Abstract
Purpose
Spontaneous notification systems are essential in a post‐marketing safety context. However, using this method, only about 6% of all adverse drug reactions are notified. To overcome this sub‐notification problem, new methods need to be developed to improve and facilitate reporting. In this sense, the use of digital media, mainly medical mobile apps, has been presented as a powerful tool, including in pharmacovigilance. We performed a scope review to identify the available apps used to report adverse drug reactions around the world to eventually identify which of them best fits the Portuguese pharmacovigilance system.
Methods
The Joanna Briggs Institute guidelines were considered, and the framework proposed by Arksey and O'Malley was followed. All the articles that met the inclusion criteria were examined for this review. When the studies lacked in information about the app, Google was used to enhance the search for further information.
Results
A final number of five articles were included, revealing seven implemented mobile apps for adverse drug reaction report (Medwatcher, VigiBIP, Yellow Card, Bijwerking, Halmed, Med Safety, and ADR PvPi). These apps are implemented in the United States, France, United Kingdom, The Netherlands, Croatia, and India. Med Safety was originally designed for multi‐region use and is implemented in 12 low and middle‐income countries.
Conclusions
Apps are easier and faster ways of reporting. The integration of such a tool in an individual care plan would allow to maintain a complete electronic health record at both individual and global level and could be eventually seen as an added value by both health professionals and patients. A country specific version of the WEB‐RADR could be a solution for Portugal, in order to introduce an app to notify ADRs at the national level, due previous successful experiences in European countries.
Keywords: adverse drug reaction, mobile apps, pharmacovigilance, spontaneous notification, underreporting
Key Points.
We found seven mobile apps for adverse drug reaction report (Medwatcher, VigiBIP, Yellow Card, Bijwerking, Halmed, Med Safety, and ADR PvPi) implemented worldwide.
Apps are easier and faster ways of reporting and allow a both‐way communication between authorities and notifiers revealing to be solid complementary instruments to the traditional channels of notification. They contribute to increase the number of reports as well as to improve the identification of safety signals.
In the future, the use of the app as a personalized intervention method may also be considered. For instance, this instrument has the potential scope of integration in an individual care plan.
1. INTRODUCTION
Pharmacovigilance is a major activity in the health area, with strong social and commercial implications, and aims to continuously monitor the benefit/risk ratio of a drug, improving the safety and quality of life of their users. 1 Spontaneous notification presents as the main methodology of routine pharmacovigilance, exhibiting several advantages, such as coverage of entire population and drug life cycle, simplicity, and cost‐effectiveness of the method. 2 , 3
Health professionals, marketing authorization holders and general population have the possibility of reporting using platforms designed for this purpose: notification forms, telephone call, electronic mail, online platforms, and mobile applications for quick adverse drug reactions (ADRs) notification. 4 , 5
ADR must be understood in its broadest concept, including harmful effects resulting from therapeutic errors, off‐label, abusive or improper drug utilization and from its ineffectiveness. 6 , 7 The consequences of its occurrence have a strong clinical and economic impact, resulting in increased morbidity and mortality, longer hospital stays and increased costs. It is estimated that ADR account for more than 5% of hospital admissions, causing around 200 000 deaths per year in Europe. 8 In Portugal, between 2000 and 2015, approximately 14.9 million admissions were registered in public hospitals, of which 5.8% were associated with at least one ADR, resulting in a total of 12.6 million days of hospitalization and a cost of 4.8 billion euros. 9 It is essential to notify, not only to avoid drug exposure of new susceptible individuals, but also to promote greater knowledge between pharmacological effects and individual susceptibilities. 10
The main limitation regarding the effectiveness of the spontaneous notification system is the underreporting of suspected ADR. It is estimated that only 6% of all ADR that occur are notified. 11 , 12 The main reasons for underreporting are already identified. 13 The participation of health professionals in ADR identification and notification programs, the improvement of education in pharmacovigilance in teaching, the reduction of the individual care workload of health seems essential to allocate time and availability for ADR notification. 14
Once it is demonstrated that the biggest failure in the system is associated with reasons such as lack of time to notify, it is urgent to consider the simplification of processes and the deconstruction of the bureaucracy associated with ADR notification. 14 , 15
A notification by the user is understood as one that is made on its own initiative, after suspicion of an ADR, without the prior interpretation of any healthcare professional. 16 With the reform of the European pharmacovigilance system (2012), citizens were given the right to report suspected ADR directly to regulatory agencies. In 2015, in the EU, 48782 notifications were registered from users, with an increase of 30% in the subsequent year. 17 The reasons for notification have been identified and include altruistic motivations, personal reasons, ADR seriousness and the dissatisfaction of the user related to the healthcare provided. 18
