Abstract
Background
The need for axillary dissection (AD) is declining, but it is still essential for many patients with nodal involvement who risk developing breast‐cancer–related lymphedema (BCRL) with lifelong consequences. Previous nonrandomized studies found axillary reverse mapping and selective axillary dissection (ARM‐SAD) a safe and feasible way to preserve the arm's lymphatic drainage.
Methods
The present two‐arm prospective randomized clinical trial was held at a single comprehensive cancer center to ascertain whether ARM‐SAD can reduce the risk of BCRL, compared with standard AD, in patients with node‐positive breast cancer. Whatever the type of breast surgery or adjuvant treatments planned, 130 patients with nodal involvement met our inclusion criteria: 65 were randomized for AD and 65 for ARM‐SAD. Twelve months after surgery, a physiatrist assessed patients for BCRL and calculated the excess volume of the operated arm. Lymphoscintigraphy was used to assess drainage impairment. Self‐reports of any impairment were also recorded.
Results
The difference in the incidence of BCRL between the two groups was 21% (95% CI, 3‐37; p = .03). A significantly lower rate of BCRL after ARM‐SAD was confirmed by a multimodal analysis that included the physiatrist's findings, excess arm volume, and lymphoscintigraphic findings, but this was not matched by a significant difference in patients' self‐reports.
Conclusions
Our findings encourage a change of surgical approach when AD is still warranted. ARM‐SAD may be an alternative to standard AD to reduce the treatment‐related morbidity.
Keywords: breast cancer, lymphedema, randomized clinical trial, reverse lymphatic mapping, selective axillary dissection
Short abstract
In this randomized clinical trial comparing axillary reverse mapping and selective axillary dissection with standard axillary dissection, breast‐cancer–related lymphedema occurred in 21% and 42% of cases, respectively. This procedure therefore can help to reduce the morbidity associated with axillary dissection.
INTRODUCTION
The need for axillary lymph node dissection (AD) in the treatment of breast cancer is declining. 1 , 2 , 3 , 4 The procedure is still essential in many patients with nodal involvement, however, for prognostic purposes and to orient adjuvant treatments. Breast‐cancer–related lymphedema (BCRL) can occur in up to 61% of patients undergoing AD, 5 sometimes causing lifelong problems, negatively affecting quality of life and body image, interfering with social life and work, and raising health care costs. 6 , 7 , 8 , 9 , 10 Hence, the interest in its prevention. Known risk factors for BCRL are: age; body mass index; number of nodes excised; number of positive nodes; and regional lymph node irradiation. 11 , 12 , 13 , 14 Axillary reverse mapping (ARM) can identify the lymphatics draining the arm, enabling the selective sparing of axillary lymph nodes to reduce the incidence of BCRL. 15 , 16 , 17
A phase 2 pilot study at the Fondazione IRCCS Istituto Nazionale Tumori in Milan (INT Milan) used a radioisotope and lymphoscintigraphy to perform ARM to establish the feasibility of selective axillary dissection (SAD) to preserve the arm's lymphatic drainage. SAD was found feasible in 75% of patients, and 9% developed BCRL after SAD, as opposed to 33% after AD. None of the patients treated with SAD developed axillary lymph node disease during a median 16‐month follow‐up. 18 We assumed that nodes spared as a result of ARM would be unlikely to affect patients' overall prognosis for three reasons. First, nodes in the central region of the axilla (including those found hot after injecting the radiotracer into the hand) were removed during SAD. 18 This region often contains the sentinel nodes and is the main site of crossover. 19 , 20 The hot nodes that were spared typically took up only about 10% of the radioactivity in the axilla and were those close to the axillary vein, which is less likely to be involved by breast cancer cell dissemination. 18 , 19 Second, very few of axillary metastases not removed during AD became clinically overt during the follow‐up. 21 , 22 A recently published study on safety aspects of SAD found a low risk of axillary failure after the procedure. 23 Third, in patients at relatively high risk, surgical radicality has little influence on survival, which depends mainly on the efficacy of systemic treatments. 1 , 24 Although SAD seemed safe and effective in our initial experience, the ARM‐SAD procedure could have selected cases with a favorable lymphatic anatomy, a subpopulation of patients naturally less likely to develop BCRL after AD. In other words, the incidence of BCRL might be inherently lower in cases in which SAD is feasible. The present randomized study compared the BCRL rates after ARM‐SAD or standard AD in a trial adequately powered to assess the efficacy of SAD in preventing BCRL.
METHODS
Study design
In a two‐arm prospective randomized trial, patients in the control arm were assigned to AD, and those in the study arm to ARM‐SAD. The end point was the difference in incidence of BCRL between the two arms. This phase 3 study was conceived and conducted at the INT Milan. It was approved by the institute's internal review board and ethics committee, and registered at clinicaltrials.gov (NCT03083314). 25
Participants
Patients met the following inclusion criteria: they had been diagnosed with operable breast cancer and were candidates for AD according to our institutional practice, irrespective of the type of breast surgery performed or adjuvant treatments administered; they could attend regular follow‐up visits, as required by the study protocol; and they gave specific written informed consent to their participation. Exclusion criteria were massive axillary metastases (cN2 American Joint Commission on Cancer); previous surgery to the contralateral axillary region; previous radiotherapy to ipsilateral or contralateral regional lymph nodes.
