Abstract
Objective
This study explored the impact of time‐restricted eating (TRE) versus standard dietary advice (SDA) on bone health.
Methods
Adults with ≥1 component of metabolic syndrome were randomized to TRE (ad libitum eating within 12 hours) or SDA (food pyramid brochure). Bone turnover markers and bone mineral content/density by dual energy x‐ray absorptiometry were assessed at baseline and 6‐month follow‐up. Statistical analyses were performed in the total population and by weight loss response.
Results
In the total population (n = 42, 76% women, median age 47 years [IQR: 31‐52]), there were no between‐group differences (TRE vs. SDA) in any bone parameter. Among weight loss responders (≥0.6 kg weight loss), the bone resorption marker β‐carboxyterminal telopeptide of type I collagen tended to decrease after TRE but increase after SDA (between‐group differences p = 0.041), whereas changes in the bone formation marker procollagen type I N‐propeptide did not differ between groups. Total body bone mineral content decreased after SDA (p = 0.028) but remained unchanged after TRE (p = 0.31) in weight loss responders (between‐group differences p = 0.028). Among nonresponders (<0.6 kg weight loss), there were no between‐group differences in bone outcomes.
Conclusions
TRE had no detrimental impact on bone health, whereas, when weight loss occurred, it was associated with some bone‐sparing effects compared with SDA.
Study Importance.
What is already known?
Weight loss achieved by conventional approaches (e.g., moderate caloric restrictions to very low‐calorie diets) may adversely affect bone health.
Time‐restricted eating (TRE) has emerged as a popular dietary intervention for weight loss and metabolic health benefits. It is, however, uncertain whether the adverse effects of conventional weight loss approaches on bone health hold true for TRE.
What does this study add?
Our results suggest no detrimental impact of a 6‐month TRE intervention on bone outcomes (bone turnover markers, total body bone mineral content or density).
When weight loss occurs, TRE might be associated with some bone‐sparing effects compared with standard dietary advice.
How might these results change the direction of research or the focus of clinical practice?
TRE may be a suitable nutritional therapeutic option for weight loss that does not compromise bone turnover and might preserve bone mass.
Future TRE studies lasting more than 6 months should assess additional bone phenotypes (bone mineral density and microstructure at clinically relevant sites, fracture risk) and explore the effects of various TRE regimens with different eating windows or timings, especially among individuals at risk for bone fragility such as postmenopausal women and the elderly.
INTRODUCTION
Intermittent fasting (IF) involves an alternation of abstinence and consumption of food and caloric beverages over a cycle of hours to days. IF has gained considerable interest as a weight management intervention that is easy to incorporate into everyday life (vs. conventional caloric restrictions with/without physical activity) and that may improve metabolic health by inducing weight loss and/or restoring the rhythmicity between anabolism and catabolism [1, 2, 3, 4]. Time‐restricted feeding (in animals) or eating (TRE in humans) is a form of IF that entails restricting eating within a window of 4 to 12 hours per 24‐hour cycle and prolonging the time spent in the fasted state to realign eating‐fasting patterns with circadian rhythms [1, 2, 3, 4]. An increasing number of randomized controlled trials (RCTs) have supported the benefits of TRE in obesity, diabetes, and cardiovascular diseases [3, 4, 5, 6, 7, 8, 9, 10, 11, 12]. It remains, however, unclear whether TRE would exert beneficial, neutral, or unfavorable effects on other organ systems such as the skeletal system.
Theoretically, TRE results in changes in physiological and metabolic parameters and/or behaviors that could influence bone health. For example, sustaining a consistent daily rhythm in eating‐fasting may improve metabolic outcomes and circadian rhythms of metabolic pathways [2, 5, 6, 9]. Preclinical and clinical data support the importance of the circadian system in bone physiology and the impact of circadian rhythm disruptions on bone fragility (for a review see [13]), therefore raising the potential that TRE may affect bone health. Furthermore, although individuals following TRE are commonly not provided guidance on caloric intake, several studies have reported inadvertent reductions in caloric intake (due to eating during a shorter time window and/or reduced consumption of energy‐dense foods commonly consumed in the evening), which have been reported to contribute to weight loss and the associated metabolic benefits [7, 8, 12].
The effects of overweight/obesity and weight loss on bone outcomes are complex [14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26]. Overweight/obesity (commonly defined using body mass index [BMI]) and related comorbidities have been associated with increased bone mass as a result of mechanical adaptations to an increased body weight and metabolic/hormonal effects. Nevertheless, individuals with overweight/obesity have also been shown to have impairments in some bone matrix and microstructural properties and an increased fracture risk at specific skeletal sites, with these unfavorable effects largely attributed to chronic inflammation and hormonal disturbances linked to increased adiposity [14, 15, 16, 17, 18, 25, 26]. Conversely, although conventional caloric restrictions result in reductions in body fat, improvements of inflammatory status, and better metabolic control that could benefit bone health, these practices have been associated with reductions in bone mass, at least in RCTs with a duration of <2 years [19, 20, 21, 22, 23, 24]. Studies on TRE have only recently started to unravel changes in bone outcomes. Total body bone mineral content (BMC) or density (BMD), assessed by dual‐energy x‐ray absorptiometry (DXA), was not adversely affected after 6 weeks [27] or 12 weeks [28, 29] of TRE. Given that a minimum monitoring time interval of 6 months is recommended for repeating bone mass measurements [19], it is possible that small changes were not captured in these shorter‐term studies. Importantly, bone metabolic activity can be assessed indirectly by determining the levels of bone turnover markers (BTMs) that provide useful information before the detection of established changes in bone mass and structure [19, 23, 24, 30, 31, 32]. The only available TRE study that reported BTMs [28] showed no changes in bone resorption (assessed by cross‐linked N‐terminal telopeptide of type I collagen) but attenuated reductions in bone formation (assessed by procollagen type 1 N‐terminal propeptide [P1NP]) after 12 weeks of TRE (vs. habitual diet). Although these results were interpreted as a potential bone protective effect of TRE, the nature (acute, persistent, or adaptive) and the clinical significance of such an effect remain uncertain.
