Abstract
Aims
Empagliflozin improves cardiovascular and renal outcomes in patients with heart failure (HF) and reduced ejection fraction (HFrEF), but its efficacy and safety across patient's age is not well established.
Methods and results
We assessed the effects of empagliflozin (10 mg daily) versus placebo, on top of standard HF therapy, in symptomatic HFrEF patients with a left ventricular ejection fraction ≤40% and increased natriuretic peptides stratified by age (<65, 65–74, ≥75 years). The primary endpoint was a composite of cardiovascular death or HF hospitalization. Key secondary endpoints included first and recurrent HF hospitalizations and slope of change in estimated glomerular filtration rate (eGFR); the latter was supported by an analysis of a renal composite endpoint (chronic dialysis or renal transplantation or profound and sustained reduction in eGFR). Of 3730 patients, 38% were <65 years, 35% were 65–74 years and 27% were ≥75 years. Compared with placebo, empagliflozin reduced the primary endpoint consistently across the three age groups (hazard ratio 0.71 [95% confidence interval 0.57–0.89] for <65 years, 0.72 [0.57–0.93] for 65–74 years, 0.86 [0.67–1.10] for ≥75 years, interaction p‐trend test = 0.24). The effects of empagliflozin were also consistent across age groups for key secondary endpoints of first and recurrent HF hospitalization (p‐trend = 0.30), the rate of decline in eGFR (p‐trend = 0.78) and the renal composite (p‐trend = 0.94). Adverse events (AEs), serious AEs and AEs leading to drug discontinuation increased with age in both treatment arms, but empagliflozin did not increase their incidence over placebo within each age group.
Conclusion
The efficacy and safety of empagliflozin in improving cardiovascular and renal outcomes in HFrEF was consistent across the spectrum of age, including older patients (aged ≥75).
Keywords: Heart failure, Age, Sodium–glucose cotransporter 2 inhibitors, Empagliflozin
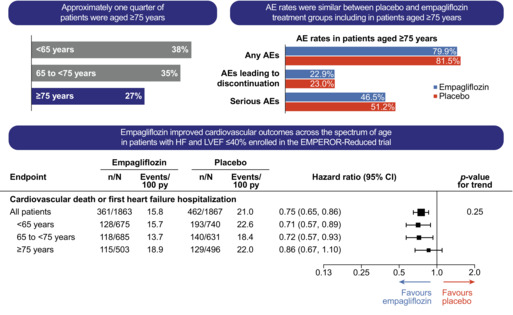
Effects of empagliflozin on cardiovascular and renal outcomes in heart failure with reduced ejection fraction according to age: a secondary analysis of EMPEROR‐Reduced. AE, adverse event; HF, heart failure; LVEF, left ventricular ejection fraction.
Introduction
The prevalence of heart failure (HF) increases with age, and HF is the most frequent cause for hospital admission in the elderly. 1 , 2 Previous studies have shown that older HF patients have worse outcomes that may partly be related to the higher burden of comorbidities and the lower use of guideline‐recommended therapies. 3 , 4 , 5 , 6 , 7 , 8 Lower prescription rates may in turn result from physicians' concerns about polypharmacy, lower tolerability, and reduced safety or impaired efficacy of these drugs in older patients. 7 , 9 , 10
The sodium–glucose cotransporter 2 inhibitors (SGLT2i) empagliflozin and dapagliflozin have been shown to improve cardiovascular and renal outcomes in HF patients with reduced ejection fraction (HFrEF). 11 , 12 A secondary analysis of the DAPA‐HF trial showed that the benefit of dapagliflozin was consistent across the spectrum of age in HFrEF. 13 The EMPEROR‐Reduced trial studied the effect of empagliflozin on cardiovascular and renal outcomes in patients with more advanced disease. In the present study, we evaluated the effect of age on the efficacy, tolerability, and safety of empagliflozin in HFrEF.
Patients and methods
The design of EMPEROR‐Reduced has been described in detail elsewhere. 12 , 14 In brief, the trial randomized 3730 symptomatic HFrEF patients with a left ventricular ejection fraction (LVEF) of 40% or less and increased levels of natriuretic peptides to either empagliflozin 10 mg daily or placebo on top of all appropriate drug and device treatments for HF. The primary endpoint was a composite of cardiovascular death or hospitalization for worsening HF, and the two secondary endpoints were first and recurrent HF hospitalizations and the rate of decline in estimated glomerular filtration rate (eGFR). The latter was supported by an analysis of a renal composite endpoint (chronic dialysis or renal transplantation or profound and sustained reduction in eGFR of ≥40%, or a sustained eGFR <15 ml/min/1.73 m2 [if baseline eGFR ≥30] or sustained eGFR <10 ml/min/1.73 m2 [if baseline eGFR <30]).
