Summary
Marstacimab, an investigational human monoclonal antibody targeting tissue factor pathway inhibitor, demonstrated safety and efficacy in preventing bleeding episodes in patients with haemophilia. This multicentre, open‐label study investigated safety, tolerability, and efficacy of long‐term weekly prophylactic marstacimab treatment in participants with severe haemophilia A and B, with or without inhibitors. Adult participants were enrolled from a previous phase Ib/II study or de novo and assigned to one of two subcutaneous (SC) marstacimab doses: once‐weekly 300 mg or a 300‐mg loading dose followed by once‐weekly 150‐mg doses, for up to 365 days. Study end‐points included safety assessments and annualised bleeding rates (ABRs). Of 20 enrolled participants, 18 completed the study. Overall, 70% of participants had treatment‐emergent adverse events, including injection site reactions, injection site haematoma, and haemarthrosis. No treatment‐related serious adverse events or thrombotic events occurred. Across all dose cohorts, mean and median on‐study ABRs ranged from 0 to 3.6 and 0 to 2.5 bleeding episodes/participant/year respectively, demonstrating comparable efficacy to that observed in the short‐term parent study. No treatment‐induced anti‐drug antibodies were detected. Once‐weekly SC marstacimab prophylaxis was well tolerated, with an acceptable safety profile, and maintained long‐term efficacy up to 365 days. (Clinicaltrials.gov identifier, NCT03363321).
Keywords: antibodies, monoclonal; clinical study; drugs, investigational; haemophilia A; haemophilia B
INTRODUCTION
Haemophilia A (haemA) and B (haemB) are X‐linked bleeding disorders characterised by deficiencies in coagulation clotting factors VIII (FVIII) and IX (FIX) respectively. 1 People with severe haemophilia (FVIII or FIX activity level <0.01 iu/ml or <1.0% of normal) experience frequent bleeding events, both spontaneous and post‐traumatic, most often in the joints (haemarthrosis) and also in muscles and soft tissues. 1 Without treatment, patients with severe haemophilia will develop progressive and debilitating arthropathy with consequent joint dysfunction, pain, and poor quality of life. 2
Standard‐of‐care treatment for haemophilia has been based on intravenous (IV) prophylactic replacement with either FVIII or FIX clotting factor concentrate for haemA or haemB respectively, to reduce or prevent bleeding events and joint disease. 1 , 2 , 3 However, patients with haemA (up to 30%) or haemB (up to 5%) may develop inhibitory antibodies in response to exogenous FVIII or FIX concentrates, decreasing the efficacy of replacement therapy. 1 , 4 , 5 In addition, venous access is limited in some patients, including children, and central venous catheters are often used, increasing the risk of infection or thrombotic complications. 1 Furthermore, patient adherence to frequent IV replacement of clotting factor is low. 6
Prophylaxis options in patients with haemA also include non‐factor therapies such as emicizumab, which mimics FVIII co‐factor activity. 1 Emicizumab is administered subcutaneously (SC) and less frequently than replacement therapies, and is associated with very low annualised bleeding rates (ABRs). 1 , 7 However, emicizumab is not indicated for patients with haemB, and its use may be associated with increased safety risks, including thrombotic microangiopathy, in patients with inhibitors who are treated with high‐dose activated pro‐thrombin complex concentrate (aPCC). 1 In addition, emicizumab should be used with caution in combination with recombinant FVIIa (rFVIIa) in patients at risk of thrombosis. 1 Emicizumab may also interfere with some clinical monitoring assays, and, although rare, its efficacy may decrease over time due to development of neutralising antibodies (NAbs). 1
Tissue factor pathway inhibitor (TFPI) antagonises early coagulation stages by inhibiting tissue factor‐activated FVII (FVIIa) and activated FX (FXa). TFPI is expressed in three isoforms, all sharing the Kunitz‐type inhibitor domain K2, which binds and inhibits activated FXa. 8 , 9 Marstacimab (formerly PF‐06741086; Pfizer Inc.), an investigational human immunoglobulin G1 monoclonal antibody, targets the K2 domain of TFPI (Figure S1). 10 It is intended to reduce the inhibition of FXa by TFPI, thereby augmenting haemostasis through the extrinsic pathway. 11
Safety and efficacy of SC marstacimab were previously evaluated in a short‐term (85‐day treatment period), phase Ib/II, multiple ascending dose study (NCT02974855) in participants with severe haemA or haemB with or without inhibitors, with weekly marstacimab doses ranging from 150 to 450 mg. 12 In that study, SC marstacimab was well tolerated and effective at reducing mean ABRs.
In the present study, we extended marstacimab prophylaxis for up to 365 days to evaluate its long‐term safety, tolerability, and efficacy as prophylactic treatment to prevent or reduce the frequency of bleeding events in adults with severe haemA or haemB with or without inhibitors.