In the present study, we aimed to explore the impact of a 6‐month RCT of TRE versus standard dietary advice (SDA, active control arm) on bone metabolism and health in a population with at least one component of the metabolic syndrome (MS). We hypothesized that the effects of weight loss on bone metabolism and health would differ according to the allocated intervention (TRE vs. SDA).
METHODS
Study participants and design
This is a secondary analysis of an open‐label 6‐month RCT that examined the impact of TRE on metabolic parameters (body weight, blood pressure, lipid profile, and glucose metabolism assessed as primary outcome), body composition, and lifestyle parameters [33]. Participants were recruited via posters, online advertisements, and social media at two study sites in Switzerland (Lausanne University Hospital, Lausanne, and Inselspital, Bern) between 2017 and 2020. Men and women were included if they were aged ≥ 18 years, they were weight stable (±2 kg within the previous 3 months), and they had at least one component of the MS according to the International Diabetes Federation consensus definition [34]. We excluded those with major illness (i.e., cardiovascular, liver, respiratory, gastrointestinal, renal, endocrine, and sleeping disorders or active cancer), prior bariatric surgery, and eating disorders, those following a diet/weight management program, and those on medication affecting the gut absorption, body weight, or hormones. Pregnant or breastfeeding women were also excluded from participation. The study was approved by the Ethics Committee of the Cantons of Vaud and Bern, Switzerland (CER‐VD 2017–00487), performed in accordance with the declaration of Helsinki and the ICH E6 Good Clinical Practice, and registered on ClinicalTrials.gov (NCT03241121) and Kofam.ch (SNCTP000002259). Participants provided their informed consent in writing before inclusion in the study.
Participants attended a 4‐week run‐in period and they underwent baseline assessments, including medical history, questionnaires (e.g., demographics, physical activity), clinical measurements, and routine blood testing (Supporting Information Figure S1). They were also asked to record their eating behavior and dietary intake with the smartphone application myCircadianClock over 4 weeks [7]. Participants who ate within a time interval > 14 hours per 24‐hour cycle (n = 54) were randomized to TRE or SDA (active control) with a 1:1 allocation ratio (Figure 1).
FIGURE 1.
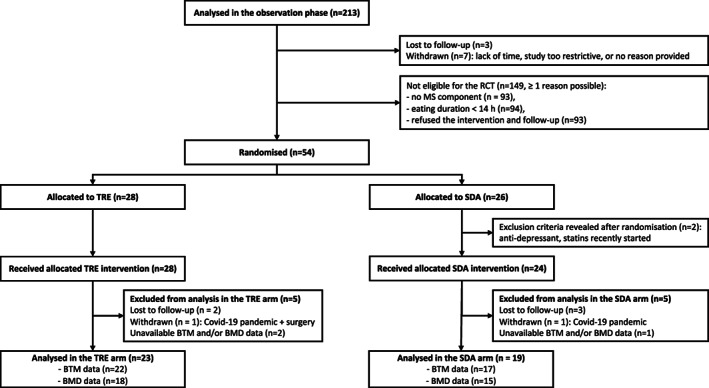
Flowchart of participants, adapted from [33]. Forty‐two participants with available BTMs and/or BMD measurements were included in the present study. BMD, bone mineral density; BTM, bone turnover marker; MS, metabolic syndrome; SDA, standard dietary advice; TRE, time‐restricted eating
Participants in the TRE group were asked to limit their eating to a 12‐hour time window of their choice, without recommendation for caloric and macronutrient intake or nutritional quality. Those in the active control group received SDA, which comprised a 10‐minute nutritional counseling at the randomization visit and the provision of a leaflet summarizing Swiss dietary recommendations for healthy eating, but no guidance in terms of the timing of their eating or dietary intake (for a detailed description see [33]). The randomized participants undertook an additional total body DXA scan and they were then followed up for 6 months, with two interim follow‐ups over the phone to reinforce the intervention and assess their compliance (Supporting Information Figure S1). At the closeout visit (6 months after randomization), participants had blood samples taken and they repeated all the assessments performed at baseline, including blood tests and total body DXA scans.
Clinical measurements, body composition, and bone mass assessment
Body weight was measured in light clothing using a digital scale at baseline and at the 6‐month follow‐up visit. Height was measured barefoot using a calibrated stadiometer. BMI was computed as weight in kilograms divided by height in meters squared. Total body composition (fat mass, fat‐free mass), BMC, and BMD were assessed at baseline and 6‐month follow‐up by DXA (GE Healthcare Lunar iDXA at Lausanne site, GE Healthcare Lunar Prodigy Advance at Bern site) following international guidelines [35].
Assessment of BTMs and bone‐related markers
Blood samples were obtained in the morning following an overnight fast (≥8 hours) at baseline and at 6‐month follow‐up. Serum β‐carboxyterminal telopeptide of type I collagen (CTX), P1NP, total 25‐hydroxyvitamin D, and parathyroid hormone were analyzed batchwise using Elecsys reagents (Roche Diagnostics, Rotkreuz, Switzerland). Insulinlike growth factor 1 (IGF‐1) levels were determined using an automated chemiluminescence‐based immunoassay (Immunodiagnostic Systems IDS‐iSYS Nordic).
Other assessments
Blood tests were analyzed for fasting glucose, high‐density lipoprotein cholesterol, and triglycerides using standard biochemistry assays. Blood pressure was measured three times with a calibrated monitor (Omron Intellisense BP monitor, Omron Healthcare) after 5 minutes of rest in the sitting position, and the average of the last two measurements was calculated. Physical activity levels were assessed by the short version of the International Physical Activity Questionnaire [36].
Statistical analysis
Given the relatively small sample size of the study, we used nonparametric tests. To account for the differences in the baseline values of the two groups, data were expressed as deltas (value at follow‐up − value at baseline) or percentage changes from baseline for all variables. The effects of allocated intervention (TRE vs. SDA) were evaluated by detecting differences in deltas/percentage changes between groups using the Mann–Whitney test. Data comparisons between pre‐ and post‐interventions within groups were carried out using the Wilcoxon signed rank test. We first assessed BTM absolute values (CTX concentration in ng/L and P1NP in μg/L). Because the reference ranges of BTMs differ according to sex, age, and menopausal status, we also normalized BTM levels by dividing the absolute concentrations by the upper bound of reference range provided by our local laboratory (CTX: men aged 30‐50 years: 158‐442 ng/L, men aged 50‐70 years: 104‐504 ng/L, men aged > 70 years: 164‐624 ng/L, premenopausal women: 62‐436 ng/L, postmenopausal women: 330‐782 ng/L; P1NP: men and premenopausal women: 15.1‐58.6 μg/L, postmenopausal women: 20.3‐76.3 μg/L). P values ≤ 0.05 were considered statistically significant. Statistical analyses were performed using Stata software, version 16.0 (StataCorp LLC, College Station, Texas).