Studies refer that users are very relevant in identifying ADR in specific populations or types of medications and so, they are also significant in detecting risk signals. 17 For the reasons mentioned, there is a growing interest in their involvement in pharmacovigilance systems. 19 However, the problem of underreporting also applies when the notifier is the user. Awareness campaigns about notification and respective explanation of the process and its importance are scarce. 20 Most citizens remain ignorant or confused towards notification systems. 21
Information systems stand out for their great potential in reducing costs, improving the quality of healthcare structures and maximize the coordination of healthcare. 22 A good clinical information system is guided for contributing positively to user safety, for greater efficiency in the workflow and for guiding decision‐making by suitable entities. 23 With the growing dispersion of information and communication technologies, several health sectors, including the pharmacovigilance one, have acquired information systems to support them. In Portugal, the first Portal RAM, an online electronic platform for inserting ADR cases developed by national authority (INFARMED, I.P.), went into production at the end of June 2012, and was later replaced by a second version that ran from November 2017 and remains active today. 24
Mobile applications (apps) emerge to provide greater quality to health services and have been designed to improve disease management and/or increasing healthy behaviors (e.g., promoting ADR reporting). As for portable equipment that can have an application installed (e.g., smartphones, tablets, or smartwatches), these contain a wide range of communication functions, such as text messages, photos, videos, telephone, and internet access, which makes them indisputably useful tools. In addition to technique, popularity and mobility factors add tremendous value to these technologies in supporting the delivery of healthcare services. 25 As currently 3.5 billion (44.9%) of people in the world use a smartphone, and the mobile technology sector is the fastest growing sector, apps prove to be extremely advantageous for optimizing healthcare services. 26
Thus, apps are emerging for fast notification of ADR. They are intended to combat underreporting and aim at improving public health and safety. They make use of the technological developments mentioned above: they allow connecting communication between notifiers and receiving entities; they take advantage of resources that are beneficial to the quality of notifications, such as the photography tool to illustrate any visible ADR; they reach a large number of users and they are strategically positioned in relation to the time factor, as they allow notification at any time of the day, as long as the user is accompanied by the mobile device, and significantly reduce the time required for the act of notification. None of them exist in Portugal.
The main objective of this research is to perform a scope review to identify the available apps used to report ADR around the world to eventually identify which of them best fits the reality and the Portuguese pharmacovigilance system.
2. METHODS
A scoping review methodology was selected to broadly and rapidly map out the available apps used to report ADR. Scoping reviews can be particularly useful tools for examine broad and emerging areas, such as the use of digital media for pharmacovigilance purposes, to identify gaps on the evidence, clarify key concepts and provide a wide overview of a topic. For this study, the Joanna Briggs Institute guidelines 27 were considered and the framework proposed by Arksey and O'Malley 28 was followed.
2.1. Step 1: identifying research questions
This study aims at identifying existing apps for adverse drug reaction reporting. Hence, the subsequent questions were made through the process:
What are the implemented mobile apps for adverse drug report?
Where are they used?
Are they contributing to minimize the sub notification issue?
2.2. Step 2: identifying relevant studies
A preliminary background search was carried out to identify pertinent keywords. These keywords were then used to search for relevant studies using three search engines (PubMed, Google Scholar, and Google Search). Both peer‐reviewed and grey literature were considered, whereas boolean operators (OR, AND) were applied throughout the searching process (Table 1). The literature search was performed in June–July 2021.
TABLE 1.
Keywords used for the identification
| No. | Keywords |
|---|---|
| 1 | “mobile phone” |
| 2 | “app” |
| 3 | “adverse drug reaction” |
| 4 | “report” |
| 5 | “pharmacovigilance” |
| 6 | “mobile phone” OR “app” |
| 7 | “mobile phone” OR “report” |
| 8 | “adverse drug reaction” OR “pharmacovigilance” |
| 9 | Combination of 1 AND 3 |
| 10 | Combination of 1 AND 3 AND 4 |
| 11 | Combination of 1 AND 3 AND 4 AND 5 |
2.3. Step 3: study selection
Inclusion and exclusion criteria were defined for the selection of the studies. Studies were selected considering the following sequence: removal of duplicates, review of title and abstract, and full text review.
After removing duplicates from the different databases, two review authors individually screened the titles and abstracts of all records identified to remove articles that were clearly irrelevant; full text articles were then examined to determine whether they met the criteria for inclusion in the review. Any divergences were resolved through discussion or the intervention of a third review author.
Moreover, only literature published in English was considered and studies were filtered based on being published between 2007 and 2021. Mobile apps have surged mainly from 2007, as this year marks the launch of the first iPhone generation. Also, in 2008, smartphones running the Google Android operating system were introduced.
Exclusion criteria for the scoping review were: (1) articles that reviewed traditional ways of ADR reporting; (2) articles that mentioned mobile apps, even in pharmacovigilance context, but whose purpose was not ADR reporting; (3) articles that only mentioned mobile apps for ADR reporting but do not explain their functionalities, characteristics; or results and (4) articles that reviewed proof‐of‐concept tests or prototype approaches to app development.
