Abstract
Background
Assessing marijuana's impact on intelligence quotient (IQ) has been hampered by a lack of evaluation of subjects before they begin to use this substance. Using data from a group of young people whom we have been following since birth, we examined IQ scores before, during and after cessation of regular marijuana use to determine any impact of the drug on this measure of cognitive function.
Methods
We determined marijuana use for seventy 17- to 20-year-olds through self-reporting and urinalysis. IQ difference scores were calculated by subtracting each person's IQ score at 9–12 years (before initiation of drug use) from his or her score at 17–20 years. We then compared the difference in IQ scores of current heavy users (at least 5 joints per week), current light users (less than 5 joints per week), former users (who had not smoked regularly for at least 3 months) and non-users (who never smoked more than once per week and no smoking in the past two weeks).
Results
Current marijuana use was significantly correlated (p < 0.05) in a dose- related fashion with a decline in IQ over the ages studied. The comparison of the IQ difference scores showed an average decrease of 4.1 points in current heavy users (p < 0.05) compared to gains in IQ points for light current users (5.8), former users (3.5) and non-users (2.6).
Interpretation
Current marijuana use had a negative effect on global IQ score only in subjects who smoked 5 or more joints per week. A negative effect was not observed among subjects who had previously been heavy users but were no longer using the substance. We conclude that marijuana does not have a long-term negative impact on global intelligence. Whether the absence of a residual marijuana effect would also be evident in more specific cognitive domains such as memory and attention remains to be ascertained.
Marijuana produces well-documented, acute cognitive changes that last for several hours after the drug has been ingested.1,2,3 Whether it produces cognitive dysfunction beyond this period of acute intoxication is much more difficult to establish. Approaches to investigating long-lasting effects include clinical assessment of long-term users,4,5,6 observations of subcultures in countries where long-term daily use of cannabis has been the cultural norm for decades7,8,9 and marijuana administration studies in which subjects with a history of use ranging from infrequent to extensive are given the drug in controlled laboratory settings after various periods of abstinence.10,11,12 As discussed in several reviews of the literature,1,13,14 the findings have been equivocal.
Most studies that examined heavy marijuana users for possible cognitive dysfunction lasting beyond the acute intoxication period assessed subjects after an abstinence period of only a day or two.10,12,15,16 The fact that cannabinoid metabolites have been detected in the urine of long-term marijuana users after weeks or even months of abstinence17,18,19 compromises the interpretation of these studies. To account for potential pre-existing differences between users and non-users, studies have typically matched the comparison group with the user group in terms of non-marijuana variables.6,20 Suggestions for improving study designs13,14 have emphasized both the need for comparison groups to be as similar as possible to the drug-using group and the need for a prolonged abstinence period. The most desirable procedure would involve a longitudinal, prospective design in which cognitive measures were available for all non-using and using subjects before and after marijuana consumption had been initiated by the users.15
The Ottawa Prenatal Prospective Study (OPPS), underway since 1978, satisfies these criteria. This study permits both within-subject and between-subject comparisons among relatively low-risk non-users and users before, during and after quitting regular marijuana use. The primary objective of the OPPS is the neuropsychologic assessment of children exposed prenatally to marijuana or cigarettes. Women who used and did not use marijuana and cigarettes volunteered to participate during their pregnancy, and their children, now between the ages of 17 and 20 years, have been assessed since birth. Details of the recruitment of the largely middle-class families, the assessment procedures and the findings for the children from birth to adolescence have been summarized elsewhere.21,22
The objectives of the current study were as follows: to determine if current, regular marijuana use is predictive of decline in IQ from pre-usage levels, to determine if a differential effect on IQ occurs with heavy versus light current, regular marijuana use, and to determine if any IQ effects persist after subjects cease using marijuana for at least 3 months.
Methods
A potential pool of 74 young adults with urinalysis results, self-reports of marijuana use and a broad measure of IQ obtained at both a preteen (9–12 years) and a young adult (17–20 years) assessment was available. Two subjects with inconsistencies between the self- report of marijuana use and the urine screening results were excluded, as were one subject who tested positive for cocaine and another who was taking methylphenidate. Consequently, the final sample comprised 70 subjects whose self-report of marijuana use and absence of hard drug use had been validated by urinalysis results.
