Abstract
Obesity is a chronic, relapsing disease associated with multiple complications and a substantial morbidity, mortality and health care burden. Pharmacological treatments for obesity provide a valuable adjunct to lifestyle intervention, which often achieves only limited weight loss that is difficult to maintain. The Semaglutide Treatment Effect in People with obesity (STEP) clinical trial programme is evaluating once‐weekly subcutaneous semaglutide 2.4 mg (a glucagon‐like peptide‐1 analogue) in people with overweight or obesity. Across STEP 1, 3, 4 and 8, semaglutide 2.4 mg was associated with mean weight losses of 14.9%‐17.4% in individuals with overweight or obesity without type 2 diabetes from baseline to week 68; 69%‐79% of participants achieved ≥10% weight loss with semaglutide 2.4 mg (vs. 12%‐27% with placebo) and 51%‐64% achieved ≥15% weight loss (vs. 5%‐13% with placebo). In STEP 5, mean weight loss was −15.2% with semaglutide 2.4 mg versus −2.6% with placebo from baseline to week 104. In STEP 2 (individuals with overweight or obesity, and type 2 diabetes), mean weight loss was −9.6% with semaglutide 2.4 mg versus −3.4% with placebo from baseline to week 68. Improvements in cardiometabolic risk factors, including high blood pressure, atherogenic lipids and benefits on physical function and quality of life were seen with semaglutide 2.4 mg. The safety profile of semaglutide 2.4 mg was consistent across trials, primarily gastrointestinal adverse events. The magnitude of weight loss reported in the STEP trials offers the potential for clinically relevant improvement for individuals with obesity‐related diseases.
Keywords: anti‐obesity drug, GLP‐1 analogue, obesity therapy, weight management
1. INTRODUCTION
Obesity is a highly prevalent, chronic, relapsing disease requiring long‐term management. 1 , 2 , 3 The clinical complications of obesity affect almost every organ system, and the impact of obesity on morbidity, mortality and health care costs is substantial. 4 , 5 , 6 , 7 The prevalence of obesity has risen globally for the past several decades, a trend predicted to continue. 8 , 9 Worldwide obesity prevalence is 13%, 10 but many countries have a much higher prevalence; for example, prevalence in adults increased from 31% to 42% between 1999‐2000 and 2017‐2018 in the USA, 11 with an increase from 10% to 40% across most European countries over a 10‐year period to 2017. 8
Lifestyle modification is the guideline‐recommended foundation of treatment for individuals with overweight or obesity, 7 , 12 , 13 , 14 but typically achieves only modest weight loss that is often regained. 15 , 16 , 17 Furthermore, this loss is challenging to maintain because of metabolic adaptation that promotes gradual weight regain. 13 , 17 Pharmacological treatments for obesity provide a valuable adjunct to lifestyle interventions, but until recently the available agents only offered moderate weight loss over that achieved with lifestyle intervention. 18 Semaglutide is a potent long‐acting glucagon‐like peptide‐1 (GLP‐1) analogue that requires once‐weekly administration. 19 The Semaglutide Treatment Effect in People with obesity (STEP) clinical trial development programme is evaluating once‐weekly subcutaneous semaglutide 2.4 mg in people with overweight or obesity. Based on data from the STEP trials, once‐weekly subcutaneous semaglutide 2.4 mg has been approved in Canada, Europe, the UK and the USA for chronic weight management in adults with overweight (with weight‐related comorbidities) or obesity. 20 , 21 , 22 , 23
The aim of this review is to describe the study designs and clinical outcomes of the published trials from the STEP programme, discuss clinical implications of the data and practicalities of using semaglutide 2.4 mg in individuals with obesity and place the STEP programme into the context of the current and future obesity pharmacotherapy landscapes.
2. PATHOPHYSIOLOGY OF OBESITY
Obesity is associated with alterations in the quantity, distribution and function of adipose tissue. Most cases result from complex interactions between genetic, metabolic, neuroendocrine, behavioural and environmental factors involved in the regulation of energy balance and fat storage. 24 , 25 In addition to these complex interactions, the imbalance in activity level and energy intake ultimately resulting in increased fat storage may be impacted by individual epigenetics and the gut microbiome. 26
3. MANAGEMENT OF OBESITY
Older pharmacological options for chronic weight management, such as orlistat, phentermine‐topiramate and naltrexone‐bupropion, typically show moderate efficacy (~3%‐9% mean weight loss over that achieved with lifestyle intervention alone). 18 , 27 , 28 , 29 , 30 , 31 Liraglutide 3.0 mg once daily administered subcutaneously was the first GLP‐1 receptor agonist (GLP‐1RA) to be approved for weight management, after demonstrating weight losses of 4%‐6% over those achieved with lifestyle intervention alone in clinical trials of 20‐56 weeks' duration. In these trials, 46%‐76% of participants lost ≥5% and 23%‐37% lost ≥10% of their baseline body weight. 18 , 32 , 33 , 34 , 35
Semaglutide is a GLP‐1 analogue shown to reduce energy intake, reduce hunger and increase feelings of satiety and fullness. 19 , 36 This effect has been shown to arise via GLP‐1 receptor activation in the central nervous system, with further indirect modulation of neuronal activity involved in appetite regulation and food intake and preference. 37 The STEP programme was designed to comprehensively explore the efficacy of once‐weekly subcutaneous semaglutide 2.4 mg in people with overweight or obesity, with each trial addressing a specific research question. For the six completed trials discussed in this review, these are: STEP 1 (large pivotal study), weight loss 38 ; STEP 2, weight loss in type 2 diabetes 39 ; STEP 3, weight loss in combination with intensive behavioural therapy 40 ; STEP 4, effect of continuing versus withdrawing semaglutide on weight‐loss maintenance 41 ; STEP 5, weight maintenance over 2 years 42 ; and STEP 8, head‐to‐head comparison of semaglutide and liraglutide. 43 In addition to these STEP trials, a regional phase IIIa trial, STEP 6, assessed the effect of semaglutide versus placebo for weight management in 401 adults from east Asia (Japan and South Korea) with obesity, with or without type 2 diabetes. 44
4. STEP TRIAL DESIGNS
Table 1 compares key aspects of the trial designs, eligibility criteria, participant demographics and baseline characteristics of the global trials.
TABLE 1.
STEP trials 1‐5 and 8: study designs and participant population baseline demographics and clinical characteristics 16 , 38 , 39 , 40 , 41 , 42 , 43
| STEP 1 16 , 38 | STEP 2 16 , 39 | STEP 3 16 , 40 | STEP 4 16 , 41 | STEP 5 16 , 42 | STEP 8 43 | |
|---|---|---|---|---|---|---|
| Weight management | Weight management in type 2 diabetes | Weight management with intensive behavioural therapy | Sustained weight management | Two‐year weight management | Semaglutide vs. liraglutide | |
| N | 1961 | 1210 | 611 | 902 enrolled; 803 randomized | 304 | 338 |
| Participants | Adults with BMI ≥30, or BMI ≥27 with ≥1 comorbidity, and without diabetes a | Adults with BMI ≥27 and type 2 diabetes, with HbA1c 7.0%‐10.0% b | Adults with BMI ≥30, or BMI ≥27 with ≥1 comorbidity, and without diabetes a | Adults with BMI ≥30, or BMI ≥27 with ≥1 comorbidity, and without diabetes a | Adults with BMI ≥30, or BMI ≥27 with ≥1 comorbidity, and without diabetes a | Adults with BMI ≥30, or BMI ≥27 with ≥1 comorbidity, and without diabetes a |
| Treatment arms and randomization ratios |
Semaglutide 2.4 mg vs. placebo 2:1 ratio |
Semaglutide 2.4 mg Semaglutide 1.0 mg Placebo 1:1:1 ratio |
Semaglutide 2.4 mg vs. placebo 2:1 ratio |
All receive semaglutide for 20 weeks (dose escalation) Then semaglutide 2.4 mg vs. placebo 2:1 ratio |
Semaglutide 2.4 mg vs. placebo 1:1 ratio |
Semaglutide 2.4 mg vs. placebo vs. liraglutide 3.0 mg vs. placebo 3:1:3:1 ratio |
| Duration of study | 68 weeks of treatment | 68 weeks | 68 weeks | 68 weeks (randomization into two arms at week 20) | 104 weeks | 68 weeks |
| Primary endpoints |
% change in body weight ≥5% weight loss |
% change in body weight ≥5% weight loss (semaglutide 2.4 mg vs. placebo) |
% change in body weight ≥5% weight loss |
% change in body weight |
% change in body weight ≥5% weight loss |
% change in body weight |
| Confirmatory secondary endpoints (in hierarchical testing order) |
≥10% weight loss ≥15% weight loss Change in: Waist circumference Systolic blood pressure Physical functioning (SF‐36 and IWQOL‐lite‐CT) |
≥10% weight loss ≥15% weight loss Change in: Waist circumference % change in body weight (semaglutide 2.4 vs. 1.0 mg) HbA1c Systolic blood pressure Physical functioning (SF‐36 and IWQOL‐lite‐CT) |
≥10% weight loss ≥15% weight loss Change in: Waist circumference Systolic blood pressure Physical functioning (SF‐36) |
Change in: Waist circumference Systolic blood pressure Physical functioning (SF‐36) |
