Abstract
Introduction
Patients with lower urinary tract symptoms (LUTS) can be subcategorized into polyuria, normal or oliguria groups. Polyuria may be caused by pathologies including diabetes mellitus (DM), chronic kidney disease (CKD), diabetes insipidus (DI), or primary polydipsia (PPD). While fluid restriction is appropriate for some, doing so in all may result in serious complications. This study investigates the prevalence of these pathologies in LUTS patients with polyuria.
Materials and Methods
Two databases were retrospectively queried for men and women who filled out a lower urinary tract symptom score (LUTSS) questionnaire, 24‐h bladder diary (24HBD) and were polyuric (>2.5 L/day). Patients were divided into four groups: poorly controlled DM, DI, an CKD grade 3 and PPD. One‐way analysis of variance compared 24HBD and LUTSS questionnaires. Pearson correlation examined LUTSS and bother with 24‐h voided volume (24 HVV), maximum voided volume (MVV) and total voids.
Results
Among 814 patients who completed a 24HBD, 176 had polyuria (22%). Of the patients with complete data, 7.8% had poorly‐controlled DM, 3.1% had DI, 4.7% had CKD grade 3% and 84.4% had PPD. Amongst the four different sub‐groups, significant differences were seen in 24 HVV (p < 0.001), nocturnal urine volume (NUV) (p < 0.001), MVV (p = 0.003), daytime voids (p = 0.05), nocturnal polyuria index (NPi) (p < 0.001) and nocturia index (Ni) (p = 0.002). Significance was also seen between LUTSS and bother subscore (r = 0.68, p < 0.001), LUTSS and total voids (r = 0.29, p = 0.001) and bother sub‐score and total voids (r = 0.21, p = 0.019).
Conclusions
22% of patients with LUTS were found to have polyuria based on a 24HBD. Within this cohort, four sub‐populations were identified as being demonstrating statistically significant differences in 24 HVV, NUV, MVV, daytime voids, NPi and Ni. Identifying the underlying etiology of polyuria should be carried out to safely treat patients with LUTS.
Keywords: lower urinary tract symptoms, nocturia, nocturnal polyuria, polyuria/LUTS, urinary bladder
1. INTRODUCTION
Patients with lower urinary tract symptoms (LUTS), may be subcategorized based on their 24‐h urinary output into polyuria, normal or oliguria groups. In prior studies, it was shown that approximately 25% of patients who presented with LUTS exhibited polyuria. 1 , 2 These subclassifications are important, as they highlight differences in LUTS patients which affect diagnostic and treatment paradigms. Patients in whom polyuria is the primary cause of symptoms will have a different diagnostic and treatment pathway than one with benign prostatic hyperplasia (BPH), or other causes.
The differential diagnosis for polyuria is multifaceted. While usually the result of habitual or health conscious driven excess water consumption, polyuria may also be caused by one of several pathologic conditions, including diabetes mellitus (DM), chronic kidney disease (CKD) or diabetes insipidus (DI). According to recent center for disease control and prevention estimates, 3 , 4 DM is present in approximately 10% of the United States population, while CKD is present in about 15% of United States adults. DI is a much rarer condition, occurring in 1 in 25,000 individuals. 5 While fluid restriction is an appropriate treatment for the majority of patients with polyuria, fluid restriction is contraindicated in conditions causing obligatory diuresis due to renal concentrating defects (arginine vasopressin deficiency [central DI] or resistance [nephrogenic DI]), solute diuresis (uncontrolled DM), and may result in serious complications. This study seeks to investigate the prevalence of these various pathologic conditions in LUTS patients with polyuria.
