Abstract
Previous evidence on postdiagnosis body fatness and mortality after breast cancer was graded as limited‐suggestive. To evaluate the evidence on body mass index (BMI), waist circumference, waist‐hip‐ratio and weight change in relation to breast cancer prognosis, an updated systematic review was conducted. PubMed and Embase were searched for relevant studies published up to 31 October, 2021. Random‐effects meta‐analyses were conducted to estimate summary relative risks (RRs). The evidence was judged by an independent Expert Panel using pre‐defined grading criteria. One randomized controlled trial and 225 observational studies were reviewed (220 publications). There was strong evidence (likelihood of causality: probable) that higher postdiagnosis BMI was associated with increased all‐cause mortality (64 studies, 32 507 deaths), breast cancer‐specific mortality (39 studies, 14 106 deaths) and second primary breast cancer (11 studies, 5248 events). The respective summary RRs and 95% confidence intervals per 5 kg/m2 BMI were 1.07 (1.05‐1.10), 1.10 (1.06‐1.14) and 1.14 (1.04‐1.26), with high between‐study heterogeneity (I 2 = 56%, 60%, 66%), but generally consistent positive associations. Positive associations were also observed for waist circumference, waist‐hip‐ratio and all‐cause and breast cancer‐specific mortality. There was limited‐suggestive evidence that postdiagnosis BMI was associated with higher risk of recurrence, nonbreast cancer deaths and cardiovascular deaths. The evidence for postdiagnosis (unexplained) weight or BMI change and all outcomes was graded as limited‐no conclusion. The RCT showed potential beneficial effect of intentional weight loss on disease‐free‐survival, but more intervention trials and well‐designed observational studies in diverse populations are needed to elucidate the impact of body composition and their changes on breast cancer outcomes.
Keywords: Body fatness, breast cancer survival, evidence grading, systematic review, weight change
What's new?
Greater body fatness and adult weight gain are established risk factors for postmenopausal breast cancer, but the impact of excess body weight on breast cancer outcomes remains unclear. In this systematic review and meta‐analysis of the Global Cancer Update Program, the independent expert panel concluded that there was strong evidence (likelihood of causality: probable) that postdiagnosis body fatness increases the risks of all‐cause mortality, breast cancer‐specific mortality and second primary breast cancer in women diagnosed with breast cancer. The findings support the development of lifestyle recommendations for breast cancer survivors to avoid obesity and be physically active, within the limits of their ability and specific medical advice.
Abbreviations
- AICR
American Institute for Cancer Research
- BMI
body mass index
- CIs
confidence intervals
- CUP Global
Global Cancer Update Program
- CUP
Continuous Update Project
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
- RCT
randomized controlled trial
- RR
relative risk
- WCRF
World Cancer Research Fund
1. INTRODUCTION
In 2020, breast cancer was the most common cancer and the leading cause of cancer death in women globally, with 2.3 million incident cases and 0.7 million deaths estimated. 1 As of the end of 2020, there were 7.8 million women worldwide who had survived at least 5 years after a breast cancer diagnosis. 1
Higher body fatness and adult weight gain have been established as risk factors for postmenopausal breast cancer, in particular hormone‐sensitive breast cancers. 2 , 3 With the increasing global prevalence of overweight and obesity, 4 it is expected that many women diagnosed with breast cancer will have excess body weight, but the impact on breast cancer outcomes has been less clear than for incidence.
Previous meta‐analyses of epidemiologic studies published up to 30 June, 2012 included in the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Third Export Report showed increased risks of all‐cause mortality and breast cancer‐specific mortality with higher postdiagnosis BMI. 5 In addition, women of normal body weight (BMI of 18.5‐24.9 kg/m2) after a breast cancer diagnosis were shown to have a more favorable overall survival than women of other weight statuses. 6
Despite these statistically significant associations, the evidence was judged as limited‐suggestive because of limitations in the design or conduct of the observational studies. In particular, the potential for residual confounding and reverse causation limited the confidence to determine causality. 5 Women with obesity may have more treatment complications and may receive less effective treatment compared with women without obesity. 7 Furthermore, cancer treatments may alter body weight and composition and could lead to adverse metabolic consequences. 8 Not all studies included in the meta‐analyses reported information on breast cancer subtypes; although the association appeared to be similar in women with hormone receptor‐positive or ‐negative breast cancers, 6 , 9 the relationship was less clear in women with aggressive tumors, such as triple‐negative breast cancers. 10 , 11
Breast cancer patients can have a long survival time and remain at risk of recurrence long after diagnosis. 12 Therefore, understanding the impact of postdiagnosis body fatness on breast cancer outcomes is important for identifying potentially actionable lifestyle targets for the growing number of women living with and beyond breast cancer. 13 , 14 We conducted updated systematic literature reviews and meta‐analyses to summarize the evidence published until and after 30 June 2012 of the previous report 5 and an independent Expert Panel evaluated the accumulated evidence. This paper presents the evidence on body fatness, weight change and breast cancer outcomes, whereas evidence on physical activity, diet and the overall summary is presented in the accompanied papers. 15 , 16 , 17
2. MATERIALS AND METHODS
The present review forms part of the on‐going Global Cancer Update Program (CUP Global), formally known as the WCRF/AICR Continuous Update Project (CUP). 18 The peer‐reviewed protocol is available online. 19 Full details on the methods and search strategies are provided in Text S1. The PRISMA checklist is available in Table S1.
2.1. Search strategy, selection criteria and data extraction
PubMed and Embase were searched for relevant publications through 31st October, 2021. The reference lists of identified articles were screened for any additional publications not identified in the primary searches.
Inclusion criteria were (1) Randomized controlled trials (RCTs), longitudinal observational studies, or pooled analyses of individual data of these studies; (2) With at least 100 women diagnosed with first primary breast cancer during adulthood; (3) Reported results on postdiagnosis BMI, waist circumference or waist‐hip‐ratio, and changes in weight or BMI in relation to all‐cause mortality, breast and nonbreast cancer‐specific mortality, breast cancer recurrence (or “recurrence/relapse‐free survival”, “disease‐free survival”, “event‐free survival”, “progression‐free survival” and “additional breast cancer events”), any second primary cancers, or cardiovascular mortality.
In the case of multiple publications from the same or overlapping populations, the publication with the largest number of events was selected for inclusion in the meta‐analysis. Study and participants' characteristics and results were extracted into the CUP Global database. Study selection and data extraction was checked by a second reviewer. Any disagreements were resolved by consensus. The quality of individual studies was not graded using a specific tool. Instead, relevant study characteristics that could be used to explore potential sources of bias were included into the CUP Global database. For all the included studies, information on potential for selection bias, information bias of exposure and outcome assessment, and residual confounding by cancer stage and treatment was retrieved after identifying the most likely influential sources of bias in cancer survival studies. 20 , 21 The potential influence of measurement error, length of follow‐up and loss to follow‐up, and adjustment for confounding factors on results was tested in subgroup meta‐analyses and meta‐regression analyses. Details on how the study authors addressed the potential biases were also included. In the Expert Panel meeting, whether the studies had serious quality issues were discussed when judging the evidence for each exposure‐outcome association.
2.2. Statistical methods for meta‐analysis
The summary relative risk (RR) estimates and their 95% confidence intervals (CIs) were calculated using the inverse variance random‐effects model. 22 When at least three (additional) studies were identified in the updated search, a linear dose‐response meta‐analysis was conducted (or updated if reviewed previously with evidence up to 30 June, 2012 5 ) if the studies reported sufficient information for analysis, otherwise the studies were descriptively synthesized. The multivariable adjusted RR estimates per exposure increment unit were pooled in a dose‐response meta‐analysis either with estimates provided in the original publications or estimated by us using the generalized weighted least‐squares regression model. 23 , 24 Standard imputations were conducted to calculate the required information when missing (0‐57% across analyses) 25 , 26 (Text S1).
In the linear dose‐response meta‐analysis, the underweight group (BMI <18.5 kg/m2 or as defined by studies) was excluded to avoid possible impact on the risk estimation. Pre‐ to postdiagnosis (≥1 year) weight change was grouped into moderate (5‐10%), or high (>10%) weight loss or gain. 27 , 28 The RR estimates for the weight change vs stable weight (±5% or as defined by studies) groups were pooled in categorical meta‐analyses.
Between‐study heterogeneity was assessed by the Cochran's Q test and I 2 statistic, 29 accompanied by visual inspection of the forest plots for consistency of associations, which contributes towards evidence grading. Pre‐defined subgroup meta‐analyses and random‐effects meta‐regression analyses were conducted to explore if a certain disease or study characteristics or aspect of risk of bias explain between‐study heterogeneity. 30 This included the exploration of the potential influence of changes in treatment regimens, over time, on the associations, by grouping the studies according to the diagnosis or treatment period: before 2000 or after 2000 for the shift to include doxorubicin/cyclophosphamide in chemotherapy, 31 and before 2005 or after 2005 for the reduced anthracycline use and the introduction of taxanes, 32 and human epidermal growth factor receptor 2 (HER2) targeted therapy—trastuzumab. 33
Small study effects such as publication bias was examined using Egger's test and visual inspection of the funnel plots for asymmetry, when there were more than 10 studies. 34 Individual studies may potentially influence the summary RR estimate and was examined by leave‐one‐out analysis. 35
Restricted cubic spline regression with three knots at 10th, 50th and 90th percentiles of distribution of the exposure was conducted and pooled in random‐effects meta‐analysis when five or more studies, each with data for at least three exposure categories, including the underweight group if presented, were available. 36 , 37 The linear and nonlinear models were compared using a likelihood ratio test. 38
A two‐tailed P value of <.1 was considered as evidence for small study effects or between‐study heterogeneity in the generally low‐powered Egger's test and meta‐regression analyses.
2.3. Evidence grading criteria
An independent Expert Panel (ELG, MJG, AAJ, EK, VL, SKC and AMT) graded the quality of the evidence into strong (subgrades evaluating likelihood of causality: convincing, probable, or substantial effect on risk unlikely) or limited (subgrades evaluating likelihood of causality: limited‐suggestive or limited‐no conclusion) level, using pre‐defined evidence grading criteria (Table S2).
3. RESULTS
Overall, 376 publications were identified reporting results on postdiagnosis body fatness and weight or BMI change and selected outcomes in breast cancer survivors; Figure 1 shows the flowchart of the search process. The meta‐analyses included 101 publications reporting postdiagnosis BMI (assessed from at‐diagnosis to on average 5.8 years postdiagnosis), waist circumference (at‐diagnosis to 7.9 years postdiagnosis), waist‐to‐hip ratio (at‐diagnosis to 2.5 years postdiagnosis) and pre‐ to postdiagnosis weight gain or loss (from 1 year before to 1 year or more after diagnosis). 27 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 In addition, 119 publications were descriptively synthesized 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 and 156 publications were excluded. 258 , 259 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 , 291 , 292 , 293 , 294 , 295 , 296 , 297 , 298 , 299 , 300 , 301 , 302 , 303 , 304 , 305 , 306 , 307 , 308 , 309 , 310 , 311 , 312 , 313 , 314 , 315 , 316 , 317 , 318 , 319 , 320 , 321 , 322 , 323 , 324 , 325 , 326 , 327 , 328 , 329 , 330 , 331 , 332 , 333 , 334 , 335 , 336 , 337 , 338 , 339 , 340 , 341 , 342 , 343 , 344 , 345 , 346 , 347 , 348 , 349 , 350 , 351 , 352 , 353 , 354 , 355 , 356 , 357 , 358 , 359 , 360 , 361 , 362 , 363 , 364 , 365 , 366 , 367 , 368 , 369 , 370 , 371 , 372 , 373 , 374 , 375 , 376 , 377 , 378 , 379 , 380 , 381 , 382 , 383 , 384 , 385 , 386 , 387 , 388 , 389 , 390 , 391 , 392 , 393 , 394 , 395 , 396 , 397 , 398 , 399 , 400 , 401 , 402 , 403 , 404 , 405 , 406 , 407 , 408 , 409 , 410 , 411 , 412 , 413
FIGURE 1.
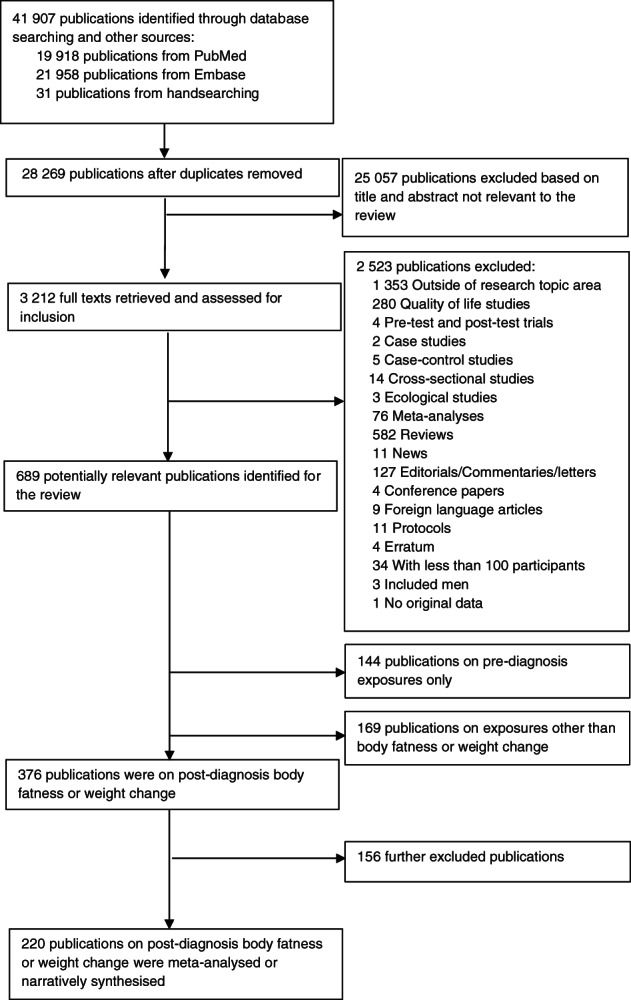
Flow chart of study selection process
The 220 included publications comprised 226 studies and included over 456 000 women diagnosed with breast cancer, of whom over 36 000 died of any causes, approximately 21 000 died of breast cancer and approximately 30 000 experienced an additional breast cancer event. Geographically, 79 publications were from North America, 27 , 39 , 41 , 46 , 47 , 49 , 50 , 51 , 53 , 54 , 56 , 57 , 58 , 61 , 62 , 63 , 64 , 69 , 71 , 72 , 73 , 74 , 77 , 79 , 80 , 81 , 84 , 87 , 88 , 91 , 93 , 95 , 98 , 99 , 100 , 107 , 109 , 111 , 113 , 114 , 115 , 117 , 118 , 124 , 126 , 127 , 128 , 132 , 140 , 146 , 147 , 149 , 150 , 154 , 165 , 166 , 168 , 170 , 171 , 174 , 175 , 177 , 178 , 179 , 183 , 184 , 190 , 192 , 200 , 201 , 203 , 211 , 217 , 225 , 235 , 248 , 253 , 256 , 257 64 from Europe, 43 , 48 , 60 , 66 , 67 , 70 , 76 , 89 , 90 , 92 , 94 , 101 , 103 , 104 , 105 , 108 , 116 , 130 , 133 , 135 , 136 , 137 , 141 , 142 , 143 , 144 , 145 , 148 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 164 , 173 , 181 , 182 , 186 , 187 , 191 , 193 , 194 , 195 , 198 , 199 , 204 , 207 , 208 , 212 , 218 , 219 , 224 , 229 , 230 , 232 , 237 , 240 , 243 , 245 , 246 , 255 50 from East or Southeast Asia 40 , 42 , 55 , 75 , 78 , 82 , 83 , 86 , 96 , 97 , 110 , 112 , 119 , 122 , 125 , 129 , 131 , 134 , 151 , 152 , 153 , 163 , 167 , 169 , 172 , 176 , 180 , 189 , 202 , 205 , 209 , 210 , 213 , 214 , 216 , 220 , 222 , 226 , 227 , 228 , 231 , 236 , 239 , 241 , 242 , 244 , 247 , 250 , 251 , 254 and 27 from international locations 45 , 52 , 59 , 68 , 102 , 106 , 120 , 121 , 123 , 138 , 221 , 238 , 252 or elsewhere. 44 , 65 , 85 , 139 , 185 , 188 , 196 , 197 , 206 , 215 , 223 , 233 , 234 , 249 One RCT was identified. 256 All others were observational studies. The publications included or excluded in each specific meta‐analysis or descriptive synthesis and the reasons for exclusion, and the corresponding study description and participants' characteristics are provided in Tables S3 and S4‐S8.
Below is an overview of the findings. Table 1 shows the summary findings and the judgment of the Expert Panel.
TABLE 1.
Evidence grades and main findings from the meta‐analyses and narrative syntheses
| Diet, nutrition, physical activity and survival in women with breast cancer | ||||||
|---|---|---|---|---|---|---|
| Increases risk | ||||||
| Exposure | Outcome | Summary of findings RR (95% confidence interval) | Conclusions | |||
| Strong evidence | Convincing | — | — | — | — | |
| Probable | Postdiagnosis greater body fatness | BMI | All‐cause mortality |
64 studies, 32 507 deaths 1.07 (1.05‐1.10) per 5 kg/m2 I 2 56%, P Egger .16 |
The evidence is substantial, generally consistent, and shows evidence of a dose‐response relationship. The observed associations are unlikely to be caused solely by chance or bias. More RCT evidence is required for the evidence to be judged “convincing.” | |
| WC |
5 studies, 983 deaths 1.18 (1.07‐1.31) per 10 cm I 2 = 55% |
|||||
| WHR |
8 studies, 2443 deaths 1.30 (1.20‐1.40) per 0.1 unit I 2 0% |
|||||
| BMI | Breast cancer‐specific mortality |
39 studies, 14 106 deaths 1.10 (1.06‐1.14) per 5 kg/m2 I 2 60%, P Egger <.001 |
||||
| WC |
3 studies, 262 deaths 1.12 (1.03‐1.22) per 10 cm I 2 0% |
|||||
| WHR |
6 studies, 1307 deaths 1.21 (1.08‐1.35) per 0.1 unit I 2 6% |
|||||
| BMI | Second primary breast cancer (BMI) |
11 studies, 5248 events 1.14 (1.04‐1.26) per 5 kg/m2 I 2 66% |
||||
| Limited evidence | Limited suggestive | Postdiagnosis greater body fatness | BMI | Recurrence |
63 studies, 29 749 deaths 1.05 (1.03‐1.08) per 5 kg/m2 I 2 54%, P Egger .46 |
The evidence is substantial, generally consistent and shows evidence of a dose‐response relationship, but is limited in methodological quality relating to outcome assessment. |
| WC | RRs ranged from 1.18 to 1.70 for the highest vs lowest WC in 5 out of the 6 studies, 2 of the 95% CIs did not include one | |||||
| WHR | 2 studies, no association | |||||
| BMI | Nonbreast cancer related mortality (BMI) |
10 studies, 2307 deaths 1.06 (0.94‐1.19) per 5 kg/m2 I 2 78%, P Egger .30 |
The evidence is substantial, but there is inconsistency. | |||
| BMI | CVD mortality (BMI) |
2 studies, 124 deaths 1.16 (0.96‐1.41) per 5 kg/m2 I 2 0% |
The evidence is sparse but is suggestive of a positive association. | |||
| Limited—no conclusion | Postdiagnosis BMI or weight change (gain or loss) |
Pre‐ to postdiagnosis weight/BMI change Any period postdiagnosis weight/BMI change Weight/BMI change during cancer treatment |
The evidence is sparse and inconsistent and is limited in methodological quality. Relating to exposure assessment. | |||
Abbreviations: BMI, Body mass index; CVD, cardiovascular disease; WC, waist circumference; WHR, waist‐hip‐ratio.