Procedures
Patients' enrollment was confirmed after a senologist had explained the aims of the trial. Randomization and data management were done by the Trial Center at the INT Milan. Computer‐generated lists of random numbers were used to allocate patients and obtain two balanced groups for comparison (1:1 allocation). No stratification factors were considered. Six to 24 hours before breast surgery, patients in the SAD group received three intradermal injections of 5 MBq 99mTc‐labeled Nanocoll in 0.1 ml saline to the back of the hand ipsilateral to the breast involved. An hour later, lymphoscintigraphy was used to identify axillary lymph nodes draining the arm. The plan was to remove at least 10 nodes on the first and second Berg levels, and preferably also on the third, including nodes in the central region of the axilla found “hot” after injecting the radiotracer. Perivascular nodes (near the axillary vein) that typically accumulate only about 10% (in counts per minute) of the radioactivity detectable in the central axilla were identified and preserved. No microsurgical procedure on lymphatics was considered. Further details of the surgical technique are available elsewhere. 18 , 23 A gamma‐ray detecting probe was used intraoperatively to identify these nodes. If no nodes were identified on ARM, or none was preserved, a standard AD was performed, but the final analysis was conducted according to the intention‐to‐treat principle. Standard AD was performed in patients in the control arm, removing at least 10 axillary nodes from at least the first and second Berg levels. All surgical procedures were performed by the same trained team.
Outcomes
The trial's primary endpoint was to compare the incidence of BCRL 12 months after SAD or AD. Patients were assessed by a physiatrist, and their arm circumference was measured, followed by clinical examination of any BCRL. The excess volume of the operated limb was calculated, and drainage impairments were assessed on lymphoscintigraphy. The examiners were blinded to the treatment group. The results of the multimodal assessment of cases of BCRL were then analyzed and compared between the two treatment groups. Patients' self‐reports of any impairment were also obtained and compared.
Physiatrist's assessment
A preliminary diagnosis of BCRL was applied to arm circumferences that had increased by ≥ 2 cm in one or more places (as in the pilot study 18 ). The clinical classification of lymphedema was based on degrees of fovea (absent, mild, marked) and consistency (soft, medium, hard), assessed on the upper arm, forearm, and hand. These features were combined to score cases of BCRL clinically on four levels, as absent, mild, moderate, or severe (including elephantiasis).
Upper limb volume
The circumference of both arms was measured, starting from the wrist and proceeding at 5‐cm intervals to the top, to calculate the volume of the arm using the formula for the volume of a truncated cone. Excess volume in the operated limb was classified as: absent; mild (1%‐5%); moderate (6%‐10%), or severe (>10%).
Lymphoscintigraphy
A total of 37 MBq of Tc99‐nanocolloid tracer were injected subcutaneously in the second, third, and fourth interdigital spaces of each hand. Planar images were acquired with the gamma camera after 20 min (early images) and 90 min (delayed images). The whole‐body acquisition technique was used to obtain a qualitative and semiquantitative assessment of dermal backflow and to compare the two arms. Drainage impairment was classified as: absent, mild, moderate, or severe. 26 , 27
Patients' self‐reports
All patients were specifically asked to assess their own perception of heaviness, swelling, and/or restricted movement. Patients self‐rated the condition of their arm on a scale of 1 to 10, where 1 was intolerable and 10 was excellent. Their ratings were then grouped into four categories of perceived impairment for inclusion in the final analysis: 10‐9 (absent), 8‐6 (mild), 5‐3 (moderate), and 2‐1 (severe).
Secondary endpoint
The safety of SAD, in terms of the rate of axillary relapses during the follow‐up due to incomplete AD, was amply investigated in an ancillary study. 23 Distant relapse‐free survival (DRFS) and breast‐cancer–related events in the SAD and AD arms are described and compared, also considering the length of follow‐up.
Sample size
Based on a pilot study involving a dichotomous assessment of the primary endpoint, the risk of lymphedema in the AD group was assumed to be 0.30 under the null hypothesis, and amounted to 0.10 under the alternative hypothesis. 18 For a power of 90% and a type I error of 0.05, 79 patients were originally planned in each arm to test an assumed 0.20 difference in risk. The sample size was later (in November 2018) reduced to 65 patients per arm because of a lower than expected recruitment rate. It was estimated that this number would still yield a satisfactory power of 83%.