PATIENTS AND METHODS
Study design
This phase II, open‐label, multicentre study investigated long‐term prophylactic treatment with marstacimab in participants with severe haemophilia, with or without inhibitory antibodies, for up to 12 months (NCT03363321). The original protocol allowed for a 6‐month duration of treatment and was subsequently amended to permit a total of 12 months of treatment with implementation of a second 6‐month treatment period for those participants who had already completed the initial 6‐month treatment period. This change was intended to extend the duration of longitudinal safety assessments but also extended the duration of access to treatment with this investigational study drug.
Marstacimab was administered by the SC route; injection volumes were 1 ml at a concentration of 150 mg/ml in a syringe and vial format. One injection of 1 ml was used to achieve a 150‐mg dose, and two injections of 1 ml each were used to achieve a 300‐mg dose. After training and demonstration of proficiency, participants were permitted to self‐administer their doses at home. The study comprised six treatment cohorts assigned to one of two dose groups (Figure 1). Participants in each cohort received treatment, as follows:
Cohort 1 (non‐inhibitor participants, 300 mg once weekly) from the previous study rolled over to Cohort 1 of the present study and continued treatment at 300 mg once weekly (300‐mg dose group).
Cohort 2 (non‐inhibitor participants, a single 300‐mg loading dose followed by 150 mg once weekly) from the previous study rolled over to Cohort 2 of the present study and continued treatment at the single 300‐mg loading dose followed by treatment at 150 mg once weekly (300‐mg/150‐mg dose group).
Cohort 3 from the previous study (non‐inhibitor participants, 450 mg once‐weekly) rolled over to Cohort 3 of the present study but were reduced to the lowest effective dose level (single 300‐mg loading dose followed by 150 mg once weekly [300‐mg/150‐mg dose group]).
Cohort 4 (inhibitor participants, 300 mg once weekly) from the previous study rolled over to Cohort 4 of the present study and continued treatment at 300 mg once weekly (300‐mg dose group).
Cohort 5 (single 300‐mg loading dose followed by 150 mg once weekly [300‐mg/150‐mg dose group]), intended for adolescent participants enrolled de novo.
Cohort 6 (single 300‐mg loading dose followed by 150 mg once weekly [300‐mg/150‐mg dose group]), intended for adult participants with inhibitors enrolled de novo.
FIGURE 1.
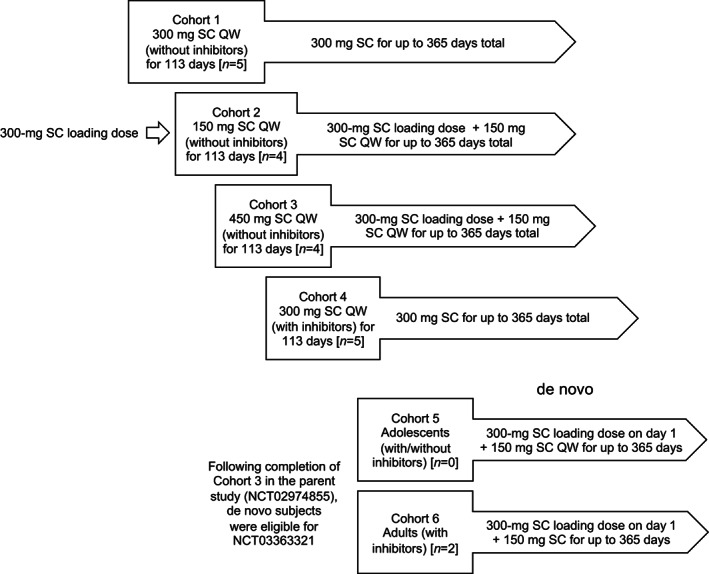
Participant marstacimab dose assignments. Participants from Cohorts 1 and 4 in the previous study continued to receive 300 mg weekly, and Cohorts 2 and 3 in the previous study received the lowest dose determined to be safe and effective (300‐mg loading dose followed by 150 mg SC weekly). Two participants were recruited de novo into the present study. Both were treated at the lowest effective dose level (300‐mg loading dose followed by 150 mg SC weekly). SC, subcutaneous; QW, once weekly
The study was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines. All study participants provided prior signed informed consent.