2. MATERIALS AND METHODS
In this multi‐institutional IRB approved study, two databases were queried to identify patients who completed a 24‐h bladder diary (24HBD) and the lower urinary tract symptom score (LUTSS) questionnaire on a mobile app* (beginning in 2014) or paper. The first database contained patients from two different medical centers between 2015 and 2021, and the second contained those seen at a veterans affairs (VA) urology clinic between 2008 and 2019. All patients included in the study presented to urologists for initial evaluation of LUTS. The LUTSS is a validated 14 item questionnaire scored on a 5‐points Likert scale (0–4) which is comprised of a total score and six sub‐scores: storage, voiding, overactive bladder, incontinence, nocturia, and bother. 6 For multiple 24HBD and/or LUTSS, only the first recorded set was analyzed. LUTSS, when combined with a 24HBD provides granular data for establishing a diagnosis of voiding dysfunction, overactive bladder, incontinence and nocturia. Additional relevant information including age, gender, primary clinical diagnosis, and relevant associated conditions including DM, DI, CKD, and primary polydipsia (PPD) was obtained from the electronic medical record. Patients who had incomplete entries or had inaccurate recording of entries were excluded.
All patients analyzed in the study met criteria for polyuria (>2.5 L/24 h), a cutoff value based on published data modified by panel consensus. 7 , 8 , 9 Other criteria for polyuria have been used as well such as >3 L/day, 10 or >40−50 ml/kg/24 h, 11 which is the threshold for DI. We have found that most patients with LUTS due to high intake do not have DI or mellitus/glycosuria, but simply drink too much fluid for a variety of reasons which may be investigated by careful history‐taking. These patients often have no structural or functional lower urinary tract abnormalities and may be treated with counseling alone. If the threshold for diagnosing polyuria among a population of LUTS patients was raised, it would likely miss a great many individuals with no endocrine abnormality and no lower tract dysfunction, who might ordinarily be futilely treated with uropharmaceuticals.
Patients were divided into four groups based on secondary clinical diagnosis: poorly controlled DM (PC‐DM), DI, CKD ≥ grade 3 and PPD. Patient groups were not mutually exclusive. Only patients from the VA database had data on level of DM control, CKD grade 3 and presence of hypertension (HTN). Patients were classified as having well‐controlled or poorly‐controlled DM according to the guidelines set by the American Diabetes Association for target A1c levels in nonpregnant adults. The 2020 Standards of Medical Care in Diabetes outlines a target A1c goal of <7%. 12 Patients with an HbA1c level of greater than 7% and/or those with glycosuria were therefore regarded as poorly‐controlled DM, while those with an HbA1c level of equal to or less than 7% without glycosuria were considered to have well‐controlled DM. Well‐controlled diabetics were excluded from sub‐group analysis, reflecting a desire to portray the most accurate representation of patients who may be harmed by fluid restriction as a primary treatment for polyuria. Individuals with CKD were selected based on grade determined from estimated glomerular filtration rate (eGFR) values, and only those with grades 3 and 4 were included. These selection criteria were used to reflect patients with more severe disease and concurrent polyuria symptoms.
PPD is usually divided into two categories: dipsogenic (an intrinsic thirst disorder) and psychogenic. In PPD, polyuria is driven by excess fluid intake, rarely due to abnormally increased thirst (dipsogenic) and more commonly due to compulsive water drinking or due to a patient's belief of positive health benefits (behavioral). 13 With other forms of polyuria, excessive output is driven by either renal disease or endocrinopathy. However, in the context of this study, we use the term PPD to define polyuria in patients without a secondary diagnosis of DM, DI, or CKD and, thus, include those with a behaviorally induced excess urine production due to commensurately excessive fluid intake.
Data was analyzed using SPSS software version 28 (SPSS Inc.), utilizing a one‐way analysis of variance (ANOVA) to compare the four groups across data derived from 24HBD and LUTSS questionnaire. Pearson correlation was run examining the relationship between LUTSS and bother with 24 HVV, maximum voided volume (MVV) and total voids. A p‐value of ≤0.05 was considered significant. In addition, the prevalence of <2 nocturia episodes and presence of HTN among PC‐DM, DI, CKD grade 3, and PPD patients was examined.
