Summary
Background
Tofacitinib is an oral Janus kinase (JAK) inhibitor and is registered for the treatment of ulcerative colitis (UC). The effectiveness of tofacitinib has been evaluated up to 12 months of treatment.
Aim
The aim of this study was to assess the effectiveness and safety of 24 months of tofacitinib use in UC patients in the Netherlands.
Methods
Patients initiating tofacitinib treatment were included in the ICC Registry, a nationwide, observational registry. Patients were prospectively evaluated for up to 24 months. The primary outcome was corticosteroid‐free clinical remission (CSFR, Simple Clinical Colitis Activity Index [SCCAI] ≤2) at week 104. Secondary outcomes included biochemical remission (C‐reactive protein (CRP) ≤5 mg/L and faecal calprotectin (FC) ≤250 μg/g), safety, and discontinuation rate.
Results
We included 110 patients of whom 104 (94.5%) were anti‐TNF experienced. After 104 weeks of tofacitinib, 31.8% (34/107) were in CSFR, 23.4% (25/107) in biochemical remission and 18.7% (20/107) in combined clinical and biochemical remission. Of the patients in CSFR at week 52, 76.5% (26/34) remained so after 104 weeks of treatment. Sixty‐one patients (55.5%) discontinued tofacitinib after a median duration of 13 weeks (IQR 7–34). The main reasons for discontinuation were non‐response (59%), loss of response (14.8%), and adverse events (18%). There were 33.9 possible tofacitinib‐related adverse events per 100 patient‐years during follow‐up. Adverse events most probably related to tofacitinib were skin reactions and headaches. There were 6.4 herpes zoster infections per 100 patient‐years.
Conclusion
Tofacitinib was effective in 31.8% of patients after 24 months of treatment.
Abstract

Effectiveness and safety of tofacitinib for ulcerative colitis: Two‐year results of the ICC Registry
1. INTRODUCTION
Tofacitinib is the first JAK inhibitor approved for the treatment of moderate to severe ulcerative colitis (UC). 1 Tofacitinib is a small molecule Janus kinase (JAK) inhibitor, which inhibits JAK1 and JAK3. By preventing phosphorylation and subsequent dimerisation of signal transducer and activator of transcription (STATs) proteins, it impairs intracellular signalling in certain immune cells, resulting in reduced inflammatory cytokine production. 2
In the phase 3 OCTAVE trial, a remission rate (defined as a total Mayo score of ≤2, with no subscore >1 and a rectal bleeding subscore of 0) in 34.3% of UC patients after 52 weeks of treatment with tofacitinib was shown. 1 This drug is administered orally and has a rapid onset of action in most patients. 3 The effect of tofacitinib cannot be reduced by the development of anti‐drug antibodies, a potential advantage compared to biologicals, such as anti‐tumour necrosis factor (aTNF) agents. 4 However, there are safety concerns about tofacitinib, such as interference with lipid metabolism, increased risk of venous thromboembolisms and infections. 5
Although in the OCTAVE study it was demonstrated that the therapeutic efficacy of tofacitinib in UC, a study population did not accurately reflect patients who initiate tofacitinib in daily practice. This is partly due to strict in‐and exclusion criteria of clinical trials and the pre‐specified follow‐up period. 6 Therefore, so‐called real‐world studies evaluating the effectiveness and safety of tofacitinib are of great importance. Several studies demonstrated the effectiveness of tofacitinib in UC patients, but the follow‐up did not exceed 1 year. 7 , 8 , 9 , 10 , 11 , 12 , 13 In the current era of many (new) therapeutic options for UC, personalisation of an individual's treatment is a beckoning perspective. As much as up to one‐third of UC patients lose therapeutic response within a year in the case of modern, complex therapies. This necessitates effectiveness and safety data over longer periods, best with standardisation of disease and clinical characterisation of included patients. In this study, we assessed the effectiveness and safety of 24 months of tofacitinib treatment in UC patients.
2. METHODS
2.1. Study design and participants
The ICC Registry is a prospective observational registry of patients with inflammatory bowel disease (IBD) starting novel IBD therapies in regular care in the Netherlands. Its rationale has previously been described in more detail. 14 Currently, 19 centres are actively recruiting and enrolling IBD patients in the ICC Registry. Patients in this study were enrolled between October 2018 and October 2019. Adult patients (aged ≥18 years) with an established diagnosis of IBD are prospectively followed‐up with scheduled outpatient visits at weeks 12, 24, 52 and 104 or discontinuation of treatment. The decision to start tofacitinib treatment was at the discretion of the treating physician. Patients initiating tofacitinib received an induction regimen of 10 mg bi‐daily (BID) for the first 8 weeks, followed by a maintenance treatment of 5 mg BID. Optional dose optimisation in case of insufficient response was at the discretion of the treating physician. There was no standard tapering regimen in case of concomitant treatment for steroids or other regimens for conventional immunosuppressants. Steroids and conventional immunosuppressants were prescribed at the initiation of the treating physician. At baseline, all patients included in the primary analysis had both clinical and objective (biochemical and/or endoscopic) disease activity.