In the present study, we analysed the efficacy and safety outcomes of empagliflozin or placebo according to three commonly used age groups (of similar size), defined as younger than 65, 65–74 and 75 or older. We investigated the effects of empagliflozin versus placebo on the primary and key secondary endpoints along with other pre‐specified endpoints including time‐to‐first HF hospitalization, cardiovascular death, all‐cause death, renal endpoints and change in health status at week 52 as evaluated by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ‐CSS). We further analysed the effect of age on the primary endpoint and the incidence of adverse events (AEs) in the placebo arm.
Statistical analysis
Analyses were performed according to the intention‐to‐treat principle for all randomized patients. For time‐to‐first‐event analyses, differences between the placebo and empagliflozin groups were assessed for statistical significance using a Cox proportional hazards model, with pre‐specified covariates of gender, geographical region, diabetes status at baseline, LVEF, and eGFR at baseline. In addition, for the primary endpoint of cardiovascular death or HF hospitalization, considering age as a continuous variable was investigated and differences between age groups in placebo arm are compared separately using a same Cox model. For the analysis of total (first and repeated) events, between‐group differences were assessed using a joint frailty model, with cardiovascular death as a competing risk. Between‐group difference in the slope of change in eGFR was analysed using a random intercept random slope model including baseline eGFR as linear covariate and sex, region, baseline LVEF, baseline diabetes status, and baseline eGFR‐by‐time, treatment‐by‐age group, and treatment‐by‐time‐by‐age group as fixed effects; the model allows for randomly varying slope and intercept between patients. For the analysis of changes in eGFR and KCCQ scores, treatment effects were assessed based on changes from baseline using a mixed model for repeated measures (MMRM). eGFR slope and changes in eGFR MMRM analyses were analysed using on‐treatment data. The MMRM and the joint frailty model included the same covariates as the Cox model. To assess the consistency of effects across subgroups, subgroup‐by‐treatment interaction terms were added in the models and trend test were performed assuming ordered age categories. Analyses for safety were performed including all patients who had received at least one dose of empagliflozin or placebo. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA). All p‐values reported are two‐sided, and p < 0.05 was considered as statistically significant. No adjustments for multiple testing were made.
Results
The mean age of patients enrolled in EMPEROR‐Reduced was 67 years (empagliflozin arm, 67.2 ± 10.8 years; placebo arm, 66.5 ± 11.2 years). The distribution of age in the study population is outlined in online supplementary Figure S1 . Out of a total of 3730 patients, 1415 (38%) were younger than 65 years, 1316 (35%) were aged 65–74, and 999 (27%) were 75 or older.
The baseline features of patients according to age are reported in Table 1 . Patients in the oldest age group were more likely to be female and to suffer from comorbidities such as arterial hypertension and atrial fibrillation. Systolic blood pressure, LVEF and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) increased with age, while body mass index (BMI) and eGFR declined. Functional capacity according to New York Heart Association class and health status by KCCQ‐CSS did not differ significantly among groups. The frequency of HF hospitalization within the preceding 12 months declined with age. Regarding HF therapies, the use of renin–angiotensin–aldosterone system inhibitors, including angiotensin‐converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), angiotensin receptor–neprilysin inhibitor (ARNi) and mineralocorticoid receptor antagonists (MRA), declined with age, while the use of beta‐blockers did not differ significantly among age groups. Inversely, use of device therapies, particularly cardiac resynchronization therapy (CRT), increased with age.
Table 1.
