Abstract
HER2‐targeted anticancer therapies may be associated with cardiovascular adverse events. This study evaluated effects of the HER2‐targeted antibody–drug conjugate trastuzumab deruxtecan (T‐DXd, DS‐8201a) on QT/QTc interval and its pharmacokinetics. Patients with heavily pretreated, metastatic HER2‐expressing breast cancer were enrolled at seven study sites in Japan. T‐DXd was administered intravenously at 6.4 mg/kg on day 1 of each 21‐day cycle. Primary end points were baseline‐adjusted QTcF interval and pharmacokinetics parameters. Key secondary end points included safety events, serum concentration of T‐DXd and DXd at the time of electrocardiographic measurements, and antitumor activity parameters. Among 51 total patients, 47 (92.2%) had HER2‐low breast cancer (immunohistochemistry 1+ or 2+ and in situ hybridization–negative/equivocal/missing). Pharmacokinetic parameters after a single dose of T‐DXd were consistent with previous studies. After multiple doses, T‐DXd showed moderate accumulation (accumulation ratio (cycle 3/cycle 1), 1.35), but DXd showed minimal accumulation (1.09). The upper bound of the 90% confidence interval for mean ΔQTcF interval was < 10 ms at all timepoints, and at mean maximum serum concentration was also < 10 ms. Based on concentration‐QT analysis, ΔQTcF increased with increasing concentrations of T‐DXd and DXd. No clinically meaningful QTcF prolongation was observed. T‐DXd had a manageable safety profile and showed antitumor activity in HER2‐low breast cancer. In this study, a T‐DXd dose of 6.4 mg/kg, higher than the 5.4‐mg/kg dose currently approved for breast cancer, was not associated with clinically relevant QTcF prolongation in heavily pretreated patients with HER2‐expressing metastatic breast cancer. This study adds to our understanding of T‐DXd for treatment of HER2‐low breast cancer.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Trastuzumab deruxtecan (T‐DXd) has been approved for the treatment of HER2‐positive and HER2‐low (United States) metastatic breast cancer and HER2‐positive metastatic gastric cancer; it is under investigation for other indications. Previous studies showed HER2‐targeted therapies may be associated with cardiovascular adverse events. In addition, previous studies of T‐DXd investigated pharmacokinetic parameters after a single dose.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study focused on the effect of T‐DXd on QT/QTc interval and the pharmacokinetic profile of T‐DXd after multiple doses in heavily pretreated patients with advanced HER2‐expressing breast cancer.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The results of this study show T‐DXd did not have a clinically meaningful impact on QTc prolongation, and its payload accumulated minimally after multiple doses, suggesting that T‐DXd is stable in systemic circulation. T‐DXd also had a manageable safety profile and antitumor activity in HER2‐positive and HER2‐low breast cancer.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The results of this study will inform physicians about the use of T‐DXd to treat HER2‐expressing tumors.
Trastuzumab deruxtecan (T‐DXd) is a novel, humanized antibody–drug conjugate (ADC) consisting of trastuzumab bound to a cytotoxic topoisomerase I inhibitor by a cleavable tetrapeptide‐based linker. 1 T‐DXd has shown antitumor activity and a manageable safety profile in treatment of human epidermal growth factor receptor 2 (HER2)–expressing or –mutated solid tumors, including HER2‐low breast cancer. 2 , 3 , 4 , 5 , 6 T‐DXd is approved in various countries worldwide for the treatment of patients with unresectable or metastatic HER2‐positive breast cancer (after ≥ 1 anti‐HER2–based regimen in the United States and Europe) or gastric cancer (after chemotherapy or a trastuzumab‐based regimen) 3 , 4 , 7 , 8 , 9 , 10 , 11 , 12 and was recently approved in the United States for the treatment of patients with unresectable or metastatic HER2‐low breast cancer (immunohistochemistry (IHC) 1+ or IHC 2+/in situ hybridization (ISH)–negative, after chemotherapy). 6 , 7
In the DS8201‐A‐J101 study (ClinicalTrials.gov number NCT02564900), the pharmacokinetic parameters of T‐DXd were evaluated after a single dose. After a single dose of T‐DXd, the total antibody concentrations were similar to concentrations of T‐DXd at all timepoints. 2 The study also showed that the serum concentration of DXd was low after the single dose of T‐DXd. 2
Consideration of the effect of T‐DXd on QT interval is important because anticancer drugs and their side effects can cause QT prolongation. 13 , 14 Specifically, many HER2‐directed therapies are potentially associated with cardiovascular adverse events (AEs). 15 , 16 Results from murine studies indicate that HER2 signaling plays a role in both apoptosis and maintenance of cardiac health, 16 and results from initial trials of trastuzumab administered concomitantly with chemotherapy showed rates of depressed left ventricular ejection fraction (LVEF) as high as 26.9%. 17 However, most new HER2‐directed therapies (e.g., T‐DXd, lapatinib, pertuzumab, and trastuzumab emtansine (T‐DM1)) have been shown to pose less cardiotoxicity risk compared with trastuzumab. 18 , 19 Cardiovascular AEs and QT risk have been well‐characterized for trastuzumab and other HER2‐targeted therapies 20 , 21 , 22 but not for the ADC T‐DXd or DXd.
Results of human ether‐a‐go‐go‐related gene (hERG) studies of DXd showed that DXd did not inhibit the hERG channel current. 23 In telemetered male cynomolgus monkeys treated with single intravenous doses of T‐DXd, no effects on the cardiovascular, respiratory, or central nervous systems were observed at dose levels up to 78.8 mg/kg. 23 Furthermore, clinically significant effects of T‐DXd on LVEF reduction or heart failure have been infrequently reported to date. 2 , 3 , 4 , 6 , 24 , 25 , 26 Nonetheless, the current International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use E14 guidelines still recommend the evaluation of effect of non‐antiarrhythmic drugs in development on QT intervals and QTc. 27 Although the cardiotoxic effects of HER2‐targeting therapies have been well‐documented in patients with HER2‐positive metastatic breast cancer, 21 , 22 cardiotoxicity in patients with HER2‐expressing breast cancer, including HER2‐low, is not well‐established. Because of the significant benefit of T‐DXd in this patient population, 6 further investigation into the drug's cardiotoxicity in patients with tumors across the spectrum of HER2 expression is warranted.