2.2. Outcomes and definitions
The primary outcome was a corticosteroid‐free clinical remission rate at week 104. Clinical remission was defined as a Simple Clinical Colitis Activity Index (SCCAI) ≤2. Secondary outcomes included biochemical remission, combined corticosteroid‐free clinical and biochemical remission, endoscopic remission, safety, and discontinuation rate. Biochemical remission was defined as a C‐reactive protein (CRP) ≤ 5 mg/L and faecal calprotectin ≤250 μg/g (FC). Endoscopic remission was defined as an endoscopic Mayo score of 0. Patients who discontinued tofacitinib due to non‐response, loss of response, adverse events or at the patients' request without clinical remission were considered a treatment failure and considered as non‐responders. Patients who discontinued tofacitinib because of pregnancy or at patients' request with long‐term sustained clinical and biochemical remission were considered censored cases. Patients were considered non‐responders in case of missing data. In case patients were lost to follow‐up as they changed treatment facilities, we contacted the new treatment facility to collect data on the remaining follow‐up visits.
Adverse events leading to therapy withdrawal were classified as severe. The remaining adverse events were labelled as possibly‐ or probably‐related by the treating physician. Infections not leading to antibiotic or antiviral medication were classified as mild infections. Infections requiring oral antibiotics of antiviral medication were classified as moderate infections and infections leading to hospitalisation and/or requiring intravenous (IV) administration of antibiotics or anti‐viral medication were classified as severe infections. Reasons for treatment discontinuation were documented by the treating physician.
2.3. Statistical methods
Analyses were performed with the intention to treat base. Cumulative drug survival was assessed using the Kaplan–Meier method. Depending on the distribution, continuous variables were presented as means with standard deviation (SD) or as median with interquartile range (IQR). Categorical variables were presented as percentages and compared by using the χ 2 test. Predictors of corticosteroid‐free clinical remission at week 104 were assessed using binary logistic regression. Variables with a p‐value of ≤0.2 in the univariate analysis were selected for the multivariable analysis. p < 0.05 was considered to be statistically significant. Statistical analyses were performed using IBM® SPSS® Statistics version 28.0.0.
2.4. Ethical consideration
This study was reviewed and approved by the Committee on Research Involving Human Subjects at the Radboud University Medical Centre (institutional review board: 2018‐4076).
3. RESULTS
3.1. Baseline characteristics
In total, 110 patients were included. Baseline characteristics are shown in Table 1. Most patients were anti‐TNF experienced (94.5%) and vedolizumab experienced (60.9%), but not ustekinumab experienced (3.6%). The mean follow‐up was 102 (7) weeks. Biochemical data at baseline were available in all patients and 96 out of 110 patients (87.3%) had biochemical disease activity at baseline (CRP results were available in 103 patients and increased in 53 patients [51.2%] and FC results were available in 88 patients and increased in 78 patients [88.6%]).
TABLE 1.
Baseline characteristics.
| Tofacitinib (n = 110) | ||
|---|---|---|
| Age (years) | Mean (SD) | 45 (14) |
| Female | N (%) | 50 (45.5) |
| Disease duration (years) | Median (IQR) | 7 (3–14) |
| Follow‐up (weeks) | Mean (SD) | 102 (7) |
| BMI | Mean (SD) | 24.8 (4.2) |
| Disease activity | ||
| SCCAI‐score | Median (IQR) | 7 (5–10) |
| CRP (mg/L), n = 103 | Median (IQR) | 5.9 (2–13) |
| FC (mg/kg), n = 88 | Median (IQR) | 1765 (633–2763) |
| Medical history | ||
| Disease location a | ||
| Proctitis | N (%) | 8 (7.3) |
| Left‐sided | N (%) | 36 (32.7) |
| Pancolitis | N (%) | 64 (58.2) |
| Unknown | N (%) | 2 (1.8) |
| Prior treatment | ||
| Prior thiopurine | N (%) | 95 (86.4) |
| Prior ≥1 anti‐TNF | N (%) | 104 (94.5) |
| Prior ≥2 anti‐TNF | N (%) | 41 (37.2) |
| Prior vedolizumab | N (%) | 67 (60.9) |
| Prior ustekinumab | N (%) | 4 (3.6) |
| JAK inhibitor | ||
| Concomitant treatment | ||
| Oral prednisone | N (%) | 41 (37.3) |
| Oral prednisone dose | mg (IQR) | 20 (15–30) |
| Budesonide | N (%) | 17 (15.5) |
| Mesalazine | N (%) | 40 (36.4) |
| Thiopurine | N (%) | 3 (2.7) |
| Methotrexate | N (%) | 2 (1.8) |
Abbreviations: BMI, body mass index; CRP, C‐reactive protein; FC, faecal calprotectin; SCCAI, Simple Clinical Colitis Activity Index.
Maximum extend until inclusion.
3.2. Clinical outcomes
Corticosteroid‐free clinical remission was achieved in 38.2% (42/110), 35.8% (39/109), 31.8% (34/107), and 31.8% (34/107) of patients at 12, 24, 52, and 104 weeks, respectively (Figure 1A). Of the patients in corticosteroid‐free clinical remission at week 24, 66.7% (26/39) remained in corticosteroid‐free clinical remission after 104 weeks of treatment. Of the patients in corticosteroid‐free clinical remission at week 52, 76.5% (26/34) remained in corticosteroid‐free clinical remission after 104 weeks of treatment. Corticosteroid‐free clinical remission rates in patients treated with at least two different classes of biologicals (e.g. not two anti‐TNF therapies) before initiating tofacitinib are displayed in Figure 1B.