Study population
The study was conducted at sites in Brazil, Chile, Croatia, Poland, South Africa, Switzerland, and the United States between 30 May 2018 and 5 August 2020. Participants from a previous short‐term, phase Ib/II, dose‐escalation study (NCT02974855) 12 and participants enrolled de novo were eligible for enrolment. Eligible participants from the previous study were males aged ≥18 and <65 years with a diagnosis of severe haemA or haemB (FVIII or FIX activity ≤1%) and did not require further screening. De novo participants could also include adolescents aged ≥12 to <18 years (Cohort 5) and adults with inhibitors, aged ≥18 to <75 years (Cohort 6) who were previously receiving on‐demand treatment and had at least six acute bleeding episodes during the 6‐month period prior to screening. De novo participants with inhibitors to FVIII or FIX had to have a positive inhibitor test result above the upper limit of normal within 6 months prior to study day 1 and be receiving a bypass agent as treatment for bleeding. Exclusion criteria included known coronary artery, thrombotic, or ischaemic disease, or known severe, uncontrolled hypercholesterolaemia; a known haemostatic defect other than haemA or haemB; a known prothrombotic condition; regular, concomitant therapy with immunomodulating drugs; current treatment with aPCC with inability to substitute treatment with rFVIIa; or renal, hepatic, haematological, or coagulation laboratory values outside of prespecified limits. Additional details regarding the use of concomitant treatments are provided in the Supporting Information.
Study assessments
The primary end‐points were safety outcomes, including treatment‐emergent adverse events (TEAEs), injection site reactions, vital signs, physical examinations, and laboratory tests including haematology, chemistry, and urine analysis. The ABR based on the frequency of treated bleeding events during the study treatment period, calculated as: ([number of bleeding events × 365.25]/treatment duration in days) was assessed as a secondary end‐point; immunogenicity, measured by the frequency of anti‐drug antibody (ADA) and NAb production against marstacimab (only positive ADA samples were tested in the NAb assay) was assessed as an exploratory end‐point.
Statistical analyses
Safety was assessed in all participants who received at least one dose of marstacimab, and efficacy was assessed in participants who received at least one dose of marstacimab and who did not have any major protocol deviations. Descriptive statistics were used to summarise safety and efficacy data, including mean, median, standard deviation, minimum and maximum.
RESULTS
Participants
Of 24 individuals screened, 20 were enrolled (Table 1). Of these, 18 were participants from the previous study 12 and two were de novo adult participants with severe haemA with inhibitors. In total, 10 were assigned to the 300‐mg/150‐mg cohorts and 10 were assigned to the 300‐mg cohorts (Table 1). All participants were male and aged 19–57 years. In the 300‐mg/150‐mg cohorts, seven participants had haemA without inhibitors, two had haemA with inhibitors, and one had haemB without inhibitors. In the 300‐mg cohorts, five participants had haemA without inhibitors and five had haemA with inhibitors. No adolescent participants were enrolled. At baseline, prior to initiation of any treatment with marstacimab, 13 participants had haemophilic arthropathy and 13 participants had target joints. The mean durations of marstacimab treatment for the 300‐mg/150‐mg cohorts and 300‐mg cohorts were 318 and 335 days respectively. A total of 18 (90.0%) participants completed the study, and two (10.0%) withdrew for reasons not related to safety. For six participants, the protocol amendment extending the duration of dosing from 6 to 12 months was not implemented in time to avoid a dose gap of >14 days in duration (range 43–77) between completion of the first 6‐month treatment period and initiation of the second 6‐month treatment period. One of the six participants had an additional 92‐day dose gap during the second 6 months of treatment while he underwent knee replacement surgery. For this participant, only the duration before the first dose gap was included in the efficacy analysis. An additional participant had a 23‐day dose gap during the second 6‐month treatment period.
TABLE 1.
