis, we deliberately included women with a screen‐detected breast cancer at first screen in the recruitment period. The reason being that exclusion could have led to underregistration of breast cancers prevalent at the start of screening in women with high density. It was a weakness that we did not have density data back to the start of screening age for all women, but among the 132 903 women screened at least twice almost three in four had the same BI‐RADS density code at their first and last screen, and only 0.5% changed density category beyond one category up or down (Table S2).
Nor did we have data on covariates, for example, body mass index, as these data are not collected at screening in Denmark. It should be noted though that in recent studies where covariables had been controlled for, only minor differences were reported between age‐adjusted and fully adjusted risk estimates. 14 , 17 We were not able to separate women recruited at age 50 to 54 by menopausal status, and it is possible that the slightly steeper gradient for women in this age group could be attributable to inclusion of premenopausal women.
If the two readers of the mammograms disagreed on the BI‐RADS density code, the highest code was used. For a shorter time period, we have seen previously that this procedure resulted as expected in assignment of more breast cancer cases to the higher BI‐RADS density categories than use of the single reader codings would have done. 20 Whether or not use of highest code will have affected the RRs in the present study depends on the effect the procedure had on the distribution of person years.
One could argue that the use of real‐world data is a limitation of the present study as over time different radiologists have participated in the reading of the mammograms. However, the use of real‐world data could be seen also as a strength of the study, because the very purpose of knowing the breast cancer risk by density is to have a basis for decisions on whether or not to take this variable into account in procedures used in actual screening programs.
4.4. Clinical implications
In Denmark, women entering the screening program at age 50 years have on average a 9.8% cumulative risk of breast cancer in their remaining life time. 21 Given the results observed in the present study for women at start of screening age, this average of 9.8% would stretch from a 4.9% cumulative risk for women with lowest breast density to a 15.8% cumulative risk for women with highest density. However, the proportion of women with highest density decreased with increasing age, and it therefore seems more reasonable to use the density distribution across all ages as the basis for calculation of the density specific cumulative risk. This calculation gives a 6.2% cumulative risk of breast cancer for women with lowest breast density, and 14.7% for women with highest density. Breast cancer is thus an important disease for all groups of women.
The notion of an increased risk of breast cancer and a lower sensitivity of screening in women with high breast density raised concern about ability of screening to decrease breast cancer mortality in women with high density. Originating from a patient initiative, the Breast Density Notification Legislation was implemented first in Connecticut and later in most US states, and it has been followed by more frequent use of supplementary ultrasound examination of women with dense breast. 22 However, there is no agreed policy in the United States for supplementary screening tests based on information on breast density. 23
In the United Kingdom, women are not notified of their density scores and are not offered supplemental imaging purely based on density. 24 In the DENSE trial in the Netherlands, women with a negative result on mammography and with Volpara density grade 4 were randomized to supplemental magnetic resonance imaging (MRI) or no further imaging. The interval cancer rates per 1000 women were 2.5 (95% CI 1.6‐3.8) and 5.0 (95% CI 4.3‐5.8), respectively, 25 and MRI screening every fourth year of women with extremely dense breasts was found to be cost‐effective. 26 In a trial in Norway, women were randomized to screening with digital breast tomosynthesis or digital mammography, and in women with Volpara density grade 4, no difference was found between the schemes in screen‐detection and false positive rates. 27
The upper limit of screening age is set to avoid overdiagnosis with screen detected breast cancers that would in absence of screening not have become clinical manifest in the women's remaining life time. 28 In line with the lower sensitivity of screening in dense than in fatty breasts, we hypothesized that past the upper age limit for screening it would be more difficult for women with dense breasts than for women with fatty breasts to detect a breast cancer. Our data indicated some support for this hypothesis; an observation that could potentially be an argument for a density‐dependent upper limit of screening age.
5. CONCLUSION
In a population‐based screening program, women with highest breast density had a 2.37‐fold risk of breast cancer compared to women with lowest density. Translated into absolute risk of breast cancer in the remaining life time for women entering the screening program at the age of 50 years, this reflected a 6.2% risk for women with the lowest density and a 14.7% risk for women with the highest density.