Baseline patient features by age groups
| Age group, years | p‐value | |||
|---|---|---|---|---|
| <65 | 65–74 | ≥75 | ||
| Patients, n (%) | 1415 (37.9) | 1316 (35.3) | 999 (26.8) | – |
| Age, years | 55.5 ± 7.6 | 69.4 ± 2.8 | 79.5 ± 3.5 | <0.0001 |
| Female sex, n (%) | 340 (24.0) | 289 (22.0) | 264 (26.4) | 0.04 |
| Race a , n (%) | <0.0001 | |||
| White | 879 (62.1) | 971 (73.8) | 779 (78.0) | |
| Black | 151 (10.7) | 64 (4.9) | 42 (4.2) | |
| Asian | 303 (21.4) | 220 (16.7) | 149 (14.9) | |
| Other or missing | 82 (5.8) | 61 (4.6) | 29 (2.9) | |
| Region, n (%) | <0.0001 | |||
| North America | 143 (10.1) | 131 (10.0) | 151 (15.1) | |
| Latin America | 609 (43.0) | 425 (32.3) | 252 (25.2) | |
| Europe | 365 (25.8) | 542 (41.2) | 446 (44.6) | |
| Asia | 192 (13.6) | 169 (12.8) | 132 (13.2) | |
| Other | 106 (7.5) | 49 (3.7) | 18 (1.8) | |
| NYHA class III/IV, n (%) | 354 (25.0) | 315 (23.9) | 261 (26.1) | 0.48 |
| Body mass index, kg/m2 | 28.5 ± 5.7 | 28.1 ± 5.2 | 26.8 ± 5.0 | <0.0001 |
| Systolic blood pressure, mmHg | 119.9 ± 15.3 | 122.8 ± 15.9 | 123.9 ± 15.4 | <0.0001 |
| LVEF, % | 26.5 ± 6.2 | 27.6 ± 5.9 | 28.5 ± 5.8 | <0.0001 |
| NT‐proBNP, pg/ml, median (IQR) | 1671 (978, 2995) | 1903 (1117, 3501) | 2255 (1412, 4098) | <0.0001* |
| HF cause, n (%) | <0.0001 | |||
| Ischaemic | 607 (42.9) | 753 (57.2) | 569 (57.0) | |
| Non‐ischaemic | 808 (57.1) | 563 (42.8) | 430 (43.0) | |
| CV history, n (%) | ||||
| HF hospitalization within 12 months | 476 (33.6) | 385 (29.3) | 290 (29.0) | 0.02 |
| Arterial hypertension | 921 (65.1) | 980 (74.5) | 797 (79.8) | <0.0001 |
| Atrial fibrillation | 333 (23.5) | 530 (40.3) | 506 (50.7) | <0.0001 |
| Diabetes mellitus | 722 (51.0) | 680 (51.7) | 454 (45.4) | 0.01 |
| eGFR, ml/min/1.73 m2 (CKD‐EPI) | 73.4 ± 22.1 | 59.0 ± 18.2 | 49.9 ± 16.5 | <0.0001 |
| KCCQ‐CSS | 70.0 ± 22.8 | 71.4 ± 21.2 | 70.8 ± 21.7 | 0.25 |
| HF therapies, n (%) | ||||
| ACEi or ARB | 1001 (70.7) | 938 (71.3) | 661 (66.2) | 0.02 |
| ARNi | 292 (20.6) | 235 (17.9) | 200 (20.0) | 0.17 |
| ACEi, ARB or ARNi | 1278 (90.3) | 1162 (88.3) | 853 (85.4) | 0.0010 |
| Beta‐blockers | 1338 (94.6) | 1256 (95.4) | 939 (94.0) | 0.29 |
| MRA | 1117 (78.9) | 925 (70.3) | 619 (62.0) | <0.0001 |
| ICD (ICD or CRT‐D) | 388 (27.4) | 451 (34.3) | 331 (33.1) | 0.0002 |
| CRT (CRT‐D or CRT‐P) | 94 (6.6) | 180 (13.7) | 164 (16.4) | <0.0001 |
Plus‐minus values are means ± standard deviation.
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CRT, cardiac resynchronization therapy; CRT‐D, cardiac resynchronization therapy with defibrillator; CRT‐P, cardiac resynchronization therapy with pacemaker; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter‐defibrillator; IQR, interquartile range; KCCQ‐CSS, Kansas City Cardiomyopathy Questionnaire‐clinical summary score; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
Race was self‐reported; patients who identified with ≥1 race or with no race were classified as other.
Based on log‐transformed value.
In the placebo arm, the incidence rate of the primary endpoint of cardiovascular death or HF hospitalization was lower in the middle‐age group compared to the youngest group (hazard ratio [HR] 0.79, 95% confidence interval [CI] 0.63, 0.99, p = 0.04), but did not differ between the oldest and the youngest groups (HR 0.89 [95% CI 0.69,1.14], p = 0.36; Figure 1 ). This difference was driven by a lower incidence rate of HF hospitalization in the middle‐age group (HR 0.71 [95% CI 0.54, 0.93], p = 0.01) versus the youngest group.
Figure 1.