*WeShare® Uro by Symptelligence Medical Informatics LLC.
3. RESULTS
A summary of the patient population is displayed in Figure 1 along with corresponding inclusion/exclusion criteria. Of the 146 men and women with polyuria who completed a 24HBD and LUTSS Questionnaire, 7.8% had PC‐DM, 3.1% had DI, 4.7% had CKD grade 3 and 84.4% had PPD. Of the 6 CKD patients, 1 was followed for suspected DI, although a diagnosis was never confirmed. Out of 28 diabetic patients, 18 were excluded from further analysis, 10 due to a lack of complete information on diabetic control and 8 due to our a priori exclusion of well controlled diabetics. Thus, subgroup percentages were calculated from a total of 128 polyuria patients. Additionally, all 10 poorly controlled diabetics maintained a diagnosis of DM before the discovery of polyuria by a urologist, and 9 of 10 were on medications for diabetic control at the time of polyuria diagnosis.
Figure 1.
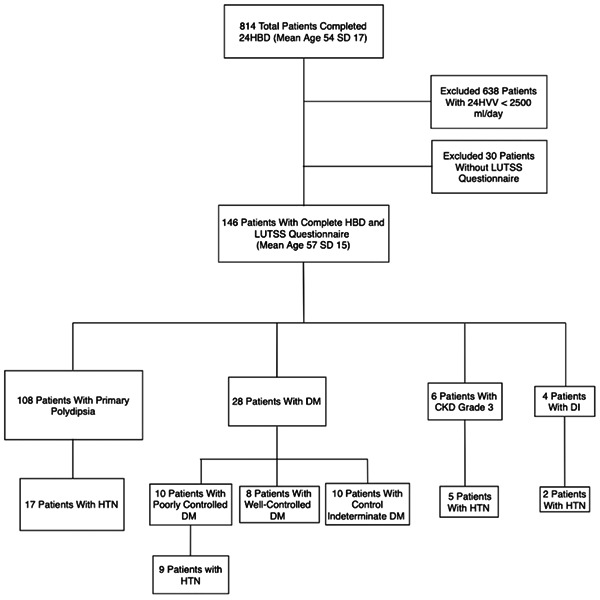
Summary of patient population with corresponding inclusion/exclusion criteria. CKD, chronic kidney disease; DI, diabetes insipidus; DM, diabetes mellitus; HTN, hypertension.
Amongst the four different sub‐groups (PC‐DM, DI, CKD‐3, and PPD), statistically significant differences were seen in one‐way ANOVA measurements of 24 h voided volume (24 HVV) (3564.3, 5107.5, 4195.8, 3330.0, respectively, p < 0.001), nocturnal urine volume (NUV) (1494.6, 1287.6, 1746.4, 711.9 respectively, p ≤ 0.001), MVV (669.9, 693.8, 772.5, 479.6 respectively, p = 0.003), daytime voids (7.5, 12.0, 7.6, 11.0 respectively, p = 0.05), nocturnal polyuria index (NPi) (0.41, 0.28, 0.42, 0.22 respectively, p < 0.001), nocturia index (Ni) (2.3, 1.8, 2.5, 1.8 respectively, p = 0.002). The prevalence of nocturia < 2 amongst all polyuria patients was 41%, amongst patients with concomitant PC‐DM was 38%, amongst patients with DI was 25%, amongst patients with CKD Grade 3 was 20% and amongst patients with PPD was 44%. Additionally, of the 55 patients who had data on hypertensive status available, the prevalence of HTN amongst all was 64%, amongst those with PC‐DM was 90%, amongst those with DI was 67%, amongst those with CKD grade 3 was 83% and amongst those with PPD was 57%.
Symptoms play a large role in guiding the management for males with LUTS. As a result, we explored the relationship between LUTSS and 24HBD. Pearson correlation was run and there was a significant relationship between LUTSS and bother score (r = 0.68 and p = <0.001), LUTSS and total voids (r = 0.29 and p = 0.001) and bother and total voids (r = 0.21 and p = 0.019). The above data is summarized in Table 1.
