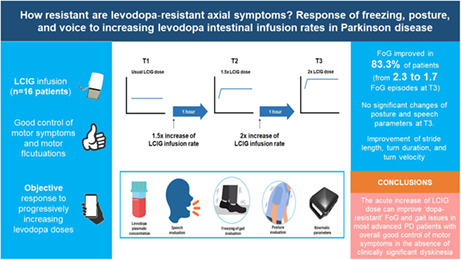
Abstract
Background and purpose
Treatment of freezing of gait (FoG) and other Parkinson disease (PD) axial symptoms is challenging. Systematic assessments of axial symptoms at progressively increasing levodopa doses are lacking. We sought to analyze the resistance to high levodopa doses of FoG, posture, speech, and altered gait features presenting in daily‐ON therapeutic condition.
Methods
We performed a pre‐/postinterventional study including patients treated with levodopa/carbidopa intestinal gel infusion (LCIG) with disabling FoG in daily‐ON condition. Patients were evaluated at their usual LCIG infusion rate (T1), and 1 h after 1.5× (T2) and 2× (T3) increase of the LCIG infusion rate by quantitative outcome measures. The number of FoG episodes (primary outcome), posture, speech, and gait features were objectively quantified during a standardized test by a blinded rater. Changes in motor symptoms, dyskinesia, and plasma levodopa concentrations were also analyzed.
Results
We evaluated 16 patients with a mean age of 69 ± 9.4 years and treated with LCIG for a mean of 2.2 ± 2.1 years. FoG improved in 83.3% of patients by increasing the levodopa doses. The number of FoG episodes significantly decreased (mean = 2.3 at T1, 1.7 at T2, 1.2 at T3; p = 0.013). Posture and speech features did not show significant changes, whereas stride length (p = 0.049), turn duration (p = 0.001), and turn velocity (p = 0.024) significantly improved on doubling the levodopa infusion rate.
Conclusions
In a short‐term evaluation, the increase of LCIG dose can improve "dopa‐resistant" FoG and gait issues in most advanced PD patients with overall good control of motor symptoms in the absence of clinically significant dyskinesia.
Keywords: axial symptoms, freezing of gait, levodopa, Parkinson disease, posture
INTRODUCTION
Freezing of gait (FoG) and other axial symptoms, encompassing disorders of speech, posture, gait, and balance, dominate the advanced phases of Parkinson disease (PD) and represent one of the major causes of patients' disability [1]. The management of these symptoms remains a challenge, because their response to dopaminergic therapies is complex [2, 3]. FoG is typically correlated with OFF periods and shows a good response to levodopa; however, FoG and other axial symptoms tend to become dopa‐resistant over time [4, 5, 6], being present also when the control of cardinal motor symptoms and fluctuations is adequate.
According to the response to levodopa, four different FoG conditions have been clinically described: “OFF‐FoG,” relieved by dopaminergic medications; “pseudo‐ON‐FoG,” appearing during a seemingly optimal ON state but improved by higher dopaminergic supply; “unresponsive‐FoG,” present in both OFF and ON states, independently from changes in dopaminergic medication; and “ON‐FoG,” a rare type of FoG induced by dopaminergic medications [7, 8]. Despite these clinically derived concepts of FoG pathophysiology, studies aiming to systematically evaluate FoG response to progressively increasing levodopa doses are lacking [9, 10]. Similarly, other axial symptoms like postural abnormalities and speech impairment have an uncertain response to levodopa that current literature does not help to clarify [4, 5, 9, 11, 12].
We hypothesize that FoG and other axial symptoms presenting in the daily‐ON condition in advanced PD patients could have in most cases a higher threshold for improvement than cardinal motor symptoms, relieved by higher doses of levodopa. Thus, in this study we evaluated PD patients treated with levodopa/carbidopa intestinal gel (LCIG) infusion to analyze the actual resistance to levodopa of FoG and other axial symptoms presenting in ON therapeutic condition by assessing their changes at progressively increased levodopa doses.
METHODS
Study population
Sixteen consecutively consenting PD patients treated with LCIG infusion continuously delivered via a percutaneous endoscopic gastrostomy with a jejunal extension were recruited from the Movement Disorder Unit of the University of Turin (Italy) and Bologna (Italy) between April and October 2021. All patients had good control of motor fluctuations and a stable LCIG infusion dose for at least 3 months.
Inclusion criteria were idiopathic PD [13], Hoehn and Yahr (H&Y) score between 2 and 3 in ON therapeutic condition [14], and a recent history of regular and disabling FoG in daily‐ON condition. To further improve the probability of including patients showing episodes of FoG during the experimental evaluation, we also screened and selected among patients reporting regular daily FoG those reporting FoG episodes several times a day in the past month, according with a score of 1 on Question 1 and score ≥ 2 on Question 2 of the New Freezing of Gait Questionnaire [15, 16].
Exclusion criteria were a diagnosis of severe cognitive decline according to a Mini‐Mental State Examination (MMSE) score ≤ 18 [17]; previous evidence of severe adverse effects with high levodopa doses, such as psychosis, hallucinations, painful dyskinesia, severe orthostatic hypotension, and digestive symptoms; inability to walk independently for 10 m in therapeutic ON condition; ineffective control of motor complications (Movement Disorder Society–Unified Parkinson's Disease Rating Scale [MDS‐UPDRS] Part IV ≥ 13) [18]; and previous or concomitant conditions other than PD known to negatively affect gait, posture, or speech.
Study design
We performed a pre‐/postinterventional study consisting of a systematic, quantitative, blinded evaluation of axial symptoms during a single session of progressively increased LCIG infusion rate. All patients were evaluated in the morning, after the morning bolus dose and at least 2 h of LCIG infusion therapy at their usual dose. The assessment was performed in three consecutive different therapeutic regimens: T1, at the patient's usual LCIG continuous infusion rate (daily‐ON); T2, 1 h after 1.5× increase of the LCIG infusion rate; and T3, 1 h after 2× increase of the LCIG infusion rate (Figure 1).