2.4. Step 4: charting the data
To identify the existing mobile apps for ADR report and their importance, the selected studies (Step 3) were uploaded to an excel spreadsheet. Data were extracted on: author, year of publication, type of literature (peer reviewed or grey literature), name of the app, geographical area (countries or regions where the app was implemented), level of implementation (if the app was created to cover a region, a country, or more areas), operating system (iOS, Android, or both), type of owner, target audience (health care professionals, public in general, or both), type of features used and significant findings.
2.5. Step 5: collating, summarizing, and reporting the results
For each study, the previous data referred in Step 4 were first included in a table. The information concerning each app was then summarized according to their similarities and differences. The features on each app and the data regarding the way they are contributing for an enhanced national/regional pharmacovigilance system was finally considered.
3. RESULTS
A total of 91 studies were identified. After duplicates removal, 84 were screened based on title and abstract, of which 19 remained for full‐text review. A final number of five articles were included in this systematic scoping review, revealing seven implemented mobile apps for ADR report (Figure 1). The articles were published in 2012 (n = 1), 2017 (n = 1), 2018 (n = 1), and 2019 (n = 2).
FIGURE 1.
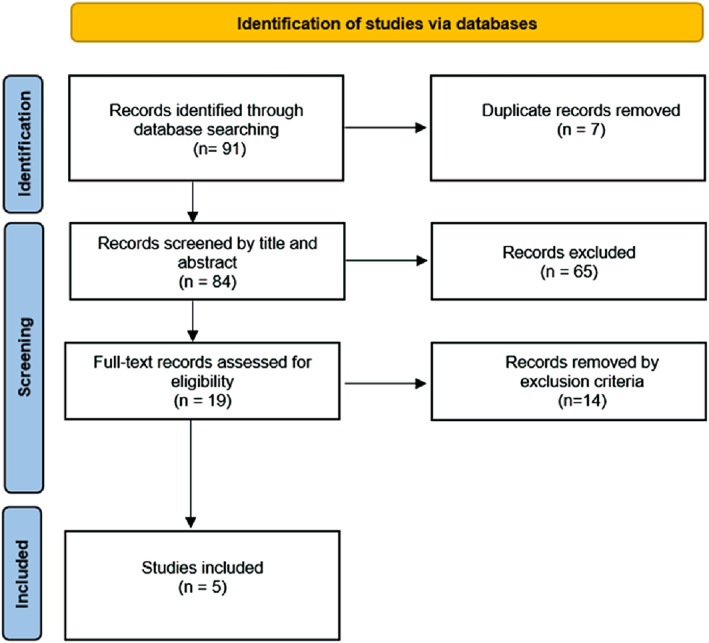
PRISMA flow diagram of the study selection process 29
Launched in September 2012 (Figure 2) and created by Boston Children's Hospital and Harvard Medical School in collaboration with the Food and Drug Administration (FDA), MedWatcher was the first mobile app enabling users to submit voluntary reports of ADR to the FDA. It was developed to overcome the limitations of traditional reporting methods (e‐mail, phone or online), since it allows an easier and faster report. 30 During the reporting process, the app allows users to upload images. Additionally, users can customize the app based on their pathology and medical products of interest and thus choose to automatically receive information about them. The app is available in English, in both iOS and Android operating systems and is free. 31 , 32 , 33 MedWatcher can be used by healthcare professionals and consumers (e.g., patients, caregivers).
FIGURE 2.
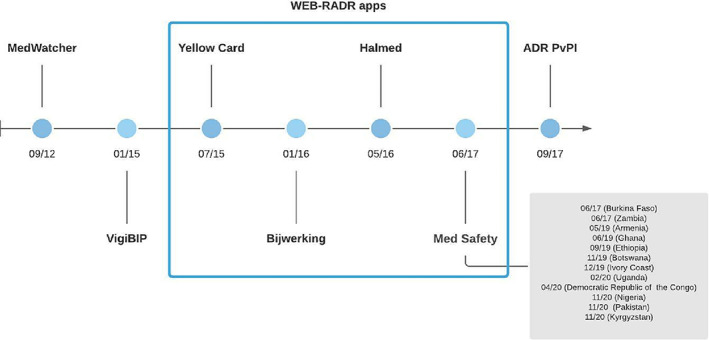
Mobile apps for adverse drug reaction reporting appearance timeline
The French app VigiBIP it was introduced in January 2015 by the Toulouse University Pharmacovigilance Centre and promoted via website. It is like MedWatcher in many aspects: a free mobile tool; available in iOS and Android; and allows healthcare professionals and public in general to report ADR and receive safety information on drugs. The following information should be included by the users: age; sex; history of the patient; name, dosage, and date of administration of the drug(s); and a short history of the ADR. It also enables photographs to be uploaded. 34
A previous study 35 comparing ADR reports received via VigiBIP, between 10 January 2015 and 1 February 2017, and those received through the classical methods suggested a particular interest in this type of approach to consumers.
