Abstract
Background
Atopic dermatitis (AD) is a prevalent inflammatory, pruritic skin disease. The Janus kinase (JAK) pathway is a treatment target.
Objectives
To assess the efficacy, safety and pharmacokinetics of topical cream brepocitinib, a small‐molecule tyrosine kinase 2 (TYK2)/JAK1 inhibitor, in participants with mild‐to‐moderate AD.
Methods
In this phase IIb, double‐blind, dose‐ranging study, participants were randomized to receive one of eight treatments for 6 weeks: brepocitinib 0·1% once daily (QD), 0·3% QD or twice daily (BID), 1·0% QD or BID, 3·0% QD, or vehicle QD or BID. The primary endpoint was the percentage change from baseline in the Eczema Area and Severity Index (EASI) total score at week 6. Adverse events (AEs) were monitored.
Results
Overall, 292 participants were enrolled and randomized. The brepocitinib 1% QD and 1% BID groups achieved statistically significantly greater (with multiplicity‐adjusted P < 0·05 due to Hochberg’s step‐up method) percentage reductions from baseline in EASI total score at week 6 [least squares mean (90% confidence interval, CI): QD: –70·1 (–82·1 to –58·0); BID: –75·0 (–83·8 to –66·2)] compared with respective vehicle [QD: –44·4 (–57·3 to –31·6); BID: –47·6 (–57·5 to –37·7)]. There was not a dose‐dependent trend in AE frequency, and there were no serious AEs or deaths.
Conclusions
Topical brepocitinib is effective and well tolerated in participants with mild‐to‐moderate AD.
What is already known about this topic?
Janus kinase (JAK) inhibitors are in development for treatment of atopic dermatitis (AD).
The tyrosine kinase 2 and JAK 1 inhibition by brepocitinib may bring a new profile for topical JAK inhibitors for treatment of mild‐to‐moderate AD.
What does this study add?
Topical brepocitinib can provide rapid, effective symptom reduction, and could offer a novel alternative to current topical treatments for mild‐to‐moderate AD.
Brepocitinib, a TYK2/JAK1 inhibitor, is being investigated as a topical cream treatment for atopic dermatitis (AD). In a phase IIb, randomised, double‐blind study, brepocitinib was effective and well tolerated in participants with mild‐to‐moderate AD, although further study with a larger population and longer treatment duration is required.

Linked Comment: S.J. Lax and A.M. Drucker. Br J Dermatol 2022; 187:838.
Plain language summary available online
Atopic dermatitis (AD) is an inflammatory, pruritic skin disease that affects up to 10% of adults and 20% of children in developed countries. 1 , 2 , 3 AD negatively impacts the health‐related quality of life (HRQoL) of patients and their caregivers/families, with greater disease severity being associated with poorer HRQoL. 4 , 5 Due to the complex pathophysiology of AD, guidelines recommend regimented hygiene, the use of moisturizers, topical steroids and calcineurin inhibitors, which can be used in combination with other treatments such as phototherapy for moderate‐to‐severe or refractory AD. 6 The ultimate goals for treatment are to repair and maintain the skin barrier, 7 , 8 to reduce sensations of itching and burning, 7 and to prevent AD relapse. 8 , 9 Despite current treatments, there is still an unmet need for safe and effective therapies to improve disease management and long‐term patient outcomes. 10
Modulating inflammatory pathways has been successful in the treatment of inflammatory skin diseases. 11 , 12 , 13 , 14 , 15 For example, topical crisaborole, a phosphodiesterase‐4 inhibitor, is approved in the USA for treatment of mild‐to‐moderate AD. 16 However, a host of cytokines have been implicated in the pathophysiology of AD, 17 , 18 , 19 many of which require activation of the Janus kinase (JAK) molecular pathway. 20 The JAK family of kinases includes JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2). 21 Inhibition of the JAK family of kinases has been shown to improve signs and symptoms of AD. 22 , 23 , 24 , 25 , 26 Clinical trials for the oral JAK inhibitors abrocitinib, baricitinib and upadacitinib have had positive results for patients with moderate‐to‐severe AD. 27 , 28 , 29 Abrocitinib and upadacitinib (JAK1 inhibitors) have recently been approved in the USA to treat moderate‐to‐severe AD. 30 , 31 Similarly, topical administration of JAK inhibitors (JAKi) has shown promise in clinical trials for the treatment of mild‐to‐moderate and moderate‐to‐severe AD. 24 , 25 , 32 , 33 In fact, topical delgocitinib, a pan‐JAKi, was approved in Japan for adults and children with AD, 34 and ruxolitinib cream, a JAK1/2i, was US Food and Drug Administration (FDA)‐approved to treat mild‐to‐moderate AD in adults and children aged ≥ 12 years. 35 Efficacy of these medications in AD indicates a role for JAK1 in AD pathogenesis, in part through interleukin (IL)‐4 and IL‐13 signalling. 36 , 37 Still, alternative topical, nonsteroidal treatments for patients with mild‐to‐moderate AD are needed to reduce systemic exposure to therapeutic agents and to provide a new mechanism of action suitable for long‐term management. 10
Brepocitinib is a small‐molecule TYK2/JAK1 inhibitor 38 that has shown promising results in dermatological indications, including alopecia areata and plaque psoriasis, when administered in an oral formulation. 39 , 40 The combination of TYK2 and JAK1 inhibitory activity within a therapeutic molecule brings the potential for inhibition of the IL‐12 and IL‐23 pathways. 38 , 41 Inhibition of IL‐23 signalling will likely reduce T‐helper (Th)17 and Th22 cell differentiation, 42 which may bring about the potential for activity in certain subtypes of AD that appear to have greater dependence on Th17 and Th22 pathways including early‐onset paediatric AD, 43 intrinsic (nonallergic) AD and AD in Asian and African American participants. 42 , 44 , 45 Oral brepocitinib has been evaluated in a phase IIa study in patients with plaque psoriasis, and it showed signs of clinical benefit. 40 The current study explores a topical cream formulation of brepocitinib in participants with mild‐to‐moderate AD. We hypothesize that topical administration of brepocitinib, given its small molecular weight 38 and amenability to topical formulation, could reduce systemic exposure and provide robust efficacy to participants with mild‐to‐moderate AD. Therefore, we aimed to assess the efficacy, safety and pharmacokinetics of topical brepocitinib cream in participants with mild‐to‐moderate AD.
