Abstract
Usutu virus (USUV) is an emerging zoonotic arbovirus in Europe, where it primarily impacts Eurasian blackbirds (Turdus merula). For mosquito‐borne viruses to persist in temperate areas, transovarial transmission in vectors or overwintering in either hosts or diapausing vectors must occur to facilitate autochthonous transmission. We undertook surveillance of hosts and vectors in 2021 to elucidate whether USUV had overwintered in the United Kingdom (UK) following its initial detection there in 2020. From 175 dead bird submissions, we detected 1 case of USUV infection, in a blackbird, from which a full USUV genome was derived. Using a molecular clock analysis, we demonstrate that the 2021 detection shared a most recent common ancestor with the 2020 Greater London, UK, USUV sequence. In addition, we identified USUV‐specific neutralizing antibodies in 10 out of 86 serum samples taken from captive birds at the index site, demonstrating in situ cryptic infection and potential sustained transmission. However, from 4966 mosquitoes, we detected no USUV RNA suggesting that prevalence in the vector community was absent or low during sampling. Combined, these results suggest that USUV overwintered in the UK, thus providing empirical evidence for the continued northward expansion of this vector‐borne viral disease. Currently, our detection indicates geographically restricted virus persistence. Further detections over time will be required to demonstrate long‐term establishment. It remains unclear whether the UK, and by extension other high‐latitude regions, can support endemic USUV infection.
Keywords: Culex pipiens, emerging infectious disease, Flavivirus, molecular clock, mosquito‐borne disease, Turdus merula
1. INTRODUCTION
By definition, emerging infectious diseases (EIDs) expand into novel geographic regions and/or hosts (Daszak et al., 2000). Once a pathogen has emerged, it requires sufficient host density to facilitate onward transmission, which, in time, may result in endemicity (Nash et al., 2001). In addition, in the case of vector‐borne diseases, there needs to be a sufficient abundance of competent vectors and the appropriate climatic conditions to enable replication and transmission cycles between hosts (Diallo et al., 2014; Rijks et al., 2016). Where these requirements are not met, a pathogen will fail to establish within a new population, although a brief period of persistence might be sustained (Calzolari et al., 2013; Manore & Beechler, 2015; Mencattelli et al., 2021). Differentiating between EID persistence and the establishment of endemism in wildlife is often complicated, especially for pathogens with cryptic transmission cycles or with a low detection rate, which may be a consequence of sampling effort or recognition of disease signs. New incursions often are readily identified through genomic monitoring (Oude Munnink et al., 2020). However, rapid molecular evolution, as has been reported for viruses, can complicate this, and detailed phylogenetic analyses are often required to differentiate new incursions from in situ evolution (Oude Munnink et al., 2020).
Usutu virus (USUV; Flaviviridae) is a zoonotic, arthropod‐borne RNA virus that has spread across Africa and into Europe since its initial detection in 1959 (Clé et al., 2019; Woodall, 1964;). In 2020, USUV lineage Africa 3.2 was detected for the first time in the United Kingdom (UK) (index site: Zoological Society of London (ZSL) London Zoo [51°32′N0°9′E]) in five Eurasian blackbirds (Turdus merula) and one house sparrow (Passer domesticus) (Folly, Lawson, et al., 2020). The introduction most likely originated from mainland Europe (Folly, Lawson, et al., 2020), where USUV lineage Africa 3.2 is prevalent (Oude Munnink et al., 2020), and may have caused a rapid onset, localized population decline of blackbirds in Greater London (Harris et al., 2022; Lawson et al., 2022). The first cases from this outbreak were detected in July 2020, which followed a relatively warm period in south‐east England, likely facilitating the replication of virus in, and transmission by, vectors (Folly, Lawson, et al., 2020). However, given that USUV is a mosquito‐borne virus and that, in temperate areas, mosquitoes are only active during the warmer months (typically April–November in the UK [NBN Atlas, 2022]), to become established, the virus must persist in hosts or vectors so that onward transmission can occur the following year when conditions are permissive for viral replication. The detection of USUV in the UK presents an important milestone in national biosecurity as it is the first mosquito‐borne zoonotic virus to emerge there (Folly, Lawson, et al., 2020). Currently, it is unclear if the UK provides a suitable climate for USUV endemicity. Critically, if USUV can establish in the UK, then it is likely that other mosquito‐borne zoonotic viruses with similar ecological niches, such as West Nile virus (WNV), may emerge there in the future (Nikolay, 2015). Of wider importance is that the establishment of a mosquito‐borne virus in the UK may indicate other high‐latitude countries that were previously considered inhospitable to emerging viral diseases from tropic and sub‐tropic regions are becoming permissive, consequent to a changing global climate (Tjaden et al., 2018).
An on‐going flavivirus surveillance programme in Great Britain, coordinated by the Animal and Plant Health Agency and the Institute of Zoology, screens samples from wild birds found dead for WNV and USUV using molecular methods (Horton et al., 2013; Linke et al., 2007). Since the detection of USUV in the UK in 2020, the programme has increased in scope following the recommendation of the UK government's Human Animal Infections and Risk Surveillance group (Human Animal Infections and Risk Surveillance Group, 2020) to include vector screening in collaboration with the UK Health Security Agency. During 2021, archived serum samples from captive birds held in the collection at ZSL London Zoo, along with mosquitoes from north‐west London and tissue samples from dead birds from across Great Britain were obtained for USUV screening. Findings from these submissions were used to elucidate whether USUV had overwintered in the UK following its initial detection.
