Abstract
Anthropogenic climate change along with the more frequent extreme weather it prompts, are having direct and indirect effects on distributions and abundance of species with consequence for community structure—especially if habitat providers are lost. Rocky shores have long been recognized as tractable experimental arenas for ecology contributing to theory. They have also emerged as important sentinel systems for tracking climate change responses of marine biodiversity and ecosystems, capitalizing on both historic broadscale surveys and time series. Combining these twin traditions is a powerful approach for better understanding and forecasting climate change impacts. Sustained observing allows extreme events to be detected and explored by in‐parallel experimentation.
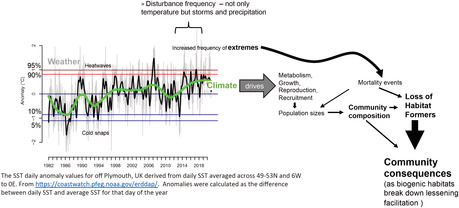
Anthropogenic climate change has been re‐shaping biogeographic patterns of species, causing shifts along terrestrial altitudinal gradients and marine depth gradients (Parmesan & Yohe, 2003), plus driving phenological changes (Moore et al., 2011). Such responses at all levels of biological organization are ultimately driven by temperature change, especially in ectothermic organisms. Within assemblages the composition and relative abundance of species with different thermal affinities are being re‐sorted (Burrows et al., 2020), often with aphasy in response from species with differing life‐history traits. Disturbance due to extreme weather events is superimposed upon these long‐term patterns of response to climate (Mieszkowska et al., 2021). Greater amplitude and more frequent return times of extreme events are already occurring and predicted to accelerate, themselves being symptoms of climate change. Both extreme events and pervasive climate change will have direct effects on individuals and hence populations, with consequences for community structure and ecosystem functioning. This is especially so when the species affected are important foundation species and/or ecosystem engineers, dominating space and providing biogenic habitat for others, often by ameliorating environmental conditions.
The direct effects of an extreme event on a foundation species and its community‐level consequences have been excellently explored by Hesketh and Harley (2022) in this issue. They report on rocky shore habitats exposed to a major heat anomaly—the 2021 Pacific Northwest Heat Dome. They used a classic combination of hypothesis‐testing surveys, in situ temperature recording, and in‐parallel experiments focusing on an important habitat‐forming, space‐occupying barnacle, Semibalanus cariosus. They measured variation in mortality across micro‐habitats with different temperature regimes modulated by topography and presence of canopy‐forming algae along a well‐characterized outer to inner coast thermal gradient. They used experimental shading to explore barnacle mortality and the consequences for assemblage diversity. The experiments were fortuitously established just before the heat dome event, but once it occurred, extensive targeted surveys showed its direct and indirect effects. There was extensive mortality, but with much variation from place to place at small spatial scales, emphasizing the importance of topographically derived refuges. No facilitation by fucoid canopy was found, unlike in other studies, but perhaps this reflected the small size of the algae involved. They emphasize that in the short term, facilitation by habitat‐forming species, including the matrix of dead barnacle shells, will ameliorate extreme events, especially under canopies or topographic shade. Such refuges will be important reservoirs of biodiversity—but with greater frequency of such events, even the benefits of facilitation will break down as habitat formers decline.