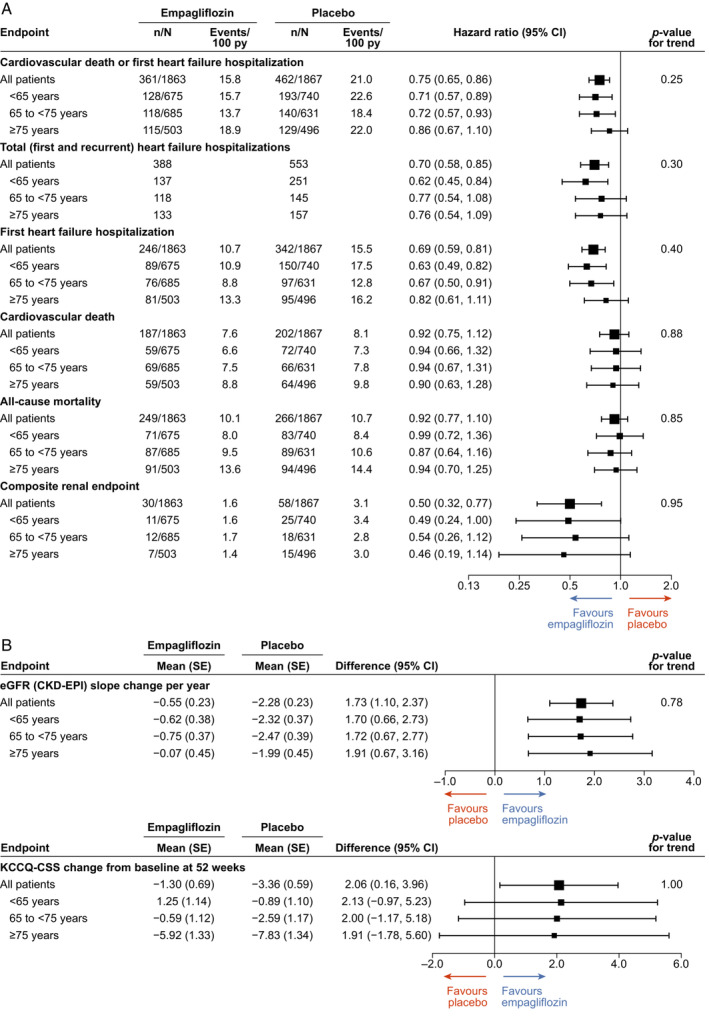
Forest plots for main cardiovascular and renal outcomes (A) and across estimated glomerular filtration rate (eGFR) and Kansas City Cardiomyopathy Questionnaire‐ clinical summary score (KCCQ‐CSS) (B) by age groups. CI, confidence interval; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; SE, standard error; py, patient‐years.
The effects of empagliflozin versus placebo on the primary endpoint of cardiovascular death or HF hospitalization was consistent across the three age groups (p for trend test = 0.25). Empagliflozin also reduced the primary endpoint compared with placebo across the spectrum of patient age taken as a continuous variable (p for interaction = 0.24; online supplementary Figure S2 ). The effects of empagliflozin compared to placebo were consistent in the three age groups also for the key secondary endpoints of first and recurrent HF hospitalization (p for trend test = 0.30) and slope of change in eGFR per year (p for trend test = 0.78). The same was true for other pre‐specified endpoints including cardiovascular death (p for trend test = 0.88), all‐cause death (p for trend test = 0.85), time‐to‐first HF hospitalization (p for trend test = 0.40) and the renal composite outcome (p for trend test = 0.95; Figure 1 ).
Concerning renal function, eGFR exhibited an initial drop after empagliflozin initiation that was similar across age groups and this initial drop was attenuated by week 12, followed thereafter by a slower rate of decline compared with placebo in all three age groups (mean slope of change: <65 years, −0.62; 65–75 years, −0.75; ≥75 years, −0.074 ml/min/1.73 m2). The effect of empagliflozin versus placebo on the change in the KCCQ‐CSS through week 52 was also consistent in the three age groups (+1.98 [95% CI 0, 3.95] in <65 years, +1.43 [95% CI –0.61, 3.48] in 65–74 years +1.12 [95% CI –1.30, 3.54] in ≥75 years, p for trend test = 0.57).
The incidence of AEs, serious AEs and AEs leading to drug discontinuation increased with age in both treatment arms (Table 2 ). Within each age group, empagliflozin did not increase the incidence of AEs compared with placebo. Regarding AEs of specific interest in patients aged 75 or older, such as hypotension, hypoglycaemia, volume depletion, urinary tract infections, hypo‐ and hyperkalaemia, empagliflozin was not associated with an increase in these AEs compared with placebo.
Table 2.