FIGURE 1.
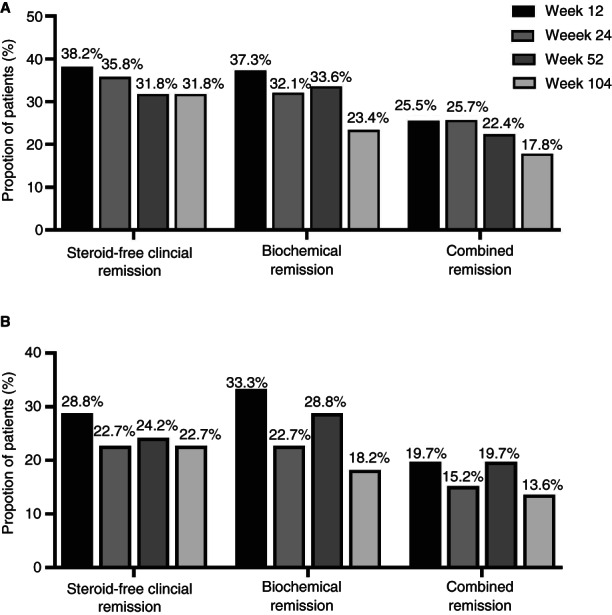
Corticosteroid‐free clinical remission rates, biochemical remission rates and combined remission rates in all patients (A) and patients treated with at least two different classes of biologicals therapies (e.g. not two anti‐TNF therapies) before initiating tofacitinib (B).
3.3. Biochemical outcomes
Biochemical remission was achieved in 37.3% (41/110), 32.1% (35/109), 33.6% (36/107) and 23.4% (25/107) of patients after 12, 24, 52 and 104 weeks respectively (Figure 1A). Of the patients in biochemical remission at week 24, 89.5% (17/35) remained in biochemical remission after 104 weeks of treatment. Of the patients in biochemical remission at week 52, 55.6% (20/36) remained in biochemical remission after 104 weeks of treatment. Biochemical remission rates in patients treated with at least two different classes of biologic therapies before initiating tofacitinib are displayed in Figure 1B.
In patients with elevated CRP concentrations at baseline (n = 53), 39.6% (n = 21), 35.8% (n = 10), 32.1% (n = 17) and 18.9% (n = 10) were in biochemical remission at week 12, 24, 52 and 104 based on CRP concentrations.
In patients with elevated FC concentrations at baseline (n = 78), 34.6% (n = 27), 25.6% (n = 20), 21.8% (n = 21) and 12.8% (n = 10) were in biochemical remission at week 12, 24, 52 and 104 based on FC concentrations.
3.4. Combined corticosteroid‐free clinical and biochemical outcomes
Combined corticosteroid‐free clinical and biochemical remission was achieved in 25.5% (28/110), 25.7% (28/109), 22.4% (24/107) and 18.7% (20/107) patients after 12, 24, 52 and 104 weeks, respectively (Figure 1A). Of the patients in combined corticosteroid‐free clinical and biochemical remission at week 24, 42.9% (12/28) remained in corticosteroid‐free clinical and biochemical remission after 104 weeks of treatment. Of the patients in combined corticosteroid‐free clinical and biochemical remission at week 52, 76.5% (26/34) remained in corticosteroid‐free clinical and biochemical remission after 104 weeks of treatment. Combined corticosteroid‐free clinical and biochemical remission rated in patients treated with at least two different classes of biologic therapies before initiating tofacitinib are displayed in Figure 1B.
3.5. Endoscopic outcomes
Out of 88 patients with documented endoscopic disease activity at baseline, 52 patients (59.1%) underwent endoscopy during follow‐up after a median treatment duration of 15 weeks (IQR 8–43). Thirty‐seven of these patients (71.2%) achieved endoscopic remission during follow‐up and 17 (32.7%) achieved corticosteroid‐free clinical remission (CSFR) after 104 weeks.
3.6. Clinical factors associated with corticosteroid‐free clinical remission
Univariate and multivariate predictors of corticosteroid‐free clinical remission at week 104 are depicted in Table 2. None of the assessed factors was predictive of achieving corticosteroid‐free clinical remission.
TABLE 2.
Univariate and multivariate predictors of corticosteroid‐free clinical remission at week 104.
| Univariate analyses | Multivariate analyses | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p‐value | OR | 95% CI | p‐value | |
| Age at inclusion | 1.01 | 0.96–1.07 | 0.67 | |||
| BMI per point | 1.13 | 0.86–1.47 | 0.39 | |||
| Sex | ||||||
| Male | Ref | |||||
| Female | 1.27 | 0.22–7.20 | 0.79 | |||
| Disease duration (year) | 1.13 | 0.94–1.37 | 0.20 | 1.11 | 0.91–1.35 | 0.31 |
| Disease location a | ||||||
| Proctitis | 0.19 | 0.01–3.72 | 0.60 | |||
| Left‐sided | 2.10 | 0.01–21.10 | 0.27 | |||
| Pancolitis | Ref | 0.53 | ||||
| Prior biological treatments | ||||||
| Vedolizumab | 2.25 | 0.36–13.97 | 0.38 | |||
| ≥2 biologic therapy | 3.95 | 0.42–37.50 | 0.23 | |||
| Clinical disease activityb | ||||||
| SCCAI per point | 0.77 | 0.56–1.06 | 0.11 | 1.13 | 0.94–1.37 | 0.20 |
| Biochemical disease activity a | ||||||
| CRP (mg/L) | 1.02 | 0.94–1.11 | 0.64 | |||
| Faecal calprotectin (μg/g) | 1.00 | 1.00–1.00 | 0.79 | |||
| Concomitant medication a | ||||||
| Corticosteroids | 2.78 | 0.47–16.35 | 0.26 | |||
At inclusion.