Rocky shores have long been used as a tractable system for experimental ecology, shaping concepts in population, community, and ecosystem ecology (Hawkins et al., 2020 for review). This is because of their accessibility and ease of experimental manipulation: they have sharp environmental gradients plus the short life spans of organisms lead to rapid response times; most algae, sessile, and sedentary animals compete for space to live—an easily quantifiable two‐dimensional resource; these attributes enable non‐destructive sampling and experimentation. Rocky shores are also emerging as sentinel systems for monitoring wider climate‐driven change in marine biodiversity and ecosystems. This is because they are cost‐effective to study on broad scales unlike offshore systems; in many locations there is little harvest from the shore (e.g., the British Isles), meaning that climate signals are not over‐ridden by over‐exploitation (e.g., fish in the English Channel, Genner et al., 2010). Changes on the seashore have long been known to mirror those offshore (Southward et al., 1995). In some parts of the world such as the Northeast Atlantic, there are extensive historic survey data that can be used as a baseline to measure change (Lima et al., 2007), including time‐series spanning warming periods (1930s to late 1950s), subsequent short‐term cooling (1960s to early 1980s), more rapid recent warming (late 1980s to mid‐2000s), including a more recent slight downturn (Burrows et al., 2020). Insights from experimental ecology can be invaluable in interpreting assemblage level responses, including unraveling the direct effects of climate from their indirect modulation by biological interactions (e.g., classic 1960s work by Connell informing time‐series analysis and modeling of warm‐water barnacles being released from competition from faster‐growing cold‐water species in warm springs by Poloczanska et al., 2008). Hesketh and Harley (2022) have amply demonstrated the melding of these twin traditions to better understand processes involved in climate change responses and their consequences at community level.
Hesketh and Harley (2022) also emphasize the importance of sustained observations or monitoring of both biota and environmental drivers in anticipation of extreme events which can cause much disturbance and mortality. Our work in the British Isles and Europe stretching back over four decades, overlapping with or re‐surveying work done by Crisp, Southward, and Lewis dating back to the 1950s, has shown gradual shifts in relative abundance of co‐existing sets of species from differing thermal evolutionary origins, with these increases in abundance driving poleward extensions of range edges (Hawkins et al., 2009). We have also seen more frequent damage from extreme weather to habitat‐forming fucoid canopy algae. In the cooler 1980s such damage was only observed infrequently in high‐shore species (Pelvetia canaliculata and Fucus spiralis). In many recent years, mid‐shore species such as F. vesiculosus and Ascophyllum nodosum, which had not shown damage in the past, have suffered at their upper limits along with their higher‐zoned counterparts. Extreme storms in the winter of 2013/2014 have also ripped off mussel beds (Mieszkowska et al., 2021).
Global change interacts with regional‐ and local‐scale chronic (press) and acute (pulse) anthropogenic impacts, including those caused by societal adaptation to climate change (e.g., reservoirs and irrigation to tackle drought; sea defenses to combat rising and stormier seas). In addition, local and regional impacts can reduce resilience of ecosystems to climate change. Conversely climate change can exacerbate local‐ and regional‐scale impacts. Managing such interactions is crucial to taking a precautionary approach to adapting to climate change. It is important to integrate understanding of how biodiversity and ecosystems respond to low‐amplitude long‐wavelength climate‐driven change with that of disturbance caused by more frequent and severe extreme events. Both are superimposed on natural disturbance regimes and fluctuations caused by stochastic recruitment events, typical of many open ecosystems such as the ocean. Understanding natural variability is essential for managing uncertainty while adapting to climate change in the short to medium term, until transition to low carbon economies occurs.
Sustained observing, coupled with in‐parallel experimentation and modeling to understand underlying processes, aid predictive power and are essential for forecasting future responses to global climate change. Work such as Hesketh and Harley's, using tractable systems contributes greatly to our understanding of the direct effects of climate change on populations and indirect consequences for communities and ecosystems.
Hawkins, S. J. , Burrows, M. T. , & Mieszkowska, N. (2023). Shoreline sentinels of global change show the consequences of extreme events. Global Change Biology, 29, 7–9. 10.1111/gcb.16477
This article is a Response to the Letter by Hesketh and Harley, https://doi.org.10.1111/gcb.16390.
DATA AVAILABILITY STATEMENT
The Seawater data that support the findings of this study are openly available in https://coastwatch.pfeg.noaa.gov/erddap/. Contact Michael T. Burrows for details of analysis.