2. MATERIALS AND METHODS
2.1. Serology of captive birds
To identify if USUV exposure and cryptic infection had occurred in captive birds at the index site of the 2020 USUV outbreak and to investigate the potential for USUV transmission pre‐2020, a serosurvey was undertaken on archived serum samples. These sera had been previously collected from live birds in the collection at ZSL London Zoo, 2017–2021 inclusive (n = 86 [2017 = 1, 2018 = 16, 2019 = 12, 2020 = 32, 2021 = 25] Table S1). Blood samples had been collected during clinical investigations of birds for ill health, or as part of routine health check examinations, conducted under the Veterinary Surgeons Act 1986, and serum was only archived when excess was available following diagnostic procedures; no blood draws were performed specifically for this study. Sera were tested using a commercially available enzyme‐linked immunosorbent assay (ELISA) (IDvet, Grabels, France), known to detect flavivirus antibodies (Folly, Waller, et al., 2020). If sufficient serum was available, positive ELISA samples were also subjected to a plaque reduction neutralization test (PRNT) using Vero cells (Mansfield et al., 2011) and an isolate of USUV 2020 Greater London, UK (GenBank accession number: MW001216) to assess antibody specificity against USUV. Serum that neutralized USUV at or above a plaque reduction threshold of 90% (PRNT90) was considered to contain USUV‐specific neutralizing antibodies.
2.2. Mosquito collection and Usutu virus screening
To determine if local vectors were infected with USUV, mosquitoes were collected from May–November 2021 inclusive, from four sites within north‐west London (Regent's Park, Hampstead Heath [two sites] and ZSL London Zoo) using a combination of five types of mosquito trap: Mosquito Magnets, BG‐Mosquitaire, BG‐Sentinel, CDC Gravid‐trap and ovitraps. These traps were selected to detect both host‐seeking ornithophilic and mammalophagic species, gravid Culex pipiens s.l. and invasive species (e.g. Aedes albopictus). Overwintering Cx. pipiens s.l. were collected using mechanical aspirators in January 2022, from a variety of areas at the index site, for viral detection. Adult female mosquitoes were identified to species level using morphology (Hawkes et al., 2020) and pooled into groups of 10 (by species and date of collection) for molecular analysis. Total RNA was extracted from mosquito pools using TRIzol (Invitrogen) according to the manufacturer's instructions and subjected to a USUV‐specific RT‐PCR (Jöst et al., 2011). To check for PCR inhibition, a subset of mosquito pools (n = 10) was diluted with a USUV positive control (1:10 dilution of USUV in RNA extract) and screened using the same USUV RT‐PCR assay.
2.3. Sequence characterisation and molecular clock analysis
Brain and kidney samples, selected as both tissues contain strong flavivirus immunolabelling and detectable viral genome, in USUV‐infected birds (Folly, Lawson, et al., 2020), were collected from 175 birds submitted for post‐mortem examination during the mosquito active season in the UK (April–November inclusive) in 2021: These comprised 151 wild birds from Great Britain and 24 captive birds from ZSL London Zoo (Table S2). Pooled brain and kidney samples from each bird were screened for USUV using the RT‐PCR assay described earlier, following total RNA extraction. Any positive samples were submitted for next‐generation sequencing (NGS). For NGS, a Nextera XT DNA library preparation kit (2 × 150‐bp reads, Illumina, San Diego, USA) was used, and sequencing was carried out on an Illumina MiSeq sequencer. Consensus sequences were generated by using a combination of the Burrows–Wheeler Aligner v0.7.13 and SAMtools v1.9 with a representative USUV genome as a scaffold. To remove bias, GenBank accession numbers (representative USUV lineage in parentheses): MN122238 (Africa 3.1), MN122254 (Africa 3.2), MN122237 (Africa 3.3) and MN122213 (Europe 3) were used to generate consensus sequences. The rationale was to use the average read depth (n = 157) to inform base calling and to remove the bias of the scaffold sequences because a whole‐genome contig was not generated using de novo assembly. The resulting sequence was then visually inspected in Tablet v1.19.09.03.
To elucidate the genomic relationship between USUV from any infected birds in 2021 with the original 2020 Greater London, UK, USUV sequence, a molecular clock analysis was undertaken. Here, constructed sequences were aligned with 88 whole‐genome USUV sequences, representing lineages Africa 3.1, 3.2 and 3.3 sampled between 2016 and 2021 from GenBank (Table S3), including the 2020 Greater London UK sequence (GenBank accession number: MW001216) in MAFFT v7.471. Other lineages of USUV were excluded from the molecular clock analysis due to the lack of whole‐genome sequences, which could confound the analysis. The alignment was imported into BEAST v1.10.4, and a Bayesian molecular clock was established with tip dates using a relaxed clock and the GTR+G nucleotide substitution model. The programme ran with 10,000,000 Markov chain Monte Carlo generations. Log files were analysed in Tracer v1.7.1 to check the effective sample size and a 10% burn‐in was included (TreeAnnotator v.1.10.4) before being annotated in FigTree v1.4.4.