Summary of adverse events and adverse events of interest by treatment status and age groups
| Age group, years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <65 | 65–75 | ≥75 | ||||||||||
| Placebo | Empagliflozin | Placebo | Empagliflozin | Placebo | Empagliflozin | |||||||
| Category of AEs, n (%) | n = 739 | Incidence rate per 100 py | n = 675 | Incidence rate per 100 py | n = 628 | Incidence rate per 100 py | n = 685 | Incidence rate per 100 py | n = 496 | Incidence rate per 100 py | n = 503 | Incidence rate per 100 py |
| Patients with any AEs | 558 (75.5) | 158.3 | 493 (73.0) | 136.4 | 501 (79.8) | 156.1 | 525 (76.6) | 148.5 | 404 (81.5) | 195.1 | 402 (79.9) | 169.2 |
| AEs leading to study drug discontinuation | 113 (15.3) | 12.6 | 92 (13.6) | 11.2 | 101 (16.1) | 13.1 | 115 (16.8) | 13.6 | 114 (23.0) | 19.8 | 115 (22.9) | 19.4 |
| Serious AEs | 340 (46.0) | 53.5 | 249 (36.9) | 38.3 | 302 (48.1) | 53.5 | 289 (42.2) | 44.1 | 254 (51.2) | 59.9 | 234 (46.5) | 51.4 |
| Hypotension | 63 (8.5) | 7.5 | 64 (9.5) | 8.3 | 45 (7.2) | 6.0 | 60 (8.8) | 7.5 | 55 (11.1) | 10.3 | 52 (10.3) | 9.2 |
| Acute renal failure | 78 (10.6) | 9.2 | 57 (8.4) | 7.2 | 60 (9.6) | 8.2 | 68 (9.9) | 8.4 | 54 (10.9) | 9.8 | 50 (9.9) | 8.9 |
| Confirmed hypoglycaemic events a | 18 (2.4) | 2.0 | 10 (1.5) | 1.2 | 5 (0.8) | 0.7 | 12 (1.8) | 1.4 | 5 (1.0) | 0.9 | 5 (1.0) | 0.8 |
| Volume depletion | 70 (9.5) | 8.4 | 66 (9.8) | 8.6 | 52 (8.3) | 7.0 | 68 (9.9) | 8.5 | 62 (12.5) | 11.8 | 63 (12.5) | 11.3 |
| UTI | 24 (3.9) | 2.7 | 13 (1.9) | 1.6 | 21 (3.3) | 2.8 | 29 (4.2) | 3.5 | 27 (5.4) | 4.7 | 27 (5.4) | 4.7 |
| Genital infections | 7 (0.9) | 0.78 | 10 (1.5) | 1.23 | 2 (0.3) | 0.26 | 10 (1.5) | 1.19 | 3 (0.6) | 0.57 | 11 (2.2) | 1.87 |
| Hyperkalaemia | 35 (4.7) | 4.0 | 30 (4.4) | 3.8 | 41 (6.5) | 5.5 | 42 (6.1) | 5.2 | 39 (7.9) | 7.1 | 29 (5.8) | 5.0 |
| Hypokalaemia | 12 (1.6) | 1.4 | 16 (2.4) | 2.0 | 11 (1.8) | 1.4 | 10 (1.5) | 1.2 | 5 (1.0) | 0.9 | 7 (1.4) | 1.2 |
AE, adverse event; py, patient‐years; UTI, urinary tract infection.
Hypoglycaemic adverse events with plasma glucose ≤70 mg/dl or requiring assistance.
Discussion
In the present secondary analysis of the EMPEROR‐Reduced trial, the efficacy and safety of empagliflozin compared with placebo, on top of appropriate medical and device HF therapies, was consistent across all age groups in HFrEF patients (Graphical Abstract). This analysis extends the findings of a similar study on dapagliflozin to a HF population with more advanced disease, and therefore, the totality of evidence on SGLT2i in HFrEF shows that these drugs are effective and safe regardless of patient age. 13
Knowing the impact of HF therapies across the spectrum of patient age is important for several reasons. First, older patients have previously been underrepresented in clinical trials, 15 , 16 including trials studying neurohormonal inhibitors in HFrEF. 17 , 18 The mean age of patients enrolled in EMPEROR‐Reduced was 67 years, with two thirds of patients being 65 years or older and one fourth of them being 75 or older. This age distribution is consistent with that reported by recent HF registries in Europe and US. 19 , 20 Two recent randomized clinical trials examining the efficacy and safety of dapagliflozin (DAPA‐HF) and vericiguat (VICTORIA) in HFrEF also enrolled patients with a mean age of 66 and 67 years, respectively. 11 , 21 The previously reported age‐related differences in HF patient characteristics were also confirmed by the present study. 6 , 7 Systolic blood pressure, NT‐proBNP, and LVEF increased with age along with the prevalence of arterial hypertension and atrial fibrillation. Older patients also had lower BMI and worse renal function than younger patients.
Second, older HFrEF patients are less frequently treated with guideline‐recommended therapies compared to younger patients according to large registries. 7 , 22 This was also seen in the present analysis, in which standard HF drug therapy declined with increasing age, with the exception of ARNi, the use of which was generally higher in EMPEROR‐Reduced compared to other contemporary HFrEF trials. 11 , 12 , 21 This finding is in contrast with the fact that the benefit of neurohormonal inhibitors/modulators, including ACEi, ARB, ARNi, beta‐blockers and MRA, and of ivabradine and dapagliflozin have been shown to be consistent across age and that older patients seem to benefit from these therapies as much as younger ones. 4 , 8 , 13 , 22 , 23 , 24 , 25 This was also true for empagliflozin in the present analysis. Evidence on the benefit of HF therapies in the elderly, provided by the present and previous studies, may thus allow an improvement in prescription rates of life‐saving therapies in these patients.