The WEB Recognising Adverse Drug Reactions (WEB‐RADR) project was launched in September 2014 with the aim of using new technologies and benefits of social media for pharmacovigilance purposes. 36 Within this project, funded by the Innovative Medicines Initiative (IMI), country‐specific mobile apps have been launched (Figure 2): in the United Kingdom (Yellow Card in July 2015), Netherlands (Bijwerking in January 2016) and in Croatia (Halmed in May 2016). All apps share similar features between them and among those previously described (i.e., MedWatcher and VigiBIP). 37 These three WEB‐RADR apps comprised more than 18 000 downloads among them by the end of December 2017, resulting in 838 suspect ADR reports. 38 Comparing the characteristics, quality, and contribution to safety signals of reports submitted via the WEB‐RADR apps with the classical methods, it was found that 78%–85% and 78%–98% of all reports were considered of at least moderate quality when submitted via the app or by classical methods, and eight potential safety signals came from app reports, of which four turned out as issued signals. 39
A generic version of the WEB‐RADR app, called Med Safety, was rolled out in 2017, in Burkina Faso (Figure 2). It resulted from a collaboration between IMI, WEB‐RADR, Medicines and Healthcare products Regulatory Agency, and the World Health Organization (WHO), and aimed at creating a cheapest approach for ADR data collection. Med Safety was originally designed for multi‐region use and can be thus adopted in low and middle‐income countries within the WHO Programme for International Drug Monitoring, where access to computers with wired internet connections can be poor and it is supplanted by the prevalence of smartphones. So far, Med Safety is already implemented in 12 countries (Figure 2): Burkina Faso, Zambia, Armenia, Ghana, Ethiopia, Botswana, Ivory Coast, Uganda, Democratic Republic of the Congo, Nigeria, Pakistan, and Kyrgyzstan. 40 , 41
The ADR PvPi app was launched in India in September 2017 (Figure 2). It is a free use app only available in the Android operating system and created by the National Coordination Centre‐Pharmacovigilance Programme of India. This app, as for MedWatcher, VigiBIP and WEB‐RADR apps, allows healthcare professionals and consumers to quickly report ADR and receive safety information on drugs. 42
Table 2 summarises the information for each app.
TABLE 2.
Literature review on mobile apps for quick adverse drug reactions report
| References | Online references that completed the app profile | Name of the app | Date of implementation | Country of implementation | Operating system (iOS, Android, or both) | Target audience (Healthcare professional, public in general, or both) | Type of features | Significant findings |
|---|---|---|---|---|---|---|---|---|
| Bahk et al 30 | 31 , 32 , 33 | MedWatcher | September 2012 | United States of America | Both | Both |
Images can be uploaded; Customizable; Two‐way communication channel |
11.4 min average are needed to conclude a report |
| Montastruc et al 35 | 34 | VigiBIP | January 2015 | France | Both | Both |
Images can be uploaded; Customizable; Two‐way communication channel |
Consumers report more frequently via the app; |
|
Pierce et al 38 Oosterhuis et al 39 |
, 37 |
WEB‐RADR apps 1. Yellow Card 2. Bijwerking 3. Halmed 4. Med Safety |
1. July 2015 2. January 2016 3. May 2016 4. Jun 2017 |
1. United Kingdom 2. The Netherlands 3. Croatia 4. Burkina Faso, Zambia, Armenia, Ghana, Ethiopia, Botswana, Ivory Coast, Uganda, Democratic Republic of the Congo, Nigeria, Pakistan, and Kyrgyzstan |
Both | Both |
Allows more free text; Images can be uploaded; Functions offline; Customizable; Interface of the app adapts to the device; Designed for multi‐region use; Two‐way communication channel. |
Patients report more frequently via app in UK and Croatia; No significant differences in sex or ages of reporters were observed; 78%–85% and 78%–98% of all reports were considered of at least moderate quality when submitted via the app or by classical methods, respectively; Eight potential safety signals came from app reports, of which four turned out as issued signals |
| Prakash et al 42 | ‐ | ADR PvPi | September 2017 | India | Android | Both |
Images can be uploaded; Customizable; Two‐way communication channel |
‐ |
As it can be concluded from data presented in Table 2, all the identified apps are quite similar in terms of characteristics: allow both operating systems and target audiences, similar launch periods and type of features. However, country specific versions of the WEB‐RADR app are the ones that were more frequently implemented in different country and cultures.
4. DISCUSSION
We identified all the apps available and used to report suspected ADR. Moreover, it was of our concern understanding if this instrument brings an additive value to the national pharmacovigilance systems.
