Abstract
Background
Neurogenic detrusor overactivity incontinence (NDOI) is often inadequately managed with oral therapy.
Objective
To assess efficacy and safety of abobotulinumtoxinA (aboBoNT‐A; Dysport®; Ipsen Ltd.) according to etiology of NDOI.
Design, Setting, and Participants
Two phase III, randomized, double‐blind studies (CONTENT1 [NCT02660138] conducted in Asia, Europe and North America; CONTENT2 [NCT02660359] conducted in the Americas, Asia, Europe and Oceania) both included patients with spinal cord injury (SCI) or multiple sclerosis (MS), with inadequately managed NDOI, regularly performing clean intermittent catheterization (CIC).
Intervention
Patients in CONTENT1 and CONTENT2 received aboBoNT‐A injections 600 U (n = 162)/800 U (n = 161), or placebo (n = 162) into the detrusor muscle.
Outcome Measurements and Statistical Analysis
Primary endpoint: mean change from baseline in number of NDOI episodes/week at Week 6. Secondary endpoints: proportion of patients with no NDOI episodes; incontinence‐related quality of life (I‐QoL); urodynamic parameters; and time‐to‐retreatment. Safety was also assessed. Statistical analyses were conducted for pooled populations by etiology (aboBoNT‐A doses vs. placebo).
Results and Limitations
Of 485 randomized patients, 341 (70%) and 144 (30%) had SCI and MS etiologies, respectively. A significant reduction was observed in mean NDOI episodes/week at Week 6 with both aboBoNT‐A doses versus placebo in the SCI (all p < 0.001) and MS (all p < 0.01) groups, as well as significant improvements in I‐QoL and urodynamic parameters. Median time‐to‐retreatment was longer in patients with MS (48–62 weeks across doses) than those with SCI (39–44 weeks). Safety data were similar between etiologies. Urinary tract infection was the most frequent adverse event; similar numbers were reported across treatment groups.
Conclusions
AboBoNT‐A was well tolerated and significantly improved continence and bladder function, and QoL, in patients with SCI or MS with NDOI performing regular CIC.
Patient Summary
AboBoNT‐A injections improved QoL, symptoms, and bladder function in patients with SCI or MS with bladder muscle overactivity that causes incontinence.
Keywords: AbobotulinumtoxinA, Botulinum toxin, multiple sclerosis, neurogenic detrusor overactivity incontinence, spinal cord injury
1. INTRODUCTION
Disruption of the nervous system controlling the lower urinary tract can cause neurogenic detrusor overactivity (NDO), a chronic condition resulting in involuntary detrusor contractions (IDCs) during bladder storage and often associated with urinary incontinence (UI; neurogenic detrusor overactivity incontinence; NDOI). 1 , 2 NDO can occur as a result of neurologic conditions, including multiple sclerosis (MS) and spinal cord injury (SCI); NDOI arises due to high detrusor pressures and/or reduced volume capacity. 1 , 2 Management of NDOI includes use of intermittent catheterization (aseptic or clean [clean intermittent catheterization, CIC]) to achieve regular bladder emptying at low pressure, and to minimize risk (from elevated pressures) of upper urinary tract deterioration. 2 , 3 , 4 Although not well characterized, renal deterioration is more common in patients with NDOI due to SCI versus an MS etiology. 5 , 6 Pharmacological therapies, typically anticholinergics, can be used to reduce detrusor contractions; however, these can be ineffective, and many patients experience adverse events (AEs). 2 , 4
Botulinum toxin type A (BoNT‐A) has shown efficacy in the treatment of NDOI. 7 , 8 , 9 , 10 , 11 , 12 BoNT‐A inhibits vesicle‐mediated neurotransmission through cleavage of synaptosome‐associated protein 25 (SNAP‐25); SNAP‐25 is integral to the soluble N‐ethyl‐maleimide‐sensitive factor attachment protein–receptor complex responsible for docking and fusion of synaptic vesicles to the nerve terminal membrane for neurotransmitter release. 13 , 14 Thus, BoNT‐A directly affects the efferent pathways of detrusor smooth muscle activity via inhibition of acetylcholine release and may inhibit afferent neurotransmitters and sensory pathways. Different BoNT‐A formulations are recommended as second‐line therapy 15 ; however, only onabotulinumtoxinA (onaBoNT‐A; Botox®, Allergan, Inc.) is approved for NDOI in the United States. 16 In a previous analysis, no difference in efficacy between MS and SCI etiologies were observed with onaBoNT‐A 200 or 300 U treatment of NDOI. 17 However, patients were not required to regularly perform CIC; the risks of new onset CIC, urinary retention and urinary tract infection (UTI) were found to be higher following onaBoNT‐A treatment in patients with MS compared with SCI; and the majority of the SCI group were also already performing CIC. 17 The clinical program presented here assessed abobotulinumtoxinA (aboBoNT‐A; Dysport®, Ipsen Ltd.) in patients with NDOI due to SCI or MS who regularly performed CIC (CONTENT1 [NCT02660138] and CONTENT2 [NCT02660359]). 18 Given the difference in patient populations between studies of onaBoNT‐A and aboBoNT‐A with respect to CIC status, a pooled analysis of data from the CONTENT studies was performed to evaluate whether etiology of NDOI had an impact on efficacy and safety of aboBoNT‐A, since it has already been shown that aboBoNT‐A 600 or 800 U was well tolerated, and improved bladder storage symptoms, quality of life (QoL), and urodynamic parameters in the overall population. 18
2. METHODS
2.1. Study designs
Full details of methodology have been published. 18 Briefly, CONTENT1 and CONTENT2 were phase III multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled studies in adults with SCI or MS and NDOI uncontrolled with oral medication and requiring regular CIC. CONTENT1 was conducted in Asia, Europe and North America, and CONTENT2 was conducted in the Americas, Asia, Europe and Oceania. Patients were randomized (2:2:1:1), stratified by etiology (SCI/MS) and prior intradetrusor BoNT‐A use (naïve/non‐naïve), to receive aboBoNT‐A 600 or 800 U or placebo in the double‐blind placebo‐controlled (DBPC) cycle and aboBoNT‐A 600 or 800 U on fulfillment of retreatment criteria (Figure S1). Patients could be reinjected ≥12 weeks after treatment if they achieved a <30% reduction in weekly NDOI episodes with no safety concerns.
2.2. Ethics approval
Both studies were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Consolidated Guideline on Good Clinical Practice, the FDA Guidance for Industry: Computerized Systems Used in Clinical Trials, and local regulatory guidelines. All patients provided written informed consent.