2.4. Immunohistochemistry
Sections of formalin‐fixed brain, liver and kidney tissues obtained during the post‐mortem examination of the blackbird that was positive for USUV on RT‐PCR were prepared to 4‐μm thickness for the detection of Flavivirus non‐structural 1 (NS1) antigen. Dewaxed and rehydrated slides were quenched for endogenous peroxidase with 3% hydrogen peroxide in methanol (VWR International, UK), before epitope unmasking using a pH9 buffer (Dako, Denmark) for 10 min at 100°C by microwave (Shandon, UK) or proteinase (Sigma‐Aldrich, UK) for 15 min at room temperature. Slides were blocked with normal goat serum (1/66 dilution; Vector Laboratories, USA) followed by incubation with EA11 mouse monoclonal anti‐Dengue virus NS1 (0.17 μg/ml or 1/6000; The Native Antigen Company, UK) or a rabbit polyclonal anti‐Kunjin virus NS1 (1/4000; Australian Centre for Disease preparedness, CSIRO, Australia) antibody. Concentration‐matched isotype controls mouse IgG or rabbit sera were used for the assessment of non‐specific labelling. Slides were then incubated with mouse‐specific EnVision+TM HRP‐labelled polymer (Dako, Denmark) with an additional normal goat serum (1/66 dilution; Vector Laboratories), followed by visualization using DAB chromogen (Sigma‐Aldrich, UK). Tris‐buffered saline–Tween (Fisher Scientific, VWR International) was used for rinsing sections between incubations and as the antibody diluent. Finally, sections were counterstained within Mayer's haematoxylin (Surgipath, UK), dehydrated and glass coverslips were mounted using DPX (TCS Biosciences, UK). Positive and negative control tissues and cell pellets were included to validate the immunostaining.
3. RESULTS
Of the 86 serum samples, 15 tested positive for flavivirus antibodies using a competition ELISA. Of these ELISA‐positive samples, 12 were screened by PRNT, and 10 serum samples from 7 birds in the ZSL London Zoo collection had USUV‐specific neutralizing antibodies (PRNT90 titre range: 10−1–10−6) (Figure 1). The clinical presentation of these seven seropositive birds included wounds (3/7) and lameness (2/7). The remaining two birds were Humboldt penguins (Spheniscus humboldti) with clinical signs consistent with aspergillosis, although this was never definitively confirmed due to the difficulties of antemortem diagnosis of this disease (Fischer & Lierz, 2015). Although it remains possible that USUV‐associated disease occurred and was not detected, USUV was considered and excluded as a differential for the cause of presentation in all clinical cases at the time of their examination and blood sampling. Flavivirus seropositivity detected in one sample obtained from a griffon vulture (Gyps fulvus) could not be associated with USUV based on the absence of specific neutralizing antibodies. No sera drawn before the 2020 UK USUV detection showed evidence of USUV exposure.
FIGURE 1.
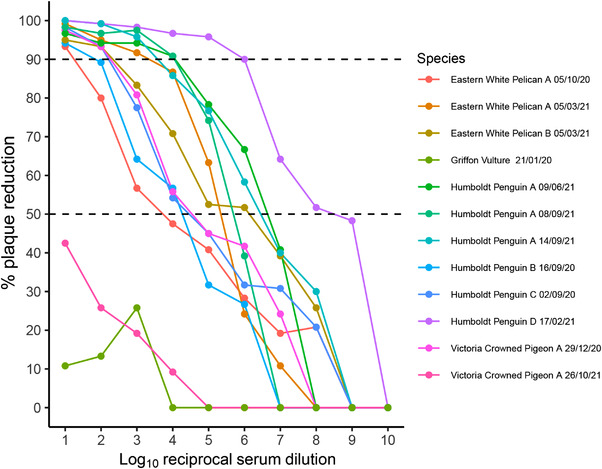
Usutu virus antibody response of reciprocal serum dilutions from captive birds at Zoological Society of London (ZSL) London Zoo. Antibody response is measured by proxy as a percentage of plaque reduction in Vero cell tissue culture when compared to a control (PRNT90 titre range: 10−1–10−6). Sampling date is detailed in the legend and serum samples that relate to the same bird have been given a unique alphabetized identifier for each species. Both 90% and 50% plaque reduction thresholds are denoted using a dashed line. Samples were considered to contain USUV‐specific neutralizing antibodies if ≥90% of plaques were reduced.
In total, 4966 mosquitoes were collected in north‐west London (Figure 2), including 4798 Cx. pipiens s.l., 88 Anopheles plumbeus and 80 Culiseta annulata, with mosquito numbers peaking in the months of July and August, as would be expected in the UK (NBN Atlas, 2022). None of the pooled samples were positive for USUV RNA, and there was no evidence of PCR inhibition in the subset of tested samples.
FIGURE 2.
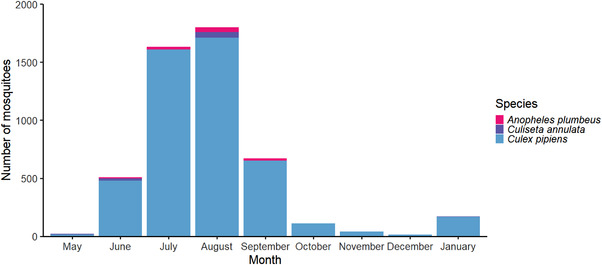
Monthly mosquito catch numbers for May–December 2021 and January 2022 from four sampling sites in north‐west London combined. The greater number of mosquitoes caught in January 2022 when compared to December 2021 is an artefact of increased sampling effort to identify overwintering female mosquitoes.
Of the 175 birds screened in 2021, only 1 tested positive for USUV RNA. This wild, juvenile male blackbird was found collapsed and unresponsive in the grounds of ZSL London Zoo in September and died before veterinary intervention. The animal was thin, with intestinal parasitism and marked splenomegaly. Systemic distribution of flavivirus NS1 antigen (indicative of virus replication) was observed using IHC, with strong cytoplasmic labelling identified in the brain (Figure 3a,b), heart, liver (Figure 3c), kidney (Figure 3d), spleen and lung, congruent with previous blackbird USUV cases (Folly, Lawson, et al., 2020; Lawson et al., 2022; Störk et al., 2021). Areas of immunolabelling were also co‐localized to areas with neuronal necrosis in the forebrain and cerebellum (Figure 3a,b).