Univariate and multivariate predictors of biochemical remission and combined corticosteroid‐free clinical and biochemical remission at week 104 are shown in Tables S1 and S2. None of the assessed factors was predictive for biochemical of combined corticosteroid‐free clinical and biochemical remission. Prior exposure to anti‐TNF therapy or ustekinumab treatment could not be assessed due to the small patient number who were naïve for anti‐TNF or with ustekinumab treatment.
3.7. Drug survival
Cumulative tofacitinib treatment survival is illustrated in Figure 2. Sixty‐one patients stopped tofacitinib treatment after a median treatment duration of 13 weeks (IQR 7–34) (Table 3). After 1 year of treatment, five patients discontinued tofacitinib therapy. These five patients constituted 9.3% of all patients treated with tofacitinib for at least 52 weeks. The most common reasons for treatment discontinuation were primary non‐response (59%), adverse events (18%) and loss of response (14.8%). One patient received euthanasia for non‐IBD‐related disease and died after 23 weeks of follow‐up. Three patients stopped treatment due to an infection (urinary tract, herpes zoster and CMV infections). Two patients discontinued therapy on their own initiative after 20 and 26 weeks of treatment after achieving corticosteroid‐free clinical remission. Of the patients discontinuing tofacitinib, 15 patients continued treatment with concomitant corticosteroids, 15 patients with mesalazine and three patients with budesonide.
FIGURE 2.
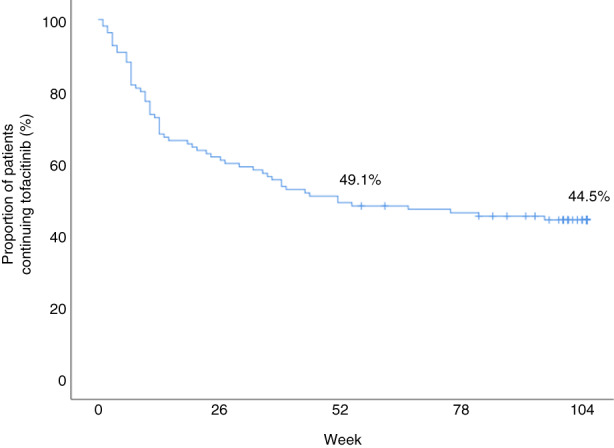
Tofacitinib drug survival in ulcerative colitis patients after 104 weeks of follow‐up.
TABLE 3.
Discontinuation within 104 weeks of treatment.
| UC, N = 61 (55.5%) | |
|---|---|
| Treatment duration—weeks, Median (IQR) | 13 (7–34) |
| Reason discontinuation, N (%) | |
| No response | 36 (59) |
| Adverse events | 11 (18) |
| Secondary loss of response | 9 (14.8) |
| Request patient | 2 (3.3) |
| Death after euthanasia (non‐IBD related) | 1 (1.6) |
| Long‐term biochemical remission | 1 (1.6) |
| No effect on arthralgia | 1 (1.6) |
3.8. Safety
During follow‐up, 13.8 moderate and 1.8 severe infections were reported per 100 patient‐years (Table 4). The most common infections reported were urinary tract infections and herpes zoster infections. After 52 weeks, one severe respiratory tract infection, five moderate infections (gynecologic, urinary tract, herpes zoster, respiratory tract and skin), two mild COVID‐19, one mild herpes zoster and one mild herpes simplex occurred. None of the patients with a herpes zoster infection was vaccinated.
TABLE 4.
Tofacitinib‐related adverse events.