3.1. Postdiagnosis body mass index
The respective linear dose‐response meta‐analyses included 64 studies that reported all‐cause mortality (52 publications, 32 507 deaths), 39 , 40 , 41 , 43 , 44 , 45 , 46 , 53 , 54 , 55 , 57 , 63 , 64 , 65 , 66 , 67 , 68 , 70 , 72 , 78 , 79 , 81 , 82 , 84 , 85 , 88 , 89 , 91 , 93 , 94 , 95 , 97 , 98 , 100 , 102 , 104 , 105 , 109 , 111 , 112 , 113 , 114 , 116 , 118 , 120 , 123 , 125 , 126 , 127 , 129 , 130 , 135 39 studies that reported breast cancer‐specific mortality (31 publications, 14 106 deaths), 57 , 63 , 64 , 65 , 74 , 75 , 77 , 78 , 79 , 82 , 86 , 91 , 93 , 95 , 96 , 98 , 99 , 100 , 101 , 102 , 103 , 106 , 109 , 110 , 117 , 118 , 122 , 125 , 126 , 128 , 137 63 studies that reported breast cancer recurrence (49 publications, 29 749 events), 40 , 41 , 43 , 44 , 45 , 46 , 48 , 51 , 54 , 55 , 56 , 59 , 63 , 64 , 65 , 66 , 68 , 69 , 70 , 72 , 73 , 74 , 76 , 79 , 83 , 84 , 85 , 88 , 89 , 92 , 94 , 96 , 102 , 104 , 106 , 110 , 112 , 115 , 116 , 120 , 121 , 122 , 123 , 124 , 125 , 127 , 130 , 131 , 136 11 studies that reported second primary breast cancer (eight publications, 5248 events), 58 , 63 , 64 , 87 , 90 , 108 , 132 , 138 10 studies that reported nonbreast cancer‐related mortality (seven publications, 2307 deaths), 57 , 63 , 64 , 65 , 77 , 95 , 106 and two studies that reported cardiovascular mortality (two publications, 124 deaths). 100 , 114
Higher postdiagnosis BMI was associated with higher risks of all‐cause mortality (summary RR per 5 kg/m2: 1.07, 95% CI: 1.05‐1.10; Figure 2), breast cancer‐specific mortality (1.10, 95% CI: 1.06‐1.14; Figure 3), breast cancer recurrence (1.05, 95% CI: 1.03‐1.08; Figure S1) and second primary breast cancer (1.14, 95% CI: 1.04‐1.26; Figure S2). There was evidence of between‐study heterogeneity for all outcomes. The I 2 was 56%, 60%, 54% and 66% (all P heterogeneity <.001), respectively. There was suggestion of positive associations between BMI (per 5 kg/m2) and nonbreast cancer‐related mortality (1.06, 95% CI: 0.94‐1.19; I 2 78%, P heterogeneity <.001) and cardiovascular mortality (1.16, 95% CI: 0.96‐1.41; I 2 0%, P heterogeneity = .38; Figures S3 and S4).
FIGURE 2.
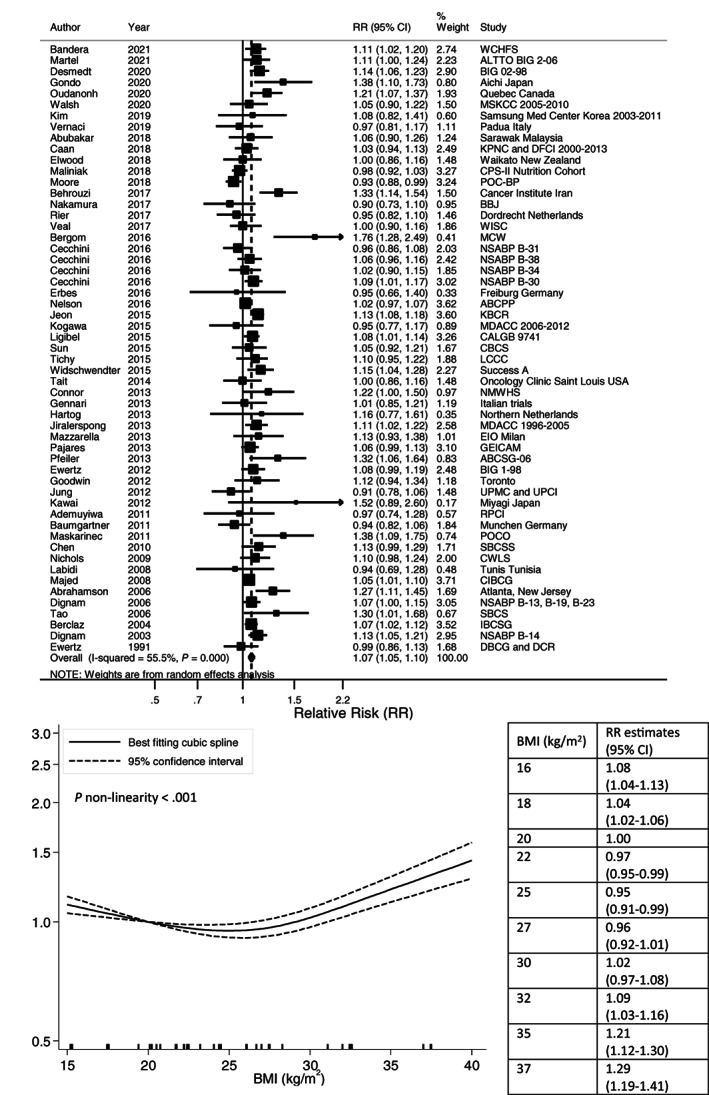
Linear and nonlinear dose‐response meta‐analyses of postdiagnosis body mass index and all‐cause mortality. Forest plot shows the linear dose‐response results for postdiagnosis body mass index (BMI) and all‐cause mortality from the inverse variance DerSimonian‐Laird random‐effects model. Diamond represents the summary relative risk (RR) estimate and its width as the 95% confidence interval (CI). Each square represents the RR estimate of each study and the horizontal line across each square represents the 95% CI of the RR estimate. The increment unit was per 5 kg/m2. Nonlinear curve was estimated using restricted cubic spline regression with three knots at 10th, 50th and 90th percentiles of distribution of the exposure and pooled in random‐effects meta‐analysis. BMI at 20 kg/m2 was selected as reference. The table shows selected BMI values and their corresponding RR (95% CI) estimated in the nonlinear dose‐response meta‐analysis.
FIGURE 3.
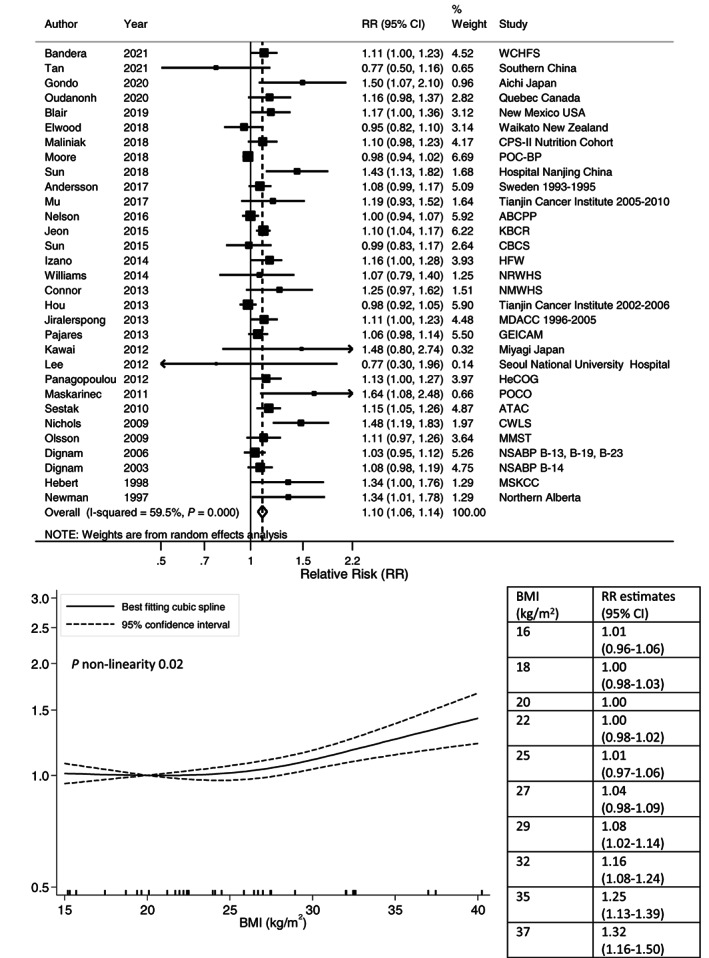
Linear and nonlinear dose‐response meta‐analyses of postdiagnosis body mass index and breast cancer‐specific mortality. Forest plot shows the linear dose‐response results for postdiagnosis body mass index (BMI) and breast cancer‐specific mortality from the inverse variance DerSimonian‐Laird random‐effects model. Diamond represents the summary relative risk (RR) estimate and its width as the 95% confidence interval (CI). Each square represents the RR estimate of each study and the horizontal line across each square represents the 95% CI of the RR estimate. The increment unit was per 5 kg/m2. Nonlinear curve was estimated using restricted cubic spline regression with three knots at 10th, 50th and 90th percentiles of distribution of the exposure and pooled in random‐effects meta‐analysis. BMI at 20 kg/m2 was selected as reference. The table shows selected BMI values and their corresponding RR (95% CI) estimated in the nonlinear dose‐response meta‐analysis.
Nonlinear dose‐response analysis detected a J‐shaped relationship with all‐cause mortality (P nonlinearity <.001; 52 studies, 27 478 deaths; 40 publications). 39 , 40 , 41 , 45 , 53 , 54 , 55 , 57 , 63 , 64 , 65 , 67 , 68 , 70 , 72 , 78 , 79 , 81 , 82 , 85 , 91 , 93 , 94 , 95 , 97 , 98 , 100 , 102 , 104 , 109 , 111 , 112 , 116 , 118 , 120 , 123 , 125 , 126 , 129 , 130 Compared with BMI arbitrary chosen at 20 kg/m2, increased all‐cause mortality risks were observed for BMI below 18 kg/m2 and above 32 kg/m2 with the most favorable survival in the upper normal to low overweight range (24‐26 kg/m2). Increased risk of breast cancer‐specific mortality was evident for BMI above 29 kg/m2 (P nonlinearity = .02; 34 studies, 13 324 deaths; 27 publications) 57 , 63 , 64 , 65 , 75 , 78 , 79 , 82 , 86 , 91 , 93 , 95 , 96 , 98 , 99 , 100 , 101 , 102 , 103 , 106 , 109 , 110 , 118 , 125 , 126 , 128 , 137 (Figures 2 and 3). The curves for breast cancer recurrence (P nonlinearity = .71; 47 studies, 28 165 events; 37 publications) 40 , 41 , 45 , 48 , 51 , 54 , 55 , 59 , 63 , 64 , 65 , 68 , 69 , 70 , 72 , 73 , 79 , 83 , 85 , 89 , 94 , 96 , 102 , 104 , 106 , 110 , 112 , 115 , 116 , 119 , 120 , 123 , 124 , 125 , 130 , 131 , 133 and second primary breast cancer (P nonlinearity = .19; nine studies, 2925 events; seven publications) 58 , 63 , 64 , 87 , 108 , 132 , 138 appeared linear (Figures S1 and S2).
Figures S5‐S7 show the RR estimates for the analyses comparing the highest to lowest BMI category, including the studies that were not available in the dose‐response meta‐analyses (Table S3). Most studies showed that higher BMI was associated with poorer survival after breast cancer.
3.2. Subgroup dose‐response meta‐analyses and meta‐regression analyses of postdiagnosis BMI
The results from the subgroup dose‐response meta‐analyses (by menopausal status, hormone receptor subtype, nodal status, geographic location, study type, exposure time, treatment period, length of follow‐up, loss to follow‐up, BMI assessment and covariate adjustment) largely resembled those of the main analyses, with few exceptions (Tables 2 and 3). However, few studies were included in some subgroups leading to wide 95% CIs and limiting the ability to assess/explain heterogeneity. Meta‐regression analyses indicated that tumor invasiveness or stage may partly explain the heterogeneity observed for breast cancer recurrence (I 2 = 54%), with the studies of invasive and in situ breast cancers showing a higher average positive association (five studies/publications) 55 , 96 , 110 , 112 , 127 compared with studies with only invasive cancers (50 studies, 41 publications), 40 , 41 , 43 , 44 , 45 , 46 , 48 , 51 , 54 , 56 , 59 , 63 , 64 , 65 , 66 , 68 , 69 , 70 , 72 , 74 , 79 , 84 , 85 , 88 , 89 , 92 , 94 , 102 , 104 , 106 , 115 , 116 , 120 , 121 , 122 , 123 , 124 , 125 , 130 , 131 , 136 and the studies of metastatic breast cancers showing inverse associations (eight studies, two publications) 70 , 121 compared with the positive associations observed for early stage disease cases (27 studies/publications) 41 , 43 , 44 , 46 , 48 , 51 , 54 , 56 , 59 , 63 , 66 , 68 , 69 , 72 , 74 , 79 , 88 , 89 , 94 , 106 , 116 , 120 , 122 , 123 , 124 , 127 , 130 (P meta‐regression = .02 and .003, respectively). A similar pattern by tumor stage was observed for all‐cause mortality (30 studies, 28 publications on early stage diseases 41 , 43 , 44 , 46 , 54 , 63 , 66 , 67 , 68 , 72 , 78 , 79 , 88 , 89 , 91 , 94 , 95 , 98 , 100 , 111 , 116 , 120 , 123 , 125 , 126 , 127 , 129 , 130 ; five studies, three publications on metastatic disease 70 , 81 , 105 ; P meta‐regression .14; Table 2). The studies with fewer breast cancer deaths (four studies/publications with <100 events 74 , 86 , 93 , 117 ; 16 studies, 15 publications with 100‐500 events 57 , 65 , 77 , 82 , 91 , 96 , 99 , 100 , 106 , 109 , 110 , 118 , 125 , 126 , 137 ) observed stronger positive associations compared with the studies with more breast cancer deaths (18 studies, 11 publications with >500 events 63 , 64 , 75 , 78 , 79 , 95 , 98 , 101 , 102 , 103 , 128 ), which may have contributed to the heterogeneity observed for breast cancer‐specific mortality (I 2 = 60%; P meta‐regression = .04; Table 3).
TABLE 2.
Subgroup meta‐analyses of postdiagnosis body mass index and breast cancer outcomes by disease characteristics
| Subgroup by disease characteristics a | All‐cause mortality Summary RR (95% CI) I 2, P heterogeneity | Breast cancer mortality Summary RR (95% CI) I 2, P heterogeneity | Breast cancer recurrence Summary RR (95% CI) I 2, P heterogeneity |
|---|---|---|---|
| Menopausal status | |||
| Premenopausal |
5 studies, 1812 deaths 1.10 (1.02‐1.20) 27%, 0.24 |
4 studies, 193 deaths 1.35 (1.08‐1.70) 21%, 0.29 |
6 studies, 2575 events 1.23 (1.06‐1.42) 77%, 0.001 |
| Postmenopausal |
10 studies, 4924 deaths 1.06 (0.99‐1.13) 68%, 0.001 P meta‐regression .52 |
6 studies, 1329 deaths 1.12 (1.07‐1.18) 0%, 0.45 P meta‐regression .14 |
9 studies, 5059 events 1.06 (1.02‐1.11) 23%, 0.24 P meta‐regression .17 |
| Hormone receptor subtype | |||
| ER+ and/or PR+ |
5 studies, 1048 deaths 1.10 (0.96‐1.26) 67%, 0.02 |
2 studies, 520 deaths 1.70 (0.57‐5.06) 65%, 0.09 |
5 studies, 2557 events 1.06 (1.01‐1.11) 0%, 0.51 |
| ER+ |
8 studies, 4640 deaths 1.11 (1.07‐1.15) 0%, 0.45 |
3 studies, 925 deaths 1.09 (1.01‐1.18) 0%, 0.75 |
7 studies, 4097 events 1.06 (1.02‐1.11) 25%, 0.24 |
| ER+/PR+ |
2 studies, 433 deaths 1.03 (0.90‐1.17) 2%, 0.31 |
— | — |
| ER− |
6 studies, 2005 deaths 1.09 (1.04‐1.14) 0%, 0.55 |
4 studies, 700 deaths 1.05 (0.97‐1.13) 1%, 0.32 P meta‐regression .50 |
6 studies, 2989 events 1.06 (1.02‐1.11) 0%, 0.91 |
| ER−/PR− |
3 studies, 235 deaths 1.20 (1.00‐1.42) 35%, 0.22 P meta‐regression .18 |
— |
2 studies, 189 events 1.22 (1.06‐1.40) 0%, 0.48 P meta‐regression .60 |
| Molecular subtype | |||
| ER+ and/or PR+/HER2− |
2 studies, 1491 deaths 1.24 (1.14‐1.33) 0%, 0.66 |
2 studies, 973 deaths 1.01 (0.78‐1.31) 86%, 0.008 |
— |
| ER+ and/or PR+/HER2+ |
3 studies, 794 deaths 1.04 (0.94‐1.16) 0%, 0.79 |
— |
2 studies, 691 events 1.01 (0.92‐1.11) 0%, 0.65 |
| Triple negative/basal‐like |
7 studies, 2364 deaths 1.03 (0.98‐1.09) 0%, 0.42 |
2 studies, 726 deaths 1.03 (0.88‐1.20) 13%, 0.28 |
5 studies, 1174 events 0.97 (0.83‐1.13) 46%, 0.12 |
| HER2+ |
4 studies, 1566 deaths 1.03 (0.96‐1.11) 19%, 0.30 P meta‐regression .12 |
— |
5 studies, 2056 events 1.06 (1.00‐1.11) 4%, 0.38 P meta‐regression .59 |
| Invasiveness of tumor | |||
| Invasive |
55 studies, 30 735 deaths 1.07 (1.04‐1.09) 58%, <0.001 |
32 studies, 13 160 deaths 1.08 (1.04‐1.13) 62%, <0.001 |
50 studies, 27 035 events 1.05 (1.03‐1.07) 38%, 0.007 |
| Invasive and in situ |
8 studies, 1576 deaths 1.12 (1.04‐1.22) 34%, 0.16 P meta‐regression .41 |
5 studies, 631 deaths 1.26 (1.09‐1.46) 41%, 0.15 P meta‐regression .21 |
5 studies, 1377 events 1.19 (1.08‐1.30) 34%, 0.20 P meta‐regression .02 |
| Nodal status | |||
| Node+ |
6 studies, 5556 deaths 1.06 (1.03‐1.10) 0%, 0.48 |
— |
6 studies, 6504 events 1.06 (1.03‐1.09) 0%, 0.68 |
| Node‐ |
6 studies, 2168 deaths 1.09 (1.04‐1.15) 0%, 0.78 P meta‐regression .35 |
5 studies, 1292 deaths 1.08 (0.98‐1.19) 45%, 0.17 |
5 studies, 2618 events 1.04 (1.00‐1.09) 0%, 0.46 P meta‐regression .50 |
| Cancer stage | |||
| Early stage/stage 0‐III/nonmetastatic |
30 studies, 20 393 deaths 1.08 (1.04‐1.12) 67%, <0.001 |
16 studies, 7856 deaths 1.11 (1.05‐1.17) 72%, <0.001 |
27 studies, 15 677 events 1.06 (1.04‐1.07) 0%, 0.54 |
| Stage 0‐II |
2 studies, 438 deaths 1.20 (1.02‐1.40) 22%, 0.26 |
— |
3 studies, 659 events 1.06 (0.77‐1.45) 80%, 0.002 |
| Locally advanced/stage III |
4 studies, 411 deaths 0.98 (0.88‐1.09) 35%, 0.21 |
— |
3 studies, 396 events 1.02 (0.87‐1.20) 52%, 0.13 |
| Stage IV/distant/metastatic |
5 studies, 761 deaths 0.95 (0.87‐1.04) 0%, 0.66 P meta‐regression .14 |
— |
8 studies, 1057 events 0.91 (0.86‐0.96) 0%, 0.74 P meta‐regression .003 |
Abbreviations: CI, Confidence interval; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; RR, relative risk.
Results not shown for the strata with only one study or the few studies with unknown information.
TABLE 3.