In this multicenter, open‐label, multiple‐dose, phase I study (DS8201‐A‐J102; ClinicalTrials.gov number NCT03366428), the effects of multiple doses of T‐DXd on QT/QTc interval and pharmacokinetics were assessed in patients with HER2‐expressing (IHC ≥ 1+ and/or ISH–positive) unresectable and/or metastatic breast cancer that is refractory or intolerable to standard treatment. Secondary end points included safety and antitumor activity.
Patients were treated with T‐DXd 6.4 mg/kg, the highest dose of T‐DXd that did not have dose‐limiting toxicities in a phase I study, 2 which was selected for this study based on its balance of efficacy, safety, and pharmacokinetics. 28 The 5.4‐mg/kg dose is currently the approved dose of T‐DXd monotherapy for breast cancer 3 , 7 , 8 , 10 , 11 , 28 ; the 6.4‐mg/kg dose is approved for gastric cancer. 4 , 7 , 8
METHODS
Study design and patients
The study protocol was approved by the ethics committees or institutional review boards at each study site. This study was conducted in compliance with its protocol, the ethical principles outlined in the Declaration of Helsinki, the International Council for Harmonization Guideline for Good Clinical Practice, and all applicable regulatory requirements. Written informed consent was provided by each patient before evaluation for eligibility.
Patients received intravenous T‐DXd at 6.4 mg/kg on day 1 of each 21‐day cycle. The dose was chosen based on efficacy, tolerability, and the pharmacokinetics profile established in a phase I study. 2 Patients included in the primary analyses either discontinued the study or completed at least three cycles, whichever came first. The first patient was enrolled on December 26, 2017, and primary analyses of pharmacokinetic end points and QTcF interval were based on a data cutoff of December 5, 2018. For safety and efficacy analyses, the patients were followed up after the primary analysis until March 26, 2021.
Primary end points were serum concentration and pharmacokinetics parameters (area under plasma concentration‐time curve over the dosing interval (AUCtau), maximum serum concentration (Cmax), time to reach Cmax (Tmax), and trough serum concentration (Ctrough)) of T‐DXd, total anti‐HER2 antibody, and MAAA‐1181a (DXd) after single and multiple dosing, and baseline‐adjusted QTcF interval. Secondary end points of interest included serious AEs (SAEs), treatment‐emergent adverse events (TEAEs), confirmed overall response rate (ORR; the sum of proportion of patients with complete response (CR) rate and partial response (PR) rate) per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, disease control rate (DCR; the sum of proportion of patients with CR, PR, and stable disease for ≥ 5 weeks from the first dosing date), clinical benefit rate (CBR; sum of proportion of patients with CR, PR, and stable disease for > 6 months), duration of response (DOR), time to response (TTR), progression‐free survival (PFS), and overall survival (OS). The efficacy end points were measured and confirmed by individual investigators.
Key inclusion and exclusion criteria
Patients were required to be ≥ 20‐year‐old women or men with pathologically documented unresectable or metastatic breast tumor with HER2 expression that was refractory to or intolerable with standard treatment or for which no standard treatment was available. HER2 expression was defined as IHC ≥ 1+ and/or ISH+, and the study included patients with HER2‐low tumors (IHC 1+ or 2+ and ISH‐negative/equivocal/missing) and HER2+ tumors (IHC 3+ or IHC 2+/ISH+). Patients were also required to have an Eastern Cooperative Oncology Group performance status of 0 or 1 and LVEF ≥ 50% assessed via echocardiography or multigated acquisition. Patients were not eligible for the study if they had any of several cardiovascular conditions or current, suspected, or a history of noninfectious interstitial lung disease (ILD)/pneumonitis. Further information on key exclusion criteria and screening procedures performed before enrollment is provided in the Supplementary Methods .
Cardiac function assessments
QTcF interval was assessed by 12‐lead electrocardiography (ECG) in triplicate for screening within 7 days before enrollment and again for baseline within 3 days before cycle 1, day 1 at 15 minutes before the planned start of administration, and 30 minutes, 2, 4, and 7 hours after the planned start time. Electrocardiography was also assessed on day 1 of cycle 3, within 15 minutes of T‐DXd administration, 15 minutes within end of infusion, and 2, 4, and 7 hours after the planned start time. Follow‐up ECGs were obtained on days 8 and 15 of cycles 1 and 3. Baseline electrocardiographic evaluation for cycle 2 as well as cycles 4 and beyond was to occur within 3 days before administration. A final ECG was obtained 7 days after the last dose. All electrocardiographic evaluations were performed in triplicate.
In addition to evaluation by the investigator, ECGs at screening, cycle 1, cycle 2, and cycle 3 (including QTcF) were reviewed by a qualified cardiologist in the central laboratory. The QTc interval calculation in this study was performed using the cardiologist assessment data. If QT prolongation was grade 3 in severity (QTc > 500 ms on 2 separate ECGs), the T‐DXd dose was delayed until resolution to grade ≤ 1 (QTc ≤ 480 ms).
The investigators assessed if another medication that the patient was receiving was potentially responsible, and the dose was adjusted or, if necessary, abnormalities in serum electrolytes were corrected. If electrocardiographic changes (grade 3, defined as QTc > 500 ms on 2 separate ECGs) were attributed to T‐DXd, the dose was reduced by 1 level.
If QT prolonged to grade 4 (QTc > 500 or > 60 ms change from baseline and torsade de pointes or polymorphic ventricular tachycardia or signs/symptoms of serious arrhythmia), T‐DXd was discontinued. If acute myocardial infarction was confirmed, T‐DXd was discontinued.
Baseline‐adjusted QTc interval calculation
The baseline QTc interval for each patient was subtracted from the QTcF interval to create a baseline‐adjusted QTcF interval for each patient at each timepoint (cycles 1 to 3). Baseline‐adjusted QTcF was calculated using time‐matched baseline. The baseline‐adjusted QTcF was averaged across each timepoint, and a pointwise 2‐sided 90% confidence interval (CI) was also calculated.