2.3. Patients
Patients aged 18–80 years with NDOI for ≥3 months before screening due to SCI (≤T1 level, occurring ≥6 months before screening) or clinically stable MS (per investigator's opinion with no exacerbations within ≥3 months before screening; Expanded Disability Status Scale assessment not performed) were enrolled. Eligible patients had an inadequate response after ≥4 weeks of oral medications to treat NDOI and routinely performed CIC for ≥4 weeks before screening. Patients receiving oral medications (e.g., anticholinergics) were to continue their regimen during the study. Exclusion criteria included previous or current tumor or malignancy affecting the spine or any other nonstable cause of SCI, medications that affect neuromuscular transmission, and unstable MS (which can affect urinary symptoms and therefore interfere with treatment effect measures). Full details of eligibility criteria have been published. 18
2.4. Assessments and outcomes
Patients recorded urinary events in a 7‐day eDiary in the week preceding study visits at Weeks 2, 6, and 12. An incontinence‐specific QoL questionnaire (22‐item Incontinence‐QoL [I‐QoL]; a 5‐point response scale) 19 was completed by patients at baseline, and Weeks 6 and 12. Urodynamic parameters (maximum cystometric capacity [MCC], maximum detrusor filling pressure [MDFP], occurrence of IDCs, volume at first IDC [V1stIDC], and detrusor compliance [DC]) were measured at baseline and Week 6.
The primary endpoint was change from baseline in number of weekly NDOI episodes at Week 6. Secondary endpoints included: proportion of patients achieving different thresholds of reduction in weekly NDOI episodes; change from baseline in I‐QoL total summary score and domain scores; proportion of patients with ≥11‐point improvement in I‐QoL total summary score; change from baseline in MCC, MDFP, V1stIDC, and DC; proportion of patients with no IDC during storage; and time to retreatment. Full details of assessments and outcomes for the pooled randomized population, have been published 18 ; outcomes according to etiology are reported here. Analyses by etiology were preplanned for the primary efficacy endpoint and for the secondary efficacy endpoints included in the hierarchical analysis.
Treatment‐emergent AEs (TEAEs) were assessed and classified as previously described. 18
2.5. Statistical analysis
Descriptive statistics are presented for data from all timepoints. A priori statistical testing was performed during the first 12 weeks of the DBPC cycle according to NDOI etiology (patients with SCI who received aboBoNT‐A 600 or 800 U vs. placebo; patients with MS who received aboBoNT‐A 600 or 800 U vs. placebo). Mixed‐model repeated measures (MMRM) analysis was conducted with change in weekly number of NDOI episodes from baseline as a response variable, and study (CONTENT1 or CONTENT2), treatment group, visit (Weeks 2, 6, and 12), treatment‐by‐visit interaction, treatment‐by‐visit‐by‐etiology group interaction, and stratification variables (etiology of NDO and prior intradetrusor BoNT‐A for NDOI) as fixed effect, with baseline value of weekly NDOI episodes as a covariate and patient as random effect.
A logistic generalized linear mixed model (GLMM) for repeated measures was fitted for NDOI responses using repeated measurements at Weeks 2, 6, and 12. The proportion of patients with no episodes of NDOI was analyzed as a response variable, the model included treatment group, stratification variables, visit, study, baseline value (weekly number of NDOI episodes), and baseline‐by‐visit, treatment‐by‐visit, and treatment‐by‐visit‐by‐etiology group interactions as fixed effect. The logistic GLMM was fitted using logit link and binomial distribution. Changes from baseline in QoL endpoints were analyzed with MMRM; I‐QoL total summary score was calculated as:
Change from baseline was calculated for urodynamic endpoints and was assessed with an analysis of covariance model. A logistic regression model was used for analysis of the proportion of patients with no IDCs at Week 6. Details of statistical analyses have been published. 18 No imputations were performed for missing data. Safety data were summarized descriptively.
3. RESULTS
3.1. Baseline characteristics
Overall, 485 patients were randomized between March 2016 and July 2019 (aboBoNT‐A 600 U: n = 162; aboBoNT‐A 800 U: n = 161; placebo: n = 162; Figure S2); NDOI was due to SCI and MS in 341 (70%) and 144 (30%) patients, respectively.
Baseline patient characteristics according to etiology are presented in Table 1. Overall, demographics were similar between etiology groups, except for: a higher proportion of males and of younger patients in the SCI group; a higher proportion of patients with MS had previously received BoNT‐A; mean number of NDOI episodes per week and mean DC were slightly higher in the MS group; and mean MDFP was slightly lower versus the SCI group. In patients with SCI, the most common neurological level was T12, trauma was the most common cause, and the majority were American Spinal Injury Association Impairment Scale (AIS) Grade A across treatment groups. The majority of patients with MS presented with relapsing remitting MS. Duration of NDOI symptoms was similar between all treatment groups for both etiologies.
Table 1.
Baseline patient and disease characteristics by NDOI etiology
| Parameter | SCI | MS | ||||
|---|---|---|---|---|---|---|
| Randomized population | Placebo (N = 113) | AboBoNT‐A 600 U (N = 114) | AboBoNT‐A 800 U (N = 114) | Placebo (N = 49) | AboBoNT‐A 600 U (N = 48) | AboBoNT‐A 800 U (N = 47) |
| Age, years | ||||||
| Median (IQR) | 41.0 (18.0) | 41.0 (20.0) | 38.0 (18.0) | 50.0 (18.0) | 45.0 (16.0) | 54.0 (20.0) |
| Age >65 years, n (%) | 6 (5) | 6 (5) | 11 (10) | 6 (12) | 2 (4) | 5 (11) |
| Male, n (%) | 76 (67) | 94 (83) | 88 (77) | 12 (25) | 12 (25) | 13 (28) |
| BoNT‐A non‐naïve, n (%) | 27 (24) | 31 (27) | 30 (26) | 16 (33) | 15 (31) | 20 (43) |
| Duration of NDOI symptoms, months | ||||||
| n | 113 | 114 | 114 | 47 | 47 | 46 |
| Median (IQR) | 76.0 (142.0) | 79.5 (118.0) | 60.5 (110.0) | 83.0 (105.0) | 94.0 (109.0) | 102.5 (142.0) |
| Anticholinergic use at baseline, n (%) | 0 | 1 (1) | 1 (1) | 0 | 0 | 0 |