FIGURE 1.
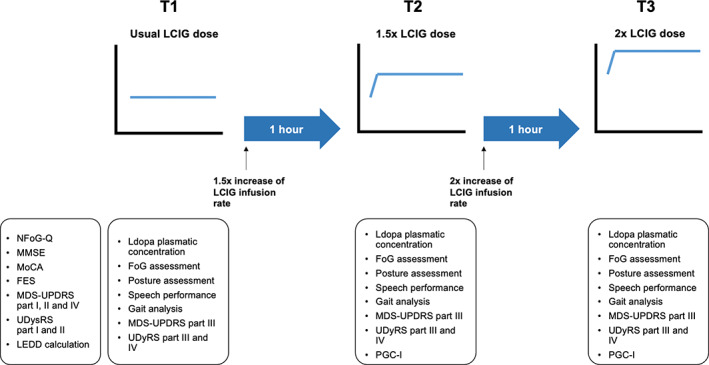
Flowchart of the assessment performed. FES, Falls Efficacy Scale; FoG, freezing of gait; LCIG, levodopa/carbidopa intestinal gel; LEDD, levodopa equivalent daily dose; MDS‐UPDRS, Movement Disorder Society–Unified Parkinson's Disease Rating Scale; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; NFoG‐Q, New Freezing of Gait Questionnaire; PGC‐I, Patient Global Impression of Change; UDysRS, Unified Dyskinesia Rating Scale
Endpoints
As primary endpoint, we assessed FoG improvement, defined as the blinded, video‐assessed reduction in the count of FoG episodes during a video‐recorded 2‐minute walk test (2MWT) performed at the usual infusion rate (T1) and after doubling the LCIG infusion rate (T3).
As secondary endpoints, we evaluated the T1 versus T3 improvement of (i) posture, objectively evaluated by the blinded software‐based calculation of postural angles from standardized patient pictures; and (ii) speech, defined as changes in monopitch, monoloudness, pauses, and rate of speech timing evaluated by vocal analysis of recorded voices while performing standardized reading performances.
As exploratory endpoint, we analyzed gait parameters in a subgroup of 12 patients performing an instrumented 2MWT and Timed Up and Go (TUG) test by using validated inertial motion sensors (Opal, APDM's Mobility Lab system).
Procedure
General evaluation of patients
Each patient was first evaluated for PD stage as per the H&Y score, FoG presence and impact as per the New Freezing of Gait Questionnaire [15], falls as per the Falls Efficacy Scale [19], PD symptoms and their fluctuations as per the MDS‐UPDRS Parts I–IV [18], axial score as per the sum of MDS‐UPDRS items 3.1, 3.3, 3.9, and 3.10–3.13 [3], PD phenotype (tremor dominant, postural instability/gait difficulty, indeterminate) [20], dyskinesia as per the Unified Dyskinesia Rating Scale (UDysRS) Parts I–IV [21], cognitive performances as per the MMSE [17] and the Montreal Cognitive Assessment [22], and levodopa equivalent daily dose calculated according to a validated conversion table [23], and with the more recent proposed levodopa equivalent dose conversions factors for opicapone and safinamide [24].
Evaluation of FoG episodes
A video‐recorded 2MWT was conducted in adequate space to allow for a 10‐m walk, back and forth in a straight line and performing tight turnings. The camera was placed vertically on a tripod, facing the patient at an adequate distance to capture the entire patient's figure and allow for detection of all FoG episodes during the video assessment. A rater expert in movement disorders not involved in the clinical assessment used videos to rate the number of FoG episodes blinded for the patient's therapeutic condition [16, 25]. This evaluation was performed at T1, T2, and T3.
Evaluation of postural abnormalities
Six photos were taken for each patient to determine the angles of forward trunk flexion/lateral trunk flexion (FTF/LTF): two photos in the frontal plane (framing the posterior side of the body) and four in the sagittal plane (two framing the right side and two the left side of the body). The first of each pair of photos was captured with the patient in a relaxed standing posture and the second one during the best effort to maintain a straight posture. Photos were collected for postural evaluation as previously reported [26]. We measured the degrees of trunk flexion on the frontal and sagittal plane according to the following protocol. Angles of FTF with lumbar fulcrum, FTF with thoracic fulcrum, and LTF were calculated according to validated software‐based methods using free software (http://www.neuroimaging.uni‐kiel.de/NeuroPostureApp) [27]. This evaluation was performed at T1, T2, and T3.
Evaluation of speech
A reading performance with a phonetically balanced text was recorded in a quiet hospital room using a digital voice recorder [4]. All speech samples were copied to a computer, edited into individual files, and analyzed by a biomedical engineer blinded to the participants' therapeutic condition using an algorithm for speech analysis [28]. Vocal signals underwent a preprocessing step using the software Praat (https://www.fon.hum.uva.nl/praat/). The recordings were first downsampled to 16 kHz and a denoising filter with Praat default hyperparameters was applied to each signal; the signal amplitude was normalized in the range 0–1 to compensate the speaker‐microphone distance. Initial and final silence regions were manually removed. Finally, we extracted five features characterized by high interpretability to describe speech samples. Measures from the entire vocal signal includes monoloudness (i.e., the SD of the signal intensity) and timing measures to detect changes in the rhythmic organization of the speech [29], namely, the number of pauses (NP), the duration of pause intervals (DPI), defined as the median duration of unvoiced intervals exceeding 30 ms, and the rate of speech timing (RST), proposed to evaluate the capability of alternating voiced, unvoiced, and paused regions [30]. After identifying and merging voiced regions, we evaluated the monopitch, that is, the SD of the fundamental frequency [29]. This evaluation was performed at T1, T2, and T3.