Statistical methods
Descriptive statistics (means with standard deviations, medians with interquartile ranges, contingency tables) were used to summarize patients' characteristics, treatments, and all data concerning BCRL. Pearson's χ2 test was used to compare the incidence of BCRL in the SAD and AD arms. For the primary end point, the comparison was also drawn with the Cochran‐Mantel‐Haenszel test on stratified data to adjust for the possible confounding effect of neoadjuvant treatments. DRFS curves were obtained with the Kaplan–Meier method and compared with the log‐rank test. All statistical analyses were conducted using SAS software 28 and R. 29
RESULTS
From June 12, 2014, to November 14, 2018, 139 patients met our inclusion criteria, but seven declined to take part in the trial, and two were not enrolled because they expressed doubts about completing the required follow‐up. The remaining 130 patients consented and were enrolled. They all met our criteria for AD: 52 had nodal involvement ascertained by fine‐needle biopsy; 42 had a previous positive sentinel lymph node biopsy (SLNB); 36 had confirmed nodal involvement and received neoadjuvant chemotherapy.
All 130 patients were randomly assigned to surgical treatment, which involved AD in 65 (the control arm) and SAD in 65 (the study arm). In four of the latter patients, no nodes were identified on ARM, or none was preserved, indicating an overall 94% feasibility of SAD. The two arms were well balanced overall, with the sole exception of more neoadjuvant treatments in the SAD arm (35% vs 20%, p = .0769). Table 1 shows details of patients' characteristics and major risk factors for BCRL.
TABLE 1.
Characteristics, treatments, and BCRL risk factors of patients enrolled, by trial arm
| SAD | AD | |||
|---|---|---|---|---|
| 65 patients | 65 patients | |||
| N | % | N | % | |
| Age, median (IQR), y | 52 (46‐60) | 51 (45‐63) | ||
| BMI, kg/m2: median (IQR) | 21.8 (19.9‐26.3) | 23.0 (20.3‐25.4) | ||
| Dominant side affected | 28 | 43.1 | 31 | 47.7 |
| Surgical treatment | ||||
| Wide excision | 30 | 46.2 | 33 | 50.8 |
| Mastectomy | 34 | 52.3 | 32 | 49.2 |
| Axillary surgery alone | 1 | 1.5 | 0 | ‐ |
| Axillary assessment by prior SLNB | 20 | 30.8 | 22 | 33.8 |
| No. of excised nodes: median (IQR) | 17.0 (14.0‐23.0) | 19.0 (16.0‐24.0) | ||
| No. of involved nodes: median (IQR) | 2.0 (1.0‐5.0) | 2.0 (1.0‐4.0) | ||
| Systemic treatment | ||||
| Neoadjuvant | 23 | 35.4 | 13 | 20.0 |
| Adjuvant | 42 | 64.6 | 52 | 80.0 |
| Radiation treatment | ||||
| Breast | 31 | 47.7 | 33 | 50.8 |
| Chest wall | 22 | 33.8 | 22 | 33.8 |
| Supra‐infraclavicular | 25 | 38.5 | 24 | 36.9 |
Abbreviations: AD, axial dissection; BCRL, breast cancer–related lymphedema; BMI, body mass index; IQR, interquartile range; SAD, selective axillary dissection; SLNB, sentinel lymph‐node biopsy.
Twelve months after surgery, 123 patients completed at least one procedure to identify any BCRL and were assessed on the study's primary end point. In particular, 111 patients were examined by a physiatrist, and their arm volume was calculated; 101 patients had lymphoscintigraphy; and 122 self‐rated the condition of their arm. Figure 1 shows the trial flow chart.
FIGURE 1.
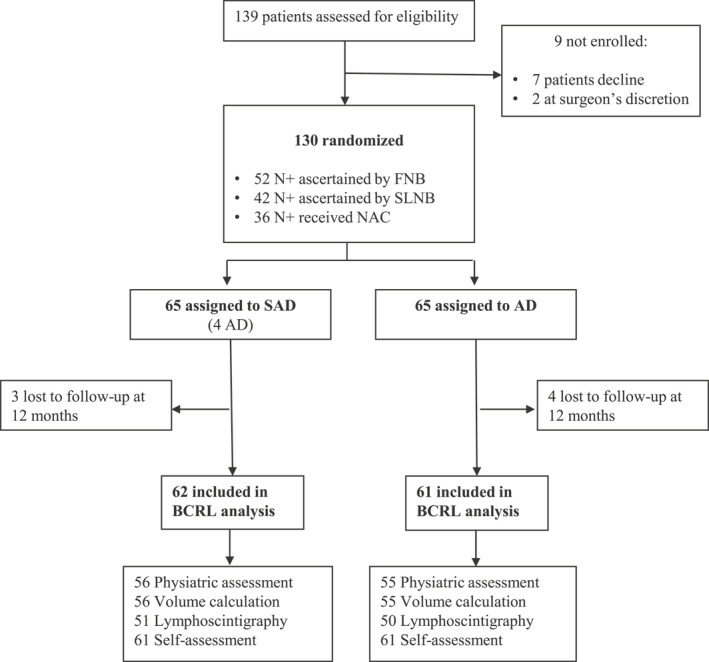
Trial flow chart. FNB indicates fine‐needle biopsy; NAC, neo‐adjuvant chemotherapy; SLNB, sentinel lymph node biopsy.