The concentration‐QT relationship using the baseline‐adjusted QTcF was quantified following linear mixed effects modeling:
where ΔQTcFit, C(T‐DXd or DXd)it, and QTc0 are the change from baseline in QTc for patient i at time t, serum concentration of T‐DXd or DXd for patient i and time t, and overall mean of QTc i=0, which is the baseline of patient i, respectively. The parameters, θ 0, θ 1, and θ 2, are the population mean intercept, the population mean slope of the assumed linear association between concentration and ΔQTcFit, and the fixed effect associated with baseline QTc i=0, respectively, and η 0i and η 1i are the random effect associated with the intercept term θ 0 and the random effect associated with the slope θ 1.
Pharmacokinetic assessments
Starting day 1 of cycle 1, blood samples were collected before administration within 10 minutes after completing the electrocardiographic measurement on days 1, 8, and 15 of cycles 1 and 3 and within 10 minutes after the infusion on day 1 of cycle 1. Samples were also collected on days 2 and 4 of cycles 1 and 3. On day 1 of cycles 2, 4, 6, and 8, samples were collected before the infusion and within 30 minutes after the infusion.
Safety assessments
Safety end points included SAEs, TEAEs, and ECG/multigated acquisition findings. All clinical AEs occurring after the patient signed the Informed Consent Form and up to the 40‐day follow‐up visit (+7 days), whether observed by the investigator or reported by the patient, were recorded as AEs. LVEF decrease and ILD/pneumonitis were assessed based on Standardized MedDRA Queries of Cardiac Failure and ILD (plus acute respiratory failure and respiratory failure), respectively. All events of potential drug‐related ILD/pneumonitis were evaluated by an external independent adjudication committee. Before each treatment, patients were assessed for toxicity based on National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Antitumor activity assessments
The following antitumor activities were assessed by the investigators: ORR (evaluated using RECIST, version 1.1), DCR, DOR, TTR, PFS, and OS. Efficacy assessments were based on tumor assessments via computed tomography or magnetic resonance imaging performed at screening and every 6 weeks in the first 24 weeks after day 1 of cycle 1 and thereafter every 12 weeks while the patient remained on study drug.
Statistical considerations
A sample size of 50 patients was determined based on a calculation that a baseline‐adjusted QTcF interval of 0 ms with an SD of 15 ms provides 99.9% probability that the upper bound of the 2‐sided 90% CI for the baseline‐adjusted QTcF interval would be < 10 ms. The estimated SD of 15 ms was derived from a phase II study with T‐DM1. 15
Pharmacokinetic parameters, based on noncompartmental analysis, were analyzed using the Pharmacokinetics Analysis Set, which included all enrolled patients who received ≥ 1 dose of T‐DXd and had measurable serum concentrations of the drug. QT intervals were analyzed using the Cardiac Safety Analysis Set, which included all enrolled patients who received ≥ 1 dose of T‐DXd, had time‐matched baseline and post‐treatment electrocardiographic data, and did not receive QTc prolongation drugs during the period when their administration is prohibited. Safety outcomes were analyzed using the Safety Analysis Set, which included all enrolled patients who received ≥ 1 dose of T‐DXd. Antitumor activities were analyzed using the Efficacy Analysis Set, which included all enrolled patients who received ≥ 1 dose of T‐DXd and had pre‐ and post‐treatment efficacy data for the target or nontarget tumors.
The statistical analysis was performed using SAS version 9.3 or higher (SAS Institute, Cary, NC). Pharmacokinetic analysis was performed using Phoenix WinNonlin version 6.4 or higher (Certara USA, Princeton, NJ).
RESULTS
Patients
Fifty‐one female patients were enrolled at seven study centers in Japan (Table S2 ). Patient disposition is shown in Figure S1 ; at data cutoff, all 51 subjects had discontinued treatment.
The median age was 56.0 years (range, 31–79), with most patients (74.5%) younger than 65 years old. HER2‐low patients (defined as IHC 1+, IHC 2+/ISH‐negative, IHC 2+/ISH‐missing, or IHC 2+/ISH‐equivocal) represented 92.2% of patients (47/51).
All patients had received cancer therapy before study enrollment. Most patients (80.4%) had an accumulated dose of anthracyclines (doxorubicin‐equivalent dose) of < 300 mg/m2, followed by 350–400 mg/m2 (7.8%), 300–350 mg/m2 (3.9%), 400–450 mg/m2 (3.9%), and 450–500 mg/m2 (2.0%). Data for accumulated dose of anthracyclines were missing for 2.0% of patients. At least 5 prior cancer therapy regimens had been received by 78.4% (40/51) of patients for locally advanced or metastatic breast cancer. Additional demographics and baseline characteristics are summarized in Table 1 .
Table 1.
Demographics and baseline characteristics
| Characteristic/category | Total (N = 51) |
|---|---|
| Age, median (range), years | 56 (31–79) |
| ≥ 65 years, n (%) | 13 (25.5) |
| Female, n (%) | 51 (100) |
| ECOG performance status, n (%) | |
| 0 | 31 (60.8) |
| 1 | 20 (39.2) |
| Estrogen receptor, n (%) | |
| Positive | 38 (74.5) |
| Negative | 13 (25.5) |
| Progesterone receptor, n (%) | |
| Positive | 23 (45.1) |
| Negative | 28 (54.9) |
| HER2 expression, n (%) | |
| HER2‐positive | |
| IHC 3+ | 2 (3.9) |
| IHC 2+/ISH‐positive | 2 (3.9) |
| HER2‐low | |
| IHC 1+ | 37 (72.5) |
| IHC 2+/ISH‐negative | 6 (11.8) |
| IHC 2+/ISH‐missing | 2 (3.9) |
| IHC 2+/ISH‐equivocal | 2 (3.9) |
| Any prior cancer therapy, n (%) | 51 (100) |
| Hormone therapy | 43 (84.3) |
| CDK4/6 inhibitors | 11 (21.6) |
| Trastuzumab | 12 (23.5) |
| Pertuzumab | 8 (15.7) |
| T‐DM1 | 8 (15.7) |
| Anthracyclines | 51 (100) |
| ≥ 5 prior cancer therapy regimens,a n (%) | 40 (78.4) |
CDK4/6, cyclin‐dependent kinase 4/6; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization; T‐DM1, trastuzumab emtansine.
For locally advanced or metastatic breast cancer.
