Perinatally transmitted HIV infections have continued to occur in Ontario despite the introduction of universal prenatal HIV counselling and voluntary testing guidelines in December 1998. We conducted a retrospective chart review of all infants diagnosed with perinatally acquired HIV infection over a 24-month period (August 1999 to July 2001), born to women who became pregnant subsequent to the introduction of these guidelines, with the purpose of determining possible reasons for the failure to eliminate perinatal transmission of HIV in Ontario. Data were extracted from the hospital charts of the infants. As part of the initial history, all HIV-infected mothers seen in our clinic were routinely questioned as to whether they received prenatal HIV counselling, whether they agreed to such testing, whether they thought such testing was performed and whether appropriate therapy was initiated and received. For ethical and legal reasons, no attempt was made to question the physicians who cared for the mothers during pregnancy regarding their antenatal care. An infant was considered to be HIV infected if HIV was detected by DNA polymerase chain reaction (PCR) or culture, or both, on 2 separate occasions.
In Ontario, about two-thirds of children diagnosed as being HIV infected receive their care in the HIV/AIDS Family Centered Care Clinic at the Hospital for Sick Children, Toronto.1 Six infants with perinatally (“vertically”) acquired HIV infection were identified (none of the infants had received blood products). For 4 infants, the diagnosis of HIV was suspected because of clinical manifestations consistent with HIV infection, and for the other 2 infants, who were asymptomatic, because of postnatally proven HIV infection in the mother. Pertinent details of each mother's history and self-reported reasons for the failure to perform prenatal HIV testing are summarized in Table 1. None of the mothers was aware that they were HIV-positive when they became pregnant, none was tested for HIV infection during pregnancy and none received antiretroviral therapy during pregnancy. There was no history of injection drug use, prostitution or sexually transmitted diseases for any of the mothers. Our results suggest that incomplete application of universal prenatal HIV counselling and voluntary testing guidelines by health care providers is largely responsible for the continued occurrence of perinatal transmission of HIV in Ontario. This finding is consistent with those of the Ontario surveillance study, which indicate that during the first quarter of 2001 only 52.5% of pregnant women underwent prenatal HIV testing (Dr. Robert Remis, Department of Public Health Sciences, Faculty of Medecine, University of Toronto, Toronto, and Ms. Carol Major, HIV Laboratory, Laboratory Services Branch, Ontario Ministry of Health and Long-Term Care, Toronto: personal communication, 2001).
Table 1
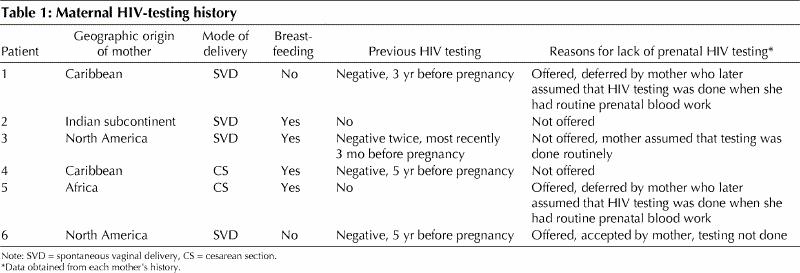
A major limitation of our study is that it relied exclusively on each mother's history and, consequently, the possibility of recall bias cannot be excluded. It is conceivable that some of the women who said they deferred HIV testing and later assumed such testing was done had in fact refused HIV testing. On the other hand, it is unlikely that women would completely forget having been counselled about HIV testing. Furthermore, even if there are some inaccuracies in our data, it is certainly clear that 6 potentially preventable perinatal HIV infections occurred in spite of the recommendations for prenatal HIV counselling and voluntary testing in Ontario.
The effectiveness of prenatal HIV counselling and subsequent voluntary HIV testing programs has been evaluated in several countries including the United Kingdom, France and the United States 2,3,4,5,6 The proportion of women who agreed to undergo HIV testing varied from 35% in Scotland2 to 86% in parts of the United States.3 Reasons for refusing HIV testing included lack of perceived risk, previous testing and lack of endorsement by the health care provider.3
In order to further reduce the rate of perinatal HIV transmission in the United States, the Institute of Medicine recently recommended that “a national policy of universal HIV testing, with patient notification, as a routine component of prenatal care” be adopted.7 These recommendations have since been supported by the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics.8 The key new element in this proposed strategy is that antenatal HIV testing would be routine, consistent with other routine antenatal tests for infectious agents including syphilis, rubella and hepatitis B. Formalized prenatal HIV counselling and written informed consent would no longer be required. With this “opt-out” strategy, physicians would be required to inform all pregnant women that HIV testing is part of routine antenatal care and that such testing would be done unless the woman specifically objected to it.
The effectiveness and acceptability of the “opt-out” strategy have been evaluated in the United Kingdom and the United States.9,10,11 In these studies, the proportion of pregnant women undergoing prenatal HIV testing increased from 33%–74% with the “opt-in” strategy to 81%–88% with the “opt-out” strategy. Surveillance data from Alberta indicate that 96% of pregnant women who received prenatal care underwent HIV testing during the first year of that province's “opt-out” prenatal HIV testing program.12
Clearly, there are important ethical issues with an “opt-out” strategy that must be considered.13 It must be clear to health care workers that under such a strategy they must explicitly inform women of the policy, its benefits and risks, and their right of refusal. Physicians must be cognizant of the stigma associated with HIV infection and some women's fear of rejection or violence by their partners and of being ostracized by their communities should their HIV status become known to others.
