Abstract
Background
The correlation between thalassemia and malignancies other than hepatocellular carcinoma (HCC) and the possible relationship between other hemoglobinopathies and tumor risk have been poorly evaluated.
Methods
Eight Italian specialized centers evaluated the incidence of malignant neoplasms in hemoglobinopathies as well as their sites and features. The study cohort included 4631 patients followed between 1970 and 2021 (transfusion‐dependent β‐thalassemia, 55.6%; non–transfusion‐dependent thalassemia, 17.7%; sickle cell disease, 17.6%; hemoglobin H disease, 8.3%).
Results
A total of 197 diagnoses of cancer were reported (incidence rate, 442 cases per 100,000 person‐years). The liver was the most frequent site of tumors in both sexes, with a higher incidence (190 cases per 100,000 person‐years) in comparison with the general population found in all types of hemoglobinopathies (except hemoglobin H disease). In recent years, tumors have become the second cause of death in patients with transfusion‐dependent thalassemia. A lower risk of breast and prostate cancer was observed in the whole group of patients with hemoglobinopathies. The first cancer diagnoses dated back to the 1980s, and the incidence rate sharply increased after the 2000s. However, although the incidence rate of cancers of all sites but the liver continued to show an increasing trend, the incidence of HCC showed stability.
Conclusions
These findings provide novel insights into the relationship between cancer and hemoglobinopathies and suggest that the overall risk is not increased in these patients. HCC has been confirmed as the most frequent tumor, but advances in chelation and the drugs that have led to the eradication of hepatitis C may explain the recent steadiness in the number of diagnoses that is reported here.
Keywords: cancer, hemoglobinopathies, hepatocellular carcinoma, neoplasm, thalassemia
Short abstract
Except for hepatocellular carcinoma, cancers are not more frequent in patients with hemoglobinopathies in comparison with the general Italian population. The incidence of hepatocellular carcinoma over time showed an increasing trend until 2012; since then, there has been a steady number of new diagnoses despite the increasing age of patients with hemoglobinopathies.
INTRODUCTION
In recent decades, the survival of patients with thalassemia has dramatically improved because of more effective iron chelation therapy and safe transfusions. However, because of the increased lifespan, new complications, including oncologic diseases, have emerged. 1 In 2009, Telfer et al. 2 reported that cancer was responsible for 6% of deaths in Cypriot patients with thalassemia, whereas hepatocellular carcinoma (HCC) was reported to be the second cause of death in Greek subjects with thalassemia between 2000 and 2015. 3 According to a large longitudinal study in Taiwan, the overall incidence of cancer was 52% higher among patients with thalassemia in comparison with controls. 4
Several factors may be implicated in the increased neoplastic risk of individuals with thalassemia. These include oxidative damage secondary to iron accumulation, immunologic abnormalities, viral infections, hydroxyurea use, and bone marrow stimulation from chronic anemia. 5 In patients with transfusion‐dependent thalassemia (TDT), iron overload is secondary to regular transfusions, whereas in patients with non–transfusion‐dependent thalassemia, iron accumulation results from increased intestinal absorption and increased release of recycled iron from the reticuloendothelial system (due to the suppression of hepatic hepcidin synthesis).
According to studies so far, HCC is the most frequent and impactful neoplasm in patients with thalassemia, in whom a causative role is played by iron overload with or without transfusion‐transmitted viral infections. 1 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13
Hemosiderosis can be treated with long‐term iron chelation therapy, and direct antiviral agents (DAAs) capable of eliciting a sustained virological response could reduce the risk of neoplastic transformation in almost all hepatitis C virus (HCV) RNA–positive patients. 14 However, data on the real reduction in the incidence of HCC in the general population are still controversial, and no data are available for patients affected by hemoglobinopathy.
There are no published data on neoplastic risk in patients with hemoglobin H (HbH) disease.
As for sickle cell disease (SCD), subjects with the hemoglobin S trait have an increased risk of renal medullary carcinoma, a rare and highly aggressive tumor associated with the chromatin remodeling factor SMARCB1. 15
More rarely, renal medullary carcinoma has been reported to be associated with SCD in the homozygous form. 16 , 17 Although there are several case reports in the literature regarding the occurrence of leukemias in patients with SCD on chronic hydroxyurea therapy, the incidence of hydroxyurea‐related leukemias in these patients remains undetermined, as does the leukemogenic risk. 18 , 19
This study was aimed at increasing the information available on the relevance of cancer in hemoglobinopathies, updating the data on HCC, and investigating whether other cancers are more frequent in these patients than the general population. The study was also aimed at evaluating the differences in the prevalence and distribution of malignant neoplasms among different types of hemoglobinopathies and at investigating the association between the occurrence of neoplasms and risk factors such as the type of hemoglobinopathy, age, sex, markers of iron accumulation, and history of bone marrow transplantation (BMT).
MATERIALS AND METHODS
This multicenter, retrospective cohort study, supported by the Italian Society of Thalassemia and Hemoglobinopathy, reports the incidences and characteristics of malignant tumors in patients with hemoglobinopathies followed at eight reference centers of the Italian Hemoglobinopathy Comprehensive Care Network. Each center considered all consecutive patients in its historical series, and each patient ended the observation period at death or at the last follow‐up. Nonmelanoma skin cancers were not considered in the incidence assessments.
The study, approved by the ethics committee of the participating centers and registered at ClinicalTrials.gov (NCT05286138), was performed according to the Good Clinical Practice guidelines and the Declaration of Helsinki. All patients alive at the time of the study signed an informed consent form.
Demographic data, clinical data, laboratory data, and data for the characterization of the neoplasia and its evolution were collected from the web‐based clinical records of the subjects who experienced cancer. For patients without neoplasia, the data collected in addition to demographic data were limited to their HCV infection and BMT status. Details on the statistics are reported in the Supporting Methods. In order to compare the incidence and mortality rates of patients with hemoglobinopathies with those of the Italian population, data from the Italian Association of Cancer Registries and the Istituto Nazionale di Statistica were taken into consideration. 20 , 21
RESULTS
The study population included 4631 patients (48% male) followed between 1970 and 2021 for a total of 161,468 person‐years of observation (Figure 1A). The group of patients most represented in the study cohort was the TDT group with 2579 subjects (55.6%), which was followed by the non–transfusion‐dependent β‐thalassemia (β‐NTDT) group (818 subjects [17.7%]), the SCD group (815 [17.6%]), the HbH group (384 [8.3%]), and others (35 [0.8%]) (Table S1). Two hundred eighty‐eight patients had undergone BMT. Figure 1B reports the distributions of the studied cohort by age and pathology.