| Tofacitinib: 109 years | |
|---|---|
| Mild infections | 24 (22.0 per 100 patient‐years) |
| Flu‐like symptoms | 4 |
| Upper respiratory tract | 4 |
| Herpes simplex | 3 |
| Herpes zoster | 3 |
| Covid‐19 | 2 |
| Fever of unknown origin | 2 |
| Dental | 1 |
| Ear | 1 |
| Gynaecologic | 1 |
| Lower respiratory tract | 1 |
| Skin | 1 |
| Urinary tract | 1 |
| Moderate infections | 15 (13.8 per 100 patient‐years) |
| Urinary tract | 6 |
| Herpes zoster | 4 |
| CMV | 3 |
| Herpes simplex | 3 |
| Flu‐like symptoms | 2 |
| Fever of unknown origin | 1 |
| Gastrointestinal | 1 |
| Gynaecologic | 1 |
| Lower respiratory tract | 1 |
| Skin | 1 |
| Throat | 1 |
| Upper respiratory tract | 1 |
| Severe infections | 2 (1.8 per 100 patient‐years) |
| Flu‐like symptoms | 1 |
| Lower respiratory tract | 1 |
| Possibly related | 37 (33.9 per 100 patient‐years) |
| Skin | 10 |
| Headache | 6 |
| Gastrointestinal | 3 |
| Respiratory | 3 |
| Cardiac | 2 |
| Musculoskeletal | 2 |
| Glaucoma | 1 |
| Genital | 1 |
| Hepatobiliary | 1 |
| Kidney and urinary tract | 1 |
| Malaise | 1 |
| Mouth | 1 |
| Nerve system | 1 |
| Oedema | 1 |
| Psychiatric | 1 |
| Sleep disturbance | 1 |
| Vascular | 1 |
| Probably related | 8 (7.3 per 100 patient‐years) |
| Musculoskeletal | 3 |
| Skin | 3 |
| Cardiac | 1 |
| Headache | 1 |
| Serious adverse events | 10 (9.2 per 100 patient‐years) |
| Severe headache | 2 |
| Dizziness | 1 |
| Gastrointestinal | 1 |
| Hepatocellular hepatitis | 1 |
| Malaise | 1 |
| Musculoskeletal | 1 |
| Nausea | 1 |
| Skin | 1 |
| Throat | 1 |
Note: Number of adverse events during treatment of ulcerative colitis patients with tofacitinib. Infections were classified as: mild infections: no antibiotics or antiviral medication; moderate infections: oral antibiotics or antiviral medication; severe infections: hospitalisation or intravenously administrated antibiotic or antiviral medication.
In total, 7.3 probably and 33.9 possible tofacitinib‐related adverse events per 100 patients years were seen during follow‐up (Table 4). The most probably related adverse events were skin reactions and headaches. Only one patient experienced possibly related headaches after 52 weeks of treatment. One patient developed hypertension during tofacitinib treatment and one patient experienced an increased heart rate after initiating tofacitinib, this was not objectified. No other cardiovascular of thromboembolic events were seen during follow‐up.
Adverse events led to therapy withdrawal in 10 patients and two of these patients, this was after 52 weeks of treatment, including a CMV infection (viremia) and a urinary tract infection at week 54 and one herpes zoster and urinary tract infection at week 81.
The mean relative difference between baseline and after induction therapy for triglycerides (n = 46), cholesterol (n = 49), HDL‐cholesterol (n = 47) and LDL‐cholesterol (n = 44) concentrations were −0.06 (95% CI: −0.32 to 0.20, p = 0.656), −0.31 (95%CI: −0.59 to 0.03, p = 0.031), −0.13 (95% CI: −0.25 to 0.01, p = 0.031) and − 0.16 (95% CI: −0.41 to 0.09, p = 0.215), respectively.
3.9. Dosage and concomitant treatment
Two patients were treated with a tofacitinib induction scheme of 5 mg BID. Both patients had cardiovascular risk factors (hypertension, hypercholesterolemia, smoking and substance abuse). All other patients initiated tofacitinib with an induction regimen of 10 mg BID. After 12, 24, 52 and 104 weeks of treatment, 32/78 (41%), 18/67 (26.9%) 12/53 (22.6%) and 9/49 (18.4%) patients were on a 10 mg BID dosing schema respectively. Of the patients withdrawing from therapy, 45 patients (73.8%) were on a 10 mg BID dosing scheme and one patient (1.6%) was on a 15 mg BID dosing scheme.
Two patients received concomitant treatment with methotrexate (MTX) at the initiation of tofacitinib therapy. One patient discontinued MTX treatment after 24 weeks, whilst in biochemical remission. The other patient continued MTX throughout 24 months of follow‐up. Out of these three patients who received concomitant treatment with thiopurines at the start of therapy, two stopped thiopurine agents within 12 weeks and the third patient within 52 weeks.
4. DISCUSSION
This is the first prospective cohort describing the effectiveness of tofacitinib in UC patients up to 24 months of treatment. In this prospective, real‐world cohort we observed that 32% of UC patients were in corticosteroid‐free clinical remission at 24 months after initiating tofacitinib treatment. Seventy‐seven per cent of patients who were in corticosteroid‐free clinical remission at 1 year, remained in corticosteroid‐free clinical remission after 24 months.
By applying non‐responder imputation, approximately 60% of UC patients included in the OCTAVE trial were in remission after 24 months treatment. 15 This percentage of patients with remission‐in‐remitters (to induction therapy with tofacitinib) is about twice that high as was observed in common practice, as represented in the current study.
Up to now, in only one relatively small, retrospective cohort remission rates of tofacitinib in UC patients after 24 months of treatment were described. 16 In 13 patients, 53.8% were in remission after 24 months (defined as a partial Mayo score ≤2), but it was unclear whether an intention to treat analysis was used. Other studies evaluated the effectiveness of tofacitinib up to 1 year of treatment. 8 , 11 , 12 Two retrospective cohorts described corticosteroid‐free clinical remission rates of 34.2% and 42% in 38 and 36 patients, respectively, after 1 year of tofacitinib treatment, which is comparable to the here presented results. 11 , 12 In another, Spanish cohort maintenance remission rates were not documented, but similar discontinuation rates of 40% (n = 45/113) of patients were reported in a time period of up to 44 weeks of follow‐up. 8
Adverse events in the current study were higher compared to other real‐world studies assessing the safety of tofacitinib. 12 , 17 These studies had a retrospective design which may have resulted in underreporting of adverse events. Although the Food and Drug Administration (FDA) has expressed concerns about the risk of cancer, cardiovascular and thromboembolic events, none were reported in 109 patient‐years of follow‐up in our cohort. This was in line with other real‐world studies, However, long‐term registries with large sample sizes are required to obtain more details regarding these uncommon but severe adverse events. Due to the risk of cardiovascular and thromboembolic events, the FDA recommends dosing tofacitinib 10 mg BID only during induction therapy for 8 weeks and subsequently dose tofacitinib 5 mg BID. Notwithstanding, in our cohort, 32/78 (41%), 18/67 (26.9%) 12/53 (22.6%) and 9/49 (18.4%) patients were on a 10 mg BID dosing scheme, at 12, 24, 52 and 104 weeks of treatment, respectively. This was due to prolonged induction therapy in case of (partially) non‐response or re‐induction in patients with loss of response. It is unclear for how many weeks patients were treated with a 10 mg BID tofacitinib as this was not part of the collected data at each visit.