Third, older HF patients have a significant burden of comorbidities. 6 This is particularly true for non‐cardiovascular comorbidities that seem to increase linearly with age. 26 Comorbidities may explain the lower prescription rates of guideline‐directed therapies as well as the higher incidence of AEs and serious AEs with age, also observed herein in both treatment arms. It is important to stress, however, that despite the higher rates of AEs, the use of guideline‐recommended therapies and SGLT2i provide a net benefit in older patients that is consistent with that observed in younger patients.
Age has long been associated with worse outcomes in HF, 4 , 5 , 6 , 7 , 8 , 13 with older patients considered more vulnerable due to the effects of ageing on the cardiovascular system and the accumulation of comorbidities. In the present study, we did not observe a graded increase in the risk of cardiovascular death or HF hospitalization with advancing age in the placebo arm; event rates were actually lower in the middle‐age group compared to younger patients but did not differ between the youngest and the oldest group. A similar trend in cardiovascular outcomes, with lower events in the middle‐age group, was also observed in some recent clinical trials and registries, 4 , 7 , 27 in which patients were receiving good background therapy and age was taken as a trichotomous variable, as was also the case herein. Such a trend was further observed for in‐hospital mortality in two nationwide registries. 28 , 29 In one of these latter registries, there was actually an inverse relationship between age and 30‐day HF readmission, with younger patients having higher readmission rates than older ones. 28 The lower use of guideline‐recommended therapies may theoretically account at least in part for the worse outcomes in elderly HF patients observed by previous studies. In OPTIMIZE‐HF, older patients were characterized by both worse outcomes and a lower use of guideline‐recommended therapies, 22 while in the V‐HeFT study, increasing age (up to 75 years) was not associated with worse survival in HF patients who received optimal therapy. 30 Yet in contrast, in the present study, patients in the youngest group had lower LVEF, higher rate of HF hospitalization within the preceding year and lower use of CRT and these findings may partly explain the observed higher rate of HF hospitalization in this group during follow‐up in the placebo arm compared with middle‐aged patients.
Empagliflozin improves renal outcomes in patients with diabetes, regardless of the presence of HF, and in patients with HFrEF, regardless of the presence of diabetes. 12 , 31 These reno‐protective effects are particularly relevant for elderly HF patients, in whom age‐related renal function worsening may be accelerated by HF and comorbidities. 32 The reno‐protective effect of empagliflozin was observed both in younger and in older HFrEF patients, as the effects of the drug compared with placebo on the slope of change in eGFR and the renal composite outcome of end‐stage kidney disease or sustained profound eGFR decrease were consistent across the three age groups. The benefits of empagliflozin did not come at the costs of worse tolerability, with AEs not being increased with empagliflozin versus placebo, even in the elderly.
Patient‐reported health status and quality of life represents an important aspect of HF and a major treatment target. 33 Evidence on the effect of ageing on health status in patients with HF is controversial. In DAPA‐HF and one other observational study, KCCQ tended to improve with increasing age, as older patients seemed to have less severe symptoms. 13 , 34 In PARADIGM‐HF, in contrast, age was inversely correlated with KCCQ scores in physical and social activity limitations. 35 In the present analysis, KCCQ‐CSS did not differ among age groups at baseline. Regarding the effects of HF therapies on patients' self‐reported health status, in DAPA‐HF, the effect of dapagliflozin on KCCQ was consistent across age groups. 13 In PARADIGM‐HF, the improvement in combined KCCQ physical and social activity score seen with sacubitril/valsartan was comparable to a difference of 9 years of ageing. 35 In a previous analysis of the EMPEROR‐Reduced trial, empagliflozin reduced the risk of cardiovascular death or HF hospitalization regardless of patients' baseline KCCQ‐CSS, improving outcomes even in patients in the worst KCCQ‐CSS tertile. 36 In addition, the drug improved significantly KCCQ‐CSS, total summary score and overall summary score compared with placebo. 36 In the present analysis, we further expand these findings showing that the benefit of empagliflozin on KCCQ‐CSS is consistent across the spectrum of age, including patients in the oldest age group. Taken together, the potentially impaired health status of the elderly does not affect their response to empagliflozin in cardiovascular or renal outcomes, while it is expected to improve with this treatment.