Gait and balance analysis
Twelve patients underwent an additional explorative instrumented evaluation of gait and balance parameters using wearable inertial sensors (Opal, APDM's Mobility Lab system) placed at multiple points in the upper and lower body (two sensors attached on the feet, two at outer surface of the thighs, one at right hip joint, one on the sternum, and one at lumbar level). The tasks consisted of a battery of standardized motion tests:
2MWT, as described in Section 2.4.2;
TUG test, with subjects asked to stand up from a chair, walk 3 m straight, turn around, walk back, and sit down, at a comfortable pace;
360° Turn Test, with patients required to start from standing position, make a turn 360° clockwise, and as soon as they return to the initial position, 360° counterclockwise; and
Sway test, with patients asked to remain in balance during an upright standing position for 30 s, with arms at rest and eyes closed.
This evaluation was performed at T1, T2, and T3.
Levodopa plasma levels
The pharmacokinetic–dynamic assessment of levodopa plasma concentration was performed for each patient by a blood venous sample (2 ml) drawn by an indwelling catheter at T1, and then at T2 and T3, 60 min after every change of the infusion rate. Blood specimens were collected and processed for plasma levodopa concentration as previously reported [31].
Clinical scale assessment
Finally, severity of motor symptoms and dyskinesia were collected at T1, T2, and T3 by means of MDS‐UPDRS Part III and UDysRS Parts III and IV, and the Patient Global Impression of Change (PGI‐C) scale was used at T2 and T3 to evaluate the patients' subjective perception of improvement, stability, or worsening of their clinical state.
Sample size calculation and statistical analysis
For the sample size calculation, we relied on a previous study with a similar experimental setting and the same outcome measure reporting an effect size of 1.1 on video‐assessed episodes of FoG [16]. Considering 80% power at 5% level of significance, we estimated that a sample size of 10 patients was sufficient to address the primary endpoint.
Descriptive statistics (mean ± SD) were used for continuous variables and frequency for categorical data. The nonparametric Freidman test was used to compare results at the three different therapeutic conditions, and Bonferroni correction for multiple comparisons was applied. All test reported were two‐tailed, and a p‐value ≤ 0.05 was considered to be statistically significant. Data were analyzed using the Statistical Package for the Social Sciences (SPSS 27).
The study conforms to World Medical Association Declaration of Helsinki principles and was approved by the local institutional review board "Città della Salute e della Scienza di Torino," and all patients gave their written informed consent to participate in the study.
RESULTS
Sixteen PD patients (10 males, 6 females) with a mean age of 69 ± 9.4 years and treated with LCIG for a mean of 2.2 ± 2.1 years were enrolled in the study. LCIG infusion mean morning dose, continuous dose, and total dose were 7.6 ± 2.6 ml, 2.8 ± 0.9 ml/h, and 49.1 ± 14.6 ml, respectively, with a mean of 15.1 ± 1.7 h of daily infusion therapy. In addition to LCIG, eight patients were treated with dopamine agonists, two with amantadine, two with opicapone, and one with safinamide. All demographic, clinical, and therapeutic data of the cohort are detailed in Table 1. All patients completed the three study phases. One patient could not complete the voice assessment due to very severe dysarthria.
TABLE 1.
Demographic and clinical features of enrolled patients
| Feature | Value |
|---|---|
| Gender, males/females | 10/6 (62.5%/37.5%) |
| Age, years | 69.0 ± 9.4 (57–80) |
| Age at onset of disease, years | 52.8 ± 8.9 (36–63) |
| Disease duration, years | 15.9 ± 6.6 (8–28) |
| LCIG duration, years | 2.2 ± 2.1 (1–8) |
| Clinical phenotype, n (%) | |
| PIGD | 8 (50%) |
| Tremor dominant | 2 (12.5%) |
| Indeterminate | 6 (37.5%) |
| LEDD, mg | 1317.9 ± 296.2 (885.3–1992.8) |
| LCIG infusion | |
| Morning dose, ml | 7.6 ± 2.6 (0–11) |
| Continuous dose, ml/h | 2.8 ± 0.9 (1.6–4.6) |
| Total daily dose, ml | 49.1 ± 14.6 (29.6–80) |
| MMSE | 25.4 ± 3.1 (19–30) |
| MoCA | 21.4 ± 4.1 (15–28) |
| New Freezing of Gait Questionnaire | 18.6 ± 5.9 (10–31) |
| Falls Efficacy Scale | 32.6 ± 18.4 (10–73) |
| MDS‐UPDRS I score | 10.5 ± 5.0 (0–20) |
| MDS‐UPDRS II score | 17.3 ± 5.0 (10–30) |
| MDS‐UPDRS IV score | 8.5 ± 2.9 (3–12) |
| MDS‐UPDRS Item 4.1 | 2.1 ± 1.4 (0–4) |
| MDS‐UPDRS Item 4.2 | 1.3 ± 0.8 (0–2) |
| MDS‐UPDRS Item 4.3 | 1.5 ± 0.6 (1–3) |
| MDS‐UPDRS Item 4.4 | 1.5 ± 0.8 (0–3) |
| UDysRS I total score | 13.6 ± 8.4 (0–25) |
| UDysRS II total score | 5.3 ± 3.0 (0–9) |
Note: Results are reported as average ± SD (range) or absolute values (percentage), as appropriate.
Abbreviations: LCIG, levodopa/carbidopa intestinal gel; LEDD, levodopa equivalent daily dose; MDS‐UPDRS, Movement Disorder Society–Unified Parkinson's Disease Rating Scale; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; PIGD, postural instability/gait difficulty; UDysRS, Unified Dyskinesia Rating Scale.
Plasma levodopa concentrations showed a significant progressive increase at each study phase, with levodopa levels ranging from a mean of 3.2 ± 2.6 μg/ml at T1 to 3.6 ± 2.7 μg/ml at T2, and 4.6 ± 2.9 μg/ml at T3 (p < 0.001).