Despite all the efforts that have been made to establish more effective spontaneous notification mechanisms, underreporting of suspected ADR remains a concern. The traditional routes of notification can still be very demanding in terms of how much time is needed to complete a report. 43
The results of our review study evidenced that apps are easier and faster ways of reporting. In fact, Medwatcher proved that is possible to reduce the notification time on at least 75%, when compared to the traditional routes. This study on Medwatcher app also found that the received reports were classified with a high average quality, measured by the vigiGrade completeness score (0.8 on a scale of 0 to 1). In order to have an accurate causality assessment, it is essential that high‐quality clinical information can be extracted from the submitted reports. Therefore, the vigiGrade completeness scale was created to measure the amount of relevant clinically information received. 44 Regarding the WEB‐RADR apps, a comparison between the quality of the app reports and those received via traditional means was conducted. For all countries (UK, the Netherlands, and Croatia) the vigiGrade completeness score was high for both the app and reference report samples, but overall lower for the app samples. The proportion of reports of at least moderate quality was high in both samples (app: 78%–85%, reference: 78%–98%), for all countries. 30 This slight loss of quality in the reports received through WEB‐RADR apps may be a result of the less extensive questionnaire that is necessary to complete a report. This, however, should be seen as encouraging since the simplified form matches up the traditional reports in terms of contribution to the detection of safety signals. 39
By decreasing the number of structured fields, and allowing for more free text, apps are not only becoming faster means of report, but also more appealing for public in general. De Vries et al conducted a study whose results suggest that people prefer to describe the ADR using their own words instead of having to choose a term between, for instance, a drop‐down menu. 45 This mainly occurs for two reasons: people can present difficulty to understand medical terms and/or may feel that none of the terms describes exactly what they are experiencing. These reasons can consequently cause a person to give up on the intention to report. 46
Previous studies have also demonstrated that lack of time and difficulties in filling out records or forms, as well as poor access to the pharmacovigilance system, play a key obstacle in spontaneous reporting. For instance, it is known that physicians, because of the high workload or because they postpone the act of reporting, choose not to report, or forget. 47 Moreover, an app would be always accessible and a quick option, capable of removing pressure and optimizing the time from the pharmacovigilance professionals (i.e., healthcare professionals in general).
Even for PV teams working on ADR collecting and processing, receiving the information through a more harmonized manner and with practically no time gap between the moment of the report and the moment of the information arrival to the PV Units would agile this process for those teams, despite the goal of having more information to deal with.
According to Fukushima et al, apps facilitates reporting through two main factors: simplicity and quality. The simplicity was enhanced by digital features, such as drop‐down menus, a defined drug list, and data file attachment capability. Defining mandatory reporting fields reduced issues of missing data and increased the overall quality of reports. However, the limited information collected by such simple reporting apps needed to be complemented by more comprehensive reporting afterward. 48
While use of the app to date seems modest in comparison with other ADR‐reporting modalities, it is reasonable to expect that app‐based reporting will grow in importance as a younger generation increasingly use their mobile devices to access the Internet all around the world. It can be expected that apps will supplement the traditional technologies for evaluating a product's risk profile, as quality seems not to significantly differ, but the profile of reporters and cases seem to present some differences. 38 , 49 All the seven sins of Inman are certainly not overcome with the use of apps, but studies point to the fact that additional information can be obtained on drug's safety profile with the use of such means, namely due to new reporters with different profiles. 50
Besides the ADR‐reporting component, all apps presented a safety information‐provision component as well. Before the implementation of WEB‐RADR apps, a European survey was conducted to assess the interest of healthcare professionals and consumers in such implementation. From those taking part of the study, 61% of the healthcare professionals and 48% of the consumers were “very interested” in the app. 46
The already implemented apps presented herein should be seen as models for future implementations, namely for the Portuguese population. In fact, Pierce et al published a set of 27 recommendations on app development based on lessons from WEB‐RADR project. 38 Design, country regulation on personal data and security, characteristics on the app target group (e.g., type of reporter, health literacy level, access to new technologies), and through what means will the app be disseminated are some important aspects that should be addressed in advance.
In the future, the use of the app as a personalized intervention method may also be considered. For instance, this instrument has the potential scope of integration in an individual care plan (ICP). According to Lopes, ICP is defined as “a person‐centred tool that constitutes a space for dialogue among all caregivers that supports and facilitates the management of pathways and the integration of care”. 51
To establish an ICP it is necessary to have an effective electronic health record capable of gathering essential data on each citizen and allowing a both‐way communication between healthcare professionals, caregivers and/or patients. Thus, an app with the aforementioned features not only could be a useful channel for consumers to receive information and safety alerts on their medication and to report suspected ADR, but also because it has the potential to be part of an electronic health record equipped with artificial intelligence apt of supervised machine learning. 52
A strength of this scoping review is that it is the first review of its type to identify and describe the apps available and used to report ADR among the world. It is important to be able to compare them and eventually to be able to create new ones, or to improve the existing based on lived experiences.
Notwithstanding the value of this research, some limitations must be acknowledged. Although this review was performed using multiple databases and grey literature, searching other databases such as Cochrane Library may have yielded other relevant published papers. In addition, as this review was limited to papers published in the English, it is possible that other potentially relevant reviews were omitted. A quality assessment of the studies included in the review was not undertaken, as authors considered that it was not relevant for its aim, and this is also why it is not always necessary for scoping reviews. 29
5. CONCLUSION
Seven different apps aiming to allow quick ADR reports were identified in this scoping review. This type of official mobile app still does not exist in Portugal. It is therefore of paramount importance to reflect about its national development, based on the existing apps described in this review. A country specific version of the WEB‐RADR could be a solution for Portugal, in order to introduce an app to notify ADRs at the national level, due previous successful experiences in European countries. This will allow to increase the notification rate, which remains low, despite all efforts conducted by the authorities.