Methods
Study design
This was a phase IIb, multicentre, randomized, double‐blind, vehicle‐controlled, parallel‐group, dose‐ranging study to evaluate the efficacy, safety, tolerability and pharmacokinetics of topical brepocitinib cream in participants with mild‐to‐moderate AD (clinicaltrials.gov: NCT03903822; EudraCT: 2018‐003050‐24). Participants were randomized at 65 sites in 10 countries (Australia, Bulgaria, Canada, Denmark, Germany, Hungary, Japan, Latvia, Poland and the USA). The study included a screening period of up to 6 weeks, a 6‐week treatment period and a 4‐week follow‐up period. The study took place between May 2019 and May 2020.
This study was conducted in compliance with the ethical principles of the Declaration of Helsinki and in compliance with all International Council for Harmonisation Good Clinical Practice guidelines. The study protocol was reviewed and approved by the Institutional Review board and/or Independent Ethics Committee at each participating centre. All participants provided written informed consent.
Participants
Male (12–75 years of age) and female (18–75 years of age) participants were included if they had a clinical diagnosis of AD for ≥ 3 months. Adolescent male participants (12–18 years of age) were eligible for enrolment only after an interim analysis of safety and tolerability in approximately 20% of adult participants after 2 weeks of treatment. At screening and baseline, participants were required to have an Investigator’s Global Assessment (IGA) score of 2 (mild) or 3 (moderate), 2–20% body surface area (BSA) involvement (not including groin, genitals or hair‐bearing scalp), Eczema Area and Severity Index (EASI) total score of 3 to 21, body weight ≥ 40 kg and a body mass index between 17·5 and 35 kg m–2. Participants must have had a peak pruritus numerical rating scale (PP‐NRS) grade ≥ 2 at baseline. Participants with other active forms of dermatitides and/or eczematous conditions were excluded. Topical corticosteroids were not permitted for use [except for hair‐bearing scalp; see Appendix S1 (Supporting Information)]. Other permitted and prohibited concomitant medications are described in Appendix S1.
Treatments
Participants were randomized equally to eight treatment arms to receive either once (QD) or twice daily (BID) application for 6 weeks: QD doses of vehicle, brepocitinib 0·1% (1 mg g–1), 0·3% (3 mg g–1), 1·0% (10 mg g–1) or 3·0% (30 mg g–1), and BID doses of vehicle, brepocitinib 0·3% or 1%. Participants and investigators were blinded to treatment but not to frequency of dosing. Randomization was performed at each investigative site using interactive response technology, which provided a randomization number and dispensable unit of treatment for each participant. Prior to baseline, participants and caregivers were instructed how and where to apply the treatment, and treatment was self‐administered or given by a caregiver to all areas affected by AD, except those on hair‐bearing scalp.
Endpoints
The primary endpoint was the percentage change from baseline in EASI total score at week 6 (EASI total score did not include scalp scoring), and the key secondary endpoint was the proportion of participants who achieved a composite of an IGA score of clear (0) or almost clear (1) and a reduction of ≥ 2 points from baseline at week 6. Additional secondary endpoints, exploratory endpoints and post hoc analyses included percentage change from baseline in EASI score at all visits; EASI total score over time; the proportion of participants achieving 75% and 90% improvement in EASI (EASI75 and EASI90, respectively) at week 6; the proportion of participants having ≥ 4‐grade reduction in weekly averages of PP‐NRS and percentage change from baseline in affected BSA at all scheduled visits; change from baseline in Patient‐Oriented Eczema Measure (POEM) at weeks 1, 2 and 6; the proportion of participants with a Dermatology Life Quality Index (DLQI) response of ≥ 4‐point improvement from baseline at weeks 1, 2 and 6 among adult participants with a baseline DLQI score ≥ 4. Safety and tolerability were assessed by measuring the incidence of treatment‐emergent adverse events (TEAEs) and significant changes in vital signs, laboratory assessments and electrocardiograms.
Pharmacokinetics
Blood samples were collected pre‐dose at baseline and weeks 1, 2, 3, 4 and 6 to assess plasma concentrations of brepocitinib. To ensure measurement consistency, blood samples were collected at the same time interval after treatment administration.
Statistical analyses
For the primary endpoint, ANCOVA was used, adjusting for baseline EASI score. Missing data from active treatment arms were imputed assuming data were missing at random, and that missing observations were similar to the corresponding vehicle arm. The analysis combined results from multiple imputations using Rubin’s rules as implemented in SAS PROC MIANALYZE, and the overall family‐wise type I error rate of the primary endpoint was controlled at the one‐sided 0·05 level using the Hochberg’s step‐up method. For all binary secondary endpoints, proportions of participants responding and the corresponding risk differences were analysed using Chan and Zhang’s unconditional exact method, 46 and no adjustments for multiplicity were made. Missing data were imputed as nonresponders for binary endpoints. Continuous secondary endpoints were assessed using ANCOVA with fixed factors of baseline values and treatment, or by mixed‐model repeated measures including fixed effects of treatment, visit week and treatment‐by‐visit interaction and visit as a categorical covariate. Exploratory endpoints were summarized descriptively, with no adjustments for multiplicity. TEAEs, laboratory tests and vital signs were summarized descriptively. Sample size determination is described in Appendix S1.
Results
Participants
In total, 500 subjects were screened, and 292 were enrolled and randomized to treatment. The mean age of participants was 40·2 years, and just over half of the randomized participants were female (53·4%). The mean (SD) EASI score at baseline was 7·3 (3·6), and approximately half of all participants had mild (2, n = 151) or moderate (3, n = 140) IGA scores at baseline. Baseline demographic and disease characteristics, stratified by treatment, are shown in Table 1.
Table 1.