FIGURE 1.
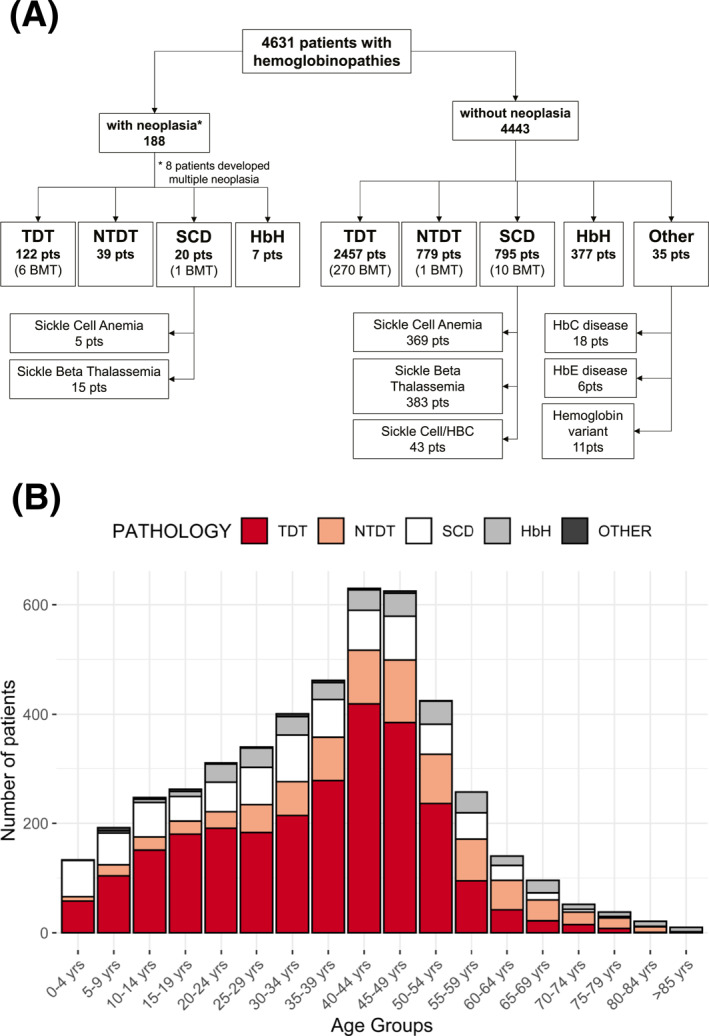
Study population. (A) Flow chart of patients with hemoglobinopathies who developed and did not develop neoplasia. Eight patients developed multiple neoplasia. (B) Distribution of the study population by age and pathology. BMT indicates bone marrow transplantation; HbC, hemoglobin C; HbE, hemoglobin E; HbH, hemoglobin H; NTDT, non–transfusion‐dependent β‐thalassemia; pts, patients; SCD, sickle cell disease; TDT, transfusion‐dependent β‐thalassemia.
Cancer cases and their characteristics
In all, 197 cancer diagnoses in 188 patients (52% male) were reported during the period of observation; this corresponded to 4.0% of the observed patients. The median age at diagnosis was 45.7 years (interquantile range 37.6–54.6 years). Table 1 reports the general characteristics of patients with hemoglobinopathies at the diagnosis of neoplasia. The distributions of tumors by disease and gender are reported in Figure 2. The liver was the most frequent site of tumor development (80 cases; 65% male) in both sexes and for all types of hemoglobinopathies (except HbH disease). No significant differences were found in the age at diagnosis of HCC when we stratified by gender (males, 46.3 years [42.1–53.7 years]; females, 51 years [46.6–54.8]) or by type of hemoglobinopathy (TDT, 47.5 years [42.6–53.3 years]; β‐NTDT, 51 years [46.4–56 years]; SCD, 50 years [42–57.3 years]). After HCC, the thyroid (8 [7.9%]), kidneys (8 [7.9%), and lungs (7 [6.9%) were the most frequent sites in men, and they were followed by non‐Hodgkin lymphoma (5 [4.9%]); together, they accounted for 28% of all male cancers. For women, the most common incident cancers were thyroid cancer (16 [16.7%]), breast cancer (11 [11.4%]), and kidney cancer (8 [8.3%]), which together represented 36% of all female cancer cases (Figure 2). When hematologic cancers were considered as a whole, they became the second most frequent tumors in men (13 [12.9%]) and the third most frequent tumors in women, and they showed the same incidence as renal cancer (8 [8.3%]).
TABLE 1.