≥10% weight loss ≥15% weight loss Change in: Waist circumference Systolic blood pressure |
≥10% weight loss ≥15% weight loss ≥20% weight loss |
| Background treatment | Lifestyle intervention Reduced‐calorie diet (500 kcal/day deficit relative to estimated energy expenditure) and increased physical activity (150 min/week) | Lifestyle intervention Reduced‐calorie diet (500 kcal/day deficit relative to estimated energy expenditure) and increased physical activity (150 min/week) | Intensive behavioural therapy Low‐calorie diet (1000‐1200 kcal/day) as meal replacements for first 8 weeks post‐randomization then a hypocaloric diet (1200‐1800 kcal/day) of conventional food for remainder of the 68 weeks. Participants were prescribed 100 min/week of physical activity at randomization, increasing by 25 min every 4 weeks, to reach 200 min/week 30 individual intensive behavioural therapy visits with a registered dietitian | Lifestyle intervention Reduced‐calorie diet (500 kcal/day deficit relative to estimated energy expenditure) and increased physical activity (150 min/week) | Lifestyle intervention Reduced‐calorie diet (500 kcal/day deficit relative to estimated energy expenditure) and increased physical activity (150 min/week) | Lifestyle intervention Reduced‐calorie diet (500 kcal/day deficit relative to estimated energy expenditure) and increased physical activity (150 min/week) |
| Sex, female, n (%) | 1453 (74.1) | 616 (50.9) | 495 (81.0) | 634 (79.0) | 236 (77.4) | Sema/lira/placebo: 102 (81.0)/97 (76.4)/66 (77.6) |
| Age, years; mean (SD) | 46 (13) | 55 (11) | 46 (13) | 46 (12) | 47 (11) | Sema/lira/placebo: 48 (14)/49 (13)/51 (12) |
| Race, n (%) | ||||||
| White | 1472 (75.1) | 751 (62.1) | 465 (76.1) | 672 (83.7) | 283 (93.1) | Sema/lira/placebo: 94 (74.6)/95 (74.8)/60 (70.6) |
| Black/African American | 111 (5.7) | 100 (8.3) | 116 (19.0) | 104 (13.0) | 12 (3.9) | Sema/lira/placebo: 25 (19.8)/20 (15.7)/19 (22.4) |
| Asian | 261 (13.3) | 317 (26.2) | 11 (1.8) | 19 (2.4) | 2 (0.7) | Sema/lira/placebo: 4 (3.2)/6 (4.7)/3 (3.5) |
| Other c | 117 (6.0) | 42 (3.5) | 19 (3.1) | 8 (1.0) | 7 (2.3) | Sema/lira/placebo: 3 (2.4)/6 (4.7)/3 (3.5) |
| Mean body weight, kg | 105.3 | 99.8 | 105.8 | 107.2 | 106.0 | 104.5 |
| HbA1c, %; mean (SD) | 5.7 (0.32) | 8.1 (0.8) | 5.7 (0.3) | 5.7 (0.3) | 5.7 (0.3) | Sema/lira/placebo: 5.5 (0.3)/5.5 (0.3)/5.6 (0.4) |
| BMI, kg/m2; mean (SD) | 37.9 (6.7) | 35.7 (6.3) | 38.0 (6.7) | 38.4 (6.9) | 38.5 (6.9) | Sema/lira/placebo: 37.0 (7.4)/37.2 (6.4)/38.8 (6.5) |
| Waist circumference, cm; mean (SD) | 114.7 (14.7) | 114.6 (14.1) | 113.0 (15.5) | 115.3 (15.5) | 115.7 (14.8) | Sema/lira/placebo: 111.8 (16.3)/113.5 (15.0)/115.4 (15.1) |
| Blood pressure, mmHg; mean (SD) | ||||||
| Systolic | 126.5 (14.3) | 130.0 (13.5) | 124.4 (14.8) | 127 (14) | 125.5 (14.5) | Sema/lira/placebo: 125 (14)/126 (16)/123 (14) |
| Diastolic | 80.3 (9.6) | 79.8 (9.0) | 80.5 (9.7) | 81 (10) | 80.1 (9.4) | Sema/lira/placebo: 81 (9)/81 (10)/79 (9) |
| Prediabetes d | 885 (43.6) | NA | 305 (49.9) | 408 (45.3) | 141 (46.4) | Sema/lira/placebo: 43 (34.1)/45 (35.4)/34 (40.0) |
| Number of comorbidities at screening, n (%) e | ||||||
| 0 | 491 (25.0) | 0 | 148 (24.2) | 214 (26.7) | NA | Sema/lira/placebo: 32 (25.4)/25 (19.7)/16 (18.8) |
| 1 | 524 (26.7) | 124 (10.2) | 146 (23.9) | 238 (29.6) | Sema/lira/placebo: 31 (24.6)/29 (22.8)/17 (20.0) | |
| 2 | 433 (22.1) | 219 (18.1) | 139 (22.7) | 171 (21.3) | Sema/lira/placebo: 25 (19.8)/29 (22.8)/21 (24.7) | |
| 3 | 279 (14.2) | 317 (26.2) | 100 (16.4) | 111 (13.8) | Sema/lira/placebo: 17 (13.5)/24 (18.9)/9 (10.6) | |
| 4 | 139 (7.1) | 290 (24.0) | 45 (7.4) | 53 (6.6) | Sema/lira/placebo: 10 (7.9)/11 (8.7)/9 (10.6) | |
| ≥5 | 95 (4.8) | 260 (21.5) | 33 (5.4) | 16 (2.0) | Sema/lira/placebo: 11 (8.7)/9 (7.1)/13 (15.3) |
Abbreviations: BMI, body mass index; HbA1c, glycated haemoglobin; IWQOL‐Lite‐CT, impact of weight on quality of life‐lite clinical trials version questionnaire; lira, liraglutide; NA, not available; SD, standard deviation; sema, semaglutide; SF‐36, short form‐36 version 2 health survey, acute version.
Key inclusion criteria for STEP 1, 3‐5 and 8 trials were age ≥18 years, BMI ≥30 kg/m2 or ≥27 kg/m2 with ≥1 weight‐related comorbidity (treated or untreated): hypertension, dyslipidaemia, obstructive sleep apnoea or cardiovascular disease, history of at least one self‐reported unsuccessful dietary effort to lose weight and no type 1 or type 2 diabetes.
Key inclusion criteria for the STEP 2 trial were age ≥18 years, type 2 diabetes diagnosed ≥180 days prior, BMI ≥27 kg/m2, HbA1c 7%‐10% (53‐86 mmol/mol) and treatment with diet and exercise alone or stable treatment with metformin, sulphonylurea, sodium‐glucose co‐transporter‐2 inhibitor, glitazone as single‐agent therapy or ≤3 agents for diabetes according to local label.
Native American, Alaska Native, Native Hawaiian, other Pacific Islander and other ethnic group. In STEP 1, this category included participants who answered ‘not applicable’, which is the way race or ethnic group was recorded in France.
For STEP 1, the presence of prediabetes was determined by investigators based on available information (e.g. medical records, concomitant medication and blood glucose variables) and in accordance with American Diabetes Association criteria.
Information collected at screening on comorbidities was based on medical history and included: type 2 diabetes (STEP 2 only), dyslipidaemia, hypertension, coronary artery disease, cerebrovascular disease, obstructive sleep apnoea, impaired glucose metabolism (not included for STEP 2), reproductive system disorders, liver disease, kidney disease, osteoarthritis, gout, thyroid disease (STEP 3 only) and asthma or chronic obstructive pulmonary disease.
In STEP 1, the largest trial (N = 1961), 38 participants were randomized to receive once‐weekly subcutaneous semaglutide 2.4 mg or matching placebo for 68 weeks. At week 68, treatments (including lifestyle intervention) were discontinued and an off‐treatment extension followed a representative subset of participants for a further year.
STEP 2 39 enrolled individuals with type 2 diabetes [glycated haemoglobin (HbA1c) 7.0%‐10.0%], and randomized them to once‐weekly subcutaneous semaglutide 2.4 mg, semaglutide 1.0 mg (the approved dose in type 2 diabetes) or placebo for 68 weeks.
STEP 3 40 was a 68‐week trial that compared once‐weekly subcutaneous semaglutide 2.4 mg with placebo as an adjunct to intensive behavioural therapy and an initial low‐calorie meal‐replacement diet for all participants.
STEP 4 41 was a 68‐week withdrawal trial, designed to assess the effect on weight change of continuing versus discontinuing once‐weekly subcutaneous semaglutide 2.4 mg after an initial 20‐week semaglutide run‐in period.
STEP 5 42 is the longest trial of the STEP programme. Participants were randomized to receive once‐weekly subcutaneous semaglutide 2.4 mg or matching placebo for 104 weeks.
STEP 6 44 was a 68‐week trial in adults from east Asia with a body mass index (BMI) of ≥27.0 kg/m 2 with ≥2 weight‐related comorbidities or a BMI of ≥35.0 kg/m 2 with ≥1 weight‐related comorbidity (one comorbidity had to be either hypertension, dyslipidaemia or, in Japan only, type 2 diabetes). Participants were randomized to semaglutide 2.4 mg, 1.7 mg or placebo.
STEP 8 43 was a head‐to‐head comparison in which participants were randomized to receive once‐weekly subcutaneous semaglutide 2.4 mg or matching placebo, or once‐daily liraglutide 3.0 mg or matching placebo for 68 weeks.
All trials followed the same dose‐escalation regimen. Participants treated with subcutaneous semaglutide 2.4 mg were initiated on a dose of 0.25 mg once weekly and the dose was escalated every 4 weeks to 0.5 mg, 1.0 mg and 1.7 mg, until the target dose of 2.4 mg was reached at week 16. If participants were unable to tolerate the 2.4 mg dose because of adverse events (AEs), lower maintenance doses were permitted if the participant would otherwise discontinue trial treatment completely. It was recommended that participants made at least one attempt to re‐escalate to the recommended target dose. 38 , 39 , 40 , 41 , 42 , 43 , 44 Diet and physical activity (lifestyle intervention) were recorded daily in a diary or by use of a smartphone application or other tools, reviewed during counselling sessions.
4.1. Estimands used to assess treatment efficacy
In line with regulatory guidance, the STEP trials were designed to report results using two estimands. 45 The treatment policy (primary) estimand assessed effects regardless of treatment discontinuation or rescue medication, while the secondary trial product estimand modelled treatment effects in all randomly assigned participants, assuming they had remained on treatment for the duration of the trials, and without initiation of rescue medication. The data reported in this review are based on the primary treatment policy estimand, since the statistical analyses were performed using this approach. Results using the two estimands were largely consistent.