Demographics and baseline disease characteristics in participants with AD
| Vehicle QD (N = 37) | 0·1% QD (N = 37) | 0·3% QD (N = 36) | 1·0% QD (N = 37) | 3·0% QD (N = 36) | Vehicle BID (N = 36) | 0·3% BID (N = 36) | 1·0% BID (N = 37) | Total (N = 292) | |
|---|---|---|---|---|---|---|---|---|---|
| Mean age, years (range) | 39·1 (13–68) | 40·8 (17–71) | 43·4 (17–70) | 38·4 (17–62) | 40·5 (19–67) | 42·3 (14–71) | 39·4 (18–74) | 38·1 (20–73) | 40·2 (13–74) |
| Female, n (%) | 20 (54·1) | 19 (51·4) | 24 (66·7) | 23 (62·2) | 15 (41·7) | 19 (52·8) | 16 (44·4) | 20 (54·1) | 156 (53·4) |
| Mean BMI, kg m–2 (SD) | 27·2 (5·05) | 26·2 (4·88) | 28·0 (6·01) | 25·3 (3·67) | 25·9 (4·40) | 26·9 (4·60) | 26·3 (4·47) | 27·1 (5·40) | 26·6 (4·86) |
| Race, n (%) | |||||||||
| White | 24 (64·9) | 22 (59·5) | 21 (58·3) | 20 (54·1) | 21 (58·3) | 20 (55·6) | 22 (61·1) | 24 (64·9) | 174 (59·6) |
| Black or African American | 8 (21·6) | 7 (18·9) | 9 (25·0) | 6 (16·2) | 4 (11·1) | 6 (16·7) | 5 (13·9) | 6 (16·2) | 51 (17·5) |
| Asian | 4 (10·8) | 8 (21·6) | 4 (11·1) | 8 (21·6) | 10 (27·8) | 9 (25·0) | 9 (25·0) | 7 (18·9) | 59 (20·2) |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 1 (2·7) | 0 | 1 (2·8) | 0 | 0 | 2 (0.7) |
| Multiracial | 0 | 0 | 2 (5·6) | 2 (5·4) | 1 (2·8) | 0 | 0 | 0 | 5 (1·7) |
| Not reported | 1 (2·7) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0·3) |
| Mean AD duration since onset, years (SD) | 15·1 (14·25) | 18·4 (14·33) | 19·8 (17·45) | 24·1 (16·38) | 23·4 (16·02) | 18·6 (14·84) | 17·9 (12·32) | 24·9 (16·22) | 20·3 (15·46) |
| IGA score, n (%) | |||||||||
| Mild (score = 2) | 18 (48·6) | 22 (59·5) | 17 (47·2) | 21 (56·8) | 17 (47·2) | 24 (66·7) | 14 (38·9) | 18 (48·6) | 151 (51·7) |
| Moderate (score = 3) | 19 (51·4) | 14 (37·8) | 19 (52·8) | 16 (43·2) | 19 (52·8) | 12 (33·3) | 22 (61·1) | 19 (51·4) | 140 (47·9) |
| Severe (score = 4) | 0 | 1 (2·7) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0·3) |
| EASI total score, mean (SD) | 7·3 (3·25) | 7·1 (3·40) | 7·3 (2·87) | 7·0 (3·89) | 7·7 (4·27) | 6·0 (2·17) | 7·6 (4·13) | 8·2 (4·09) | 7·3 (3·58) |
| BSA, mean % (SD) | 10·3 (5·64) | 8·8 (5·65) | 9·6 (4·56) | 9·1 (5·01) | 8·6 (5·32) | 7·8 (4·71) | 9·1 (5·50) | 10·2 (5·43) | 9·2 (5·24) |
| PP‐NRS grade, mean (SD) | 6·0 (1·94) | 6·1 (1·98) | 6·3 (2·07) | 6·2 (2·03) | 5·7 (1·91) | 6·1 (2·04) | 5·7 (1·88) | 5·8 (2·44) | 6·0 (2·03) |
| Participants with PP‐NRS ≥ 4, n (%) | 33 (89·2) | 30 (81·1) | 32 (88·9) | 31 (83·8) | 28 (77·8) | 30 (83·3) | 30 (83·3) | 27 (73·0) | 241 (82·5) |
| POEM score, mean (SD) | 14·9 (5·16) | 16·9 (4·74) | 16·5 (5·72) | 18·4 (4·89) | 17·0 (6·11) | 17·5 (5·81) | 14·6 (5·67) | 16·4 (6·26) | 16·5 (5·62) |
| DLQI score, mean (SD) | 8·8 (5·64) | 9·8 (5·32) | 11·8 (6·86) | 9·0 (6·11) | 9·2 (5·37) | 10·0 (6·20) | 8·7 (5·37) | 9·4 (6·01) | 9·6 (5·88) |
| Participants with DLQI ≥ 4, n (%) | 29 (78·4) | 34 (91·9) | 30 (83·3) | 30 (81·1) | 31 (86·1) | 29 (80·6) | 28 (77·8) | 29 (78·4) | 240 (82·2) |
AD, atopic dermatitis; BID, twice daily; BMI, body mass index; BSA, body surface area; DLQI, dermatology life quality index; EASI, eczema area and severity index; IGA, Investigator’s Global Assessment; N, number of participants with observed data; n, number of participants with response; POEM, Patient‐Oriented Eczema Measure; PP‐NRS, peak pruritus numerical rating scale; QD, once daily.
Overall, 240 participants (82·2%) completed treatment. The discontinuation rates were highest (> 20%) in the vehicle QD and BID groups and in the lowest brepocitinib dose group (0·1% QD). The most frequent reasons for discontinuation were ‘withdrawal by subject’ (n = 19) and ‘adverse event’ (n = 16) across both brepocitinib and vehicle groups. Worsening AD was the cause of six discontinuations: four in the vehicle groups, and two in the 0·1% QD group. Participant disposition is shown in the CONSORT diagram in Figure S1 (see Supporting Information).
Efficacy outcomes
Primary endpoint
At week 6, the brepocitinib 1% QD and 1% BID groups had statistically significantly greater least squares (LS) mean percentage reductions from baseline in EASI total score [LS mean (90% confidence interval, CI): QD: –70·1 (–82·1 to –58·0); BID: –75·0 (–83·8 to –66·2)] compared with the respective vehicle groups [LS mean (90% CI): QD: –44·4 (–57·3 to –31·6); BID: –47·6 (–57·5 to –37·7)] (Figure 1). All other brepocitinib groups had numerically greater reductions from baseline in EASI, compared with the respective vehicle and dosing frequency, but reductions were not statistically significant.
Figure 1.