Subgroup meta‐analyses of postdiagnosis body mass index and breast cancer outcomes by study characteristics
| Subgroup by study characteristics a | All‐cause mortality Summary RR (95% CI) I 2, P heterogeneity | Breast cancer mortality Summary RR (95% CI) I 2, P heterogeneity | Breast cancer recurrence Summary RR (95% CI) I 2, P heterogeneity |
|---|---|---|---|
| Geographic location | |||
| East or Southeast Asia |
8 studies, 6375 deaths 1.13 (1.05‐1.21) 33%, 0.17 |
8 studies, 4847 deaths 1.12 (1.00‐1.26) 69%, 0.002 |
9 studies, 3406 events 1.13 (1.00‐1.27) 78%, <0.001 |
| Europe |
13 studies, 5555 deaths 1.04 (0.99‐1.10) 32%, 0.14 |
3 studies, 1593 deaths 1.10 (1.03‐1.17) 0%, 0.82 |
14 studies, 7498 events 1.05 (1.00‐1.10) 45%, 0.04 |
| Multi‐national |
8 studies, 5460 deaths 1.08 (1.05‐1.12) 0%, 0.59 |
5 studies, 1145 deaths 1.10 (1.01‐1.19) 44%, 0.18 |
15 studies, 9913 events 1.04 (1.00‐1.08) 68%, 0.003 |
| North America |
32 studies, 14 435 deaths 1.06 (1.03‐1.10) 62%, <0.001 P meta‐regression .22 |
22 studies, 6204 deaths 1.11 (1.05‐1.16) 64%, <0.001 P meta‐regression .81 |
22 studies, 8163 events 1.05 (1.03‐1.08) 13%, 0.29 P meta‐regression .73 |
| Study type | |||
| Secondary analysis of clinical trials |
22 studies, 11 069 deaths 1.08 (1.06‐1.11) 2%, 0.43 |
10 studies, 2871 deaths 1.08 (1.04‐1.12) 0%, 0.46 |
30 studies, 17 098 events 1.05 (1.03‐1.08) 51%, 0.01 |
| Prospective cohort studies |
12 studies, 10 840 deaths 1.07 (1.02‐1.12) 40%, 0.07 |
8 studies, 3932 deaths 1.15 (1.04‐1.27) 50%, 0.06 |
9 studies, 6798 events 1.05 (1.02‐1.09) 0%, 0.67 |
| Retrospective cohort studies |
20 studies, 5199 deaths 1.06 (1.00‐1.12) 67%, <0.001 |
9 studies, 3336 deaths 1.08 (1.00‐1.18) 69%, 0.001 |
22 studies, 5044 events 1.04 (0.98‐1.11) 62%, <0.001 P meta‐regression .67 |
| Follow‐up of cases from case‐control studies |
5 studies, 1565 deaths 1.16 (1.07‐1.26) 27%, 0.25 |
4 studies, 775 deaths 1.16 (0.99‐1.35) 69%, 0.02 |
— |
| Population‐based studies/Follow‐up of cases from a noncancer cohort |
2 studies, 1622 deaths 1.04 (0.91‐1.18) 84%, 0.012 |
3 studies, 547 deaths 1.10 (1.02‐1.19) 0%, 0.97 |
— |
| Pooled analyses |
3 studies (1 publication), 2212 deaths 1.02 (0.97‐1.07) P meta‐regression .43 |
3 studies (1 publication), 1131 deaths 1.00 (0.94‐1.07) P meta‐regression .91 |
— |
| Exposure time b | |||
| Before chemotherapy |
40 studies, 22 641 deaths 1.06 (1.03‐1.09) 56%, <0.001 |
24 studies, 8213 deaths 1.09 (1.04‐1.15) 61%, 0.001 |
40 studies, 21 643 events 1.06 (1.03‐1.08) 52%, <0.001 |
| During chemotherapy |
2 studies, 723 deaths 1.16 (0.99‐1.37) 68%, 0.08 |
— | — |
| After chemotherapy |
6 studies, 3345 deaths 1.04 (0.96‐1.12) 63%, 0.05 |
4 studies, 1072 deaths 1.12 (1.06‐1.19) 0%, 0.93 P meta‐regression .50 |
3 studies, 1572 events 1.05 (1.00‐1.11) 0%, 0.69 |
| Before hormonal therapy |
2 studies, 991 deaths 1.16 (0.97‐1.40) 60%, 0.11 P meta‐regression .41 |
7 studies, 2375 events 1.02 (0.89‐1.17) 86%, 0.001 P meta‐regression .50 |
|
| Treatment period | |||
| ≤2000 |
16 studies, 11 045 deaths 1.09 (1.05‐1.12) 43%, 0.05 |
12 studies, 2956 deaths 1.18 (1.08‐1.28) 55%, 0.02 |
17 studies, 13 519 events 1.05 (1.02‐1.08) 33%, 0.10 |
| >2000 |
16 studies, 4761 deaths 1.08 (1.01‐1.14) 66%, <0.001 P meta‐regression .63 |
7 studies, 2555 deaths 1.08 (0.98‐1.18) 74%, 0.01 P meta‐regression .28 |
16 studies, 4041 events 1.09 (1.03‐1.16) 41%, 0.04 P meta‐regression .10 |
| ≤2005 |
26 studies, 15 404 deaths 1.08 (1.06‐1.11) 21%, 0.19 |
16 studies, 4487 deaths 1.14 (1.08‐1.21) 43%, 0.05 |
27 studies, 19 817 events 1.05 (1.03‐1.07) 23%, 0.16 |
| >2005 |
2 studies, 154 deaths 0.98 (0.87‐1.11) 0%, 0.71 P meta‐regression .20 |
— |
3 studies, 365 events 0.97 (0.86‐1.09) 0%, 0.78 P meta‐regression .23 |
| Length of follow‐up | |||
| ≤5 years |
13 studies, 2820 deaths 1.07 (1.00‐1.14) 35%, 0.12 |
5 studies, 1254 deaths 1.14 (0.97‐1.34) 57%, 0.05 |
19 studies, 4326 events 1.02 (0.95‐1.10) 62%, 0.002 |
| 5‐10 years |
35 studies, 20 706 deaths 1.07 (1.03‐1.10) 65%, <0.001 |
21 studies, 9478 deaths 1.11 (1.06‐1.16) 67%, <0.001 |
30 studies, 16 409 events 1.06 (1.03‐1.09) 57%, <0.001 |
| >10 years |
12 studies, 8123 deaths 1.09 (1.04‐1.13) 45%, 0.06 P meta‐regression .91 |
10 studies, 2750 deaths 1.08 (1.01‐1.15) 50%, 0.07 P meta‐regression .87 |
8 studies, 6983 events 1.05 (1.04‐1.07) 0%, 0.65 P meta‐regression .65 |
| Loss to follow‐up | |||
| Complete/<10% loss |
18 studies, 7171 deaths 1.05 (1.00‐1.11) 64%, <0.001 |
10 studies, 3676 deaths 1.11 (1.03‐1.19) 70%, <0.001 |
17 studies, 5944 events 1.08 (1.03‐1.13) 41%, 0.04 |
| ≥10% loss |
1 study, 189 deaths 1.38 (1.10‐1.73) |
2 studies, 137 deaths 1.08 (0.56‐2.09) 83%, 0.02 |
2 studies, 329 events 1.14 (0.82‐1.58) 55%, 0.14 |
| No description |
45 studies, 25 147 deaths 1.08 (1.05–1.10) 42%, 0.005 P meta‐regression .10 |
27 studies, 10 293 deaths 1.09 (1.05‐1.13) 40%, 0.04 P meta‐regression .96 |
44 studies, 23 476 events 1.04 (1.02‐1.07) 57%, <0.001 P meta‐regression .20 |
| Number of events | |||
| <100 |
5 studies, 329 deaths 1.06 (0.96‐1.17) 0%, 0.75 |
4 studies, 211 deaths 1.25 (1.01‐1.56) 24%, 0.27 |
5 studies, 364 events 0.92 (0.74‐1.15) 61%, 0.04 |
| 100‐500 |
30 studies, 7759 deaths 1.09 (1.05‐1.14) 50%, 0.001 |
16 studies, 3425 deaths 1.15 (1.09‐1.21) 43%, 0.04 |
23 studies, 5971 events 1.09 (1.04‐1.15) 35%, 0.06 |
| >500 |
22 studies, 24 419 deaths 1.06 (1.03‐1.09) 63%, <0.001 P meta‐regression .61 |
18 studies, 10 470 deaths 1.05 (1.01‐1.09) 58%, 0.008 P meta‐regression .04 |
28 studies, 23 414 events 1.04 (1.02‐1.07) 64%, <0.001 P meta‐regression .21 |
| BMI assessment | |||
| Measured |
23 studies, 12 539 deaths 1.07 (1.03‐1.10) 54%, 0.002 |
9 studies, 1719 deaths 1.08 (0.99‐1.18) 66%, 0.01 |
25 studies, 13 911 events 1.04 (1.01‐1.08) 45%, 0.02 |
| Self‐reported |
9 studies, 3710 deaths 1.08 (1.01‐1.16) 58%, 0.02 |
10 studies, 2050 deaths 1.14 (1.08‐1.20) 11%, 0.34 |
5 studies, 1048 events 1.09 (1.01‐1.19) 21%, 0.28 |
| From records |
22 studies, 11 879 deaths 1.07 (1.03‐1.11) 53%, 0.003 P meta‐regression .48 |
14 studies, 8582 deaths 1.10 (1.04‐1.16) 59%, 0.002 P meta‐regression .44 |
25 studies, 10 948 events 1.04 (1.00‐1.08) 62%, <0.001 P meta‐regression .32 |
| Covariate adjustment | |||
| Age, disease, treatment, comorbidity, smoking, alcohol or physical activity |
6 studies, 4030 deaths 1.02 (0.97‐1.08) 53%, 0.09 |
5 studies, 1632 deaths 1.06 (0.98‐1.14) 49%, 0.14 |
‐ |
| Age, disease, treatment, comorbidity or smoking |
10 studies, 4727 deaths 1.11 (1.06‐1.17) 26%, 0.21 |
8 studies, 1818 deaths 1.13 (1.04‐1.24) 38%, 0.14 |
6 studies, 4422 events 1.06 (1.02‐1.10) 0%, 0.90 |
| Age, disease, treatment |
30 studies, 12 336 deaths 1.07 (1.04‐1.11) 32%, 0.07 |
16 studies, 7830 deaths 1.09 (1.04‐1.14) 55%, 0.01 |
38 studies, 12 754 events 1.06 (1.02‐1.10) 68%, <0.001 |
| Not adjusted for age, disease characteristics, or treatment |
18 studies, 11 414 deaths 1.06 (1.02‐1.11) 70%, <0.001 P meta‐regression .31 |
10 studies, 2826 deaths 1.12 (1.00‐1.24) 69%, 0.001 P meta‐regression .84 |
19 studies, 12 573 events 1.05 (1.02‐1.08) 24%, 0.16 P meta‐regression .93 |
Abbreviations: CI, Confidence interval; RR, relative risk.
Results not shown for the strata with only one study or the few studies with unknown information, except for loss to follow‐up that was mostly not described in the studies.
Exposure time relative to cancer treatment was defined according to when the exposure assessment was conducted in the studies. Some study participants might not have received the treatment.
3.3. Postdiagnosis waist circumference and waist‐to‐hip ratio
The respective linear dose‐response meta‐analyses of postdiagnosis waist circumference included five studies reporting on all‐cause mortality (983 deaths) 39 , 71 , 72 , 112 , 118 and three on breast cancer‐specific mortality (262 deaths), 71 , 117 , 118 and of postdiagnosis waist‐to‐hip ratio included eight studies reporting on all‐cause mortality (2443 deaths) 39 , 55 , 60 , 71 , 107 , 109 , 112 , 118 and six on breast cancer‐specific mortality (1307 deaths). 49 , 60 , 71 , 107 , 109 , 118
Higher waist circumference and waist‐to‐hip ratio were associated with higher risks of all‐cause mortality and breast cancer‐specific mortality. The summary RRs per 10 cm increase in waist circumference were 1.18 (95% CI: 1.07‐1.31; I 2 = 55%) and 1.12 (95% CI: 1.03‐1.22; I 2 = 0%), and per 0.1 unit increase in waist‐to‐hip ratio were 1.30 (95% CI: 1.20‐1.40; I 2 = 0%) and 1.21 (95% CI: 1.08‐1.35; I 2 = 6%), respectively.
Nonlinear dose‐response meta‐analyses including the same studies showed linear relationships (P nonlinearity = .47, .08 and .73, respectively; Figures S8‐S11).
Both studies with 39 , 49 , 55 , 71 , 107 and without 39 , 60 , 72 , 109 , 112 , 118 BMI adjustment on average showed a positive association (Figures S12‐S14). No further subgroup and sensitivity analyses were conducted because of the low number of included studies.
Five 112 , 143 , 151 , 207 , 255 out of six studies reported higher breast cancer recurrence with higher waist circumference 72 , 112 , 143 , 151 , 207 , 255 (Hazard ratios for the highest vs lowest category ranged from 1.18 to 1.76; Figure S15). No clear trend of association with breast cancer recurrence was observed for waist‐to‐hip ratio (two studies and four publications). 42 , 55 , 112 , 214
3.4. Postdiagnosis weight and BMI change
One weight loss intervention trial, of 338 postmenopausal, stage I‐IIIa, hormone receptor‐positive breast cancer survivors receiving adjuvant letrozole and with BMI at least 24 kg/m2, was identified. 256 The results suggested improved survival (but the CIs were wide) in women randomized into the 24‐month lifestyle intervention group compared with the education group (HRs 0.71, 95% CI: 0.41‐1.24 for disease‐free survival; 0.86, 0.35‐2.14 for overall survival; 52 breast cancer events, 19 deaths, 8 years median follow‐up). Weight loss in the intervention group was not sustainable (−5.5% vs −0.6% at 12 months; −3.7% vs −0.4% at 24 months; −2.0% vs −1.6% at 36 months). Additional landmark analysis of weight loss up to 24 months in disease‐free participants showed attenuated results. 256
In observational studies, postdiagnosis weight or BMI change were evaluated for the timeframes from before diagnosis to one or more years after diagnosis (pre‐ to postdiagnosis; 10 27 , 39 , 42 , 50 , 51 , 52 , 55 , 100 , 107 , 146 and 2 178 , 225 publications, respectively), for any period postdiagnosis (7 120 , 132 , 138 , 149 , 172 , 218 , 240 and 1 211 publications, respectively), or specifically during cancer treatment (5 39 , 147 , 191 , 204 , 257 and 7 84 , 132 , 160 , 177 , 200 , 236 , 254 publications, respectively), and these exposures were separately reviewed.
Categorical meta‐analyses of percentage weight change from before to after diagnosis were possible in seven studies reporting results on all‐cause and breast cancer‐specific mortality (three publications, 2784 total deaths, 1752 breast cancer deaths), 50 , 52 , 107 but substantial between‐study heterogeneity was present in some analyses. There was a suggestion that weight loss (unknown causes) across the investigated timeframes, was associated with higher all‐cause mortality compared with stable weight (RRs: 1.15‐5.29; Figures S16‐S18).
Meta‐analysis was not possible for BMI change. There was a suggestion that, compared with stable BMI, pre‐ to postdiagnosis BMI gain 178 , 225 was associated with higher all‐cause mortality, breast cancer‐specific mortality and breast cancer recurrence, and that BMI gain after neoadjuvant chemotherapy was associated with higher all‐cause mortality among nonmetastatic survivors. 177 , 236 Only two studies each reported results (Figure S19).
No clear trend of association was observed for other investigated postdiagnosis changes of weight or BMI and breast cancer outcomes (Figures S16‐S20).
3.5. Sensitivity analyses and tests of publication bias
The overall summary RR estimates remained materially unchanged in leave‐one‐out sensitivity analyses. There was evidence of small study effects in the analysis of BMI and breast cancer‐specific mortality (Egger P = .001; Figure S21), with the average positive associations being stronger in studies of fewer compared with more events (Table 3). No evidence of publication bias was detected in analyses of other pre‐specified outcomes (Egger P values ≥.19).
3.6. Evidence grading
Table 1 shows the summary of findings and the corresponding evidence grades (Table S2).
The evidence on body fatness (BMI, waist circumference, waist‐to‐hip ratio) and all‐cause mortality, breast cancer‐specific mortality and second primary breast cancer (BMI only) was substantial, consistent in general and across different study designs and populations and showed evidence of a dose‐response relationship, which was unlikely to be caused by chance or bias. This evidence was graded as strong (subgrade: probable), but not convincing, as more supporting evidence from RCTs is needed.
The evidence for body fatness and breast cancer recurrence was limited in quality relating to outcome assessment but suggestive of a positive dose‐response relationship. The evidence suggesting an increased risk of nonbreast cancer‐related mortality (BMI only) and cardiovascular mortality (BMI only) with greater body fatness was also limited suggestive.
The evidence on weight or BMI change was sparse and inconsistent and was limited in quality relating to exposure assessment and no conclusions could be made.
4. DISCUSSION
The epidemiologic evidence on body fatness, weight change and breast cancer prognosis was systematically synthesized and independently evaluated. The estimated increase in risk per 5 kg/m2 higher postdiagnosis BMI was 7% for all‐cause mortality, 10% for breast cancer‐specific mortality and 14% for second primary breast cancer. There was high, partially explained, between‐study heterogeneity, but the positive associations were generally consistent across several study and disease subgroups. However, most subgroups included a low number of studies and will require further analyses in the future.
Plausible biological mechanisms underpinning the observed associations of increased body fatness with poorer survival have been widely studied. 414 , 415 , 416 , 417 Increased conversion of androgens to oestrogens by aromatase in adipose fat, decreased levels of sex‐hormone binding globulin and the resulting increased circulating oestrogen levels may drive breast cancer progression in postmenopausal women with obesity. 418 Other potential mediators include insulin resistance and associated increased insulin levels 419 ; chronic sub‐clinical inflammation 420 ; and altered adipokine levels, 421 including increased leptin that is pro‐inflammatory and decreased adiponectin and the consequent reduced anti‐inflammatory and insulin‐sensitising effects. 422 These inter‐connected signaling pathways could promote tumor growth and proliferation in women with obesity. In the present review, there was evidence that the association with breast cancer recurrence were, on average, inverse among women with metastatic disease but positive among women with early‐stage diseases. The results may reflect disease characteristics (tumor biology and treatment responses) rather than host factors (obesity and its metabolic consequences) may have greater impact on recurrence and survival in these distinctive survivors. Further studies are needed to elucidate such findings.
The present nonlinear analysis of postdiagnosis BMI and all‐cause mortality revealed a J‐shaped relationship suggesting a more favorable survival in women of high normal to low overweight (24‐26 kg/m2) compared with women of low normal weight (arbitrary chosen at 20 kg/m2). This observation relates somewhat to the phenomenon of the “obesity paradox” that has been reported in other cancer patients. 423 The exact causes are unclear but could include metabolic advantage, collider stratification bias (selection bias), confounding and/or reverse causation bias. 424 In addition, BMI does not reflect fat distribution and cannot distinguish lean from fat mass. 425 , 426 Analyses conducted for waist circumference and waist‐to‐hip ratio in the present review showed positive associations with all‐cause and breast cancer‐specific mortality, which may be independent of BMI. Sarcopenia and sarcopenic obesity may also be independent prognostic factors for breast cancer, 427 but further investigations are needed to study in more detail associations with body shape and composition. 428
Taken together, the evidence was graded as strong (subgrade: probable) for body fatness and all‐cause mortality, breast cancer‐specific mortality and second primary breast cancer. The evidence for BMI and higher risk of breast cancer recurrence was graded as limited‐suggestive since the outcomes were inconsistently defined across the studies, 429 and could be misclassified and/or incompletely ascertained in observational studies that used participants' reported or registry record linkage data. 430 , 431 The evidence for BMI and higher risks of nonbreast cancer‐related mortality and cardiovascular mortality was also graded as limited‐suggestive. There was a paucity of data on cardiovascular mortality, despite cardiovascular deaths being the most common cause of nonbreast cancer deaths in breast cancer patients, 432 , 433 and high BMI may increase cardiovascular events in general. 434 More research is needed to investigate these important outcomes.
Relatively few numbers of studies investigated weight or BMI change and the results were substantially heterogeneous. Weight loss after cancer diagnosis may be associated with higher all‐cause mortality, but the intentionality of weight loss was unclear and could be related to cancer cachexia. 435 In the pooling project, the positive association with large weight loss was only restricted to the under or normal weight individuals, those with comorbidities, and who ever smoked, 52 hence the overall results could be affected by reverse causation. In addition, we found no apparent associations between weight or BMI gain and breast cancer outcomes across the timeframes, unlike a recent published meta‐analysis that reported negative prognostic impact with large weight gain overall. 436 The association may be distorted, as chemotherapy may cause weight gain particularly in patients treated with older regimens that often incorporated high doses of corticosteroids. 437 The evidence was judged as limited‐no conclusion.
We only identified one small weight loss intervention trial publishing results on survival outcomes, which suggested beneficial effects of intervention through diet, physical activity and behavioral change compared with education. 256 Whether sustainable, intentional weight loss can reverse the adverse pathological sequelae and improve survival outcomes in breast cancer patients with overweight and obesity requires elucidation. Findings on the combined influence of body fatness and physical activity could provide important lifestyle and cancer care information.
The substantial body of evidence (226 studies with over 456 000 women) accumulated over the years was comprehensively and systematically synthesized, and independently graded using standardized criteria. All postdiagnosis data were pooled, and subgroup analyses were conducted by timeframe relative to cancer treatment to better account for any influence from treatment on body measurements. Other subgroup analyses were conducted, however, individual studies may not report enough, comparable information required for inclusion in a dose‐response meta‐analysis. For the analyses by breast cancer subtype, the low numbers of studies, coupled with the low numbers of the less common breast cancers, that were differently assessed and classified in the studies, had hindered definitive conclusions on these associations. As shown, recent published meta‐analyses with largely the same included studies reported conflicting results, 436 , 438 , 439 suggesting further investigation in studies using comparable classification is warranted.