During the preteen period and before initiation of marijuana use, IQ was measured by means of the Wechsler Intelligence Scale for Children-III (WISC).23 When the subjects were young adults, IQ was evaluated with the Wechsler Adult Intelligence Scale-III (WAIS).24 The outcome variable for the examination of potential marijuana effects was an IQ difference score, derived by subtracting the preteen WISC IQ score from the young adult WAIS IQ score. Thus a positive difference score reflects an increase in IQ over the approximately 10-year period, whereas a negative score reflects a decrease.
Marijuana use was determined by 2 procedures that were part of an extensive neuropsychologic battery given to the 17– to 20-year-olds. The first consisted of a questionnaire completed by the subject, which asked for details of current and past marijuana use, as well as other drug use. The second was a urine sample analyzed for the presence of cannabinoids, amphetamines, opiates, cocaine and cotinine (a metabolite of nicotine). All metabolite concentrations were adjusted for creatinine to control for urine dilution. Although these procedures did not assess the strength of the marijuana used by the OPPS subjects, an estimate was suggested by Health Canada's analysis of marijuana seized by police between 1996 and 1999, which revealed an average of 5% to 6% tetrahydrocannabinol (THC).
Marijuana measures treated as continuous variables were self-report of mean number of joints currently smoked per week, self-report of length of time (months) that marijuana had been smoked and total estimated number of joints smoked (mean number of joints smoked per week multiplied by number of weeks of use). The mean number of joints currently smoked per week was also treated as a categorical variable, as follows: the subjects were grouped as light current regular users, heavy current regular users, former regular users or non-users.
Categorization of the current marijuana users as light or heavy users was based on both the self-report and the urinalysis data. The urinalysis data were bimodally distributed: 11 subjects had cannabinoid to creatinine ratios between 4 and 54 ng/mg, and 13 subjects had ratios between 147 and 705 ng/mg. These 2 groups of subjects were used to validate the categorization based on self-reports. Defining heavy regular use as at least 5 joints per week (n = 15) and light regular use of any amount less than 5 joints at least once a week (n = 9) optimized concordance with the bimodal urine division as indicated by χ2 analysis. Eight (73%) of the 11 subjects with the lower metabolite values smoked fewer than 5 joints per week, and 12 (92%) of the 13 subjects with the higher metabolite values smoked an average of 5 or more joints per week (p = 0.001).
Of the 70 subjects, 37 were non-users who had never used marijuana regularly (where regular use was defined as at least once a week) and who had not used any marijuana in the past 2 weeks; 9 were former users who had smoked marijuana regularly in the past but had not smoked for at least 3 months before the young adult assessment; 9 were light current users; and 15 were heavy current users.
The assessments were conducted in laboratories at Carleton University, Ottawa. Given that the testing sessions commenced in the early morning and that all subjects reported no use of marijuana on the day of testing, it is unlikely that the subjects were assessed while in an acute state of intoxication.
The validity of self-reporting for current marijuana use was examined with 2 approaches. The initial selection of the 70 subjects involved a criterion of concordance between self-reports of marijuana use and urine screening results (see above). The second measure of concordance was a high correlation between reported current marijuana use and the cannabinoid to creatinine ratio found with urinalysis (r = 0.70, p < 0.001). Although self-reports of earlier use could not be directly confirmed pharmacologically, their reliability is enhanced by the validity of the self-reporting for current marijuana use.
In examining the relation between marijuana use and IQ difference scores, we considered a variety of potentially confounding variables, including variables related to socioeconomic status, such as family income and parental education; the subject's education level (number of years of education at the time of the young adult assessment); age and sex of the subject; mother's age at the time of the subject's birth; maternal use of cigarettes, marijuana and alcohol during pregnancy; and the subject's use of tobacco and alcohol and exposure to secondhand marijuana smoke. In the subsequent analyses, we controlled for any potential confounding factor that was related to both the marijuana independent variable (at ? = 0.1) and the IQ difference score (at ? = 0.05).25
Hierarchical regression (a statistical approach to measure the impact of marijuana use after considering potential confounders) was used to examine the predictive relation of quantity (both mean number of joints per week and total joints over lifetime) and duration (period of use) of current marijuana use to the IQ difference score. Differential effects on the IQ difference score of light current use, heavy current use and former use as contrasted to non-use were examined with Dunnett's 2-sided multiple comparison procedure26 with analysis of variance (ANOVA) and analysis of covariance (ANCOVA) when required to control for confounding variables.