Pharmacokinetics
All 51 patients (Pharmacokinetics Analysis Set; cutoff date of December 5, 2018) were included in the analysis for cycle 1, and of 47 patients still on treatment at cycle 3, 37 patients were eligible for inclusion in the cycle 3 analysis. The AUCtau of T‐DXd increased in cycle 3 compared with cycle 1 (Table 2 ). The accumulation ratio was 1.35 (SD, 0.15), which represents a moderate increase of 35% in the AUCtau in cycle 3 compared with cycle 1. Thus, T‐DXd almost reached steady‐state by cycle 3. In contrast, DXd showed minimal accumulation, with an accumulation ratio of 1.09.
Table 2.
Pharmacokinetics parameters for T‐DXd, total anti‐HER2 antibody, and DXd by cycle
| Patients (N = 51) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (range) | ||||||||
| Cmax, μg/mL | AUCtau, μg.day/mL | AUClast, μg.day/mL | AUCinf, μg.day/mL | Ctrough, μg/mL | t 1/2, day | CL, mL/day/kg | Vss, mL/kg | Tmax, hour | |
| T‐DXd | |||||||||
| Cycle 1 n = 51 | 179 (112) | 677 (141) | 677 (143) | 731 (169) | 6.03 (2.96) | 5.82 (1.11) | 9.18 (2.00) | 60.9 (11.0) | 2.08 (1.55–7.02) |
| Cycle 3 n = 37 | 154 (23.3) | 905 (189) | — | — | 11.8 (4.33) | 7.40 (1.48) | 7.40 (1.75) | 66.3 (12.8) | 2.12 (0.70–7.15) |
| AR, cycle 3‐cycle 1 | — | 1.35 (0.150) | — | — | — | — | — | — | — |
| Total anti‐HER2 antibody | |||||||||
| Cycle 1 n = 51 | 165 (110) | 752 (185) | 753 (190) | 846 (281) | 8.61 (5.72) | 6.35 (2.01) | — | — | 2.07 (1.48–7.02) |
| Cycle 3 n = 37 | 142 (27) | 1,030 (256) | — | — | 16.7 (7.97) | 8.27 (1.97) | — | — | 2.07 (0.60–7.15) |
| AR, cycle 3‐cycle 1 | — | 1.36 (0.219) | — | — | — | — | — | — | — |
| Cmax, ng/mL | AUCtau, ng.day/mL | AUClast ng.day/mL | AUCinf, ng.day/mL | Ctrough, ng/mL | t 1/2, day | CL mL/day/kg | Vss mL/kg | Tmax, hour | |
|---|---|---|---|---|---|---|---|---|---|
| DXd | |||||||||
| Cycle 1 n = 51 | 12.6 (4.49) | 39.0 (11.2) | 39.3 (11.3) | 40.4 (10.9) | 0.296 (0.128) | 5.74 (1.29) | — | — | 6.93 (3.88–191.47) |
| Cycle 3 n = 37 | 9.6 (3.89) | 41.5 (13.8) | — | — | 0.409 (0.150) | 6.57 (1.81) | — | — | 6.92 (1.95–70.65) |
| AR, cycle 3‐cycle 1 | — | 1.09 (0.194) | — | — | — | — | — | — | — |
AR, accumulation ratio; AUCinf, area under the serum concentration‐time curve up to infinity; AUClast, area under the serum concentration‐time curve up to the last quantifiable time; AUCtau, area under the serum concentration‐time curve during the dosing interval; CL, total body clearance; Cmax, maximum serum concentration; Ctrough, trough serum concentration; HER2, human epidermal growth factor receptor 2; t1/2, terminal elimination half‐life; T‐DXd, trastuzumab deruxtecan; Tmax, time to reach Cmax; Vss, volume of distribution at steady‐state.
The Cmax of T‐DXd was essentially unchanged in cycle 3 compared with cycle 1 (Table 2 ). On the other hand, the Ctrough values of T‐DXd almost doubled from cycle 1 to cycle 3 (6.03 vs. 11.8; Table 2 ). The median Tmax of T‐DXd and the total anti‐HER2 antibody load were also highly similar between cycles 1 and 3, at ~ 2 hours for both (Table 2 ). In contrast, the mean terminal elimination half‐life (t 1/2) of T‐DXd increased from cycle 1 to cycle 3 (5.82 days vs. 7.40 days; Table 2 ). The total anti‐HER2 antibody load had a similar pattern to T‐DXd for AUCtau, Cmax, Ctrough, Tmax, and t 1/2. In general, much lower systemic exposure was observed in DXd for all pharmacokinetics parameters (Table 2 , Figure S2 ).
QTc interval
Forty‐nine patients, comprising the Cardiac Safety Analysis Set, were included in this analysis with a cutoff date of December 5, 2018. Two patients were excluded because of concomitant QT‐prolonging agents; the first patient received sulfamethoxazole trimethoprim twice, and the second patient received ephedrine three times. Four patients experienced a notable electrocardiographic change with a > 30‐ms increase in QTcF during the study (Table S3 ). No patients had a QTcF increase > 60 ms, and the maximum QTcF value remained < 480 ms (Table S3 ).
A slight increase in the mean QTcF was observed up to 7 hours after dose administration at cycles 1 and 3 (Figure 1 ), which corresponds approximately to the Tmax of DXd after the T‐DXd administration of cycles 1 and 3 (Table 2 ). The upper boundary of the 90% CI was < 10 ms at all timepoints (Figure 1 ).
Figure 1.
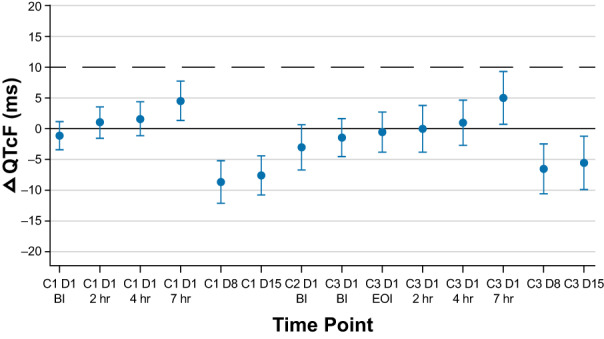
Mean change from baseline over time in time‐matched, baseline‐adjusted ΔQTcF and 2‐sided 90% confidence interval. Two patients received prohibited QTc prolongation drugs and were thus excluded from this analysis. ΔQTcF, change in Fridericia‐corrected QT interval; BI, before infusion; C, cycle; D, day; EOI, end of infusion.