Several limitations in relation to the evidence require discussion. First, survival benefit may present in studies that recruited participants who were well enough to survive the cancer years after diagnosis. Second, most studies did not have repeated body measurements to account for postdiagnosis weight change 440 , 441 or changes in muscle and fat mass 442 perhaps because of chemotherapy or changes in lifestyle or hormonal metabolism. 443 Third, reverse causation because of undetected disease outcome that leads to changes in exposure was possible, but most studies included only early stage (I‐III) breast cancer survivors, who on average showed positive associations that were not observed among metastatic breast cancer survivors in the present subgroup meta‐analyses across the outcomes. Fourth, uncontrolled or residual confounding, from lifestyle factors for which information were often missing, tumor stage and treatment that could lack details and pre‐diagnosis body fatness that may drive the development of aggressive tumors, 444 was possible. However, studies adjusted at least for age, tumor stage, cancer treatment, and either comorbidities or smoking, on average, showed positive associations. Consistent positive associations were also observed among the secondary analysis of clinical trials 45 , 48 , 54 , 59 , 63 , 64 , 68 , 70 , 88 , 102 , 103 , 104 , 106 , 116 , 120 , 121 , 123 , 147 , 159 , 161 , 162 , 164 , 181 , 191 , 193 , 199 , 203 , 211 , 221 , 222 , 238 , 252 , 253 , 283 which were expected to have better treatment protocols and management. The evidence of greater body fatness causing increased mortality was unlikely to be caused solely by chance or bias, and was graded as strong probable evidence. To reach the strong convincing grade, more definitive evidence from RCTs (body composition, weight control or weight loss trials 445 ) is needed.
The present review supports the advice for women who have completed primary treatment for breast cancer to follow the WCRF/AICR Cancer Prevention Recommendations to eat a healthy diet, be physically active and maintain a healthy weight if it fits with the specific medical advice given by their cancer management team. 5 Adherence to such lifestyle recommendations, in line with the recently released American Cancer Society Guideline on Diet and Activity for Cancer Survivors 2022, 446 has shown to lower all‐cause mortality. 447
Continual research effort is needed to inform whether specific recommendations are needed for different breast cancer survivors.
5. CONCLUSIONS
There was strong probable evidence that higher postdiagnosis body fatness increases risks of all‐cause mortality, breast cancer‐specific mortality and second primary breast cancer in women diagnosed with breast cancer. The evidence for breast cancer recurrence, nonbreast cancer‐related mortality and cardiovascular mortality was limited suggestive. For postdiagnosis weight or BMI change, the evidence was limited‐no conclusion. Intervention trials, well‐designed observational studies and biological mechanistic studies in diverse populations are needed to elucidate the impact of body composition and distribution and their changes on outcomes across the cancer continuum.
AUTHOR CONTRIBUTIONS
Konstantinos K. Tsilidis and Doris S. M. Chan are co‐principal investigators of CUP Global at Imperial College London. Konstantinos K. Tsilidis was part of the Expert Panel but was not involved with judging the evidence after becoming a co‐principal investigator of CUP Global. Teresa Norat and Doris S. M. Chan wrote the protocol based on the advice from the Protocol Expertise Group and implemented the study with Konstantinos K. Tsilidis. Doris S. M. Chan and Neesha Nanu did the literature search. Rita Vieira, Leila Abar, Katia Balducci, Margarita Cariolou and Neesha Nanu did the study selections and data extraction. Nerea Becerra‐Tomas did the study selections, data extraction and checked the data. Doris S. M. Chan and Rita Vieira checked, analyzed and interpreted the data. Dagfinn Aune and Georgios Markozannes were CUP Global team members who revised the manuscript. Darren C. Greenwood was statistical adviser. Anne McTiernan (AMcT), Steven K. Clinton (SC), Edward L. Giovannucci (EG), Ellen Kampman (EK), Alan A. Jackson (AJ), Konstantinos K. Tsilidis (KT), Marc J. Gunter (MG) and Vivien Lund (VL) (lay member) were the Expert Panel members who provided judgments on the evidence and advised on the interpretation of the review. Elio Riboli and Amanda J. Cross were Expert Panel observers. Kate Allen, Nigel T. Brockton, Helen Croker, Daphne Katsikioti, Deirdre McGinley‐Gieser, Panagiota Mitrou and Martin Wiseman were the CUP Global Secretariat members who provided overall coordination for the work and convened and facilitated discussions with the Expert Panel. Doris S. M. Chan drafted the original manuscript. All authors reviewed and provided comments on the manuscript. Doris S. M. Chan is the guarantor and has full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
This stduy was funded by the World Cancer Research Fund network of charities (American Institute for Cancer Research [AICR]; World Cancer Research Fund [WCRF]; Wereld Kanker Onderzoek Fonds [WKOF]) (CUP GLOBAL Special Grant 2018). Konstantinos K. Tsilidis, Doris S. M. Chan, Rita Vieira, Dagfinn Aune, Katia Balducci, Margarita Cariolou, Georgios Markozannes and Nerea Becerra‐Tomás are supported by the World Cancer Research Fund network of charities. Leila Abar and Neesha Nanu were previously supported by the World Cancer Research Fund network of charities. Teresa Norat was supported by the World Cancer Research Fund network of charities as principal investigator of the WCRF/AICR Continuous Update Project (CUP) and by WCRF International as the CUP Global scientific advisor. Dr. McTiernan was supported by grants from the Breast Cancer Research Foundation (BCRF‐19‐107/BCRF‐20‐107/BCRF‐21‐107). The funders of this study had no role in the decisions about the design and conduct of the study; collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The process used was based on the method developed by WCRF International's Methodology Task Force for the WCRF/AICR Second Expert Report. The CUP Global Secretariat, led by WCRF International, provided overall coordination for the work and convened and facilitated discussions with the Expert Panel who provided judgments on the evidence. The views expressed in this review are the opinions of the authors. They may differ from those in future updates of the evidence related to food, nutrition, physical activity and cancer incidence and survival.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
APPENDIX S1 Supporting Information
ACKNOWLEDGEMENTS
We thank Teresa Norat for leading the WCRF/AICR Continuous Update Project (CUP) as principal investigator from 2007 to 2020. We thank the Protocol Expertise Group: Annie Anderson (University of Dundee), Steven Clinton (The Ohio State University), Ellen Copson (Southampton University), Wendy Demark‐Wahnefried (UAB Comprehensive Cancer Center, Birminham, AL), John Mathers (Newcastle University), Anne McTiernan (Fred Hutchinson Cancer Research Center), Andrew Renehan (University of Manchester), Lesley Turner (patient representative), Franzel van Duijnhoven (Wageningen University) and Galina Velikova (University of Leeds), for their expert opinion on the review protocol. We thank the CUP Global team members: Sonia Chemlal, Jakub Sobiecki, Britta Talumaa and Victoria White, for their contribution to the literature search and data extraction; and database managers: Rui Vieira, Christophe Stevens, Yusuf O. Anifowoshe and Lam Teng for implementing and updating the CUP Global database. We also acknowledge the input of Isobel Bandurek and Susannah Brown as past CUP Global Secretariat members.
Chan DSM, Vieira R, Abar L, et al. Postdiagnosis body fatness, weight change and breast cancer prognosis: Global Cancer Update Program (CUP global) systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):572‐599. doi: 10.1002/ijc.34322
Funding information American Institute for Cancer Research, Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018; Breast Cancer Research Foundation, Grant/Award Number: BCRF‐19‐107/BCRF‐20‐107/BCRF‐21‐107; Wereld Kanker Onderzoek Fonds, Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018; World Cancer Research Fund, Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018
DATA AVAILABILITY STATEMENT
Only publicly available data were used in our study. Data sources and handling of these data are described in the Materials and Methods section. Further details are available from the corresponding author upon request.
REFERENCES
- 1. Ferlay JEM, Lam F, Colombet M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2020. [Google Scholar]
- 2. Chan DSM, Abar L, Cariolou M, et al. World Cancer Research Fund international: continuous update project‐systematic literature review and meta‐analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30:1183‐1200. [DOI] [PubMed] [Google Scholar]
- 3. Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collaboration NCDRF . Worldwide trends in body‐mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population‐based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627‐2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Cancer Research Fund/American Institute for Cancer Research , 2018. Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Expert Report.
- 6. Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer‐systematic literature review and meta‐analysis of 82 follow‐up studies. Ann Oncol. 2014;25:1901‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee K, Kruper L, Dieli‐Conwright CM, Mortimer JE. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep. 2019;21:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buch K, Gunmalm V, Andersson M, Schwarz P, Brons C. Effect of chemotherapy and aromatase inhibitors in the adjuvant treatment of breast cancer on glucose and insulin metabolism‐a systematic review. Cancer Med. 2019;8:238‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta‐analysis. Breast Cancer Res Treat. 2012;134:769‐781. [DOI] [PubMed] [Google Scholar]
- 10. Chan DS, Norat T. Obesity and breast cancer: not only a risk factor of the disease. Curr Treat Options Oncol. 2015;16:22. [DOI] [PubMed] [Google Scholar]
- 11. Mei L, He L, Song Y, et al. Association between obesity with disease‐free survival and overall survival in triple‐negative breast cancer: a meta‐analysis. Medicine (Baltimore). 2018;97:e0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan H, Gray R, Braybrooke J, et al. 20‐year risks of breast‐cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836‐1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. U.S. Cancer Statistics Data Visualizations Tool, based on 2019 submission data (1999‐2017): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, ed. June 2020. www.cdc.gov/cancer/dataviz. Accessed May 2021.
- 14. Breast cancer burden in EU‐27 . https://ecis.jrc.ec.europa.eu. Accessed May 2021.
- 15. Tsilidis KK, Cariolou M, Becerra‐Tomás N, et al. Post‐diagnosis body fatness, recreational physical activity, dietary factors and breast cancer prognosis: global cancer update Programme (CUP global) summary of evidence grading. Int J Cancer. 2023;152(4):635‐644. 10.1002/ijc.34320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Becerra‐Tomás N, Balducci K, Abar L, et al. Post‐diagnosis dietary factors, supplement use and breast cancer prognosis: global cancer update Programme (CUP global) systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):616‐634. 10.1002/ijc.34321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cariolou M, Abar L, Aune D, et al. Post‐diagnosis recreational physical activity and breast cancer prognosis: global cancer update Programme (CUP global) systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):600‐615. 10.1002/ijc.34324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Global Cancer Update Programme (CUP Global). 2022. https://www.wcrf.org/diet-activity-and-cancer/global-cancer-update-programme/about-the-global-cancer-update-programme/. Accessed September 2022.
- 19. Imperial College London CUP Global Team . Continuous Update Project on diet and cancer: Protocol for the data collection and systematic literature reviews on the role of diet, nutrition and physical activity on outcomes after diagnosis of breast cancer., ed. Version 3, 2019. https://www.imperial.ac.uk/school-public-health/epidemiology-and-biostatistics/research/cancer-and-nutritional-epidemiology/global-cancer-update-programme/. Accessed July 2022.
- 20. Chubak J, Boudreau DM, Wirtz HS, McKnight B, Weiss NS. Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst. 2013;105:1456‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Savitz DA, Wellenius GA, Trikalinos TA. The problem with mechanistic risk of bias assessments in evidence synthesis of observational studies and a practical alternative: assessing the impact of specific sources of potential bias. Am J Epidemiol. 2019;188:1581‐1585. [DOI] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 23. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135:1301‐1309. [DOI] [PubMed] [Google Scholar]
- 24. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose‐response data. Stata J. 2006;6:40‐57. [Google Scholar]
- 25. Aune D, Greenwood DC, Chan DS, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non‐linear dose‐response meta‐analysis of prospective studies. Ann Oncol. 2012;23:843‐852. [DOI] [PubMed] [Google Scholar]
- 26. Bekkering GE, Harris RJ, Thomas S, et al. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta‐analysis? Am J Epidemiol. 2008;167:1017‐1026. [DOI] [PubMed] [Google Scholar]
- 27. Nechuta S, Chen WY, Cai H, et al. A pooled analysis of post‐diagnosis lifestyle factors in association with late estrogen‐receptor‐positive breast cancer prognosis. Int J Cancer. 2016;138:2088‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML. Weight gain after breast cancer diagnosis and all‐cause mortality: systematic review and meta‐analysis. J Natl Cancer Inst. 2015;107:djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 30. Stram DO. Meta‐analysis of published data using a linear mixed‐effects model. Biometrics. 1996;52:536‐544. [PubMed] [Google Scholar]
- 31. Verrill M. Chemotherapy for early‐stage breast cancer: a brief history. Br J Cancer. 2009;101(Suppl 1):S2‐S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30:2232‐2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pinto AC, Ades F, de Azambuja E, Piccart‐Gebhart M. Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast. 2013;22(Suppl 2):S152‐S155. [DOI] [PubMed] [Google Scholar]
- 34. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta‐analysis. Res Synth Methods. 2010;1:112‐125. [DOI] [PubMed] [Google Scholar]
- 36. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta‐analyses. Stat Med. 2010;29:1282‐1297. [DOI] [PubMed] [Google Scholar]
- 37. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med. 2000;19:1831‐1847. [DOI] [PubMed] [Google Scholar]
- 39. Abrahamson PE, Gammon MD, Lund MJ, et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1871‐1877. [DOI] [PubMed] [Google Scholar]
- 40. Abubakar M, Sung H, Bcr D, et al. Breast cancer risk factors, survival and recurrence, and tumor molecular subtype: analysis of 3012 women from an indigenous Asian population. Breast Cancer Res. 2018;20:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ademuyiwa FO, Groman A, O'Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple‐negative breast cancer. Cancer. 2011;117:4132‐4140. [DOI] [PubMed] [Google Scholar]
- 42. Bao PP, Cai H, Peng P, et al. Body mass index and weight change in relation to triple‐negative breast cancer survival. Cancer Causes Control. 2016;27:229‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baumgärtner AK, Häusler A, Seifert‐Klauss V, Schuster T, Schwarz‐Boeger U, Kiechle M. Breast cancer after hormone replacement therapy: does prognosis differ in perimenopausal and postmenopausal women? Breast. 2011;20:448‐454. [DOI] [PubMed] [Google Scholar]
- 44. Behrouzi B, Mohagheghi MA, Sadighi S. Demographic characteristics, survival and prognostic factors of early breast cancer patients with type 2 diabetes mellitus: a hospital‐based cohort study. Asian Pac J Cancer Prev. 2017;18:2485‐2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berclaz G, Li S, Price KN, et al. Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol. 2004;15:875‐884. [DOI] [PubMed] [Google Scholar]
- 46. Bergom C, Kelly T, Bedi M, et al. Association of Locoregional Control with High Body Mass Index in women undergoing breast conservation therapy for early‐stage breast cancer. Int J Radiat Oncol Biol Phys. 2016;96:65‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bernstein L, Deapen D, Cerhan JR, et al. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91:1654‐1662. [DOI] [PubMed] [Google Scholar]
- 48. Biganzoli E, Desmedt C, Fornili M, et al. Recurrence dynamics of breast cancer according to baseline body mass index. Eur J Cancer. 2017;87:10‐20. [DOI] [PubMed] [Google Scholar]
- 49. Borugian MJ, Sheps SB, Kim‐Sing C, et al. Waist‐to‐hip ratio and breast cancer mortality. Am J Epidemiol. 2003;158:963‐968. [DOI] [PubMed] [Google Scholar]
- 50. Bradshaw PT, Ibrahim JG, Stevens J, et al. Postdiagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology. 2012;23:320‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Caan BJ, Kwan ML, Hartzell G, et al. Pre‐diagnosis body mass index, post‐diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19:1319‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caan BJ, Kwan ML, Shu XO, et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev. 2012;21:1260‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caan BJ, Cespedes Feliciano EM, Prado CM, et al. Association of Muscle and Adiposity Measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4:798‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cecchini RS, Swain SM, Costantino JP, et al. Body mass index at diagnosis and breast cancer survival prognosis in clinical trial populations from NRG oncology/NSABP B‐30, B‐31, B‐34, and B‐38. Cancer Epidemiol Biomarkers Prev. 2016;25:51‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen X, Lu W, Zheng W, et al. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat. 2010;122:823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Christiansen N, Chen L, Gilmore J, Pechar D, Szabo S. Association between African American race and outcomes in patients with nonmetastatic triple‐negative breast cancer: a retrospective analysis by using results from the Georgia cancer specialist database. Clin Breast Cancer. 2012;12:270‐275. [DOI] [PubMed] [Google Scholar]
- 57. Connor AE, Baumgartner RN, Pinkston C, Baumgartner KB. Obesity and risk of breast cancer mortality in Hispanic and non‐Hispanic white women: the New Mexico Women's health study. J Womens Health (Larchmt). 2013;22:368‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cook LS, White E, Schwartz SM, McKnight B, Daling JR, Weiss NS. A population‐based study of contralateral breast cancer following a first primary breast cancer (Washington, United States). Cancer Causes Control. 1996;7:382‐390. [DOI] [PubMed] [Google Scholar]
- 59. Crozier JA, Moreno‐Aspitia A, Ballman KV, Dueck AC, Pockaj BA, Perez EA. Effect of body mass index on tumor characteristics and disease‐free survival in patients from the HER2‐positive adjuvant trastuzumab trial N9831. Cancer. 2013;119:2447‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dal Maso L, Zucchetto A, Talamini R, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188‐2194. [DOI] [PubMed] [Google Scholar]
- 61. Dawood S, Broglio K, Gonzalez‐Angulo AM, et al. Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res. 2008;14:1718‐1725. [DOI] [PubMed] [Google Scholar]
- 62. Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez‐Angulo AM. Impact of body mass index on survival outcome among women with early stage triple‐negative breast cancer. Clin Breast Cancer. 2012;12:364‐372. [DOI] [PubMed] [Google Scholar]
- 63. Dignam JJ, Wieand K, Johnson KA, Fisher B, Xu L, Mamounas EP. Obesity, tamoxifen use, and outcomes in women with estrogen receptor‐positive early‐stage breast cancer. J Natl Cancer Inst. 2003;95:1467‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dignam JJ, Wieand K, Johnson KA, et al. Effects of obesity and race on prognosis in lymph node‐negative, estrogen receptor‐negative breast cancer. Breast Cancer Res Treat. 2006;97:245‐254. [DOI] [PubMed] [Google Scholar]
- 65. Elwood JM, Tin Tin S, Kuper‐Hommel M, Lawrenson R, Campbell I. Obesity and breast cancer outcomes in chemotherapy patients in New Zealand: a population‐based cohort study. BMC Cancer. 2018;18:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Erbes T, Stickeler E, Rücker G, et al. BMI and pathologic complete response to neoadjuvant chemotherapy in breast cancer: a study and meta‐analysis. Clin Breast Cancer. 2016;16:e119‐e132. [DOI] [PubMed] [Google Scholar]
- 67. Ewertz M, Gillanders S, Meyer L, Zedeler K. Survival of breast cancer patients in relation to factors which affect the risk of developing breast cancer. Int J Cancer. 1991;49:526‐530. [DOI] [PubMed] [Google Scholar]