REFERENCES
- Burrows, M. T. , Hawkins, S. J. , Moore, J. J. , Adams, L. , Sugden, H. , Firth, L. , & Mieszkowska, N. (2020). Global‐scale species distributions predict temperature‐related changes in species composition of rocky shore communities in Britain. Global Change Biology, 26, 2093–2105. 10.1111/gcb.14968 [DOI] [PubMed] [Google Scholar]
- Genner, M. J. , Sims, D. W. , Southward, A. J. , Budd, G. C. , Masterson, P. , Mchugh, M. , Rendle, P. , Southall, E. J. , Wearmouth, V. J. , & Hawkins, S. J. (2010). Body size‐dependent responses of a marine fish assemblage to climate change and fishing over a century‐long scale. Global Change Biology, 16, 517–527. 10.1111/j.1365-2486.2009.02027.x [DOI] [Google Scholar]
- Hawkins, S. J. , Pack, K. E. , Hyder, K. , Benedetti‐Cecchi, L. , & Jenkins, S. R. (2020). Rocky shores as tractable test systems for experimental ecology. Journal of the Marine Biological Association of the United Kingdom, 100, 1017–1041. 10.1017/S0025315420001046 [DOI] [Google Scholar]
- Hawkins, S. J. , Sugden, H. E. , Mieszkowska, N. , Moore, P. , Poloczanska, E. , Leaper, R. , Herbert, R. J. H. , Genner, M. J. , Moschella, P. S. , Thompson, R. C. , Jenkins, S. R. , Southward, A. J. , & Burrows, M. T. (2009). Consequences of climate driven biodiversity changes for ecosystem functioning of north European rocky shores. Marine Ecology Progress Series, 396, 245–259. 10.3354/meps08378 [DOI] [Google Scholar]
- Hesketh, A. V. , & Harley, C. D. (2022). Extreme heatwave drives topography‐dependent patterns of mortality in a bed‐forming intertidal barnacle, with implications for associated community structure. Global Change Biology, 29, 165–178. 10.1111/gcb.16390 [DOI] [PubMed] [Google Scholar]
- Lima, F. P. , Ribeiro, P. A. , Queiroz, N. , Xavier, R. , Tarroso, P. , Hawkins, S. J. , & Santos, A. M. (2007). Modelling past and present geographical distribution of the marine gastropod Patella rustica as a tool for exploring responses to environmental change. Global Change Biology, 13, 2065–2077. 10.1111/j.1365-2486.2007.01424.x [DOI] [Google Scholar]
- Mieszkowska, N. , Burrows, M. T. , Hawkins, S. J. , & Sudgen, H. (2021). Impacts of pervasive climate change and extreme events on rocky intertidal communities: Evidence from long‐term data. Frontiers in Marine Ecology, 8, 642764. 10.3389/fmars.2021.642764 [DOI] [Google Scholar]
- Moore, P. J. , Thompson, R. C. , & Hawkins, S. J. (2011). Phenological changes in intertidal con‐specific gastropods in response to climate warming. Global Change Biology, 17, 709–719. 10.1111/j.1365-2486.2010.02270.x [DOI] [Google Scholar]
- Parmesan, C. , & Yohe, G. (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421, 37–42. 10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Poloczanska, E. S. , Hawkins, S. J. , Southward, A. J. , & Burrows, M. T. (2008). Modeling the response of populations of competing species to climate change. Ecology, 89, 3138–3149. 10.1890/07-1169.1 [DOI] [PubMed] [Google Scholar]
- Southward, A. J. , Hawkins, S. J. , & Burrows, M. T. (1995). Seventy years' observations of changes in distribution and abundance of zooplankton and intertidal organisms in the western English Channel in relation to rising sea temperature. Journal of Thermal Biology, 20, 127–155. 10.1016/0306-4565(94)00043-1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Seawater data that support the findings of this study are openly available in https://coastwatch.pfeg.noaa.gov/erddap/. Contact Michael T. Burrows for details of analysis.