The concentration‐QT analysis showed a trend of slight increase in QTcF change with increasing concentrations of T‐DXd and DXd (Figures 2 a,b ). The relationship between ΔQTcF and concentrations of T‐DXd was much smaller than that observed for the relationship between ΔQTcF and concentrations of DXd (difference between ΔQTcF interval estimate of cycle 1 and cycle 3, 0.1 vs. 2.0, respectively). The upper bound of the 90% CI for the relationship between concentrations of DXd and ΔQTcF was slightly over 10 ms at the highest concentration of DXd.
Figure 2.
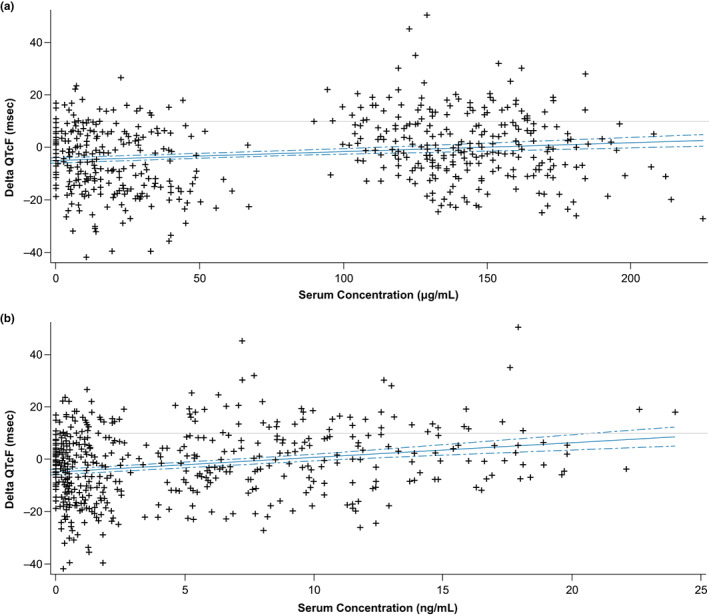
Relationship between ΔQTcF and concentration of (a) T‐DXd and (b) DXd. QTcF, change in Fridericia‐corrected QT interval; T‐DXd, trastuzumab deruxtecan. The baseline QTcF interval for each patient was subtracted from QTcF interval to create a baseline‐adjusted QTcF interval for each patient at each timepoint (cycle 1 to cycle 3). Solid lines represent the model, predicted baseline‐adjusted ΔQTcF at a given concentration; the dotted lines represent the 90% confidence interval of the model. (a) ΔQTcF, DS‐8201a = −5.34 + 0.044 × concentration. (b) ΔQTcF, MAAA‐1181a = −4.94 + 0.65 × concentration.
At the observed Cmax values for T‐DXd and DXd, the estimated mean change in QTcF using the linear model equation indicated the value was < 10 ms (Table 3 ). Both QT and concentration‐QT analysis indicated that T‐DXd administered at 6.4 mg/kg was not associated with clinically meaningful QTcF prolongation (i.e., change from baseline of > 10 ms; Figures 2 a,b ).
Table 3.
Relationship between QTcF interval and concentration of T‐DXd and DXd
| QTcF interval (maximum serum concentration) | T‐DXd (N = 49a) |
|---|---|
| T‐DXd | |
| At mean Cmax on cycle 1 | |
| ΔQTcF interval | 1.3 |
| 90% CI | −1.4 to 4.0 |
| At mean Cmax on cycle 3 | |
| ΔQTcF interval | 1.4 |
| 90% CI | −1.4 to 4.1 |
| DXd | |
| At mean Cmax on cycle 1 | |
| ΔQTcF interval | 2.6 |
| 90% CI | −0.3 to 5.4 |
| At mean Cmax on cycle 3 | |
| ΔQTcF interval | 0.4 |
| 90% CI | −1.8 to 2.7 |
CI, confidence interval; Cmax, maximum serum concentration; QTcF, QT corrected using Fridericia's formula; T‐DXd, trastuzumab deruxtecan.
The estimated value and its 90% CIs are calculated based on the model where concentration and baseline (difference between individual value and mean value) are fixed covariates and measurement time is a fixed factor and random effects for the intercept and slope are included.
Two patients were excluded because of concomitant QT‐prolonging agents.
Safety
The data cutoff date for safety analyses was March 26, 2021, and used the Safety Analysis Set (N = 51). The median duration of treatment was 7.0 months (range, 0.7–26.5), and the median total number of cycles initiated was 10.0 cycles (range, 1–36). All patients experienced at least one TEAE. Grade 3 or 4 drug‐related events occurred in 41 (80.4%) patients. The most common (> 50%) any‐grade drug‐related TEAEs included nausea (n = 42, 82.4%), neutrophil count decreased (n = 36, 70.6%), white blood cell count decreased (n = 33, 64.7%), and anemia (n = 31, 60.8%; Table S4 ). The most common grade ≥3 drug‐related TEAEs (> 10%) were neutrophil count decreased (n = 26, 51.0%), white blood cell count decreased (n = 16, 31.4%), anemia (n = 7, 13.7%), and lymphocyte count decreased (n = 7, 13.7%; Table S4 ).
SAEs occurred in eight (15.7%) patients. The four (7.8%) drug‐related SAEs were nausea (n = 2, 3.9%), ILD (n = 1, 2.0%), and pneumonitis (n = 1, 2.0%). Out of 51 patients, dose interruption, dose reduction, or study withdrawal due to AEs were required in 35 (68.6%), 7 (13.7%), and 14 (27.5%) patients, respectively. There were no TEAEs resulting in death.
Five (9.8%) patients experienced grade 1 QT prolongation (450–< 480 ms), but all recovered and continued in the study. There were no SAEs, no patients required medication, and no action was taken with T‐DXd because of QT prolongation. One patient had a treatment‐related grade 2 decreased ejection fraction, and the patient was withdrawn from the study because of the event.
There were 13 subjects (25.5%) who had ILD/pneumonitis events adjudicated as drug‐related by an independent ILD adjudication committee. Most (11/13; 84.6%) ILD/pneumonitis events were grade 1 or 2; 2 (15.4%) events were grade 3.