Participant demographics and baseline characteristics
| Marstacimab dose group | |||||||
|---|---|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Cohort 6 | Total 300 mg (n = 10) | Total 300‐mg loading + 150 mg (n = 10) | |
| Parameter | 300 mg → 300 mg without inhibitors (n = 5) | 300‐mg loading + 150 mg → 300‐mg loading + 150 mg without inhibitors (n = 4) | 450 mg → 300‐mg loading + 150 mg without inhibitors (n = 4) | 300 mg → 300 mg with inhibitors (n = 5) | De novo 300‐mg loading + 150 mg with inhibitors (n = 2) | ||
| Age, years | |||||||
| Mean (SD) | 33.0 (9.7) | 31.3 (10.6) | 41.8 (16.2) | 42.0 (5.2) | 20.0 (1.4) | 37.5 (8.8) | 33.2 (14.1) |
| Median (range) | 28.0 (27–50) | 29.5 (22–44) | 45.5 (19–57) | 40.0 (37–49) | 20.0 (19–21) | 37.5 (27–50) | 29.5 (19–57) |
| 18–44, n (%) | 4 (80.0) | 4 (100.0) | 2 (50.0) | 3 (60.0) | 2 (100.0) | 7 (70.0) | 8 (80.0) |
| 45–64, n (%) | 1 (20.0) | 0 | 2 (50.0) | 2 (40.0) | 0 | 3 (30.0) | 2 (20.0) |
| Race, n (%) | |||||||
| White | 3 (60.0) | 2 (50.0) | 4 (100.0) | 3 (60.0) | 2 (100.0) | 6 (60.0) | 8 (80.0) |
| Black | 2 (40.0) | 2 (50.0) | 0 | 2 (40.0) | 0 | 4 (40.0) | 2 (20.0) |
| Ethnicity, n (%) | |||||||
| Hispanic or Latino | 3 (60.0) | 2 (50.0) | 1 (25.0) | 1 (20.0) | 0 | 4 (40.0) | 3 (30.0) |
| Not Hispanic or Latino | 2 (40.0) | 2 (50.0) | 3 (75.0) | 4 (80.0) | 2 (100.0) | 6 (60.0) | 7 (70.0) |
| Height, cm, mean (SD) | — | — | — | — | 179.5 (0.7) | — | 179.5 (0.7) |
| Weight, kg, mean (SD) | 73.8 (15.6) | 63.2 (11.3) | 86.7 a | 82.1 (12.0) | 66.6 (12.0) | 77.5 (13.9) | 67.5 (12.7) |
| BMI, kg/m2, mean (SD) | — | — | — | — | 20.7 (3.9) | — | 20.7 (3.9) |
| Type of haemophilia, n (%) | |||||||
| Haemophilia A | 5 (100.0) | 3 (75.0) | 4 (100.0) | 5 (100.0) | 2 (100.0) | 10 (100.0) | 9 (90.0) |
| Haemophilia B | 0 | 1(25.0) | 0 | 0 | 0 | 0 | 1 (10.0) |
| Haemophilic arthropathy, n (%) | 5 (100.0) | 2 (50.0) | 1 (25.0) | 3 (60.0) | 2 (100.0) | 8 (80.0) | 5 (50.0) |
| Target joints, n (%) | 5 (100.0) | 2 (50.0) | 1 (25.0) | 3 (60.0) | 2 (100.0) | 8 (80.0) | 5 (50.0) |
Abbreviations: BMI, body mass index; SD, standard deviation.
SD not available.
Safety
No participants died during the long‐term study. One participant experienced two serious AEs (SAEs), including a Grade 3 cerebral haemorrhage (intracerebral haemorrhage [ICH]) that was diagnosed on study day 76 following a traumatic skull fracture sustained on study day 73, and a Grade 2 generalised tonic–clonic seizure; these AEs were not considered by the investigators to be related to treatment. The ICH was treated as a bleed event in the study efficacy analysis. There were no interruptions in marstacimab treatment (300‐mg cohort) for this participant, and concomitant treatment for the ICH included FVIII replacement, with initial doses of 57 and 28 iu/kg, and tranexamic acid.
In the 300‐mg/150‐mg cohorts, a total of 24 TEAEs occurred in seven (70.0%) participants (Table 2); four had Grade 1, two had Grade 2, and one had a Grade 3 TEAEs. One treatment‐related injection site reaction of Grade 2 severity was reported in one participant.
TABLE 2.
Summary of treatment‐emergent adverse events
| Marstacimab dose group | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Cohort 6 | Total 300 mg (n = 10) | Total 300‐mg loading + 150 mg (n = 10) |
| 300 mg → 300 mg without inhibitors (n = 5) | 300‐mg loading + 150 mg → 300‐mg loading + 150 mg without inhibitors (n = 4) | 450 mg → 300‐mg loading + 150 mg without inhibitors (n = 4) | 300 mg → 300 mg with inhibitors (n = 5) | De novo 300‐mg loading + 150 mg with inhibitors (n = 2) | |||
| Participants with any AE, n (%) a | 5 (100.0) | 2 (50.0) | 4 (100.0) | 2 (40.0) | 1 (50.0) | 7 (70.0) | 7 (70.0) |
| Participants with SAEs, n (%) | 1 (20.0) | 0 | 0 | 0 | 0 | 1 (10.0) | 0 |
| Participants with TRAEs, n (%) | 2 (40.0) | 0 | 0 | 0 | 1 (50.0) | 2 (20.0) | 1 (10.0) |
| Injection site haematoma | 1 (20.0) | 0 | 0 | 0 | 0 | 1 (10.0) | 0 |
| Injection site reaction, n (%) | 1 (20.0) | 0 | 0 | 0 | 1 (50.0) | 1 (10.0) | 1 (10.0) |
| Participants with serious TRAEs, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AEs in >5% of participants overall, n (%) | |||||||
| Haemarthrosis | 2 (40.0) | 1 (25.0) | 0 | 0 | 0 | 2 (20.0) | 1 (10.0) |
| Injection site reaction | 1 (20.0) | 0 | 0 | 0 | 1 (50) | 1 (10.0) | 1 (10.0) |
| Arthralgia | 1 (20.0) | 0 | 0 | 0 | 1 (50) | 1 (10.0) | 1 (10.0) |
| Haematoma | 0 | 1 (0.25.0) | 1 (25.0) | 0 | 0 | 0 | 2 (20.0) |
Abbreviations: AE, adverse event; SAE, serious AE; TRAE, treatment‐related AE.