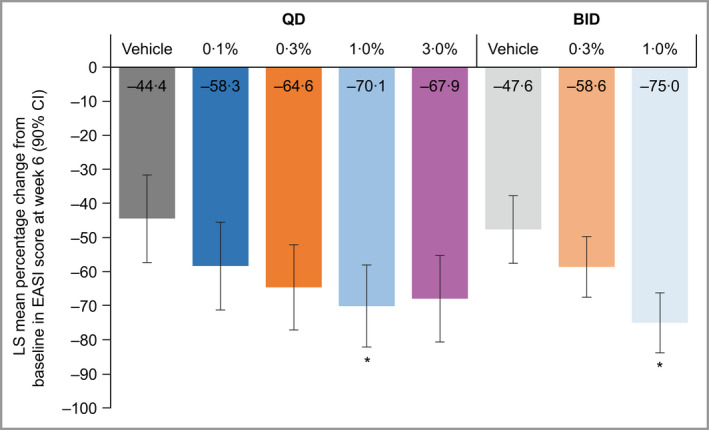
Percentage decrease from baseline in EASI score at week 6, in participants with AD. Multiplicity‐adjusted (Hochberg’s step‐up) one‐sided P‐value: *P ≤ 0·05 vs. respective vehicle. Analysis by ANCOVA on the full analysis set contained fixed factors of treatment and baseline value. AD, atopic dermatitis; BID, twice daily; CI, confidence interval; EASI, Eczema Area and Severity Index; LS, least squares; QD, once daily. [Colour figure can be viewed at wileyonlinelibrary.com]
Key secondary endpoint
In five of the six active treatment groups, the proportion of participants achieving IGA scores of clear (0) or almost clear (1) as well as at least a 2‐point reduction in IGA score was significantly higher [percentage (90% CI): 0·1% QD: 29·7 (18·5–43·3); 0·3% QD: 33·3 (21·3–47·0); 1% QD: 40·5 (28·0–54·4); 3% QD: 44·4 (30·2–59·1); 0·3% BID: 33·3 (21·3–47·0)], compared with the respective vehicle [QD: 10·8 (4·8–22·2); BID: 13·9 (6·9–25·4)] (Figure 2). The 1% BID group [27·0 (15·5–40·2)] was numerically greater but did not reach statistical significance, compared with vehicle BID.
Figure 2.
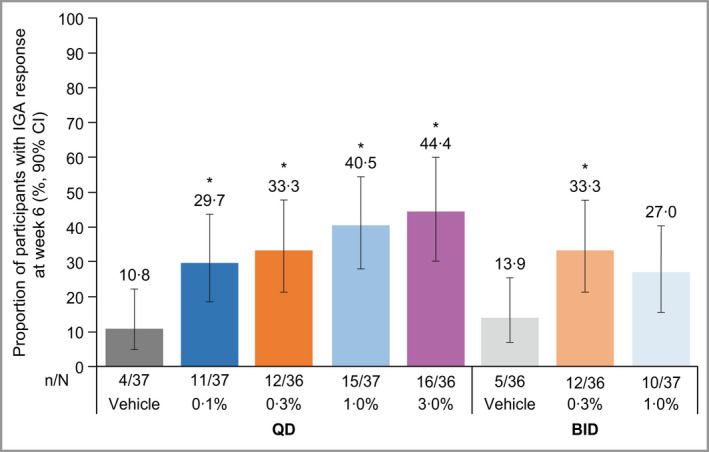
Proportion of participants with AD, with IGA ‘clear’ or ‘almost clear’ and with ≥ 2‐point reduction from baseline at week 6. Unadjusted, one‐sided P‐values: *P < 0·05 vs. respective vehicle. Analysis by exact Chan and Zhang method 46 on the full analysis set using nonresponder imputation. AD, atopic dermatitis; BID, twice daily; CI, confidence interval; IGA, Investigator’s Global Assessment; QD, once daily. [Colour figure can be viewed at wileyonlinelibrary.com]
Secondary and exploratory endpoints
Across all groups, including vehicle, there was a steady trend of improvement in EASI score (Figure S2a; see Supporting Information) and in percentage change from baseline in EASI total score from week 1 (Figure S2b). As early as week 1, and at all subsequent time points, the 1% QD, 3% QD and 1% BID groups had significantly greater percentage changes from baseline in EASI score, compared with respective vehicle (Figure S2b), with the 3% QD group generally achieving the greatest reductions from baseline throughout the study (Figure S2a). On withdrawal of treatment, the percentage change in EASI total score worsened from week 6 values in all groups.
A statistically significantly higher proportion of participants achieved EASI75 at week 6 in the 0·3% BID [percentage (90% CI): 36·1 (22·9–50·0)] and 1% BID [51·4 (37·0–65·7)] groups, compared with vehicle BID [16·7 (7·5–30·2)] (Figure S3a; see Supporting Information). For EASI90, statistically significantly higher proportions of participants in the 0·3% QD [27·8 (15·9–40·9)], 1·0% QD [37·8 (24·8–52·0)], 3·0% QD [41·7 (29·0–56·0)] and 1·0% BID [27·0 (15·5–40·2)] groups achieved the endpoint, compared with the respective vehicles [QD: 10·8 (4·8–22·2); BID: 8·3 (3·1–18·9)] (Figure S3b).
Regarding PP‐NRS, all active treatment groups had numerically higher proportions of participants with ≥ 4‐grade reduction in weekly averages of PP‐NRS by week 3 and through week 6, compared with either vehicle. However, the higher dose groups showed separation from their respective vehicles by week 1 (Figure S4; see Supporting Information) and improvements in pruritus were reported as early as day 2 and day 3 in the brepocitinib 1·0% and 3·0% QD groups (Table S1; see Supporting Information). At week 6, the 1% QD [percentage (90% CI): 45·2 (29·7–60·1)], 3% QD [50·0 (33·3–66·7)] and 1% BID [40·7 (24·8–58·3)] groups had statistically significantly higher proportions of participants with ≥ 4‐grade reduction in weekly averages of PP‐NRS, compared with respective vehicles [QD: 18·2 (8·2–31·3); BID: 16·7 (8·3–30·8)] (Figure 3). Except for the vehicle QD and 0·1% QD groups, the proportion of participants experiencing a PP‐NRS response decreased across all groups from week 6 at follow‐up [percentage (90% CI): vehicle QD: 9·1 (3·4–20·2); 0·1% QD: 30·0 (18·2–45·5); 0·3% QD: 21·9 (12·1–36·2); 1% QD: 19·4 (8·8–32·7); 3% QD: 21·4 (9·8–36·6); vehicle BID: 20·0 (9·1–33·9); 0·3% BID: 20·0 (9·1–33·9)] (Figure S4).
Figure 3.