- 68. Ewertz M, Gray KP, Regan MM, et al. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the breast international group 1‐98 trial. J Clin Oncol. 2012;30:3967‐3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Flatt SW, Thomson CA, Gold EB, et al. Low to moderate alcohol intake is not associated with increased mortality after breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:681‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gennari A, Nanni O, Puntoni M, et al. Body mass index and prognosis of metastatic breast cancer patients receiving first‐line chemotherapy. Cancer Epidemiol Biomarkers Prev. 2013;22:1862‐1867. [DOI] [PubMed] [Google Scholar]
- 71. George SM, Bernstein L, Smith AW, et al. Central adiposity after breast cancer diagnosis is related to mortality in the health, eating, activity, and lifestyle study. Breast Cancer Res Treat. 2014;146:647‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin‐ and obesity‐related variables in early‐stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30:164‐171. [DOI] [PubMed] [Google Scholar]
- 73. Habel LA, Daling JR, Newcomb PA, et al. Risk of recurrence after ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 1998;7:689‐696. [PubMed] [Google Scholar]
- 74. Hebert JR, Hurley TG, Ma Y. The effect of dietary exposures on recurrence and mortality in early stage breast cancer. Breast Cancer Res Treat. 1998;51:17‐28. [DOI] [PubMed] [Google Scholar]
- 75. Hou G, Zhang S, Zhang X, Wang P, Hao X, Zhang J. Clinical pathological characteristics and prognostic analysis of 1,013 breast cancer patients with diabetes. Breast Cancer Res Treat. 2013;137:807‐816. [DOI] [PubMed] [Google Scholar]
- 76. Imkampe AK, Bates T. Impact of a raised body mass index on breast cancer survival in relation to age and disease extent at diagnosis. Breast J. 2010;16:156‐161. [DOI] [PubMed] [Google Scholar]
- 77. Izano M, Satariano WA, Tammemagi MC, et al. Long‐term outcomes among African‐American and white women with breast cancer: what is the impact of comorbidity? J Geriatr Oncol. 2014;5:266‐275. [DOI] [PubMed] [Google Scholar]
- 78. Jeon YW, Kang SH, Park MH, Lim W, Cho SH, Suh YJ. Relationship between body mass index and the expression of hormone receptors or human epidermal growth factor receptor 2 with respect to breast cancer survival. BMC Cancer. 2015;15:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early‐stage breast cancer patients. Ann Oncol. 2013;24:2506‐2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jung SY, Sereika SM, Linkov F, Brufsky A, Weissfeld JL, Rosenzweig M. The effect of delays in treatment for breast cancer metastasis on survival. Breast Cancer Res Treat. 2011;130:953‐964. [DOI] [PubMed] [Google Scholar]
- 81. Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. 2012;23:103‐112. [DOI] [PubMed] [Google Scholar]
- 82. Kawai M, Minami Y, Nishino Y, Fukamachi K, Ohuchi N, Kakugawa Y. Body mass index and survival after breast cancer diagnosis in Japanese women. BMC Cancer. 2012;12:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kim HJ, Kwon H, Lee JW, et al. Metformin increases survival in hormone receptor‐positive, HER2‐positive breast cancer patients with diabetes. Breast Cancer Res. 2015;17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kogawa T, Fouad TM, Wei C, et al. Association of Body mass index changes during neoadjuvant chemotherapy with pathologic complete response and clinical outcomes in patients with locally advanced breast cancer. J Cancer. 2015;6:310‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Labidi SI, Mrad K, Mezlini A, et al. Inflammatory breast cancer in Tunisia in the era of multimodality therapy. Ann Oncol. 2008;19:473‐480. [DOI] [PubMed] [Google Scholar]
- 86. Lee KH, Keam B, Im SA, et al. Body mass index is not associated with treatment outcomes of breast cancer patients receiving neoadjuvant chemotherapy: korean data. J Breast Cancer. 2012;15:427‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li CI, Daling JR, Porter PL, Tang MT, Malone KE. Relationship between potentially modifiable lifestyle factors and risk of second primary contralateral breast cancer among women diagnosed with estrogen receptor‐positive invasive breast cancer. J Clin Oncol. 2009;27:5312‐5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ligibel JA, Cirrincione CT, Liu M, et al. Body mass index, PAM50 subtype, and outcomes in node‐positive breast cancer: CALGB 9741 (Alliance). J Natl Cancer Inst. 2015;107:djv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111:329‐342. [DOI] [PubMed] [Google Scholar]
- 90. Majed B, Moreau T, Asselain B. Overweight, obesity and breast cancer prognosis: optimal body size indicator cut‐points. Breast Cancer Res Treat. 2009;115:193‐203. [DOI] [PubMed] [Google Scholar]
- 91. Maliniak ML, Patel AV, McCullough ML, et al. Obesity, physical activity, and breast cancer survival among older breast cancer survivors in the cancer prevention study‐II nutrition cohort. Breast Cancer Res Treat. 2018;167:133‐145. [DOI] [PubMed] [Google Scholar]
- 92. Marret H, Perrotin F, Bougnoux P, et al. Low body mass index is an independent predictive factor of local recurrence after conservative treatment for breast cancer. Breast Cancer Res Treat. 2001;66:17‐23. [DOI] [PubMed] [Google Scholar]
- 93. Maskarinec G, Pagano I, Lurie G, Bantum E, Gotay CC, Issell BF. Factors affecting survival among women with breast cancer in Hawaii. J Womens Health (Larchmt). 2011;20:231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mazzarella L, Disalvatore D, Bagnardi V, et al. Obesity increases the incidence of distant metastases in oestrogen receptor‐negative human epidermal growth factor receptor 2‐positive breast cancer patients. Eur J Cancer. 2013;49:3588‐3597. [DOI] [PubMed] [Google Scholar]
- 95. Moore AH, Trentham‐Dietz A, Burns M, et al. Obesity and mortality after locoregional breast cancer diagnosis. Breast Cancer Res Treat. 2018;172:647‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mu L, Zhu N, Zhang J, Xing F, Li D, Wang X. Type 2 diabetes, insulin treatment and prognosis of breast cancer. Diabetes Metab Res Rev. 2017;33:e2823. [DOI] [PubMed] [Google Scholar]
- 97. Nakamura K, Okada E, Ukawa S, et al. Characteristics and prognosis of Japanese female breast cancer patients: the BioBank Japan project. J Epidemiol. 2017;27:S58‐s64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nelson SH, Marinac CR, Patterson RE, et al. Impact of very low physical activity, BMI, and comorbidities on mortality among breast cancer survivors. Breast Cancer Res Treat. 2016;155:551‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Newman SC, Lees AW, Jenkins HJ. The effect of body mass index and oestrogen receptor level on survival of breast cancer patients. Int J Epidemiol. 1997;26:484‐490. [DOI] [PubMed] [Google Scholar]
- 100. Nichols HB, Trentham‐Dietz A, Egan KM, et al. Body mass index before and after breast cancer diagnosis: associations with all‐cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18:1403‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Olsson A, Garne JP, Tengrup I, Zackrisson S, Manjer J. Body mass index and breast cancer survival in relation to the introduction of mammographic screening. Eur J Surg Oncol. 2009;35:1261‐1267. [DOI] [PubMed] [Google Scholar]
- 102. Pajares B, Pollán M, Martín M, et al. Obesity and survival in operable breast cancer patients treated with adjuvant anthracyclines and taxanes according to pathological subtypes: a pooled analysis. Breast Cancer Res. 2013;15:R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Panagopoulou P, Gogas H, Dessypris N, Maniadakis N, Fountzilas G, Petridou ET. Survival from breast cancer in relation to access to tertiary healthcare, body mass index, tumor characteristics and treatment: a Hellenic cooperative oncology group (HeCOG) study. Eur J Epidemiol. 2012;27:857‐866. [DOI] [PubMed] [Google Scholar]
- 104. Pfeiler G, Stöger H, Dubsky P, et al. Efficacy of tamoxifen ± aminoglutethimide in normal weight and overweight postmenopausal patients with hormone receptor‐positive breast cancer: an analysis of 1509 patients of the ABCSG‐06 trial. Br J Cancer. 2013;108:1408‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock M, Levin MD. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast. 2017;31:9‐15. [DOI] [PubMed] [Google Scholar]
- 106. Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411‐3415. [DOI] [PubMed] [Google Scholar]
- 107. Shariff‐Marco S, Gomez SL, Sangaramoorthy M, et al. Impact of neighborhoods and body size on survival after breast cancer diagnosis. Health Place. 2015;36:162‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Storm HH, Andersson M, Boice JD Jr, et al. Adjuvant radiotherapy and risk of contralateral breast cancer. J Natl Cancer Inst. 1992;84:1245‐1250. [DOI] [PubMed] [Google Scholar]
- 109. Sun X, Nichols HB, Robinson W, Sherman ME, Olshan AF, Troester MA. Post‐diagnosis adiposity and survival among breast cancer patients: influence of breast cancer subtype. Cancer Causes Control. 2015;26:1803‐1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sun L, Zhu Y, Qian Q, Tang L. Body mass index and prognosis of breast cancer: An analysis by menstruation status when breast cancer diagnosis. Medicine (Baltimore). 2018;97:e11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tait S, Pacheco JM, Gao F, Bumb C, Ellis MJ, Ma CX. Body mass index, diabetes, and triple‐negative breast cancer prognosis. Breast Cancer Res Treat. 2014;146:189‐197. [DOI] [PubMed] [Google Scholar]
- 112. Tao MH, Shu XO, Ruan ZX, Gao YT, Zheng W. Association of overweight with breast cancer survival. Am J Epidemiol. 2006;163:101‐107. [DOI] [PubMed] [Google Scholar]
- 113. Tichy JR, Deal AM, Anders CK, Reeder‐Hayes K, Carey LA. Race, response to chemotherapy, and outcome within clinical breast cancer subtypes. Breast Cancer Res Treat. 2015;150:667‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Veal CT, Hart V, Lakoski SG, et al. Health‐related behaviors and mortality outcomes in women diagnosed with ductal carcinoma in situ. J Cancer Surviv. 2017;11:320‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Warren LE, Ligibel JA, Chen YH, Truong L, Catalano PJ, Bellon JR. Body mass index and Locoregional recurrence in women with early‐stage breast cancer. Ann Surg Oncol. 2016;23:3870‐3879. [DOI] [PubMed] [Google Scholar]
- 116. Widschwendter P, Friedl TW, Schwentner L, et al. The influence of obesity on survival in early, high‐risk breast cancer: results from the randomized SUCCESS a trial. Breast Cancer Res. 2015;17:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Williams PT. Significantly greater reduction in breast cancer mortality from post‐diagnosis running than walking. Int J Cancer. 2014;135:1195‐1202. [DOI] [PubMed] [Google Scholar]
- 118. Bandera EV, Qin B, Lin Y, et al. Association of Body Mass Index, central obesity, and Body composition with mortality among black breast cancer survivors. JAMA Oncol. 2021;7:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ko SM, Lee J, Bae SJ, et al. Body mass index and absolute lymphocyte count predict disease‐free survival in Korean breast cancer patients. Br J Cancer. 2021;125:119‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Martel S, Lambertini M, Agbor‐Tarh D, et al. Body mass index and weight change in patients with HER2‐positive early breast cancer: exploratory analysis of the ALTTO BIG 2‐06 trial. J Natl Compr Canc Netw. 2021;19:181‐189. [DOI] [PubMed] [Google Scholar]
- 121. Polley MC, Dickler MN, Sinnwell J, et al. A clinical calculator to predict disease outcomes in women with hormone receptor‐positive advanced breast cancer treated with first‐line endocrine therapy. Breast Cancer Res Treat. 2021;189:15‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tan X, Huang D, Zhang F, et al. Evaluation of the body mass index in breast cancer prognosis in a cohort of small‐stature overweight patients: multi‐center study in China. Gland Surg. 2021;10:23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Desmedt C, Fornili M, Clatot F, et al. Differential benefit of adjuvant docetaxel‐based chemotherapy in patients with early breast cancer according to baseline Body mass index. J Clin Oncol. 2020;38:2883‐2891. [DOI] [PubMed] [Google Scholar]
- 124. Fadelu T, Damuse R, Lormil J, et al. Body mass index, chemotherapy‐related weight changes, and disease‐free survival in Haitian women with nonmetastatic breast cancer. JCO Glob Oncol. 2020;6:1656‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gondo N, Sawaki M, Hattori M, et al. Impact of BMI for clinical outcomes in Japanese breast cancer patients. Jpn J Clin Oncol. 2020;50:230‐240. [DOI] [PubMed] [Google Scholar]
- 126. Oudanonh T, Nabi H, Ennour‐Idrissi K, Lemieux J, Diorio C. Progesterone receptor status modifies the association between body mass index and prognosis in women diagnosed with estrogen receptor positive breast cancer. Int J Cancer. 2020;146:2736‐2745. [DOI] [PubMed] [Google Scholar]
- 127. Walsh SM, Zabor EC, Flynn J, Stempel M, Morrow M, Gemignani ML. Breast cancer in young black women. Br J Surg. 2020;107:677‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Blair CK, Wiggins CL, Nibbe AM, et al. Obesity and survival among a cohort of breast cancer patients is partially mediated by tumor characteristics. NPJ Breast Cancer. 2019;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kim JY, Kang D, Nam SJ, et al. Clinical features and outcomes of invasive breast cancer: age‐specific analysis of a modern hospital‐based registry. J Glob Oncol. 2019;5:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Vernaci G, Dieci MV, Manfrin S, et al. BMI is an independent prognostic factor for late outcome in patients diagnosed with early breast cancer: a landmark survival analysis. Breast. 2019;47:77‐84. [DOI] [PubMed] [Google Scholar]
- 131. Zhang JY, Liao YH, Lin Y, et al. Effects of tea consumption and the interactions with lipids on breast cancer survival. Breast Cancer Res Treat. 2019;176:679‐686. [DOI] [PubMed] [Google Scholar]
- 132. Flanagan MR, Tang MC, Baglia ML, Porter PL, Malone KE, Li CI. Relationship between anthropometric factors and risk of second breast cancer among women with a history of ductal carcinoma In situ. JNCI Cancer Spectr. 2018;2:pky020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hamy AS, Pierga JY, Sabaila A, et al. Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease‐free survival in HER2‐positive breast cancer. Ann Oncol. 2017;28:2233‐2240. [DOI] [PubMed] [Google Scholar]
- 134. Xiao Y, Zhang S, Hou G, Zhang X, Hao X, Zhang J. Clinical pathological characteristics and prognostic analysis of diabetic women with luminal subtype breast cancer. Tumour Biol. 2014;35:2035‐2045. [DOI] [PubMed] [Google Scholar]
- 135. Hartog H, Boezen HM, de Jong MM, Schaapveld M, Wesseling J, van der Graaf WT. Prognostic value of insulin‐like growth factor 1 and insulin‐like growth factor binding protein 3 blood levels in breast cancer. Breast. 2013;22:1155‐1160. [DOI] [PubMed] [Google Scholar]
- 136. Barba M, Sperati F, Stranges S, et al. Fasting glucose and treatment outcome in breast and colorectal cancer patients treated with targeted agents: results from a historic cohort. Ann Oncol. 2012;23:1838‐1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Andersson TM, Crowther MJ, Czene K, Hall P, Humphreys K. Mammographic density reduction as a prognostic marker for postmenopausal breast cancer: results using a joint longitudinal‐survival modeling approach. Am J Epidemiol. 2017;186:1065‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Brooks JD, John EM, Mellemkjaer L, et al. Body mass index, weight change, and risk of second primary breast cancer in the WECARE study: influence of estrogen receptor status of the first breast cancer. Cancer Med. 2016;5:3282‐3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Al Jarroudi O, Abda N, Seddik Y, Brahmi SA, Afqir S. Overweight: is it a prognostic factor in women with triple‐negative breast cancer? Asian Pac J Cancer Prev. 2017;18:1519‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Arce‐Salinas C, Aguilar‐Ponce JL, Villarreal‐Garza C, et al. Overweight and obesity as poor prognostic factors in locally advanced breast cancer patients. Breast Cancer Res Treat. 2014;146:183‐188. [DOI] [PubMed] [Google Scholar]
- 141. Barba M, Pizzuti L, Sperduti I, et al. Body mass index and treatment outcomes in metastatic breast cancer patients treated with Eribulin. J Cell Physiol. 2016;231:986‐991. [DOI] [PubMed] [Google Scholar]
- 142. Barnett GC, Shah M, Redman K, Easton DF, Ponder BA, Pharoah PD. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol. 2008;26:3310‐3316. [DOI] [PubMed] [Google Scholar]
- 143. Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014;147:159‐165. [DOI] [PubMed] [Google Scholar]
- 144. Bonsang‐Kitzis H, Chaltier L, Belin L, et al. Beyond axillary lymph node metastasis, BMI and menopausal status are prognostic determinants for triple‐negative breast cancer treated by neoadjuvant chemotherapy. PLoS One. 2015;10:e0144359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Buono G, Crispo A, Giuliano M, et al. Combined effect of obesity and diabetes on early breast cancer outcome: a prospective observational study. Oncotarget. 2017;8:115709‐115717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Caan BJ, Emond JA, Natarajan L, et al. Post‐diagnosis weight gain and breast cancer recurrence in women with early stage breast cancer. Breast Cancer Res Treat. 2006;99:47‐57. [DOI] [PubMed] [Google Scholar]
- 147. Camoriano JK, Loprinzi CL, Ingle JN, Therneau TM, Krook JE, Veeder MH. Weight change in women treated with adjuvant therapy or observed following mastectomy for node‐positive breast cancer. J Clin Oncol. 1990;8:1327‐1334. [DOI] [PubMed] [Google Scholar]
- 148. Carmichael AR, Bendall S, Lockerbie L, Prescott RJ, Bates T. Does obesity compromise survival in women with breast cancer? Breast. 2004;13:93‐96. [DOI] [PubMed] [Google Scholar]
- 149. Cespedes Feliciano EM, Kroenke CH, Bradshaw PT, et al. Postdiagnosis weight change and survival following a diagnosis of early‐stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:44‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chang S, Alderfer JR, Asmar L, Buzdar AU. Inflammatory breast cancer survival: the role of obesity and menopausal status at diagnosis. Breast Cancer Res Treat. 2000;64:157‐163. [DOI] [PubMed] [Google Scholar]
- 151. Chen HL, Ding A, Wang ML. Impact of central obesity on prognostic outcome of triple negative breast cancer in Chinese women. Springerplus. 2016;5:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Cho WK, Choi DH, Park W, et al. Effect of Body mass index on survival in breast cancer patients according to subtype, metabolic syndrome, and treatment. Clin Breast Cancer. 2018;18:e1141‐e1147. [DOI] [PubMed] [Google Scholar]
- 153. Chung IY, Lee JW, Lee JS, et al. Interaction between body mass index and hormone‐receptor status as a prognostic factor in lymph‐node‐positive breast cancer. PLoS One. 2017;12:e0170311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Clough‐Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow‐up. J Clin Oncol. 2010;28:380‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Contiero P, Berrino F, Tagliabue G, et al. Fasting blood glucose and long‐term prognosis of non‐metastatic breast cancer: a cohort study. Breast Cancer Res Treat. 2013;138:951‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Copson ER, Cutress RI, Maishman T, et al. Obesity and the outcome of young breast cancer patients in the UK: the POSH study. Ann Oncol. 2015;26:101‐112. [DOI] [PubMed] [Google Scholar]
- 157. Crispo A, Grimaldi M, D'Aiuto M, et al. BMI and breast cancer prognosis benefit: mammography screening reveals differences between normal weight and overweight women. Breast. 2015;24:86‐89. [DOI] [PubMed] [Google Scholar]
- 158. Deluche E, Leobon S, Desport JC, Venat‐Bouvet L, Usseglio J, Tubiana‐Mathieu N. Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer. 2018;26:861‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ewertz M, Jensen MB, Gunnarsdóttir K, et al. Effect of obesity on prognosis after early‐stage breast cancer. J Clin Oncol. 2011;29:25‐31. [DOI] [PubMed] [Google Scholar]
- 160. Fedele P, Orlando L, Schiavone P, et al. BMI variation increases recurrence risk in women with early‐stage breast cancer. Future Oncol. 2014;10:2459‐2468. [DOI] [PubMed] [Google Scholar]
- 161. Fontanella C, Lederer B, Gade S, et al. Impact of body mass index on neoadjuvant treatment outcome: a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res Treat. 2015;150:127‐139. [DOI] [PubMed] [Google Scholar]
- 162. Furlanetto J, Eiermann W, Marmé F, et al. Higher rate of severe toxicities in obese patients receiving dose‐dense (dd) chemotherapy according to unadjusted body surface area: results of the prospectively randomized GAIN study. Ann Oncol. 2016;27:2053‐2059. [DOI] [PubMed] [Google Scholar]
- 163. Gao YC, S.; Ren, X.B. ; Zhou, L. The effect of obesity on the overall survival in node‐positive breast cancer patients. Tumor 2010; 30: 5. [Google Scholar]