Patients with hemoglobinopathies who developed tumors during their lifetime: General characteristics and iron overload–related comorbidities
| HCC (n = 78) | All with HCC excluded (n = 110) | All (n = 188) | |
|---|---|---|---|
| Patient characteristics a | |||
| No. of patients (% of males) b | 78 (65) | 110 (42.7) | 188 (52.1) |
| No. of tumors (% of males) | 80 (65) | 117 (41.9) | 197 (51.3) |
| Age at diagnosis, years | 48.4 (42.9–54.6) | 42.2 (34.6–54.4) | 45.7 (37.7–54.6) |
| Deaths, No. (%) | 54 (67.5) | 51 (43.6) | 105 (5.3) |
| Neoplasia‐related deaths, No. (%) | 50 (62.5) | 40 (34.2) | 90 (45.7) |
| Multiple tumor foci, No. | 22 | 16 | 38 |
| Metastasis, No. | 5 | 30 | 35 |
| Histological confirmation, No. | 41 | 90 | 131 |
| Cirrhosis, No. (%) | 63/75 (84) | — | — |
| Steatosis, No. (%) | 12/56 (21.4) | ||
| Anti‐HCV–positive, No. (%) | 64/78 (82.1) | 43/109 (39.4) | 107/187 (57.2) |
| HCV RNA–positive, No. (%) | 30/68 (44.1) | 16/98 (16.3) | 46/166 (27.7) |
| Anti‐HBV, No. (%) | 14/67 (20.9) | 14/109 (12.8) | 28/176 (15.9) |
| HBV DNA–positive, No. (%) | 1/25 (4) | 0/22 (0) | 1/47 (2.1) |
| Hydroxyurea, No. (%) | 10/71 (14.1) | 22/110 (20) | 32/181 (17.7) |
| Obesity, No. (%) | 5/67 (7.5) | 8/110 (7.3) | 13/177 (7.3) |
| Smoke, No. (%) | 21/51 (41.2) | 27/79 (34.2) | 48/130 (36.9) |
| Alcohol, No. (%) | 17/56 (30) | 32/140 (22.8) | 49/196 (25) |
| Ferritin at diagnosis, median and interquartile range, ng/ml | 786 (408–1904) | 586 (293–1325) | 648 (349–1656) |
| Ferritin peak before diagnosis, ng/ml | 2704 (1802–4858) | 1467 (665–3046) | 2096 (1094–4143) |
| LIC at diagnosis, mg/g dw | 5.2 (2.9–9.5) | 3.8 (2.1–10.2) | 4.5 (2.3–10.2) |
| LIC peak before diagnosis, mg/g dw | 10.5 (5.5–18.1) | 9 (4.5–16.9) | 10.1 (5–17.8) |
| Heart MRI (T2*), ms | 36 (27.7–41) | 33.3 (25.5–39.4) | 34.2 (27–40.5) |
| Pancreas MRI (T2*), ms | 9.7 (7.8–12.6) | 16.7 (8.7–39.5) | 11.6 (8.2–17.7) |
| α‐Fetoprotein, median and interquartile range, µg/L | 3.18 (1.83–53.5) | — | |
| [range, 1.06–4314] | |||
| ALT, median and interquartile range, U/L | 54 (25–83) | ||
| [range, 10–215] | |||
| Iron overload–related comorbidities, No. (%) | |||
| N = 0 | 13 (17) | 59 (52.7) | 72 (38) |
| N = 1 | 17 (21.8) | 31 (27.7) | 48 (25.3) |
| N = 2 | 19 (24.4) | 16 (14.3) | 35 (18.4) |
| N = 3 | 17 (21.8) | 5 (4.5) | 22 (11.5) |
| N = 4 | 9 (11.5) | — | 9 (4.7) |
| N = 5 | 3 (3.8) | 1 (0.9) | 4 (2.1) |
| N ≥ 1 | 65 (83) | 53 (47.3) | 118 (62) |
| Hypogonadism | 48 (64) | 27 (24.8) | 75 (40.1) |
| Hypothyroidism | 33 (44.6) | 18 (16.1) | 51 (27.4) |
| Hypoparathyroidism | 12 (17.1) | 3 (2.7) | 15 (8.2) |
| Diabetes | 19 (27.1) | 9 (8) | 28 (15.3) |
| Hypoadrenalism | 2 (3.1) | 1 (0.9) | 1.7% |
| Cardiopathy | 43 (57.3) | 25 (22.5) | 15% |
Abbreviations: ALT, alanine aminotransferase; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; LIC, liver iron concentration; MRI, magnetic resonance imaging; No., Number.
Percentages have been calculated with respect to the total number of compiled fields.
The first tumor diagnosis has been considered for patients with multiple tumors.
FIGURE 2.
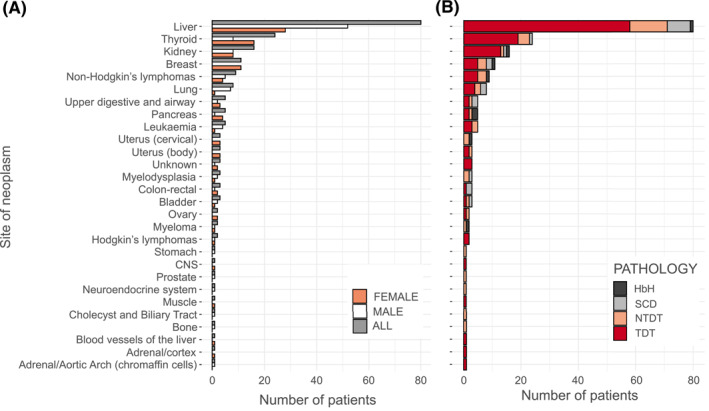
Tumors in patients with hemoglobinopathies: (A) distribution of tumors by gender and (B) distribution of tumors by type of hemoglobinopathy. CNS, Central Nervous System; HbH indicates hemoglobin H; NTDT, non–transfusion‐dependent β‐thalassemia; SCD, sickle cell disease; TDT, transfusion‐dependent β‐thalassemia.
The crude incidence rate of all cancers was 122 cases per 100,000 people. It was 49.5 per 100,000 people for HCC, 14.8 per 100,000 for thyroid cancer, 12.8 per 100,000 for breast tumors, 9.9 per 100,000 for kidney neoplasia, 5.6 per 100,000 for non‐Hodgkin lymphoma, and 4.9 per 100,000 for lung cancer (Table S2). The incidence rate value for all hematologic malignancies combined was 11.7 per 100,000.
Although the distributions of tumors were similar in patients with TDT, β‐NTDT, and SCD, only one case of HCC was reported among patients with HbH disease (Figure 2B).
As in the general population, papillary thyroid cancer was the most common type of thyroid cancer (83% of all histologically characterized thyroid tumors).
As for the 288 patients who underwent BMT, malignant tumors occurred in seven individuals (2.4%), and in two cases, they were oral squamous cell carcinomas typically related to chronic graft‐vs‐host disease. In addition, HCC was diagnosed in two other patients; both had severe liver iron overloads, and one had a history of HCV.
Incidence rates (with comparisons to the general population)
The first cancer diagnoses dated back to the 1980s, and the incidence rate sharply increased after the 2000s (Figure 3A,B). However, although the incidence rate of cancers of all sites but the liver has continued to show an increasing trend in the last decade, the incidence of HCC has shown stability.
FIGURE 3.