5. CLINICAL EVIDENCE FROM THE STEP PROGRAMME
5.1. Demographics and baseline characteristics
STEP 1, 3, 4, 5 and 8 showed broadly similar participant demographics and baseline characteristics (Table 1). 38 , 40 , 41 , 42 , 43 In contrast, STEP 2 had a lower proportion of female participants (51% vs. 74%‐81% in the other trials), a greater mean age (55 years vs. 46‐49 years) and a higher mean HbA1c (8.1% vs. 5.5%‐5.7%), which is to be expected for a trial enrolling individuals with type 2 diabetes. 39 Mean BMI was lower in STEP 2 than the other trials (35.7 kg/m2 vs. 37.5‐38.4 kg/m2). 38 , 39 , 40 , 41 , 42 , 43 The STEP 6 trial population comprised mainly Japanese participants (90%) with the remainder from South Korea. Mean body weight and BMI was lower for this population than in other STEP trials (87.5 kg and 31.9 kg/m2, respectively). 44
5.2. Efficacy outcomes
Table 2 presents results of the primary and selected key secondary and exploratory endpoints across the trials. All trials met their primary endpoints. Across the 68‐week long trials in individuals with overweight or obesity with comorbidities without type 2 diabetes (STEP 1, 3, 4 and 8), semaglutide 2.4 mg was associated with a mean weight loss of 14.9%‐17.4% from baseline to week 68 (Table 2). 38 , 40 , 41 , 43 Furthermore, >84% of participants were receiving the full dose of semaglutide at week 68.
TABLE 2.
Efficacy results from the STEP 1‐5 and 8 trials a
| STEP 1 38 | STEP 2 39 | STEP 3 40 | STEP 4 41 | STEP 5 42 | STEP 8 43 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight management | Weight management in type 2 diabetes | Weight management with intensive behavioural therapy | Sustained weight management | Two‐year weight management | Semaglutide vs. liraglutide | |||||||||
| Semaglutide 2.4 mg (n = 1306) | Placebo (n = 655) | Semaglutide 2.4 mg (n = 404) | Semaglutide 1.0 mg (n = 403) | Placebo (n = 403) | Semaglutide 2.4 mg (n = 407) | Placebo (n = 204) | Semaglutide 2.4 mg (n = 535) | Placebo (n = 268) | Semaglutide 2.4 mg (n = 152) | Placebo (n = 152) | Semaglutide 2.4 mg (n = 126) | Liraglutide 3.0 mg (n = 127) | Placebo (n = 85) b | |
| % weight change | −14.9 | −2.4 | −9.6 | −7.0 | −3.4 | −16.0 | −5.7 | Baseline to week 20: −10.6; week 20‐68: −7.9; week 0‐68: −17.4 | Week 20‐68: 6.9; week 0‐68: −5.0 | −15.2 | −2.6 | −15.8 | −6.4 | −1.9 |
| Treatment difference | −12.4 |
2.4 mg vs. 1.0 mg −2.7 2.4 mg vs. placebo −6.2 |
−10.3 | −14.8 | −12.6 | Sema vs. lira −9.4 | ||||||||
| % of participants with weight reduction of: c | ||||||||||||||
| ≥5% | 86.4 | 31.5 | 68.8 | 57.1 | 28.5 | 86.6 | 47.6 | 88.7 | 47.6 | 77.1 | 34.4 | 87.2 | 58.1 | 29.5 |
| ≥10% | 69.1 | 12.0 | 45.6 | 28.7 | 8.2 | 75.3 | 27.0 | 79.0 | 20.4 | 61.8 | 13.3 | 70.9 | 25.6 | 15.4 |
| ≥15% | 50.5 | 4.9 | 25.8 | 13.7 | 3.2 | 55.8 | 13.2 | 63.7 | 9.2 | 52.1 | 7.0 | 55.6 | 12.0 | 6.4 |
| ≥20% | 32.0 | 1.7 | 13.1 | 4.7 | 1.6 | 35.7 | 3.7 | 39.6 | 4.8 | 36.1 | 2.3 | 38.5 | 6.0 | 2.6 |
| Change from baseline to end of treatment in: | ||||||||||||||
| Waist circumference change, cm | −13.54 | −4.13 | −9.4 | −6.7 | −4.5 | −14.6 | −6.3 | −6.4 | 3.3 | −14.4 | −5.2 | −13.2 | −6.6 | −2.0 |
| Treatment difference | −9.42 |
2.4 mg vs. 1.0 mg –2.7 2.4 mg vs. placebo −4.9 |
−8.3 | −9.7 | −9.2 | Sema vs. lira −6.6 | ||||||||
| Systolic blood pressure, mmHg | −6.16 | −1.06 | −3.9 | −2.9 | −0.5 | −5.6 | −1.6 | 0.5 | 4.4 | −5.7 | −1.6 | −5.7 | −2.9 | 3.2 |
| Treatment difference | −5.10 |
2.4 mg vs. 1.0 mg −1.0 2.4 mg vs. placebo −3.4 |
−3.9 | −3.9 | −4.2 | Sema vs. lira −2.8 | ||||||||
| Diastolic blood pressure, mmHg | −2.83 | −0.42 | −1.6 | −0.6 | −0.9 | −3.0 | −0.8 | 0.3 | 0.9 | −4.4 | −0.8 | −5.0 | −0.5 | 0.7 |
| Treatment difference | −2.41 |
2.4 mg vs. 1.0 mg −0.9 2.4 mg vs. placebo −0.7 |
−2.2 | −0.6 | −3.7 | Sema vs. lira −4.5 | ||||||||
| SF‐36 physical functioning score | 2.21 | 0.41 | 2.5 | 2.4 | 1.0 | 2.4 | 1.6 | 1.0 | −1.5 | NA | NA | NA | NA | NA |
| Treatment difference | 1.80 |
2.4 mg vs. 1.0 mg 0.1 2.4 mg vs. placebo 1.5 |
0.8 | 2.5 | – | – | ||||||||
| IWQOL‐Lite‐CT physical function score | 14.67 | 5.25 | 10.1 | 8.7 | 5.3 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Treatment difference | 9.43 |
2.4 mg vs. 1.0 mg 1.4 2.4 mg vs. placebo 4.8 |
– | – | – | – | ||||||||
| Body weight, kg | −15.3 | −2.6 | −9.7 | −6.9 | −3.5 | −16.8 | −6.2 | −7.1 | 6.1 | −16.1 | −3.2 | −15.3 | −6.8 | −1.6 |
| Treatment difference | −12.7 |
2.4 mg vs. 1.0 mg −2.7 2.4 mg vs. placebo −6.1 |
−10.6 | −13.2 | −12.9 | Sema vs. lira −8.5 | ||||||||
| BMI, kg/m2 | −5.54 | −0.92 | −3.5 | −2.5 | −1.3 | −6.0 | −2.2 | −2.6 | 2.2 | −5.9 | −1.6 | NA | NA | NA |
| Treatment difference | −4.61 |
2.4 mg vs. 1.0 mg −1.0 2.4 mg vs. placebo −2.3 |
−3.8 | −4.7 | −12.6 | − | ||||||||
| HbA1c, % | −0.45 | −0.15 | −1.6 | −1.5 | −0.4 | −0.51 | −0.27 | −0.1 | 0.1 | −0.4 | −0.1 | −0.2 | −0.1 | 0.1 |
| Treatment difference | −0.29 |
2.4 mg vs. 1.0 mg −0.2 2.4 mg vs. placebo −1.2 |
−0.24 | −0.2 | −0.3 | Sema vs. lira −0.2 | ||||||||
| Fasting plasma glucose, mg/dl | −8.35 | −0.48 | −38.0 | −32.2 | −1.4c | −6.73 | −0.65 | −0.8 | 6.7 | −7.6 | 1.7 | −8.3 | −4.3 | 3.3 |
| Treatment difference | −7.87 |
2.4 mg vs. 1.0 mg −5.7 2.4 mg vs. placebo −36.6 |
−6.09 | −7.5 | −9.3 | Sema vs. lira −3.9 | ||||||||
| Lipid levels, % change d | ||||||||||||||
| Total cholesterol | −3.0 | 0 | −1.0 | −2.0 | −1.0 | −3.8 | 2.1 | 5 | 11 | −3.3 | 1.4 | −7.1 | −0.1 | −3.3 |
| Treatment difference | −3.0 |
2.4 mg vs. 1.0 mg 1.0^l 2.4 mg vs. placebo −1.0 |
−5.8 | −6 | −4.6 | Sema vs. lira −7.0 | ||||||||
| HDL cholesterol | 5.0 | 1.0 | 7.0 | 5.0 | 4.0 | 6.5 | 5.0 | 18 | 18 | 9.6 | 8.1 | −0.3 | 1.9 | −0.9 |
| Treatment difference | 4.0 |
2.4 mg vs. 1.0 mg 2.0 2.4 mg vs. placebo 3.0 |
1.5 | 0 | 1.3 | Sema vs. lira −2.2 | ||||||||
| LDL cholesterol | −3.0 | 1.0 | 0 | −1.0 | 0 | −4.7 | 2.6 | 1 | 8 | −6.1 | −2.7 | −6.5 | 0.9 | −1.1 |
| Treatment difference | −4.0 |
2.4 mg vs. 1.0 mg 1.0 2.4 mg vs. placebo 0 |
−7.1 | −6 | −3.4 | Sema vs. lira −7.3 | ||||||||
| VLDL cholesterol | −22.0 | −7.0 | −21.0 | −17.0 | −10.0 | −22.5 | −6.6 | −6 | 15 | −18.9 | 3.3 | −20.7 | −10.9 | −4.1 |
| Treatment difference | −16.0 |
2.4 mg vs. 1.0 mg −5.0 2.4 mg vs. placebo −12.0 |
−17.0 | −18 | −21.5 | Sema vs. lira −11.0 | ||||||||
| Free fatty acids | −17.0 | −7.0 | −16.0 | −14.0 | −1.0 | −11.9 | 4.0 | −18 | −14 | 0.3 | 7.0 | −12.6 | −8.8 | 2.6 |
| Treatment difference | −11.0 |
2.4 mg vs. 1.0 mg −3.0 2.4 mg vs. placebo −16.0 |
−15.3 | −5 | −6.2 | Sema vs. lira −4.2 | ||||||||
| Triglycerides | −22.0 | −7.0 | −22.0 | −17.0 | −9.0 | −22.5 | −6.5 | −6 | 15 | −19.0 | −3.7 | −20.7 | −11.0 | −3.2 |
| Treatment difference | −16.0 |
2.4 mg vs. 1.0 mg −6.0 2.4 mg vs. placebo −14.0 |
−6.5 | −18 | −21.9 | Sema vs. lira −11.0 | ||||||||
Abbreviations: BMI, body mass index; HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; IWQOL‐Lite‐CT, impact of weight on quality of life‐lite clinical trials version questionnaire; LDL, low‐density lipoprotein; NA, not available; SF‐36, short form‐36 version 2 health survey, acute version; VLDL, very low‐density lipoprotein.