Table 1.
Phenotypes of polyuria patients
| Poorly Controlled Diabetes Mellitus | Diabetes Insipidus | CKD Grade 3 | Primary Polydipsia | p Value | |
|---|---|---|---|---|---|
| n = 10/28a | n = 4 | n = 6 | n = 108 | ||
| (7.8%) | (3.1%) | (4.7%) | (8 4.4%) | ||
| Age | 64.3 | 60.5 | 68.3 | 56.2 | 0.08 |
| 24 HVV (ml/24 h) | 3564.3 | 5107.5 | 4195.8 | 3330.0 | <0.001b |
| NUV | 1494.6 | 1287.6 | 1746.4 | 711.9 | <0.001b |
| MVV (ml) | 669.9 | 693.8 | 772.5 | 479.6 | 0.003b |
| Daytime voids | 7.5 | 12.0 | 7.6 | 11.0 | 0.05b |
| Nighttime voids | 2.1 | 2.3 | 3.2 | 2.1 | 0.707 |
| Total voids | 9.6 | 14.3 | 10.8 | 13.1 | 0.159 |
| UPG | 2.6 | 2.7 | 2.3 | 2.3 | 0.635 |
| NPi | 0.41 | 0.28 | 0.42 | 0.22 | <0.001b |
| Ni | 2.3 | 1.8 | 2.5 | 1.0 | 0.002b |
| LUTSS | |||||
| Total score | 23.6 | 23.0 | 29.0 | 23.7 | 0.580 |
| Bother score | 2.2 | 2.5 | 2.7 | 2.2 | 0.781 |
Abbreviations: MVV, Maximum Voided Volume; NI, Nocturia Index; NPI, Nocturia Polyuria Index; NUV, Nocturnal Urine Volume; UPG, Urge Perception Grade; VV, Voided Volume.
Poorly controlled diabetics represent 10/28 total diabetic patients;
Denotes statistical significance.
4. DISCUSSION
Recent studies have documented that approximately 25% of patients with LUTS referred to urologists have polyuria, 1 , 2 yet none of the guidelines specifically address the diagnosis and treatment of polyuria in these patients. The 2021 american urological association (AUA) guidelines for the management of BPH estimates the prevalence of BPH at approximately 60% in men at age 60 and 80% in men at age 80. 14 Additionally, the guideline document states, “because BPH is nearly ubiquitous and because LUTS in men is commonly associated with and/or caused by BPE/BPO, a compromise terminology is often used referring to ‘LUTS most likely associated with BPE/BPO and BPH’, or ‘LUTS secondary to BPH’.” While the panel does state that with extensive evaluation, some men will be found to have other causes contributing to their symptoms, they only stress the importance for a more definitive diagnosis “as treatments being considered specifically for BPO become more invasive and risky.” These statements imply that in men, LUTS may be primarily assumed to be associated with BPH, and that this is often thought to be due to underlying anatomic obstruction, namely BPO. However, it has been reported that a significant portion of those presenting with LUTS may actually be suffering from polyuria, and it is likely that, in many, the polyuria is the primary cause of their symptoms. 1 Additionally, the AUA guideline for diagnosis and treatment of overactive bladder (OAB) in adults, which is conventionally thought to be a leading component of LUTS in females, recommends investigating other causes which may mimic symptoms of OAB, namely excess fluid intake. 15 Therefore, an investigation into causes of polyuria, a major consequence of excess fluid intake, is warranted.