FIGURE 3.
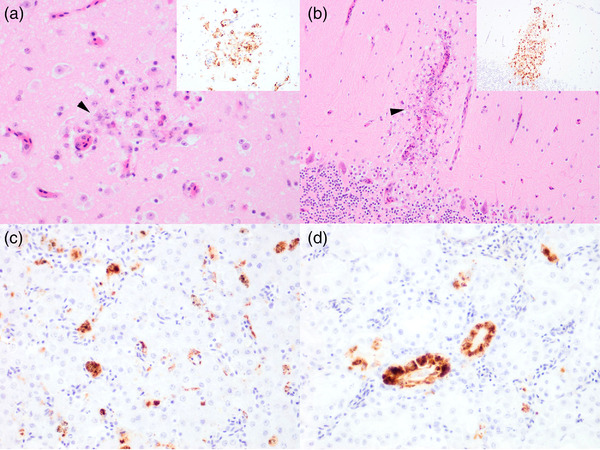
Detection of flavivirus NS1 antigen and colocalization with histopathology in Eurasian blackbird (Turdus merula) tissues: (a) cerebrum, (b) cerebellum, (c) liver and (d) kidney, from the Usutu virus RT‐PCR positive bird submitted in 2021 from Greater London, UK, using immunohistochemistry on formalin‐fixed paraffin‐embedded tissue sections at ×200 magnification. Strong immunolabelling is observed in all assessed tissues, indicated by darker red/brown areas. Neuronal necrosis (arrowhead) can be detected in areas colocalized with virus antigen positive neurons and capillaries (shown in insets) of the forebrain (a) and the molecular layer of the cerebellum (b). Antibody used: anti‐DENV NS1. Images taken at ×200 magnification
A 10,922 base pair consensus genome sequence (GenBank accession number: OM202464) obtained from the USUV‐positive blackbird in 2021, using non‐fixed tissue, was constructed using a range of representative USUV lineages (as described earlier). The consensus sequences generated across scaffolds were identical and unique from the scaffolds. The generated 2021 Greater London UK USUV sequence formed a strongly supported clade with other Africa 3.2 sequences and shares a most recent common ancestor (MRCA) with the 2020 Greater London UK sequence dated December 2018 (95% confidence interval February 2017–May 2020) (Figure 4), with the two sharing 99.86% sequence identity. To exclude bias, the 2020 Greater London UK sequence was not used to create the consensus. The mean rate of molecular evolution across the USUV lineage Africa 3 phylogeny was calculated at 2.84 × 10−4 substitutions per site per year.
FIGURE 4.
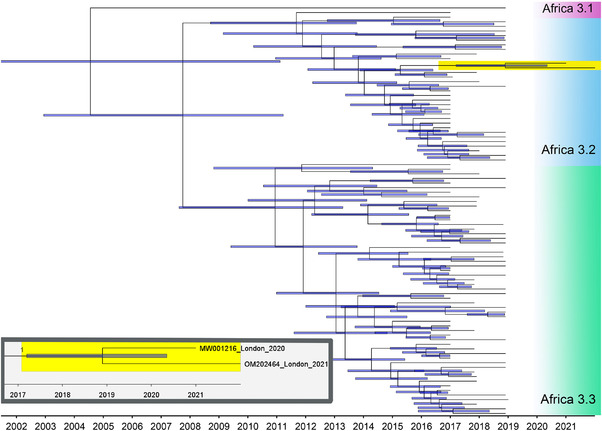
Molecular clock of whole‐genome Usutu virus Africa 3 lineages, including the 2020 Greater London UK (GenBank accession number: MW001216) and 2021 Greater London UK (GenBank accession number: OM202464) USUV sequences (highlighted in yellow). The blue bars indicate 95% confidence intervals of node dates. The two sequences from Greater London, UK, share 99.86% sequence identity and a most recent common ancestor dated December 2018 (95% confidence interval February 2017–May 2020). The mean rate of molecular evolution across the phylogeny was 2.84 × 10−4 substitutions per site per year. Magnified inset shows relationship and GenBank accession numbers for the 2020 and 2021 Greater London UK USUV sequences. Branch label of inset indicates posterior probability.
4. DISCUSSION
We detected active USUV infection in a single blackbird from a location in north‐west London in 2021. The recovered viral genome was most closely related to the 2020 Greater London UK USUV detection, with the two sharing an MRCA prior to the initial detection of USUV in the UK. There was no evidence of USUV RNA circulating in collected mosquitoes in the field sites in north‐west London during the mosquito active season of 2021, nor in overwintering samples collected at ZSL London Zoo in January 2022. However, we identified recent (late 2020 onwards) USUV exposure in archived sera from captive birds at ZSL London Zoo. Combined, our results suggest that, following its incursion in 2020, USUV persisted in the UK.