Results are estimated data for the treatment policy estimand (effects assessed regardless of treatment discontinuation or rescue intervention); data and treatment differences are reported for the randomized period [i.e. baseline (week 20 in STEP 4) to week 68 for STEP 1, 2, 3, 4 and 8, and from baseline to week 104 for STEP 5].
Pooled placebo data.
Observed percentages of participants who had body weight reductions of ≥5%, ≥10%, ≥15% and ≥20% from baseline to week 68 (week 104 for STEP 5) during the in‐trial observation period, based on the number of participants for whom data were available at the week 68 visit (week 104 for STEP 5).
For STEP 1 and 2, lipid parameters were initially analysed on a log scale as estimated ratio of end of treatment value to baseline. For interpretation, these data are expressed as relative percentage change and were calculated using the formula (estimated ratio − 1) × 100.
In STEP 1, mean weight loss with semaglutide plus usual lifestyle intervention was 14.9% (vs. 2.4% with placebo), whereas in STEP 3, mean weight loss with semaglutide plus intensive behavioural therapy was 16.0% (vs. 5.7% with placebo). 38 , 40 In STEP 4, the mean decrease in body weight during the 20‐week run‐in period with semaglutide treatment was 10.6%. Individuals randomized to continue semaglutide lost an additional 7.9% in body weight from weeks 20 to 68, whereas individuals who switched to placebo experienced a mean 6.9% increase. 41 In STEP 8, mean weight loss was greater with semaglutide 2.4 mg than with liraglutide 3.0 mg from baseline to week 68 (15.8% vs. 6.4%). 43
In the STEP 1, 3, 4 and 8 trials (the 68‐week long trials in participants without diabetes), weight loss of ≥5% (a threshold widely accepted as indicating clinically meaningful response to therapy) 46 was achieved by 86%‐89% of participants receiving semaglutide 2.4 mg versus 29%‐48% receiving placebo (Table 2). 38 , 40 , 41 , 43 In these trials, 69%‐79% of participants achieved ≥10% weight loss with semaglutide 2.4 mg (vs. 12%‐27% with placebo), 51%‐64% achieved ≥15% weight loss (vs. 5%‐13% with placebo), and 32%‐40% achieved ≥20% weight loss (vs. 2%‐5% with placebo) (Table 2). 38 , 40 , 41 , 43
Among participants with type 2 diabetes in the STEP 2 trial, the reduction in body weight with semaglutide 2.4 mg was 9.6% (vs. 7.0% for semaglutide 1.0 mg and 3.4% for placebo) from baseline to week 68 (Table 2). In STEP 2, 69% of participants achieved ≥5% weight loss with semaglutide 2.4 mg (vs. 57% with semaglutide 1.0 mg and 29% with placebo). In addition, participants were more likely to probably lose ≥10%, ≥15% and ≥20% body weight with semaglutide 2.4 mg versus placebo (46% vs. 8%, 26% vs. 3% and 13 vs. 2%, respectively) (Table 2). 39
In STEP 5, mean weight loss from baseline to week 104 was 15.2% with semaglutide 2.4 mg versus 2.6% with placebo. Participants were more likely to probably lose ≥5%, ≥10% and ≥15% and ≥20% body weight with semaglutide versus placebo (77% vs. 34%, 62% vs. 13%, 52% vs. 7.0% and 36% vs. 2%, respectively). 42
In STEP 6 (east Asian population with or without diabetes), mean weight loss from baseline to week 68 was 13.2% with semaglutide 2.4 mg, 9.6% with semaglutide 1.7 mg and 2.1% with placebo. As in the other STEP trials, a larger proportion of participants achieved ≥5%, ≥10% and ≥15% reduction in baseline bodyweight with semaglutide 2.4 mg compared with placebo (83% vs. 21%, 61% vs. 5% and 41% vs. 3%, respectively). 44
Secondary endpoint results from the STEP trials indicated improvement in cardiometabolic risk factors including waist circumference, blood pressure, lipids and C‐reactive protein with semaglutide 2.4 mg, as well as benefits on physical function and quality of life from baseline to week 68 (Table 2). 38 , 39 , 40 , 41 , 42 , 43 Improvements in body composition with semaglutide 2.4 mg were observed in a subpopulation of participants assessed by dual‐energy X‐ray absorptiometry in STEP 1, 38 and with reductions in visceral fat areas with semaglutide 2.4 mg observed in a subpopulation of participants assessed by computed tomography scan in STEP 6. 44 Changes in glycaemic status (shift from prediabetes to normoglycaemia; tested while on treatment) were observed in STEP 1 and STEP 6. Further analyses of participants with baseline prediabetes from the STEP 1, 3 and 4 trials showed that treatment with semaglutide resulted in significantly more participants with normoglycaemia at week 68. 47
In STEP 2, mean reduction in HbA1c from baseline to week 68 with semaglutide 2.4 mg was 1.6%, versus 1.5% with semaglutide 1.0 mg and 0.4% with placebo. More patients receiving semaglutide decreased use of concomitant glucose‐lowering medications. Furthermore, urine albumin‐to‐creatinine ratios were improved with semaglutide in STEP 2. 39
5.3. Safety
The safety profile of once‐weekly subcutaneous semaglutide 2.4 mg was broadly consistent across the STEP trials. AEs, serious AEs, AEs leading to discontinuation, AEs reported in ≥10% of participants in any trial and safety focus area AEs are detailed in Table 3. 38 , 39 , 40 , 41 , 42 , 43
TABLE 3.
Number and proportion of participants with adverse events in the STEP 1‐5 and 8 trials
| STEP 1 38 | STEP 2 39 | STEP 3 40 | STEP 4, 41 , a | STEP 5 42 | STEP 8 43 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight management | Weight management in type 2 diabetes | Weight management with intensive behavioural therapy | Sustained weight management | Two‐year weight management | Semaglutide vs. liraglutide | |||||||||
| n (%) of participants with AEs | Semaglutide 2.4 mg (n = 1306) | Placebo (n = 655) | Semaglutide 2.4 mg (n = 403) | Semaglutide 1.0 mg (n = 402) | Placebo (n = 402) | Semaglutide 2.4 mg (n = 407) | Placebo (n = 204) | Semaglutide 2.4 mg (n = 535) | Placebo (n = 268) | Semaglutide 2.4 mg (n = 152) | Placebo (n = 152) | Semaglutide 2.4 mg (n = 126) | Liraglutide 3.0 mg (n = 127) | Placebo b (n = 85) |
| Any AE | 1171 (89.7) | 566 (86.4) | 353 (87.6) | 329 (81.8) | 309 (76.9) | 390 (95.8) | 196 (96.1) | 435 (81.3) | 201 (75.0) | 146 (96.1) | 136 (89.5) | 120 (95.2) | 122 (96.1) | 81 (95.3) |
| Serious AEs | 128 (9.8) | 42 (6.4) | 40 (9.9) | 31 (7.7) | 37 (9.2) | 37 (9.1) | 6 (2.9) | 41 (7.7) | 15 (5.6) | 12 (7.9) | 18 (11.8) | 10 (7.9) | 14 (11.0) | 6 (7.1) |
| AE leading to discontinuation | 92 (7.0) | 20 (3.1) | 25 (6.2) | 20 (5.0) | 14 (3.5) | 24 (5.9) | 6 (2.9) | 13 (2.4) | 6 (2.2) | 9 (5.9) | 7 (4.6) | 4 (3.2) | 16 (12.6) | 3 (3.5) |
| GI disorders leading to discontinuation | 59 (4.5) | 5 (0.8) | 17 (4.2) | 14 (3.5) | 4 (1.0) | 14 (3.4) | 0 | NA | NA | 6 (3.9) | 1 (0.7) | 1 (0.8) | 8 (6.3) | 1 (1.2) |
| Fatal events | 1 (0.1) | 1 (0.2) | 1 (0.2) | 1 (0.2) | 1 (0.2) | 0 | 0 | 1 (0.2) | 1 (0.4) | 1 (0.7) | 0 | 0 | 0 | 0 |
| GI AEs reported in ≥10% of participants c | ||||||||||||||
| Nausea | 577 (44.2) | 114 (17.4) | 136 (33.7) | 129 (32.1) | 37 (9.2) | 237 (58.2) | 45 (22.1) | 75 (14.0) | 13 (4.9) | 81 (53.3) | 33 (21.7) | 77 (61.1) | 75 (59.1) | 19 (22.4) |
| Diarrhoea | 412 (31.5) | 104 (15.9) | 86 (21.3) | 89 (22.1) | 48 (11.9) | 147 (36.1) | 45 (22.1) | 77 (14.4) | 19 (7.1) | 53 (34.9) | 36 (23.7) | 35 (27.8) | 23 (18.1) | 22 (25.9) |
| Vomiting | 324 (24.8) | 43 (6.6) | 88 (21.8) | 54 (13.4) | 11 (2.7) | 111 (27.3) | 22 (10.8) | 55 (10.3) | 8 (3.0) | 46 (30.3) | 7 (4.6) | 32 (25.4) | 26 (20.5) | 5 (5.9) |
| Constipation | 306 (23.4) | 62 (9.5) | 70 (17.4) | 51 (12.7) | 22 (5.5) | 150 (36.9) | 50 (24.5) | 62 (11.6) | 17 (6.3) | 47 (30.9) | 17 (11.2) | 49 (38.9) | 40 (31.5) | 20 (23.5) |
| Safety focus areas | ||||||||||||||
| GI disorders | 969 (74.2) | 314 (47.9) | 256 (63.5) | 231 (57.5) | 138 (34.3) | 337 (82.8) | 129 (63.2) | 224 (41.9) | 70 (26.1) | 125 (82.2) | 82 (53.9) | 106 (84.1) | 105 (82.7) | 47 (55.3) |
| Gall‐bladder‐related disorders | 34 (2.6) | 8 (1.2) | 1 (0.2) | 4 (1.0) | 3 (0.7) | 20 (4.9) | 3 (1.5) | 15 (2.8) | 10 (3.7) | 4 (2.6) | 2 (1.3) | 1 (0.8) | 4 (3.1) | 1 (1.2) |
| Hepatic disorders | 31 (2.4) | 20 (3.1) | 10 (2.5) | 10 (2.5) | 14 (3.5) | 8 (2.0) | 4 (2.0) | 11 (2.1) | 4 (1.5) | 3 (2.0) | 3 (2.0) | 2 (1.6) | 1 (0.8) | 3 (3.5) |
| Acute pancreatitis | 3 (0.2) | 0 | 1 (0.2) | 0 | 1 (0.2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.8) | 0 |
| Cardiovascular disorders | 107 (8.2) | 75 (11.5) | 6 (1.5) | 6 (1.5) | 5 (1.2) | 40 (9.8) | 22 (10.8) | 26 (4.9) | 30 (11.2) | 17 (11.2) | 32 (21.1) | 16 (12.7) | 18 (14.2) | 9 (10.6) |
| Allergic reactions | 96 (7.4) | 54 (8.2) | 26 (6.5) | 22 (5.5) | 18 (4.5) | 35 (8.6) | 19 (9.3) | 26 (4.9) | 11 (4.1) | 23 (15.1) | 8 (5.3) | 9 (7.1) | 11 (8.7) | 10 (11.8) |