Primary end point
A year after surgery for breast cancer, 12 (21%) patients randomized to SAD, as opposed to 23 (42%) treated with AD, had an increase in arm circumference on the treated side. The difference between the two rates was 21% (95% CI, 3‐37) and reached statistical significance (p = .0253). When assessed separately in patients given neoadjuvant or adjuvant treatments, the difference between the two groups in the incidence of BCRL was again in favor of SAD: 13.6% among patients given neoadjuvant chemotherapy and 19.0% among those given adjuvant chemotherapy. The overall stratified test neared statistical significance (p = .0511). Taken together, these findings rule out any substantial confounding effect of different medical treatment.
Comparing the two arms on the four severity levels of BCRL, women in the SAD arm fared significantly better on physical features (p = .0105), excess volume (p = .0006), and lymphoscintigraphic findings (p < .0001). We also found higher rates of major impairment (severe BCRL) in the AD arm when physical features (3/28 vs 1/16), excess volume (8/50 vs 2/30), and lymphoscintigraphic findings (8/37 vs 1/13) were considered separately. There was no significant difference between the two arms in the women's self‐reports, however. Figure 2 summarizes the findings of the multimodal analysis at 12 months after surgery.
FIGURE 2.
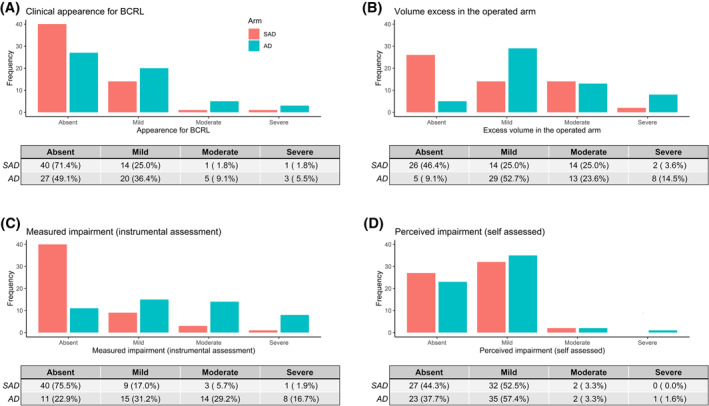
Comparison of BCRL rates in SAD vs AD arm at 12 months after surgery. Multimodal assessment of primary endpoint: (A) Clinical appearance of upper limb. (B) Excess volume of upper limb. (C) Lymphoscintigraphic findings. (D) Patients' self‐ratings. AD indicates axial dissection; BCRL, breast‐cancer–related lymphedema; SAD, selective axillary dissection.
Secondary end point
With a median follow‐up of 44.1 months (interquartile range, 30.7‐59.6), the breast‐cancer–related events did not differ significantly between the two arms in terms of ipsilateral breast tumor recurrences, contralateral breast cancers, isolated axillary relapses, distant relapses, or deaths (Table 2). Figure 3 shows the DRFS curves.
TABLE 2.
Secondary endpoint: breast‐cancer–related events, by trial arm, after a median follow‐up of 44.1 mo
| SAD | AD | |||
|---|---|---|---|---|
| 61 patients | 62 patients | |||
| N | % | N | % | |
| Ipsilateral breast tumor recurrence | 0 | ‐ | 0 | ‐ |
| Contralateral breast cancer | 1 | 1.5 | 1 | 1.5 |
| Isolated axillary relapse | 1 | 1.5 | 0 | 0 |
| Distant relapse | 6 | 9.2 | 8 | 12.3 |
| Death | 3 | 4.6 | 2 | 3.1 |
Abbreviations: AD, axial dissection; IQR, interquartile range; SAD, selective axillary dissection.
FIGURE 3.
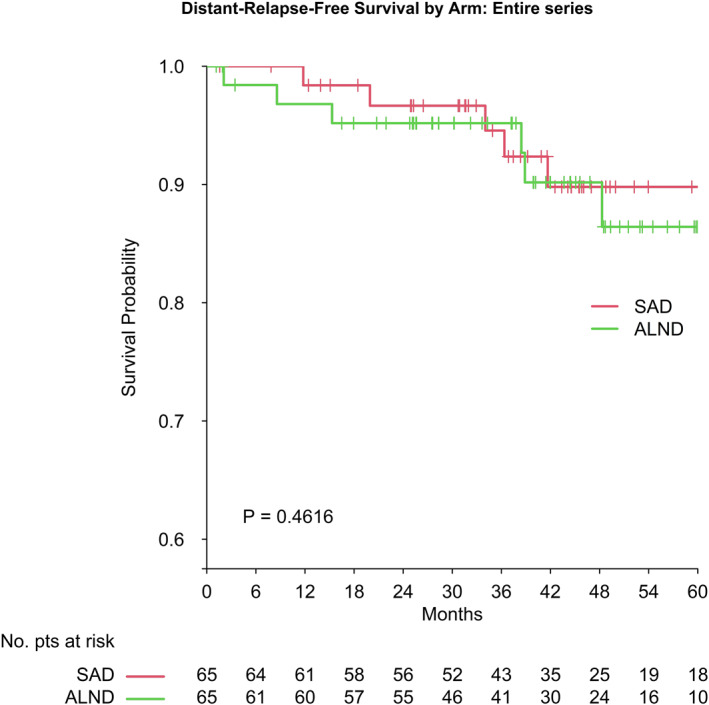
Distant‐relapse‐free survival: SAD vs AD. AD indicates axial dissection; SAD, selective axillary dissection.