The 2021 AUA guidelines for the surgical management of BPH do not recommend the routine use of bladder diaries in the initial workup of men with LUTS. The 2014 guideline panel did recommend use of bladder diaries for men experiencing > 2 nocturia episodes. Data from the present study, however, show that 41% of polyuria patients did not experience > 2 nocturnal voids. Therefore, based on the current and past AUA guidelines, failure to utilize bladder diaries in the initial workup of patients with LUTS would fail to identify 18%−41% of these polyuria patients who may benefit from a polyuria specific treatment pathway. The data in the past and present studies reinforce the appropriateness of phenotyping patients based on urinary output and exploring polyuria specific algorithms for patient management.
Polyuria patients with LUTS may simply experience symptoms due to excess fluid intake. However, restricting fluids in a patient with underlying pathologic conditions like DM, DI, or CKD could be very harmful and result in severe dehydration, electrolyte imbalances, coma and even death. 5 , 16 Determination of the root causes of polyuria necessitates an exploration of the physiological mechanism behind their current disease etiology.
DM may cause an osmotic diuresis, as excess blood glucose cannot be properly reabsorbed by the kidneys and is excreted in the urine. 17 , 18 Water passively follows and results in increased urine production. In uncontrolled DM, the osmotic diuresis caused by hyperglycemia further concentrates blood glucose as water is excreted in urine along with excess glucose, expressed as symptoms of polydipsia and polyuria. The loss of renal homeostatic mechanisms results in further dehydration, hypovolemia and diabetic complications as hyperglycemia worsens which can be greatly exacerbated with fluid restriction. 16 , 19
In DI, there are two distinct forms: Central (CDI), in which there is inadequate production of ADH, and nephrogenic (NDI), in which the kidneys cannot properly respond to ADH. 5 In both cases, the result is increased production of dilute urine. CDI may be hereditary or acquired, although the majority of cases are acquired. Causes of acquired CDI include prior head trauma, neurosurgery, brain tumors, or autoimmune, inflammatory and vascular issues involving the brain. 20 Congenital causes of NDI result from hereditary mutations in AQP2/V2R genes, symptoms commonly presenting early in childhood. 5 Adults generally present with the acquired form of NDI which may be drug induced, most commonly due to lithium toxicity. In rat models of CKD, a similar downregulation of AVPR2 and AQP2 genes was seen. 21 In DI, the danger of fluid restriction is highlighted by the effects of the water deprivation test, where hypotonic polyuria persists despite restriction of fluids, and, on a prolonged basis, may result in hypovolemia and hypernatremia. Severe hypernatremia is a serious consequence which may lead to seizures, coma and death. All DI patients in the present study were identified as having NDI as a result of lithium renal toxicity. The possibility of concomitant DI was investigated in the CKD patients within this study due to their polyuric output, however, upon further inquiry into their medical records, only one patient was explicitly followed for potential DI, but a diagnosis was never confirmed.
PPD results in prolonged excessive fluid intake and is usually classified as either psychogenic or dipsogenic, but to this classification, we add a 3rd category—behavioral. Psychogenic polydipsia, which was first noted in schizophrenic patients, 22 is largely seen in schizoaffective disorder, bipolar disorder, and psychotic depression. 13 Behavioral polydipsia often stems from the perception that it is healthy to drink even and beyond what thirst dictates—“8 glasses per day (1920 mL).” 13 , 23 , 24 Add to this, the obligatory water intake that is part of the solid food component of the average diet and practically everyone would have polyuria.
Dipsogenic polydipsia is seen in patients with disease in the hypothalamus, thus affecting the thirst mechanism. Fluid consumption in these patients will result in increased urine production, but rarely causes hyponatremia. 23 There is no established treatment protocol for patients with PPD, 13 and fluid restriction may not be adequate due to issues of noncompliance. Unfortunately, our database did not classify patients according to the schemata that was just described, so patients were classified as having PPD when no concurrent secondary diagnosis could be identified.