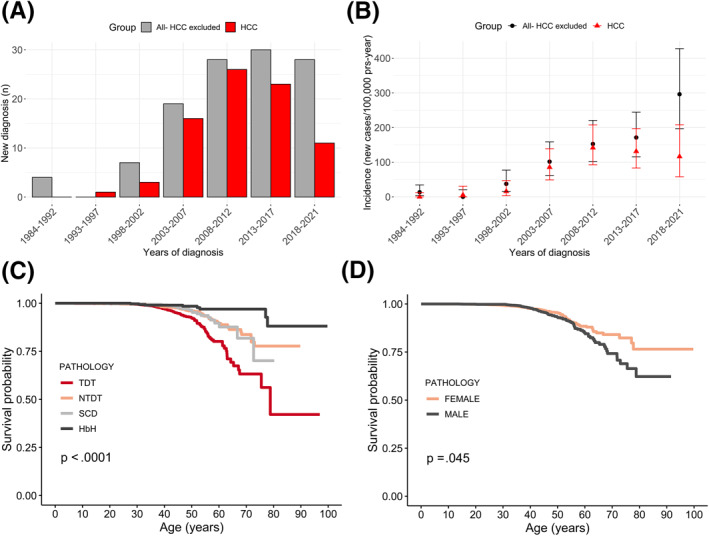
(A, B) Tumors by time period in patients with hemoglobinopathies. New cases of cancer (with HCC excluded) and HCC from 1984 to 2021 are reported as (A) counts and (B) incidences. Non–age‐standardized incidence rates are reported in the bar plots. (C,D) Probability of cancer survival. Kaplan–Maier curves are used to report the probability of cancer survival by (C) pathology and (D) gender. HbH indicates hemoglobin H; HCC, hepatocellular carcinoma; NTDT, non–transfusion‐dependent β‐thalassemia; SCD, sickle cell disease; TDT, transfusion‐dependent β‐thalassemia.
The overall age‐adjusted incidence rate of cancer in patients with hemoglobinopathies was estimated to be 442 cases per 100,000 person‐years (95% CI, 340–700 cases per 100,000 person‐years), which was not statistically different from that of the general Italian population (632 cases per 100,000 person‐years) both when hemoglobinopathies were considered altogether and when each subgroup of patients was considered.
However, the age‐adjusted incidence rate of HCC was almost 9 times lower in the general population (22 cases per 100,000 person‐years) versus patients with hemoglobinopathies (190 cases per 100,000 person‐years; 95% CI, 123–269 cases per 100,000 person‐years; p < .001). Patients with TDT had the highest HCC incidence rate and therefore showed the greatest difference from the general population (445 cases per 100,000 person‐years; 95% CI, 132–826 cases per 100,000 person‐years; p < .001); they were followed by patients with β‐NTDT (102 cases per 100,000 person‐years; 95% CI, 39–198 cases per 100,000 person‐years; p < .001). The comparison of the age‐adjusted incidence rates of HCC in patients with SCD (32 cases per 100,000 person‐years; 95% CI, –33.5 to 748.5 cases per 100,000 person‐years) and the general population did not reach statistical significance. No other malignancies had a higher incidence in patients with hemoglobinopathies in comparison with the general population. On the contrary, breast cancer in women and prostate cancer in men were significantly less frequent in subjects with hemoglobinopathies than the general population; 11 and 1 cases were observed for breast and prostate cancer, respectively, whereas 37 and 6 cases were expected in light of the age‐specific incidence rate of the Italian population.
Risk factors for any type of neoplasia and HCC
The probability of developing any type of neoplasm or HCC was significantly higher (p < .001) in subjects with TDT, as shown in Figure 3C,D. In addition, male gender, anti‐HCV antibody positivity, and HCV RNA detection emerged as significant risk factors for HCC from a univariate analysis. All of them, excluding anti‐HCV positivity, were confirmed to be significant by a multivariate analysis (Table S3).
Other detailed specifications of the patients who developed HCC are reported in Table S4. Fourteen (17.9%; 10 with TDT, 3 with β‐NTDT, and 1 with SCD) were negative for anti‐HCV. In all those with known data, there was a history of elevated liver iron concentrations (LICs) and/or serum ferritin. Their median age at diagnosis was 55.1 years (interquartile range [IQR], 54.1–58.1 years), and only one patient was under 45 years. They were older than those who developed HCC and were positive for anti‐HCV and negative for HCV RNA (median age, 47.3 years; IQR, 52.7–68 years; p = .021) or positive for HCV RNA (median age, 45.3 years; IQR, 50.9–78.8 years; p = .011). Nineteen patients who were negative for HCV RNA at the time when the neoplasm was diagnosed (13 of 18 with cirrhosis; information was not available for one patient) had been previously treated with antivirals and achieved a sustained virological response. In 11 of these patients, viral clearance had been acquired with DAAs (Table 2). However, antiviral therapy with particular reference to DAAs (p < .001) was demonstrated to be protective against HCC occurrence (Figure S1).
TABLE 2.
Characteristics of HCV RNA–negative patients previously treated with antivirals who achieved a sustained virological response and developed HCC
| Patient | Gender | Diagnosis | Age at HCC, years | Cirrhosis | Therapy type | HCC after therapy, years | Last ferritin (peak) (ng/ml) before HCC | Last LIC (peak) (mg/g dw) before HCC |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | TDT | 50 | Yes | DAA | 4 | 653 (773) | 4.1 (5) |
| 2 | Male | TDT | 46 | Yes | DAA | 4 | 290 (9100) | 5.5 (23.3) |
| 3 | Male | TDT | 40 | Yes | DAA | 1 | 81 (2250) | 1.2 (5.5) |
| 4 | Male | SCD | 43 | Yes | DAA | 2 | 727 (2710) | 11.89 (11.89) |
| 5 | Female | TDT | 55 | No | DAA | 5 | 351 (3998) | 2.4 (6.6) |
| 6 | Female | TDT | 47 | No | IFN + RIBA | 3 | 241 (1850) | 2 (3.89) |
| 7 | Male | TDT | 53 | Yes | DAA | 5 | 543 (NA) | 3.88 (—) |
| 8 | Female | TDT | 51 | Yes | IFN + RIBA | 4 | 929 (1632) | 9.5 (—) |
| 9 | Male | TDT | 39 | — | IFN | 6 | — (—) | — (—) |
| 10 | Male | TDT | 39 | Yes | DAA | 1 | 395 (3890) | 2.9 |
| 11 | Female | SCD | 57 | Yes | DAA | 4 | 272 (—) | — (—) |
| 12 | Male | SCD | 32 | No | NA | >3 | 5686 (7147) | 10.4 (20.5) |
| 13 | Male | TDT | 52 | Yes | DAA | 2 | 854 (2098) | 5.53 (5.53) |
| 14 | Male | TDT | 44 | Yes | IFN + RIBA | 3 | 483 (2384) | 7.44 (18.22) |
| 15 | Male | TDT | 46 | Yes | IFN | 18 | 788 (4851) | 6.96 (6.96) |
| 16 | Male | TDT | 46 | Yes | IFN + RIBA | 3 | 357 (9200) | 5.28 (21.4) |
| 17 | Male | TDT | 43 | No | DAA | 1 | 204 (—) | 1.4 (10.37) |
| 18 | Female | TDT | 37 | Yes | DAA | 2 | 225 (5000) | 2.04 (13.2) |
| 19 | Female | TDT | 51 | No | IFN + RIBA | 8 | 643 (1631) | 2.73 (13.86) |
Abbreviations: DAA, direct antiviral agent; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IFN, interferon; LIC, liver iron concentration; NA, not available; RIBA, ribavirin; SCD, sickle cell disease; TDT, transfusion‐dependent β‐thalassemia.