Only 10%–30% of children with perinatally acquired HIV infection develop symptoms that lead to a diagnosis of HIV during infancy.14,15 Consequently, it is likely that most of the children who were perinatally infected subsequent to the implementation of the Ontario guidelines remain undiagnosed at the present time. It is our position that the continued occurrence of perinatally transmitted HIV infections is unacceptable in view of the very high efficacy of preventive measures that are currently available for HIV-infected pregnant women.16 We recommend that an “opt-out” strategy for prenatal HIV testing, with patient notification and counselling, be adopted in Ontario and all other provinces and territories as a routine component of prenatal care to further reduce the rate of perinatal transmission of HIV in Canada.
β See related article page 909
Footnotes
This article has been peer reviewed.
Contributors: Dr. Bitnun was the principal author of the manuscript; he proposed the study, collected the data, aided in the interpretation of the data and wrote the manuscript. Drs. Read and King aided in the interpretation of the data and were responsible for manuscript review, correction and advice. Ms. Arneson assisted in data collection as well as manuscript review, correction and advice.
Competing interests: None declared.
Correspondence to: Dr. Stanley E. Read, Chief, Division of Infectious Diseases, Department of Pediatrics, The Hospital for Sick Children, 555 University Ave., Toronto ON M5G 1X8; fax 416 813-5032; sread@sickkids.on.ca
References
- 1.Remis RS, Major C, Wallace E, Schiedel L, Whittingham EP. Report on HIV/AIDS in Ontario, 1999. Toronto: Ontario Ministry of Health and Long- Term Care; 2000.
- 2.Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Prescott RJ. Uptake and acceptability of antenatal HIV testing: randomized controlled trial of different methods of offering the test. BMJ 1998;316:262-7. [DOI] [PMC free article] [PubMed]
- 3.Fernandez MI, Wilson TE, Ethier KA, Walter EB, Gay CL, Moore J, for the Perinatal Guidelines Evaluation Project. Acceptance of HIV testing during prenatal care. Public Health Rep 2000;115:460-8. [DOI] [PMC free article] [PubMed]
- 4.Carusi D, Learman LA, Posner SF. Human immunodeficiency virus test refusal in pregnancy: a challenge to voluntary testing. Obstet Gynecol 1998;91:540-5. [DOI] [PubMed]
- 5.Joo E, Carmack A, Garcia-Buñuel E, Kelly CJ. Implementation of guidelines for HIV counseling and voluntary testing of pregnant woman. Am J Public Health 2000;90:273-6. [DOI] [PMC free article] [PubMed]
- 6.Rey D, Carrieri MP, Obadia Y, Pradier C, Moatti JP. Mandatory prenatal screening for the human immunodeficiency virus: the experience in south-east France of a national policy, 1992-1994. Br J Obstet Gynecol 1998;105:269-74. [DOI] [PubMed]
- 7.Institute of Medicine, Committee on Perinatal Transmission of HIV and Commission on Behavioral and Social Sciences and Education. Reducing the odds: preventing perinatal transmission of HIV in the United States. Washington: National Academy Press; 1999. [PubMed]
- 8.American Academy of Pediatrics and American College of Obstetricians and Gynecologists. Human immunodeficiency virus screening. Pediatrics 1999; 104:128. [PubMed]
- 9.Lo B, Wolf L, Sengupta S. Ethical issues in early detection of HIV infection to reduce vertical transmission. J Acquir Immune Defic Syndr Hum Retrovirol 2000; 25:S136-43. [DOI] [PubMed]
- 10.Simpson WM, Johnstone FD, Goldberg DJ, Gormley SM, Hart GJ. Antenatal HIV testing: assessment of a routine voluntary approach. BMJ 1999;318: 1660-1. [DOI] [PMC free article] [PubMed]
- 11.Blott M, Yearwood J, Gerval M, Welch J, Zuckerman M. Routine antenatal HIV testing is acceptable to woman. BMJ 1999;319:1069-70. [PubMed]
- 12.Bureau of HIV/AIDS, STD and TB, Centre for Infectious Disease Prevention and Control, Health Canada. HIV/AIDS Epi Update: Perinatal transmission of HIV. 2001 May. Available: www.hc-sc.gc.ca/hpb/lcdc/bah/epi/peri_e.html (modified 2001 June 5) (accessed 2002 Feb 11).
- 13.Stringer E, Stringer J, Cliver S, Goldenberg R, Goepfert A. Active refusal increases human immunodeficiency virus screening in an urban prenatal clinic system. Obstet Gynecol 2001;97:S58. [DOI] [PubMed]
- 14.Auger I, Thomas P, De Gruttola V, Morse D, Moore D, Williams R, et. al. Incubation periods for paediatric AIDS patients. Nature 1988;336:575-7. [DOI] [PubMed]
- 15.The French Pediatric HIV Infection Study Group and European Collaborative Study. Morbidity and mortality in European children vertically infected by HIV-1. J Acquir Immune Defic Syndr Hum Retrovirol 1997;14:442-50. [DOI] [PubMed]
- 16.Mofenson LM, McIntyre JA. Advances and research directions in the prevention of mother-to-child HIV-1 transmission. Lancet 2000;355:2237-44. [DOI] [PubMed]