DISCUSSION
This two‐arm randomized clinical trial found the incidence of BCRL lower after SAD than after AD. When BCRL did occur, it was more often severe after AD. Blinded assessments of lymphedema a year after surgery all indicated a significantly lower incidence of BCRL in the SAD group. These objective benefits were not matched by patients' self‐ratings, however, which were similar for the two groups. The hypothesized efficacy of SAD in reducing the risk of BCRL was nonetheless confirmed. As for the safety of this approach, there were no differences between the SAD and AD groups in terms of breast‐cancer–related events or DRFS rates at 5 years.
Five randomized clinical trials with the same aim as ours have been published so far 30 , 31 , 32 , 33 , 34 : two found that ARM procedures significantly reduced the incidence of BCRL at 12 months 30 , 31 ; one found better results only 24 months after surgery 34 ; and two (on small samples) found no significant differences. 32 , 33 One of these latter two trials was a Dutch study that enrolled 107 patients at four dedicated breast cancer centers, instead of the 280 calculated to be needed to obtain a power of 0.8, and it was terminated prematurely after it emerged that the Z0011 and AMAROS trials hampered patient enrollment. Only 83 of the 107 patients were assessed at 12 months, when 9/39 treated with AD had developed BCRL, as opposed to 4/44 treated with ARM‐SAD (p = .080). Based on the intention‐to‐treat principle, the analysis did not consider the learning curve of participating centers with few cases, which could mean that less expert surgeons failed to preserve ARM lymphatics and nodes, and consequently performed standard AD. 33 As reported in our own initial experience, ARM‐SAD procedures were completed successfully in 63% of our first 30 patients and in 86% of the next 30. 18 The figure rose to 94% in the present series, indicating the need to gain confidence with this node‐sparing procedure. A significant difference in favor of ARM emerged when patients answered the World Health Organization's WHOQOL‐BREF questionnaire validated for patients with breast cancer. 35 Our patients' self‐ratings were similar between the two treatment arms, possibly because the simple, unvalidated questionnaire we administered could not adequately capture patients' impressions, an aspect that may represent a limitation of our study.
Our study has several strengths. All of our patients were treated by the same well‐trained surgical team. It is the first study to compare the efficacy of ARM‐SAD vs standard AD in preventing BCRL at 1 year after surgery using blinded physiatrist assessments, excess volume calculations, and qualitative and semiquantitative lymphoscintigraphic findings. These objective parameters were used in our multimodal analysis of the morbidity associated with axillary dissection, in terms of the arm's physical features (fovea and consistency), excess volume, and lymphatic drainage impairment. This type of impairment is associated with functional capacities and functional reserves that vary from one individual to another. Our study is also the first to consider even subclinical and minimal BCRL. These strengths of our approach enabled a thorough comparison of the morbidity following ARM‐SAD vs standard AD, even in a sample of only 130 patients, as they were uniformly treated and assessed on the primary endpoint in a strictly blinded manner.
A limitation of this study lies in that some patients did not complete the follow‐up, but the missing results were balanced between the two groups. Another possible limitation stems from the ARM‐SAD procedure considered here. SAD cannot be done at the same time as SLNB because the same radioisotope is used in both cases. The need for a separate procedure led to a long accrual period because most patients treated at our institute underwent AD at the same session if a frozen‐section SLNB was found positive. The use of intraoperative frozen‐section SLNB is declining but may still hinder a more extensive use of SAD in clinical practice. On the other hand, the use of different tracers to visualize lymphatics and nodes during reverse mapping has already been tested. The first experiences with the ARM technique used methylene blue dye injected subcutaneously at the medial intramuscular crease between the biceps and triceps. 36 , 37 More recently, a similar approach involved using indocyanine green dye and intraoperative visualization with a near‐infrared fluorescent imaging system that may significantly improve lymphatics detection during ARM. 38 , 39 Such methods are easily reproducible, afford a good view of the lymphatics to be spared, and can be adopted when the indication for AD is established during the same procedure. 31
All patients enrolled in our trial were node positive: 52 were cN1, ascertained on ultrasound‐guided fine‐needle biopsy; 42 were cN0, but had a prior positive SLNB; and 36 had been given neoadjuvant chemotherapy for positive nodes. More patients in the SAD arm than in the AD arm had received neoadjuvant treatments, but this did not substantially bias the overall findings, as shown by a lower incidence of BCRL after SAD in both the adjuvant and the neoadjuvant treatment subgroups. The p value was borderline, then no longer significant in the stratified analysis. The study was not originally powered for this kind of analysis, however, which was needed post hoc to deal with the imbalance between the two trial arms by type of adjuvant treatment.