Mortality data
Mortality due to malignancy in subjects with hemoglobinopathies was also similar to that in the general population except for HCC (Table S2). The crude mortality rate of all cancers was 55.4 per 100,000 people. The median survival time after HCC was 4 years (95% CI, 2.4–5.8 years), and the 5‐year survival rate was 39.5% (95% CI, 29.2%–53.5%). Figure 4A shows the Kaplan–Meier survival curves of our cohort and those cohorts reported in 2004 and 2014.
FIGURE 4.
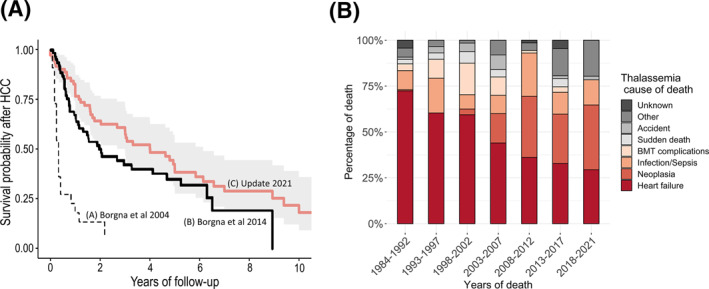
(A) Survival after the diagnosis of HCC (Kaplan Meier Curve C): a comparison with the 2004 and 2014 cohorts of Borgna‐Pignatti et al. 1 , 6 (Kaplan Meier Curve A and B, respectively) (B) Causes of death in thalassemia. The distribution of causes of death by 5‐year periods is shown for patients with transfusion‐dependent β‐thalassemia and non–transfusion‐dependent β‐thalassemia. BMT indicates bone marrow transplantation; HCC, hepatocellular carcinoma.
Table 1 reports the collected causes of death, whereas Figure 4B and Figure S2 report the distributions of causes of death over time by 5‐year periods. There was a progressive reduction in the percentage of deaths due to heart failure, whereas, after the 2000s, deaths due to neoplasia increased and became comparable to those due to hearth failure.
DISCUSSION
The first important finding of our work is that there is no evidence to date that thalassemia or other forms of hemoglobinopathy are associated with increased overall cancer risk or mortality in the age groups considered. Indeed, some types of cancer mainly represented in the general population seem to be statistically less frequent in subjects with hemoglobinopathies, as already reported by Brunson et al. 22 for SCD.
Why this is the case remains unknown. Among these is breast cancer, whose correlation with TDT is still controversial in the literature because some studies argue that iron accumulation and related oxidative stress as well as hormone replacement therapy may be determinants of increased risk. 23
Even thyroid cancer has, in our study, the same incidence in patients with hemoglobinopathies and in the general population, and the prevalence of the papillary variant is not different from what occurs in subjects without hemoglobinopathies. This finding contrasts with an Italian study reporting an 8% rate of nodules (31% malignant) in a cohort of 195 patients with TDT, whereas in the general population, thyroid nodules are present in 4%–7% of individuals, with only 5% being malignant. 24
Likewise, leukemia and Hodgkin and non‐Hodgkin lymphomas do not appear to be more frequent among patients with hemoglobinopathies in comparison with the general population. This stands in contrast to what emerged in a large Asian study, which estimated a 5.32‐fold higher risk in hemoglobinopathies than in the general population with the highest probability of hematologic tumors in transfusion‐dependent patients, 4 and in several reports on patients with SCD. 22 , 25 , 26 , 27 , 28 , 29 In this respect, a 72% increased risk of hematologic malignancies in patients with SCD in comparison with the general population was reported by Brunson et al. 22 Chronic inflammation and hydroxyurea have been considered as possible eventual causative factors, although there are no definitive findings so far. 30 , 31
Moreover, the largest lentiviral vector–mediated β‐globin replacement gene therapy trial in SCD reported two cases of adult patients diagnosed with myeloid neoplasms, both considered unrelated to the gene therapy procedure, 32 as previously reported for patients with SCD undergoing BMT. 33 , 34
However, when we consider all hemoglobinopathies, BMT does not seem to be a major risk factor for cancer in our study, whereas the types of tumors detected in this group of patients indicate that the well‐known risk of graft‐vs‐host disease–related cancers adds to that of a history of HCV and hepatic iron accumulation before BMT. 35
Only three cases of renal carcinoma have been described in the literature so far, with the prevalence of the disease among subjects with thalassemia being superimposable to that of the general population, but the age at diagnosis has been significantly younger. 36 Underlying risk factors that have been called into consideration are iron overload, reactive oxygen species, and hemosiderin deposits in renal tubules. However, our results are the opposite of these results, as we report that subjects with hemoglobinopathies, compared with a general population of the same age and geographical origin, have the same risk of developing renal carcinoma.