RESULTS
Baseline characteristics
A total of 42 participants (76% women) with available BTMs and/or bone mass measurements were included in the present study (Figure 1; Table 1). At baseline, participants had a median age of 47 (interquartile range [IQR]: 31‐52) years and a median BMI of 27.8 (IQR: 24.9‐30.6) kg/m2. Approximately one in three participants was classified as having obesity (31%) and MS (29%) according to the International Diabetes Foundation definition [34]. There were no significant differences between intervention groups (TRE vs. SDA) in demographic, anthropometric, and body composition characteristics or lifestyle and metabolic factors at baseline. Overall, participants had baseline levels for CTX and P1NP within the reference range for age, sex, and menopausal status (Table 2) and a median total body BMD z score of 0.9 (range from −0.7 to 3.0). Baseline values of BTMs, bone‐related hormones, and total body BMC/BMD did not differ by intervention group (Table 2).
TABLE 1.
Baseline characteristics of the study population
| All (n = 42) | TRE (n = 23) | SDA (n = 19) | p value a | |
|---|---|---|---|---|
| Demographics | ||||
| Age (y) | 47 (31‐52) | 47 (32‐57) | 45 (27‐50) | 0.29 |
| Women, n (%) | 32 (76%) | 18 (78%) | 14 (74%) | 0.73 |
| Menopause, n (%) | 9 (28%) | 6 (33%) | 3 (21%) | 0.46 |
| Anthropometrics and body composition | ||||
| BMI (kg/m2) | 27.8 (24.9‐30.6) | 27.9 (25.5‐31.4) | 26.7 (23.8‐30.6) | 0.48 |
| Lean body mass (kg) | 42.8 (40.3‐48.3) | 43.7 (41.0‐48.3) | 42.3 (38.7‐47.7) | 0.45 |
| Total body fat (kg) | 27.7 (21.5‐36.7) | 27.5 (21.8‐37.9) | 28.7 (20.2‐34.8) | 0.45 |
| Total body fat (%) | 38.1 (32.2‐46.0) | 39.7 (32.2‐46.0) | 37.1 (32.5‐45.5) | 0.68 |
| Lifestyle factors | ||||
| Physical activity (MET‐minutes/wk) | 1173 (636‐1635) | 1377 (594‐2094) | 1036 (678‐1314) | 0.36 |
| Eating time window (h/d) | 15.3 (14.7‐16.0) | 15.4 (14.7‐16.0) | 15.1 (14.6‐15.6) | 0.30 |
| Current smokers, n (%) | 4 (10%) | 2 (9%) | 2 (11%) | 0.84 |
| Alcohol (units/wk) | 3.2 (0.6‐7.3) | 2.1 (0.6‐7.3) | 3.3 (0.8‐7.7) | 0.75 |
| Metabolic parameters | ||||
| Metabolic syndrome, n (%) b | 12 (29%) | 7 (30%) | 5 (26%) | 0.77 |
| Obesity, n (%) c | 13 (31%) | 6 (26%) | 7 (37%) | 0.45 |
| Systolic BP (mm Hg) | 127 (118‐132) | 128 (114‐132) | 124 (119‐134) | 0.87 |
| Diastolic BP (mm Hg) | 79 (75‐87) | 79 (75‐88) | 80 (74‐83) | 0.82 |
| HDL‐cholesterol (mmol/L) | 1.4 (1.2‐1.7) | 1.4 (1.2‐1.7) | 1.4 (1.2‐1.7) | 0.96 |
| Triglycerides (mmol/L) | 1.1 (0.9‐1.6) | 1.0 (0.9‐1.6) | 1.1 (0.8‐1.4) | 0.69 |
| Glucose (mmol/L) | 5.1 (4.8‐5.4) | 5.2 (4.9‐5.4) | 5.0 (4.8‐5.4) | 0.64 |
Note: Data are presented as median (interquartile range: 25th percentile‐75th percentile) for continuous variables or as n (%) for categorical variables.
Abbreviations: BP, blood pressure; HDL, high‐density lipoprotein; MET, metabolic equivalents; SDA, standard dietary advice; TRE, time‐restricted eating.
p values for the between‐group comparison at baseline.
Metabolic syndrome definition according to the International Diabetes Foundation [34].
Obesity defined as BMI ≥ 30 kg/m2.
TABLE 2.