The integration of such a tool in an ICP would allow to maintain a complete electronic health record and could be eventually seen as an added value by both health professionals and patients.
AUTHOR CONTRIBUTIONS
Edna Ribeiro Parracha: Study design, data collection and analysis, and article writing. Ana Margarida Advinha: Study design, data analysis and article review. Manuel José Lopes: Study design, data analysis and article review; Sofia Oliveira‐Martins: Study design, data analysis and article writing. All authors read and approved the final manuscript.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENT
The authors would like to thank Professor Carolino Monteiro (Faculty of Pharmacy of the University of Lisbon).
Parracha ER, Advinha AM, Lopes MJ, Oliveira‐Martins S. Mobile apps for quick adverse drug reaction report: A scoping review. Pharmacoepidemiol Drug Saf. 2023;32(1):19‐27. doi: 10.1002/pds.5542
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. World Health Organization. Regulation and Prequalification. Accessed 7 September 2021. https://www.who.int/teams/regulation‐prequalification/regulation‐and‐safety/pharmacovigilance.
- 2. Sousa‐Ferreira PB, Torre C. Notificação Espontânea [Spontaneous Notification]. In: de Oliveira Martins S , ed. Farmacovigilância em Portugal: 25 anos [Pharmacovigilance in Portugal: 25 years]. INFARMED ‐ Autoridade Nacional do Medicamento e Produtos de Saúde, I.P.; 2019:171 (in Portuguese). [Google Scholar]
- 3. European Medicines Agency . Module VI – Collection, management and submission of reports of suspected adverse reactions to medicinal products (Rev 2). Guideline on good pharmacovigilance practices (GVP). 2017:144.
- 4. Lindquist M. Vigibase, the WHO global ICSR database system: basic facts. Drug Inf J. 2008;42:409‐419. doi: 10.1177/009286150804200501 [DOI] [Google Scholar]
- 5. Guideline on good pharmacovigilance practices (GVP) . Module IX Addendum I – methodological aspects of signal detection from spontaneous reports of suspected adverse reactions. 2017. Accessed 10 September 2021. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-ix-addendum-i-methodological-aspects-signal_en.pdf.
- 6. Farmacovigilância RN. Qual a perceção da Indústria Farmacêutica em relação à Farmacovigilância. [Pharmacovigilance. What is the perception of the pharmaceutical industry regarding the pharmacovigilance]. Ordem dos Farmacêuticos. 2016; https://www.ordemfarmaceuticos.pt/fotos/editor2/Colegios_de_Especialidade/Titulo_Especialidade/Especialidade_AR/Especialistas_Anteriores/2016/2016_Nuno_Jorge_Mangorrinha_Henriques_Amorim_Romao.pdf [Google Scholar]
- 7. Coleman JJ, Pontefract SK. Adverse drug reactions. Clin Med (Lond). 2016;16(5):481‐485. doi: 10.7861/clinmedicine.16-5-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Defer G, Le Caignec F, Fedrizzi S, et al. Dedicated mobile application for drug adverse reaction reporting by patients with relapsing remitting multiple sclerosis (Vigip‐SEP study): study protocol for a randomized controlled trial. Trials. 2018;19:174. doi: 10.7861/clinmedicine.16-5-48110.1186/s13063-018-2560-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sousa‐Pinto B, Marques B, Lopes F, Freitas A. Frequency and impact of adverse events in inpatients: a nationwide analysis of episodes between 2000 and 2015. J Med Syst. 2018;42(3):48. [DOI] [PubMed] [Google Scholar]
- 10. Rosa Pereira MR, Fonseca M, Maria VA. O Médico [the physician]. In: de Oliveira Martins S, ed. Farmacovigilância em Portugal: 25 anos [Pharmacovigilance in Portugal: 25 years]. INFARMED ‐ Autoridade Nacional do Medicamento e Produtos de Saúde, I.P.; 2019:293‐299 (in Portuguese). [Google Scholar]
- 11. Herdeiro MT, Ferreira M, Ribeiro‐Vaz I, Junqueira Polónia J, Costa‐Pereira A. O sistema português de farmacovigilância [The portuguese system of pharmacovigilance]. Acta Med Port. 2012;25(4):241‐249. [PubMed] [Google Scholar]
- 12. Montané E, Santesmases J. Adverse drug reactions. Reacciones adversas a medicamentos. Med Clin (Barc). 2020;154(5):178‐184. doi: 10.1016/j.medcli.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 13. Lopez‐Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under‐reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32(1):19‐31. doi: 10.2165/00002018-200932010-00002 [DOI] [PubMed] [Google Scholar]
- 14. Khalili M, Mesgarpour B, Sharifi H, Daneshvar Dehnavi S, Haghdoost AA. Interventions to improve adverse drug reaction reporting: a scoping review. Pharmacoepidemiol Drug Saf. 2020;29(9):965‐992. doi: 10.1002/pds.4966 [DOI] [PubMed] [Google Scholar]