Changes in FoG episodes, postural angles, and speech
Considering that 12 patients showed at least one FoG episode at T1, the rate of patients with a reduction of FoG episodes was 75% at T2 (n = 9/12) and 83.3% at T3 (n = 10/12). Three patients showed an increase of FoG episodes at T2 and one patient at T3.
On average, we observed in the entire cohort of 16 patients a significant progressive improvement of FoG episodes at each study phase (p = 0.013), ranging from a mean of 2.3 ± 2.3 at T1 to 1.7 ± 2.3 at T2 and 1.2 ± 2.2 at T3 (Table 2, Figure 2).
TABLE 2.
Axial symptoms outcomes
| Symptom type | Outcome | T1 | T2 | T3 | p |
|---|---|---|---|---|---|
| Freezing of gait | Episodes, n | 2.3 ± 2.3 | 1.7 ± 2.3 | 1.2 ± 2.2 | 0.013 a |
| Type of episodes b |
0.1 ± 0.3 start 2.2 ± 2.2 turn 0 linear |
0.1 ± 0.3 start 1.4 ± 2 turn 0.2 ± 0.8 linear |
0.1 ± 0.2 start 0.7 ± 1 turn 0.4 ± 1.5 linear |
n.a. | |
| Pattern of episodes b |
1.8 ± 1.7 trembling 0.3 ± 0.5 shuffling 0.3 ± 0.6 akinesia |
1.3 ± 1.9 trembling 0.2 ± 0.5 shuffling 0.3 ± 0.8 akinesia |
0.6 ± 0.9 trembling 0.2 ± 0.8 shuffling 0.4 ± 1.1 akinesia | n.a. | |
| Posture | LTF relaxed, ° | 7.0 ± 15.5 | 6.3 ± 13.3 | 6.1 ± 12.2 | 0.74 |
| LTF straight, ° | 3.9 ± 8.5 | 4.9 ± 10.9 | 4.6 ± 9.2 | 0.195 | |
| Thoracic FTF relaxed, ° | 44.0 ± 10.3 | 43.4 ± 7.5 | 43.0 ± 10.6 | 0.819 | |
| Lumbar FTF relaxed, ° | 13.9 ± 14.5 | 13.3 ± 14.2 | 13.5 ± 17.0 | 0.164 | |
| Thoracic FTF straight, ° | 39.3 ± 8.6 | 41.3 ± 7.4 | 39.1 ± 7.2 | 0.121 | |
| Lumbar FTF straight, ° | 11.0 ± 11.8 | 10.8 ± 12.0 | 11.0 ± 12.9 | 0.725 | |
| Speech | Monopitch, Hz | 40.9 ± 27.2 | 48.3 ± 28.7 | 43.9 ± 30.5 | 0.57 |
| Monoloudness, dB | 15.0 ± 3.9 | 15.0 ± 3.5 | 15.6 ± 3.1 | 0.247 | |
| Pauses, n | 242.1 ± 77.7 | 243.1 ± 85.3 | 233.6 ± 75.9 | 0.766 | |
| DPI, ms | 0.1 ± 0.2 | 0.1 ± 0.02 | 0.1 ± 0.1 | 0.766 | |
| RST, intervals/min | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.155 | |
| MDS‐UPDRS Item 3.1 (speech) | 1.6 ± 0.7 | 1.7 ± 0.7 | 1.4 ± 0.6 | 0.039 a |
Note: Results are reported as average ± SD.
Abbreviations: DPI, duration of pause intervals; FTF, forward trunk flexion; LTF, lateral trunk flexion; MDS‐UPDRS, Movement Disorder Society–Unified Parkinson's Disease Rating Scale; n.a., not applicable; RST, rate of speech timing.
Statistically significant.
Types and patterns of freezing of gait episodes have been reported based on video analysis according to the classification proposed in Nutt et al. [8].
FIGURE 2.
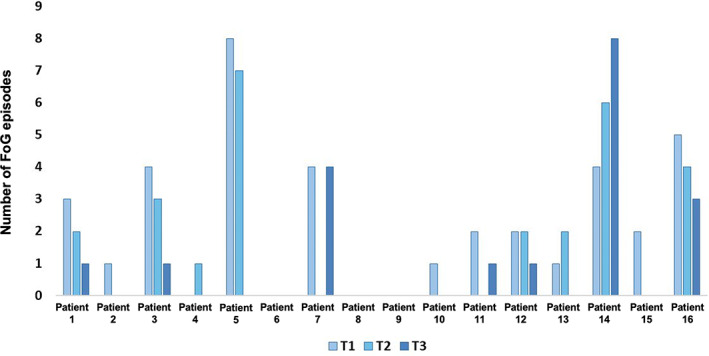
Freezing of gait (FoG) episodes for each patient at different timepoints. T1, patient's usual levodopa/carbidopa intestinal gel (LCIG) continuous infusion rate (daily‐ON); T2, 1 h after 1.5× increase of the LCIG infusion rate; T3, 1 h after 2× increase of the LCIG infusion rate
Regarding posture, we found no significant improvement in relaxed or in straight standing position for lumbar FTF (p = 0.164 relaxed; p = 0.725 straight), thoracic FTF (p = 0.819 relaxed; p = 0.121 straight), and LTF (p = 0.74 relaxed; p = 0.195 straight) at different infusion rates (Table 2). No significant differences were found also regarding speech parameters, namely monopitch (p = 0.570), monoloudness (p = 0.247), NP (p = 0.766), DPI (p = 0.766), and RST (p = 0.155; Table 2).
Motor symptoms, dyskinesia, and patient global impression
Motor symptoms evaluated by the MDS‐UPDRS Part III did not show statistically significant changes from T1 to T3 (p = 0.091), with scores ranging from a mean of 31.7 ± 11.7 at T1 to 29 ± 11.7 at T2 and 27.3 ± 13.4 at T3. Similarly, the axial score showed a slight improvement from a mean of 8.8 ± 3.3 at T1 to 8.1 ± 3.6 at T2, and 7.9 ± 3.9 at T3 in the absence of a statistically significant difference (p = 0.159).