| Injection‐site reactions | 65 (5.0) | 44 (6.7) | 12 (3.0) | 6 (1.5) | 10 (2.5) | 22 (5.4) | 12 (5.9) | 14 (2.6) | 6 (2.2) | 10 (6.6) | 15 (9.9) | 0 | 14 (11.0) | 5 (5.9) |
| Malignant neoplasms | 14 (1.1) | 7 (1.1) | 5 (1.2) | 7 (1.7) | 8 (2.0) | 3 (0.7) | 1 (0.5) | 6 (1.1) | 1 (0.4) | 2 (1.3) | 4 (2.6) | 3 (2.4) | 3 (2.4) | 1 (1.2) |
| Psychiatric disorders | 124 (9.5) | 83 (12.7) | 24 (6.0) | 23 (5.7) | 15 (3.7) | 60 (14.7) | 24 (11.8) | 46 (8.6) | 35 (13.1) | 26 (17.1) | 25 (16.4) | 7 (5.6) | 19 (15.0) | 9 (10.6) |
| Acute renal failure | 3 (0.2) | 2 (0.3) | 4 (1.0) | 2 (0.5) | 2 (0.5) | 0 | 0 | 1 (0.2) | 1 (0.4) | 0 | 0 | 1 (0.8) | 0 | 1 (1.2) |
| Hypoglycaemia | 8 (0.6) | 5 (0.8) | 23 (5.7) | 22 (5.5) | 12 (3.0) | 2 (0.5) | 0 | 3 (0.6) | 3 (1.1) | 4 (2.6) | 0 | 0 | 1 (0.8) | 0 |
Abbreviations: AE, adverse event; GI, gastrointestinal; NA, not available.
Data are reported for the randomized period (i.e. weeks 20‐68).
Pooled placebo data.
AE in ≥5% of participants in STEP 4.
AEs reported for semaglutide 2.4 mg were typical of the GLP‐1RA class in general and were primarily gastrointestinal (GI) events. Most events were transient, and mild or moderate in severity. No new safety concerns arose from the STEP trials. The occurrence of cholelithiasis was consistent with the known associations between rapid weight loss and increased risk of cholelithiasis, and with previous reports of gallbladder‐related disorders with GLP‐1RAs. 48 , 49 , 50 There were no notable increases in the incidence of acute pancreatitis with semaglutide 2.4 mg. In the STEP 2 trial, participants were excluded if they had uncontrolled and potentially unstable diabetic retinopathy or maculopathy. In terms of AEs, diabetic retinopathy events were reported in 4.0% of patients receiving semaglutide 2.4 mg, 2.7% with semaglutide 1.0 mg and 2.7% with placebo. 39
GI AEs generally led to more participants discontinuing treatment in the semaglutide 2.4 mg groups compared with placebo groups (0.8%‐4.5% vs. 0%‐1.2%, respectively), but overall, few participants discontinued treatment for this reason (Table 3). 38 , 39 , 40 , 41 , 42 , 43 No new safety signals were observed with longer‐term semaglutide 2.4 mg treatment in STEP 5. 42 In STEP 8, rates of AEs (which were mostly GI‐related) were similar with semaglutide 2.4 mg and liraglutide 3.0 mg (95.2% vs. 96.1%). Of note, rates of discontinuation due to of AEs were lower with semaglutide than with liraglutide (3.2% vs. 12.6%). 43
STEP 2 was the only trial to include a once‐weekly semaglutide 1.0 mg treatment arm in addition to the placebo arm. A slightly higher proportion of participants experienced GI AEs in the semaglutide 2.4 mg group versus the semaglutide 1.0 mg group (63.5% vs. 57.5%, respectively, and vs. 34.3% in the placebo group). 39 In the STEP 6 trial, which included once‐weekly semaglutide 1.7 mg, GI AEs were reported in fewer participants in the semaglutide 2.4 mg group compared with the 1.7 mg group (59% and 64%, respectively). 44
6. CLINICAL IMPLICATIONS OF THE STEP PROGRAMME
6.1. Clinical implications of the trial outcomes for weight management
Across the STEP trials, once‐weekly subcutaneous semaglutide 2.4 mg demonstrated mean weight loss of 14.9%‐17.4% at 68 weeks in participants without diabetes with the mean baseline weight of 100‐107 kg, 38 , 40 , 41 , 43 offering the potential for clinically relevant improvement for individuals with obesity‐related diseases. Weight loss with behavioural interventions often plateaus at a level of 5%‐10% and is associated with a high risk of relapse, which may be related to metabolic adaptation. 17 , 51 Although weight loss of 5%‐10% is linked to improvements in cardiovascular (CV) risk factors, glycaemia and quality of life, reductions in body weight beyond 10% provide further improvement in these outcomes and are associated with remission of type 2 diabetes and reductions in CV events. 52 , 53 , 54 , 55 As such, weight loss of ≥10%‐15% is recommended in people with complications of overweight and obesity, such as gastroesophageal reflux disease, osteoarthritis, obstructive sleep apnoea, non‐alcoholic steatohepatitis and diabetes. 7 , 56 As obesity is a chronic and relapsing disease, weight regain after initial loss is common. With lifestyle intervention alone, the initial weight lost is typically regained gradually, even when the intervention continues. 57 , 58 , 59 This is reflected in the STEP 4 results, where the mean weight change after 48 weeks among participants who switched from semaglutide 2.4 mg to placebo was +6.9%, even though lifestyle intervention was continued (vs. −7.9% among those who continued semaglutide plus lifestyle intervention). 41
Semaglutide 2.4 mg for 2 years resulted in substantial and sustained changes in body weight versus placebo in STEP 5 (−15.2% vs. −2.6%), 42 demonstrating encouraging long‐term maintenance of weight loss.
The mean weight loss in patients with type 2 diabetes in STEP 2 was less than that observed in STEP 1 (which employed a similar study design but with a population of adults with obesity, and without type 2 diabetes). 38 , 39 It is well known that weight reductions in people with type 2 diabetes are often less than in people without diabetes, 60 although the full reasons for this remain unknown. In STEP 2, semaglutide 2.4 mg provided only a small numerical improvement over 1.0 mg in glycaemic parameters (HbA1c levels and targets, fasting plasma glucose, and reduction in use of concomitant oral glucose‐lowering mediations). However, the benefit of the higher dose is seen in the context of greater weight loss. 39
STEP 3 assessed the impact of semaglutide 2.4 mg in combination with intensive behavioural therapy and an initial low‐calorie meal‐replacement diet, reporting mean weight loss of 16.0% (vs. 5.7% in the placebo group). 40 However, in the other STEP trials, a less intensive lifestyle intervention programme was used, and similar levels of weight loss were achieved, suggesting that an intensive approach may not contribute significant additional weight loss. Rather, the results show the effectiveness of semaglutide 2.4 mg in those with overweight or obesity, probably through its ability to control appetite, energy intake and food cravings.
The STEP 6 results indicate that semaglutide 2.4 mg is an effective weight management option also in people from east Asia with obesity, and further illustrates a semaglutide‐induced reduction in visceral fat, which are known to be a strong predictor of weight‐related comorbidities, particularly in people from east Asia. 44
STEP 8 is the first direct comparison of semaglutide with another weight‐management medication in a randomized trial. Semaglutide 2.4 mg demonstrated better efficacy than liraglutide as well as offering more convenient once‐weekly rather than once‐daily injections. 43 Although direct comparisons with other treatments cannot be made because of methodological differences between trials, overall the weight loss with other approved medications appears to be more modest than that seen with semaglutide 2.4 mg in the STEP trials. 18 , 27 , 28 , 29 , 30 , 31
The STEP trials showed both short‐ and longer‐term improvements in cardiometabolic risk factors in participants with overweight or obesity. Earlier data from PIONEER 6 and SUSTAIN 6 suggested a CV benefit of semaglutide at lower doses in individuals with type 2 diabetes. 61 Together, these findings indicate a potential role for semaglutide in the management of cardiometabolic risk factors. The ongoing SELECT trial (NCT03574597) will report on CV outcomes with semaglutide 2.4 mg in a population without diabetes who are at high CV risk. 62
The safety profile of semaglutide 2.4 mg was as expected for the GLP‐1RA class and was similar to that seen in other trials of subcutaneous semaglutide in individuals with type 2 diabetes. 63 In clinical practice, the extensive experience with this molecule at the 1.0 mg dose in type 2 diabetes and with the GLP‐1RA class overall should ensure a certain degree of confidence in the safety profile. This includes the rate of severe or blood glucose‐confirmed hypoglycaemia, which in STEP 2 was reported in similar proportions of subjects receiving semaglutide 2.4 mg and 1.0 mg (5.7% and 5.5%, respectively). 39
The encouraging data from validated patient‐reported outcomes (Short Form‐36 Version 2 Health Survey, Acute Version in STEP 1‐4 and Impact of Weight on Quality of Life‐Lite Clinical Trials Version questionnaire in STEP 1 and 2) point towards the benefits of semaglutide 2.4 mg being directly perceived by study participants.