Antitumor activity
Antitumor activity was assessed in the Efficacy Analysis Set (N = 51), with a cutoff date of March 26, 2021 (Table 4 ). Investigator‐assessed confirmed ORR was achieved by 43.1% (95% CI, 29.3–57.8) of total patients. DCR was achieved by 43 patients (84.3%; 95% CI, 71.4–93.0), and the CBR was achieved by 25 patients (49.0%; 95% CI, 34.8–63.4). The median TTR was 3.0 months (range, 1.2–16.6), corresponding to the second postbaseline scan. The median DOR was 8.5 months (95% CI, 5.1–15.4), the median PFS was 8.1 months (95% CI, 5.6–10.2), and the median OS was 27.1 months (95% CI, 20.5–not evaluable) for the overall patient population. Among the 47 patients with HER2‐low breast cancer, the investigator‐assessed confirmed ORR was 42.6% (95% CI, 28.3–57.8), and the median PFS was also 8.1 months (95% CI, 5.6–9.9; Table 4 ).
Table 4.
Summary of antitumor activities
| HER2‐positive (IHC 3+ or ISH‐positive) n = 4 | HER2‐low (IHC 1+ or IHC 2+/ISH‐negative or ‐missing) n = 47 | Total N = 51 | |
|---|---|---|---|
| Confirmeda ORR (95% CI), % | 50.0 (6.8–93.2) | 42.6 (28.3–57.8) | 43.1 (29.3–57.8) |
| CR, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PR, n (%) | 2 (50.0) | 20 (42.6) | 22 (43.1) |
| Stable disease, n (%) | 1 (25.0) | 22 (46.8) | 23 (45.1) |
| Non‐CR/non‐PD, n (%) | 1 (25.0) | 2 (4.3) | 3 (5.9) |
| PD, n (%) | 0 (0.0) | 3 (6.4) | 3 (5.9) |
| NE, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unconfirmedb ORR (95% CI), % | 50.0 (6.8–93.2) | 48.9 (34.1–63.9) | 49.0 (34.8–63.4) |
| TTR, median (range), months | 2.0 (1.4–2.6) | 3.0 (1.2–16.6) | 3.0 (1.2–16.6) |
| CBR (95% CI), % | 50.0 (6.8–93.2) | 48.9 (34.1–63.9) | 49.0 (34.8–63.4) |
| DOR, median (range), months | — | 7.6 (5.1–15.4) | 8.5 (5.1–15.4) |
| DCR (95% CI), % | 50.0 (6.8–93.2) | 87.2 (74.3–95.2) | 84.3 (71.4–93.0) |
| PFS, median (95% CI), months | 11.14c | 8.1 (5.6–9.9) | 8.1 (5.6–10.2) |
| OS, median (95% CI), months | — | 24.7 (18.4–NE) | 27.1 (20.5–NE) |
CBR, clinical benefit rate; CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization; NE, not evaluable; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; PD, progressive disease; PR, partial response; TTR, time to response.
Confirmed by investigator.
Not confirmed by investigator.
95% CI could not be calculated because of the small sample size.
DISCUSSION
In this study, the effect of T‐DXd on QT/QTc interval was assessed in patients with HER2‐expressing unresectable and/or metastatic breast cancer, along with pharmacokinetic parameters after multiple doses. T‐DXd administered at 6.4 mg/kg was not associated with clinically relevant QTcF prolongation; there were no occurrences of clinically relevant QTcF prolongation at any timepoint, although a tendency of QT prolongation for up to 7 hours was observed. Furthermore, even at the upper bound of the 90% CI for ΔQTcF at the observed mean Cmax for T‐DXd and DXd, there were no instances of clinically relevant QTcF prolongation. Although 5 patients experienced mild QTcF prolongation, it was grade 1 (450–< 480 ms) in all cases and therefore deemed not clinically relevant. None of the patients required medication, and no action was taken with T‐DXd because of TEAEs of electrocardiography QT prolongation. All patients recovered and continued with the study. These results are consistent with those of studies of trastuzumab, 21 pertuzumab, 22 and T‐DM1, 15 in which QT prolongation in patients with HER2‐positive metastatic breast cancer was not significantly affected. In contrast with HER2‐targeted small molecules, such as the tyrosine kinase inhibitor lapatinib, antibody‐based therapies are not expected to affect ion channels in the heart because of their high specificity and large size. 21 , 22 Although an increased risk of LVEF decline and congestive heart failure has been observed with trastuzumab in patients with HER2‐positive breast cancer with or after anthracycline treatment, 20 there was no clear evidence of heart failure or LVEF decline in the current study, in which all patients had previously received anthracyclines.