| Daily CIC frequency at screening | ||||||
| n | 110 | 112 | 110 | 46 | 44 | 47 |
| Median (IQR) | 5.0 (1.6) | 5.3 (2.2) | 5.0 (2.0) | 4.2 (2.6) | 3.9 (2.3) | 4.6 (3.3) |
| Prior UTI within 6 months, n (%) | 15 (13) | 20 (18) | 26 (23) | 10 (20) | 8 (17) | 12 (26) |
| Duration of stable SCI injury or MS, months | ||||||
| Median (IQR) | 78.0 (164.0) | 80.5 (119.0) | 78.0 (136.0) | 206.0 (172.0) | 131.5 (173.5) | 208.0 (206.0) |
| Neurological level of SCI, n (%) | ||||||
| T1 | 6 (5) | 6 (5) | 1 (1) | |||
| T2 | 3 (3) | 4 (4) | 6 (5) | |||
| T3 | 8 (7) | 6 (5) | 5 (4) | |||
| T4 | 10 (9) | 10 (9) | 13 (11) | |||
| T5 | 3 (3) | 7 (6) | 8 (7) | |||
| T6 | 11 (10) | 12 (11) | 8 (7) | |||
| T7 | 9 (8) | 6 (5) | 2 (2) | |||
| T8 | 9 (8) | 9 (8) | 9 (8) | |||
| T9 | 9 (8) | 4 (4) | 5 (4) | |||
| T10 | 11 (10) | 2 (2) | 15 (13) | |||
| T11 | 12 (11) | 10 (9) | 10 (9) | |||
| T12 | 13 (12) | 17 (15) | 18 (16) | |||
| L1 | 3 (3) | 10 (9) | 2 (2) | |||
| L2 | 1 (1) | 4 (4) | 4 (4) | |||
| L3 | 0 | 1 (1) | 3 (3) | |||
| L4 | 1 (1) | 2 (2) | 2 (2) | |||
| L5 | 3 (3) | 2 (2) | 2 (2) | |||
| S1 | 1 (1) | 2 (2) | 0 | |||
| Missing | 0 | 0 | 1 (1) | |||
| Cause of SCI, n (%) | ||||||
| Traumatic | 101 (89) | 101 (89) | 99 (87) | |||
| Vascular/infarction | 3 (3) | 1 (1) | 4 (4) | |||
| Focal mechanical compression | 3 (3) | 3 (3) | 4 (4) | |||
| Other | 6 (5) | 9 (8) | 7 (6) | |||
| AIS grade, n (%) | ||||||
| A | 61 (54) | 69 (61) | 74 (65) | |||
| B | 14 (12) | 10 (9) | 11 (10) | |||
| C | 16 (14) | 14 (12) | 14 (12) | |||
| D | 17 (15) | 16 (14) | 11 (10) | |||
| E | 4 (4) | 2 (2) | 3 (3) | |||
| Missing | 1 (1) | 3 (3) | 1 (1) | |||
| Classification of MS, n (%) | ||||||
| Relapsing remitting | 25 (51) | 27 (56) | 25 (53) | |||
| Secondary progressive | 14 (29) | 17 (35) | 13 (28) | |||
| Primary progressive | 6 (12) | 3 (6) | 7 (15) | |||
| Relapsing progressive | 2 (4) | 1 (2) | 1 (2) | |||
| Other | 2 (4) | 0 | 1 (2) | |||
| NDOI episodes per week | ||||||
| n | 110 | 112 | 110 | 46 | 44 | 47 |
| Median (IQR) | 27.0 (17.0) | 26.0 (16.0) | 28.0 (19.0) | 34.5 (22.2) | 30.5 (32.1) | 30.3 (19.0) |
| I‐QoL Total summary score | ||||||
| n | 113 | 111 | 114 | 49 | 46 | 47 |
| Median (IQR) | 36.4 (29.5) | 33.0 (34.1) | 28.4 (30.7) | 39.8 (36.4) | 34.1 (30.7) | 37.5 (25.0) |
| Urodynamic population | n = 106 | n = 108 | n = 102 | n = 42 | n = 45 | n = 44 |
| MCC, mL | ||||||
| n | 106 | 108 | 102 | 41 | 44 | 43 |
| Median (IQR) | 187.0 (178.0) | 222.0 (185.5) | 252.5 (213.0) | 213.0 (156.0) | 190.5 (208.5) | 229.0 (223.0) |
| MDFP, cm H2O | ||||||
| n | 102 | 104 | 97 | 36 | 42 | 40 |
| Median (IQR) | 57.0 (47.0) | 53.5 (37.5) | 60.0 (54.0) | 38.0 (36.0) | 47.5 (31.0) | 46.5 (40.0) |
| V1stIDC, mL | ||||||
| n | 102 | 101 | 98 | 38 | 42 | 37 |
| Median (IQR) | 134.5 (153.0) | 147.0 (126.0) | 202.0 (207.0) | 138.5 (147.0) | 144.0 (156.0) | 130.0 (128.0) |
| DC, mL/cm H2O | ||||||
| n | 102 | 104 | 97 | 35 | 43 | 40 |
| Median (IQR) | 26.0 (34.0) | 21.0 (27.5) | 23.0 (29.0) | 31.0 (37.0) | 23.0 (25.0) | 24.5 (43.0) |
Note: Data shown are for the pooled randomized population.
Abbreviations: AboBoNT‐A, abobotulinumtoxinA; AIS, American Spinal Injury Association (ASIA) Impairment Scale; BoNT‐A, botulinum toxin type A; CIC, clean intermittent catheterization; DC, detrusor compliance; I‐QoL, incontinence quality of life; IQR, interquartile range; MCC, maximum cystometric capacity; MDFP, maximum detrusor filling pressure; MS, multiple sclerosis; N, number of patients in treatment group; n, number of patients in category; NDOI, neurogenic detrusor overactivity incontinence; SCI, spinal cord injury; U, units; UTI, urinary tract infection; V1stIDC, volume at first IDC.
3.2. Efficacy
Statistically significant reductions from baseline in number of weekly NDOI episodes at Weeks 2, 6, and 12 were observed with both aboBoNT‐A doses versus placebo in both etiologies (Figure 1A,B). For the primary endpoint analysis at Week 6, mean change from baseline in weekly number of NDOI episodes was −19.5, −20.9, and −11.1 (SCI group) and −23.4, −29.7, and −13.0 (MS group) for aboBoNT‐A 600 U, 800 U, and placebo, respectively.
Figure 1.
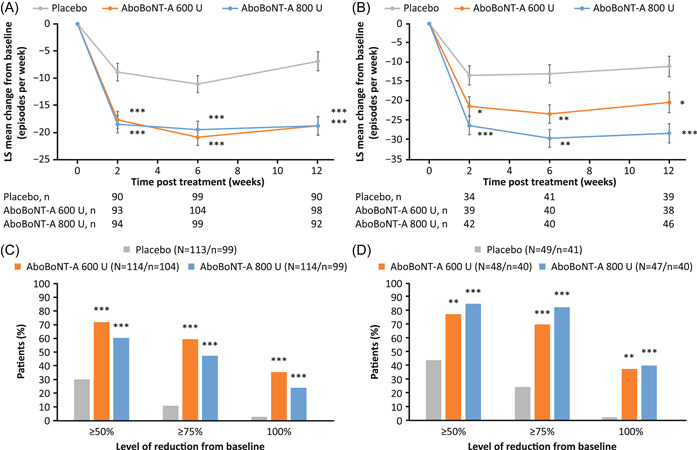
Change in weekly number NDOI episodesa in patients with (A) SCI and (B) MS, and proportion of patientsb with different thresholds of improvements from baseline at Week 6 with (C) SCI and (D) MS. *p < 0.05; **p < 0.01; ***p < 0.001. Data in (A) and (B) are LS mean ± SE. Data shown are for the randomized pooled population (DBPC period). aBased on the MMRM model to assess difference in change from baseline between each aboBoNT‐A dose group (600 or 800 U) versus placebo at each timepoint (Weeks 2, 6, and 12) with treatment group, visits (Weeks 2, 6, and 12), treatment by‐visit interaction, etiology of NDOI [SCI or MS], prior intradetrusor use [BoNT‐A‐naïve or BoNT‐A‐non‐naïve]), treatment‐by‐visit‐by‐etiology group interaction, study as fixed effect variables, study baseline value as covariate, and patient as a random effect. bThe outcomes are based on logistic GLMM model to assess difference between each aboBoNT‐A dose group (600 or 800 U) versus placebo with treatment group, stratification factors, visit (Weeks 2, 6, and 12), treatment‐by‐visit interaction, study baseline‐by‐visit interaction, treatment‐by‐visit‐etiology group interaction, study as fixed effect, and study baseline covariate and patient as random effect. Week 6 data only are presented. AboBoNT‐A, abobotulinumtoxinA; BoNT‐A, botulinum toxin type A; DBPC, double‐blind placebo‐controlled; LS, least square; GLMM, generalized linear mixed model; MMRM, mixed‐model repeated measures; MS, multiple sclerosis; N, number of patients in treatment group; n, number of patients with data; NDOI, neurogenic detrusor overactivity incontinence; SCI, spinal cord injury; SE, standard error; U, units
The proportions of patients achieving reductions from baseline in NDOI episodes of ≥50%, ≥75% or 100% by Week 6 were significantly higher for aboBoNT‐A 600 and 800 U groups versus placebo in both etiologies (Figure 1C,D). Of patients who received aboBoNT‐A 800 U, a greater proportion of patients with MS achieved each of these thresholds versus patients with SCI.