- 15. Hergy F, Araújo A. Eudravigilance – Transmissão Eletrónica de Informação Em Farmacovigilância [EudraVigilance – Eletronic Transmission of Information in Pharmacovigilance]. In: de Oliveira Martins S, ed. Farmacovigilância em Portugal: 25 anos [Pharmacovigilance in Portugal: 25 years]. INFARMED ‐ Autoridade Nacional do Medicamento e Produtos de Saúde, I.P.; 2019:47‐54 (in Portuguese). [Google Scholar]
- 16. World Health Organization . Safety Monitoring of medicinal products: Reporting system for the general public. 2012. Accessed 22 March 2021. https://www.who.int/publications/i/item/9789241503198
- 17. Inácio P, Cavaco A, Airaksinen M. The value of patient reporting to the pharmacovigilance system: a systematic review. Br J Clin Pharmacol. 2017;83(2):227‐246. doi: 10.1111/bcp.13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Margraff F, Bertram D. Adverse drug reaction reporting by patients: an overview of fifty countries. Drug Saf. 2014;37(6):409‐419. doi: 10.1007/s40264-014-0162-y [DOI] [PubMed] [Google Scholar]
- 19. Inch J, Watson MC, Anakwe‐Umeh S. Patient versus healthcare professional spontaneous adverse drug reaction reporting: a systematic review. Drug Saf. 2012;35(10):807‐818. doi: 10.1007/BF03261977 [DOI] [PubMed] [Google Scholar]
- 20. Corrêa‐Nunes AM. O sistema de farmacovigilância em Portugal (sua criação e desenvolvimento) [The pharmacovigilance system in Portugal (its establishment and development)]. Cad Saude Publica. 1998;14(4):725‐733. (in Portuguese). doi: 10.1590/s0102-311x1998000400014 [DOI] [PubMed] [Google Scholar]
- 21. Al Dweik R, Stacey D, Kohen D, Yaya S. Factors affecting patient reporting of adverse drug reaction: a systematic review. Br J Clin Pharmacol. 2017;83(4):875‐883. doi: 10.1111/bcp.13159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fichman RG, Kohli R, Krishnan R. Editorial overview—the role of information Systems in Healthcare: current research and future trends. Inf Syst Res. 2011;22(3):419‐428. doi: 10.1287//isre.110.0382 [DOI] [Google Scholar]
- 23. Islam MM, Poly TN, Li YJ. Recent advancement of clinical information systems: opportunities and challenges. Yearb Med Inform. 2018;27(1):83‐90. doi: 10.1055/s-0038-1667075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bragança F, Carmona R. Organização do Sistema Nacional de Farmacovigilância [Organization of the National Pharmacovigilance System]. In: de Oliveira Martins S, ed. Farmacovigilância em Portugal: 25 anos [Pharmacovigilance in Portugal: 25 years]. INFARMED ‐ Autoridade Nacional do Medicamento e Produtos de Saúde, I.P.; 2019:57‐64 (in Portuguese). [Google Scholar]
- 25. Kao CK, Liebovitz DM. Consumer Mobile health apps: current state, barriers, and future directions. PMR. 2017;9(5S):S106‐S115. doi: 10.1016/j.pmrj.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 26. Free C, Phillips G, Galli L, et al. The effectiveness of mobile‐health technology‐based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peters, MD , Godfrey CM, Mcinerney P, Soares CB, Khalil H, Parker D. The Joanna Briggs Institute Reviewers'. Manual 2015: Methodology for JBI scoping reviews. Joanne Briggs Inst [Internet]. 2015;1–24. Accessed 12 April 2021. http://joannabriggs.org/assets/docs/sumari/ReviewersManual_Mixed-Methods-Review-Methods-2014-ch1.pdf
- 28. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract. 2005;8(1):19‐32. [Google Scholar]
- 29. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 30. Bahk CY, Goshgarian M, Donahue K, et al. Increasing patient engagement in pharmacovigilance through online community outreach and Mobile reporting applications: an analysis of adverse event reporting for the Essure device in the US. Pharmaceut Med. 2015;29(6):331‐340. doi: 10.1007/s40290-015-0106-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Food and Drug Adminitration . MedWatcher Mobile App. Accessed 25 April 2021. https://www.fda.gov/medical-devices/medical-device-reporting-mdr-how-report-medical-device-problems/medwatcher-mobile-app
- 32. The MassDevice Life Sciences Network . There's an app fot that: FDA crowdsources adverse event reporting. Accessed 25 April 2021. https://www.massdevice.com/fdas-new-medwatcher-mobile-app-aims-crowd-source-adverse-event-reporting/
- 33. Boston Childrens's Hospital. MedWatcher, Track and report side effects of drugs, vaccines and medical devices . Accessed 25 April 2021. https://accelerator.childrenshospital.org/about/healthmap/