Changes in BTMs, bone‐related hormones, and total BMC/BMD with TRE and SDA in the total population
| TRE | SDA | TRE | SDA | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow‐up | p value | Baseline | Follow‐up | p value | Percentage change | Percentage change | ||
| BTMs and bone‐related hormones a | |||||||||
| CTX | |||||||||
| Concentration (ng/L) | 391 (312, 466) | 366 (285, 462) | 0.13 | 395 (312, 486) | 413 (304, 534) | 0.45 | −4.0 (−16.7, 7.9) | 0.0 (−5.0, 22.1) | 0.13 |
| Normalized level (%) b | 78.2 (59.6, 89.7) | 67 (57.1, 84.6) | 0.09 | 78.1 (58.4, 93.1) | 85.1 (56.0, 120.8) | 0.34 | −3.9 (−14.5, 3.5) c | 0.0 (−2.9, 14.0) c | 0.09 |
| P1NP | |||||||||
| Concentration (μg/L) | 49.7 (39.8, 65.7) | 49.1 (38.1, 62.4) | 0.88 | 49.4 (43.3, 58.6) | 50.0 (37.2, 58.4) | 0.65 | −0.4 (−8.9, 14.8) | 1.1 (−24.4, 15.0) | 0.71 |
| Normalized level (%) b | 83.1 (61.8, 93.9) | 75.9 (65, 95.6) | 0.94 | 75.9 (69.6, 100) | 84.8 (62.5, 87.4) | 0.67 | −0.4 (−7.5, 8.7) c | 0.7 (−11.6, 11.1) c | 0.80 |
| PTH (pmol/L) | 4.15 (3.50, 5.04) | 4.16 (3.55, 4.63) | 0.59 | 3.83 (3.01, 4.34) | 3.91 (3.7, 4.6) | 0.27 | −1.7 (−16.8, 13.4) | 1.6 (−5.2, 19.9) | 0.21 |
| Vitamin D (nmol/L) | 51.9 (40.9, 68.7) | 48.5 (38.1, 57.8) | 0.53 | 52.5 (39.7, 69.7) | 52.6 (43.3, 61.8) | 0.44 | −5.6 (−42.6, 12.7) | 11.1 (−18.5, 42.7) | 0.38 |
| IGF‐1 (μg/L) | 157 (131, 205) | 151 (129, 204) | 0.03 | 168 (141, 189) | 176 (127, 193) | 0.74 | −8.2 (−14.3, −3.4) | −2.8 (−9.5, 16.0) | 0.16 |
| BMC and BMD by DXA d | |||||||||
| Total body BMC (g) | 2443 (2341, 2923) | 2464 (2333, 2901) | 0.68 | 2348 (2291, 2911) | 2380 (2298, 2872) | 0.21 | 0.1 (−0.5, 0.8) | −0.3 (−1, 0.4) | 0.22 |
| Total body BMD (g/cm d ) | 1.218 (1.172, 1.352) | 1.234 (1.162, 1.348) | 0.07 | 1.203 (1.148, 1.221) | 1.206 (1.150, 1.227) | 0.82 | 0.9 (−0.3, 1.6) | 0.0 (−0.9, 0.9) | 0.10 |
Note: Data are presented as median (interquartile range: 25th percentile, 75th percentile).
Abbreviations: BMC, bone mineral content; BMD, bone mineral density; BTM, bone turnover marker; CTX, β‐carboxyterminal telopeptide of type I collagen; DXA, dual‐energy x‐ray absorptiometry; IGF‐1, insulinlike growth factor 1; PTH, parathyroid hormone; P1NP, procollagen type I N‐terminal propeptide; SDA, standard dietary advice; TRE, time‐restricted eating.
Available BTM data for 39 participants (TRE: n = 22; SDA: n = 17).
BTM levels were normalized by dividing the absolute concentration value by the upper bound of reference range for age, sex, and menopausal status provided by our local laboratory.
Delta in normalized data (in percentage points).
Available BMD data in 33 participants (TRE: n = 18; SDA: n = 15).
Compliance with the study intervention and changes in body weight
Compliance with the study intervention and changes in main study outcomes have been previously described in detail [33]. In brief, for the participants included in this secondary analysis, the TRE group reduced their median eating window from 15.4 (IQR: 14.7‐16.0) h/d to 12.0 (11.8‐12.7) h/d (p < 0.0001), whereas no significant changes were seen in the eating window of the SDA group. Body weight significantly decreased with TRE (baseline median 79.1 [IQR: 68.0‐85.6] kg, follow‐up 77.7 [67.5‐84.0] kg, p = 0.035), but not with SDA (baseline 74.5 [68.0‐83.6] kg, follow‐up 72.4 [67.3‐84.3] kg, p = 0.13). Similar to our previous report [33], there was no significant between‐group difference in weight loss (p = 0.85). A closer look at individual body weight data revealed that participants responded variably to either intervention. Very few individuals lost >5 to 10 kg of body weight, although most participants experienced smaller body weight reductions, and a few others even gained some weight (Figure 2). In the present study, the median weight loss for both intervention arms pooled together was 0.6 kg. Physical activity levels did not change significantly in the TRE (baseline median 1377 [IQR: 594‐2094] metabolic equivalent task [MET]‐min/wk, follow‐up 618 [396‐1653] MET‐min/wk, p = 0.16) or the SDA groups (baseline median 1036 [IQR: 678‐1314] MET‐min/wk, follow‐up 1356 [462‐1800] MET‐min/wk, p = 0.88).
FIGURE 2.
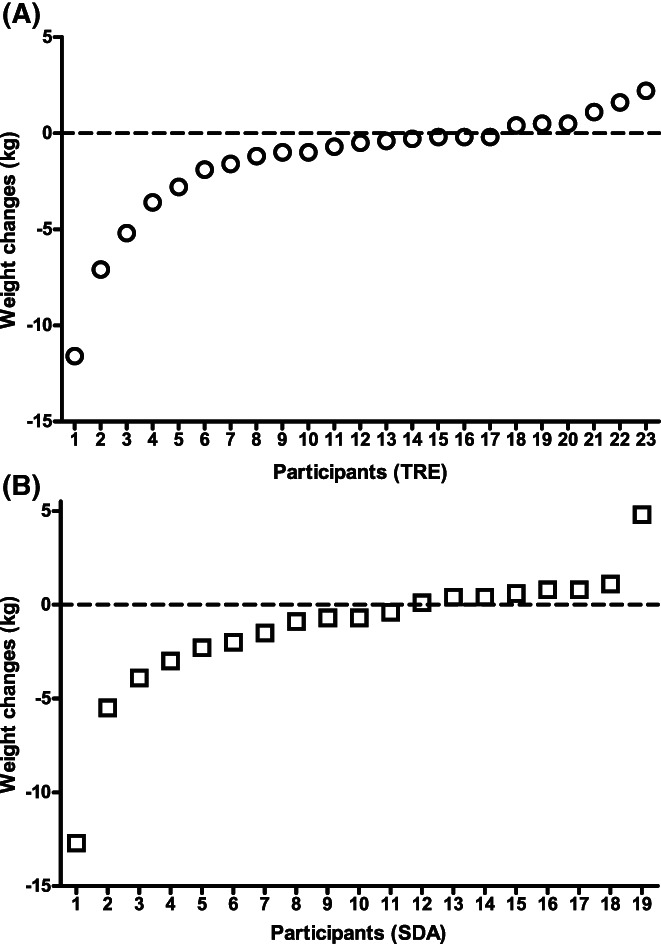
Individual body weight changes in response to 6 months of (A) TRE and (B) SDA, based on [33] and recomputed for participants included in this analysis. SDA, standard dietary advice; TRE, time‐restricted eating
Changes in BTMs, bone‐related hormones, and bone mass
Changes in BTMs, bone‐related hormones, and bone mass by DXA were compared within and between groups. In the total study population, there were no between‐group differences (TRE vs. SDA) in any bone outcome (Table 2). Given that smoking is a well‐established factor of osteoporosis, we repeated this analysis in nonsmokers (i.e., after excluding those who were current smokers, n = 4), and the results did not change (data not shown).