Each adverse event at the preferred term level in each treatment group was counted once.
In the 300‐mg dose cohorts, at total of 15 TEAEs occurred in seven (70.0%) participants (Table 2); five participants had Grade 1, one had Grade 2, and one had Grade 3 TEAEs. Two treatment‐related events of Grade 1/2 severity were reported in two participants (injection site reaction and injection site haematoma; one participant each). One participant from the de novo 300‐mg/150‐mg inhibitor cohort had a Grade 2 treatment‐related injection site reaction. No instances of allergic reaction were reported.
No clinically relevant laboratory test abnormalities were found. As in the prior study, during the treatment period, at various time points a mean reduction in the range of 0.0888 to –0.582 g/L in fibrinogen levels was observed relative to baseline across the dose cohorts. In all instances, fibrinogen levels remained within the reference range of the central laboratory. No consistent pattern was observed in the physical examination findings.
No thrombotic events were reported, and no participants discontinued from the study because of TEAEs.
Efficacy
In total, 20 participants were included in the efficacy analysis. For one participant with two dose gaps of >30 days, only the duration before the first dose gap was included. All participants remained within their assigned dosing cohort throughout their participation in the study. The efficacy attained in the 3‐month parent study 12 was maintained over the duration of the present study, irrespective of weekly maintenance dose (150 or 300 mg), the presence of inhibitors, and whether the participant had haemA or haemB. As shown in Table 3, across all treatment cohorts, the mean on‐study ABR ranged from 0 to 3.6 bleeding episodes/participant/year (median ranged from 0 to 2.5), compared with a mean pre‐treatment ABR of 14.0 to 22.0 bleeding episodes/participant/year (median ranged from 14.0 to 20.0). The overall mean on‐study ABRs for the 300‐mg and 300‐mg/150‐mg dose groups were 1.5 and 2.7 bleeding episodes/participant/year respectively. Nine out of 18 participants who completed the study had no bleeding events.
TABLE 3.
Summary of pre‐study versus on‐study annualised bleeding rates
| Study phase | Marstacimab dose group | ||||||
|---|---|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Cohort 6 | Total 300 mg (n = 10) | Total 300‐mg loading + 150 mg (n = 10) | |
| 300 mg → 300 mg without inhibitors (n = 5) | 300‐mg loading + 150 mg → 300‐mg loading + 150 mg without inhibitors (n = 4) | 450 mg → 300‐mg loading + 150 mg without inhibitors (n = 4) | 300 mg → 300 mg with inhibitors (n = 5) | De novo 300‐mg loading + 150 mg with inhibitors (n = 2) | |||
| Pre‐treatmenta ABR, mean (SD) | 22.0 (7.9) | 14.0 (1.6) | 22.0 (13.6) | 18.4 (1.7) | 15.0 (4.2 | 20.2 (5.7) | 17.4 (9.0) |
| Median (range) | 20.0 (12.0–30.0) | 14.0 (12.0–16.0) | 17.0 (12.0–42.0) | 18.0 (16.0–20.0) | 15.0 (12.0–18.0) | 19.0 (12.0–30.0) | 15.0 (12.0–42.0) |
| On‐study ABR, mean (SD) | 3.0 (2.8) | 3.6 (7.2) | 1.9 (1.5) | 0 | 2.5 (3.5) | 1.5 (2.4) | 2.7 (4.5) |
| Median (range) | 2.0 (0–6.0) | 0 (0–14.4) | 2.1 (0–3.5) | 0 (0–0) | 2.5 (0–5.0) | 0 (0–6.0) | 1.0 (0–14.4) |
Abbreviations: ABR, annualised bleeding rate (bleeding episodes/participant/year); SD, standard deviation.
Pre‐treatment summarised data up to 6 months prior to participation in the long‐term study for de novo participants, and up to 6 months prior to participation in the prior phase Ib/II short‐term multiple ascending dose study for rollover participants.
In the 300‐mg/150‐mg dose group, one participant with haemA who had rolled over from the previous study had the maximum on‐study ABR of 14.4 compared with an ABR of 4.3 in the prior study. The study investigator judged that the participant's high ABR was related to the participant's history of cyclical increases in ABR due to increased activity. While the study did not incorporate any formal assessments of lifestyle activity, this individual tested negative for ADAs, had no apparent decline in marstacimab plasma concentrations over the course of the study (data not shown), and showed no consistent pattern for attenuation of treatment effect on pharmacodynamic parameters, including prothrombin fragment 1 + 2, peak thrombin generation, and thrombin generation lag time (Table S1).