- 164. Gennari A, Amadori D, Scarpi E, et al. Impact of body mass index (BMI) on the prognosis of high‐risk early breast cancer (EBC) patients treated with adjuvant chemotherapy. Breast Cancer Res Treat. 2016;159:79‐86. [DOI] [PubMed] [Google Scholar]
- 165. Gonzalez‐Angulo AM, Broglio K, Kau SW, et al. Women age < or =35 years with primary breast carcinoma: disease features at presentation. Cancer. 2005;103:2466‐2472. [DOI] [PubMed] [Google Scholar]
- 166. Haakinson DJ, Leeds SG, Dueck AC, et al. The impact of obesity on breast cancer: a retrospective review. Ann Surg Oncol. 2012;19:3012‐3018. [DOI] [PubMed] [Google Scholar]
- 167. Hao S, Liu Y, Yu KD, Chen S, Yang WT, Shao ZM. Overweight as a prognostic factor for triple‐negative breast cancers in Chinese women. PLoS One. 2015;10:e0129741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer‐specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012;23:1771‐1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. He Y, Peng L, Huang Y, Liu C, Zheng S, Wu K. Blood cadmium levels associated with short distant metastasis‐free survival time in invasive breast cancer. Environ Sci Pollut Res Int. 2017;24:28055‐28064. [DOI] [PubMed] [Google Scholar]
- 170. Herlevic VC, Mowad R, Miller JK, Darensburg NA, Li BD, Kim RH. Breast cancer outcomes in a population with high prevalence of obesity. J Surg Res. 2015;198:371‐376. [DOI] [PubMed] [Google Scholar]
- 171. Horn PL, Thompson WD. Risk of contralateral breast cancer: associations with factors related to initial breast cancer. Am J Epidemiol. 1988;128:309‐323. [DOI] [PubMed] [Google Scholar]
- 172. Jeon YW, Lim ST, Choi HJ, Suh YJ. Weight change and its impact on prognosis after adjuvant TAC (docetaxel‐doxorubicin‐cyclophosphamide) chemotherapy in Korean women with node‐positive breast cancer. Med Oncol. 2014;31:849. [DOI] [PubMed] [Google Scholar]
- 173. Karpińska A, Safranow K, Kładny J, Sulżyc‐Bielicka V. The influence of obesity on results of AT (doxorubicin plus docetaxel) neoadjuvant chemotherapy In locally advanced breast cancer patients. Pol Przegl Chir. 2015;87:231‐237. [DOI] [PubMed] [Google Scholar]
- 174. Katoh A, Watzlaf VJ, D'Amico F. An examination of obesity and breast cancer survival in post‐menopausal women. Br J Cancer. 1994;70:928‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kmet LM, Cook LS, Weiss NS, Schwartz SM, White E. Risk factors for colorectal cancer following breast cancer. Breast Cancer Res Treat. 2003;79:143‐147. [DOI] [PubMed] [Google Scholar]
- 176. Kobayashi T, Tomomatsu J, Fukada I, et al. Eribulin‐induced liver dysfunction as a prognostic indicator of survival of metastatic breast cancer patients: a retrospective study. BMC Cancer. 2016;16:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Kogawa T, Fujii T, Fouad TM, et al. Impact of change in body mass index during neoadjuvant chemotherapy and survival among breast cancer subtypes. Breast Cancer Res Treat. 2018;171:501‐511. [DOI] [PubMed] [Google Scholar]
- 178. Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370‐1378. [DOI] [PubMed] [Google Scholar]
- 179. Kumar NB, Cantor A, Allen K, Cox CE. Android obesity at diagnosis and breast carcinoma survival: evaluation of the effects of anthropometric variables at diagnosis, including body composition and body fat distribution and weight gain during life span, and survival from breast carcinoma. Cancer. 2000;88:2751‐2757. [DOI] [PubMed] [Google Scholar]
- 180. Kwak M, Kim C. Disparities by age, sex, tumor stage, diagnosis path, and area‐level socioeconomic status in survival time for major cancers: results from the Busan cancer registry. J Korean Med Sci. 2017;32:1974‐1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ladoire S, Dalban C, Roché H, et al. Effect of obesity on disease‐free and overall survival in node‐positive breast cancer patients in a large French population: a pooled analysis of two randomised trials. Eur J Cancer. 2014;50:506‐516. [DOI] [PubMed] [Google Scholar]
- 182. Larsen SB, Kroman N, Ibfelt EH, Christensen J, Tjønneland A, Dalton SO. Influence of metabolic indicators, smoking, alcohol and socioeconomic position on mortality after breast cancer. Acta Oncol. 2015;54:780‐788. [DOI] [PubMed] [Google Scholar]
- 183. Litton JK, Gonzalez‐Angulo AM, Warneke CL, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol. 2008;26:4072‐4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Liu YL, Saraf A, Catanese B, et al. Obesity and survival in the neoadjuvant breast cancer setting: role of tumor subtype in an ethnically diverse population. Breast Cancer Res Treat. 2018;167:277‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Loi S, Milne RL, Friedlander ML, et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1686‐1691. [DOI] [PubMed] [Google Scholar]
- 186. Majed B, Dozol A, Ribassin‐Majed L, Senouci K, Asselain B. Increased risk of contralateral breast cancers among overweight and obese women: a time‐dependent association. Breast Cancer Res Treat. 2011;126:729‐738. [DOI] [PubMed] [Google Scholar]
- 187. Martel S, Poletto E, Ferreira AR, et al. Impact of body mass index on the clinical outcomes of patients with HER2‐positive metastatic breast cancer. Breast. 2018;37:142‐147. [DOI] [PubMed] [Google Scholar]
- 188. Mason BH, Holdaway IM, Stewart AW, Neave LM, Kay RG. Season of tumour detection influences factors predicting survival of patients with breast cancer. Breast Cancer Res Treat. 1990;15:27‐37. [DOI] [PubMed] [Google Scholar]
- 189. Moon HG, Han W, Noh DY. Underweight and breast cancer recurrence and death: a report from the Korean breast cancer society. J Clin Oncol. 2009;27:5899‐5905. [DOI] [PubMed] [Google Scholar]
- 190. Mowad R, Chu QD, Li BD, Burton GV, Ampil FL, Kim RH. Does obesity have an effect on outcomes in triple‐negative breast cancer? J Surg Res. 2013;184:253‐259. [DOI] [PubMed] [Google Scholar]
- 191. Mutschler NS, Scholz C, Friedl TWP, et al. Prognostic impact of weight change during adjuvant chemotherapy in patients with high‐risk early breast cancer: results from the ADEBAR study. Clin Breast Cancer. 2018;18:175‐183. [DOI] [PubMed] [Google Scholar]
- 192. Nomura AM, Marchand LL, Kolonel LN, Hankin JH. The effect of dietary fat on breast cancer survival among Caucasian and japanese women in Hawaii. Breast Cancer Res Treat. 1991;18(Suppl 1):S135‐S141. [DOI] [PubMed] [Google Scholar]
- 193. Pfeiler G, Königsberg R, Fesl C, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG‐12 trial. J Clin Oncol. 2011;29:2653‐2659. [DOI] [PubMed] [Google Scholar]
- 194. Pizzuti L, Natoli C, Gamucci T, et al. Anthropometric, clinical and molecular determinants of treatment outcomes in postmenopausal, hormone receptor positive metastatic breast cancer patients treated with fulvestrant: results from a real word setting. Oncotarget. 2017;8:69025‐69037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Pukkala E, Kyyrönen P, Sankila R, Holli K. Tamoxifen and toremifene treatment of breast cancer and risk of subsequent endometrial cancer: a population‐based case‐control study. Int J Cancer. 2002;100:337‐341. [DOI] [PubMed] [Google Scholar]
- 196. Robinson PJ, Bell RJ, Davis SR. Obesity is associated with a poorer prognosis in women with hormone receptor positive breast cancer. Maturitas. 2014;79:279‐286. [DOI] [PubMed] [Google Scholar]
- 197. Sahin S, Erdem GU, Karatas F, et al. The association between body mass index and immunohistochemical subtypes in breast cancer. Breast. 2017;32:227‐236. [DOI] [PubMed] [Google Scholar]
- 198. Sánchez L, Lana A, Hidalgo A, et al. Risk factors for second primary tumours in breast cancer survivors. Eur J Cancer Prev. 2008;17:406‐413. [DOI] [PubMed] [Google Scholar]
- 199. Scholz C, Andergassen U, Hepp P, et al. Obesity as an independent risk factor for decreased survival in node‐positive high‐risk breast cancer. Breast Cancer Res Treat. 2015;151:569‐576. [DOI] [PubMed] [Google Scholar]
- 200. Schvartsman G, Gutierrez‐Barrera AM, Song J, Ueno NT, Peterson SK, Arun B. Association between weight gain during adjuvant chemotherapy for early‐stage breast cancer and survival outcomes. Cancer Med. 2017;6:2515‐2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Senie RT, Rosen PP, Rhodes P, Lesser ML, Kinne DW. Obesity at diagnosis of breast carcinoma influences duration of disease‐free survival. Ann Intern Med. 1992;116:26‐32. [DOI] [PubMed] [Google Scholar]
- 202. Song EJ, Lee CW, Jung SY, et al. Prognostic impact of skeletal muscle volume derived from cross‐sectional computed tomography images in breast cancer. Breast Cancer Res Treat. 2018;172:425‐436. [DOI] [PubMed] [Google Scholar]
- 203. Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor‐positive operable breast cancer. Cancer. 2012;118:5937‐5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Thivat E, Thérondel S, Lapirot O, et al. Weight change during chemotherapy changes the prognosis in non metastatic breast cancer for the worse. BMC Cancer. 2010;10:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tomiguchi M, Yamamoto Y, Yamamoto‐Ibusuki M, et al. Fibroblast growth factor receptor‐1 protein expression is associated with prognosis in estrogen receptor‐positive/human epidermal growth factor receptor‐2‐negative primary breast cancer. Cancer Sci. 2016;107:491‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Turkoz FP, Solak M, Petekkaya I, et al. The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J BUON. 2013;18:335‐341. [PubMed] [Google Scholar]
- 207. Wisse A, Tryggvadottir H, Simonsson M, et al. Increasing preoperative body size in breast cancer patients between 2002 and 2016: implications for prognosis. Cancer Causes Control. 2018;29:643‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Wolters R, Schwentner L, Regierer A, Wischnewsky M, Kreienberg R, Wöckel A. Endocrine therapy in obese patients with primary breast cancer: another piece of evidence in an unfinished puzzle. Breast Cancer Res Treat. 2012;131:925‐931. [DOI] [PubMed] [Google Scholar]
- 209. Wu SY, Tan Y, Guan YS. Clinical features and prognosis of patients with first‐episode liver metastasis of different molecular subtypes of breast cancer. Zhonghua Gan Zang Bing Za Zhi. 2016;24:422‐428. [DOI] [PubMed] [Google Scholar]
- 210. Yan M, Wang J, Xuan Q, Dong T, He J, Zhang Q. The relationship between tamoxifen‐associated nonalcoholic fatty liver disease and the prognosis of patients with early‐stage breast cancer. Clin Breast Cancer. 2017;17:195‐203. [DOI] [PubMed] [Google Scholar]
- 211. Yerushalmi R, Dong B, Chapman JW, et al. Impact of baseline BMI and weight change in CCTG adjuvant breast cancer trials. Ann Oncol. 2017;28:1560‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Zewenghiel L, Lindman H, Valachis A. Impact of body mass index on the efficacy of endocrine therapy in patients with metastatic breast cancer: a retrospective two‐center cohort study. Breast. 2018;40:136‐140. [DOI] [PubMed] [Google Scholar]
- 213. Zhang Y, Qu Q, Mao Y, Shen K. Effect of body mass index on disease‐free and overall survival in Chinese women with breast cancer. Zhonghua Zhong Liu Za Zhi. 2015;37:395‐399. [PubMed] [Google Scholar]
- 214. Zhang M, Cai H, Bao P, et al. Body mass index, waist‐to‐hip ratio and late outcomes: a report from the Shanghai breast cancer survival study. Sci Rep. 2017;7:6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Almeida NR, Brenelli FP, Dos Santos CC, et al. Comparative study of surgical and oncological outcomes in oncoplastic versus non oncoplastic breast‐conserving surgery for breast cancer treatment. JPRAS Open. 2021;29:184‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Anwar SL, Cahyono R, Prabowo D, et al. Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer. 2021;21:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Cárdenas‐Cárdenas E, Tenorio‐Torres A, Méndez JP, et al. Different body mass indexes and their relation to prognosis of early‐stage breast cancer in postmenopausal Mexican‐mestizo women. Women Health. 2021;61:210‐217. [DOI] [PubMed] [Google Scholar]
- 218. Jung AY, Hüsing A, Behrens S, et al. Postdiagnosis weight change is associated with poorer survival in breast cancer survivors: a prospective population‐based patient cohort study. Int J Cancer. 2021;148:18‐27. [DOI] [PubMed] [Google Scholar]
- 219. Ligorio F, Zambelli L, Bottiglieri A, et al. Hormone receptor status influences the impact of body mass index and hyperglycemia on the risk of tumor relapse in early‐stage HER2‐positive breast cancer patients. Ther Adv Med Oncol. 2021;13:17588359211006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Lin YC, Cheng HH, Chen SC, Shen WC, Huang YT. Pre‐treatment high body mass index is associated with poor survival in Asian premenopausal women with localized breast cancer. J Cancer. 2021;12:4488‐4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Modi ND, Tan JQE, Rowland A, et al. The obesity paradox in early and advanced HER2 positive breast cancer: pooled analysis of clinical trial data. NPJ Breast Cancer. 2021;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Nakatsukasa K, Niikura N, Kashiwabara K, et al. Secondary endpoints analysis in patients with estrogen receptor‐positive metastatic breast cancer treated with everolimus and exemestane enrolled in Oral Care‐BC. BMC Cancer. 2021;21:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Orlandini LF, Pimentel FF, Andrade JM, Reis F, Mattos‐Arruda L, Tiezzi DG. Obesity and high neutrophil‐to‐lymphocyte ratio are prognostic factors in non‐metastatic breast cancer patients. Braz J Med Biol Res. 2021;54:e11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Saleh K, Carton M, Dieras V, et al. Impact of body mass index on overall survival in patients with metastatic breast cancer. Breast. 2021;55:16‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Shang L, Hattori M, Fleming G, et al. Impact of post‐diagnosis weight change on survival outcomes in Black and White breast cancer patients. Breast Cancer Res. 2021;23:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Ahn HR, Kang SY, Youn HJ, Jung SH. Hyperglycemia during adjuvant chemotherapy as a prognostic factor in breast cancer patients without diabetes. J Breast Cancer. 2020;23:398‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Chen B, Lai J, Guo L, et al. Adverse effects of being underweight on young female breast cancer patients with lymph node metastases. J Cancer. 2020;11:1976‐1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Engkakul T, Thnogtang N, Nimmannit A, Chuthapisith S, Akewanlop C. Impact of obesity on outcomes of operable breast cancer: a retrospective cohort study. Asian Pac J Cancer Prev. 2020;21:953‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Godina C, Ottander E, Tryggvadottir H, Borgquist S, Isaksson K, Jernström H. Prognostic impact of menopausal hormone therapy in breast cancer differs according to tumor characteristics and treatment. Front Oncol. 2020;10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Krasniqi E, Pizzuti L, Barchiesi G, et al. Impact of BMI on HER2+ metastatic breast cancer patients treated with pertuzumab and/or trastuzumab emtansine. Real‐World Evidence J Cell Physiol. 2020;235:7900‐7910. [DOI] [PubMed] [Google Scholar]
- 231. Liu G, Luo J. TIMP‐2 gene rs4789936 polymorphism is associated with increased risk of breast cancer and poor prognosis in southern Chinese women. Aging (Albany NY). 2020;12:19325‐19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Tiainen S, Masarwah A, Oikari S, et al. Tumor microenvironment and breast cancer survival: combined effects of breast fat, M2 macrophages and hyaluronan create a dismal prognosis. Breast Cancer Res Treat. 2020;179:565‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Al‐Saleh K, Abd El‐Aziz N, Ali A, et al. A multicenter study of the impact of Body mass index (BMI) on the incidence of pathologic complete response (pCR) among Saudi patients with locally advanced breast cancer (LABC) post neoadjuvant chemotherapy (NAC). Gulf J Oncolog. 2019;1:33‐42. [PubMed] [Google Scholar]
- 234. Ayoub NM, Yaghan RJ, Abdo NM, Matalka II, Akhu‐Zaheya LM, Al‐Mohtaseb AH. Impact of obesity on Clinicopathologic characteristics and disease prognosis in pre‐ and postmenopausal breast cancer patients: a retrospective institutional study. J Obes. 2019;2019:3820759‐3820711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Cacho‐Díaz B, Spínola‐Maroño H, Reynoso N, González‐Aguilar A, Mohar‐Betancourt A. Role of overweight, obesity, and comorbidities in the prognosis of patients with breast cancer with Brain metastases. Clin Breast Cancer. 2019;19:e394‐e398. [DOI] [PubMed] [Google Scholar]
- 236. Fang Q, Huang J, Gan L, Shen K, Chen X, Wu B. Weight gain during neoadjuvant chemotherapy is associated with worse outcome among the patients with operable breast cancer. J Breast Cancer. 2019;22:399‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Fasching PA, Gass P, Häberle L, et al. Prognostic effect of Ki‐67 in common clinical subgroups of patients with HER2‐negative, hormone receptor‐positive early breast cancer. Breast Cancer Res Treat. 2019;175:617‐625. [DOI] [PubMed] [Google Scholar]
- 238. Franzoi MA, Eiger D, Ameye L, et al. Clinical implications of Body mass index in metastatic breast cancer patients treated with Abemaciclib and endocrine therapy. J Natl Cancer Inst. 2021;113:462‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Kim JY, Lee DW, Lee KH, et al. Prognostic role of body mass index is different according to menopausal status and tumor subtype in breast cancer patients. Breast Cancer Res Treat. 2019;176:453‐460. [DOI] [PubMed] [Google Scholar]
- 240. Tryggvadottir H, Ygland Rödström M, Markkula A, et al. The impact of body size changes on recurrence risk depends on age and estrogen receptor status in primary breast cancer. Cancer Causes Control. 2019;30:1157‐1170. [DOI] [PubMed] [Google Scholar]
- 241. Wang K, Wu YT, Zhang X, et al. Clinicopathologic and prognostic significance of Body mass index (BMI) among breast cancer patients in Western China: a retrospective multicenter cohort based on Western China clinical cooperation group (WCCCG). Biomed Res Int. 2019;2019:3692093‐3692014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Wang X, Hui TL, Wang MQ, Liu H, Li RY, Song ZC. Body mass index at diagnosis as a prognostic factor for early‐stage invasive breast cancer after surgical resection. Oncol Res Treat. 2019;42:195‐201. [DOI] [PubMed] [Google Scholar]
- 243. Bouvard B, Chatelais J, Soulié P, et al. Osteoporosis treatment and 10 years' oestrogen receptor+ breast cancer outcome in postmenopausal women treated with aromatase inhibitors. Eur J Cancer. 2018;101:87‐94. [DOI] [PubMed] [Google Scholar]
- 244. Hwang KT, Chu AJ, Kim J, et al. Prognostic influence of preoperative mammographic breast density in operable invasive female breast cancer. Sci Rep. 2018;8:16075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Strand F, Humphreys K, Holm J, et al. Long‐term prognostic implications of risk factors associated with tumor size: a case study of women regularly attending screening. Breast Cancer Res. 2018;20:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Pizzuti L, Marchetti P, Natoli C, et al. Fasting glucose and body mass index as predictors of activity in breast cancer patients treated with everolimus‐exemestane: the EverExt study. Sci Rep. 2017;7:10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Ohara M, Akimoto E, Noma M, et al. Prognostic impact of progesterone receptor status combined with body mass index in breast cancer patients treated with adjuvant aromatase inhibitor. Oncol Lett. 2015;10:3286‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Zeichner SB, Koru‐Sengul T, Shah N, et al. Improved clinical outcomes associated with vitamin D supplementation during adjuvant chemotherapy in patients with HER2+ nonmetastatic breast cancer. Clin Breast Cancer. 2015;15:e1‐e11. [DOI] [PubMed] [Google Scholar]
- 249. Kaviani A, Neishaboury M, Mohammadzadeh N, Ansari‐Damavandi M, Jamei K. Effects of obesity on presentation of breast cancer, lymph node metastasis and patient survival: a retrospective review. Asian Pac J Cancer Prev. 2013;14:2225‐2229. [DOI] [PubMed] [Google Scholar]
- 250. Xing P, Li JG, Jin F, et al. Prognostic significance of body mass index in breast cancer patients with hormone receptor‐positive tumours after curative surgery. Clin Invest Med. 2013;36:E297‐E305. [DOI] [PubMed] [Google Scholar]
- 251. Zheng Z, Cao H, Qu S, Liu Y, Piao Y, Xie X. Clinical features and prognosis of obese breast cancer patients: a retrospective study*. Chinese‐German J Clin Oncol. 2013;12:5‐415. [Google Scholar]
- 252. Greenlee H, Unger JM, LeBlanc M, Ramsey S, Hershman DL. Association between Body mass index and cancer survival in a pooled analysis of 22 clinical trials. Cancer Epidemiol Biomarkers Prev. 2017;26:21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Rosner GL, Hargis JB, Hollis DR, et al. Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from cancer and leukemia group B study 8541. J Clin Oncol. 1996;14:3000‐3008. [DOI] [PubMed] [Google Scholar]