Dyskinesia showed a significant increase between T1 and T3, with UDysRS Part III ranging from a mean of 7.1 ± 5 at T1 to 7.6 ± 4.4 at T2 and 9.4 ± 5 at T3 (p = 0.005), and UDysRS Part IV from 5.4 ± 3.0 at T1 to 5.9 ± 3.2 at T2 and 6.6 ± 4 at T3 (p = 0.001; Figure 3).
FIGURE 3.
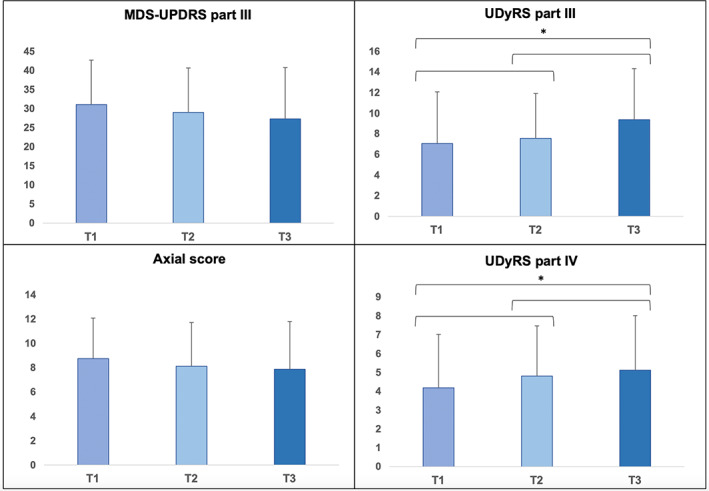
Motor, axial, and dyskinesia scores. *Statistically significant. MDS‐UPDRS, Movement Disorder Society–Unified Parkinson's Disease Rating Scale; T1, patient's usual levodopa/carbidopa intestinal gel (LCIG) continuous infusion rate (daily‐ON); T2, 1 h after 1.5× increase of the LCIG infusion rate; T3, 1 h after 2× increase of the LCIG infusion rate; UDysRS, Unified Dyskinesia Rating Scale
Regarding patient's impression of treatment efficacy, the self‐administered PGI‐C score at T3 reported eight cases of improvement from T1 (score < 4), three cases of no change (score = 4), and five cases of worsening (score > 4). The mean PGI‐C score was 3.9 ± 1 at T2 and 3.8 ± 1.3 at T3.
No significant side effects were observed during the study, with the exception of two cases of discomfort due to transient nausea and lipothymia not preventing the completion of the study protocol. Additionally, one patient experienced an episode of fall during the 2MWT.
Exploratory analysis of gait and balance
Twelve patients underwent gait and balance analysis using wearable inertial sensors. Significant improvements were observed between T1 and T3 for stride length during the 2MWT (p = 0.049), turn duration (p = 0.001), and turn velocity during the TUG test (p = 0.024). A trend of improvement between T1 and T3 was found for gait speed and double support during the 2MWT, for turn angle during the TUG test, and for turn velocity during the 360° Turn Test; step duration during the 2MWT, sit to stand duration during the TUG test, sway area, and acceleration showed a trend of worsening without reaching statistical significance (Table 3).
TABLE 3.
Gait and balance exploratory analysis
| Measure | T1 | T2 | T3 | p |
|---|---|---|---|---|
| 2MWT–gait speed, m/s | 0.7 ± 1.6 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.205 |
| 2MWT–double support, %GCT | 26.4 ± 6 | 26.2 ± 5.8 | 25.6 ± 5.5 | 0.125 |
| 2MWT–step duration, s | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.717 |
| 2MWT–stride length, m | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.049 a |
| TUG–duration, s | 16.3 ± 3.6 | 17.6 ± 4.6 | 17.3 ± 8.1 | 0.297 |
| TUG–angle, ° | 175.8 ± 7.2 | 168.8 ± 7.9 | 156.8 ± 36.6 | 0.064 |
| TUG–turn duration, s | 2.9 ± 0.5 | 2.6 ± 0.5 | 2.4 ± 0.5 | 0.001 a |
| TUG–turn velocity, m/s | 132.2 ± 25.7 | 148.1 ± 41.8 | 156.5 ± 59.9 | 0.024 a |
| TUG–sit to stand duration, s | 1 ± 0.2 | 1.2 ± 0.6 | 1.2 ± 0.7 | 0.558 |
| 360°–turn duration, s | 5.0 ± 1.5 | 4.7 ± 1.5 | 4.3 ± 1.0 | 0.076 |
| 360°–turn velocity, m/s | 119.3 ± 37.8 | 127.2 ± 26.3 | 131.7 ± 36.0 | 0.455 |
| Sway–area, m2 | 20.1 ± 34.6 | 26.0 ± 36.0 | 32.2 ± 43.2 | 0.105 |
| Sway–acceleration, m/s2 | 0.6 ± 1.0 | 0.8 ± 1.1 | 0.9 ± 1.2 | 0.105 |
Note: Results are reported as average ± SD.
Abbreviations: 2MWT, 2‐minute walk test; GCT, gait cycle time; TUG, Timed Up and Go.
Statistically significant.
DISCUSSION
In this study, we quantitatively analyzed the response of FoG, posture, and speech to increased levodopa doses in 16 LCIG‐treated PD patients presenting disabling axial symptoms despite good control of cardinal motor symptoms and fluctuations. FoG episodes, quantified by a movement disorder expert‐blinded for the therapeutic condition, significantly improved after doubling the levodopa infusion rate, whereas the objective analysis of posture and speech did not show significant changes. Gait analysis, performed in a subgroup of 12 patients, showed a significant improvement in stride length, turn duration, and turn velocity after doubling the levodopa infusion rate. As expected, the MDS‐UPDRS part III score did not differ in the three therapeutic conditions, as all patients had good control of main motor symptoms in the daily‐On condition. Dyskinesia showed mild yet statistically significant worsening from T1 to T3, with UDysRS Part III scores ranging from 7.1 to 9.4; the change we observed was below the threshold of 2.7 points to discriminate a clinically important worsening of dyskinesia [32], and the therapy increase was well tolerated in all patients, except for two cases of transient malaise.