Immunogenicity
None of the participants had positive ADA samples at baseline or after treatment and, consequently, no NAb testing was conducted. Therefore, the incidence of treatment‐induced ADA and NAb was zero for both the 150‐ and 300‐mg dose groups.
DISCUSSION
In this open‐label phase II study of once‐weekly marstacimab in participants with haemophilia with or without inhibitors, long‐term treatment (up to 365 days) was generally safe and well tolerated at both dose levels. Moreover, long‐term marstacimab treatment in participants with haemA was associated with maintenance of efficacy observed in the short‐term parent study. 12
No thrombotic events or treatment‐related SAEs occurred, and the most common AEs were of Grade 1 severity. The SC dosing of marstacimab was generally well tolerated; Grades 1 and 2 injection site reactions were reported in one participant each. As in the prior study, a reduction from baseline in fibrinogen was observed in both dosing groups. This reduction was modest and not associated with any clinical findings, and values for fibrinogen remained within the reference range. These observations are in agreement with those of earlier marstacimab studies, including a phase I study in healthy volunteers with single SC marstacimab doses ranging from 30 to 300 mg and a phase Ib/II study in adults with severe haemA or haemB (with or without inhibitors), with doses of marstacimab of up to 450 mg once weekly, which were found to be safe and well tolerated. 12 , 13
Compared with the parent study, long‐term marstacimab treatment maintained efficacy in a self‐administered, extended‐treatment setting in which the majority (13/20) of the participant population had target joints at baseline with a predisposition to frequent bleeding episodes. In the parent study, mean on‐study ABR ranged from 0.7 to 4.2 bleeding episodes/participant/year, 12 whereas in this study, mean on‐study ABR ranged from 0 to 3.6 bleeding episodes/participant/year.
While the prophylactic use of IV clotting factor and use of emicizumab for prophylaxis in patients with haemA continue to transform the patient experience, an unmet need persists among patients with severe haemophilia. 2 Factor replacement is associated with the development of NAbs, and the IV administration route and frequency of infusion are burdensome to patients and can lead to reduced adherence. 2 A subset of patients continue to experience bleeding episodes with emicizumab prophylaxis, and ADAs can adversely impact efficacy. 7 , 14 , 15 Furthermore, circulating levels of emicizumab can interfere with commonly used clinical assays of coagulation. 16
The mean on‐study ABRs for the respective treatment groups of 1.5 bleeding episodes/participant/year (total for 300‐mg cohorts) and 2.7 bleeding episodes/participant/year (total for 300‐mg/150‐mg cohorts) in this study are comparable with efficacy findings from recent studies of non‐factor replacement therapies in development for the treatment of haemophilia. Phase III trials of emicizumab prophylaxis in participants with haemA reported ABRs of 1.3–2.9 bleeding episodes/participant/year, 7 , 17 , 18 , 19 versus 23.3 bleeding episodes/participant/year with no prophylaxis. 17 In phase II trials, prophylaxis treatment with the anti‐TFPI antibody concizumab in participants with haemA and haemB with inhibitors resulted in an estimated ABR of 4.5 bleeding episodes/participant/year, compared with 20.4 bleeding episodes/participant/year in the setting of on‐demand treatment with FVIIa. 20 In participants with severe haemA without inhibitors, concizumab treatment resulted in an estimated ABR of 7.0 bleeding episodes/participant/year. 20
While no thrombotic episodes have been observed in association with marstacimab treatment of haemophilia, clinical experience remains limited. The use of other novel non‐factor therapies has been associated with thromboembolic events, requiring safety precautions for concomitant use with clotting factors and bypass agents. 1 , 21 , 22 , 23 , 24 Although emicizumab was generally well tolerated in clinical trials, 7 , 17 , 18 , 19 thrombotic microangiopathy and thrombosis occurred in participants receiving high cumulative doses of aPCC for breakthrough bleeding events while receiving emicizumab. 17 Other anti‐TFPI agents in development (e.g., concizumab and befovacimab) have been associated with thrombosis and the risk of coagulation. 21 , 23 , 24 Non‐fatal thromboembolic events occurred in three participants in phase III clinical trials of concizumab, which led to the temporary pausing of those trials. 23 , 25 A phase II dose‐escalation study of the investigational anti‐TFPI antibody, befovacimab, was terminated early because of thrombosis. 21 , 26 In a phase II trial of the investigational RNA interference therapeutic, fitusiran, fatal cerebral sinus thrombosis occurred in one participant after repeated infusions of high‐dose FVIII for >24 h, and the trial was temporarily put on hold. 22 An ongoing phase III trial in participants with severe haemA or haemB with or without inhibitors (NCT03938792) will more comprehensively establish the thrombotic safety profile of marstacimab.