Treating physicians face challenges with the positioning of JAK inhibitors in UC patients. Tofacitinib is currently mostly prescribed after anti‐TNF failure in UC patients due to experience, price and safety concerns. Considering the lack of head‐to‐head trials, the exact positioning of tofacitinib after anti‐TNF failure remains an ongoing field of uncertainty and thus research. A meta‐analysis evaluating second‐line pharmacotherapies after anti‐TNF failure for patients with moderate‐to‐severe UC showed that tofacitinib was significantly superior to vedolizumab and adalimumab for induction of clinical remission. 18 Also, comparative real‐world studies showed that tofacitinib was shown to be superior to vedolizumab in achieving biochemical remission up to 1 year of treatment. 19 To determine the optimal positioning of JAK inhibitors in individual patients several aspects should be taken into account including disease severity, immunogenicity, prior anti‐TNF exposure, and patient characteristics such as age and comorbidity.
One of the strengths of this study was the prospective follow‐up of 24 months and its relatively large sample size. Since patients were included in both tertiary and secondary centres and no restricting inclusion criteria other than objectified disease activity and lack of drug‐associated contraindications were used, the presented cohort was representative of patients initiating tofacitinib in daily clinical care. The limitation of this study was the lack of systematic endoscopic evaluation of disease activity, randomisation and direct treatment (strategy) comparison, successively. Since the endoscopic assessment was not mandatory, treating physicians performed endoscopy at their own discretion. This may have led to bias since some centres did not as per standard perform endoscopy for ascertainment of endoscopic healing.
In conclusion, tofacitinib was a relatively effective and safe treatment modality in approximately one‐third (31.8%) of UC patients after 24 months of treatment.
5. AUTHOR CONTRIBUTIONS
Tessa Straatmijer: Conceptualization (supporting); data curation (equal); formal analysis (lead); project administration (equal); writing – original draft (lead); writing – review and editing (equal). Fiona D.M. van Schaik: Data curation (equal); writing – review and editing (equal). Alexander Bodelier: Data curation (equal); writing – review and editing (equal). Marijn C. Visschedijk: Data curation (equal); writing – review and editing (equal). Annemarie C. De Vries: Data curation (equal); writing – review and editing (equal). Cyriel Ponsioen: Data curation (equal); methodology (supporting); writing – review and editing (equal). Marie Pierik: Conceptualization (equal); data curation (equal); methodology (equal); writing – review and editing (equal). Ad A van Bodegraven: Data curation (equal); writing – review and editing (equal). Rachel L West: Data curation (equal); writing – review and editing (equal). Nanne de Boer: Data curation (equal); writing – review and editing (equal). Nidhi Srivastava: Data curation (equal); writing – review and editing (equal). Tessa Romkens: Data curation (equal); writing – review and editing (equal). Jildou Hoekstra: Data curation (equal); writing – review and editing (equal). Bas Oldenburg: Data curation (equal); writing – review and editing (equal). Gerard Dijkstra: Data curation (equal); writing – review and editing (equal). C. Janneke van der Woude: Data curation (equal); writing – review and editing (equal). Mark Lowenberg: Data curation (equal); writing – review and editing (equal). Zlatan Mujagic: Data curation (equal); writing – review and editing (equal). Vince Biemans: Data curation (equal); writing – review and editing (equal). Andrea E. van der Meulen‐de Jong: Conceptualization (equal); data curation (equal); methodology (equal); supervision (lead); writing – original draft (supporting); writing – review and editing (equal). Marjolijn Duijvenstein: Conceptualization (equal); data curation (equal); methodology (equal); supervision (lead); writing – original draft (supporting); writing – review and editing (equal).