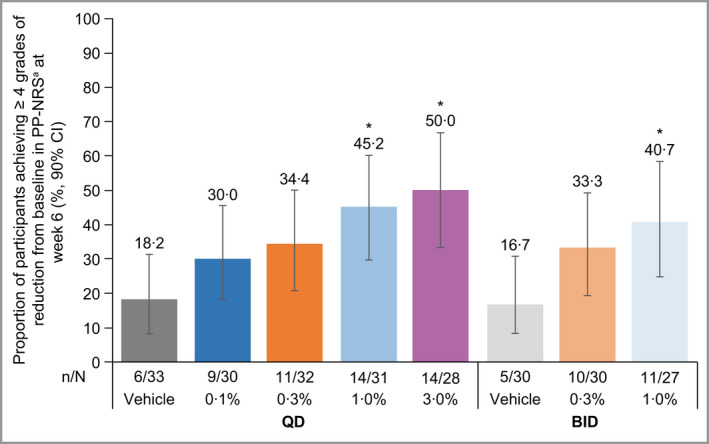
Proportion of participants with AD achieving ≥ 4‐grade reduction from baseline in PP‐NRS at week 6 among participants with grade ≥ 4 at baseline. aAnalysis by exact Chan and Zhang method 46 on the full analysis set using nonresponder imputation. Unadjusted, one‐sided P‐values: *P < 0·05 vs. respective vehicle. aProportion calculated based on the number of participants with PP‐NRS grade ≥ 4 at baseline. AD, atopic dermatitis; BID, twice daily; CI, confidence interval; PP‐NRS, peak pruritus numerical rating scale; QD, once daily. [Colour figure can be viewed at wileyonlinelibrary.com]
The decrease in affected BSA was greater in all active treatment groups compared with respective vehicles. At week 6, the 0·3% QD [LS mean (90% CI): –54·2 (–75·2 to –33·3)], 1·0% QD [–60·6 (–81·0 to –40·1)], 3·0% QD [–51·4 (–73·7 to –29·1)] and 1·0% BID [–63·0 (–73·2 to –52·8)] groups had significantly greater decrease in percentage change from baseline in affected BSA, compared with respective vehicles (Figure S5; see Supporting Information).
Improvement in AD symptom severity measured by POEM was reported soon after initiation of therapy, with statistically significant improvements in change from baseline in POEM at week 1 in the brepocitinib 0·3%, 1·0% and 3·0% QD groups, compared with vehicle. At week 6, the change from baseline in POEM was significantly different from vehicle in all brepocitinib groups (Figure S6; see Supporting Information).
Dermatology‐specific HRQoL measured by the DLQI was also generally numerically improved in the brepocitinib groups at all time points, compared with vehicle. At week 6, up to 76·7% of participants reported a ≥ 4‐point improvement in DLQI, with a significantly greater proportion of participants in the brepocitinib 0·3% QD, 1·0% QD, 3·0% QD, 0·3% BID and 1·0% BID groups reporting a ≥ 4‐point improvement, compared with vehicle (Figure S7; see Supporting Information).
Safety and tolerability outcomes
Overall, 108 participants (37·0%) experienced TEAEs during the study. The vehicle groups had the highest proportions of participants with TEAEs (47·2–48·6%) and study discontinuations due to TEAEs [50% of all discontinuations due to TEAEs (eight of 16 participants) were in the vehicle groups] (Table 2). The most common TEAEs leading to discontinuation related to worsening of AD [System Organ Class (SOC): skin and subcutaneous tissue disorders]. There were twice as many discontinuations in this SOC in the vehicle groups (n = 8) compared with brepocitinib groups (n = 4). There was not a dose‐dependent trend in the frequency of AEs, and there were no serious TEAEs or deaths. Two participants experienced severe TEAEs: one participant in the 0·3% BID group experienced treatment‐related erythema (which resolved after 12 days), and one participant in the vehicle BID group experienced treatment‐related contact dermatitis (which resolved after 4 days). Both participants discontinued from the study.
Table 2.
Summary of TEAEs in participants with AD, all causalities
| Participants, n (%) | Vehicle QD (N = 37) | 0·1% QD (N = 37) | 0·3% QD (N = 36) | 1.0% QD (N = 37) | 3·0% QD (N = 36) | Vehicle BID (N = 36) | 0·3% BID (N = 36) | 1·0% BID (N = 37) | Total (N = 292) |
|---|---|---|---|---|---|---|---|---|---|
| TEAEs | 18 (48·6) | 17 (45·9) | 11 (30·6) | 12 (32·4) | 10 (27·8) | 17 (47·2) | 9 (25·0) | 14 (37·8) | 108 (37·0) |
| Treatment‐emergent SAEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe TEAEs | 0 | 0 | 0 | 0 | 0 | 1 (2·8) | 1 (2·8) | 0 | 2 (0.7) |
| Discontinued from study due to TEAE | 3 (8·1) | 2 (5·4) | 1 (2·8) | 2 (5·4) | 2 (5·6) | 5 (13·9) | 1 (2·8) | 0 | 16 (5·5) |
| TEAEs reported in ≥ 5 participants in total | |||||||||
| Nasopharyngitis | 1 (2·7) | 2 (5·4) | 2 (5·6) | 3 (8·1) | 4 (11·1) | 2 (5·6) | 2 (5·6) | 2 (5·4) | 18 (6·2) |
| Worsening of AD | 3 (8·1) | 3 (8·1) | 1 (2·8) | 0 | 1 (2·8) | 3 (8·3) | 0 | 2 (5·4) | 13 (4·5) |
AD, atopic dermatitis; BID, twice daily; QD, once daily; N, number of participants with observed data; n, number of participants with response; SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
The most common TEAEs were nasopharyngitis and worsening AD, with the highest proportion of participants experiencing worsening of AD in the vehicle groups (8·1–8·3%) (Table 2). There were no clinically significant trends in haemoglobin, platelet, lymphocyte or neutrophil counts or creatine kinase levels. Additionally, no clinically meaningful trends in liver function tests were observed.
There were three cases of creatine kinase elevation (≥ 2–3× the upper limit of normal; one participant each in the vehicle BID, 0·1% QD and 0·3% QD groups). One participant, in the 0·3% QD group, was discontinued from the study due to an elevated alanine aminotransferase level (131 U L–1, day 15). None of these changes in laboratory values were deemed as treatment‐related by the investigators, nor did they appear to be associated with systemic concentrations. This is based on both measured drug levels at trough and by the lack of haematological changes that are sensitive markers for JAK pharmacology in these participants.
Pharmacokinetics
Overall, plasma brepocitinib concentrations were low and highly variable (Figure S8; see Supporting Information). At dosing levels above 0·3% in QD groups and in both BID brepocitinib groups, there was a dose‐related increase in brepocitinib plasma concentration. A higher proportion of participants receiving doses of 1·0% QD, 3·0% QD or 1·0% BID had brepocitinib concentrations above the lower limit of quantification (0·200 ng mL–1), compared with the lower dosing groups (Figure S8).