6.2. Key clinical considerations for using once‐weekly subcutaneous semaglutide 2.4 mg in clinical practice
Currently, once‐weekly subcutaneous semaglutide 2.4 mg is approved for use in Canada, Europe, the UK and the USA as an adjunct to a reduced calorie diet and increased physical activity for chronic weight management in adults with an initial BMI of ≥30 or ≥27 kg/m2 in the presence of ≥1 weight‐related comorbid condition. 20 , 21 , 22 , 23
Figure 1 illustrates key considerations for the use of once‐weekly subcutaneous semaglutide 2.4 mg in clinical practice. Semaglutide should be initiated at a dose of 0.25 mg once weekly and then escalated every 4 weeks according to the dose‐escalation schedule in Figure 1, until the maintenance dose of 2.4 mg once weekly is reached. 20 , 21 This escalation schedule is designed to minimize GI AEs, but if a patient does not tolerate a dose during the escalation period, the subsequent escalation step can be delayed for a further 4 weeks, after which it should be re‐escalated to 2.4 mg. If needed, this re‐escalation step can be postponed (e.g. for 4 more weeks) and the escalation can be stopped at a lower maintenance dose where AEs are tolerable. 20 In patients with type 2 diabetes, blood glucose should be monitored prior to starting and during treatment with semaglutide 2.4 mg. 20
FIGURE 1.
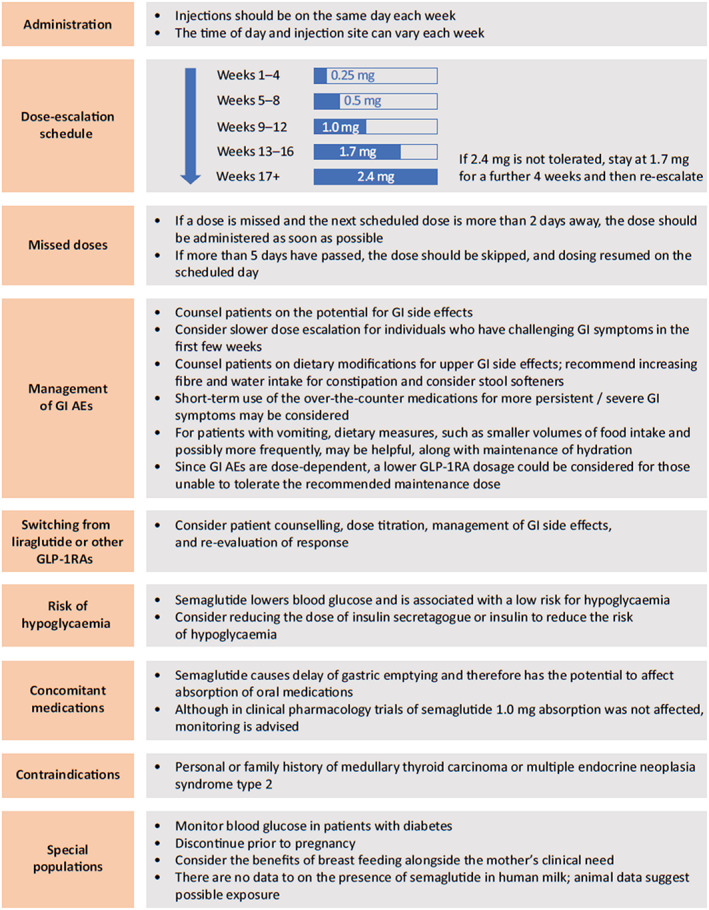
Key considerations for using once‐weekly subcutaneous semaglutide 2.4 mg in clinical practice (approved in Canada, Europe, the UK and the USA). 20 , 21 , 22 , 23 Abbreviations: AE, adverse event; GI, gastrointestinal; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist
The STEP trials excluded participants with renal impairment (estimated glomerular filtration rate <15 ml/min/1.73 m2; except STEP 2, <30 ml/min/1.73 m2 or <60 ml/min/1.73 m2 with SGLT2i). 38 , 39 , 40 , 41 , 42 , 43 , 44 However, the prescribing information states that no dose adjustment of semaglutide is recommended for patients with renal impairment. 20
There is limited evidence from trials and real‐world studies on strategies for managing GI side effects of GLP‐1RA treatment. Expert recommendations based on clinical experience centre around education and explanation, escalation to an appropriate dose and effective management. 64 Patients should be counselled about the possibility of GI side effects and advised that they are typically transient and mild‐to‐moderate in severity. For mild and short‐term GI side effects, this should include providing advice on dietary modifications and recommending that patients increase their fibre and water intake for constipation and consider stool softeners. For more persistent or severe GI symptoms, pausing of dose escalation is recommended, and short‐term use of over‐the‐counter medications may be considered.
The lack of clinical trial data on switching between liraglutide 3.0 mg and semaglutide 2.4 mg in individuals with overweight or obesity precludes the development of formal evidence‐based recommendations. However, in the context of the use of GLP‐1RAs in patients with type 2 diabetes, recommendations include patient counselling, starting with the recommended dose titration (considering history of GI events with the previous GLP‐1RA), management of GI side effects and re‐evaluation of response. 65 , 66
Semaglutide 2.4 mg has shown clinically meaningful bodyweight reductions in obesity both in the primarily white population of the STEP 1‐5 and 8 trials, as well as in the east Asian participants of the STEP 6 trial. Furthermore, post hoc analyses from STEP 2 indicate that the response to semaglutide 2.4 mg was not influenced by race or ethnicity. 67 However, a regional Phase IIIa trial in Brazil, China, Hong Kong and South Korea is ongoing and will further add to our knowledge on ethnicity as a potential clinical consideration.
Semaglutide regulates blood glucose via the incretin pathway, stimulating insulin and inhibiting glucagon secretion in a glucose‐dependent manner, leading to lower blood glucose levels with low risk for hypoglycaemia. 68 In STEP 2, insulin treatment was only allowed for rescue therapy and those taking sulphonylureas were to reduce the dose by 50% to reduce the risk of hypoglycaemia. Severe or blood glucose‐confirmed hypoglycaemia was reported in 5.7% versus 3.0% of participants treated with semaglutide 2.4 mg versus placebo. 39 In the SUSTAIN trials in patients with type 2 diabetes, severe or blood glucose‐confirmed hypoglycaemia occurred among participants in the semaglutide groups at a similar or lower level than in comparator groups. 63 Clinicians may wish to consider reducing the dose of insulin secretagogue or insulin to reduce the risk of hypoglycaemia. 20 , 21
Semaglutide causes delay of gastric emptying and therefore has the potential to affect absorption of oral medications. 20 , 21 While absorption was not affected in clinical pharmacology trials of semaglutide 1.0 mg, monitoring is advised. 20 , 21
7. FUTURE LANDSCAPE OF OBESITY PHARMACOTHERAPY
In addition to the published STEP trials, the programme includes three further ongoing global phase IIIa studies in adolescents with obesity (STEP TEENS), 69 in individuals with heart failure and obesity (STEP‐HFpEF) 70 and in individuals with heart failure and obesity with type 2 diabetes (STEP‐HFpEF‐DM) 71 (Figure 2). In addition, as previously mentioned, a regional phase IIIa trial is ongoing in Brazil, China, Hong Kong and South Korea (STEP 7). 72 Phase IIIb trials in the programme are investigating weight management in knee osteoarthritis (STEP 9) 73 and reversal of prediabetes to normoglycaemia (STEP 10). 74
FIGURE 2.
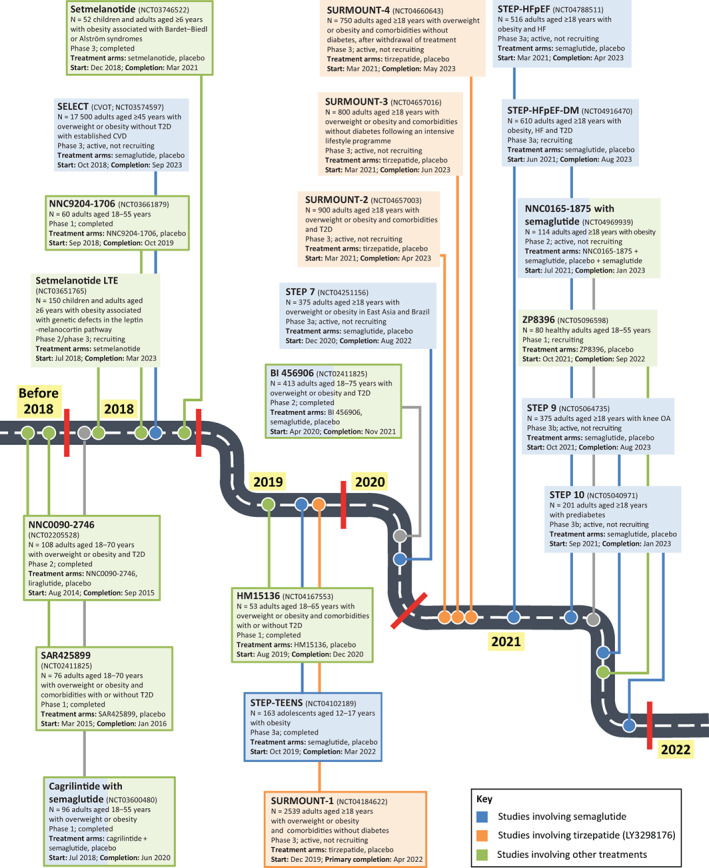
Future landscape of obesity pharmacotherapy. Ongoing trials with semaglutide, and selected ongoing and completed trials with other investigational agents. Studies in closed boxes are completed, while those in open boxes are ongoing. Abbreviations: CVD, cardiovascular disease; CVOT, cardiovascular outcomes trial; HF, heart failure; LTE, long‐term extension; OA, osteoarthritis; T2D, type 2 diabetes
Beyond the STEP programme, the SELECT trial is an ongoing event‐driven placebo‐controlled CV outcomes trial of semaglutide 2.4 mg once daily in people with overweight or obesity without diabetes and with established CV disease. 62 SELECT is the first CV trial to evaluate superiority in reduction of major adverse CV events for an obesity pharmacotherapy in this population.