Results
Analyses in which number of joints smoked per week was used both as a continuous and as a categorical variable revealed significant associations of this variable with the IQ difference score.
When number of joints smoked per week was treated as a continuous variable, regression analyses revealed a significant negative association with the IQ difference score (r = –0.24, p < 0.05) after accounting for potentially confounding variables. In these analyses, no predictive relation with the IQ difference score was found for the self-reported period of marijuana use or the estimated total number of joints smoked.
For analyses in which number of joints smoked per week was treated as a categorical variable, ANOVA with Dunnett's procedure26 indicated that the mean IQ difference score for the heavy current user group was significantly different from that for non-users (–4.0 v. 2.6, p < 0.05), whereas no significant differences were evident in comparisons with the light current users and former users (5.8 v. 2.6 and 3.5 v. 2.6 respectively) (Table 1). The characteristics of the 4 groups (light current users, heavy current users, former users and non-users) are presented in Table 1. Of particular importance to the present study is the fact that preteen IQ, assessed before marijuana use, did not differ across the groups. Although some characteristics did differ across the 4 groups (such as father's and mother's education), none of these was associated with the IQ difference score; therefore, they were not used as covariates.
Table 1
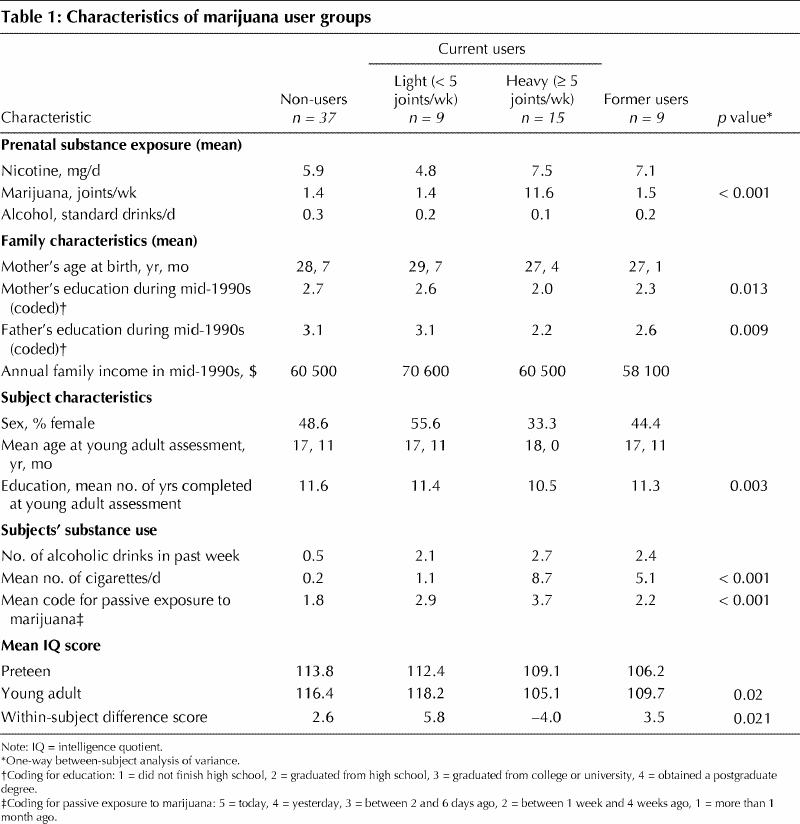
Although there was no overall difference in IQ difference score between former users and non-users, a subgroup of former users, those who had used at least 5 joints per week (heavy use), was analyzed separately; again, there was no significant difference relative to non-users (t-test, p = 0.7). This lack of a negative impact among the former heavy users is striking, as they had smoked, on average, an estimated 5793 joints over 3.2 years (mean of 37 joints per week); in contrast, the current heavy users had smoked, on average, an estimated 2386 joints over 3.1 years (mean of 14 joints per week).