7. FUNDING INFORMATION
N de Boer has served as a speaker for AbbVie, Takeda and MSD. He has served as a consultant and principal investigator for Takeda and/or TEVA Pharma B.V. He has received research grants from Dr. Falk, MLDS and Takeda. All outside the submitted work. A.C. de Vries served as an advisory board member of Jansen, Abbvie, Galapagos and Takeda. ISR grant from Pfizer, Jansen and Takeda. Mark Löwenberg has served as a speaker and/or principal investigator for: Abbvie, Alimentiv, Bristol Myers Squibb, Celgene, Covidien, Dr. Falk, Ferring Pharmaceuticals, Galapagos, Gilead, GlaxoSmithKline, Janssen‐Cilag, Merck Sharp & Dohme, Pfizer, Protagonist therapeutics, Receptos, Takeda, Tillotts, Tramedico. He has received research grants from AbbVie, Merck Sharp & Dohme, Dr Falk, Achmea healthcare, Galapagos and ZonMW. Marijn Visschedijk has served as speaker for Janssen‐Cilag. N de Boer has served as a speaker for AbbVie, Takeda and MSD. He has served as a consultant and principal investigator for Takeda and/or TEVA Pharma B.V. He has received research grants from Dr. Falk, MLDS and Takeda. All outside the submitted work. Fiona DM van Schaik served as a consultant for Takeda, Galapagos and Dr Falk. AAvB has received speaker or consultancy or advisory board fees from AbbVie, BMS/Celgene, Ferring, Janssen/J&J, Galapagos, Pfizer, Takeda, and TEVA. Ferring, Janssen, and Takeda supported educational programmes. He participated in Tofacitinib phase 2 and phase 3 registration studies. Research grants were provided by Pfizer, TEVA, Zon‐MW and Zuyderland MC. Vince B.C. Biemans: none.
The ICC fellowship is sponsored by AbbVie, Pfizer, Takeda, Celgene, Janssen Pharmaceutica, MLDS Teva Pharmaceutical Industries, Cablon Medical, Ferring pharmaceuticals, Mundipharma, Dr. Falk Pharma, Sandoz and Tramedico.
Independent Investigator Sponsored Research Grant (Pfizer Tracking Number #57944353).
8.
9. COLLABORATORS
Malena Schlotter, Martine van Workum, Dirk de Jong, Willemijn van Dop, Frank Hoentjen, S. van der Marel, Hayat El Ghabzouri, Kamila Talhaoui, Nynke Boontje, Herma Fidder, Meike Hirdes, Rob H Creemers, Annemarie van der Spek, Jael Smid, Noortje Festen, Carmen Horjus, Bindia Jharap, Wout Mares, Michael van der Voorn, Marthe François‐Verweij, Toos Schakel‐van den Berge, Jeroen Maljaars, Rosaline Theeuwen, Denise van den Berg, Suzanne Gerretsen, Xenia Yocarini, Geert D'Haens, Mark Lowenberg, Joep Grootjans, Krisztina Gecse, Gerd Bouma, Petra Waaijenberg, Bart Muskens.
10. MANUSCRIPT STATEMENT
The manuscript, including figures and tables, has not been previously published and is not under consideration elsewhere.
Supporting information
Appendix S1
6. ACKNOWLEDGEMENT
Declaration of personal interests: Fiona D. M. van Schaik: Advisory Boards Takeda, Galapagos; Alexander G. L. Bodelier has participated in advisory boards of Takeda, Janssen Pharmaceutica, Ferring and Mundipharma; Marijn Visschedijk: has served on the advisory board for Janssen‐Cilag and a speakers fee from Takeda, outside the submitted work; Annemarie C. de Vries: has participated in advisory board and/or received financial compensation from the following companies: Jansen, Takeda, Abbvie and Tramedico; Cyriel Y. Ponsioen: received grants or contracts from Takeda, Pliant, and Gilead Sciences; consulting fees from Pliant and Shire (Takeda); and payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Takeda, Tillotts Pharma, and Pfizer outside of the submitted work; Marieke Pierik: has served on advisory boards, or as speaker or consultant for Abbvie, Janssen‐Cilag, MSD, Takeda, Ferring, Dr Falk, and Sandoz and has received unrestricted grants from, Janssen Cilag, Abbvie and Takeda outside the submitted work; Ad A. van Bodegraven: has served as consultant or speaker for AbbVie, ARENA, Ferring, Janssen, MSD, Pfizer, Takeda, TEVA, Tramedico, VIFOR, and Dutch Ministry of Health (ZonMW). He has received (unrestricted) research grants from Aventis and Ferring, Pfizer, TEVA, and the Dutch Ministry of Health. He performed as (local) principal investigator in studies sponsored by Schering‐Plough, Roche, Teva, Janssen, MSD, Pfizer, ARENA and Centocor; Nanne K. H. de Boer: has served as a speaker for AbbVie and MSD and has served as consultant and/or principal investigator for TEVA Pharma BV and Takeda. He has received a (unrestricted) research grant from Dr. Falk, TEVA Pharma BV, MLDS and Takeda. All outside the submitted work; Bas Oldenburg: has served as speaker for Galapagos, MSD, Janssen and received unrestricted grants from Takeda, Pfizer, Galapagos, Ferring, Celltrion and Abbvie; Gerard Dijkstra: has received grant support from DSM nutritional products LTD and speaker's fees from Janssen Pharmaceuticals, Abbvie and Takeda, outside of the submitted work; Mark Löwenberg: has served as speaker and/or principal investigator for: Abbvie, Alimentiv, Bristol Myers Squibb, Celgene, Covidien, Dr. Falk, Ferring Pharmaceuticals, Galapagos, Gilead, GlaxoSmithKline, Janssen‐Cilag, Merck Sharp & Dohme, Pfizer, Protagonist therapeutics, Receptos, Takeda, Tillotts, Tramedico. He has received research grants from AbbVie, Merck Sharp & Dohme, Dr Falk, Achmea healthcare, Galapagos and ZonMW; Andrea E. van der Meulen‐de Jong: has received a presentation fee from Janssen and served on the advisory board of Takeda and Galapagos, outside of the submitted work.; Marjolijn Duijvestein: reports advisory fees from Echo Pharma and Robarts Clinical Trials, Inc., speaker fees from Janssen, Merck & Co., Inc., Pfizer, Takeda and Tillotts Pharma, and nonfinancial support from Dr. Falk Pharma. All authors approved the last version of the manuscript.