Discussion
This study supports the further evaluation of topical brepocitinib as a novel treatment for mild‐to‐moderate AD. JAK inhibition, a new mechanism of action in the treatment of AD, is being assessed in clinical trials, 27 but this study focuses on the evaluation of the TYK2/JAK1 inhibition. Topical brepocitinib cream was significantly more effective than vehicle, as assessed by the investigator through multiple measures (EASI total score, IGA responder rates and reduction in affected BSA) and by patient‐reported outcomes (≥ 4‐point improvement in the PP‐NRS and DLQI and improvement in POEM). In particular, a dose–response trend was observed. Improving both the physician‐reported clinical signs of AD and the patient‐reported symptoms and HRQoL outcomes is critical, as highlighted in the Patient‐Focused Drug Development initiative by the FDA. 47 Clinically relevant reductions in pruritus were observed as early as day 2, 24 h after the first administration of brepocitinib treatment. Furthermore, consistent with improvements in AD symptoms and severity, dermatology‐specific HRQoL was improved with topical brepocitinib, despite high scores at baseline, with meaningful improvements of ≥ 4 points in up to 76·7% of adult participants at week 6. In general, and across all endpoints, there did not appear to be a major advantage of BID dosing, compared with QD, suggesting that QD application may be adequate and could support greater adherence than BID application.
Topical brepocitinib was well tolerated in this population, with mostly mild TEAEs reported and half of discontinuations being among participants in the vehicle groups. Additionally, most TEAEs of worsening AD were reported in the vehicle groups. Low rates of TEAEs, compared with oral formulations of JAK inhibitors for AD, 23 , 28 reflect low systemic exposure with this topical formulation. Indeed, brepocitinib plasma concentrations were low with many participants not having levels above the lower limit of quantification. Low systemic exposure with topical application also eliminated the clinically significant haematological trends that have been observed with oral JAK inhibitors. 48 , 49 , 50
This topical cream formulation of brepocitinib offers an alternative to petrolatum‐based ointments. The vehicle groups saw minimal improvements in the stringent IGA and EASI90 endpoints, highlighting the efficacy of brepocitinib itself within the cream formulation.
Limitations of this study should be acknowledged. In order to test an extensive dose range given a lack of previous experience with topical brepocitinib, the group sizes were small and contributed to variability on some endpoints. The effect size needs to be confirmed in larger studies. In addition, the treatment period spanned only 6 weeks. Further assessment over a longer treatment and follow‐up period would be required to confirm persistence of efficacy and the long‐term safety profile. This study’s strengths included treatment using brepocitinib as a monotherapy without any rescue therapy, thereby providing added robustness to the results. Additionally, a range of brepocitinib doses and two different dosing frequencies were examined, facilitating dose and regimen selection for future studies. The patient‐reported endpoints in this study also provided a multidimensional view of the patient symptoms and impacts from their own perspective, all of which showed improvement with brepocitinib treatment.
The results of this study showed that topical brepocitinib is effective and well tolerated in participants with mild‐to‐moderate AD. Further studies are required to evaluate brepocitinib using a larger sample size and a longer treatment period.
Author contributions
Megan N. Landis: Writing – review and editing (equal). Mark Arya: Writing – review and editing (equal). Stacy Smith: Data curation (equal); validation (equal); writing – review and editing (equal). Zoe D Draelos: Data curation (equal); validation (equal); writing – review and editing (equal). Lisa Usdan: Writing – review and editing (equal). Sanela Tarabar: Writing – review and editing (equal). Vivek Pradhan: Writing – review and editing (equal). Sudeepta Aggarwal: Writing – review and editing (equal). Christopher Banfield: Writing – review and editing (equal). Elena Peeva: Writing – review and editing (equal). Michael S. Vincent: Methodology (equal); writing – review and editing (equal). Vanja Sikirica: Writing – review and editing (equal). Jason Xenakis: Writing – review and editing (equal). Jean S. Beebe: Data curation (equal); methodology (equal); validation (equal); writing – review and editing (equal).
Funding sources
This study was sponsored by Pfizer Inc. Pfizer Inc. funded the study including the development of the protocol, implementation of the study, data collection and analysis and supported the manuscript preparation.
Conflicts of interest
M.N.L. has served as Principal Investigator and/or Sub‐Investigator for Amgen, Celgene, Cutanea, Eli Lilly, Foamix, Galderma, Incyte, Janssen, Johnson & Johnson, Kadmon, Leo Pharma, Novartis, Novum, Pfizer Inc and Symbio; and has received fees from Amgen, Celgene, Cutanea, Foamix, Galderma, Incyte, Johnson & Johnson, Kadmon, Leo Pharma, Novartis, Novum and Symbio. S.S. has performed clinical research studies for AbbVie, Aclaris Therapeutics, Allergan, Arcutis, Boehringer Ingelheim, Brickell Biotech, Croma Pharma, Dermira, Eli Lilly, Endo Pharmaceuticals, Evolus, Galderma, Glenmark Pharmaceuticals, Leo Pharma, Nielsen Biosciences, Novartis, Pfizer Inc, Prollenium Medical Technologies, Revance Therapeutics and Sun Pharmaceutical Industries; and has received fees and honoraria as a consultant to Brickell Biotech, Galderma, Nielsen Biosciences, Prollenium Medical Technologies, Scarless Laboratories and Teoxane. Z.D. received a research grant from Pfizer Inc to conduct the research detailed in this manuscript. S.T., V.P., S.A., C.B., E.P., M.S.V. and J.X. are employees and stockholders of Pfizer Inc. V.S. and J.S.B. were employees of Pfizer Inc at the time this work was conducted and hold shares in Pfizer Inc. M.A. and L.U. have no conflicts of interest to declare.
Ethics statement
This study was conducted in compliance with the ethical principles of the Declaration of Helsinki and in compliance with all International Council for Harmonisation Good Clinical Practice guidelines. The study protocol was reviewed and approved by the Institutional Review board and/or Independent Ethics Committee at each participating centre. All participants provided written informed consent.
Supporting information
Appendix S1 Supplementary Methods: Concomitant medications; Sample size determination; Participant selection.
Figure S1 Participant disposition (CONSORT diagram).
Figure S2 For selected doses: (a) LS mean of change from baseline in EASI score and (b) LS mean percentage change from baseline in EASI score in participants with AD.
Figure S3 Proportion of participants with atopic dermatitis achieving (a) EASI75 and (b) EASI90 at week 6.