Interpretation
In the present work, the use of commensurable IQ measures obtained before and after initiation of marijuana use permitted examination of the consequences of marijuana use in the context of pre-drug performance. Of all the marijuana and non-marijuana variables considered, only the quantity of current marijuana use, in terms of number of joints smoked per week, was negatively related to change in IQ from preteen to young adult. Not associated with change in IQ were duration of marijuana use, the total quantity of marijuana used and former use of marijuana. In addition, variables such as socioeconomic status (family income and parental education), age of mother at time of subject's birth, subject's prenatal exposure to drugs (nicotine, marijuana and alcohol), preteen IQ score, age, sex, academic history, other drug use and passive marijuana exposure were not predictive of change in IQ score.
The IQ difference score for the heavy current users differed from that for non-users, but no such differences were apparent between light current users and non-users. The clinical significance for an individual of such an effect on IQ scores is difficult to ascertain, but the impact on society might be substantial. IQ scores are considered normally distributed, with a mean of 100 and a standard deviation of 15, and it is therefore estimated that 2.3% of individuals will score 70 or below (2 standard deviations [SD]), and 6.7% will score 77.5 or below (1.5 SD) on global intelligence tests. These are cutoff points at which intervention and special education have typically been provided.27 Any factors in a population that result in a 4-point decrease in IQ, as was found with the heavy current marijuana users, would increase to 5.5% the proportion of individuals with an IQ of 70 or below and to 11.0% those with an IQ of 77.5 or below. A corresponding decrease in proportions would be expected on the other end of the distribution (people with higher IQ scores). For comparison, an IQ decrement of 5 points has been observed in children exposed prenatally to 3 alcoholic drinks per day,28 of 3.75 points in offspring exposed prenatally to cocaine27 and of 2.6 points after low lead exposure.29
The IQ deficit among heavy current users in the present study likely reflected residue of the drug in their bodies.10 Assuming use of at least 5 joints per week by subjects in this group and given the elimination half-life of THC in the plasma of long-term marijuana users,30,31 such quantities and patterns of smoking are likely to result in an accumulation of THC in the body.
Although the heavy current users experienced a decrease in IQ score, their scores were still above average at the young adult assessment (mean 105.1). If we had not assessed preteen IQ, these subjects would have appeared to be functioning normally. Only with knowledge of the change in IQ score does the negative impact of current heavy use become apparent.
There were no differences in IQ score at the preteen assessment among the future groups of users and the future non-users. This finding suggests that, at least in a low-risk, white, predominantly middle-class sample, IQ score before any marijuana use is not a predictor of future marijuana use.
We investigated the possibility of a longer-lasting deficit, perhaps representing a neurotoxic consequence on the central nervous system (CNS), using data for the former users. The mean IQ difference score for the former users did not differ significantly from that for the non-users, which suggests a lack of long-term effects. Similarly, there was no negative impact on IQ difference among former heavy users relative to non-users (in contrast to the situation for current heavy users). This lack of a long-lasting negative impact suggests the absence of any CNS alteration as reflected by global IQ performance.
Both the negative effects of use of at least 5 joints weekly and the lack of long-term effects found in this study should be interpreted cautiously. The relatively small number of subjects for whom data were available, the length of time that the drug was used, the estimated total number of joints smoked and the young age of the subjects may serve, individually or collectively, to moderate effects. Smoking at least 5 joints weekly should not be interpreted as a definitive threshold, as subjects were at low risk for other factors that could have a negative synergistic effect on IQ score. It is also important to emphasize that broad intellectual functioning may be less vulnerable to the consequences of marijuana use than more specific cognitive domains, such as attention and memory.7,13,14
The popularity of marijuana among youth has been increasing during the past 4 years,32,33 and pressure on governmental agencies to assess the medical uses of the drug and to reassess the legal status of the drug has been growing.34 These trends emphasize the need to continue investigating the cognitive consequences of both current and previous marijuana use.