Moreover, our work is in line with other works published on this topic, insofar as it indicates that, for the age group considered, HCC is clearly the most frequent type of tumor in patients with TDT and β‐NTDT, although it does not represent 90% of tumor manifestations as reported, for example, by De Sanctis et al. 37 but the lower percentage of 41% (65% in males and 28% in females). In addition, our analysis confirms that patients with SCD also have an increased risk of developing HCC, which is the most common neoplasia in this group of patients. HCC is instead rare in subjects with HbH disease, probably because of the lower probability of developing chronic hepatitis secondary to transfusions and lesser hepatic iron overload due to the lower duodenal iron absorption in comparison with β‐NTDT. 38
Both univariate and multivariate analyses have confirmed that significant risk factors for HCC include a diagnosis of TDT, male gender, and HCV RNA positivity. The increased risk in males compared with females (hazard ratio, 2.8; 95% CI, 1.6–4.7) is not different from that in the normal population. 39
Notably, our analysis confirms that HCC is still possible in patients who have achieved a sustained virological response both with DAAs and with interferon plus ribavirin, and this may occur more frequently in patients with cirrhosis, as observed in the general population. 40 , 41 However, the Kaplan–Meier survival analysis clearly demonstrates that a sustained virological response protects against HCC, and the protection is greater if viral clearance is achieved with DAAs, as already shown by Ricchi et al. 41
An analysis of patient survival after HCC indicates that it has progressively increased in comparison with the previous studies by Borgna‐Pignatti et al., 1 , 6 and it is approximately 39% 5 years after the diagnosis. This finding might not be solely related to advances in therapies because 5‐year survival in the general Italian population is still approximately 20% (or approximately 30% according to Bannon et al. 42 ).
Actually, two main factors have led to earlier diagnoses of HCC with a greater chance for a cure: the increased awareness of the risk of HCC and the dissemination of guidelines indicating the need for liver ultrasound twice a year in adult patients with cirrhosis, HCV and/or hepatitis B virus infections, and an LIC ≥ 5 mg/g dw (β‐NTDT) or an LIC ≥7 mg/g dw with or without serum ferritin values ≥ 1000 ng/ml (TDT). 43
In this respect, our study confirms once again that even patients without a history of HCV can develop HCC, and it shows that the age at diagnosis tends to be higher in comparison with patients with a history of HCV. Thus, the analysis of our data supports an extension of the existing indications for biannual ultrasound in patients aged 45 years or older with β‐thalassemia and a history of (or current) significant hepatic iron accumulation even without a history of chronic HCV.
In addition, the analysis of the incidence of HCC over time shows an increasing trend until 2012, which is followed by a steady number of new diagnoses despite the increasing age of the patients. Advances in chelation with renewed attention to hepatic iron overload and the new drugs that have led to the eradication of HCV even in these patients may explain the relative steadiness of the number of diagnoses of HCC that we have reported in this study. The increase in the incidence of the other cancers over time and the finding that they have become the second most common cause of death in recent years mirror at least in part what has been observed in the general population, where a clear correlation between age and the risk of developing neoplasia has been found. Notably, our data suggest that iron may not play a substantial role in the genesis of tumors other than HCC: Currently, all cancers except HCC are not more frequent in patients with hemoglobinopathies than the general population, and they occur in patients showing a significantly lower serum ferritin life peak and limited complications from iron accumulation in comparison with patients with HCC. These observations support the possibility that the risk of developing cancer will not increase in patients with hemoglobinopathies in comparison with the general population as they grow older.
AUTHOR CONTRIBUTIONS
Raffaella Origa: Conception and design; acquisition of data; analysis and interpretation of data; and writing, review, and/or revision of the manuscript. Barbara Gianesin: Analysis and interpretation of data. Filomena Longo: Conception and design; acquisition of data; analysis and interpretation of data; and writing, review, and/or revision of the manuscript. Rosario Di Maggio: Conception and design; acquisition of data; analysis and interpretation of data; and writing, review, and/or revision of the manuscript. Elena Cassinerio: Conception and design; acquisition of data; analysis and interpretation of data; and writing, review, and/or revision of the manuscript. Maria Rita Gamberini: Acquisition of data; analysis and interpretation of data; and writing, review, and/or revision of the manuscript. Valeria Maria Pinto: Conception and design; acquisition of data; analysis and interpretation of data; and writing, review, and/or revision of the manuscript. Antonella Quarta: Conception and design, acquisition of data, and analysis and interpretation of data. Maddalena Casale: Conception and design; acquisition of data; analysis and interpretation of data; and writing, review, and/or revision of the manuscript. Giorgio La Nasa: Study supervision and writing, review, and/or revision of the manuscript. Giovanni Caocci: Acquisition of data and writing, review, and/or revision of the manuscript. Antonio Piroddi: Acquisition of data and writing, review, and/or revision of the manuscript. Andrea Piolatto: Acquisition of data and writing, review, and/or revision of the manuscript. Alessandra Di Mauro: Acquisition of data and writing, review, and/or revision of the manuscript. Claudia Romano: Acquisition of data and writing, review, and/or revision of the manuscript. Antonia Gigante: Writing, review, and/or revision of the manuscript. Susanna Barella: Study supervision and writing, review, and/or revision of the manuscript. Aurelio Maggio: Study supervision and writing, review, and/or revision of the manuscript. Giovanna Graziadei: Study supervision and writing, review, and/or revision of the manuscript. Silverio Perrotta: Study supervision and writing, review, and/or revision of the manuscript. Gian Luca Forni: Conception and design; analysis and interpretation of data; study supervision; and writing, review, and/or revision of the manuscript.
CONFLICTS OF INTEREST
Filomena Longo reports acting as an independent contractor for Bristol‐Myers Squibb and Vertex Pharmaceuticals. The other authors made no disclosures.
Supporting information
Supporting Information 1
ACKNOWLEDGMENTS
This article is dedicated to the memory of our beloved Dr Antonella Quarta, who could not see it finished. We continue to miss her competence, her humanity, and her kindness every day. We also thank the For Anemia Foundation and ALT Ferrara for their support.
Open Access Funding provided by $INSTITUTION within the CRUI‐CARE Agreement.
Origa R, Gianesin B, Longo F, et al. Incidence of cancer and related deaths in hemoglobinopathies: A follow‐up of 4631 patients between 1970 and 2021. Cancer. 2023;129(1):107‐117. doi: 10.1002/cncr.34509
The first two authors contributed equally to this article.
†Coauthor Antonella Quarta, MD, died on September 25, 2021.