Statistically significant improvements from baseline in I‐QoL total summary scores at Week 6 were observed for both aboBoNT‐A doses versus placebo in the SCI and MS groups (all p < 0.001; Table 2; Figure 2). Overall, >60% of patients achieved ≥11‐point improvement in I‐QoL total summary scores at Week 6 (all p < 0.05 vs. placebo) regardless of etiology. A higher proportion of patients with MS than SCI reached this threshold. In addition, within the MS group, improvements from baseline of ≥11 points were greater with aboBoNT‐A 800 U compared with aboBoNT‐A 600 U.
Table 2.
Summary of efficacy and urodynamic outcomes at Week 6 by NDOI etiology
| Randomized population | SCI | MS | ||||
|---|---|---|---|---|---|---|
| Placebo | AboBoNT‐A | AboBoNT‐A | Placebo | AboBoNT‐A | AboBoNT‐A | |
| (N = 114) | 600 U (N = 114) | 800 U (N = 113) | (N = 49) | 600 U (N = 48) | 800 U (N = 47) | |
| Weekly NDO episodes, change from baseline a | ||||||
| LS mean [SE] (n) | −11.1 [1.6] (99) | −20.9 [1.5] (104) | −19.5 [1.6] (99) | −13.0 [2.3] (41) | −23.4 [2.4] (40) | −29.7 [2.3] (40) |
| Difference to placebo (95% CI) | −9.8 (−14.0, −5.6)*** | −8.4 (−12.6, −4.2)*** | −10.4 (−16.9, −3.9)* | −16.8 (−23.2, −10.3)*** | ||
| Weekly NDO episodes, threshold of improvement from baseline b | ||||||
| 50% reduction, n (%) | 30 (30) | 75 (72) | 60 (61) | 18 (44) | 31 (78) | 34 (85) |
| Odds ratio versus placebo (95% CI) | 5.8 (3.2, 10.7)*** | 3.8 (2.1, 6.9)*** | 4.6 (1.7, 12.3)** | 7.7 (2.6, 22.4)*** | ||
| ≥75% reduction, n (%) | 11 (11) | 62 (60) | 47 (48) | 10 (24) | 28 (70) | 33 (83) |
| Odds ratio versus placebo (95% CI) | 10.4 (5.1, 21.3)*** | 7.2 (3.5, 14.8)*** | 6.8 (2.6, 17.9)*** | 14.8 (5.0, 43.5)*** | ||
| 100% reduction in UI, n (%) | 3 (3) | 37 (36) | 24 (24) | 1 (2) | 15 (38) | 16 (40) |
| Odds ratio versus placebo (95% CI) | 18.5 (5.5, 61.7)*** | 12.4 (3.7, 42.3)*** | 20.0 (3.1, 131.0)** | 25.5 (3.9, 165.4)*** | ||
| I‐QoL total summary score, change from baseline a | ||||||
| LS mean [SE] (n) | 4.7 [2.1] (101) | 19.9 [2.0] (105) | 18.2 [2.0] (106) | 8.8 [3.0] (48) | 23.2 [2.1] (42) | 27.7 [3.0] (44) |
| Difference to placebo (95% CI) | 15.2 (9.65, 20.67)*** | 13.5 (7.9, 19.0)*** | 14.4 (6.0, 22.8)*** | 18.9 (10.7, 27.2)*** | ||
| I‐QoL domain scores, change from baseline a | ||||||
| Avoidance and limiting behavior | ||||||
| LS mean [SE] (n) | 4.5 [2.1] (101) | 19.7 [2.0] (105) | 17.6 [2.0] (106) | 8.7 [3.0] (48) | 22.6 [3.1] (42) | 30.7 [3.1] (44) |
| Difference to placebo (95% CI) | 15.2 (9.7, 20.8)*** | 13.1 (7.6, 18.7)*** | 13.9 (5.4, 22.3)** | 22.0 (13.7, 30.3)*** | ||
| Psychological impact | ||||||
| LS mean [SE] (n) | 5.1 [2.2] (101) | 19.8 [2.2] (105) | 18.2 [2.2] (106) | 10.1 [3.2] (48) | 21.8 [3.3] (42) | 24.0 [3.2] (44) |
| Difference to placebo (95% CI) | 14.8 (8.9, 20.7)*** | 13.1 (7.2, 19.0)*** | 11.7 (2.7, 20.6)* | 13.9 (5.0, 22.7)** | ||
| Social embarrassment | ||||||
| LS mean [SE] (n) | 4.8 [2.4] (101) | 20.4 [2.3] (105) | 18.5 [2.3] (106) | 7.1 [3.4] (48) | 26.2 [3.6] (42) | 29.7 [3.5] (44) |
| Difference to placebo (95% CI) | 15.7 (9.3, 22.0)*** | 13.7 (7.4, 20.1)*** | 19.1 (9.5, 28.8)*** | 22.7 (13.1, 32.2)*** | ||
| I‐QoL total summary score, ≥11‐point increase from baseline b | ||||||
| n (%) | 28 (28) | 67 (64) | 61 (58) | 19 (40) | 28 (67) | 33 (75) |
| Odds ratio versus placebo (95% CI) | 4.4 (2.4, 8.0)*** | 3.1 (1.7, 5.6)*** | 2.8 (1.2, 6.8)* | 4.5 (1.8, 11.2)** | ||
| Time to retreatment, weeks | ||||||
| Median (95% CI) | 19.3 (16.7, 24.1) | 43.7 (36.3, 53.0) | 39.4 (29.1, 43.7) | 24.4 (19.0, 36.9) | 61.9 (26.0, NE) | 48.3 (34.0, 62.7) |
| Urodynamic population | n = 106 | n = 108 | n = 102 | n = 42 | n = 45 | n = 44 |
| MCC (mL), change from baseline c | ||||||
| LS mean [SE] (n) | −8.0 [16.2] (89) | 160.8 [15.5] (98) | 174.9 [16.0] (92) | 1.5 [24.2] (39) | 169.7 [24.4] (38) | 174.4 [23.5] (41) |
| Difference to placebo (95% CI) | 168.8 (125.8, 211.8)*** | 182.8 (139.1, 226.6)*** | 168.3 (101.0, 235.5)*** | 173.0 (107.3, 238.6)*** | ||
| MDFP (cm H 2 O), change from baseline c | ||||||
| LS mean [SE] (n) | −0.9 [2.6] (81) | −30.5 [2.5] (92) | −33.3 [2.5] (86) | −10.9 [4.2] (31) | −35.1 [4.0] (33) | −36.4 [3.9] (36) |
| Difference to placebo (95% CI) | −29.6 (−36.5, −22.8)*** | −32.4 (−39.3, −25.4)*** | −24.2 (−35.5, −12.9)*** | −25.5 (−36.6, −14.5)*** | ||
| V1stIDC (mL), change from baseline c | ||||||
| LS mean [SE] (n) | −9.5 [16.8] (87) | 160.5 [16.4] (92) | 173.7 [16.8] (89) | 44.0 [25.8] (36) | 158.3 [25.6] (36) | 212.8 [26.0] (35) |