Because all patients enrolled were pragmatically considered candidates for AD according to our institution's established criteria, the study population was rather heterogeneous, with a variable risk of subsequent nodal involvement. This enabled us to explore the efficacy of axillary dissection in different situations. Our approach could extend the number of potential candidates for ARM‐SAD, but could also interfere with the assessment of its safety: this surgical approach goes further than axillary sampling, but differs somewhat from a complete AD.
This trial was not sized for a survival analysis and the follow‐up was too short for such purposes, but our assumptions on the safety of SAD seem to be confirmed by our findings regarding breast‐cancer–related events and DRFS after a median follow‐up of 44.1 months, the longest after ARM reported so far.
Our findings support the effectiveness of ARM‐SAD in reducing the incidence of BCRL, but our patients' perceptions did not improve as a result, so this surgical technique should not be considered a good reason for extending the indications for axillary surgery. The American College of Surgeons Oncology Group Z0011 trial, and the European Organization for Research and Treatment of Cancer AMAROS trial led to a lower use of AD, 1 , 2 but more efforts are still needed to avoid unnecessary AD, particularly after neoadjuvant chemotherapy. 3 , 40 Meanwhile, ARM‐SAD can significantly reduce the expected sequelae of axillary surgery, and is now a feasible, effective, and putatively safe alternative to standard AD. Although evidence on the safety of this somewhat less radical surgical procedure is still limited, our findings encourage a change of approach when AD is still warranted.
AUTHOR CONTRIBUTIONS
Massimiliano Gennaro: Study design, patient enrollment, surgical treatment, data interpretation, and article draft. Marco Maccauro: Study design, primary end point evaluation, and data acquisition and interpretation. Luigi Mariani: statistical analysis, data interpretation. Chiara Listorti: surgical treatment, secondary end point evaluation, and data acquisition and interpretation. Carmela Sigari: primary end point evaluation. Annarita De Vivo: follow‐up evaluation. Marco Chisari: follow‐up evaluation and data acquisition. Ilaria Maugeri: surgical treatment, secondary end point evaluation, and data acquisition and interpretation. Alice Lorenzoni: primary outcome evaluation. Gianluca Aliberti: primary outcome evaluation. Gianfranco Paride Scaperrotta: patient enrollment and secondary end point evaluation. Augusto Caraceni: primary and secondary end point evaluation. Giancarlo Pruneri: critical manuscript revision for important intellectual content. Secondo Folli: surgical treatment and critical manuscript revision for important intellectual content. All authors give final approval of the version to be published. Massimiliano Gennaro is responsible for the overall content as guarantor.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
Remembering Salvatore Lo Vullo. This study was supported by the Italian Ministry of Health (Grant No. RF‐2013‐02355260 to M.G.).
Open access funding provided by BIBLIOSAN.
Gennaro M, Maccauro M, Mariani L, et al. Occurrence of breast‐cancer–related lymphedema after reverse lymphatic mapping and selective axillary dissection versus standard surgical treatment of axilla: a two‐arm randomized clinical trial. Cancer. 2022;128(24):4185‐4193. doi: 10.1002/cncr.34498
REFERENCES
- 1. Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10‐year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017;318(10):918‐926. doi: 10.1001/jama.2017.11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981‐22023 AMAROS): a randomised, multicentre, open‐label, phase 3 non‐inferiority trial. Lancet Oncol. 2014;15(12):1303‐1310. doi: 10.1016/S1470-2045(14)70460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martelli G, Barretta F, Miceli R, et al. Sentinel node biopsy alone or with axillary dissection in breast cancer patients after primary chemotherapy: long‐term results of a prospective interventional study. Ann Surg. 2020;Publish Ahead of Print. doi: 10.1097/SLA.0000000000004562 [DOI] [PubMed] [Google Scholar]
- 4. Fisher CS, Margenthaler JA, Hunt KK, Schwartz T. The Landmark Series: axillary management in breast cancer. Ann Surg Oncol. 2020;27(3):724‐729. doi: 10.1245/s10434-019-08154-5 [DOI] [PubMed] [Google Scholar]