REFERENCES
- 1. Borgna‐Pignatti C, Vergine G, Lombardo T, et al. Hepatocellular carcinoma in the thalassaemia syndromes. Br J Haematol. 2004;124(1):114‐117. doi: 10.1046/j.1365-2141.2003.04732.x [DOI] [PubMed] [Google Scholar]
- 2. Telfer PT, Warburton F, Christou S, et al. Improved survival in thalassemia major patients on switching from desferrioxamine to combined chelation therapy with desferrioxamine and deferiprone. Haematologica. 2009;94(12):1777‐1778. doi: 10.3324/haematol.2009.009118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voskaridou E, Kattamis A, Fragodimitri C, et al. National registry of hemoglobinopathies in Greece: updated demographics, current trends in affected births, and causes of mortality. Ann Hematol. 2019;98(1):55‐66. doi: 10.1007/s00277-018-3493-4 [DOI] [PubMed] [Google Scholar]
- 4. Chung W‐S, Lin C‐LC‐L, Lin C‐LC‐L, Kao C‐H. Thalassaemia and risk of cancer: a population‐based cohort study. J Epidemiol Community Health. 2015;69(11):1066‐1070. doi: 10.1136/jech-2014-205075 [DOI] [PubMed] [Google Scholar]
- 5. Hodroj MH, Bou‐Fakhredin R, Nour‐Eldine W, Noureldine HA, Noureldine MHA, Taher AT. Thalassemia and malignancy: an emerging concern? Blood Rev. 2019;37:100585. doi: 10.1016/j.blre.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 6. Borgna‐Pignatti C, Garani MC, Forni GL, et al. Hepatocellular carcinoma in thalassaemia: an update of the Italian Registry. Br J Haematol. 2014;167(1):121‐126. doi: 10.1111/bjh.13009 [DOI] [PubMed] [Google Scholar]
- 7. Zanella S, Garani MC, Borgna‐Pignatti C. Malignancies and thalassemia: a review of the literature. Ann N Y Acad Sci. 2016;1368(1):140‐148. doi: 10.1111/nyas.13005 [DOI] [PubMed] [Google Scholar]
- 8. Restivo Pantalone G, Renda D, Valenza F, et al. Hepatocellular carcinoma in patients with thalassaemia syndromes: clinical characteristics and outcome in a long term single centre experience. Br J Haematol. 2010;150(2):245‐247. doi: 10.1111/j.1365-2141.2010.08180.x [DOI] [PubMed] [Google Scholar]
- 9. Mancuso A, Rigano P, Renda D, et al. Hepatocellular carcinoma on cirrhosis‐free liver in a HCV‐infected thalassemic. Am J Hematol. 2005;78(2):158‐159. doi: 10.1002/ajh.20289 [DOI] [PubMed] [Google Scholar]
- 10. Mancuso A, Sciarrino E, Concetta Renda M, Maggio A. A prospective study of hepatocellular carcinoma incidence in thalassemia. Hemoglobin. 2006;30(1):119‐124. doi: 10.1080/03630260500455565 [DOI] [PubMed] [Google Scholar]
- 11. Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127(5):S79‐S86. doi: 10.1016/j.gastro.2004.09.019 [DOI] [PubMed] [Google Scholar]
- 12. Maakaron JE, Cappellini MD, Graziadei G, Ayache JB, Taher AT. Hepatocellular carcinoma in hepatitis‐negative patients with thalassemia intermedia: a closer look at the role of siderosis. Ann Hepatol. 2013;12(1):142‐146. doi: 10.1016/s1665-2681(19)31397-3 [DOI] [PubMed] [Google Scholar]
- 13. Fragatou S, Tsourveloudis I, Manesis G. Incidence of hepatocellular carcinoma in a thalassemia unit. Hemoglobin. 2010;34(3):221‐226. doi: 10.3109/03630269.2010.485071 [DOI] [PubMed] [Google Scholar]
- 14. Origa R, Ponti ML, Filosa A, et al. Treatment of hepatitis C virus infection with direct‐acting antiviral drugs is safe and effective in patients with hemoglobinopathies. Am J Hematol. 2017;92(12):1349‐1355. doi: 10.1002/ajh.24911 [DOI] [PubMed] [Google Scholar]
- 15. Msaouel P, Tannir NM, Walker CL. A model linking sickle cell hemoglobinopathies and SMARCB1 loss in renal medullary carcinoma. Clin Cancer Res. 2018;24(9):2044‐2049. doi: 10.1158/1078-0432.CCR-17-3296 [DOI] [PubMed] [Google Scholar]
- 16. Alvarez O, Rodriguez MM, Jordan L, Sarnaik S. Renal medullary carcinoma and sickle cell trait: a systematic review. Pediatr Blood Cancer. 2015;62(10):1694‐1699. doi: 10.1002/pbc.25592 [DOI] [PubMed] [Google Scholar]
- 17. Alvarez OA. Renal medullary carcinoma: the kidney cancer that affects individuals with sickle cell trait and disease. J Oncol Pract. 2017;13(7):424‐425. doi: 10.1200/JOP.2017.023820 [DOI] [PubMed] [Google Scholar]
- 18. Nevitt SJ, Jones AP, Howard J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst Rev. 2017;2017(4):CD002202. doi: 10.1002/14651858.CD002202.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez A, Duez P, Dedeken L, Cotton F, Ferster A. Hydroxyurea (hydroxycarbamide) genotoxicity in pediatric patients with sickle cell disease. Pediatr Blood Cancer. 2018;65(7):e27022. doi: 10.1002/pbc.27022 [DOI] [PubMed] [Google Scholar]
- 20. Aiom, AIRTUM, SIAPEC‐IAP . I Numeri Del Cancro in Italia. Intermedia; 2020. [Google Scholar]
- 21. StatBase . Istituto Nazionale di Statistica. Accessed December 15, 2021. https://www.istat.it/it/dati‐analisi‐e‐prodotti/banche‐dati/statbase
- 22. Brunson A, Keegan THM, Bang H, Mahajan A, Paulukonis S, Wun T. Increased risk of leukemia among sickle cell disease patients in California. Blood. 2017;130(13):1597‐1599. doi: 10.1182/blood-2017-05-783233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bletsa G, Zagouri F, Gounaris A, et al. Beta‐thalassemia major: does it confer particularities to the breast? Breast J. 2013;19(1):124‐125. doi: 10.1111/tbj.12066 [DOI] [PubMed] [Google Scholar]