AUTHOR CONTRIBUTIONS
Elsebeth Lynge: Conceptualization, methodology, funding acquisition, writing original draft. Ilse Vejborg: Data curation, writing review and editing. Mads Nielsen: Methodology, writing review and editing. Martin Lillholm: Methodology, writing review and editing. George Napolitano: Formal analysis, methodology, visualization, writing review and editing. My von Euler‐Chelpin: Data curation, formal analysis, project administration, writing review and editing. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
Our study was financially supported by the Independent Research Council Denmark (9039‐00053B). The funder has no role in the undertaking and/or reporting of the study.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
ETHICS STATEMENT
Approval by Faculty of Health and Medical Sciences, University of Copenhagen, Ref. No.: 514‐0238/18‐3000, served as ethical clearance. According to Danish legislation, informed consent is not required for register‐based studies without contact to patients, relatives and/or treating physicians.
Supporting information
Table S1. Rate ratio (RR) and 95% confidence interval (in parentheses) of breast cancer incidence by BI‐RADS density code at first screen in recruitment period, Capital Region, Denmark, 2012 to 2020, by age at recruitment. Baseline = All women
Table S2. Number of women aged 50 to 69 at recruitment; density at recruitment and density at last screen. Women with missing density on first and/or last screen excluded
Lynge E, Vejborg I, Lillholm M, Nielsen M, Napolitano G, von Euler‐Chelpin M. Breast density and risk of breast cancer. Int J Cancer. 2023;152(6):1150‐1158. doi: 10.1002/ijc.34316
Funding information Danmarks Frie Forskningsfond, Grant/Award Number: 9039‐00053B
DATA AVAILABILITY STATEMENT
Data from our study are stored in Statistics Denmark, which can be accessed given the relevant data permits. Further information is available from the corresponding author upon request.
REFERENCES
- 1. McCormack VA, dos Santos SI. Breast density and parenchymal patterns as markers of breast cancer risk: a meta‐analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159‐1169. doi: 10.1158/1055-9965.epi-06-0034 [DOI] [PubMed] [Google Scholar]
- 2. Boyd NF. Mammographic density and risk of breast cancer. Am Soc Clin Oncol Educ Book. 2013;e57‐e62. doi: 10.1200/EdBook_AM.2013.33.e57 [DOI] [PubMed] [Google Scholar]
- 3. Kerlikowski K. The mammogram that cried Wolfe. N Engl J Med. 2007;356(3):297‐300. doi: 10.1056/NEJMe068244 [DOI] [PubMed] [Google Scholar]
- 4. BI‐RADS, 4th ed. Accessed July 2, 2018. http://www.radiologyassistant.nl/en/p53b4082c92130/bi-rads-for-mammography-and-ultrasound-2013.html
- 5. Lynge E, Vejborg I, Andersen Z, von Euler‐Chelpin M, Napolitano G. Mammographic density and screening sensitivity, breast cancer incidence and associated risk factors in Danish breast cancer screening. J Clin Med. 2019 Nov 19;8(11):2021. doi: 10.3390/jcm8112021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007. Jan 18;356(3):227‐236. [DOI] [PubMed] [Google Scholar]
- 7. Danish Quality Database for Breast Cancer Screening . Assessed April 8, 2020. https://www.sundhed.dk/content/cms/78/4678_dkms_rapport_2019-(2).pdf
- 8. Danish Breast Cancer Group . Guidelines for Women With Concern About a Hereditary Risk of Breast Cancer [in Danish]. Accessed August 16, 2022. https://www.dmcg.dk/Kliniske-retningslinjer/kliniske-retningslinjer-opdelt-paa-dmcg/brystcancer/arvelig-mammacancer--henvisningskriterier-til-genetisk-radgivning-indikation-for-tilbud-om-surveillance-og-profylaktisk-kirurgi/
- 9. Danish Pathology Registration System. Accessed June 31, 2021. https://www.patobank.dk/snomed/
- 10. Biesheuvel C, Barratt A, Howard K, Houssami N, Irwig L. Effects of study methods and biases on estimates of invasive breast cancer overdetection with mammography screening: a systematic review. Lancet Oncol. 2007;8:1129‐1138. [DOI] [PubMed] [Google Scholar]
- 11. Leconte I, Feger C, Galant C, et al. Mammography and subsequent whole‐breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol. 2003;180(6):1675‐1679. doi: 10.2214/ajr.180.6.1801675 [DOI] [PubMed] [Google Scholar]
- 12. Accessed July 1, 2021. https://wexnermedical.osu.edu/blog/facts-about-dense-breast-tissue