- 5. Shah C, Arthur D, Riutta J, Whitworth P, Vicini FA. Breast‐cancer related lymphedema: a review of procedure‐specific incidence rates, clinical assessment AIDS, treatment paradigms, and risk reduction. Breast J. 2012;18(4):357‐361. doi: 10.1111/j.1524-4741.2012.01252.x [DOI] [PubMed] [Google Scholar]
- 6. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta‐analysis. Lancet Oncol. 2013;14(6):500‐515. doi: 10.1016/S1470-2045(13)70076-7 [DOI] [PubMed] [Google Scholar]
- 7. Degnim AC, Miller J, Hoskin TL, et al. A prospective study of breast lymphedema: frequency, symptoms, and quality of life. Breast Cancer Res Treat. 2012;134(3):915‐922. doi: 10.1007/s10549-012-2004-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheville A, Lee M, Moynihan T, et al. The impact of arm lymphedema on healthcare utilization during long‐term breast cancer survivorship: a population‐based cohort study. J Cancer Surviv. 2020;14(3):347‐355. doi: 10.1007/s11764-019-00851-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naoum GE, Roberts S, Brunelle CL, et al. Quantifying the impact of axillary surgery and nodal irradiation on breast cancer‐related lymphedema and local tumor control: long‐term results from a prospective screening trial. J Clin Oncol. 2020;38(29):3430‐3438. doi: 10.1200/JCO.20.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vignes S, Fau‐Prudhomot P, Simon L, Sanchez‐Bréchot ML, Arrault M, Locher F. Impact of breast cancer‐related lymphedema on working women. Support Care Cancer. 2020;28(1):79‐85. doi: 10.1007/s00520-019-04804-2 [DOI] [PubMed] [Google Scholar]
- 11. Zou L, Liu FH, Shen PP, et al. The incidence and risk factors of related lymphedema for breast cancer survivors post‐operation: a 2‐year follow‐up prospective cohort study. Breast Cancer. 2018;25(3):309‐314. doi: 10.1007/s12282-018-0830-3 [DOI] [PubMed] [Google Scholar]
- 12. Nguyen TT, Hoskin TL, Habermann EB, Cheville AL, Boughey JC. Breast cancer‐related lymphedema risk is related to multidisciplinary treatment and not surgery alone: results from a large cohort study. Ann Surg Oncol. 2017;24(10):2972‐2980. doi: 10.1245/s10434-017-5960-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McLaughlin SA, Brunelle CL, Taghian A. Breast cancer‐related lymphedema: risk factors, screening, management, and the impact of locoregional treatment. J Clin Oncol. 2020;38(20):2341‐2350. doi: 10.1200/JCO.19.02896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah C, Wilkinson JB, Baschnagel A, et al. Factors associated with the development of breast cancer‐related lymphedema after whole‐breast irradiation. Int J Radiat Oncol Biol Phys. 2012;83(4):1095‐1100. doi: 10.1016/j.ijrobp.2011.09.058 [DOI] [PubMed] [Google Scholar]
- 15. Noguchi M. Axillary reverse mapping for breast cancer. Breast Cancer Res Treat. 2010;119(3):529‐535. doi: 10.1007/s10549-009-0578-8 [DOI] [PubMed] [Google Scholar]
- 16. Ahmed M, Rubio IT, Kovacs T, Klimberg VS, Douek M. Systematic review of axillary reverse mapping in breast cancer. Br J Surg. 2016;103(3):170‐178. doi: 10.1002/bjs.10041 [DOI] [PubMed] [Google Scholar]
- 17. Wijaya WA, Peng J, He Y, Chen J, Cen Y. Clinical application of axillary reverse mapping in patients with breast cancer: a systematic review and meta‐analysis. Breast. 2020;53:189‐200. doi: 10.1016/j.breast.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gennaro M, Maccauro M, Sigari C, et al. Selective axillary dissection after axillary reverse mapping to prevent breast‐cancer‐related lymphoedema. Eur J Surg Oncol. 2013;39(12):1341‐1345. doi: 10.1016/j.ejso.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 19. Clough KB, Nasr R, Nos C, Vieira M, Inguenault C, Poulet B. New anatomical classification of the axilla with implications for sentinel node biopsy. Br J Surg. 2010;97(11):1659‐1665. doi: 10.1002/bjs.7217 [DOI] [PubMed] [Google Scholar]
- 20. Han C, Yang B, Zuo WS, Zheng G, Yang L, Zheng MZ. The feasibility and oncological safety of axillary reverse mapping in patients with breast cancer: a systematic review and meta‐analysis of prospective studies. PLoS One. 2016;11(2):e0150285. doi: 10.1371/journal.pone.0150285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martelli G, Boracchi P, Ardoino I, et al. Axillary dissection versus no axillary dissection in older patients with T1N0 breast cancer: 15‐year results of a randomized controlled trial. Ann Surg. 2012;256(6):920‐924. doi: 10.1097/SLA.0b013e31827660a8 [DOI] [PubMed] [Google Scholar]