Figure S4 Proportion of participants with AD achieving ≥ 4‐grade reduction from baseline in PP‐NRS through week 6 and follow‐up among participants with grade ≥ 4 at baseline.
Figure S5 Percentage change from baseline in BSA score at week 6 in participants with AD.
Figure S6 Change from baseline in POEM in participants with AD.
Figure S7 Proportion of participants with AD achieving ≥ 4‐grade reduction in DLQI from baseline at week 6 among participants with grade ≥ 4 at baseline (full analysis set, nonresponder imputation).
Figure S8 Median brepocitinib plasma concentration in participants with AD.
Table S1 Proportion of participants achieving ≥ 4‐grade reduction in severity of PP‐NRS over time.
Acknowledgments
The authors would like to thank the investigators and participants who made this study possible. Medical writing support, under the guidance of the authors, was provided by Eric Comeau PhD, of CMC Connect, McCann Health Medical Communications, and was funded by Pfizer Inc., New York, NY, USA in accordance with Battisti WP, Wager E, Baltzer L et al.; for the International Society for Medical Publication Professionals. Good publication practice for communicating company‐sponsored medical research: GPP3 guidelines. Ann Intern Med 2015; 163:461–4; https://doi.org/10.7326/M15‐0288.
This trial is registered with clinicaltrials.gov (NCT03903822) and with EudraCT (2018‐003050‐24).
Plain language summary available online
Data availability
On request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information.
References
- 1. Eichenfield LF, Tom WL, Chamlin SL et al. Guidelines of care for the management of atopic dermatitis: part 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014; 70:338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387:1109–22. [DOI] [PubMed] [Google Scholar]
- 3. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population‐based study. J Allergy Clin Immunol 2013; 132:1132–8. [DOI] [PubMed] [Google Scholar]
- 4. Hebert AA, Stingl G, Ho LK et al. Patient impact and economic burden of mild‐to‐moderate atopic dermatitis. Curr Med Res Opin 2018; 34:2177–85. [DOI] [PubMed] [Google Scholar]
- 5. Huang J, Choo YJ, Smith HE et al. Quality of life in atopic dermatitis in Asian countries: a systematic review. Arch Dermatol Res 2022; 314:445–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eichenfield LF, Tom WL, Berger TG et al. Guidelines of care for the management of atopic dermatitis: part 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71:116–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmitt J, Csötönyi F, Bauer A et al. Determinants of treatment goals and satisfaction of patients with atopic eczema. J Dtsch Dermatol Ges 2008; 6:458–65. [DOI] [PubMed] [Google Scholar]
- 8. Wong ITY, Tsuyuki RT, Cresswell‐Melville A et al. Guidelines for the management of atopic dermatitis (eczema) for pharmacists. Can Pharm J (Ott) 2017; 150:285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sidbury R, Tom WL, Bergman JN et al. Guidelines of care for the management of atopic dermatitis: section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol 2014; 71:1218–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cork MJ, Danby SG, Ogg GS. Atopic dermatitis epidemiology and unmet need in the United Kingdom. J Dermatolog Treat 2020; 31:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Solimani F, Pollmann R, Schmidt T et al. Therapeutic targeting of Th17/Tc17 cells leads to clinical improvement of lichen planus. Front Immunol 2019; 10:1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang J, Tsai TF, Lee MG et al. The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: a phase 3, randomized, double‐blind, placebo‐controlled study. J Dermatol Sci 2017; 88:36–45. [DOI] [PubMed] [Google Scholar]
- 13. Papp K, Gordon K, Thaçi D et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med 2018; 379:1313–21. [DOI] [PubMed] [Google Scholar]
- 14. Kimball AB, Tzellos T, Calimlim BM et al. Achieving hidradenitis suppurativa response score is associated with significant improvement in clinical and patient‐reported outcomes: post hoc analysis of pooled data from PIONEER I and II. Acta Derm Venereol 2018; 98:932–7. [DOI] [PubMed] [Google Scholar]
- 15. Kimball AB, Okun MM, Williams DA et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016; 375:422–34. [DOI] [PubMed] [Google Scholar]
- 16. Pfizer Inc . Highlights of Prescribing Information: EUCRISA® (crisaborole) ointment. Revised March 2020. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/207695s007s009s010lbl.pdf (last accessed 1 July 2022).
- 17. Neis MM, Peters B, Dreuw A et al. Enhanced expression levels of IL‐31 correlate with IL‐4 and IL‐13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol 2006; 118:930–7. [DOI] [PubMed] [Google Scholar]
- 18. Klonowska J, Glen J, Nowicki RJ et al. New cytokines in the pathogenesis of atopic dermatitis – new therapeutic targets. Int J Mol Sci 2018; 19:3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hassan Z, Luvsannyam E, Patel D et al. Review of prominent cytokines as superior therapeutic targets for moderate‐to‐severe atopic dermatitis. Cureus 2020; 12:e9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanimoto A, Ogawa Y, Oki C et al. Pharmacological properties of JTE‐052: a novel potent JAK inhibitor that suppresses various inflammatory responses in vitro and in vivo . Inflamm Res 2015; 64:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamaoka K, Saharinen P, Pesu M et al. The Janus kinases (Jaks). Genome Biol 2004; 5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gooderham MJ, Forman SB, Bissonnette R et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol 2019; 155:1371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silverberg JI, Simpson EL, Thyssen JP et al. Efficacy and safety of abrocitinib in patients with moderate‐to‐severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol 2020; 156:863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guttman‐Yassky E, Silverberg JI, Nemoto O et al. Baricitinib in adult patients with moderate‐to‐severe atopic dermatitis: a phase 2 parallel, double‐blinded, randomized placebo‐controlled multiple‐dose study. J Am Acad Dermatol 2019; 80:913–21. [DOI] [PubMed] [Google Scholar]
- 25. Bissonnette R, Papp KA, Poulin Y et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol 2016; 175:902–11. [DOI] [PubMed] [Google Scholar]
- 26. Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol 2015; 73:395–9. [DOI] [PubMed] [Google Scholar]
- 27. Pfizer Inc . FDA grants priority review and EMA accepts regulatory submission for Pfizer’s abrocitinib, an oral once‐daily JAK1 inhibitor, for patients 12 and up with moderate to severe atopic dermatitis. 27 October 2020. Available at: https://investors.pfizer.com/investor‐news/press‐release‐details/2020/FDA‐Grants‐Priority‐Review‐and‐EMA‐Accepts‐Regulatory‐Submission‐for‐Pfizers‐Abrocitinib‐an‐Oral‐Once‐Daily‐JAK1‐Inhibitor‐for‐Patients‐12‐and‐Up‐with‐Moderate‐to‐Severe‐Atopic‐Dermatitis/default.aspx (last accessed 1 July 2022).