Taken together, the results from the STEP clinical trial programme show semaglutide 2.4 mg to be the most effective drug currently approved for weight loss in adults with overweight or obesity. Among new molecules on the horizon for the treatment of overweight and obesity, the most promising appears to be tirzepatide (LY3298176), an incretin mimetic with a dual action targeting both the GLP‐1 receptor and the glucose‐dependent insulinotropic polypeptide (GIP) receptor 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 (Figure 2 and Supplementary Figure 1). Tirzepatide has shown promising results on obesity with a side effect profile close to semaglutide. A second GLP‐1/GIP receptor dual agonist, NNC0090‐2746, is at an earlier stage of development. 86 Other potential future obesity treatments include the GLP‐1/GIP/glucagon receptor triple agonist, NNC9204‐1706 87 ; the dual GLP‐1 receptor/glucagon receptor agonists, BI 456906 and SAR425899 88 , 89 ; the glucagon analogue, HM15136 90 ; the amylin analogues, ZP8396 91 and cagrilintide in combination with semaglutide 92 ; the Y2R agonist, NNC0165‐1875 in combination with semaglutide 93 ; and the melanocortin 4 receptor agonist, setmelanotide 94 , 95 (Figure 2).
8. CONCLUSIONS
A growing body of data from the STEP clinical trial programme has demonstrated the efficacy and tolerability of once‐weekly subcutaneous semaglutide 2.4 mg in individuals with overweight or obesity. Across the STEP trials, once‐weekly subcutaneous semaglutide 2.4 mg was consistently associated with mean weight losses of 14.9%‐17.4% in participants without diabetes, and improvements in cardiometabolic risk factors, physical function and quality of life. Furthermore, the safety profile of semaglutide 2.4 mg was as expected for the GLP‐1RA class. Results of other trials within the STEP programme are awaited, together with data from SELECT, which will provide valuable information on the impact of weight reduction with semaglutide 2.4 mg on decreasing CV risk.
AUTHOR CONTRIBUTIONS
Drafting the manuscript (NCB, MJD, IL, FKK); critical revision of the manuscript for important intellectual content (NCB, MJD, IL, FKK); responsibility for content (NCB, MJD, IL, FKK).
CONFLICTS OF INTEREST
NCB declared no conflict of interest. MJD is a consultant, advisory board member and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi‐Aventis; advisory board member and speaker for AstraZeneca; advisory board member for Gilead Sciences Ltd, Janssen, Lexicon, Pfizer and Servier; speaker for Mitsubishi Tanabe Pharma Corporation, Napp Pharmaceuticals and Takeda Pharmaceuticals International Inc.; received grants to support investigator and investigator‐initiated trials from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk and Sanofi‐Aventis. MJD is co‐funded by the NIHR Leicester Biomedical Research Centre. IL obtained research funding, advisory/consulting fees, and/or other support from AstraZeneca, Bayer, Boehringer Ingelheim, GI Dynamics, Intarcia, Intercept, Janssen, Eli Lilly, Mannkind, Merck, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi, TARGETPharma, Valeritas and Zealand Pharma. FKK served on scientific advisory panels and/or been part of speaker bureaus for, served as a consultant to and/or received research support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, Lupin, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Pharmacosmos, Sanofi, ShouTi, Zealand Pharma and Zucara; minority shareholder in Antag Therapeutics.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14863.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
The authors thank Paul Barlass, PhD, of Axis, a division of Spirit Medical Communications Group Ltd., and Sarah Stowell, a contract writer working on behalf of Axis who provided medical writing assistance under the direction of the authors, funded by Novo Nordisk A/S, Denmark, in accordance with Good Publication Practice 3 (GPP3) guidelines (www.ismpp.org/gpp3).
Bergmann NC, Davies MJ, Lingvay I, Knop FK. Semaglutide for the treatment of overweight and obesity: A review. Diabetes Obes Metab. 2023;25(1):18‐35. doi: 10.1111/dom.14863
Funding information Novo Nordisk A/S; Novo Nordisk, Grant/Award Number: GPP3
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Frühbeck G, Busetto L, Dicker D, et al. The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obes Facts. 2019;12(2):131‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NCD Risk Factor Collaboration (NCD‐RisC) . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bray GA, Kim KK, Wilding JPH, World Obesity Federation . Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715‐723. [DOI] [PubMed] [Google Scholar]
- 4. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pereira‐Miranda E, Costa PRF, Queiroz VAO, Pereira‐Santos M, Santana MLP. Overweight and obesity associated with higher depression prevalence in adults: a systematic review and meta‐analysis. J Am Coll Nutr. 2017;36(3):223‐233. [DOI] [PubMed] [Google Scholar]
- 6. Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1‐203. [DOI] [PubMed] [Google Scholar]
- 8. Agha M, Agha R. The rising prevalence of obesity: part A: impact on public health. Int J Surg Oncol (N Y). 2017;2(7):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janssen F, Bardoutsos A, Vidra N. Obesity prevalence in the long‐term future in 18 European countries and in the USA. Obes Facts. 2020;13(5):514‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed March 31, 2022.
- 11. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017‐2018. NCHS Data Brief. 2020;360:1‐8. [PubMed] [Google Scholar]
- 12. Durrer Schutz D, Busetto L, Dicker D, et al. European practical and patient‐centred guidelines for adult obesity management in primary care. Obes Facts. 2019;12(1):40‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen MD, Ryan DH, Apovian CM, et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985‐3023. [DOI] [PubMed] [Google Scholar]
- 14. Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wadden TA, Tronieri JS, Butryn ML. Lifestyle modification approaches for the treatment of obesity in adults. Am Psychol. 2020;75(2):235‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kushner RF, Calanna S, Davies M, et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity (Silver Spring). 2020;28(6):1050‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sumithran P, Prendergast LA, Delbridge E, et al. Long‐term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597‐1604. [DOI] [PubMed] [Google Scholar]
- 18. Singh AK, Singh R. Pharmacotherapy in obesity: a systematic review and meta‐analysis of randomized controlled trials of anti‐obesity drugs. Expert Rev Clin Pharmacol. 2020;13(1):53‐64. [DOI] [PubMed] [Google Scholar]
- 19. Blundell J, Finlayson G, Axelsen M, et al. Effects of once‐weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Novo Nordisk . WEGOVY (semaglutide) highlights of prescribing information. https://www.novo-pi.com/wegovy.pdf. Accessed November 30, 2021.
- 21. Medicines & Healthcare Products Regulatory Agency . Wegovy 2.4 mg, solution for injection in pre‐filled pen – summary of product characteristics. https://mhraproducts4853.blob.core.windows.net/docs/4b161649ffc66e938bc76f4e45a4d3797af54914. Accessed March 31, 2022.
- 22. European Medicines Agency . Wegovy – summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/wegovy. Accessed March 31, 2022.
- 23. Health Canada . Wegovy – product monograph. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=101171. Accessed March 31, 2022.