The present study represents a secondary analysis of a large randomized controlled trial and as such, its findings should be interpreted with caution. However, it should be stressed that the results observed in the different age groups were in accordance with those in the whole study population.
In conclusion, in the present secondary analysis of a large randomized clinical trial, the efficacy and safety of empagliflozin compared to placebo in improving cardiovascular and renal outcomes in HFrEF patients are consistent across the spectrum of age, including patients aged 75 or older. This finding is particularly important for elderly HF patients and especially those who currently receive limited prescription and titration of neurohormonal inhibitors or other guideline‐recommended therapies.
Supporting information
Appendix S1. Supporting information.
Acknowledgements
Administrative support was provided by Elevate Scientific Solutions, and graphical support was provided by 7.4 Limited and were supported financially by Boehringer Ingelheim.
Funding
The EMPEROR‐Reduced trial was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance.
Conflict of interest: G.F. reports lecture fees and/or Committee Member contributions in trials sponsored by Bayer, Medtronic, Vifor, Servier, Novartis, Amgen and Boehringer Ingelheim and Research support from the European Union. S.D.A. reports grants and personal fees from Vifor Int. and Abbott Vascular, and personal fees from AstraZeneca, Bayer, Brahms, Boehringer Ingelheim, Cardiac Dimensions, Novartis, Occlutech, Servier, and Vifor Int. J.B. reports consulting fees from Boehringer Ingelheim, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V‐Wave Ltd., and Vifor. D.F. reports lecture and/or advisory board fees from Abbott, Bayer, Boehringer Ingelheim, Leo, Novartis, Roche and Orion. J.P.F. is a consultant for Boehringer Ingelheim. N.G., M.B. and T.I. are employees of Boehringer Ingelheim. S.P. is a consultant for Boehringer Ingelheim. F.Z. has recently received steering committee or advisory board fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cardior, CVRx, Janssen, Livanova, Merck, Mundipharma, Novartis, Novo Nordisk, and Vifor Fresenius. M.P. reports personal fees from Abbvie, Actavis, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Moderna, Novartis, Reata, Relypsa, Salamandra.
References
- 1. Zarrinkoub R, Wettermark B, Wändell P, Mejhert M, Szulkin R, Ljunggren G, et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15:995–1002. [DOI] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al.; American Heart Association Advocacy Coordinating Committee, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Stroke Council . Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Filippatos G, Parissis JT. Heart failure diagnosis and prognosis in the elderly: the proof of the pudding is in the eating. Eur J Heart Fail. 2011;13:467–71. [DOI] [PubMed] [Google Scholar]
- 4. Jhund PS, Fu M, Bayram E, Chen CH, Negrusz‐Kawecka M, Rosenthal A, et al.; PARADIGM‐HF Investigators and Committees . Efficacy and safety of LCZ696 (sacubitril‐valsartan) according to age: insights from PARADIGM‐HF. Eur Heart J. 2015;36:2576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, Normand SLT, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murad K, Goff DC, Morgan TM, Burke GL, Bartz TM, Kizer JR, et al. Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality: the Cardiovascular Health Study. JACC Heart Fail. 2015;3:542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lainščak M, Milinković I, Polovina M, Crespo‐Leiro MG, Lund LH, Anker SD, et al.; European Society of Cardiology Heart Failure Long‐Term Registry Investigators Group . Sex‐ and age‐related differences in the management and outcomes of chronic heart failure: an analysis of patients from the ESC HFA EORP Heart Failure Long‐Term Registry. Eur J Heart Fail. 2020;22:92–102. [DOI] [PubMed] [Google Scholar]
- 8. Ferreira JP, Rossello X, Eschalier R, McMurray JJV, Pocock S, Girerd N, et al. MRAs in elderly HF patients: individual patient‐data meta‐analysis of RALES, EMPHASIS‐HF, and TOPCAT. JACC Heart Fail. 2019;7:1012–21. [DOI] [PubMed] [Google Scholar]
- 9. Rich MW. Pharmacotherapy of heart failure in the elderly: adverse events. Heart Fail Rev. 2012;17:589–95. [DOI] [PubMed] [Google Scholar]
- 10. Wastesson JW, Morin L, Tan ECK, Johnell K. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin Drug Saf. 2018;17:1185–96. [DOI] [PubMed] [Google Scholar]
- 11. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al.; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 12. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al.; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24. [DOI] [PubMed] [Google Scholar]
- 13. Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, Tereshchenko S, et al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA‐HF. Circulation. 2020;141:100–11. [DOI] [PubMed] [Google Scholar]
- 14. Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, et al.; EMPEROR‐Reduced Trial Committees and Investigators . Evaluation of the effect of sodium‐glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR‐Reduced trial. Eur J Heart Fail. 2019;21:1270–1278. [DOI] [PubMed] [Google Scholar]