The USUV sequences recovered in 2020 and 2021 from Greater London form a strongly supported clade with other USUV Africa 3.2 genomes. Moreover, the two sequences are most closely related to each other when compared with other Africa 3.2 sequences and share a recent MRCA, indicating that USUV has overwintered in the UK. Blackbirds systemically infected with USUV are unlikely to survive the winter (Giglia et al., 2021), but overwintering could be through the recrudescent viraemia of chronically infected birds that survived infection (Kuchinsky et al., 2021) or, more likely, through the infection of mosquitoes that undergo diapause at the index site. Although a new incursion of a highly similar virus remains a theoretical explanation, the presence of competent mosquito vectors at the index site during the winter months supports a mechanism for virus overwintering. During the initial USUV outbreak in the UK, USUV RNA was detected in mosquito vectors caught at the index site (Lawson et al., 2022). In contrast, no USUV RNA was detected in any mosquito vectors in 2021. Although more geographically dispersed, with four monitored sites in north‐west London, the sampling effort for vectors was higher in 2021 compared to 2020. Therefore, it is likely that if USUV was circulating and established in north‐west London, viral RNA would have been detected (Kampen et al., 2021), especially as the most prevalent species sampled was Cx. pipiens s.l., a known USUV vector (Hernández‐Triana et al., 2018). Consequently, it is likely that our sampling effort was not sufficient to detect USUV in mosquito vectors, suggesting that USUV was not present at high prevalence in mosquitoes in this geographic area. Although the overall number of wild bird USUV detections in the UK to date is small, it is worth noting that only one bird was recovered with USUV infection in 2021, compared to six in the previous year. As with the original USUV detections in 2020, this bird was recovered from ZSL London Zoo. It is likely that the detections of USUV, all arising at ZSL London Zoo, are due to the disproportionately high surveillance carried out by staff in a relatively small geographic area, rather than USUV being a highly localized disease. Although some emerging diseases quickly become established in suitable populations, this is not always the case as a combination of factors need to be in place to support on‐going transmission. The annual temperature in south‐east England in 2021 was not significantly different from average (Met Office, 2022). However, in 2020, the temperature was 1–1.5°C warmer than average, which may have been permissive for USUV replication within mosquitoes and subsequent transmission (Met Office, 2022). It is not yet clear if the UK climate is a barrier for the establishment of USUV, even if it is permissive for successful overwintering. Climate has been shown to impact the emergence of vector‐borne diseases, with outbreaks followed by periods of prolonged absence being relatively common in regions where climatic conditions are not permissive for disease establishment (Cadar et al., 2017; Hubálek & Halouzka, 1999). Consequently, as USUV is endemic in mainland Europe, future incursions to the UK, and by extension to countries with similar climatic conditions, are feasible and whether these are likely to become endemic remains unclear (National Veterinary Institute, SVA, 2019).
The USUV sequences obtained from Greater London in 2020 and 2021 share high homology and have an MRCA dated to late 2018. This date is prior to the original UK outbreak and is a biologically realistic timeframe for the delimitation of sequences given that our molecular clock analysis calculated a rate of molecular evolution broadly similar to previous comprehensive USUV phylogenies that exhibit rapid intra‐lineage radiation (Engel et al., 2016; Oude Munnink et al., 2020). Our detections are therefore most likely to have originated from a single incursion event and have differentiated through subsequent autochthonous transmission. During the index site investigation, 10 birds in the ZSL London Zoo collection had evidence of flavivirus‐specific antibodies, of which 7 birds with sufficient sera for further testing showed USUV antibody specificity. All sera determined by PRNT to have USUV‐specific antibodies were collected after the initial USUV detection in 2020, with the earliest antibody response detected in a Humboldt penguin sample from 2 September 2020, concurrent with the initial USUV outbreak. In addition, one Humboldt penguin, which had USUV‐specific antibodies in serum drawn on 16 September 2020, was also sampled in April of 2020 (prior to the first detection of USUV in the UK) and neither antibodies to USUV, nor any flavivirus‐specific antibodies, were detected in this earlier sample. These serology results are consistent with an initial USUV emergence in the UK in summer 2020. Consequently, it is likely that the seropositive birds may have been exposed to circulating USUV in 2020 or 2021.
Of the bird sera which were positive for flavivirus antibodies, two were from Abdim's storks (Ciconia abdimii), which appears to be a preferred host for Cx. pipiens s.l. at ZSL London Zoo (Lawson et al., 2022). We screened brain and kidney samples collected post‐mortem from three Abdim's storks which died during 2021 for USUV RNA, all of which tested negative, and there is no evidence that this species was affected by USUV‐associated disease. One serum sample from a griffon vulture was positive using competition ELISA but had no detectable USUV‐specific antibody response using PRNT. It is likely that this animal had prior exposure to another flavivirus, the identity and timing of which is impossible to ascertain from a single serology result. For reference, this animal was born between 2001 and 2003 at an unknown location and has been at ZSL London Zoo since 2007, prior to which it was housed at a separate zoological collection in south‐east England. It is important to note that there was no indication of infectious disease in this bird at the time of blood‐sampling, which was conducted during treatment for an injury.
Our results demonstrate exposure to USUV for both captive and wild birds in or since late‐2020 and provide a valuable insight into the diversification of this emerging virus at the index site, where native‐wild, and exotic‐captive birds co‐exist. In essence, broad host diversity may be a driver of viral evolution, as the virus adapts to disparate host species (Longdon et al., 2014). Elucidating complete transmission networks, which incorporate hosts exhibiting both clinical disease and subclinical infection, is valuable to inform our understanding of drivers of viral emergence, evolution and, by extension, epidemiology.
Although the risk to public health is considered low (Human Animal Infections and Risk Surveillance Group, 2020), the persistence and autochthonous transmission of USUV in the UK presents a risk to native wildlife, especially blackbird populations (Harris et al., 2022; Lawson et al., 2022; Lühken et al., 2017). Our results provide empirical evidence that mosquito‐borne diseases, which can be geographically restricted due to climatic requirements, are shifting to higher latitudes where they are persisting in native wildlife. Understanding if the UK and countries with similar climatic conditions are permissive for the prolonged establishment of USUV, and other emerging arboviruses is critical to elucidate the long‐term impact of viral emergence on public and wildlife health.