Our findings have several important clinical implications. The first is that there is a large cohort of patients who present with LUTS who have concurrent polyuria (22% in the present series) and this has been corroborated by a number of other studies. 1 , 2 Additionally, polyuria patients fall into distinct pathologic phenotypes characterized by abnormal 24 HVV, NUV, MVV, daytime voids, NPi, and Ni. While a physician may be inclined to fluid restrict a patient presenting with polyuria, doing so in someone with DM, DI, or CKD could cause patient harm. Of note, the discovery of polyuria in patients of this cohort did not illuminate any previously undiagnosed conditions of uncontrolled DM, DI, or CKD. Therefore, inquiry into a patient's medical history for one of these conditions seems to be sufficient before pursuing treatment for polyuria via fluid restriction. The presence of these subclassifications within the polyuria cohort, however, highlights the necessity of developing more specific treatment algorithms for those in whom fluid restriction is contraindicated.
There are a number of weaknesses of the present study including its retrospective nature. Additionally, only 55 of the 146 patients had data regarding DM control, CKD grade and presence of HTN, limiting the ability of the study to fully classify all patients within these particular categories. For grade of CKD, eGFR was the sole determinant. However, the accuracy of GFR‐estimating equations to identify progression of kidney disease has been called into question given biological variability of its main determinant (serum creatinine) and lack of prospective studies of the ability of GFR‐estimating equations to monitor progression. 25
5. CONCLUSION
In this series 814 patients referred for evaluation of LUTS, 22% were found to have polyuria based on a 24HBD. Within this cohort, approximately 10% had conditions that could cause serious harm if empiric fluid restriction is recommended including DI and PC‐DM. Further, in some patients the polyuria was the sole cause of LUTS; there was no underlying lower urinary tract abnormalities. These findings magnify the importance identifying polyuria as a cause of LUTS as it may alter treatment options available for each patient.
CONFLICTS OF INTEREST
Author Jeffrey P. Weiss reports the following: Ferring—Consultant (C), Advisor (A). Institute for Bladder and Prostate Research—C, A. Evidera—C, A. Author Jerry G. Blaivas reports the following: Symptelligence—C, A. The remaining authors declares no conflict of interests.
ETHICS STATEMENT
This study was review and deemed exempt by the New York Harbor Healthcare System Institutional Review Board (IRB), (New York, NY, USA). Owing to the retrospective design and deidentified collection of data, informed consent was not required. All study procedures followed the standards set by the Declaration of Helsinki and its amendments as well as the Health Insurance Portability and Accountability Act. Owing to the retrospective design and deidentified collection of data, informed consent was not required per New York Healthcare System Institutional Review Board (New York, NY, USA).
ACKNOWLEDGMENT
This study received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Fisch GZ, Fang AH, Miller CD, et al. Polyuria in patients with lower urinary tract symptoms: prevalence and etiology. Neurourol Urodyn. 2023;42:256‐262. 10.1002/nau.25078
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Blaivas JG, Li ESW, Dayan L, et al. Overactive bladder phenotypes: development and preliminary data. Can J Urol. 2021;28(3):10699‐10704. [PubMed] [Google Scholar]
- 2. Clemens JQ, Wiseman JB, Smith AR, et al. Prevalence, subtypes, and correlates of nocturia in the symptoms of lower urinary tract dysfunction research network cohort. Neurourol Urodyn. 2020;39(4):1098‐1107. 10.1002/nau.24338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html [Google Scholar]
- 4. Centers for Disease Control and Prevention . Chronic Kidney Disease in the United States, 2021. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2021. https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html