- 15. Cherubini A, Oristrell J, Pla X, Ruggiero C, Ferretti R, Diestre G, et al. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch Intern Med. 2011;171:550–6. [DOI] [PubMed] [Google Scholar]
- 16. Tahhan AS, Vaduganathan M, Greene SJ, Fonarow GC, Fiuzat M, Jessup M, et al. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol. 2018;3:1011–9. [DOI] [PubMed] [Google Scholar]
- 17. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al.; PARADIGM‐HF Investigators and committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 18. Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost‐Brama A, et al.; SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet. 2010;376:875–85. [DOI] [PubMed] [Google Scholar]
- 19. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, et al.; Heart Failure Association (HFA) of the European Society of Cardiology (ESC) . European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail. 2016;18:613–25. [DOI] [PubMed] [Google Scholar]
- 20. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol. 2018;72:351–66. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al.; VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–93. [DOI] [PubMed] [Google Scholar]
- 22. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al.; OPTIMIZE‐HF Investigators and Hospitals . Age‐ and gender‐related differences in quality of care and outcomes of patients hospitalized with heart failure (from OPTIMIZE‐HF). Am J Cardiol. 2009;104:107–15. [DOI] [PubMed] [Google Scholar]
- 23. Cohen‐Solal A, McMurray JJV, Swedberg K, Pfeffer MA, Puu M, Solomon SD, et al.; CHARM Investigators . Benefits and safety of candesartan treatment in heart failure are independent of age: insights from the Candesartan in Heart Failure‐Assessment of Reduction in Mortality and morbidity programme. Eur Heart J. 2008;29:3022–8. [DOI] [PubMed] [Google Scholar]
- 24. Kotecha D, Manzano L, Krum H, Rosano G, Holmes J, Altman DG, et al.; Beta‐Blockers in Heart Failure Collaborative Group . Effect of age and sex on efficacy and tolerability of β blockers in patients with heart failure with reduced ejection fraction: individual patient data meta‐analysis. BMJ. 2016;353:i1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tavazzi L, Swedberg K, Komajda M, Böhm M, Borer JS, Lainscak M, et al.; SHIFT Investigators . Efficacy and safety of ivabradine in chronic heart failure across the age spectrum: insights from the SHIFT study. Eur J Heart Fail. 2013;15:1296–303. [DOI] [PubMed] [Google Scholar]
- 26. Mogensen UM, Ersbøll M, Andersen M, Andersson C, Hassager C, Torp‐Pedersen C, et al. Clinical characteristics and major comorbidities in heart failure patients more than 85 years of age compared with younger age groups. Eur J Heart Fail. 2011;13:1216–23. [DOI] [PubMed] [Google Scholar]
- 27. Regan JA, Kitzman DW, Leifer ES, Kraus WE, Fleg JL, Forman DE, et al. Impact of age on comorbidities and outcomes in heart failure with reduced ejection fraction. JACC Heart Fail. 2019;7:1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elbadawi A, Dang A, Elgendy IY, Thakker R, Albaeni A, Omer MA, et al. Age‐specific trends and outcomes of hospitalizations with acute heart failure in the United States. Int J Cardiol. 2021;330:98–105. [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez F, Wang Y, Johnson CE, Foody JM. National patterns of heart failure hospitalizations and mortality by sex and age. J Card Fail. 2013;19:542–9. [DOI] [PubMed] [Google Scholar]
- 30. Hughes CV, Wong M, Johnson G, Cohn JN. Influence of age on mechanisms and prognosis of heart failure. The V‐HeFT VA Cooperative Study Group. Circulation. 1993;87(6 Suppl):VI111–7. [PubMed] [Google Scholar]
- 31. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al.; EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 32. Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, et al. Evaluation of kidney function throughout the heart failure trajectory – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:584–603. [DOI] [PubMed] [Google Scholar]
- 33. Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, et al. Association of serial Kansas City Cardiomyopathy Questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardiol. 2017;2:1315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Masoudi FA, Rumsfeld JS, Havranek EP, House JA, Peterson ED, Krumholz HM, et al.; Cardiovascular Outcomes Research Consortium . Age, functional capacity, and health‐related quality of life in patients with heart failure. J Card Fail. 2004;10:368–73. [DOI] [PubMed] [Google Scholar]
- 35. Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, et al. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM‐HF trial. JAMA Cardiol. 2018;3:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Butler J, Anker SD, Filippatos G, Khan MS, Ferreira JP, Pocock SJ, et al.; EMPEROR‐Reduced Trial Committees and Investigators . Empagliflozin and health‐related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR‐Reduced trial. Eur Heart J. 2021;42:1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