Straatmijer T, van Schaik FDM, Bodelier AGL, Visschedijk M, de Vries AC, Ponsioen CY, et al. Effectiveness and safety of tofacitinib for ulcerative colitis: two‐year results of the ICC Registry. Aliment Pharmacol Ther. 2023;57:117–126. 10.1111/apt.17248
Andrea E. van der Meulen‐de Jong and Marjolijn Duijvestein are shared last authors.
The Handling Editor for this article was Professor Cynthia Seow, and it was accepted for publication after full peer‐review.
8.1. DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 2. Hodge JA, Kawabata TT, Krishnaswami S, et al. The mechanism of action of tofacitinib ‐ an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:318–28. [PubMed] [Google Scholar]
- 3. Hanauer S, Panaccione R, Danese S, Cheifetz A, Reinisch W, Higgins PDR, et al. Tofacitinib Induction Therapy Reduces Symptoms Within 3 Days for Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2019;17:139–47. [DOI] [PubMed] [Google Scholar]
- 4. Ha C, Mathur J, Kornbluth A. Anti‐TNF levels and anti‐drug antibodies, immunosuppressants and clinical outcomes in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2015;9:497–505. [DOI] [PubMed] [Google Scholar]
- 5. Administration FaD. FDA requires warnings about increased risk of serious heart‐related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Silver Spring: Food and Drug Administration; 2021. [Google Scholar]
- 6. Ha C, Ullman TA, Siegel CA, et al. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012;10:1002–7;quiz e78. [DOI] [PubMed] [Google Scholar]
- 7. Biemans VBC, Sleutjes JAM, de Vries AC, Bodelier AGL, Dijkstra G, Oldenburg B, et al. Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51:880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaparro M, Garre A, Mesonero F, et al. Tofacitinib in Ulcerative Colitis: Real‐world Evidence From the ENEIDA Registry. J Crohns Colitis. 2020;15:35–42. [DOI] [PubMed] [Google Scholar]
- 9. Hoffmann P, Globig AM, Thomann AK, Grigorian M, Krisam J, Hasselblatt P, et al. Tofacitinib in Treatment‐Refractory Moderate to Severe Ulcerative Colitis: Real‐World Experience from a Retrospective Multicenter Observational Study. J Clin Med. 2020;9:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Honap S, Chee D, Chapman TP, Patel M, Kent AJ, Ray S, et al. Real‐world Effectiveness of Tofacitinib for Moderate to Severe Ulcerative Colitis: A Multicentre UK Experience. J Crohns Colitis. 2020;14:1385–93. [DOI] [PubMed] [Google Scholar]
- 11. Lair‐Mehiri L, Stefanescu C, Vaysse T, Laharie D, Roblin X, Rosa I, et al. Real‐world evidence of tofacitinib effectiveness and safety in patients with refractory ulcerative colitis. Dig Liver Dis. 2020;52:268–73. [DOI] [PubMed] [Google Scholar]
- 12. Straatmijer T, van Gennep S, Duijvestein M, Ponsioen CIJ, Gecse KB, D'Haens GR, et al. Real‐world clinical and endoscopic outcomes after one year tofacitinib treatment in ulcerative colitis. Eur J Gastroenterol Hepatol. 2021;33:1288–97. [DOI] [PubMed] [Google Scholar]
- 13. Weisshof R, Aharoni Golan M, Sossenheimer PH, el Jurdi K, Ollech JE, Pekow J, et al. Real‐World Experience with Tofacitinib in IBD at a Tertiary Center. Dig Dis Sci. 2019;64:1945–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biemans VBC, van der Meulen‐de Jong AE, van der Woude CJ, et al. Ustekinumab for Crohn's Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J Crohns Colitis. 2020;14:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandborn WJ, Lawendy N, Danese S, Su C, Loftus EV Jr, Hart A, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open‐label, long‐term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther. 2022;55:464–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernández Martínez A, Navajas Hernández P, Martín Rodríguez MDM, et al. Efficacy and safety of tofacitinib in the treatment of ulcerative colitis: real‐life experience in Andalusia. Rev Esp Enferm Dig. 2022;114:516–21. [DOI] [PubMed] [Google Scholar]
- 17. Deepak P, Alayo QA, Khatiwada A, et al. Safety of Tofacitinib in a Real‐World Cohort of Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2021;19(8):1592–601.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First‐ and Second‐Line Pharmacotherapies for Patients With Moderate to Severely Active Ulcerative Colitis: An Updated Network Meta‐Analysis. Clin Gastroenterol Hepatol. 2020;18:2179–2191.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Straatmijer T, Biemans VBC, Visschedijk M, Hoentjen F, de Vries A, van Bodegraven AA, et al. Superior Effectiveness of Tofacitinib Compared to Vedolizumab in Anti‐TNF‐experienced Ulcerative Colitis Patients: A Nationwide Dutch Registry Study. Clin Gastroenterol Hepatol. 2022;In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