- 5. Kalra S, Zargar A, Jain S, et al. Diabetes insipidus: the other diabetes. Indian J Endocrinol Metab. 2016;20(1):9‐21. 10.4103/2230-8210.172273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blaivas JG, Tsui JF, Mekel G, et al. Validation of the lower urinary tract symptom score. Can J Urol. 2015;22(5):7952‐7958. [PubMed] [Google Scholar]
- 7. van Haarst EP, Heldeweg EA, Newling DW, Schlatmann TJ. The 24‐h frequency‐volume chart in adults reporting no voiding complaints: defining reference values and analysing variables. BJU Int. 2004;93(9):1257‐1261. [DOI] [PubMed] [Google Scholar]
- 8. Haylen BT, Ashby D, Sutherst JR, Frazer MI, West CR. Maximum and average urine flow rates in normal Male and female populations‐the liverposl nomograms. Br J Urol. 1989;64(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 9. Weiss JP, Blaivas JG. NOCTURIA. J Urol. 2000;163(1):5‐12. [PubMed] [Google Scholar]
- 10. Nigro N, Grossmann M, Chiang C, Inder WJ. Polyuria‐polydipsia syndrome: a diagnostic challenge. Intern Med J. 2018;48(3):244‐253. 10.1111/imj.13627 [DOI] [PubMed] [Google Scholar]
- 11. Hui C, Khan M, Radbel JM. Diabetes Insipidus. [Updated 2021 Jun 16]. StatPearls [Internet]. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK470458/ [PubMed] [Google Scholar]
- 12. American Diabetes Association . Standards of Medical Care in Diabetes‐2020 Abridged for Primary Care Providers. Clinical Diabetes. 2020;38(1):10‐38. 10.2337/cd20-as01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kotagiri R, Kutti Sridharan G. Primary Polydipsia. 2021 Jul 31. StatPearls [Internet]. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 14. Lerner Lori B, McVary Kevin T, Barry Michael J, et al. 2021. “Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA GUIDELINE.” [DOI] [PubMed]
- 15. Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non‐neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(suppl 6):2455‐2463. 10.1016/j.juro.2012.09.079 [DOI] [PubMed] [Google Scholar]
- 16. Molinaro R, Dauscher C. Complications resulting from uncontrolled diabetes. MLO Med Lab Obs. 2017;49(2):20. [PubMed] [Google Scholar]
- 17. Zerbe RL, Vinicor F, Robertson GL. Plasma vasopressin in uncontrolled diabetes mellitus. Diabetes. 1979;28(5):503‐508. 10.2337/diab.28.5.503 [DOI] [PubMed] [Google Scholar]
- 18. BRODSKY WA, RAPOPORT S, WEST CD. The mechanism of glycosuric diuresis in diabetic man. J Clin Invest. 1950;29(8):1021‐1032. 10.1172/JCI102333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Ness‐Otunnu R, Hack JB. Hyperglycemic crisis. J Emerg Med. 2013;45(5):797‐805. 10.1016/j.jemermed.2013.03.040 [DOI] [PubMed] [Google Scholar]
- 20. Di Iorgi N, Napoli F, Allegri AEM, et al. Diabetes insipidus—diagnosis and management. Hormone Research in Paediatrics. 2012;77(2):69‐84. 10.1159/000336333 [DOI] [PubMed] [Google Scholar]
- 21. Moeller HB, Rittig S, Fenton RA. Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocr Rev. 2013;34(2):278‐301. 10.1210/er.2012-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dundas B, Harris M, Narasimhan M. Psychogenic polydipsia review: etiology, differential, and treatment. Curr Psychiatry Rep. 2007;9(3):236‐241. 10.1007/s11920-007-0025-7 [DOI] [PubMed] [Google Scholar]
- 23. Christ‐Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54. 10.1038/s41572-019-0103-2 [DOI] [PubMed] [Google Scholar]
- 24. May DL. Patient perceptions of self‐induced water intoxication. Arch Psychiatr Nurs. 1995;9(5):295‐304. 10.1016/s0883-9417(95)80049-2 [DOI] [PubMed] [Google Scholar]
- 25. Lamb EJ, Brettell EA, Cockwell P, et al. The eGFR‐C study: accuracy of glomerular filtration rate (GFR) estimation using creatinine and cystatin C and albuminuria for monitoring disease progression in patients with stage 3 chronic kidney disease—prospective longitudinal study in a multiethnic population. BMC Nephrol. Published 2014 January 14, 2014. doi:10.1186/1471‐2369‐15‐13 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