AUTHOR CONTRIBUTIONS
Arran J. Folly, Becki Lawson, Andrew A. Cunningham, Jolyon M. Medlock and Nicholas Johnson conceived the experiment; Arran J. Folly, Sanam Sewgobind, Luis M. Hernández‐Triana, Karen L. Mansfield, Fabian Z. X. Lean, Becki Lawson, Katharina Seilern‐Moy, Simon Spiro, Ethan Wrigglesworth, Paul Pearce‐Kelly, Trent Herdman, Colin Johnston, Morgan Berrell, Alexander G. C. Vaux, Jolyon M. Medlock and Nicholas Johnson undertook the investigation, methods and data curation; Arran J. Folly, Sanam Sewgobind, Luis M. Hernández‐Triana, Becki Lawson, Katharina Seilern‐Moy, Simon Spiro and Nicholas Johnson undertook formal analysis; Arran J. Folly, Becki Lawson, Andrew A. Cunningham, Jolyon M. Medlock and Nicholas Johnson were responsible for project administration, supervision and securing funding; Arran J. Folly wrote the first draft; all authors reviewed, edited and agreed to the final version.
CONFLICT OF INTEREST
The authors declare there are no competing interests.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. Brain and kidney samples used in this investigation were taken from birds found dead, or euthanased for welfare reasons, that were submitted for post‐mortem examination. All serology samples had been collected during clinical investigations of captive birds for ill health, or as part of routine health check examinations, conducted by the veterinary team at ZSL London Zoo under the Veterinary Surgeons Act 1986: As such no sera samples were collected specifically for the purpose of this study.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
The authors would like to thank members of the public and participants in the British Trust for Ornithology's Garden BirdWatch scheme, who report to the Garden Wildlife Health project (www.gardenwildilfehealth.org); APHA regional laboratories, the Predatory Bird Monitoring Scheme and the Institute of Zoology's Disease Risk Analysis and Health Surveillance project, for wild bird submissions to the GB flavivirus surveillance scheme. The authors would also like to thank Sophie Myers and Sophie Harding for assisting with mosquito collection, histology scientists at APHA for performing IHC and the Protein Expression Facility, University of Queensland, Australia for producing the Kunjin NS1 protein for the generation of polyclonal antiserum. This study was supported by grants SV3045 and SE0560 from Department for Environment, Food & Rural Affairs (Defra) and the Devolved Administrations of Scotland and Wales and the European Union Horizon 2020 Research and Innovation Program under grant agreement No. 871029 EVA‐GLOBAL. Financial support for the Garden Wildlife Health project comes in part from the Defra, the Welsh Government and the APHA Diseases of Wildlife Scheme Scanning Surveillance Programme (Project ED1058), and from the Garfield Weston Foundation and the Universities Federation for Animal Welfare. BL and AAC receive financial support from Research England.
Folly, A. J. , Sewgobind, S. , Hernández‐Triana, L. M. , Mansfield, K. L. , Lean, F. Z. X. , Lawson, B. , Seilern‐Moy, K. , Cunningham, A. A. , Spiro, S. , Wrigglesworth, E. , Pearce‐Kelly, P. , Herdman, T. , Johnston, C. , Berrell, M. , Vaux, A. G. C. , Medlock, J. M. , & Johnson, N. (2022). Evidence for overwintering and autochthonous transmission of Usutu virus to wild birds following its redetection in the United Kingdom. Transboundary and Emerging Diseases, 69, 3684–3692. 10.1111/tbed.14738
DATA AVAILABILITY STATEMENT
Data used for this study can be found at 10.6084/m9.figshare.20438991.
REFERENCES
- Cadar, D. , Lühken, R. , van der Jeugd, H. , Garigliany, M. , Ziegler, U. , Keller, M. , Lahoreau, J. , Lachmann, L. , Becker, N. , Kik, M. , Oude Munnink, B. B. , Bosch, S. , Tannich, E. , Linden, A. , Schmidt, V. , Koopmans, M. P. , Rijks, J. , Desmecht, D. , Groschup, M. H. , … Schmidt‐Chanasit, J. (2017). Widespread activity of multiple lineages of Usutu virus, Western Europe, 2016. Eurosurveillance, 22, 30452. 10.2807/1560-7917.es.2017.22.4.30452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzolari, M. , Bonilauri, P. , Bellini, R. , Albieri, A. , Defilippo, F. , Tamba, M. , Tassinari, M. , Gelati, A. , Cordioli, P. , Angelini, P. , & Dottori, M. (2013). Usutu virus persistence and West Nile virus inactivity in the Emilia‐Romagna region (Italy) in 2011. PLoS One, 8, e63978. 10.1371/journal.pone.0063978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clé, M. , Beck, C. , Salinas, S. , Lecollinet, S. , Gutierrez, S. , Van de Perre, P. , Baldet, T. , Foulongne, V. , & Simonin, Y. (2019). Usutu virus: A new threat? Epidemiology and Infection, 147, e232. 10.1017/S0950268819001213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak, P. , Cunningham, A. A. , & Hyatt, A. D. (2000). Emerging infectious diseases of wildlife – Threats to biodiversity and human health. Science, 287, 443–449. 10.1126/science.287.5452.443 [DOI] [PubMed] [Google Scholar]