| Difference to placebo (95% CI) | 170.0 (125.0, 215.0)*** | 183.1 (137.4, 228.9)*** | 114.4 (43.4, 185.4)** | 168.8 (97.3, 240.4)*** | ||
| DC (mL/cm H 2 O), change from baseline c | ||||||
| LS mean [SE] (n) | −6.4 [7.8] (83) | 18.7 [7.5] (92) | 6.6 [7.7] (86) | 4.5 [12.7] (31) | 33.7 [12.0] (34) | 61.1 [11.6] (37) |
| Difference to placebo (95% CI) | 25.1 (4.4, 45.8)* | 13.0 (−8.1, 34.1) | 29.2 (−5.0, 63.4) | 56.6 (23.3, 89.9)*** | ||
| No IDC during storage d | ||||||
| n (%) | 2 (2) | 41 (42) | 45 (50) | 6 (17) | 18 (50) | 26 (67) |
| Odds ratio versus placebo (95% CI) | 30.6 (7.1, 131.6)*** | 42.9 (9.9, 185.0)*** | 4.8 (1.6, 14.5)** | 10.1 (3.3, 30.7)*** | ||
Note: *p < 0.05; **p < 0.01; ***p < 0.001 vs. placebo.
Abbreviations: AboBoNT‐A, abobotulinumtoxinA; BoNT‐A, botulinum toxin type A; cm H2O, centimeters of water; CI, confidence interval; DBPC, double‐blind placebo‐controlled; DC, detrusor compliance; IDC, involuntary detrusor contraction; GLMM, generalized linear mixed model; I‐QoL, 22‐item incontinence‐related quality of life; LS, least squares; MCC, maximum cystometric capacity; MDFP, maximum detrusor filling pressure; MMRM, mixed model repeated measures; MS, multiple sclerosis; N, number of patients in treatment group; n, number of patients in category; NDO, neurogenic detrusor overactivity; NDOI, neurogenic detrusor overactivity incontinence; NE, not estimated; SCI, spinal cord injury; SE, standard error; U, units; V1stIDC, volume at first IDC.
Based on MMRM with treatment group, visits (Weeks 2, 6, and 12 for weekly NDOI episodes; Weeks 6 and 12 for I‐QoL), treatment by‐visit interaction, treatment‐by‐visit‐by‐etiology group interaction, stratification factors (etiology of NDOI [SCI or MS], prior intradetrusor use [BoNT‐A naïve or non‐naïve]) and study as fixed effects, study baseline value as covariate, and patient as a random effect. Only the results for each aboBoNT‐A dose versus placebo by NDO etiology at Week 6 are presented.
Based on logistic GLMM with treatment group, stratification factors (etiology of NDOI [SCI or MS], prior intradetrusor use [BoNT‐A naïve or non‐naïve]), visit (Weeks 6 and 12 only), treatment‐by‐visit interaction, treatment‐by‐visit‐by‐etiology group interaction, study baseline‐by‐visit interaction, study baseline value and study as fixed effect. The denominator is the number of patients with available data as indicated under “Weekly NDO episodes, change from baseline” or “I‐QoL total summary score, change from baseline,” as relevant. Only the results for each aboBoNT‐A dose versus placebo by NDO etiology at Week 6 are presented.
Based on analysis of covariance model used with treatment group, stratification factors (etiology of NDOI [SCI or MS], prior intradetrusor use[BoNT‐A naïve or non‐naïve]), study baseline value, study and the treatment‐by‐etiology group interaction as fixed effect variables.
Based on logistic regression model with treatment group, stratification factors (etiology of NDOI [SCI or MS], prior intradetrusor use [BoNT‐A naïve or non‐naïve]), study, and treatment‐by‐etiology group interaction as fixed variables. Denominator values for the placebo, aboBoNT‐A 600 and 800 U groups, respectively, were n = 87, 98, 90 for patients with SCI and n = 35, 36, and 39 for patients with MS.
Figure 2.
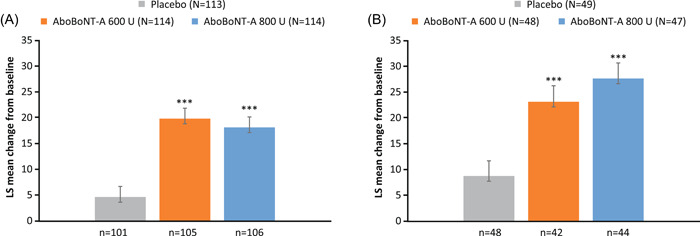
Change from baseline to Week 6 in I‐QoL total summary scorea in patients with (A) SCI and (B) MS. ***p < 0.001 vs. placebo. Data are LS mean ± SE. Data shown are for the randomized population. aBased on the MMRM model to assess difference in change from baseline between each aboBoNT‐A dose group (600 or 800 U) versus placebo with treatment group, visits (Weeks 6 and 12), treatment by‐visit interaction, stratification factors (etiology of NDOI [SCI or MS], prior intradetrusor use [BoNT‐A‐naïve or BoNT‐A‐non‐naïve]), treatment‐by‐visit‐by‐etiology group interaction, study as fixed effect variables, study baseline value as covariates, and patient as a random effect. The statistical model includes Weeks 6 and 12; only Week 6 results are presented. AboBoNT‐A, abobotulinumtoxinA; BoNT‐A, botulinum toxin type A; I‐QoL, incontinence quality of life; LS, least squares; MMRM, mixed‐model repeated measures; MS, multiple sclerosis; N, number of patients in treatment group; n, number of patients with data; NDOI, neurogenic detrusor overactivity incontinence; SCI, spinal cord injury; SE, standard error; U, units
Significant improvements from baseline in all I‐QoL domain scores were observed for both etiologies with either aboBoNT‐A dose versus placebo at Week 6 (all p < 0.05; Table 2).