Acknowledgments
This work was supported by a grant to Peter Fried from the National Institute on Drug Abuse, Washington, DC. The authors thank Heather Linttell for her testing of the subjects over the past 15 years and all the families who have participated in the Ottawa Prenatal Prospective Study for the past 2 decades. The urinalysis was carried out under the direction of Dr. Sherry Perkins, Head, Division of Biochemistry, Ottawa Hospital, Ottawa.
Footnotes
This article has been peer reviewed.
Contributors: Peter Fried has been the Director of Ottawa Prenatal Prospective Study since its inception. He designed the protocol for the study, as well as the questionnaire that was administered to the subjects. He also made a substantial contribution to the writing and editing of the manuscript. Barbara Watkinson was the primary statistical analyst and made a substantial contribution to the writing and editing of the manuscript. Deborah James assisted in the analyses and made a substantial contribution to the writing and editing of the manuscript. Robert Gray was in charge of data management and extrapolated the data reported in this manuscript. He also assisted in writing the manuscript.
Competing interests: None declared.
Correspondence to: Dr. P.A. Fried, Department of Psychology, Carleton University, 1125 Colonel By Drive, Ottawa ON K1S 5B6; fax 613 520-3667; peter_fried@carleton.ca
References
- 1.Hall W, Solowij N, Lemon J. The health and psychological consequences of cannabis use. Canberra: Australian Government Publishing Service; 1994.
- 2.Klonoff H. Acute psychological effects of marijuana in man, including acute cognitive, psychomotor and perceptual effects on driving. In: Fehr KO, Kalant H, editors. Cannabis and health hazards. Toronto: Addiction Research Foundation; 1983. p. 433-74.
- 3.Beardsley PM, Kelly TH. Acute effects of cannabis on human behavior and central nervous system functions. In: Kalant H, Corrigall W, Hall W, Smart R, editors. The health effects of cannabis. Toronto: Addiction Research Foundation; 1999. p. 129-69.
- 4.Kolansky H, Moore RT. Toxic effects of chronic marihuana use. JAMA 1972; 222:35-41. [PubMed]
- 5.Lundqvist T. Specific thought patterns in chronic cannabis smokers observed during treatment. Life Sci 1995;56:2141-4. [DOI] [PubMed]
- 6.Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. Am J Dis Child 1989;143:1214-9. [DOI] [PubMed]
- 7.Rubin V, Comitas L. Psychological assessment. In: Rubin V, Comitas L, editors. Ganja in Jamaica: a medical anthropological study of chronic marijuana use. The Hague: Mouton; 1975. p. 111-9.
- 8.Carter WE, Coggins W, Doughty PL. Cannabis in Costa Rica: a study of chronic marihuana use. Philadelphia: Institute for the Study of Human Issues; 1980.
- 9.Varma VJ, Malhotra AK, Dang R, Das K, Nehra R. Cannabis and cognitive functions: a prospective study. Drug Alcohol Depend 1988;21:147-52. [DOI] [PubMed]
- 10.Pope HG Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA 1996;275:521-7. [PubMed]
- 11.Jones RT, Benowitz N. The 30 day trip — clinical studies of cannabis tolerance and dependence. In: Braude MC, Szara S, editors. The pharmacology of marijuana. New York: Raven Press; 1976. p. 627-42.
- 12.Chait LD. Subjective and behavioral effects of marijuana the morning after smoking. Psychopharmacology (Berl) 1990;100:328-33. [DOI] [PubMed]
- 13.Pope HG Jr, Gruber AJ, Yurgelun-Todd D. The residual neuropsychological effects of cannabis: the current status of research. Drug Alcohol Depend 1995; 38:25-34. [DOI] [PubMed]
- 14.Solowij N. Long-term effects of cannabis on the central nervous system. In: Kalant H, Corrigall W, Hall W, Smart R, editors. The health effects of cannabis. Toronto: Addiction Research Foundation; 1999. p. 195-265.