- 254. Liu LN, Lin YC, Miaskowski C, Chen SC, Chen ML. Association between changes in body fat and disease progression after breast cancer surgery is moderated by menopausal status. BMC Cancer. 2017;17:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Buono G, Crispo A, Giuliano M, et al. Metabolic syndrome and early stage breast cancer outcome: results from a prospective observational study. Breast Cancer Res Treat. 2020;182:401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Goodwin PJ, Segal RJ, Vallis M, et al. The LISA randomized trial of a weight loss intervention in postmenopausal breast cancer. NPJ Breast Cancer. 2020;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Raghavendra A, Sinha AK, Valle‐Goffin J, Shen Y, Tripathy D, Barcenas CH. Determinants of weight gain during adjuvant endocrine therapy and Association of Such Weight Gain with Recurrence in long‐term breast cancer survivors. Clin Breast Cancer. 2018;18:e7‐e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Abe R, Kumagai N, Kimura M, Hirosaki A, Nakamura T. Biological characteristics of breast cancer in obesity. Tohoku J Exp Med. 1976;120:351‐359. [DOI] [PubMed] [Google Scholar]
- 259. Al Saeed EF, Ghabbban AJ, Tunio MA. Impact of BMI on Locoregional control among Saudi patients with breast cancer after breast conserving surgery and modified radical mastectomy. Gulf J Oncolog. 2015;1:7‐14. [PubMed] [Google Scholar]
- 260. Albain KS, Green S, LeBlanc M, Rivkin S, O'Sullivan J, Osborne CK. Proportional hazards and recursive partitioning and amalgamation analyses of the southwest oncology group node‐positive adjuvant CMFVP breast cancer data base: a pilot study. Breast Cancer Res Treat. 1992;22:273‐284. [DOI] [PubMed] [Google Scholar]
- 261. Allin KH, Nordestgaard BG, Flyger H, Bojesen SE. Elevated pre‐treatment levels of plasma C‐reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res. 2011;13:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast‐conserving therapy in five National Surgical Adjuvant Breast and bowel project protocols of node‐negative breast cancer. J Clin Oncol. 2009;27:2466‐2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Artac M, Bozcuk H, Afacan B, Ozdogan M, Samur M. The impact of waist‐to‐hip ratio on clinical outcomes in metastatic breast cancer patients treated with aromatase inhibitors. Breast. 2008;17:418‐422. [DOI] [PubMed] [Google Scholar]
- 264. Artac M, Bozcuk H, Kiyici A, Eren OO, Boruban MC, Ozdogan M. Serum leptin level and waist‐to‐hip ratio (WHR) predict the overall survival of metastatic breast cancer (MBC) patients treated with aromatase inhibitors (AIs). Breast Cancer. 2013;20:174‐180. [DOI] [PubMed] [Google Scholar]
- 265. Bastarrachea J, Hortobagyi GN, Smith TL, Kau SW, Buzdar AU. Obesity as an adverse prognostic factor for patients receiving adjuvant chemotherapy for breast cancer. Ann Intern Med. 1994;120:18‐25. [DOI] [PubMed] [Google Scholar]
- 266. Bayraktar S, Hernadez‐Aya LF, Lei X, et al. Effect of metformin on survival outcomes in diabetic patients with triple receptor‐negative breast cancer. Cancer. 2012;118:1202‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Bergmann A, Bourrus NS, de Carvalho CM, et al. Arm symptoms and overall survival in Brazilian patients with advanced breast cancer. Asian Pac J Cancer Prev. 2011;12:2939‐2942. [PubMed] [Google Scholar]
- 268. Biglia N, Peano E, Sgandurra P, et al. Body mass index (BMI) and breast cancer: impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol Endocrinol. 2013;29:263‐267. [DOI] [PubMed] [Google Scholar]
- 269. Björner S, Rosendahl AH, Simonsson M, et al. Body mass index influences the prognostic impact of combined nuclear insulin receptor and estrogen receptor expression in primary breast cancer. Front Endocrinol (Lausanne). 2017;8:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Boivin L, Diguisto C, Chas M, et al. Outcomes of patients with breast cancer in function of their body mass index. Gynecol Obstet Fertil Senol. 2017;45:215‐223. [DOI] [PubMed] [Google Scholar]
- 271. Borgquist S, Hjertberg M, Henningson M, Ingvar C, Rose C, Jernström H. Given breast cancer, is fat better than thin? Impact of the estrogen receptor beta gene polymorphisms. Breast Cancer Res Treat. 2013;137:849‐862. [DOI] [PubMed] [Google Scholar]
- 272. Boyd NF, Campbell JE, Germanson T, Thomson DB, Sutherland DJ, Meakin JW. Body weight and prognosis in breast cancer. J Natl Cancer Inst. 1981;67:785‐789. [PubMed] [Google Scholar]
- 273. Bradshaw PT, Ibrahim JG, Gammon MD. A Bayesian proportional hazards regression model with non‐ignorably missing time‐varying covariates. Stat Med. 2010;29:3017‐3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Brewster AM, Do KA, Thompson PA, et al. Relationship between epidemiologic risk factors and breast cancer recurrence. J Clin Oncol. 2007;25:4438‐4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Brooks JD, John EM, Mellemkjær L, et al. Body mass index and risk of second primary breast cancer: the WECARE study. Breast Cancer Res Treat. 2012;131:571‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Burkheimer E, Starks L, Khan M, et al. The impact of obesity on treatment choices and outcomes in operable breast cancer. Am J Surg. 2019;217:474‐477. [DOI] [PubMed] [Google Scholar]
- 277. Cakar B, Muslu U, Erdogan AP, et al. The role of Body mass index in triple negative breast cancer. Oncol Res Treat. 2015;38:518‐522. [DOI] [PubMed] [Google Scholar]
- 278. Cespedes Feliciano EM, Kwan ML, Kushi LH, et al. Body mass index, PAM50 subtype, recurrence, and survival among patients with nonmetastatic breast cancer. Cancer. 2017;123:2535‐2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Chapman JA, Pritchard KI, Goss PE, et al. Competing risks of death in younger and older postmenopausal breast cancer patients. World J Clin Oncol. 2014;5:1088‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Chen CH, Lo YF, Tsai HP, et al. Low body mass index is an independent risk factor of locoregional recurrence in women with breast cancer undergoing breast conserving therapy. Chang Gung Med J. 2009;32:553‐562. [PubMed] [Google Scholar]
- 281. Coates RJ, Clark WS, Eley JW, Greenberg RS, Huguley CM Jr, Brown RL. Race, nutritional status, and survival from breast cancer. J Natl Cancer Inst. 1990;82:1684‐1692. [DOI] [PubMed] [Google Scholar]
- 282. Crujeiras AB, Cueva J, Vieito M, et al. Association of breast cancer and obesity in a homogeneous population from Spain. J Endocrinol Invest. 2012;35:681‐685. [DOI] [PubMed] [Google Scholar]
- 283. de Azambuja E, McCaskill‐Stevens W, Francis P, et al. The effect of body mass index on overall and disease‐free survival in node‐positive breast cancer patients treated with docetaxel and doxorubicin‐containing adjuvant chemotherapy: the experience of the BIG 02‐98 trial. Breast Cancer Res Treat. 2010;119:145‐153. [DOI] [PubMed] [Google Scholar]
- 284. Demirkan B, Alacacioglu A, Yilmaz U. Relation of body mass index (BMI) to disease free (DFS) and distant disease free survivals (DDFS) among Turkish women with operable breast carcinoma. Jpn J Clin Oncol. 2007;37:256‐265. [DOI] [PubMed] [Google Scholar]
- 285. Donegan WLJ, S.; Koehler, M.R. The prognostic implications of obesity for the surgical cure of breast cancer. Breast dis Breast 1978:4:14‐17. [Google Scholar]
- 286. Eralp Y, Smith TL, Altundağ K, et al. Clinical features associated with a favorable outcome following neoadjuvant chemotherapy in women with localized breast cancer aged 35 years or younger. J Cancer Res Clin Oncol. 2009;135:141‐148. [DOI] [PubMed] [Google Scholar]
- 287. Ewertz M, Machado SG, Boice JD Jr, Jensen OM. Endometrial cancer following treatment for breast cancer: a case‐control study in Denmark. Br J Cancer. 1984;50:687‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Ewertz M. Breast cancer in Denmark. Incidence, risk factors, and characteristics of survival. Acta Oncol. 1993;32:595‐615. [DOI] [PubMed] [Google Scholar]
- 289. Fan Y, Ding X, Xu B, et al. Prognostic significance of single progesterone receptor positivity: a comparison study of estrogen receptor negative/progesterone receptor positive/Her2 negative primary breast cancer with triple negative breast cancer. Medicine (Baltimore). 2015;94:e2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Farr A, Stolz M, Baumann L, et al. The effect of obesity on pathological complete response and survival in breast cancer patients receiving uncapped doses of neoadjuvant anthracycline‐taxane‐based chemotherapy. Breast. 2017;33:153‐158. [DOI] [PubMed] [Google Scholar]
- 291. Gnant M, Pfeiler G, Stöger H, et al. The predictive impact of body mass index on the efficacy of extended adjuvant endocrine treatment with anastrozole in postmenopausal patients with breast cancer: an analysis of the randomised ABCSG‐6a trial. Br J Cancer. 2013;109:589‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early‐stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42‐51. [DOI] [PubMed] [Google Scholar]
- 293. Gordon NH, Crowe JP, Brumberg DJ, Berger NA. Socioeconomic factors and race in breast cancer recurrence and survival. Am J Epidemiol. 1992;135:609‐618. [DOI] [PubMed] [Google Scholar]
- 294. Guo Q, Burgess S, Turman C, et al. Body mass index and breast cancer survival: a Mendelian randomization analysis. Int J Epidemiol. 2017;46:1814‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Hebert JR, Augustine A, Barone J, Kabat GC, Kinne DW, Wynder EL. Weight, height and body mass index in the prognosis of breast cancer: early results of a prospective study. Int J Cancer. 1988;42:315‐318. [DOI] [PubMed] [Google Scholar]
- 296. Hyun SH, Ahn HK, Lee JH, et al. Body mass index with tumor 18F‐FDG uptake improves risk stratification in patients with breast cancer. PLoS One. 2016;11:e0165814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Iwase T, Nakamura R, Yamamoto N, Yoshi A, Itami M, Miyazaki M. The effect of molecular subtype and body mass index on neo‐adjuvant chemotherapy in breast cancer patients. Breast. 2014;23:264‐272. [DOI] [PubMed] [Google Scholar]
- 298. Jeon SJ, Lee JI, Jeon MJ, Lee M. Prognostic effects of adjuvant chemotherapy‐induced amenorrhea and subsequent resumption of menstruation for premenopausal breast cancer patients. Medicine (Baltimore). 2016;95:e3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Kamby C, Ejlertsen B, Andersen J, et al. Body size and menopausal status in relation to the pattern of spread in recurrent breast cancer. Acta Oncol. 1989;28:795‐799. [DOI] [PubMed] [Google Scholar]
- 300. Karatas F, Erdem GU, Sahin S, et al. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast. 2017;32:237‐244. [DOI] [PubMed] [Google Scholar]
- 301. Kawai M, Tomotaki A, Miyata H, et al. Body mass index and survival after diagnosis of invasive breast cancer: a study based on the Japanese National Clinical Database‐Breast Cancer Registry. Cancer Med. 2016;5:1328‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Kimura M. Obesity as prognostic factors in breast cancer. Diabetes Res Clin Pract. 1990;10(Suppl 1):S247‐S251. [DOI] [PubMed] [Google Scholar]
- 303. Kneubil MC, Brollo J, Botteri E, et al. Breast cancer subtype approximations and loco‐regional recurrence after immediate breast reconstruction. Eur J Surg Oncol. 2013;39:260‐265. [DOI] [PubMed] [Google Scholar]
- 304. Kwan ML, John EM, Caan BJ, et al. Obesity and mortality after breast cancer by race/ethnicity: the California breast cancer survivorship consortium. Am J Epidemiol. 2014;179:95‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Kyogoku S, Hirohata T, Takeshita S, Nomura Y, Shigematsu T, Horie A. Survival of breast‐cancer patients and body size indicators. Int J Cancer. 1990;46:824‐831. [DOI] [PubMed] [Google Scholar]
- 306. Lara‐Medina F, Pérez‐Sánchez V, Saavedra‐Pérez D, et al. Triple‐negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;117:3658‐3669. [DOI] [PubMed] [Google Scholar]
- 307. Lee Y, Lee SA, Choi JY, et al. Prognosis of breast cancer is associated with one‐carbon metabolism related nutrients among Korean women. Nutr J. 2012;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Lethaby AE, Mason BH, Harvey VJ, Holdaway IM. Survival of women with node negative breast cancer in the Auckland region. N Z Med J. 1996;109:330‐333. [PubMed] [Google Scholar]
- 309. Levine EG, Raczynski JM, Carpenter JT. Weight gain with breast cancer adjuvant treatment. Cancer. 1991;67:1954‐1959. [DOI] [PubMed] [Google Scholar]
- 310. Loehberg CR, Almstedt K, Jud SM, et al. Prognostic relevance of Ki‐67 in the primary tumor for survival after a diagnosis of distant metastasis. Breast Cancer Res Treat. 2013;138:899‐908. [DOI] [PubMed] [Google Scholar]
- 311. Markkula A, Bromée A, Henningson M, et al. Given breast cancer, does breast size matter? Data from a prospective breast cancer cohort. Cancer Causes Control. 2012;23:1307‐1316. [DOI] [PubMed] [Google Scholar]
- 312. McLaughlin VH, Trentham‐Dietz A, Hampton JM, Newcomb PA, Sprague BL. Lifestyle factors and the risk of a second breast cancer after ductal carcinoma in situ. Cancer Epidemiol Biomarkers Prev. 2014;23:450‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. McNee RK, Mason BH, Neave LM, Kay RG. Influence of height, weight, and obesity on breast cancer incidence and recurrence in Auckland. New Zealand Breast Cancer Res Treat. 1987;9:145‐150. [DOI] [PubMed] [Google Scholar]
- 314. Menon KV, Hodge A, Houghton J, Bates T. Body mass index, height and cumulative menstrual cycles at the time of diagnosis are not risk factors for poor outcome in breast cancer. Breast. 1999;8:328‐333. [DOI] [PubMed] [Google Scholar]
- 315. Mohle‐Boetani JC, Grosser S, Whittemore AS, Malec M, Kampert JB, Paffenbarger RS Jr. Body size, reproductive factors, and breast cancer survival. Prev Med. 1988;17:634‐642. [DOI] [PubMed] [Google Scholar]
- 316. Morrison VA, McCall L, Muss HB, et al. The impact of actual body weight‐based chemotherapy dosing and body size on adverse events and outcome in older patients with breast cancer: results from cancer and leukemia group B (CALGB) trial 49907 (Alliance A151436). J Geriatr Oncol. 2018;9:228‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Mousa U, Onur H, Utkan G. Is obesity always a risk factor for all breast cancer patients? c‐erbB2 expression is significantly lower in obese patients with early stage breast cancer. Clin Transl Oncol. 2012;14:923‐930. [DOI] [PubMed] [Google Scholar]
- 318. Papatestas AE, Miller SR, Pertsemlidis D, Fagerstrom R, Lesnick G, Aufses AH. Association between prognosis and hormone receptors in women with breast cancer. Cancer Detect Prev. 1986;9:303‐310. [PubMed] [Google Scholar]
- 319. Perez CA, Zumsteg ZS, Gupta G, et al. Black race as a prognostic factor in triple‐negative breast cancer patients treated with breast‐conserving therapy: a large, single‐institution retrospective analysis. Breast Cancer Res Treat. 2013;139:497‐506. [DOI] [PubMed] [Google Scholar]
- 320. Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable‐fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345‐2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Pizzuti L, Sergi D, Sperduti I, et al. Body mass index in HER2‐negative metastatic breast cancer treated with first‐line paclitaxel and bevacizumab. Cancer Biol Ther. 2018;19:328‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920‐2926. [DOI] [PubMed] [Google Scholar]
- 323. Pritchard KI, Shepherd LE, Chapman JA, et al. Randomized trial of tamoxifen versus combined tamoxifen and octreotide LAR therapy in the adjuvant treatment of early‐stage breast cancer in postmenopausal women: NCIC CTG MA.14. J Clin Oncol. 2011;29:3869‐3876. [DOI] [PubMed] [Google Scholar]
- 324. Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock M, Levin MD. Changes in body composition and muscle attenuation during taxane‐based chemotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat. 2018;168:95‐105. [DOI] [PubMed] [Google Scholar]
- 325. Robinson B, Currie M, Phillips E, et al. Body mass index (BMI): association with clinicopathological factors and outcome of women with newly diagnosed breast cancer in New Zealand. N Z Med J. 2017;130:46‐56. [PubMed] [Google Scholar]
- 326. Rohan TE, Hiller JE, McMichael AJ. Dietary factors and survival from breast cancer. Nutr Cancer. 1993;20:167‐177. [DOI] [PubMed] [Google Scholar]
- 327. Rossi L, Stevens D, Pierga JY, et al. Impact of adjuvant chemotherapy on breast cancer survival: a real‐world population. PLoS One. 2015;10:e0132853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Sarkissyan M, Wu Y, Vadgama JV. Obesity is associated with breast cancer in African‐American women but not Hispanic women in South Los Angeles. Cancer. 2011;117:3814‐3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Saxe GA, Rock CL, Wicha MS, Schottenfeld D. Diet and risk for breast cancer recurrence and survival. Breast Cancer Res Treat. 1999;53:241‐253. [DOI] [PubMed] [Google Scholar]
- 330. Schuetz F, Diel IJ, Pueschel M, et al. Reduced incidence of distant metastases and lower mortality in 1072 patients with breast cancer with a history of hormone replacement therapy. Am J Obstet Gynecol. 2007;196(342):e1‐e9. [DOI] [PubMed] [Google Scholar]
- 331. Sendur MA, Aksoy S, Zengin N, Altundag K. Efficacy of adjuvant aromatase inhibitor in hormone receptor‐positive postmenopausal breast cancer patients according to the body mass index. Br J Cancer. 2012;107:1815‐1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Sendur MA, Aksoy S, Ozdemir NY, et al. Effect of body mass index on the efficacy of adjuvant tamoxifen in premenopausal patients with hormone receptor‐positive breast cancer. J BUON. 2016;21:27‐34. [PubMed] [Google Scholar]
- 333. Sestak I, Dowsett M, Ferree S, Baehner FL, Cuzick J. Retrospective analysis of molecular scores for the prediction of distant recurrence according to baseline risk factors. Breast Cancer Res Treat. 2016;159:71‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Shachar SS, Deal AM, Weinberg M, et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving Taxane‐based chemotherapy. Clin Cancer Res. 2017;23:658‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Shu XO, Zheng Y, Cai H, et al. Soy food intake and breast cancer survival. Jama. 2009;302:2437‐2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Singh AK, Pandey A, Tewari M, et al. Obesity augmented breast cancer risk: a potential risk factor for Indian women. J Surg Oncol. 2011;103:217‐222. [DOI] [PubMed] [Google Scholar]
- 337. Slaoui M, Mouh FZ, Ghanname I, Razine R, El Mzibri M, Amrani M. Outcome of breast cancer in Moroccan young women correlated to clinic‐pathological features, risk factors and treatment: a comparative study of 716 cases in a single institution. PLoS One. 2016;11:e0164841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Sohrabi A, Sandoz J, Spratt JS, Polk HC Jr. Recurrence of breast cancer. Obesity, tumor size, and axillary lymph node metastases. Jama. 1980;244:264‐265. [DOI] [PubMed] [Google Scholar]
- 339. Sparano JA, Wang M, Zhao F, et al. Race and hormone receptor‐positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104:406‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Su Y, Cai H, Zheng Y, et al. Associations of the transforming growth factor β/Smad pathway, Body mass index, and physical activity with breast cancer outcomes: results from the Shanghai breast cancer study. Am J Epidemiol. 2016;184:501‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Suissa S, Pollak M, Spitzer WO, Margolese R. Body size and breast cancer prognosis: a statistical explanation of the discrepancies. Cancer Res. 1989;49:3113‐3116. [PubMed] [Google Scholar]
- 342. Tammemagi CM, Nerenz D, Neslund‐Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. Jama. 2005;294:1765‐1772. [DOI] [PubMed] [Google Scholar]
- 343. Tamura N, Tsuda H, Yoshida M, et al. Clinicopathological predictive factors for ipsilateral and contralateral events following initial surgery to treat ductal carcinoma in situ. Breast Cancer. 2016;23:510‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Tartter PI, Papatestas AE, Ioannovich J, Mulvihill MN, Lesnick G, Aufses AH Jr. Cholesterol and obesity as prognostic factors in breast cancer. Cancer. 1981;47:2222‐2227. [DOI] [PubMed] [Google Scholar]
- 345. SGt T, Knuiman MW, Sleeper LA, et al. Six‐year results of the eastern cooperative oncology group trial of observation versus CMFP versus CMFPT in postmenopausal patients with node‐positive breast cancer. J Clin Oncol. 1989;7:879‐889. [DOI] [PubMed] [Google Scholar]
- 346. Venturelli E, Orenti A, Fabricio ASC, et al. Observational study on the prognostic value of testosterone and adiposity in postmenopausal estrogen receptor positive breast cancer patients. BMC Cancer. 2018;18:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Villaseñor A, Ballard‐Barbash R, Baumgartner K, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL study. J Cancer Surviv. 2012;6:398‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Vitolins MZ, Kimmick GG, Case LD. BMI influences prognosis following surgery and adjuvant chemotherapy for lymph node positive breast cancer. Breast J. 2008;14:357‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. von Drygalski A, Tran TB, Messer K, et al. Obesity is an independent predictor of poor survival in metastatic breast cancer: retrospective analysis of a patient cohort whose treatment included high‐dose chemotherapy and autologous stem cell support. Int J Breast Cancer. 2011;2011:523276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Wu X, Ye Y, Barcenas CH, et al. Personalized prognostic prediction models for breast cancer recurrence and survival incorporating multidimensional data. J Natl Cancer Inst. 2017;109:djw314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Zekri J, Farag K, Allithy A. Obesity and outcome of post‐menopausal women receiving adjuvant letrozole for breast cancer. Ecancermedicalscience. 2018;12:821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Zhou P, Jiang YZ, Hu X, et al. Clinicopathological characteristics of patients with HER2‐positive breast cancer and the efficacy of trastuzumab in the People's Republic of China. Onco Targets Ther. 2016;9:2287‐2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Ballinger TJ, Jiang G, Kassem N, Radovich M, Schneider BP. Impact of Body mass index on presence of ctDNA and disease recurrence after neoadjuvant chemotherapy for triple‐negative breast cancer: Analysis from BRE12‐158. Clin Cancer Res. 2021;27:1195‐1199. [DOI] [PubMed] [Google Scholar]