There were statistically significant improvements from baseline in all urodynamic parameters at Week 6 with both doses of aboBoNT‐A versus placebo regardless of etiology, except for DC with aboBoNT‐A 800 U in the SCI group and with aboBoNT‐A 600 U in the MS group. Change from baseline in MCC and MDFP was greater for both etiology groups versus placebo; magnitude of improvements were similar (Table 2; Figure 3A–D). The proportion of patients with no IDCs during storage with aboBoNT‐A 600 U, 800 U, and placebo, respectively, was 42%, 50%, and 2% in the SCI group and 50%, 67%, and 17% in the MS group (Figure 3E,F). Changes from baseline in DC were significantly greater with aboBoNT‐A 800 U versus placebo in the MS group (Table 2).
Figure 3.
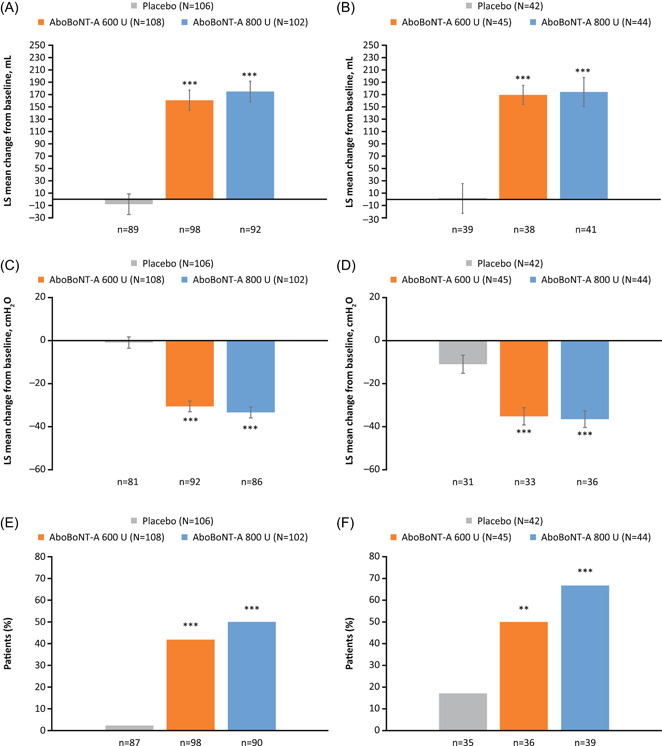
Change from baseline in MCC and MDFP,a and proportion of patients with no IDCb at Week 6 in patients with SCI (A, C, E) and MS (B, D, F). **p < 0.01; ***p < 0.001 vs. placebo. Data for (A)–(D) are LS mean ± SE. Data shown are for urodynamic pooled population (DBPC period). aBased on an analysis of covariance model to assess difference in change from baseline between each aboBoNT‐A dose group (600 or 800 U) versus placebo at Week 6, with treatment group, stratification factors (etiology of NDOI [SCI or MS], prior intradetrusor use [BoNT‐A naïve or non‐naïve]), study baseline value, study and the treatment‐by‐etiology group interaction as fixed effect variables; bBased on logistic regression model to assess difference between each aboBoNT‐A dose group (600 or 800 U) versus placebo at Week 6, with treatment group, stratification factors (etiology of NDOI [SCI or MS], prior intradetrusor use [BoNT‐A naïve or non‐naïve]), study, and treatment‐by‐etiology group interaction as fixed variables. AboBoNT‐A, abobotulinumtoxinA; BoNT‐A, botulinum toxin type A; DBPC, double‐blind placebo‐controlled; IDC, involuntary detrusor contraction; LS, least squares; MCC, maximum cystometric capacity; MDFP, maximum detrusor filling pressure; MS, multiple sclerosis; N, number of patients in treatment group; n, number of evaluable patients; NDOI, neurogenic detrusor overactivity incontinence; SCI; spinal cord injury; SE, standard error; U, units
Median time to retreatment was over 39 weeks in all aboBoNT‐A groups, for both etiologies, versus 19–24 weeks with placebo (Table 2). Regardless of aboBoNT‐A dose, over 35% and 52% of patients with SCI and MS, respectively, did not receive retreatment until 48 weeks after their initial aboBoNT‐A treatment.
3.3. Safety
Proportions of patients with TEAEs were similar across etiologies, and no clear difference in the TEAE profile was observed between treatment groups (Table 3). With both SCI and MS etiologies, the most frequent TEAE across treatment groups (including placebo), was symptomatic UTI, consistent with the rates of prior UTI at baseline. Serious UTIs (defined as any UTI that results in death, is life‐threatening, results in or prolongs hospitalization, results in a persistent or significant disability, congenital anomaly, or any other important medical event that may jeopardize the patient) were reported in the first 12 weeks in the SCI group, by three, one and two patients, respectively, with aboBoNT‐A 600 U, 800 U, and placebo, and by one, two and one patients, respectively, in the MS group. The remaining two TEAEs occurring in ≥5% of patients during the DBPC treatment cycle were hematuria in patients with SCI and MS, and bacteriuria in patients with MS (Table 3).
Table 3.
Summary of TEAEs up to Week 12 and over the full DBPC treatment cycle reported in ≥5% of patients in any treatment group by etiology
| SCI | MS | |||||
|---|---|---|---|---|---|---|
| TEAE | Placebo | AboBoNT‐A 600 U | AboBoNT‐A 800U | Placebo | AboBoNT‐A 600 U | AboBoNT‐A 800 U |
| (N = 112) | (N = 113) | (N = 114) | (N = 49) | (N = 47) | (N = 48) | |
| During first 12 weeks of DBPC cycle, n (%) | ||||||
| Any TEAE | 45 (40) | 54 (48) | 51 (45) | 21 (43) | 20 (43) | 17 (35) |
| Any serious TEAE | 4 (4) | 6 (5) | 5 (4) | 0 | 3 (6) | 1 (2) |
| TEAEs with incidence ≥5% in any group | ||||||
| UTIa | 17 (15) | 15 (13) | 18 (16) | 10 (20) | 8 (17) | 6 (13) |
| Hematuria | 4 (4) | 6 (5) | 3 (3) | 1 (2) | 1 (2) | 3 (6) |
| During full DBPC cycle, n (%) | ||||||
| Any TEAE | 53 (47) | 62 (55) | 62 (54) | 25 (51) | 26 (55) | 26 (54) |
| Any serious TEAE | 7 (6) | 11 (10) | 9 (8) | 1 (2) | 7 (15) | 5 (10) |
| TEAEs with incidence ≥5% in any group | ||||||
| UTIa | 21 (19) | 21 (19) | 30 (26) | 11 (22) | 12 (26) | 14 (29) |
| Bacteriuria | 0 | 3 (3) | 0 | 0 | 3 (6) | 1 (2) |
| Hematuria | 4 (4) | 8 (7) | 3 (3) | 1 (2) | 1 (2) | 3 (6) |
| Prior UTIb | 15 (13) | 20 (18) | 26 (23) | 10 (20) | 8 (17) | 12 (26) |
Note: Data shown are for the pooled safety population (DBPC period).