Serum concentration of T‐DXd reached steady‐state within the duration of this study. After a single dose of T‐DXd, the pharmacokinetic parameters assessed in this study were consistent with those of previous studies. The accumulation of T‐DXd was moderate, and based on the data, steady‐state was achieved by cycle 3. Accumulation of DXd was minimal, possibly because of reduced release from decreased numbers of HER2‐expressing tumor cells after multiple doses of T‐DXd. A similar phenomenon was reported for the ADC brentuximab vedotin. 29
ILD/pneumonitis is an important identified risk associated with T‐DXd that requires careful monitoring and active management. In general, the incidence of anticancer drug‐related ILD/pneumonitis in patients from Japan is higher than other countries, and a similar trend has been observed in T‐DXd clinical trials. 3 , 4 , 8 , 24 , 30 , 31 , 32 In this small study using a higher T‐DXd dose, 13 of 51 (25.5%) patients had events adjudicated as being drug‐related ILD/pneumonitis, with the majority reported as either grade 1 (5/13 (38.5%)) or grade 2 (6/13 (46.2%)); 2 patients had grade 3 ILD/pneumonitis. None of the events were associated with an outcome of death. A dose of 6.4 mg/kg may have contributed to the overall incidence of ILD/pneumonitis of 25.5%, but this incidence is consistent with data from patients with breast cancer treated in Japan with 5.4 mg/kg of T‐DXd (23.3%). 8 Data from ongoing studies in addition to completed studies will continue to elucidate patient risk factors for ILD/pneumonitis. 3 , 4 , 24 , 31 , 32 , 33 Since this trial was initiated, increased recognition of ILD/pneumonitis as an AE of special interest with T‐DXd has led to improved monitoring, management, and diagnosis. 25
This study was conducted with a dose of 6.4 mg/kg, which is higher than the currently approved dose for metastatic breast cancer (5.4 mg/kg). 7 The recommended dose for breast cancer had not been determined at the beginning of this study. Preliminary data from a phase I study showed both 5.4‐ and 6.4‐mg/kg doses showed favorable benefit–risk profile in patients with HER2‐expressing metastatic solid tumors. 2 Additionally, the recommended dose of T‐DXd for gastric cancer has been determined to be 6.4 mg/kg; this dose has been used for a phase II T‐DXd gastric cancer trial. 4 In a post hoc analysis of pooled clinical trial data, 6.4‐mg/kg dosing in gastric cancer resulted in similar T‐DXd exposure to 5.4‐mg/kg dosing in breast cancer. 34 Therefore, 6.4 mg/kg was chosen, so the results of this study can be translated across multiple indications. Finally, several phase II and III studies of T‐DXd treatment in 5.4‐ and 6.4‐mg/kg doses in patients with HER2‐positive, −low, or ‐mutated tumors are currently ongoing. 35
This study showed that in patients with HER2‐low breast cancer, T‐DXd 6.4 mg/kg demonstrated antitumor activity without substantially greater toxicity when compared with the 5.4‐mg/kg dose currently approved for patients with HER2‐positive and HER2‐low advanced breast cancer. 7 These data from 47 patients are consistent with results reported from the DS8201‐A‐J101 study, which included 54 patients with HER2‐low breast cancer treated at either the 5.4‐mg/kg or 6.4‐mg/kg dose levels. The confirmed response rate by independent central review in that study was 37.0% (95% CI, 24.3–51.3) and median DOR was 10.4 months (95% CI, 8.8–NE). 31 In the phase III DESTINY‐Breast04 trial, confirmed response rate was 52.3% (95% CI, 47.1–57.4) with T‐DXd 5.4 mg/kg vs. 16.3% (95% CI, 11.3–22.5) with physician's choice of chemotherapy in patients with metastatic or unresectable HER2‐low breast cancer who previously received 1 or 2 lines of chemotherapy. 6
In conclusion, the results of this study characterized the pharmacokinetic profile of T‐DXd after multiple doses and demonstrated that T‐DXd treatment did not have a clinically meaningful impact on the QTc interval or other cardiac toxicities. T‐DXd also demonstrated a manageable safety profile and antitumor activity in heavily pretreated patients with metastatic HER2‐expressing breast cancer, including HER2‐low disease.
FUNDING
This study was funded by Daiichi Sankyo Co, Ltd.
CONFLICT OF INTEREST
A.S. reports grants and personal fees from Daiichi Sankyo, AstraZeneca, Eisai, and Chugai Pharmaceutical; grants from Taiho Pharmaceutical and Mochida Pharmaceutical; and personal fees from Eli Lilly, Novartis, Pfizer, and Kyowa Kirin outside the submitted work. T.T. reports grants and personal fees from Daiichi Sankyo, Chugai, Kyowa Kirin, and Eisai; grants from Ono Pharmaceutical, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Taiho, and Novartis; and personal fees from Pfizer, Eli Lilly, and Celltrion Healthcare outside the submitted work. S.T. reports grants and personal fees from Daiichi Sankyo during the conduct of the study and grants and personal fees from Eisai, Novartis, Taiho, Merck Sharp & Dohme, AstraZeneca, Chugai, Bayer, Ono Pharmaceutical, and Bristol Myers Squibb outside the submitted work. J.W. reports grants from Daiichi Sankyo during the conduct of the study and personal fees from Daiichi Sankyo outside the submitted work. E.T. reports personal fees from AstraZeneca, Eli Lilly, and Chugai Pharmaceuticals outside the submitted work. T.K., K.K., E.K., Y.F., and D.S. are employees and equity owners of Daiichi Sankyo Co. Ltd. T.Y. reports grants and other fees from Chugai, Kyowa Kirin, Taiho, and Nippon Kayaku and other fees from Eli Lilly, Daiichi Sankyo, Eisai, Novartis Pharma, AstraZeneca, and Pfizer outside the submitted work. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
A.S. and D.S. wrote the manuscript. T.S., T.K., E.K., Y.F., and D.S. designed the research. A.S., T.T., S.T., Y.S., J.W., E.T., and T.Y. performed the research. A.S., S.T., T.K., K.K., and E.K. analyzed the data. T.T., T.K., K.K., and E.K. contributed new reagents/analytical tools.
Supporting information
Data S1
ACKNOWLEDGMENTS
The authors thank the patients and their families and caregivers for participating in this study and all site personnel. Under the guidance of the authors, medical writing assistance and editorial support was provided by Irene Park, PhD, and Rachel Hood, PhD, of ApotheCom and was funded by Daiichi Sankyo, Co. Ltd.
Clinical trial registration number
DATA AVAILABILITY STATEMENT
Anonymized individual participant data and applicable supporting clinical trial documents may be available upon request at (https://vivli.org). In cases where clinical trial data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of the company and our clinical study patients. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi‐sankyo.