- 354. Behrouzi B, Zokaasadi M, Mohagheghi MA, Emami AH, Sadighi S. The effect of metformin on survival outcomes of non‐metastatic breast cancer patients with type 2 diabetes. Asian Pac J Cancer Prev. 2021;22:611‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Iwase T, Parikh A, Dibaj SS, et al. The prognostic impact of Body composition for locally advanced breast cancer patients who received neoadjuvant chemotherapy. Cancers (Basel). 2021;13:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Jeon YW, Park HS, Ko Y, et al. Intermuscular fat density as a novel prognostic factor in breast cancer patients treated with adjuvant chemotherapy. Breast Cancer Res Treat. 2021;189:759‐768. [DOI] [PubMed] [Google Scholar]
- 357. Kennard K, Buckley ME, Sizer LM, et al. Metabolic syndrome: does this influence breast cancer outcomes in the triple‐negative population? Breast Cancer Res Treat. 2021;186:53‐63. [DOI] [PubMed] [Google Scholar]
- 358. Kim SW, Chun M, Jung YS, Oh YT, Noh OK, Cho O. Impact of Body mass index on local recurrence according to intrinsic subtype approximation in Korean women with early stage invasive breast cancer receiving contemporary treatments. J Cancer. 2021;12:4648‐4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Kreklau A, Nel I, Kasimir‐Bauer S, Kimmig R, Frackenpohl AC, Aktas B. An observational study on breast cancer survival and lifestyle related risk factors. In Vivo. 2021;35:1007‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Liu YS, Wu PE, Chou WC, et al. Body mass index and type 2 diabetes and breast cancer survival: a Mendelian randomization study. Am J Cancer Res. 2021;11:3921‐3934. [PMC free article] [PubMed] [Google Scholar]
- 361. Tong Y, Gao W, Wu J, et al. Comprehensive association analysis of 21‐gene recurrence score and obesity in Chinese breast cancer patients. Front Oncol. 2021;11:619840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Bayraktar S, Zhou JZ, Bassett R, et al. Clinical outcome and toxicity from taxanes in breast cancer patients with BRCA1 and BRCA2 pathogenic germline mutations. Breast J. 2020;26:1572‐1582. [DOI] [PubMed] [Google Scholar]
- 363. Caleffi M, Crivelatti I, Burchardt NA, et al. Breast cancer survival in Brazil: how much health care access impact on cancer outcomes? Breast. 2020;54:155‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Cantini L, Pistelli M, Merloni F, et al. Body mass index and hormone receptor status influence recurrence risk in HER2‐positive early breast cancer patients. Clin Breast Cancer. 2020;20:e89‐e98. [DOI] [PubMed] [Google Scholar]
- 365. Corona SP, Giudici F, Jerusalem G, et al. Impact of BMI on the outcome of metastatic breast cancer patients treated with everolimus: a retrospective exploratory analysis of the BALLET study. Oncotarget. 2020;11:2172‐2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. da Silva JL, de Paula BHR, Small IA, Thuler LCS, de Melo AC. Sociodemographic, clinical, and pathological factors influencing outcomes in locally advanced triple negative breast cancer: a Brazilian cohort. Breast Cancer (Auckl). 2020;14:1178223420962488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Di Cosimo S, Porcu L, Agbor‐Tarh D, et al. Effect of body mass index on response to neo‐adjuvant therapy in HER2‐positive breast cancer: an exploratory analysis of the NeoALTTO trial. Breast Cancer Res. 2020;22:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Huh J, Park B, Lee H, et al. Prognostic value of skeletal muscle depletion measured on computed tomography for overall survival in patients with non‐metastatic breast cancer. J Breast Cancer. 2020;23:80‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Patel V, James M, Frampton C, Robinson B, Davey V, Timmings L. Body mass index and outcomes in breast cancer treated with breast conservation. Int J Radiat Oncol Biol Phys. 2020;106:369‐376. [DOI] [PubMed] [Google Scholar]
- 370. Schmidt G, Schneider C, Gerlinger C, et al. Impact of body mass index, smoking habit, alcohol consumption, physical activity and parity on disease course of women with triple‐negative breast cancer. Arch Gynecol Obstet. 2020;301:603‐609. [DOI] [PubMed] [Google Scholar]
- 371. Tong Y, Wu J, Huang O, et al. IGF‐1 interacted with obesity in prognosis prediction in HER2‐positive breast cancer patients. Front Oncol. 2020;10:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Abdel‐Rahman O. Outcomes of metastatic breast cancer patients in relationship to disease‐free interval following primary treatment of localized disease; a pooled analysis of two clinical trials. Breast J. 2019;25:823‐828. [DOI] [PubMed] [Google Scholar]
- 373. Kus T, Cinkir HY, Aktas G, Abali H. Hepatosteatosis may predict late recurrence of breast cancer: a single‐center observational study. Curr Probl Cancer. 2019;43:100461. [DOI] [PubMed] [Google Scholar]
- 374. Lee JW, Kim SY, Lee HJ, Han SW, Lee JE, Lee SM. Prognostic significance of abdominal‐to‐Gluteofemoral adipose tissue distribution in patients with breast cancer. J Clin Med. 2019;8:1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Omarini C, Palumbo P, Pecchi A, et al. Predictive role of Body composition parameters In operable breast cancer patients treated with neoadjuvant chemotherapy. Cancer Manag Res. 2019;11:9563‐9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Yao D, Wang Z, Cai H, Li Y, Li B. Relationship between red cell distribution width and prognosis in patients with breast cancer after operation: a retrospective cohort study. Biosci Rep. 2019;39:BSR20190740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Zhang M, Zhang X, Liu J, et al. Body mass index and diabetes are important prognostic signatures for bilateral breast cancer prognosis. J Cell Biochem. 2019;120:7363‐7374. [DOI] [PubMed] [Google Scholar]
- 378. Desmedt C, Demicheli R, Fornili M, et al. Potential benefit of intra‐operative administration of ketorolac on breast cancer recurrence according to the patient's body mass index. J Natl Cancer Inst. 2018;110:1115‐1122. [DOI] [PubMed] [Google Scholar]
- 379. Espelund U, Renehan AG, Cold S, et al. Prognostic relevance and performance characteristics of serum IGFBP‐2 and PAPP‐A in women with breast cancer: a long‐term Danish cohort study. Cancer Med. 2018;7:2391‐2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Wu R, Liu T, Yang P, et al. Association of 15‐hydroxyprostaglandin dehydrogenate and poor prognosis of obese breast cancer patients. Oncotarget. 2017;8:22842‐22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Yue CF, Feng PN, Yao ZR, et al. High serum uric acid concentration predicts poor survival in patients with breast cancer. Clin Chim Acta. 2017;473:160‐165. [DOI] [PubMed] [Google Scholar]
- 382. Zhang S, Lei R, Wu J, et al. Role of high mobility group A1 and body mass index in the prognosis of patients with breast cancer. Oncol Lett. 2017;14:5719‐5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Asaga S, Kinoshita T, Hojo T, Suzuki J, Jimbo K, Tsuda H. Prognostic factors for triple‐negative breast cancer patients receiving preoperative systemic chemotherapy. Clin Breast Cancer. 2013;13:40‐46. [DOI] [PubMed] [Google Scholar]
- 384. Fei F, Messina C, Slaets L, et al. Tumour size is the only predictive factor of distant recurrence after pathological complete response to neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancers: a sub‐study of EORTC 10994/BIG 1‐00 phase III trial. Eur J Cancer. 2015;51:301‐309. [DOI] [PubMed] [Google Scholar]
- 385. Osman MA, Hennessy BT. Obesity correlation with metastases development and response to first‐line metastatic chemotherapy in breast cancer. Clin Med Insights Oncol. 2015;9:105‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Huober J, Cole BF, Rabaglio M, et al. Symptoms of endocrine treatment and outcome in the BIG 1‐98 study. Breast Cancer Res Treat. 2014;143:159‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Lee SA, Sung H, Han W, Noh DY, Ahn SH, Kang D. Serum adiponectin but not leptin at diagnosis as a predictor of breast cancer survival. Asian Pac J Cancer Prev. 2014;15:6137‐6143. [DOI] [PubMed] [Google Scholar]
- 388. Vici P, Sperati F, Maugeri‐Saccà M, et al. p53 status as effect modifier of the association between pre‐treatment fasting glucose and breast cancer outcomes in non diabetic, HER2 positive patients treated with trastuzumab. Oncotarget. 2014;5:10382‐10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Ampil F, Caldito G, Li B, et al. Morbid obesity does not disadvantage patients with in situ or early‐stage carcinoma undergoing breast‐conserving surgery. Anticancer Res. 2013;33:3867‐3869. [PubMed] [Google Scholar]
- 390. Pande M, Thompson PA, Do KA, et al. Genetic variants in the vitamin D pathway and breast cancer disease‐free survival. Carcinogenesis. 2013;34:587‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Natori A, Hayashi N, Soejima K, et al. A comparison of epidemiology, biology, and prognosis of inflammatory breast cancer in Japanese and US populations. Clin Breast Cancer. 2013;13:460‐464. [DOI] [PubMed] [Google Scholar]
- 392. Del Fabbro E, Parsons H, Warneke CL, et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist. 2012;17:1240‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Melhem‐Bertrandt A, Chavez‐Macgregor M, Lei X, et al. Beta‐blocker use is associated with improved relapse‐free survival in patients with triple‐negative breast cancer. J Clin Oncol. 2011;29:2645‐2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Eberlein T, Simon R, Fisher S, Lippman ME. Height, weight, and risk of breast cancer relapse. Breast Cancer Res Treat. 1985;5:81‐86. [DOI] [PubMed] [Google Scholar]
- 395. Heasman KZ, Sutherland HJ, Campbell JA, Elhakim T, Boyd NF. Weight gain during adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 1985;5:195‐200. [DOI] [PubMed] [Google Scholar]
- 396. Bao PP, Zhao ZG, Gao YT, et al. Association of type 2 diabetes genetic variants with breast cancer survival among Chinese women. PLoS One. 2015;10:e0117419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Chen Y, Dorjgochoo T, Bao PP, et al. Menopausal symptoms among breast cancer patients: a potential indicator of favorable prognosis. PLoS One. 2013;8:e75926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Kim JY, Han W, Moon HG, et al. Prognostic effect of preoperative serum estradiol level in postmenopausal breast cancer. BMC Cancer. 2013;13:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Fowble B, Hanlon A, Freedman G, Nicolaou N, Anderson P. Second cancers after conservative surgery and radiation for stages I‐II breast cancer: identifying a subset of women at increased risk. Int J Radiat Oncol Biol Phys. 2001;51:679‐690. [DOI] [PubMed] [Google Scholar]
- 400. Chen CM, Chang HT, Mok KT, et al. Analysis of prognostic factors in Chinese women with breast cancer in southern Taiwan. Zhonghua Yi Xue Za Zhi (Taipei). 1999;62:717‐723. [PubMed] [Google Scholar]
- 401. Sheean P, Gomez‐Perez S, Joyce C, et al. Myosteatosis at diagnosis is adversely associated with 2‐year survival in women with estrogen receptor‐negative metastatic breast cancer. Breast Cancer Res Treat. 2021;190:121‐132. [DOI] [PubMed] [Google Scholar]
- 402. Cespedes Feliciano EM, Popuri K, Cobzas D, et al. Evaluation of automated computed tomography segmentation to assess body composition and mortality associations in cancer patients. J Cachexia Sarcopenia Muscle. 2020;11:1258‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Iwase T, Sangai T, Fujimoto H, et al. Quality and quantity of visceral fat tissue are associated with insulin resistance and survival outcomes after chemotherapy in patients with breast cancer. Breast Cancer Res Treat. 2020;179:435‐443. [DOI] [PubMed] [Google Scholar]
- 404. Bradshaw PT, Cespedes Feliciano EM, Prado CM, et al. Adipose tissue distribution and survival among women with nonmetastatic breast cancer. Obesity (Silver Spring). 2019;27:997‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Iwase T, Sangai T, Nagashima T, et al. Impact of body fat distribution on neoadjuvant chemotherapy outcomes in advanced breast cancer patients. Cancer Med. 2016;5:41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Makari‐Judson G, Judson CH, Mertens WC. Longitudinal patterns of weight gain after breast cancer diagnosis: observations beyond the first year. Breast J. 2007;13:258‐265. [DOI] [PubMed] [Google Scholar]
- 407. Costa LJ, Varella PC, del Giglio A. Weight changes during chemotherapy for breast cancer. Sao Paulo Med J. 2002;120:113‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Marinho LA, Rettori O, Vieira‐Matos AN. Body weight loss as an indicator of breast cancer recurrence. Acta Oncol. 2001;40:832‐837. [DOI] [PubMed] [Google Scholar]
- 409. Kumar NB, Allen K, Cantor A, et al. Weight gain associated with adjuvant tamoxifen therapy in stage I and II breast cancer: fact or artifact? Breast Cancer Res Treat. 1997;44:135‐143. [DOI] [PubMed] [Google Scholar]
- 410. Goodwin PJ, Panzarella T, Boyd NF. Weight gain in women with localized breast cancer: a descriptive study. Breast Cancer Res Treat. 1988;11:59‐66. [DOI] [PubMed] [Google Scholar]
- 411. Swenerton KD, Legha SS, Smith T, et al. Prognostic factors in metastatic breast cancer treated with combination chemotherapy. Cancer Res. 1979;39:1552‐1562. [PubMed] [Google Scholar]
- 412. Feigelson HS, Bodelon C, Powers JD, et al. Body mass index and risk of second cancer among women with breast cancer. J Natl Cancer Inst. 2021;113:1156‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Minicozzi P, Berrino F, Sebastiani F, et al. High fasting blood glucose and obesity significantly and independently increase risk of breast cancer death in hormone receptor‐positive disease. Eur J Cancer. 2013;49:3881‐3888. [DOI] [PubMed] [Google Scholar]
- 414. Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297‐309. [DOI] [PubMed] [Google Scholar]
- 415. Hursting SD, Digiovanni J, Dannenberg AJ, et al. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res (Phila). 2012;5:1260‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Lohmann AE, Goodwin PJ, Chlebowski RT, Pan K, Stambolic V, Dowling RJ. Association of obesity‐related metabolic disruptions with cancer risk and outcome. J Clin Oncol. 2016;34:4249‐4255. [DOI] [PubMed] [Google Scholar]
- 417. Picon‐Ruiz M, Morata‐Tarifa C, Valle‐Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Bhardwaj P, Au CC, Benito‐Martin A, et al. Estrogens and breast cancer: mechanisms involved in obesity‐related development, growth and progression. J Steroid Biochem Mol Biol. 2019;189:161‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Rose DP, Vona‐Davis L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr Relat Cancer. 2012;19:R225‐R241. [DOI] [PubMed] [Google Scholar]
- 420. Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: new insights into breast cancer development and progression. Am Soc Clin Oncol Educ Book. 2013;33:46‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Yoon YS, Kwon AR, Lee YK, Oh SW. Circulating adipokines and risk of obesity related cancers: a systematic review and meta‐analysis. Obes Res Clin Pract. 2019;13:329‐339. [DOI] [PubMed] [Google Scholar]
- 422. Himbert C, Delphan M, Scherer D, Bowers LW, Hursting S, Ulrich CM. Signals from the adipose microenvironment and the obesity‐cancer link‐a systematic review. Cancer Prev Res (Phila). 2017;10:494‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Lee DH, Giovannucci EL. The obesity paradox in cancer: epidemiologic insights and perspectives. Curr Nutr Rep. 2019;8:175‐181. [DOI] [PubMed] [Google Scholar]
- 424. Karampela I, Chrysanthopoulou E, Christodoulatos GS, Dalamaga M. Is there an obesity paradox in critical illness? Epidemiologic and metabolic considerations. Curr Obes Rep. 2020;9:231‐244. [DOI] [PubMed] [Google Scholar]
- 425. James FR, Wootton S, Jackson A, Wiseman M, Copson ER, Cutress RI. Obesity in breast cancer: what is the risk factor? Eur J Cancer. 2015;51:705‐720. [DOI] [PubMed] [Google Scholar]
- 426. Bandera EV, Fay SH, Giovannucci E, et al. World Cancer Research Fund international continuous update project P. the use and interpretation of anthropometric measures in cancer epidemiology: a perspective from the world cancer research fund international continuous update project. Int J Cancer. 2016;139:2391‐2397. [DOI] [PubMed] [Google Scholar]
- 427. Aleixo GFP, Williams GR, Nyrop KA, Muss HB, Shachar SS. Muscle composition and outcomes in patients with breast cancer: meta‐analysis and systematic review. Breast Cancer Res Treat. 2019;177:569‐579. [DOI] [PubMed] [Google Scholar]
- 428. Christakoudi S, Tsilidis KK, Evangelou E, Riboli E. A Body shape index (ABSI), hip index, and risk of cancer in the UK Biobank cohort. Cancer Med. 2021;10:5614‐5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127‐2132. [DOI] [PubMed] [Google Scholar]
- 430. Warren JL, Yabroff KR. Challenges and opportunities in measuring cancer recurrence in the United States. J Natl Cancer Inst. 2015;107:djv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Department of Health. Improving Outcomes: a Strategy for Cancer. London: COI, 2011. 98p. [Google Scholar]
- 432. Afifi AM, Saad AM, Al‐Husseini MJ, Elmehrath AO, Northfelt DW, Sonbol MB. Causes of death after breast cancer diagnosis: a US population‐based analysis. Cancer. 2020;126:1559‐1567. [DOI] [PubMed] [Google Scholar]
- 433. Riihimaki M, Thomsen H, Brandt A, Sundquist J, Hemminki K. Death causes in breast cancer patients. Ann Oncol. 2012;23:604‐610. [DOI] [PubMed] [Google Scholar]
- 434. Dwivedi AK, Dubey P, Cistola DP, Reddy SY. Association between obesity and cardiovascular outcomes: updated evidence from meta‐analysis studies. Curr Cardiol Rep. 2020;22:25. [DOI] [PubMed] [Google Scholar]
- 435. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Cancer‐Associated Cachexia Nat Rev Dis Primers. 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 436. Pang Y, Wei Y, Kartsonaki C. Associations of adiposity and weight change with recurrence and survival in breast cancer patients: a systematic review and meta‐analysis. Breast Cancer. 2022;29:575‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. van den Berg MM, Winkels RM, de Kruif JT, et al. Weight change during chemotherapy in breast cancer patients: a meta‐analysis. BMC Cancer. 2017;17:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Harborg S, Zachariae R, Olsen J, et al. Overweight and prognosis in triple‐negative breast cancer patients: a systematic review and meta‐analysis. NPJ Breast Cancer. 2021;7:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Lohmann AE, Soldera SV, Pimentel I, et al. Association of Obesity with breast cancer outcome in relation to cancer subtypes: a meta‐analysis. J Natl Cancer Inst. 2021;113:1465‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12:282‐294. [DOI] [PubMed] [Google Scholar]
- 441. Pedersen B, Delmar C, Lorincz T, Falkmer U, Gronkjaer M. Investigating changes in weight and Body composition among women in adjuvant treatment for breast cancer: a scoping review. Cancer Nurs. 2019;42:91‐105. [DOI] [PubMed] [Google Scholar]
- 442. Trestini I, Carbognin L, Monteverdi S, et al. Clinical implication of changes in body composition and weight in patients with early‐stage and metastatic breast cancer. Crit Rev Oncol Hematol. 2018;129:54‐66. [DOI] [PubMed] [Google Scholar]
- 443. Makari‐Judson G, Braun B, Jerry DJ, Mertens WC. Weight gain following breast cancer diagnosis: implication and proposed mechanisms. World J Clin Oncol. 2014;5:272‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579‐591. [DOI] [PubMed] [Google Scholar]
- 445. Chlebowski RT, Reeves MM. Weight loss randomized intervention trials in female cancer survivors. J Clin Oncol. 2016;34:4238‐4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Rock CL, Thomson CA, Sullivan KR, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72:230‐262. [DOI] [PubMed] [Google Scholar]
- 447. Inoue‐Choi M, Robien K, Lazovich D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22:792‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 Supporting Information
Data Availability Statement
Only publicly available data were used in our study. Data sources and handling of these data are described in the Materials and Methods section. Further details are available from the corresponding author upon request.