- Diallo, D. , Sall, A. A. , Diagne, C. T. , Faye, O. , Faye, O. , Ba, Y. , Hanley, K. A. , Buenemann, M. , Weaver, S. C. , & Diallo, M. (2014). Zika virus emergence in mosquitoes in Southeastern Senegal, 2011. PLoS One, 9, e109442. 10.1371/journal.pone.0109442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, D. , Jöst, H. , Wink, M. , Börstler, J. , Bosch, S. , Garigliany, M. M. , Jöst, A. , Czajka, C. , Lühken, R. , Ziegler, U. , Groschup, M. H. , Pfeffer, M. , Becker, N. , Cadar, D. , & Schmidt‐Chanasit, J. (2016). Reconstruction of the evolutionary history and dispersal of Usutu virus a neglected emerging arbovirus in Europe and Africa. mBio, 7, e01938–15. 10.1128/mBio.01938-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, D. , & Lierz, M. (2015). Diagnostic procedures and available techniques for the diagnosis of aspergillosis in birds. Journal of Exotic Pet Medicine, 24, 283–295. 10.1053/j.jepm.2015.06.016 [DOI] [Google Scholar]
- Folly, A. J. , Lawson, B. , Lean, F. Z. X. , McCracken, F. , Spiro, S. , John, S. K. , Heaver, J. P. , Seilern‐Moy, K. , Masters, N. , Hernández‐Triana, L. M. , Phipps, L. P. , Nuñez, A. , Fooks, A. R. , Cunningham, A. A. , Johnson, N. , & McElhinney, L. M. (2020). Detection of Usutu virus infection in wild birds in the United Kingdom, 2020. Eurosurveillance, 25, 2001732. 10.2807/1560-7917.ES.2020.25.41.2001732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folly, A. J. , Waller, E. S. L. , McCracken, F. , McElhinney, L. M. , Roberts, H. , & Johnson, N. (2020). Equine seroprevalence of West Nile virus antibodies in the UK in 2019. Parasites & Vectors, 13, 596. 10.1186/s13071-020-04481-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglia, G. , Agliani, G. , Oude Munnink, B. B. , Sikkema, R. S. , Mandara, M. T. , & Lepri, E. (2021). Pathology and pathogenesis of Eurasian blackbirds (Turdus merula) naturally infected with Usutu virus. Viruses, 13, 1481. 10.3390/v13081481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S. J. , Massimino, D. , Balmer, D. E. , Kelly, L. , Nobel, D. G. , Pearce‐Higgins, J. W. , Woodcock, P. , Wotton, S. , & Gillings, S. (2022). The breeding bird survey 2021. BTO research report 745. British Trust for Ornithology. [Google Scholar]
- Hawkes, F. M. , Medlock, J. M. , Vaux, A. G. C. , Cheke, R. A. , & Gibson, G. (2020). Wetland mosquito survey handbook. Assessing suitability of British wetlands for mosquitoes. Natural Resources Institute. NRI‐PHE‐UoG_Wetland_Mosquito_Survey_Handbook_v1‐indexed.pdf (wetlandlife.org) [accessed 01 July 2022]. [Google Scholar]
- Hernández‐Triana, L. M. , de Marco, M. F. , Mansfield, K. L. , Thorne, L. , Lumley, S. , Marston, D. , Fooks, A. R. , & Johnson, N. (2018). Assessment of vector competence of UK mosquitoes for Usutu virus of African origin. Parasites & Vectors, 11, 381. 10.1186/s13071-018-2959-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, D. L. , Lawson, B. , Egbetade, A. , Jeffries, C. , Johnson, N. , Cunningham, A. A. , & Fooks, A. R. (2013). Targeted surveillance for Usutu virus in British birds (2005‐2011). Veterinary Record, 172, 17. 10.1136/vr.101275 [DOI] [PubMed] [Google Scholar]
- Hubálek, Z. , & Halouzka, J. (1999). West Nile fever‐ a re‐emerging mosquito‐borne viral disease in Europe. Emerging Infectious Diseases, 5, 643–650. 10.3201/eid0505.990505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Animal Infections and Risk Surveillance Group . (2020). Qualitative assessment of the risk that Usutu virus presents to the UK population. Public Health England; HAIRS risk assessment: Usutu virus ‐ GOV.UK (www.gov.uk) [accessed 01 July 2022]. [Google Scholar]
- Jöst, H. , Bialonski, A. , Maus, D. , Sambri, V. , Eiden, M. , Groschup, M. H. , Günther, S. , Becker, N. , & Schmidt‐Chanasit, J. (2011). Isolation of Usutu virus in Germany. The American Journal of Tropical Medicine and Hygiene, 85, 551–553. 10.4269/ajtmh.2011.11-0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampen, H. , Tews, B. A. , & Werner, D. (2021). First evidence of West Nile Virus overwintering in mosquitoes in Germany. Viruses, 13, 2463. 10.3390/v13122463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinsky, S. C. , Frere, F. , Heitzman‐Breen, N. , Golden, J. , Vázquez, A. , Honaker, C. F. , Siegel, P. B. , Ciupe, S. M. , Leroith, T. , & Duggal, N. K. (2021). Pathogenesis and shedding of Usutu virus in juvenile chickens. Emerging Microbes & Infections, 10, 725–738. 10.1080/22221751.2021.1908850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, B. , Robinson, R. A. , Briscoe, A. G. , Cunningham, A. A. , Fooks, A. R. , Heaver, J. P. , Hernández‐Triana, L. M. , John, S. K. , Johnson, N. , Johnston, C. , Lean, F. Z. X. , Macgregor, S. K. , Masters, N. J. , McCracken, F. , McElhinney, L. M. , Medlock, J. M. , Pearce‐Kelly, P. , Seilern‐Moy, K. , Spiro, S. , … Folly, A. J. (2022). Identifying the signal of disease emergence: Combining host and vector data shows potential impact of an Usutu virus outbreak on UK wild birds. Scientific Reports, 12, 10298. 10.1038/s41598-022-13258-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke, S. , Ellerbrok, H. , Niedrig, M. , Nitsche, A. , & Pauli, G. (2007). Detection of West Nile virus lineage 1 and 2 by real‐time PCR. Journal of Virological Methods, 146, 355–358. 10.1016/j.jviromet.2007.05.021 [DOI] [PubMed] [Google Scholar]