Given the variable body weight responses to both interventions, we then explored the effects of TRE and SDA on bone parameters in weight loss responders (weight loss greater than the median 0.6 kg) and nonresponders (weight loss <0.6 kg) separately.
In weight loss responders, changes in CTX (reflecting bone resorption) absolute concentrations (p = 0.050) and normalized levels (p = 0.041) differed significantly between groups (Figure 3A). This was largely due to a trend toward reduced bone resorption after TRE (p = 0.075; for all participants CTX levels remained within the reference range at the 6‐month follow‐up) and a nonsignificant increase in bone resorption after SDA (p = 0.14; 33% of the participants exceeded normal CTX values at the 6‐month follow‐up; Supporting Information Figure S2). There were no significant within‐group changes or between‐group differences for bone formation, as reflected by changes in P1NP absolute concentrations and normalized levels (Figure 3B). In further exploratory analyses of weight loss responders, we found no between‐group differences in calcium balance reflected in parathyroid hormone or vitamin D responses (Supporting Information Figure S3A‐S3B) or in IGF‐1 changes (Supporting Information Figure S3C). The differential CTX responses to TRE/SDA were accompanied by group differences in total body BMC changes (p = 0.028; Figure 3C), which decreased in the SDA group (p = 0.028) but not in the TRE group (p = 0.31; Supporting Information Figure S2). No further differences were observed between TRE and SDA arms for total body (Figure 3D) or regional BMD (data not shown) changes in weight loss responders.
FIGURE 3.
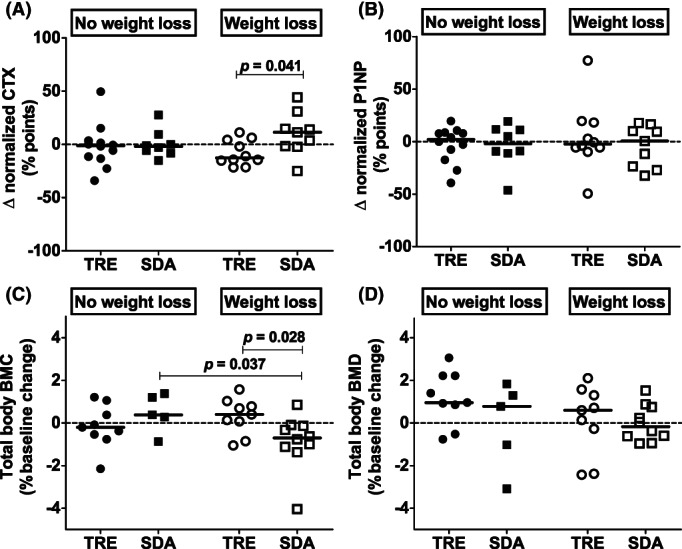
Changes in BTMs and total body BMC and BMD in weight loss responders and nonresponders by group. Individual data are shown, and solid black lines represent the median in each group: TRE weight loss nonresponders (black circles), SDA weight loss nonresponders (black squares), TRE weight loss responders (white circles), SDA weight loss responders (white squares). Only significant p values (≤0.05) are indicated for legibility. Normalized BTM levels were calculated by dividing the absolute value for CTX/P1NP by the upper bound of reference range for age, sex, and menopausal status provided by our local laboratory. BMC, bone mineral content; BMD, bone mineral density; CTX, β‐carboxyterminal telopeptide of type I collagen; P1NP, procollagen type 1 N‐terminal propeptide; SDA, standard dietary advice; TRE, time‐restricted eating
In contrast, among weight loss nonresponders, there were no differences between the TRE and SDA groups for any of the bone parameters assessed (Figure 3 and Supporting Information Figure S3), apart from different IGF‐1 responses (i.e., increased after SDA and decreased after TRE although these changes did not reach significance within groups; p = 0.025; Supporting Information Figure S3).
DISCUSSION
In the present study, we found no overall detrimental effects of 6 months of TRE on bone health outcomes. Those who lost weight following the control intervention (SDA) experienced small, albeit nonsignificant, increases in CTX levels without parallel changes in P1NP levels and a small loss of total body BMC. Conversely, when weight loss was induced by TRE, CTX levels tended to decrease and total body BMC was preserved. Although these findings suggest a possible benefit of TRE on bone during weight loss, it should be noted that our results reflect bone responses to a TRE intervention with a mildly restricted eating window (12 hours) that resulted in modest weight loss and apply to a population generally at a low risk for bone fragility and thus possibly less susceptible to bone catabolism.
Bone is a dynamic tissue that goes through continuous remodeling over the life‐span to maintain the structural/mechanical integrity of the skeleton and mineral homeostasis. During the remodeling process, old or damaged bone is removed by osteoclasts (bone resorption) and replaced with newly synthesized bone by osteoblasts (bone formation). Physiologically, these two processes are tightly coupled so that bone properties are preserved after each remodeling cycle. Among other factors (e.g., age, menopausal status, certain medications), weight loss may derail bone remodeling. In a meta‐analysis on diet‐induced weight loss, Zibellini et al. reported early increases (within 2‐3 months) in surrogate markers of bone resorption with less clear effects on markers of bone formation (increases in osteocalcin levels, no changes in P1NP levels) [19]. Hip BMD decreased by 1.0% to 1.5% in interventions with a duration ≥6 months, and total body BMD was reduced in interventions of 6 months (but not in interventions with a longer duration), whereas spine BMD remained largely unaltered.