- 34. Bulletins d'Informations de Pharmacologie . Déclaration en ligne d'effet indésirable: déclaration de pharmacovigilance au CRPV de Tououse [Online adverse reaction declaration: pharmacovigilance declaration to the CRPV in Tououse] Accessed 25 April 2021. https://www.bip31.fr/
- 35. Montastruc F, Bagheri H, Lacroix I, et al. Adverse drug reaction reports received through the Mobile app, VigiBIP®: a comparison with classical methods of reporting. Drug Saf. 2018;41(5):511‐514. doi: 10.1007/s40264-017-0630-2 [DOI] [PubMed] [Google Scholar]
- 36. de Vries ST, Wong L, Sutcliffe A, et al. Factors influencing the use of a Mobile app for reporting adverse drug reactions and receiving safety information: a qualitative study. Drug Saf. 2017;40(5):443‐455. doi: 10.1007/s40264-016-0494-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. WEB‐RADR: Recognising Adverse Drug Reactions . Working together to improve pharmacovigilance through new technology. Accessed 25 April 2021. https://web-radr.eu/
- 38. Pierce CE, de Vries ST, Bodin‐Parssinen S, et al. Recommendations on the use of mobile applications for the collection and communication of pharmaceutical product safety information: lessons from IMI WEB‐RADR. Drug Saf. 2019;42(4):477‐489. doi: 10.1007/s40264-019-00813-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oosterhuis I, Taavola H, Tregunno PM, et al. Characteristics, quality and contribution to signal detection of spontaneous reports of adverse drug reactions via the WEB‐RADR Mobile application: a descriptive cross‐sectional study. Drug Saf. 2018;41(10):969‐978. doi: 10.1007/s40264-018-0679-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. WEB‐RADR: Med Safety App. Accessed 27 April 2021. https://web-radr.eu/mobile-apps/med-safety/
- 41. Uppsala Reports: Med Safety app: an international mobile tool for drug safety. Accessed 27 April 2021. https://www.uppsalareports.org/articles/med-safety-app-an-international-mobile-tool-for-drug-safety
- 42. Prakash J, Joshi K, Malik D, et al. "ADR PvPI" android mobile app: report adverse drug reaction at any time anywhere in India. Indian J Pharmacol. 2019;51(4):236‐242. doi: 10.4103/ijp.IJP_595_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steurbaut S, Hanssens Y. Pharmacovigilance: empowering healthcare professionals and patients. Int J Clin Pharmacol. 2014;36(5):859‐862. doi: 10.1007/s11096-014-0004-0 [DOI] [PubMed] [Google Scholar]
- 44. Bergvall T, Norén GN, Lindquist M. vigiGrade: a tool to identify well‐documented individual case reports and highlight systematic data quality issues. Drug Saf. 2014;37(1):65‐77. doi: 10.1007/s40264-013-0131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Vries ST, Harrison J, Revelle P, et al. Use of a patient‐friendly terms list in the adverse drug reaction report form: a database study. Drug Saf. 2019;42(7):881‐886. doi: 10.1007/s40264-019-00800-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Vries ST, Denig P, Lasheras Ruiz C, et al. Interest in a Mobile app for two‐way risk communication: a survey study among European healthcare professionals and patients. Drug Saf. 2018;41(7):697‐712. doi: 10.1007/s40264-018-0648-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vallano A, Cereza G, Pedròs C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60(6):653‐658. doi: 10.1111/j.1365-2125.2005.02504.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fukushima A, Iessa N, Balakrishnan MR, Pal SN. Smartphone‐based mobile applications for adverse drug reactions reporting: global status and country experience. BMC Med Inform Decis Mak. 2022. May 2;22(1):118. doi: 10.1186/s12911-022-01832-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Satwika MV, Sushma DS, Jaiswal V, Asha S, Pal T. The role of advanced technologies supplemented with traditional methods in pharmacovigilance sciences. Recent Pat Biotechnol. 2021;15(1):34‐50. doi: 10.2174/1872208314666201021162704 [DOI] [PubMed] [Google Scholar]
- 50. Inman WH. Attitudes to adverse drug reaction reporting. Br J Clin Pharmacol. 1996;41(5):434‐435. [PubMed] [Google Scholar]
- 51. Lopes MJ. The individual care plan as electronic health record: a tool for management, integration of care, and better health results. Exploring the role of ICTs in healthy aging. IGI Global. 2020;1‐12. doi: 10.4018/978-1-7998-1937-0.ch001 [DOI] [Google Scholar]
- 52. Hussain R. Big data, medicines safety and pharmacovigilance. J Pharm Policy Pract. 2021;14:48. doi: 10.1186/s40545-021-00329-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