Strengths of this study include its long‐term follow‐up of up to 365 days and the inclusion of participants with and those without inhibitory antibodies. However, the study is limited by its small sample size of 20 participants, including only one participant with haemB. There is also a potential efficacy bias, as a participant not experiencing sufficient efficacy in the parent study may be less inclined to continue or resume marstacimab treatment. Experience with on‐demand treatment of bleeding episodes against a background of marstacimab prophylaxis was limited, as 11 of 20 participants reported no bleeding episodes and the overall ABR was quite low.
In conclusion, in patients with severe haemA (and in a single patient with haemB), with or without inhibitors, long‐term once‐weekly treatment with SC marstacimab was well tolerated, with an acceptable safety profile. Marstacimab was associated with maintenance of efficacy observed in the short‐term parent study.
AUTHOR CONTRIBUTIONS
Steven Arkin contributed to the study design. Johnny Mahlangu served as Principal Investigator. Jose Luis Lamas, Juan Cristobal Morales, and Daniel R. Malan served as Study Investigators. Johnny Mahlangu, Jose Luis Lamas, and Juan Cristobal Morales enrolled patients. Johnny Mahlangu participated in the collection and assembly of data, and Johnny Mahlangu and Eunhee Hwang participated in data analysis. All authors participated in data interpretation. Johnny Mahlangu, Steven Arkin, and John Teeter contributed to manuscript preparation. All authors contributed to critical review and revision of the manuscript and provided final approval of the manuscript for submission.
FUNDING INFORMATION
This study was sponsored by Pfizer Inc.
CONFLICT OF INTEREST
Johnny Mahlangu has served as a consultant or on advisory boards for Baxalta, BioMarin, CSL Behring, Catalyst Biosciences, Novo Nordisk, Roche and Spark; served on speakers’ bureaus for Novo Nordisk, Pfizer, Roche, Sanofi, Springer, and Takeda; and received research grants from Baxalta, BioMarin, Catalyst Biosciences, CSL, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, Spark, Roche, and uniQure. Jose Luis Lamas and Daniel R. Malan report no conflicts to disclose. Juan Cristobal Morales has served on speakers’ bureaus for Novo Nordisk and Roche; served on advisory boards for BioMarin and Novo Nordisk, and received fellowship support from Novo Nordisk, Roche, and Takeda. John Teeter, Robert J. Charnigo, Eunhee Hwang, and Steven Arkin are employees of Pfizer Inc. and may own stock/options in the company.
Supporting information
Table S1
Figure S1
ACKNOWLEDGEMENTS
This study was sponsored by Pfizer Inc. Medical writing and editorial support were provided by Ellen Grünewald, PhD, and Michael Morren, RPh, MBA, of Peloton Advantage, an OPEN Health company, and were funded by Pfizer Inc.
Mahlangu J, Luis Lamas J, Cristobal Morales J, Malan DR, Teeter J, Charnigo RJ, et al. Long‐term safety and efficacy of the anti‐tissue factor pathway inhibitor marstacimab in participants with severe haemophilia: Phase II study results. Br J Haematol. 2023;200(2):240–248. 10.1111/bjh.18495
DATA AVAILABILITY STATEMENT
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information.
REFERENCES
- 1. Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158. [DOI] [PubMed] [Google Scholar]
- 2. Balkaransingh P, Young G. Novel therapies and current clinical progress in hemophilia A. Ther Adv Hematol. 2018;9:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valentino LA. Considerations in individualizing prophylaxis in patients with haemophilia A. Haemophilia. 2014;20:607–15. [DOI] [PubMed] [Google Scholar]
- 4. Astermark J. Overview of inhibitors. Semin Hematol. 2006;43:S3–7. [DOI] [PubMed] [Google Scholar]
- 5. Lorenzo JI, Lopez A, Altisent C, Aznar JA. Incidence of factor VIII inhibitors in severe haemophilia: the importance of patient age. Br J Haematol. 2001;113:600–3. [DOI] [PubMed] [Google Scholar]
- 6. Thornburg CD, Carpenter S, Zappa S, Munn J, Leissinger C. Current prescription of prophylactic factor infusions and perceived adherence for children and adolescents with haemophilia: a survey of haemophilia healthcare professionals in the United States. Haemophilia. 2012;18:568–74. [DOI] [PubMed] [Google Scholar]
- 7. Mahlangu J, Oldenburg J, Paz‐Priel I, Negrier C, Niggli M, Mancuso ME, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379:811–22. [DOI] [PubMed] [Google Scholar]
- 8. Broze GJ Jr, Girard TJ. Tissue factor pathway inhibitor: structure‐function. Front Biosci (Landmark Ed). 2012;17:262–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wood JP, Ellery PE, Maroney SA, Mast AE. Biology of tissue factor pathway inhibitor. Blood. 2014;123:2934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Apgar J, Parng C, Benard S, Juo S, Tam M, Tu C, et al. X‐ray crystallography and hemostatic activities of a neutralizing anti‐TFPI antibody, marstacimab [poster]. Presented at: Annual Congress of the International Society on Thrombosis and Haemostasis; July 12–14, 2020.