Abbreviations: AboBoNT‐A, abobotulinumtoxinA; DBPC, double‐blind placebo‐controlled; NDOI, neurogenic detrusor overactivity incontinence; N, number of patients in treatment group; n, number of patients with event; TEAE, treatment‐emergent adverse event; U, units; UTI, urinary tract infection.
UTI was defined as a positive urine culture result with a bacteriuria count of >105 colony forming unit/mL, leukocyturia of >5/high power field and symptoms suggestive of a UTI (may be atypical symptoms in NDOI population). If a patient experienced more than one event in a category, the patient is counted only once in that category.
Within 6 months before screening.
4. DISCUSSION
In this pooled analysis of the CONTENT studies, patients with NDOI who were regularly performing CIC experienced significantly improved bladder symptoms, urodynamic parameters, and I‐QoL with aboBoNT‐A treatment versus placebo, in both SCI and MS etiologies.
Overall, the majority of patients who participated in the CONTENT studies had SCI. Other than demographic differences related to the etiology, there were limited differences in baseline disease characteristics between etiologies, perhaps due to the requirement that all enrolled patients performed CIC routinely for ≥4 weeks, resulting in a more homogeneous study population. However, as may be expected in view of the duration and extent of neurological impairment in the SCI group, a higher MDFP and lower DC was observed relative to patients with MS. A slightly higher number of NDOI episodes at baseline were recorded in the MS group, which could be due to these patients having a greater ability to sense the occurrence of NDOI episodes than patients with SCI.
Efficacy of aboBoNT‐A 600 and 800 U treatment on reduction in NDOI episodes was demonstrated in both SCI and MS (with a potential dose–response effect observed in the latter); magnitudes of improvement were comparable to a previous pooled analysis of phase III studies investigating the efficacy of onaBoNT‐A in patients with NDOI according to SCI or MS. 17
Median time‐to‐retreatment appeared to be longer in patients with MS versus SCI, and a potential dose–response effect was observed. This could be due to greater sensitivity in patients with MS to detect differences in the level of UI between catheterizations and pad or condom changes, which may also have led to later reporting of a reduction in UI episodes of at least 30% (the threshold for retreatment) versus the SCI group.
Although this study did not formally compare the difference in response to aboBoNT‐A treatment between SCI and MS groups, aboBoNT‐A appeared to be more efficacious at 800 U for reducing UI episodes in patients with MS, which was not observed in the SCI group. A greater placebo effect was observed in the MS group compared with the SCI group across efficacy parameters, consistent with previous studies. 17 , 20 , 21 These findings are theorized to be due to patients with MS retaining a greater degree of bladder sensitivity than patients with SCI. Indeed, the higher center descending pathways that are responsible for the inhibition of bladder contractions may be more intact in an MS population than an SCI population. The majority of patients with SCI (60%) in the present study were AIS Grade A (no motor or sensory function below the level of injury).
Improvements in symptoms with aboBoNT‐A in the present study were reflected in urodynamic parameters, and thus bladder function, in a similar manner for the SCI and MS groups, consistent with results of onaBoNT‐A treatment in patients with NDOI, with improvements observed to similar extents. 17 This trend was confirmed across urodynamic endpoints with onaBoNT‐A, consistent with the pooled analysis of previous aboBoNT‐A phase III studies. 18 However, the majority of the MS group were not performing CIC before treatment in onaBoNT‐A studies, so significant increases in post‐void residual urine, urinary retention, and instigation of CIC were observed, all of which were associated with an increased incidence of UTIs. 17 In the present analysis, the safety profile of aboBoNT‐A was similar for patients with SCI and MS, all of whom were regularly performing CIC, which will have avoided the risk of elevated post‐void residual urine volume and urinary retention AEs.
5. LIMITATIONS
Although significance differences were observed between aboBoNT‐A groups and placebo for each etiology, the sample size was not powered for etiology subgroup analyses, and no imputations to account for missing data were performed, which may limit the conclusions of this analysis. 22 Furthermore, the number of patients enrolled with MS was small compared to those with SCI. In addition, findings are specific to patients with NDOI due to SCI and MS who perform regular CIC.
6. CONCLUSION
Pooled analysis of the phase III CONTENT1/CONTENT2 studies confirmed that aboBoNT‐A treatment improves symptoms of NDOI in patients with SCI or MS who have an inadequate response to oral therapy and regularly perform CIC. A reduced number of NDOI episodes, and improved bladder storage capacity and filling pressure, and I‐QoL were observed. AboBoNT‐A was well‐tolerated with no apparent adverse impact on safety, including risk of UTIs.
AUTHOR CONTRIBUTIONS
Pierre Denys: conception and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; supervision. Juan Carlos Castaño Botero: acquisition of data; drafting of the manuscript. Ricardo Luis Vita Nunes: acquisition of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. Barton Wachs, Cristiano Mendes Gomes, Grigory Krivoborodov, Le Mai Tu, Giulio Del‐Popolo: acquisition of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. Catherine Thompson: conception and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. Claire Vilain: analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. Magali Volteau: conception and design; statistical analysis of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. Michael Kennelly: conception and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; supervision; acquisition of data.
CONFLICTS OF INTEREST
Pierre Denys: Consultancy: Allergan, Taris, Medtronic, Coloplast. Grant research study: Ipsen and Allergan. Juan Carlos Castaño Botero: Astellas (consultant and lecturer fees), Boston Scientific (consultant and lecturer fees) Medtronic (consultant fees, trial investigator), Ipsen (trial investigator). Ricardo Luis Vita Nunes: Grant/Research study: Ipsen; Consultancy: Astellas Pharma, Zambon; Lectures: Astellas, Zambon, Zodiac, Aché, Coloplast. Barton Wachs: Nothing to disclose. Cristiano Mendes Gomes: Grant/Research study: Ipsen; Consultancy: Astellas Pharma, Boston Scientific; Lectures: Astellas, Boston Scientific, Zodiac. Grigory Krivoborodov: Lectures: Astellas, Coloplast, Pierre Fabre, Braun, Medtronic. Le Mai Tu: Consultancy and Lectures: Astellas Pharma, Pfizer. Giulio Del‐Popolo: Grant/Research study: Ipsen, Wellspect, B Braun, Hollister; Consultant: Coloplast, Wellspect, Pierre Fabre, Braun. Catherine Thompson: Employee of June Pharma under contract by Ipsen. Claire Vilain and Magali Volteau: Employee of Ipsen. Michael Kennelly: Research Grants: Allergan, Amphora, Axonics, Boston Scientific, Coloplast, Cook Myosite, Dignify Therapeutics, Ipsen, Taris, Uro1, FemPulse, EBT Medical; Consultancy Fees: Allergan, Boston Scientific, Coloplast, Laborie, Urovant.
Supporting information
Supplementary information.
Supplementary information.
Supplementary information.
Supplementary information.