To our knowledge, the response of FoG to progressively increased levodopa doses has never been systematically investigated, and data provided in our study offer experimental validity to the hypothesis of the existence of four different FoG types clinically described so far, namely OFF‐FoG, pseudo‐ON‐FoG, unresponsive‐FoG, and ON‐FoG [7, 8]. In our study, the number of FoG episodes improved in all but two patients with a twofold increase of the LCIG infusion rate. The two patients who did not improve doubling the infusion rate showed substantial differences; one patient improved from T1 to T2 (from four to zero FoG episodes) and then worsened from T2 to T3 (from zero to four FoG episodes); the other patient showed a linear increase in FoG episodes from T1 to T3 (four episodes at T1, six at T2, and eight at T3; Figure 2).
These data suggest that, in most advanced patients treated with LCIG, episodes of FoG presenting in a practical daily‐ON condition can benefit from an increase in the levodopa dose, pointing toward a relative rather than an absolute FoG resistance to levodopa. Conversely, in a few cases, our data support the hypothesis of the existence of rare cases of FoG induced by high doses of levodopa, with a different threshold for each patient.
A role of maladaptive plasticity caused by long‐term levodopa treatment, resulting in a mismatch between understimulated motor loops and activated nonmotor loops, could be implicated in the difficulty of managing axial symptoms in advanced PD phases, so that increasingly higher dopamine concentrations are required to overcome thresholds of postsynaptic receptors within the motor circuitry [33]. Thus, a new activation of aberrant loops could be overcome by a substantial increase in the dopaminergic dose or by a different type of stimulation of the dopaminergic networks (e.g., deep brain stimulation or LCIG). A certain degree of efficacy in treating FoG by continuous dopaminergic stimulation has already been described as superior to the pulsatile effect typical of oral therapy even in the absence of significant dose changes [34, 35, 36].
Another interesting hypothesis is the existence of a fifth FoG type, the so‐called “triphasic‐ON” FoG, arising in both ON and supra‐ON states, but with the presence of a narrow ON therapeutic window in the transition between these two states where FoG improves, difficult to achieve with oral therapy due to the unpredictability of actual levodopa plasma levels [9].
In parallel with the improvement of FoG, the gait analysis in our study demonstrated an improvement of stride length, turn duration, and turn velocity, confirming the strict relationship between FoG and specific gait parameters [37, 38, 39] and supporting the theory of a higher threshold for the improvement of axial motor symptoms when compared with appendicular symptoms like limb tremor, rigidity, and bradykinesia.
Despite a small trend in postural angles, our study suggests that other axial symptoms like postural abnormalities and speech impairment cannot be satisfactorily improved (at least acutely) by increasing the levodopa dose in patients with already adequate control of motor symptoms and fluctuations. The complex multilevel dysfunction underlying postural abnormalities (i.e., abnormal sensory–motor integration, cognitive dysfunctions, musculoskeletal system involvement) could explain the scarce improvement early after the change of therapeutic doses and should be probably better analyzed after a few days of treatment at increased levodopa doses [40, 41]. Interestingly, previous studies did not show any difference in terms of posture angles also after chronic treatment with LCIG [42], making the treatment of these symptoms a challenge and providing clues for a different physiopathology among different axial symptoms.
Likely, the analysis of speech did not show significant differences in the three study phases. No data about the effect of the LCIG therapy on speech are currently available, and evidence about oral levodopa indicates various and unpredictable modifications in phonation and language articulation in mild to moderate PD in both the short and long term [43, 44]. Current data on oral levodopa treatment proved a great variability of speech alteration profile, rendering it difficult to understand the effect of levodopa on speech in PD. At a group level, a trend toward a greater improvement in phonation and articulation can be observed with levodopa treatment, especially in the early stages of the disease [44]. In later disease stages, the effect of nondopaminergic mechanisms on speech, particularly prosody and fundamental frequency variability, could be more prominent and justify the mixed literature results on the effect of dopaminergic therapy on speech [45, 46]. Likely, our study on advanced PD patients proved a high heterogeneity in baseline and dose‐augmented assessments, indicating variable improvement and worsening of different speech parameters varying from patient to patient, thus preventing the observation of a group trend toward an efficacy of higher LCIG infusion rates on speech. On the other hand, studies including patients treated with deep brain stimulation of the subthalamic nucleus showed that the benefit on motor symptoms provided by the chronic stimulation is typically not associated with improvement of speech, which rather may deteriorate as a consequence of increased dysarthria due to the current spreading to adjacent areas, such as the corticobulbar tract or pallidofugal and cerebellar fibers [45].
The limitations of this work are mainly related to the lack of a long‐term evaluation of the efficacy and tolerability of high doses of levodopa to overcome axial symptoms, and the relatively small sample size, adequate for the assessment of FoG episodes but probably not sufficient to disclose possible significant differences in posture and speech, also considering the heterogeneous presentation of these symptoms in our cohort. Moreover, due to difficulties in patients' access and assessment reliability, we could not evaluate patients in OFF condition after an overnight withdrawal of dopaminergic therapy. This information would have allowed quantifying the response to levodopa with the standard infusion rate. Finally, this study analyzed a specific cohort of PD patients presenting an advanced disease stage treated with LCIG and a heterogeneous cognitive profile. These aspects should be carefully considered for the generalizability of our findings to all PD patients. Finally, the progressive increase of levodopa gel infusion during the same session did not guarantee a perfectly matched increase in plasma levodopa concentrations among patients and an accumulation effect from running the three assessments in a single session cannot be ruled out; to have more precise knowledge of the pharmacokinetic effect of our progressive levodopa dose increase, we analyzed plasma levodopa concentrations immediately before the start of each assessment (at baseline and 1 h after every infusion rate increase), finding moderate intersubject variability but also a statistically significant increase of concentrations at a group level.