- Longdon, B. , Brockhurst, M. A. , Russell, C. A. , Welch, J. J. , & Jiggins, F. M. (2014). The evolution and genetics of virus hosts shifts. PLOS Pathogens, 10, e1004395. 10.1371/journal.ppat.1004395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lühken, R. , Jöst, H. , Cadar, D. , Thomas, S. M. , Bosch, S. , Tannich, E. , Becker, N. , Zeigler, U. , Lachmann, L. , & Schmidt‐Chanasit, J. (2017). Distribution of Usutu virus in Germany and its effect on breeding bird populations. Emerging Infectious Diseases, 23, 1994–2001. 10.3201/eid2312.171257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manore, C. A. , & Beechler, B. R. (2015). Inter‐epidemic and between‐season persistence of Rift Valley fever: Vertical transmission or cryptic cycling? Transboundary and Emerging Diseases, 62, 13–23. 10.1111/tbed.12082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield, K. L. , Horton, D. L. , Johnson, N. , Li, L. , Barrett, A. D. , Smith, D. J. , Galbraith, S. E. , Solomon, T. , & Fooks, A. R. (2011). Flavivirus‐induced antibody cross reactivity. Journal of General Virology, 92, 2821–2829. 10.1099/vir.0.031641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencattelli, G. , Iapaolo, F. , Monaco, F. , Fusco, G. , de Martinis, C. , Portanti, O. , Di Gennaro, A. , Curini, V. , Polci, A. , Berjaoui, S. , Di Felice, E. , Rosà, R. , Rizzoli, A. , & Savini, G. (2021). West Nile virus lineage 1 in Italy: Newly introduced or a re‐occurrence of a previously circulating strain? Viruses, 14, 64. 10.3390/v14010064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Met Office . (2022). Climate summaries. Met Office. metoffice.gov.uk/research/climate/maps‐and‐data/summaries/index [accessed 24 June 2022]. [Google Scholar]
- Nash, D. , Mostashari, F. , Fine, A. , Miller, J. , O'Leary, D. , Murray, K. , Huang, A. , Rosenberg, A. , Greenberg, A. , Sherman, M. , Wong, S. , Campbell, G. L. , Roehrig, J. T. , Gubler, D. J. , Shieh, W. , Zaki, S. , Smith, P. , & Layton, M. (2001). The outbreak of West Nile virus infection in the New York city area in 1999. The New England Journal of Medicine, 344, 1807–1814. 10.1056/NEJM200106143442401 [DOI] [PubMed] [Google Scholar]
- NBN Atlas‐ Mosquito Recording Scheme . (2022). Website at https://www.nbnatlas.org [accessed 24 June 2022]
- Nikolay, B. (2015). A review of West Nile and Usutu virus co‐circulation in Europe: How much do transmission cycles overlap? Transactions of the Royal Society of Tropical Medicine and Hygiene, 109, 609–618. 10.1093/trstmh/trv066 [DOI] [PubMed] [Google Scholar]
- Oude Munnink, B. B. , Münger, E. , Nieuwenhuijse, D. F. , Kohl, R. , van der Linden, A. , Schapendonk, C. M. E. , van der Jeugd, H. , Kik, M. , Rijks, J. M. , Reusken, C. B. E. M. , & Koopmans, M. (2020). Genomic monitoring to understand the emergence and spread of Usutu virus in the Netherlands, 2016–2018. Scientific Reports, 10, 2798. 10.1038/s41598-020-59692-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijks, J. M. , Kik, M. L. , Slaterus, R. , Foppen, R. , Stroo, A. , IJzer, J. , Stahl, J. , Gröne, A. , Koopmans, M. G. P. , van der Jeugd, H. P. , & Reusken, C. B. E. M. (2016). Widespread Usutu virus outbreak in birds in the Netherlands, 2016. Eurosurveillance, 21, 30391. 10.2807/1560-7917.ES.2016.21.45.30391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Störk, T. , de le Roi, M. , HaverKamp, A. K. , Jesse, S. T. , Peters, M. , Fast, C. , Gregor, K. M. , Könenkamp, L. , Steffen, I. , Ludlow, M. , Beineke, A. , Hansmann, F. , Wohlsein, P. , Osterhaus, A. D. M. E. , & Baumgärtner, W. (2021). Analysis of avian Usutu virus infections in Germany from 2011 to 2018 with a focus on dsRNA detection to demonstrate viral infections. Scientific Reports, 11, 4191. 10.1038/s41598-021-03638-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden, N. B. , Caminade, C. , Beierkuhnlein, C. , & Thomas, S. M. (2018). Mosquito‐borne diseases: Advances in modelling climate‐change impacts. Trends in Parasitology, 34, 227–245. 10.1016/j.pt.2017.11.006 [DOI] [PubMed] [Google Scholar]
- National Veterinary Institute, SVA . (2019). Wildlife disease surveillance in Sweden. National Veterinary Institute, SVA. wildlife‐disease‐surveillance‐in‐sweden‐2019.pdf (sva.se) [accessed 26 July 2022]. [Google Scholar]
- Woodall, J. P. (1964). The viruses isolated from arthropods at the East African virus research institute in the 26 years ending December 1963. Proceedings of the East African Academy, 2, 141–146. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Data used for this study can be found at 10.6084/m9.figshare.20438991.