In our study, SDA motivated participants in the control group to change their eating behavior. Our previous results suggest that some participants became more mindful of their eating habits, as indicated by their increased intake of unprocessed or minimally processed food and reduced consumption of processed food [33]. As a result of these dietary changes, some individuals in the control group experienced weight loss, which was accompanied by a nonsignificant increase in bone resorption, and small losses of total body BMC. In line with our findings, several other weight loss interventions resulting in comparable amounts of weight loss (3‐5 kg) have demonstrated unfavorable changes in BTMs and reductions in BMC/BMD (total body or at clinical sites) [37, 38, 39, 40]. Available literature provides further insights into the complex effects of weight loss on bone health. These appear dependent on several factors, with more pronounced effects observed with greater weight loss [41], when weight loss is achieved through caloric restrictions only (i.e., exercise may attenuate some of the negative effects of diet‐induced weight loss on bone) [22, 23], in case of suboptimal intakes of bone‐promoting nutrients [42, 43], and when the population under investigation is already susceptible to bone fragility (i.e., postmenopausal women and the elderly) [21, 22, 40].
Unlike conventional weight loss interventions, TRE deemphasizes food quantity and/or quality and allows ad libitum energy intake by simply focusing on the timing of food consumption. Thus, it has been suggested to be a less laborious, more flexible dietary approach compared with other weight loss approaches to improve metabolic health [2, 3, 11]. In our study, participants significantly lost weight after 6 months of TRE, albeit with substantial interindividual variability. We found that weight loss responders with TRE tended to have reduced bone resorption (CTX) whereas no change occurred in bone formation (P1NP). As opposed to the bone loss observed in weight loss responders with SDA, total body BMC/BMD remained unaltered in weight loss responders after TRE. It is unclear why bone responses to weight loss differed between the interventions in our study. Given that there were no pronounced differences in body composition, metabolic, and lifestyle factors between the intervention groups, we hypothesize that prolonged fasting (as per study design) and resynchronizing meals with circadian rhythms underpin the small benefits of TRE on bone during weight loss. This hypothesis is based on evidence that bone is responsive to circadian rhythmicity [13] and that TRE metabolic benefits may be at least partially explained by realignment of meal timing with circadian oscillators independently of weight loss [3, 6]; nevertheless, the exact mechanisms that may mediate bone preservation during TRE‐induced weight loss require elucidation in future studies.
Our study extends recent RCTs with shorter duration (6‐12 weeks) that have not shown undesirable effects of TRE on bone metabolism and/or total body bone mass in individuals with overweight/obesity [28, 29] or in the elderly [27]. Similarly, another form of IF, alternate day fasting (ADF), that involves 24 hours of complete fasting or ~25% of energy requirements followed by 24 hours of feasting has not been reported to affect BTMs or total body bone mass after 3 weeks [44] or 6 months [45]. In contrast, in another RCT, there was a significant decrease in lumbar spine BMD (−0.9%) within the ADF group, but no significant change in the control group (−0.5%) or between groups [46]. Although the latter study is dissimilar from our and other studies because ADF resulted in substantial reductions in caloric intake (by 37%) and rapid weight loss (3.5 kg in 4 weeks) in nonobese individuals, it indicates similar BMD changes in response to longer‐term periods of milder (continuous) energy restrictions [19, 24]. Taken together, most studies suggest that IF/TRE may not have adverse bone effects; however, the study by Stekovic et al. suggests that bone loss might occur if IF/TRE regimens are accompanied by severe caloric restriction [46], underpinning the importance of future studies of sufficient duration to explore this scenario.
Our study is strengthened by its RCT design, its pragmatic nature (i.e., an easy to follow, real‐world‐based intervention), a longer‐term duration (6 months) compared with the 4‐ to 12‐week duration of most available TRE interventions (for a review see [3]), and the assessment of BTMs (CTX, P1NP) according to international standards [47]. However, some limitations should also be acknowledged. The sample size was relatively small, and the study had few men and postmenopausal women, precluding meaningful comparisons of bone responses to TRE according to sex and menopausal status. In an effort to control for the effects of age, sex, and menopausal status, we presented normalized levels of BTMs for these factors and confirmed that our results were consistent for absolute concentrations and normalized levels. Overall, our findings apply to individuals at low fragility risk and thus may be less generalizable to populations at higher risk. BMD at reference sites for assessing osteoporotic risk (i.e., hip, lumbar spine) is unavailable and would have provided additional clinically relevant information. Finally, the lack of detailed information regarding changes in lifestyle factors (i.e., caloric intake and macronutrient distribution, intake of dietary calcium and vitamin D, and exercise characteristics, including frequency and mode [resistance/aerobic exercise]) that may affect bone parameters is a limitation of this work.
In conclusion, the present study suggests that, overall, 6 months of TRE does not have negative effects on bone metabolism (assessed by BTMs and bone‐related hormones) or bone loss (assessed by DXA total body BMC/BMD). When weight loss occurs, TRE might even be associated with small bone‐sparing effects compared with SDA. Future studies of longer duration (>6 months) assessing multiple bone phenotypes (e.g., BMD at clinically relevant sites, bone microstructure and fracture risk) are needed to confirm these findings and explore the effects of various TRE regimens (e.g., different eating windows, different timings), especially among individuals at risk for bone fragility such as postmenopausal women and the elderly.
AUTHOR CONTRIBUTIONS
Conceptualization, Maria Papageorgiou, Emmanuel Biver, Tinh‐Hai Collet; methodology, Maria Papageorgiou, Emmanuel Biver, Serge L. Ferrari, Tinh‐Hai Collet; data collection, Maria Papageorgiou, Julie Mareschal, Alexandra Hemmer, Emma Biolley, Nathalie Schwab, Elena Gonzalez Rodriguez, Daniel Aeberli, Didier Hans, Tinh‐Hai Collet; software (original study), Emily N.C. Manoogian, Satchidiananda Panda, Tinh‐Hai Collet; data analysis and interpretation, Maria Papageorgiou, Emmanuel Biver, Nicholas Edward Phillips, Elena Gonzalez Rodriguez, Nicolas Rodondi, Serge L. Ferrari, Tinh‐Hai Collet; data visualization, Maria Papageorgiou, Emmanuel Biver, Nicholas Edward Phillips, Serge L. Ferrari, Tinh‐Hai Collet; writing—original draft preparation, Maria Papageorgiou, Tinh‐Hai Collet; writing—review and editing, all; project administration, Maria Papageorgiou, Nathalie Schwab, Caroline Pot, Tinh‐Hai Collet; funding acquisition, Maria Papageorgiou, Nicholas Edward Phillips, Tinh‐Hai Collet. All authors have read and agreed to the published version of the manuscript.