- 11. Pittman DD, Rakhe S, Bowley SR, Jasuja R, Barakat A, Murphy JE. Hemostatic efficacy of marstacimab alone or in combination with bypassing agents in hemophilia plasmas and a mouse bleeding model. Res Pract Thromb Haemost. 2022;6:e12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahlangu J, Lamas JL, Morales JC, Malan DR, Zupancic‐Salek S, Wang M, et al. A phase 1b/2 study of the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of PF‐06741086, an anti‐TFPI monoclonal antibody, in patients with severe hemophilia A or B [abstract OC 11.2]. Res Pract Thromb Haemost. 2019;3(suppl 1):85–6.30656280 [Google Scholar]
- 13. Cardinal M, Kantaridis C, Zhu T, Sun P, Pittman DD, Murphy JE, et al. A first‐in‐human study of the safety, tolerability, pharmacokinetics and pharmacodynamics of PF‐06741086, an anti‐tissue factor pathway inhibitor mAb, in healthy volunteers. J Thromb Haemost. 2018;16:1722–31. [DOI] [PubMed] [Google Scholar]
- 14. Barg AA, Budnik I, Avishai E, Brutman‐Barazani T, Bashari D, Misgav M, et al. Emicizumab prophylaxis: prospective longitudinal real‐world follow‐up and monitoring. Haemophilia. 2021;27:383–91. [DOI] [PubMed] [Google Scholar]
- 15. Valsecchi C, Gobbi M, Beeg M, Adams T, Castaman G, Schiavone L, et al. Characterization of the neutralizing anti‐emicizumab antibody in a patient with hemophilia A and inhibitor. J Thromb Haemost. 2021;19:711–8. [DOI] [PubMed] [Google Scholar]
- 16. MASAC Document 258—Recommendation on the Use and Management of Emicizumab‐kxwh (Hemlibra®) for Hemophilia A with and without Inhibitors . 2020. https://www.hemophilia.org/sites/default/files/document/files/258_emicizumab.pdf. Accessed 15 October 2021.
- 17. Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377:809–18. [DOI] [PubMed] [Google Scholar]
- 18. Pipe SW, Shima M, Lehle M, Shapiro A, Chebon S, Fukutake K, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open‐label, non‐randomised phase 3 study. Lancet Haematol. 2019;6:e295–305. [DOI] [PubMed] [Google Scholar]
- 19. Shima M, Nogami K, Nagami S, Yoshida S, Yoneyama K, Ishiguro A, et al. A multicentre, open‐label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors. Haemophilia. 2019;25:979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shapiro AD, Angchaisuksiri P, Astermark J, Benson G, Castaman G, Chowdary P, et al. Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results. Blood. 2019;134:1973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lenting PJ. Laboratory monitoring of hemophilia A treatments: new challenges. Blood Adv. 2020;4:2111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Machin N, Ragni MV. An investigational RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and B. J Blood Med. 2018;9:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Figueiredo M. Novo Nordisk pauses 3 clinical trials of concizumab amid safety concerns. 2020. https://hemophilianewstoday.com/2020/03/18/novo‐nordisk‐pauses‐three‐clinical‐trials‐of‐concizumab‐due‐to‐safety‐concerns/. Accessed 4 May 2021.
- 24. Mancuso ME, Ingham SJM, Kunze M. Befovacimab, an anti‐tissue factor pathway inhibitor antibody: early termination of the multiple‐dose, dose‐escalating Phase 2 study due to thrombosis. Haemophilia. 2022;28:702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Novo Nordisk resumes the phase 3 clinical trials investigating concizumab (anti‐TFPI mAB) in haemophilia A and B with or without inhibitors [press release] . 2020. https://ml‐eu.globenewswire.com/Resource/Download/32302d78‐439f‐4a97‐a655‐3f7b96e2cc76. Accessed 4 May 2021.
- 26. Pasi KJ, Rangarajan S, Georgiev P, Mant T, Creagh MD, Lissitchkov T, et al. Targeting of antithrombin in hemophilia A or B with RNAi therapy. N Engl J Med. 2017;377:819–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information.