- 24. Mechanick JI, Hurley DL, Garvey WT. Adiposity‐based chronic disease as a new diagnostic term: the American Association of Clinical Endocrinologists and American College of Endocrinology position statement. Endocr Pract. 2017;23(3):372‐378. [DOI] [PubMed] [Google Scholar]
- 25. Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387(10031):1947‐1956. [DOI] [PubMed] [Google Scholar]
- 26. Theilade S, Christensen MB, Vilsbøll T, Knop FK. An overview of obesity mechanisms in humans: endocrine regulation of food intake, eating behaviour and common determinants of body weight. Diabetes Obes Metab. 2021;23(Suppl 1):17‐35. [DOI] [PubMed] [Google Scholar]
- 27. Broom I, Wilding J, Stott P, Myers N, UK Multimorbidity Study Group . Randomised trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK multimorbidity study. Int J Clin Pract. 2002;56(7):494‐499. [PubMed] [Google Scholar]
- 28. Lindgärde F. The effect of orlistat on body weight and coronary heart disease risk profile in obese patients: the Swedish multimorbidity study. J Intern Med. 2000;248(3):245‐254. [DOI] [PubMed] [Google Scholar]
- 29. Rössner S, Sjöström L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. Obes Res. 2000;8(1):49‐61. [DOI] [PubMed] [Google Scholar]
- 30. Hollander PA, Elbein SC, Hirsch IB, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1‐year randomized double‐blind study. Diabetes Care. 1998;21(8):1288‐1294. [DOI] [PubMed] [Google Scholar]
- 31. Finer N, James WP, Kopelman PG, Lean ME, Williams G. One‐year treatment of obesity: a randomized, double‐blind, placebo‐controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. Int J Obes Relat Metab Disord. 2000;24(3):306‐313. [DOI] [PubMed] [Google Scholar]
- 32. Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double‐blind, placebo‐controlled study. Lancet. 2009;374(9701):1606‐1616. [DOI] [PubMed] [Google Scholar]
- 33. Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low‐calorie‐diet‐induced weight loss: the SCALE maintenance randomized study. Int J Obes (Lond). 2013;37(11):1443‐1451. [DOI] [PubMed] [Google Scholar]
- 34. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11‐22. [DOI] [PubMed] [Google Scholar]
- 35. Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE sleep apnea randomized clinical trial. Int J Obes (Lond). 2016;40(8):1310‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23(3):754‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilding JPH, Batterham RL, Calanna S, et al. Once‐weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989‐1002. [DOI] [PubMed] [Google Scholar]
- 39. Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double‐blind, double‐dummy, placebo‐controlled, phase 3 trial. Lancet. 2021;397(10278):971‐984. [DOI] [PubMed] [Google Scholar]
- 40. Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403‐1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garvey WT, Batterham RL, Bhatta M, et al. Two‐year effect of semaglutide 2.4 mg once‐weekly in adults with overweight or obesity (STEP 5): a randomized, double‐blind, placebo controlled, phase 3 trial. Nat Med. 2022; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kadowaki T, Isendahl J, Khalid U, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double‐blind, double‐dummy, placebo‐controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10(3):193‐206. [DOI] [PubMed] [Google Scholar]
- 45. Wharton S, Astrup A, Endahl L, et al. Estimating and reporting treatment effects in clinical trials for weight management: using estimands to interpret effects of intercurrent events and missing data. Int J Obes (Lond). 2021;45(5):923‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring). 2015;23(12):2319‐2320. [DOI] [PubMed] [Google Scholar]
- 47. Perreault L, Davies M, Frias JP, et al. Changes in glucose metabolism and glycemic status with once‐weekly subcutaneous semaglutide 2.4 mg among participants with prediabetes in the STEP program. Diabetes Care. 2022;dc211785. doi: 10.2337/dc21-1785 Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stokes CS, Lammert F. Excess body weight and gallstone disease. Visc Med. 2021;37(4):254‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Faillie JL, Yu OH, Yin H, Hillaire‐Buys D, Barkun A, Azoulay L. Association of bile duct and gallbladder diseases with the use of incretin‐based drugs in patients with type 2 diabetes mellitus. JAMA Intern Med. 2016;176(10):1474‐1481. [DOI] [PubMed] [Google Scholar]
- 50. Nauck MA, Muus Ghorbani ML, Kreiner E, Saevereid HA, Buse JB, LEADER Publication Committee on behalf of the LEADER Trial Investigators . Effects of liraglutide compared with placebo on events of acute gallbladder or biliary disease in patients with type 2 diabetes at high risk for cardiovascular events in the LEADER randomized trial. Diabetes Care. 2019;42(10):1912‐1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hall KD, Kahan S. Maintenance of lost weight and long‐term management of obesity. Med Clin North Am. 2018;102(1):183‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6(2):187‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dambha‐Miller H, Day AJ, Strelitz J, Irving G, Griffin SJ. Behaviour change, weight loss and remission of type 2 diabetes: a community‐based prospective cohort study. Diabet Med. 2020;37(4):681‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aminian A, Zajichek A, Tu C, et al. How much weight loss is required for cardiovascular benefits? Insights from a metabolic surgery matched‐cohort study. Ann Surg. 2020;272(4):639‐645. [DOI] [PubMed] [Google Scholar]
- 56. Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399(10322):394‐405. [DOI] [PubMed] [Google Scholar]
- 57. Wadden TA, Neiberg RH, Wing RR, et al. Four‐year weight losses in the look AHEAD study: factors associated with long‐term success. Obesity (Silver Spring). 2011;19(10):1987‐1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139‐1148. [DOI] [PubMed] [Google Scholar]
- 59. Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155‐161. [DOI] [PubMed] [Google Scholar]
- 60. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687‐699. [DOI] [PubMed] [Google Scholar]
- 61. Husain M, Bain SC, Jeppesen OK, et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab. 2020;22(3):442‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ryan DH, Lingvay I, Colhoun HM, et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J. 2020;229:61‐69. [DOI] [PubMed] [Google Scholar]
- 63. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcomes with once‐weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1‐7 trials. Diabetes Metab. 2019;45(5):409‐418. [DOI] [PubMed] [Google Scholar]
- 64. Wharton S, Davies M, Dicker D, et al. Managing the gastrointestinal side effects of GLP‐1 receptor agonists in obesity: recommendations for clinical practice. Postgrad Med. 2022;134(1):14‐19. [DOI] [PubMed] [Google Scholar]
- 65. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon‐like peptide‐1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jain AB, Ali A, Gorgojo Martínez JJ, et al. Switching between GLP‐1 receptor agonists in clinical practice: expert consensus and practical guidance. Int J Clin Pract. 2021;75(2):e13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lingvay I, Faerch L, Koroleva A, et al. Influence of baseline characteristics on weight loss with semaglutide 2.4 mg in adults with overweight/obesity and type 2 diabetes (STEP 2). Presented at the American Diabetes Association (ADA) virtual meeting, June 25–29, 2021.
- 68. Donath MY, Burcelin R. GLP‐1 effects on islets: hormonal, neuronal, or paracrine? Diabetes Care. 2013;36(Suppl 2):S145‐S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. ClinicalTrials.gov . A research study on how well semaglutide works in adolescents with overweight or obesity (NCT04102189). https://clinicaltrials.gov/ct2/show/NCT04102189. Accessed November 30, 2021.
- 70. ClinicalTrials.gov . Research study to investigate how well semaglutide works in people living with heart failure and obesity (STEP‐HFpEF) (NCT04788511). https://clinicaltrials.gov/ct2/show/NCT04788511. Accessed November 30, 2021.
- 71. ClinicalTrials.gov . Research study to look at how well semaglutide works in people living with heart failure, obesity and type 2 diabetes (STEP HFpEF DM) (NCT04916470). https://clinicaltrials.gov/ct2/show/NCT04916470. Accessed November 30, 2021.
- 72. ClinicalTrials.gov . Research study of how well semaglutide works in people living with overweight or obesity (STEP 7) (NCT04251156). https://clinicaltrials.gov/ct2/show/NCT04251156. Accessed March 31, 2022.
- 73. ClinicalTrials.gov . Research study looking at how well semaglutide works in people suffering from obesity and knee osteoarthritis (NCT05064735). https://clinicaltrials.gov/ct2/show/NCT05064735. Accessed November 30, 2021.
- 74. ClinicalTrials.gov . Research study looking at how well semaglutide works in people living with obesity and prediabetes (STEP 10) (NCT05040971). https://clinicaltrials.gov/ct2/show/NCT05040971. Accessed November 30, 2021.
- 75. Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP‐1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP‐1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS‐1): a double‐blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143‐155. [DOI] [PubMed] [Google Scholar]
- 77. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503‐515. [DOI] [PubMed] [Google Scholar]
- 78. Ludvik B, Giorgino F, Jódar E, et al. Once‐weekly tirzepatide versus once‐daily insulin degludec as add‐on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS‐3): a randomised, open‐label, parallel‐group, phase 3 trial. Lancet. 2021;398(10300):583‐598. [DOI] [PubMed] [Google Scholar]
- 79. ClinicalTrials.gov . A study of tirzepatide (LY3298176) in participants with type 2 diabetes who have obesity or are overweight (SURMOUNT‐2) (NCT04657003). https://clinicaltrials.gov/ct2/show/NCT04657003. Accessed November 30, 2021.
- 80. ClinicalTrials.gov . A study of tirzepatide (LY3298176) in participants after a lifestyle weight loss program (SURMOUNT‐3) (NCT04657016). https://clinicaltrials.gov/ct2/show/NCT04657016. Accessed November 30, 2021.
- 81. ClinicalTrials.gov . A study of tirzepatide (LY3298176) in participants with obesity or overweight for the maintenance of weight loss (SURMOUNT‐4) (NCT04660643). https://clinicaltrials.gov/ct2/show/NCT04660643. Accessed November 30, 2021.
- 82. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP‐1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo‐controlled and active comparator‐controlled phase 2 trial. Lancet. 2018;392(10160):2180‐2193. [DOI] [PubMed] [Google Scholar]
- 83. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205‐216. doi: 10.1056/NEJMoa2206038 [DOI] [PubMed] [Google Scholar]
- 84. ClinicalTrials.gov . A study of tirzepatide (LY3298176) once a week versus insulin glargine once a day in participants with type 2 diabetes and increased cardiovascular risk (SURPASS‐4) (NCT03730662). https://clinicaltrials.gov/ct2/show/NCT03730662. Accessed June 24, 2022.
- 85. ClinicalTrials.gov . A study of tirzepatide (LY3298176) versus placebo in participants with type 2 diabetes inadequately controlled on insulin glargine with or without metformin (SURPASS‐5). (NCT04039503). https://clinicaltrials.gov/ct2/show/NCT04039503. Accessed June 24, 2022.
- 86. Frias JP, Bastyr EJ 3rd, Vignati L, et al. The sustained effects of a dual GIP/GLP‐1 receptor agonist, NNC0090‐2746, in patients with type 2 diabetes. Cell Metab. 2017;26(2):343‐352.e2. [DOI] [PubMed] [Google Scholar]
- 87. ClinicalTrials.gov . Research study of a new medicine (NNC9204‐1706) in people with overweight or obesity (NCT03661879). https://clinicaltrials.gov/ct2/show/NCT03661879. Accessed March 25, 2022.
- 88. Tillner J, Posch MG, Wagner F, et al. A novel dual glucagon‐like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo‐controlled first‐in‐human and first‐in‐patient trials. Diabetes Obes Metab. 2019;21(1):120‐128. [DOI] [PubMed] [Google Scholar]
- 89. ClinicalTrials.gov . A study to test whether different doses of BI 456906 are effective in treating adults with type 2 diabetes (NCT04153929). https://clinicaltrials.gov/ct2/show/NCT04153929. Accessed November 30, 2021.
- 90. Kim JK, Kim JY, Lee SM, et al. Potential of a novel long‐acting glucagon analog, HM15136, for the treatment of obesity. Diabetes. 2019;68(Suppl 1):991. [Google Scholar]
- 91. ClinicalTrials.gov . A first‐in‐human study looking at the safety of ZP8396 and how it works in the body of healthy trial participants (NCT05096598). https://clinicaltrials.gov/ct2/show/NCT05096598. Accessed November 30, 2021.
- 92. Enebo LB, Berthelsen KK, Kankam M, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet. 2021;397(10286):1736‐1748. [DOI] [PubMed] [Google Scholar]
- 93. ClinicalTrials.gov . A research study to investigate how well NNC0165‐1875 in combination with semaglutide works in people with obesity (NCT04969939). https://clinicaltrials.gov/ct2/show/NCT04969939. Accessed March 25, 2022.
- 94. ClinicalTrials.gov . Long‐term extension trial of setmelanotide (NCT03651765). https://clinicaltrials.gov/ct2/show/NCT03651765. Accessed November 30, 2021.
- 95. ClinicalTrials.gov . Setmelanotide (RM‐493), melanocortin‐4 receptor (MC4R) agonist, in Bardet‐Biedl Syndrome (BBS) and Alström Syndrome (AS) patients with moderate to severe obesity (NCT03746522). https://clinicaltrials.gov/ct2/show/NCT03746522. Accessed November 30, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