- 13. Pettersson A, Graff RE, Ursin G, et al. Mammographic density phenotypes and risk of breast cancer: a meta‐analysis. J Natl Cancer Inst. 2014;106(5):dju078. doi: 10.1093/jnci/dju078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masala G, Ambrogetti D, Assedi M, Bendinelli B, Caini S, Palli D. Mammographic breast density and breast cancer risk in a Mediterranean population: a nested case‐control study in the EPIC Florence cohort. Breast Cancer Res Treat. 2017;164(2):467‐473. doi: 10.1007/s10549-017-4274-9 [DOI] [PubMed] [Google Scholar]
- 15. Burnside ES, Warren LM, Myles J, et al. Quantitative breast density analysis to predict interval and node‐positive cancers in pursuit of improved screening protocols: a case‐control study. Br J Cancer. 2021;125:884‐892. doi: 10.1038/s41416-021-01466-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Azam S, Eriksson M, Sjölander A, et al. Mammographic density change and risk of breast cancer. J Natl Cancer Inst. 2020;112(4):391‐399. doi: 10.1093/jnci/djz149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim EY, Chang Y, Ahn J, et al. Mammographic breast density, its changes, and breast cancer risk in premenopausal and postmenopausal women. Cancer. 2020;126(21):4687‐4696. doi: 10.1002/cncr.33138 [DOI] [PubMed] [Google Scholar]
- 18. McCarthy AM, Ehsan S, Appel S, et al. Risk factors for an advanced breast cancer diagnosis within 2 years of a negative mammogram. Cancer. 2021;127:3334‐3342. doi: 10.1002/cncr.33661 [DOI] [PubMed] [Google Scholar]
- 19. Bond‐Smith D, Stone J. Methodological challenges and updated findings from a meta‐analysis of the association between mammographic density and breast cancer. Cancer Epidemiol Biomarkers Prev. 2019;28(1):22‐31. doi: 10.1158/1055-9965.EPI-17-1175 [DOI] [PubMed] [Google Scholar]
- 20. Euler‐Chelpin MV, Lillholm M, Napolitano G, Vejborg I, Nielsen M, Lynge E. Screening mammography: benefit of double reading by breast density. Breast Cancer Res Treat. 2018;171(3):767‐776. doi: 10.1007/s10549-018-4864-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Accessed July 1, 2021. https://www‐dep.iarc.fr/NORDCAN/english/Graph13l.asp?cancer=200&female=2&country%5B%5D=208&year=2016&year=2015&type=1&incidence=1&age_from=11&age_to=16&orientation=2&grid=1&line=2&submit=%A0%A0%A0Execute%A0%A0%A0
- 22. Butler RS, Hooley RJ. Screening breast ultrasound: update after 10 years of breast density notification laws. AJR Am J Roentgenol. 2020;214(6):1424‐1435. doi: 10.2214/AJR.19.22275 [DOI] [PubMed] [Google Scholar]
- 23. Accessed December 12, 2021. https://www.cancer.gov/types/breast/breast-changes/dense-breasts
- 24. Varghese J, Gohari S, Regrag F, et al. Breast density notification: current UK national practice. Clin Breast Cancer. 2022 Jan;22(1):e101‐e107. doi: 10.1016/j.clbc.2021.04.013. Epub 2021 Jun 5 [DOI] [PubMed] [Google Scholar]
- 25. Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381(22):2091‐2102. doi: 10.1056/NEJMoa1903986 [DOI] [PubMed] [Google Scholar]
- 26. Geuzinge HA, Bakker MF, Heijnsdijk EAM, et al. Cost‐effectiveness of magnetic resonance imaging screening for women with extremely dense breast tissue. J Natl Cancer Inst. 2021;113(11):1476‐1483. doi: 10.1093/jnci/djab119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moshina N, Aase HS, Danielsen AS, et al. Comparing screening outcomes for digital breast tomosynthesis and digital mammography by automated breast density in a randomized controlled trial: results from the to‐be trial. Radiology. 2020;297(3):522‐531. doi: 10.1148/radiol.2020201150 [DOI] [PubMed] [Google Scholar]
- 28. Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599‐1614. doi: 10.1001/jama.2015.12783 Erratum in: JAMA. 2016 Apr 5;315(13):1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Rate ratio (RR) and 95% confidence interval (in parentheses) of breast cancer incidence by BI‐RADS density code at first screen in recruitment period, Capital Region, Denmark, 2012 to 2020, by age at recruitment. Baseline = All women
Table S2. Number of women aged 50 to 69 at recruitment; density at recruitment and density at last screen. Women with missing density on first and/or last screen excluded
Data Availability Statement
Data from our study are stored in Statistics Denmark, which can be accessed given the relevant data permits. Further information is available from the corresponding author upon request.