ACKNOWLEDGMENTS
The authors thank all patients involved in the study, as well as their caregivers, care team, investigators, and research staff in participating institutions. This study was sponsored by Ipsen.
The authors thank Shaun Hall, MSc, and Nicola Winstone, DPhil, of Ashfield MedComms, an Ashfield Health company, and Kirsteen Munn, contracted to Ashfield MedComms, for providing medical writing support, which was sponsored by Ipsen, Slough, UK, in accordance with Good Publication Practice guidelines.
Denys P, Castaño Botero JC, Vita Nunes RL, et al. AbobotulinumtoxinA is Effective in Patients with Urinary Incontinence Due to Neurogenic Detrusor Overactivity Regardless of Spinal Cord Injury or Multiple Sclerosis Etiology: Pooled Analysis of Two Phase III Randomized Studies (CONTENT1 and CONTENT2). Neurourol Urodyn. 2023;42:153‐167. 10.1002/nau.25062
A full list of study investigators can be found in the Supporting Information Materials.
CLINICAL TRIAL REGISTRATION: The data are based on the pooled analyses of the CONTENT1 [(NCT02660138]) and CONTENT2 [(NCT02660359]) clinical trials.
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to patient‐level study data that underlie the results reported in this publication. Additional relevant study documents, including the clinical study report, study protocol with any amendments, annotated case report form, statistical analysis plan and dataset specifications may also be made available. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of study participants.
Where applicable, data from eligible studies are available 6 months after the studied medicine and indication have been approved in the US and EU or after the primary manuscript describing the results has been accepted for publication, whichever is later.
Further details on Ipsen's sharing criteria, eligible studies and process for sharing are available here (https://vivli.org/members/ourmembers/).
Any requests should be submitted to www.vivli.org for assessment by an independent scientific review board.
REFERENCES
- 1. Gajewski JB, Schurch B, Hamid R, et al. An International Continence Society (ICS) report on the terminology for adult neurogenic lower urinary tract dysfunction (ANLUTD). Neurourol Urodyn. 2018;37(3):1152‐1161. [DOI] [PubMed] [Google Scholar]
- 2. Haab F. Chapter 1: the conditions of neurogenic detrusor overactivity and overactive bladder. Neurourol Urodyn. 2014;33(Suppl 3):S2‐S5. [DOI] [PubMed] [Google Scholar]
- 3. Biardeau X, Corcos J. Intermittent catheterization in neurologic patients: update on genitourinary tract infection and urethral trauma. Ann Phys Rehabil Med. 2016;59(2):125‐129. [DOI] [PubMed] [Google Scholar]
- 4. Stöhrer M, Blok B, Castro‐Diaz D, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol. 2009;56(1):81‐88. [DOI] [PubMed] [Google Scholar]
- 5. Taweel WA, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Betts CD, D'Mellow MT, Fowler CJ. Urinary symptoms and the neurological features of bladder dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993;56(3):245‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Del Popolo G, Filocamo MT, Li Marzi V, et al. Neurogenic detrusor overactivity treated with English botulinum toxin A: 8‐year experience of one single centre. Eur Urol. 2008;53(5):1013‐1019. [DOI] [PubMed] [Google Scholar]
- 8. Ruffion A, Capelle O, Paparel P, Leriche B, Leriche A, Grise P. What is the optimum dose of type A botulinum toxin for treating neurogenic bladder overactivity? BJU Int. 2006;97(5):1030‐1034. [DOI] [PubMed] [Google Scholar]
- 9. Ehren I, Volz D, Farrelly E, et al. Efficacy and impact of botulinum toxin A on quality of life in patients with neurogenic detrusor overactivity: a randomised, placebo‐controlled, double‐blind study. Scand J Urol Nephrol. 2007;41(4):335‐340. [DOI] [PubMed] [Google Scholar]
- 10. Grosse J, Kramer G, Stöhrer M. Success of repeat detrusor injections of botulinum A toxin in patients with severe neurogenic detrusor overactivity and incontinence. Eur Urol. 2005;47(5):653‐659. [DOI] [PubMed] [Google Scholar]
- 11. Grosse J, Kramer G, Jakse G. Comparing two types of botulinum‐A toxin detrusor injections in patients with severe neurogenic detrusor overactivity: a case‐control study. BJU Int. 2009;104(5):651‐656. [DOI] [PubMed] [Google Scholar]
- 12. Grise P, Ruffion A, Denys P, Egon G, Chartier Kastler E. Efficacy and tolerability of botulinum toxin type A in patients with neurogenic detrusor overactivity and without concomitant anticholinergic therapy: comparison of two doses. Eur Urol. 2010;58(5):759‐766. [DOI] [PubMed] [Google Scholar]
- 13. Ozcakir S, Sivrioglu K. Botulinum toxin in poststroke spasticity. Clin Med Res. 2007;5(2):132‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53(1):3‐9. [DOI] [PubMed] [Google Scholar]
- 15. Blok B, Castro‐Diaz D, Del Popolo G, Groen J, Hamid R, Karsenty G, Kessler TM, Pannek J. EAU Guidelines. Edn. presented at the EAU Annual Congress Amsterdam. 2020.
- 16.Allergan, Inc. Highlights of prescribing information: Botox®. 2017.
- 17. Ginsberg D, Cruz F, Herschorn S, et al. OnabotulinumtoxinA is effective in patients with urinary incontinence due to neurogenic detrusor overactivity [corrected] regardless of concomitant anticholinergic use or neurologic etiology. Adv Ther. 2013;30(9):819‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kennelly M, Cruz F, Herschorn S, et al. Efficacy and safety of abobotulinumtoxinA in patients with neurogenic detrusor overactivity incontinence performing regular clean intermittent catheterization: pooled results from two phase 3 randomized studies (CONTENT1 and CONTENT2). Eur Urol. 2022;82(2):223‐232. [DOI] [PubMed] [Google Scholar]
- 19. Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DE. Quality of life of persons with urinary incontinence: development of a new measure. Urology. 1996;47(1):67‐71. [DOI] [PubMed] [Google Scholar]
- 20. Ginsberg D, Gousse A, Keppenne V, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012;187(6):2131‐2139. [DOI] [PubMed] [Google Scholar]
- 21. Cruz F, Herschorn S, Aliotta P, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double‐blind, placebo‐controlled trial. Eur Urol. 2011;60(4):742‐750. [DOI] [PubMed] [Google Scholar]
- 22. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Supplementary information.
Supplementary information.
Supplementary information.
Data Availability Statement
Qualified researchers may request access to patient‐level study data that underlie the results reported in this publication. Additional relevant study documents, including the clinical study report, study protocol with any amendments, annotated case report form, statistical analysis plan and dataset specifications may also be made available. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of study participants.
Where applicable, data from eligible studies are available 6 months after the studied medicine and indication have been approved in the US and EU or after the primary manuscript describing the results has been accepted for publication, whichever is later.
Further details on Ipsen's sharing criteria, eligible studies and process for sharing are available here (https://vivli.org/members/ourmembers/).
Any requests should be submitted to www.vivli.org for assessment by an independent scientific review board.