FUNDING INFORMATION
This research was funded by the European Calcified Tissue Society (2021 ECTS Fellowship to Maria Papageorgiou), the Swiss National Science Foundation (Ambizione grant no. PZ00P3‐167826 to Tinh‐Hai Collet), the Swiss Society of Endocrinology and Diabetes (2017 Young Investigator prize to Tinh‐Hai Collet), and the Strategic Focal Area Personalized Health and Related Technologies of the Swiss Federal Institute of Technology in Zürich Domain (grant no 2018‐427 to Nicholas Edward Phillips). The funders had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
CONFLICT OF INTEREST
Emmanuel Biver has received fees from Nestlé for advisory boards outside the submitted work. Daniel Aeberli has received funds from Alfred und Anneliese Sutter‐Stöttner Stiftung, 6302 Zug Switzerland. Satchidananda Panda is the author of the books “The Circadian Code” and “The Circadian Diabetes Code.” Tinh‐Hai Collet's research is supported by grants from the Leenaards Foundation, the Vontobel Foundation, the SwissLife Jubiläumsstiftung Foundation, the Medical Board of the Geneva University Hospitals (HUG), the Nutrition 2000plus Foundation, and the Swiss Multiple Sclerosis Society. The other authors declared no conflict of interest.
Supporting information
Appendix S1 Supplementary Figures
Additional Supporting Information may be found in the online version of this article.
ACKNOWLEDGMENTS
The authors wish to thank all the participants of the study, the clinical teams who referred them to the study, and the staff at the Clinical Trial Unit, the Biobank, and the Lab at Lausanne University Hospital (CHUV) and Inselspital/University of Bern. We would also like to thank Dr. O. Golaz, Dr. I. Kern, and Dr. L. Peurière for their help with blood sample analysis. Open access funding provided by Universite de Geneve.
Papageorgiou M, Biver E, Mareschal J, et al. The effects of time‐restricted eating and weight loss on bone metabolism and health: a 6‐month randomized controlled trial. Obesity (Silver Spring). 2023;31(Suppl. 1):85‐95. doi: 10.1002/oby.23577
Funding information European Calcified Tissue Society, Grant/Award Number: 2021 ECTS Fellowship to M.P.; Strategic Focal Area Personalized Health and Related Technologies (PHRT) of the Swiss Federal Institute of Technology in Zürich (ETH) Domain, Grant/Award Number: 2018‐427 to N.E.P; Swiss National Science Foundation, Grant/Award Number: Ambizione grant no. PZ00P3‐167826 to T.‐H.C.; Swiss Society of Endocrinology and Diabetes, Grant/Award Number: 2017 Young Investigator prize to T.‐H.C
Contributor Information
Maria Papageorgiou, Email: maria.papageorgiou@unige.ch.
Tinh‐Hai Collet, Email: tinh-hai.collet@hcuge.ch.
DATA AVAILABILITY STATEMENT
The data presented in this study are available upon reasonable request to the corresponding author. The data are not publicly available for confidentiality reasons.
REFERENCES
- 1. Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018;362:770‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaix A, Manoogian ENC, Melkani GC, Panda S. Time‐restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39:291‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varady KA, Cienfuegos S, Ezpeleta M, Gabel K. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol. 2022;18:309‐321. [DOI] [PubMed] [Google Scholar]
- 4. Manoogian ENC, Chow LS, Taub PR, Laferrere B, Panda S. Time‐restricted eating for the prevention and management of metabolic diseases. Endocr Rev. 2022;43:405‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeb F, Wu X, Chen L, et al. Effect of time‐restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr 2020;123:1216‐1226. [DOI] [PubMed] [Google Scholar]
- 6. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time‐restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212‐1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22:789‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antoni R, Robertson TM, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time‐restricted feeding intervention on energy intake, adiposity and metabolic physiology in free‐living human subjects. J Nutr Sci. 2018;7:e22. [Google Scholar]
- 9. Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten‐hour time‐restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 2020;31:92‐104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hutchison AT, Regmi P, Manoogian ENC, et al. Time‐restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring) 2019;27:724‐732. [DOI] [PubMed] [Google Scholar]
- 11. Pellegrini M, Cioffi I, Evangelista A, et al. Effects of time‐restricted feeding on body weight and metabolism. A systematic review and meta‐analysis. Rev Endocr Metab Disord 2020;21:17‐33. [DOI] [PubMed] [Google Scholar]
- 12. Gabel K, Hoddy KK, Haggerty N, et al. Effects of 8‐hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging 2018;4:345‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swanson CM, Kohrt WM, Buxton OM, et al. The importance of the circadian system & sleep for bone health. Metabolism 2018;84:28‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrari SL, Abrahamsen B, Napoli N, et al. Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int 2018;29:2585‐2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong S, Chin K‐Y, Suhaimi F, Ahmad F, Ima‐Nirwana S. The relationship between metabolic syndrome and osteoporosis: a review. Nutrients. 2016;8:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Compston JE, Watts NB, Chapurlat R, et al.; Glow Investigators . Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 2011;124:1043‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patsch JM, Kiefer FW, Varga P, et al. Increased bone resorption and impaired bone microarchitecture in short‐term and extended high‐fat diet–induced obesity. Metabolism 2011;60:243‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zibellini J, Seimon RV, Lee CMY, et al. Does diet‐induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta‐analysis of clinical trials. J Bone Miner Res 2015;30:2168‐2178. [DOI] [PubMed] [Google Scholar]
- 20. Soltani S, Hunter GR, Kazemi A, Shab‐Bidar S. The effects of weight loss approaches on bone mineral density in adults: a systematic review and meta‐analysis of randomized controlled trials. Osteoporos Int. 2016;27:2655‐2671. [DOI] [PubMed] [Google Scholar]