- 15.Block RI, Farnham S, Braverman S, Noyes R Jr, Ghoneim MM. Long-term marijuana use and subsequent effects on learning and cognitive functions related to school achievement: preliminary study. NIDA Res Monogr 1990; 101: 96-111. [PubMed]
- 16.Block RI, Ghoneim MM. Effects of chronic marijuana use on human cognition. Psychopharmacology (Berl) 1993;110:219-28. [DOI] [PubMed]
- 17.Ellis GM Jr, Mann MA, Judson BA, Schramm NT, Taschian A. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clin Pharmacol Ther 1985;38:572-8. [DOI] [PubMed]
- 18.Cridland JS, Rottanberg D, Robins AH. Apparent half-life of excretion of cannabinoids in man. Hum Toxicol 1983;2:641-4. [DOI] [PubMed]
- 19.Heustis MA, Mitchell JM, Cone EJ. Detection times of marijuana metabolites in urine by immunoassay and GC–MS. J Anal Toxicol 1995;19:443-9. [DOI] [PubMed]
- 20.Millsaps CL, Azrin RL, Mittenberg W. Neuropsychological effects of chronic cannabis use on the memory and intelligence of adolescents. J Child Adolesc Subst Abuse 1994;3:47-55.
- 21.Fried PA. Behavioral evaluation of the older infant and child. In: Slikker W Jr, Chang LW, editors. Handbook of developmental neurotoxicology. San Diego: Academic Press; 1998. p. 469-86.
- 22.Fried PA, Smith AM. A literature review of the consequences of prenatal marijuana exposure. An emerging theme of deficiency in aspects of executive function. Neurotoxicol Teratol 2001;23:1-11. [DOI] [PubMed]
- 23.Wechsler D. Wechsler Intelligence scale for Children. 3rd ed. New York: The Psychological Corporation; 1991.
- 24.Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. San Antonio (TX): The Psychological Corporation; 1997.
- 25.Jacobson SW, Jacobson JL. Prospective, longitudinal assessment of developmental neurotoxicity. Environ Health Perspect 1996;104:275-83. [DOI] [PMC free article] [PubMed]
- 26.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 1955;50:1096-121.
- 27.Lester BM, LaGasse LL, Seifer R. Cocaine exposure and children: the meaning of subtle effects. Science 1998;282:633-4. [DOI] [PubMed]
- 28.Streissguth AP, Barr HM, Sampson PD, Darby BL, Martin DC. IQ at age 4 in relation to maternal alcohol use and smoking during pregnancy. Dev Psychol 1989;25:3-11.
- 29.Bellinger DC. Interpreting the literature on lead and child development: the neglected role of the “experimental system.” Neurotoxicol Teratol 1995;17:201-12. [DOI] [PubMed]
- 30.Johansson E, Agurell S, Hollister LE, Halldin MM. Prolonged apparent half-life of D1-tetrahydrocannabinol in plasma of chronic marijuana users. J Pharm Pharmacol 1988;40:374-5. [DOI] [PubMed]
- 31.Martin BR, Cone EJ. Chemistry and pharmacology of cannabis. In: Kalant H, Corrigal W, Hall W, Smart R, editors. The health effects of cannabis. Toronto: Addiction Research Foundation; 1999. p. 21-68.
- 32.Ogborne AC, Smart RG, Adlaf EM. Self-reported medical use of marijuana: a survey of the general population. CMAJ 2000:162;1685-6. Available: www.cma.ca/cmaj/vol-162/issue-12/1685.htm [PMC free article] [PubMed]
- 33.Johnston LD, O'Malley PM, Bachman JG. The Monitoring the Future national survey results on adolescent drug use: overview of key findings, 2000. Bethesda (MD): National Institute on Drug Abuse; 2001. NIH Publ No. 01-4923. Available: http://monitoringthefuture.org/pubs/monographs/overview2000.pdf (accessed 20 Feb 2002)
- 34.Marijuana: federal smoke clears, a little [editorial] CMAJ 2001;164:1397. Available: www.cma.ca/cmaj/vol-164/issue-10/1397.asp [PMC free article] [PubMed]