These shortcomings notwithstanding, we demonstrated that in most cases of advanced PD treated with LCIG with good control of motor symptoms, the increase in the levodopa plasma concentrations can improve FoG, suggesting that pseudo‐ON‐FoG could be the most common FoG type in these patients. Although the increase of levodopa doses could be a viable option to overcome apparently dopa‐resistant FoG, our findings do not endorse the attempt to increase levodopa for treating postural and speech abnormalities when the control of other symptoms is adequate. We cannot recommend the practice of an increase of LCIG doses to overcome FoG episodes according to data provided by our clinical trial due to the limited time of the assessment with high LCIG infusion rates. However, the proof of improving FoG episodes provided by higher plasma levodopa concentrations should foster new clinical studies to evaluate the long‐term tolerance and efficacy of higher levodopa or LCIG doses in patients with apparently dopa‐resistant FoG. Accordingly, in patients treated with LCIG with severe FoG, the use of "on‐demand" extra doses in specific moments when they need better walking performance or when they are experiencing incapacitating FoG episodes could be an interesting option to explore. Pending further studies with larger sample size and prolonged follow‐up and considering the low generalizability of our findings, which are related to a subgroup of advanced PD patients treated with LCIG and with different clinical and cognitive issues, we believe that this study indicates the existence of a higher threshold for the efficacy of levodopa on FoG than on cardinal motor symptoms in advanced PD. An approach based on the increase of levodopa doses in therapeutically optimized patients with a seeming unresponsive‐FoG is worth further exploring.
AUTHOR CONTRIBUTIONS
Gabriele Imbalzano and Domiziana Rinaldi: Conceptualization, data curation, formal analysis, investigation, visualization, writing–original draft preparation. Giovanna Calandra‐Buonaura, Manuela Contin, Federica Amato, Giulia Giannini, and Luisa Sambati: Execution, resources, data curation, validation, writing–original draft preparation. Claudia Ledda, Alberto Romagnolo, and Gabriella Olmo: Formal analysis, review and critique. Pietro Cortelli and Maurizio Zibetti: Methodology, organization. Leonardo Lopiano and Carlo Alberto Artusi: Conceptualization, resources, project administration, supervision, validation, writing–review & editing. All authors reviewed the manuscript, provided critical comments to improve the draft, and approved the final version of the manuscript.
CONFLICT OF INTEREST
G.I. and D.R. have received travel grants from Abbvie. C.L. has received travel grants from Bial and Merz. A.R. has received travel grants from Chiesi Farmaceutici, and speaker honoraria from Bial. M.Z. has received honoraria for lecturing and travel grants from Medtronic, Bial Pharma, and AbbVie. L.L. has received honoraria for lecturing and travel grants from Medtronic, UCB Pharma, and AbbVie. C.A.A. has received honoraria from Ralpharma and Zambon for scientific support, and speaker honoraria from Ecupharma and Bial. None of the other authors has any conflict of interest to disclose.
ACKNOWLEDGMENT
We thank the patients and their families for participating in this study. Open Access Funding provided by Universita degli Studi di Torino within the CRUI‐CARE Agreement.
Imbalzano G, Rinaldi D, Calandra‐Buonaura G, et al. How resistant are levodopa‐resistant axial symptoms? Response of freezing, posture, and voice to increasing levodopa intestinal infusion rates in Parkinson disease. Eur J Neurol. 2023;30:96‐106. doi: 10.1111/ene.15558
Gabriele Imbalzano and Domiziana Rinaldi equally contributed as first author to this work.
Leonardo Lopiano and Carlo Alberto Artusi equally contributed as last author to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in an anonymized dataset from the corresponding author upon reasonable request.
REFERENCES
- 1. Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol. 2015;11:98110. [DOI] [PubMed] [Google Scholar]
- 2. Lau B, Meier N, Serra G, et al. Axial symptoms predict mortality in patients with Parkinson disease and subthalamic stimulation. Neurology. 2019;92:e2559‐e2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bouça‐Machado R, Pona‐Ferreira F, Gonçalves N, et al. Outcome measures for evaluating the effect of a multidisciplinary intervention on axial symptoms of Parkinson's disease. Front Neurol. 2020;11:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fabbri M, Guimarães I, Cardoso R, et al. Speech and voice response to a levodopa challenge in late‐Stage Parkinson's disease. Front Neurol. 2017;8:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kataoka H, Ueno S. Can postural abnormality really respond to levodopa in Parkinson's disease? J Neurol Sci. 2017;377:179‐184. [DOI] [PubMed] [Google Scholar]
- 6. Fabbri M, Coelho M, Abreu D, et al. Do patients with late‐stage Parkinson's disease still respond to levodopa? Parkinsonism Relat Disord. 2016;26:10‐16. [DOI] [PubMed] [Google Scholar]
- 7. Espay AJ, Fasano A, van Nuenen BF, et al. "On" state freezing of gait in Parkinson disease: a paradoxical levodopa‐induced complication. Neurology. 2012;78:4547‐4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nutt JG, Bloem BR, Giladi N. Freezing of gait: moving forward on a mysterious clinical phenomen. Lancet Neurol. 2011;10:734‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morales‐Briceño H, Tsui D, Griffith J, Martin AJ, Mahant N, Fung VSC. "On‐State" Freezing of Gait: Insights and Treatment With Levodopa Intestinal Gel Infusion. Mov Disord. 2020;35:895‐896. [DOI] [PubMed] [Google Scholar]