- 21. Papageorgiou M, Kerschan‐Schindl K, Sathyapalan T, Pietschmann P. Is weight loss harmful for skeletal health in obese older adults? Gerontology. 2020;66:2‐14. [DOI] [PubMed] [Google Scholar]
- 22. Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one‐year randomized controlled trial. J Clin Endocrinol Metab. 2008;93:2181‐2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Villareal DT, Fontana L, Weiss EP, et al. Bone mineral density response to caloric restriction–induced weight loss or exercise‐induced weight loss. Arch Intern Med. 2006;166:2502‐2510. [DOI] [PubMed] [Google Scholar]
- 24. Villareal DT, Fontana L, Das SK, et al. Effect of two‐year caloric restriction on bone metabolism and bone mineral density in non‐obese younger adults: a randomized clinical trial. J Bone Miner Res. 2016;31:40‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prieto‐Alhambra D, Premaor MO, Fina Aviles F, et al. The association between fracture and obesity is site‐dependent: a population‐based study in postmenopausal women. J Bone Miner Res. 2012;27:294‐300. [DOI] [PubMed] [Google Scholar]
- 26. Armstrong ME, Cairns BJ, Banks E, et al. Different effects of age, adiposity and physical activity on the risk of ankle, wrist and hip fractures in postmenopausal women. Bone. 2012;50:1394‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martens CR, Rossman MJ, Mazzo MR, et al. Short‐term time‐restricted feeding is safe and feasible in non‐obese healthy midlife and older adults. Geroscience 2020;42:667‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lobene AJ, Panda S, Mashek DG. Time‐restricted eating for 12 weeks does not adversely alter bone turnover in overweight adults. Nutrients. 2021;13:1155. doi: 10.3390/nu13041155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lowe DA, Wu N, Rohdin‐Bibby L, et al. Effects of time‐restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity. JAMA Intern Med 2020;180:1491‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Papageorgiou M, Elliott‐Sale KJ, Parsons A, et al. Effects of reduced energy availability on bone metabolism in women and men. Bone 2017;105:191‐199. [DOI] [PubMed] [Google Scholar]
- 31. Papageorgiou M, Martin D, Colgan H, et al. Bone metabolic responses to low energy availability achieved by diet or exercise in active eumenorrheic women. Bone. 2018;114:181‐188. [DOI] [PubMed] [Google Scholar]
- 32. Grinspoon SK, Baum HB, Kim V, Coggins C, Klibanski A. Decreased bone formation and increased mineral dissolution during acute fasting in young women. J Clin Endocrinol Metab. 1995;80:3628‐3633. [DOI] [PubMed] [Google Scholar]
- 33. Phillips NE, Mareschal J, Schwab N, et al. The effects of time‐restricted eating versus standard dietary advice on weight, metabolic health and the consumption of processed food: a pragmatic randomised controlled trial in community‐based adults. Nutrients 2021;13:1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640‐1645. [DOI] [PubMed] [Google Scholar]
- 35. Petak S, Barbu CG, Yu EW, et al. The official positions of the International Society for Clinical Densitometry: body composition analysis reporting. J Clin Densitom. 2013;16:508‐519. [DOI] [PubMed] [Google Scholar]
- 36. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35:1381‐1395. [DOI] [PubMed] [Google Scholar]
- 37. Ramsdale SJ, Bassey EJ. Changes in bone mineral density associated with dietary‐induced loss of body mass in young women. Clin Sci (Lond). 1994;87:343‐348. [DOI] [PubMed] [Google Scholar]
- 38. Rector RS, Loethen J, Ruebel M, Thomas TR, Hinton PS. Serum markers of bone turnover are increased by modest weight loss with or without weight‐bearing exercise in overweight premenopausal women. Appl Physiol Nutr Metab. 2009;34:933‐941. [DOI] [PubMed] [Google Scholar]
- 39. Lucey AJ, Paschos GK, Cashman KD, Martinez JA, Thorsdottir I, Kiely M. Influence of moderate energy restriction and seafood consumption on bone turnover in overweight young adults. Am J Clin Nutr. 2008;87:1045‐1052. [DOI] [PubMed] [Google Scholar]
- 40. Chao D, Espeland MA, Farmer D, et al. Effect of voluntary weight loss on bone mineral density in older overweight women. J Am Geriatr Soc. 2000;48:753‐759. [DOI] [PubMed] [Google Scholar]
- 41. Harper C, Pattinson AL, Fernando HA, Zibellini J, Seimon RV, Sainsbury A. Effects of obesity treatments on bone mineral density, bone turnover and fracture risk in adults with overweight or obesity. Horm Mol Biol Clin Investig. 2016;28:133‐149. [DOI] [PubMed] [Google Scholar]
- 42. Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM. Diets higher in dairy foods and dietary protein support bone health during diet‐ and exercise‐induced weight loss in overweight and obese premenopausal women. J Clin Endocrinol Metab. 2012;97:251‐260. [DOI] [PubMed] [Google Scholar]
- 43. Jensen LB, Kollerup G, Quaade F, Sorensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res. 2001;16:141‐147. [DOI] [PubMed] [Google Scholar]
- 44. Templeman I, Smith HA, Chowdhury E, et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci Transl Med. 2021;13:eabd8034. doi: 10.1126/scitranslmed.abd8034 [DOI] [PubMed] [Google Scholar]
- 45. Barnosky A, Kroeger CM, Trepanowski JF, et al. Effect of alternate day fasting on markers of bone metabolism: an exploratory analysis of a 6‐month randomized controlled trial. Nutr Healthy Aging. 2017;4:255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stekovic S, Hofer SJ, Tripolt N, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non‐obese humans. Cell Metab. 2019;30:462‐476.e6. [DOI] [PubMed] [Google Scholar]
- 47. Vasikaran S, Cooper C, Eastell R, et al. International osteoporosis foundation and international federation of clinical chemistry and laboratory medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med. 2011;49:1271‐1274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary Figures
Additional Supporting Information may be found in the online version of this article.
Data Availability Statement
The data presented in this study are available upon reasonable request to the corresponding author. The data are not publicly available for confidentiality reasons.