- 24. Govoni MR, Sprocati M, Fabbri E, Zanforlin N, De Sanctis V. Papillary thyroid cancer in thalassaemia. Pediatr Endocrinol Rev. 2011;8(suppl 2):314‐321. [PubMed] [Google Scholar]
- 25. Ribeil J. Primary myelofibrosis in untreated sickle cell disease: are adult patients at higher risk for developing hematological myeloid neoplasms? Am J Hematol. 2022;97(1):4‐6. doi: 10.1002/ajh.26371 [DOI] [PubMed] [Google Scholar]
- 26. Schultz WH, Ware RE. Malignancy in patients with sickle cell disease. Am J Hematol. 2003;74(4):249‐253. doi: 10.1002/ajh.10427 [DOI] [PubMed] [Google Scholar]
- 27. Seminog OO, Ogunlaja OI, Yeates D, Goldacre MJ. Risk of individual malignant neoplasms in patients with sickle cell disease: English national record linkage study. J R Soc Med. 2016;109(8):303‐309. doi: 10.1177/0141076816651037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Maule J, Neff JL, et al. Myeloid neoplasms in the setting of sickle cell disease: an intrinsic association with the underlying condition rather than a coincidence; report of 4 cases and review of the literature. Mod Pathol. 2019;32(12):1712‐1726. doi: 10.1038/s41379-019-0325-6 [DOI] [PubMed] [Google Scholar]
- 29. Halawi R, Cappellini MD, Taher A. A higher prevalence of hematologic malignancies in patients with thalassemia: background and culprits. Am J Hematol. 2017;92(5):414‐416. doi: 10.1002/ajh.24682 [DOI] [PubMed] [Google Scholar]
- 30. Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17‐year, single‐center trial (LaSHS). Blood. 2010;115(12):2354‐2363. doi: 10.1182/blood-2009-05-221333 [DOI] [PubMed] [Google Scholar]
- 31. Montalembert M, Voskaridou E, Oevermann L, et al. Real‐life experience with hydroxyurea in patients with sickle cell disease: results from the prospective ESCORT‐HU cohort study. Am J Hematol. 2021;96(10):1223‐1231. doi: 10.1002/ajh.26286 [DOI] [PubMed] [Google Scholar]
- 32. BlueBirdBio . bluebird bio provides updated findings from reported case of acute myeloid leukemia (AML) in LentiGlobin for sickle cell disease (SCD) gene therapy program. Business Wire. March 10, 2021. Accessed September 28, 2021. https://www.businesswire.com/news/home/20210310005286/en/bluebird‐bio‐Provides‐Updated‐Findings‐from‐Reported‐Case‐of‐Acute‐Myeloid‐Leukemia‐AML‐in‐LentiGlobin‐for‐Sickle‐Cell‐Disease‐SCD‐Gene‐Therapy‐Program [Google Scholar]
- 33. Eapen M, Brazauskas R, Walters MC, et al. Effect of donor type and conditioning regimen intensity on allogeneic transplantation outcomes in patients with sickle cell disease: a retrospective multicentre, cohort study. Lancet Haematol. 2019;6(11):e585‐e596. doi: 10.1016/S2352-3026(19)30154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ghannam JY, Xu X, Maric I, et al. Baseline TP53 mutations in adults with SCD developing myeloid malignancy following hematopoietic cell transplantation. Blood. 2020;135(14):1185‐1188. doi: 10.1182/blood.2019004001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caocci G, Orofino MG, Vacca A, et al. Long‐term survival of beta thalassemia major patients treated with hematopoietic stem cell transplantation compared with survival with conventional treatment. Am J Hematol. 2017;92(12):1303‐1310. doi: 10.1002/ajh.24898 [DOI] [PubMed] [Google Scholar]
- 36. Ricchi P, Ammirabile M, Spasiano A, et al. Renal cell carcinoma in adult patients with thalassaemia major: a description of three cases. Br J Haematol. 2014;165(6):887‐888. doi: 10.1111/bjh.12809 [DOI] [PubMed] [Google Scholar]
- 37. De Sanctis V. Concise review on the frequency, major risk factors and surveillance of hepatocellular carcinoma (HCC) in β‐thalassemias: past, present and future perspectives. Mediterr J Hematol Infect Dis. 2020;12(1):e2020006. doi: 10.4084/mjhid.2020.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Origa R, Cazzola M, Mereu E, et al. Differences in the erythropoiesis–hepcidin–iron store axis between hemoglobin H disease and β‐thalassemia intermedia. Haematologica. 2015;100(5):e169‐e171. doi: 10.3324/haematol.2014.115733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5 suppl 1):S5‐S16. doi: 10.1053/j.gastro.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 40. Nagaoki Y, Imamura M, Teraoka Y, et al. Impact of viral eradication by direct‐acting antivirals on the risk of hepatocellular carcinoma development, prognosis, and portal hypertension in hepatitis C virus–related compensated cirrhosis patients. Hepatol Res. 2020;50(11):1222‐1233. doi: 10.1111/hepr.13554 [DOI] [PubMed] [Google Scholar]
- 41. Ricchi P, Costantini S, Spasiano A, Cinque P, Esposito S, Filosa A. Hepatocellular carcinoma in patients with thalassemia in the post‐DAA era: not a disappearing entity. Ann Hematol. 2021;100(7):1907‐1910. doi: 10.1007/s00277-021-04511-1 [DOI] [PubMed] [Google Scholar]
- 42. Bannon F, Di Carlo V, Harewood R, et al. Survival trends for primary liver cancer, 1995–2009: analysis of individual data for 578,740 patients from 187 population‐based registries in 36 countries (CONCORD‐2). Ann Cancer Epidemiol. 2019;3:6. doi: 10.21037/ace.2019.07.01 [DOI] [Google Scholar]
- 43. Moukhadder HM, Halawi R, Cappellini MD, Taher AT. Hepatocellular carcinoma as an emerging morbidity in the thalassemia syndromes: a comprehensive review. Cancer. 2017;123(5):751‐758. doi: 10.1002/cncr.30462 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1