- 22. Agresti R, Martelli G, Sandri M, et al. Axillary lymph node dissection versus no dissection in patients with T1N0 breast cancer: a randomized clinical trial (INT09/98). Cancer. 2014;120(6):885‐893. doi: 10.1002/cncr.28499 [DOI] [PubMed] [Google Scholar]
- 23. Gennaro M, Listorti C, Mariani L, et al. Oncological safety of selective axillary dissection after axillary reverse mapping in node‐positive breast cancer. Eur J Surg Oncol. 2021;47(7):1606‐1610. doi: 10.1016/j.ejso.2020.10.031 [DOI] [PubMed] [Google Scholar]
- 24. Fisher B, Anderson S, Bryant J, et al. Twenty‐year follow‐up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233‐1241. doi: 10.1056/NEJMoa022152 [DOI] [PubMed] [Google Scholar]
- 25. Gennaro M. Selective axillary lymph node dissection vs complete axillary dissection: a randomized clinical trial to assess the prevention of lymphedema in breast cancer treatment. https://clinicaltrials.gov/ct2/show/NCT03083314?cond=Lymphedema%26cntry=IT%26draw=2%26rank=5
- 26. Maccauro M, Villa G, Manzara A, et al. Lymphoscintigraphy for the evaluation of limb lymphatic flow disorders: report of technical procedural standards from an Italian Nuclear Medicine expert panel. Rev Esp Med Nucl Imagen Mol (Engl Ed). 2019;38(5):335‐340. doi: 10.1016/j.remn.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 27. Kim YH, Hwang JH, Bae JH, Choi JY. Predictive value of lymphoscintigraphy in patients with breast cancer‐related lymphedema undergoing complex decongestive therapy. Breast Cancer Res Treat. 2019;173(3):735‐741. doi: 10.1007/s10549-018-5041-2 [DOI] [PubMed] [Google Scholar]
- 28.SAS 9.4, SAS Institute. [Google Scholar]
- 29. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 30. Yue T, Zhuang D, Zhou P, et al. A prospective study to assess the feasibility of axillary reverse mapping and evaluate its effect on preventing lymphedema in breast cancer patients. Clin Breast Cancer. 2015;15(4):301‐306. doi: 10.1016/j.clbc.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 31. Yuan Q, Wu G, Xiao SY, et al. Identification and preservation of arm lymphatic system in axillary dissection for breast cancer to reduce arm lymphedema events: a randomized clinical trial. Ann Surg Oncol. 2019;26(11):3446‐3454. doi: 10.1245/s10434-019-07569-4 [DOI] [PubMed] [Google Scholar]
- 32. Faisal M, Sayed MG, Antonious K, Abo Bakr A, Farag SH. Prevention of lymphedema via axillary reverse mapping for arm lymph‐node preservation following breast cancer surgery: a randomized controlled trial. Patient Saf Surg. 2019;13(1):35. doi: 10.1186/s13037-019-0217-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beek MA, Gobardhan PD, Klompenhouwer EG, et al. A patient‐ and assessor‐blinded randomized controlled trial of axillary reverse mapping (ARM) in patients with early breast cancer. Eur J Surg Oncol. 2020;46(1):59‐64. doi: 10.1016/j.ejso.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 34. Abdelhamid MI, Bari AA, Farid MI, Nour H. Evaluation of axillary reverse mapping (ARM) in clinically axillary node negative breast cancer patients ‐ randomised controlled trial. Int J Surg. 2020;75:174‐178. doi: 10.1016/j.ijsu.2020.01.152 [DOI] [PubMed] [Google Scholar]
- 35. The Whoqol Group. Development of the World Health Organization WHOQOL‐BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28(3):551‐558. doi: 10.1017/s0033291798006667 [DOI] [PubMed] [Google Scholar]
- 36. Thompson M, Korourian S, Henry‐Tillman R, et al. Axillary reverse mapping (ARM): a new concept to identify and enhance lymphatic preservation. Ann Surg Oncol. 2007;14(6):1890‐1895. doi: 10.1245/s10434-007-9412-x [DOI] [PubMed] [Google Scholar]
- 37. Nos C, Lesieur B, Clough KB, Lecuru F. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Ann Surg Oncol. 2007;14(9):2490‐2496. doi: 10.1245/s10434-007-9450-4 [DOI] [PubMed] [Google Scholar]
- 38. Noguchi M, Yokoi M, Nakano Y. Axillary reverse mapping with indocyanine fluorescence imaging in patients with breast cancer. J Surg Oncol. 2010;101(3):217‐221. doi: 10.1002/jso.21473 [DOI] [PubMed] [Google Scholar]
- 39. Abbaci M, Conversano A, De Leeuw F, Laplace‐Builhé C, Mazouni C. Near‐infrared fluorescence imaging for the prevention and management of breast cancer‐related lymphedema: a systematic review. Eur J Surg Oncol. 2019;45(10):1778‐1786. doi: 10.1016/j.ejso.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 40. Pilewskie M, Morrow M. Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol. 2017;3(4):549‐555. doi: 10.1001/jamaoncol.2016.4163 [DOI] [PMC free article] [PubMed] [Google Scholar]