References
- 1. Ogitani, Y. et al. DS‐8201a, a novel HER2‐targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T‐DM1. Clin. Cancer Res. 22, 5097–5108 (2016). [DOI] [PubMed] [Google Scholar]
- 2. Doi, T. et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS‐8201), a HER2‐targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro‐oesophageal tumours: a phase 1 dose‐escalation study. Lancet Oncol. 18, 1512–1522 (2017). [DOI] [PubMed] [Google Scholar]
- 3. Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2‐positive breast cancer. N. Engl. J. Med. 382, 610–621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2‐positive gastric cancer. N. Engl. J. Med. 382, 2419–2430 (2020). [DOI] [PubMed] [Google Scholar]
- 5. Tsurutani, J. et al. Targeting HER2 with trastuzumab deruxtecan: a dose‐expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 10, 688–701 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2‐low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Enhertu (fam‐trastuzumab deruxtecan‐nxki) for injection, for intravenous use . Prescribing information (Daiichi Sankyo, Inc, Basking Ridge, NJ, 2022). [Google Scholar]
- 8. Enhertu . Prescribing information (Daiichi Sankyo Co., Ltd, Tokyo, 2020). [Google Scholar]
- 9. Enhertu (fam‐trastuzumab deruxtecan‐nxki) for injection, for intravenous use. Prescribing information – Israel (Daiichi Sankyo Europe GmbH, Pfaffenhofen, 2021). [Google Scholar]
- 10. Enhertu . Summary of product characteristics (Daiichi Sankyo Europe GmbH, Pfaffenhofen, 2022). [Google Scholar]
- 11. Enhertu . Summary of product characteristics (UxDaiichi Sankyo UK Ltd, 2022). [Google Scholar]
- 12. Enhertu . Product monograph (AstraZeneca Canada Inc, Mississauga, 2022). [Google Scholar]
- 13. Coppola, C. , Rienzo, A. , Piscopo, G. , Barbieri, A. , Arra, C. & Maurea, N. Management of QT prolongation induced by anti‐cancer drugs: target therapy and old agents. Different algorithms for different drugs. Cancer Treat. Rev. 63, 135–143 (2018). [DOI] [PubMed] [Google Scholar]
- 14. Porta‐Sanchez, A. et al. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: a systematic review. J. Am. Heart Assoc. 6, e007724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupta, M. et al. Effects of trastuzumab emtansine (T‐DM1) on QT interval and safety of pertuzumab plus T‐DM1 in patients with previously treated human epidermal growth factor receptor 2‐positive metastatic breast cancer. Clin. Pharmacol. Drug Dev. 2, 11–24 (2013). [DOI] [PubMed] [Google Scholar]
- 16. Ponde, N.F. , Lambertini, M. & de Azambuja, E. Twenty years of anti‐HER2 therapy‐associated cardiotoxicity. ESMO Open 1, e000073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buzdar, A.U. et al. Fluorouracil, epirubicin, and cyclophosphamide (FEC‐75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC‐75 plus trastuzumab as neoadjuvant treatment for patients with HER2‐positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol. 14, 1317–1325 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruddy, K.J. , Patel, S.R. , Higgins, A.S. , Armenian, S.H. & Herrmann, J. Cardiovascular health during and after cancer therapy. Cancers (Basel). 12, 3737 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ponde, N. et al. Trastuzumab emtansine (T‐DM1)‐associated cardiotoxicity: pooled analysis in advanced HER2‐positive breast cancer. Eur. J. Cancer 126, 65–73 (2020). [DOI] [PubMed] [Google Scholar]
- 20. Van Leeuwen, M.T. et al. Cardiovascular toxicity of targeted therapies for cancer: an overview of systematic reviews. JNCI Cancer Spectr. 4, pkaa076 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu, N. , Redfern, C.H. , Gordon, M. , Eppler, S. , Lum, B.L. & Trudeau, C. Trastuzumab, in combination with carboplatin and docetaxel, does not prolong the QT interval of patients with HER2‐positive metastatic or locally advanced inoperable solid tumors: results from a phase Ib study. Cancer Chemother. Pharmacol. 74, 1251–1260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garg, A. et al. Exposure‐response analysis of pertuzumab in HER2‐positive metastatic breast cancer: absence of effect on QTc prolongation and other ECG parameters. Cancer Chemother. Pharmacol. 72, 1133–1141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ENHERTU . NDA/BLA multi‐disciplinary review and evaluation (US Food and Drug Administration, Rockville, MD, 2019). <https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761139Orig1s000MultidisciplineR.pdf>. [Google Scholar]
- 24. Siena, S. et al. Trastuzumab deruxtecan (DS‐8201) in patients with HER2‐expressing metastatic colorectal cancer (DESTINY‐CRC01): a multicentre, open‐label, phase 2 trial. Lancet Oncol. 22, 779–789 (2021). [DOI] [PubMed] [Google Scholar]
- 25. Bardia, A. , Harnden, K. , Mauro, L. , Pennisi, A. , Armitage, M. & Soliman, H. Clinical practices and institutional protocols on prophylaxis, monitoring, and management of selected adverse events associated with trastuzumab deruxtecan. Oncologist 27, 637–645 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cortés, J. et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386, 1143–1154 (2022). [DOI] [PubMed] [Google Scholar]
- 27. E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non‐Antiarrhythmic Drugs (US Food & Drug Administration, Rockville, MD, 2005). <https://www.fda.gov/media/71372/download>. [Google Scholar]
- 28. Yin, O. et al. Population pharmacokinetics of trastuzumab deruxtecan in patients with HER2‐positive breast cancer and other solid tumors. Clin. Pharmacol. Ther. 109, 1314–1325 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li, H. , Han, T.H. , Hunder, N.N. , Jang, G. & Zhao, B. Population pharmacokinetics of brentuximab vedotin in patients with CD30‐expressing hematologic malignancies. J. Clin. Pharmacol. 57, 1148–1158 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kubo, K. et al. Consensus statement for the diagnosis and treatment of drug‐induced lung injuries. Respir. Investig. 51, 260–277 (2013). [DOI] [PubMed] [Google Scholar]
- 31. Modi, S. et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2‐low‐expressing advanced breast cancer: results from a phase Ib study. J. Clin. Oncol. 38, 1887–1896 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li, B.T. et al. Trastuzumab deruxtecan in HER2‐mutant non‐small‐cell lung cancer. N. Engl. J. Med. 386, 241–251 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamura, K. et al. Trastuzumab deruxtecan (DS‐8201a) in patients with advanced HER2‐positive breast cancer previously treated with trastuzumab emtansine: a dose‐expansion, phase 1 study. Lancet Oncol. 20, 816–826 (2019). [DOI] [PubMed] [Google Scholar]
- 34. Kobayashi, Y . et al. Pharmacometric analysis to justify a higher recommended dose for trastuzumab deruxtecan in the treatment of subjects with metastatic gastric cancer versus breast cancer. Presented at: PAGE 2022 Meeting, 2022; Ljubljana, Slovenia.
- 35. Trastuzumab deruxtecan clinical trials . ClinicalTrials.gov <https://clinicaltrials.gov/ct2/results?recrs=&cond=&term=trastuzumab+deruxtecan&cntry=&state=&city=&dist=>.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
Anonymized individual participant data and applicable supporting clinical trial documents may be available upon request at (https://vivli.org). In cases where clinical trial data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of the company and our clinical study patients. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi‐sankyo.