- 28. Guttman‐Yassky E, Thaçi D, Pangan AL et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16‐week results from a randomized, placebo‐controlled trial. J Allergy Clin Immunol 2020; 145:877–84. [DOI] [PubMed] [Google Scholar]
- 29. Simpson EL, Lacour JP, Spelman L et al. Baricitinib in patients with moderate‐to‐severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol 2020; 183:242–55. [DOI] [PubMed] [Google Scholar]
- 30. AbbVie Inc . RINVOQ® (upadacitinib). Full prescribing information. Revised April 2022. Available at: https://www.rxabbvie.com/pdf/rinvoq_pi.pdf (last accessed 1 July 2022).
- 31. Pfizer Inc . CIBINQO (abrocitinib). Full prescribing information. Revised January 2022. Available at: https://cdn.pfizer.com/pfizercom/USPI_Med_Guide_CIBINQO_Abrocitinib_tablet.pdf (last accessed 1 July 2022).
- 32. Nakagawa H, Nemoto O, Igarashi A et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol 2019; 144:1575–83. [DOI] [PubMed] [Google Scholar]
- 33. Nakagawa H, Nemoto O, Igarashi A et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double‐blind, vehicle‐controlled study and an open‐label, long‐term extension study. J Am Acad Dermatol 2020; 82:823–31. [DOI] [PubMed] [Google Scholar]
- 34. Japan Tobacco . JT receives approvals of CORECTIM® ointment 0.25% and CORECTIM® ointment 0.5% for the treatment of pediatric atopic dermatitis in Japan. 23 March 2021. Available at: https://www.jt.com/media/news/2021/pdf/20210323_E1.pdf (last accessed 1 July 2022).
- 35. Incyte Corporation . OPZELURA™ (ruxolitinib). Full prescribing information. Revised July 2022. Available at: https://www.opzelura.com/prescribing‐information.pdf (last accessed 1 July 2022).
- 36. Bieber T, Simpson EL, Silverberg JI et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med 2021; 384:1101–12. [DOI] [PubMed] [Google Scholar]
- 37. Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol 2021; 148:927–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fensome A, Ambler CM, Arnold E et al. Design and optimization of a series of 4‐(3‐azabicyclo[3.1.0]hexan‐3‐yl)pyrimidin‐2‐amines: dual inhibitors of TYK2 and JAK1. Bioorg Med Chem 2020; 28:115481. [DOI] [PubMed] [Google Scholar]
- 39. King B, Guttman‐Yassky E, Peeva E et al. A phase 2a randomized, placebo‐controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24‐week results. J Am Acad Dermatol 2021; 85:379–87. [DOI] [PubMed] [Google Scholar]
- 40. Forman SB, Pariser DM, Poulin Y et al. TYK2/JAK1 inhibitor PF‐06700841 in patients with plaque psoriasis: phase IIa, randomized, double‐blind, placebo‐controlled trial. J Invest Dermatol 2020; 140:2359–70 e5. [DOI] [PubMed] [Google Scholar]
- 41. Sohn SJ, Barrett K, Van Abbema A et al. A restricted role for TYK2 catalytic activity in human cytokine responses revealed by novel TYK2‐selective inhibitors. J Immunol 2013; 191:2205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guttman‐Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol 2017; 48:68–73. [DOI] [PubMed] [Google Scholar]
- 43. Brunner PM, Israel A, Zhang N et al. Early‐onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22‐centered inflammation and lipid alterations. J Allergy Clin Immunol 2018; 141:2094–106. [DOI] [PubMed] [Google Scholar]
- 44. Sanyal RD, Pavel AB, Glickman J et al. Atopic dermatitis in African American patients is TH2/TH22‐skewed with TH1/TH17 attenuation. Ann Allergy Asthma Immunol 2019; 122:99–110. [DOI] [PubMed] [Google Scholar]
- 45. Chan TC, Sanyal RD, Pavel AB et al. Atopic dermatitis in Chinese patients shows TH2/TH17 skewing with psoriasiform features. J Allergy Clin Immunol 2018; 142:1013–17. [DOI] [PubMed] [Google Scholar]
- 46. Chan IS, Zhang Z. Test‐based exact confidence intervals for the difference of two binomial proportions. Biometrics 1999; 55:1202–9. [DOI] [PubMed] [Google Scholar]
- 47. US Food and Drug Administration . CDER patient‐focused drug development. 27 July 2022. Available at: https://www.fda.gov/drugs/development‐approval‐process‐drugs/cder‐patient‐focused‐drug‐development (last accessed 27 June 2022).
- 48. Genovese MC, Kalunian K, Gottenberg JE et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease‐modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA 2019; 322:315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kay J, Harigai M, Rancourt J et al. Changes in selected haematological parameters associated with JAK1/JAK2 inhibition observed in patients with rheumatoid arthritis treated with baricitinib. RMD Open 2020; 6:e001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takeuchi T, Tanaka Y, Tanaka S et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double‐blind, placebo‐controlled trial (RAJ4) in Japan. Ann Rheum Dis 2019; 78:1305–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary Methods: Concomitant medications; Sample size determination; Participant selection.
Figure S1 Participant disposition (CONSORT diagram).
Figure S2 For selected doses: (a) LS mean of change from baseline in EASI score and (b) LS mean percentage change from baseline in EASI score in participants with AD.
Figure S3 Proportion of participants with atopic dermatitis achieving (a) EASI75 and (b) EASI90 at week 6.
Figure S4 Proportion of participants with AD achieving ≥ 4‐grade reduction from baseline in PP‐NRS through week 6 and follow‐up among participants with grade ≥ 4 at baseline.
Figure S5 Percentage change from baseline in BSA score at week 6 in participants with AD.
Figure S6 Change from baseline in POEM in participants with AD.
Figure S7 Proportion of participants with AD achieving ≥ 4‐grade reduction in DLQI from baseline at week 6 among participants with grade ≥ 4 at baseline (full analysis set, nonresponder imputation).
Figure S8 Median brepocitinib plasma concentration in participants with AD.
Table S1 Proportion of participants achieving ≥ 4‐grade reduction in severity of PP‐NRS over time.
Data Availability Statement
On request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information.