- 10. Cui CK, Lewis SJG. Future therapeutic strategies for freezing of gait in Parkinson's disease. Front Hum Neurosci. 2021;15:741918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Srivanitchapoom P, Hallett M. Camptocormia in Parkinson's disease: definition, epidemiology, pathogenesis and treatment modalities. J Neurol Neurosurg Psychiatry. 2016;87:75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roediger J, Artusi CA, Romagnolo A, et al. Effect of subthalamic deep brain stimulation on posture in Parkinson's disease: A blind computerized analysis. Parkinsonism Relat Disord. 2019;62:122‐127. [DOI] [PubMed] [Google Scholar]
- 13. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30:1591‐1601. [DOI] [PubMed] [Google Scholar]
- 14. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427‐442. [DOI] [PubMed] [Google Scholar]
- 15. Nieuwboer A, Rochester L, Herman T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture. 2009;30:459‐463. [DOI] [PubMed] [Google Scholar]
- 16. Barthel C, Nonnekes J, van Helvert M, et al. The laser shoes: A new ambulatory device to alleviate freezing of gait in Parkinson disease. Neurology. 2018;90:e164‐e171. [DOI] [PubMed] [Google Scholar]
- 17. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689‐1707. [DOI] [PubMed] [Google Scholar]
- 18. Martínez‐Martín P, Rodríguez‐Blázquez C, Alvarez M, et al. Parkinson's disease severity levels and MDS‐Unified Parkinson's Disease Rating Scale. Parkinsonism Relat Disord. 2015;21:50‐54. [DOI] [PubMed] [Google Scholar]
- 19. Mehdizadeh M, Martinez‐Martin P, Habibi SA, et al. Reliability and validity of fall efficacy scale‐international in people with Parkinson's disease during on‐ and off‐drug phases. Parkinsons Dis. 2019;2019:6505232‐6505237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov Disord. 2013;28:668‐670. [DOI] [PubMed] [Google Scholar]
- 21. Goetz CG, Nutt JG, Stebbins GT. The Unified Dyskinesia Rating Scale: presentation and clinimetric profile. Mov Disord. 2008;23:2398‐2403. [DOI] [PubMed] [Google Scholar]
- 22. Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738‐1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649‐2653. [DOI] [PubMed] [Google Scholar]
- 24. Schade S, Mollenhauer B, Trenkwalder C. Levodopa equivalent dose conversion factors: an updated proposal including opicapone and safinamide. Mov Disord Clin Pract. 2020;7:343‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morris TR, Cho C, Dilda V, et al. A comparison of clinical and objective measures of freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. 2012;18:572‐577. [DOI] [PubMed] [Google Scholar]
- 26. Tinazzi M, Gandolfi M, Ceravolo R, et al. Postural abnormalities in Parkinson's disease: an epidemiological and clinical multicenter study. Mov Disord Clin Pract. 2019;6:576‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Margraf NG, Wolke R, Granert O, et al. Consensus for the measurement of the camptocormia angle in the standing patient. Parkinsonism Relat Disord. 2018;52:1‐5. [DOI] [PubMed] [Google Scholar]
- 28. Amato F, Borzì L, Olmo G, Orozco‐Arroyave JR. An algorithm for Parkinson's disease speech classification based on isolated words analysis. Health Inf Sci Syst. 2021;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rusz J, Tykalová T, Novotný M, Růžička E, Dušek P. Distinct patterns of speech disorder in early‐onset and late‐onset de‐novo Parkinson's disease. NPJ Parkinsons Dis. 2021;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rusz J, Tykalova T, Ramig LO, Tripoliti E. Guidelines for speech recording and acoustic analyses in dysarthrias of movement disorders. Mov Disord. 2021;36:803‐814. [DOI] [PubMed] [Google Scholar]
- 31. Baruzzi A, Contin M, Albani F, Riva R. Simple and rapid micromethod for the determination of levodopa and 3‐O‐methyldopa in human plasma by high‐performance liquid chromatography with coulometric detection. J Chromatogr. 1986;375:165‐169. [Google Scholar]
- 32. Mestre TA, Beaulieu‐Boire I, Aquino CC, et al. What is a clinically important change in the Unified Dyskinesia Rating Scale in Parkinson's disease? Parkinsonism Relat Disord. 2015;21:1349‐1354. [DOI] [PubMed] [Google Scholar]
- 33. Nonnekes J, Bereau M, Bloem BR. Freezing of gait and its levodopa paradox. JAMA Neurol. 2020;77:287‐288. [DOI] [PubMed] [Google Scholar]
- 34. Zibetti M, Angrisano S, Dematteis F, et al. Effects of intestinal levodopa infusion on freezing of gait in parkinson disease. J Neurol Sci. 2018;385:105‐108. [DOI] [PubMed] [Google Scholar]
- 35. Cossu G, Ricchi V, Pilleri M, et al. Levodopa‐carbidopa intrajejunal gel in advanced Parkinson disease with "on" freezing of gait. Neurol Sci. 2015;36:1683‐1686. [DOI] [PubMed] [Google Scholar]
- 36. Rispoli V, Golfrè Andreasi N, Penna G, Preda F, Contini E, Sensi M. Levodopa/carbidopa intestinal gel infusion therapy: focus on gait and balance. Mov Disord Clin Pract. 2018;5:542‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chee R, Murphy A, Danoudis M, Georgiou‐Karistianis N, Iansek R. Gait freezing in Parkinson's disease and the stride length sequence effect interaction. Brain. 2009;132(Pt 8):2151‐2160. [DOI] [PubMed] [Google Scholar]
- 38. Nieuwboer A. Cueing for freezing of gait in patients with Parkinson's disease: a rehabilitation perspective. Mov Disord. 2008;23(Suppl 2):S475‐S481. [DOI] [PubMed] [Google Scholar]
- 39. Diep C, O'Day J, Kehnemouyi Y, et al. Gait parameters measured from wearable sensors reliably detect freezing of gait in a stepping in place task. Sensors (Basel). 2021;21(8):2661. doi: 10.3390/s21082661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tinazzi M, Geroin C, Gandolfi M, et al. Pisa syndrome in Parkinson's disease: An integrated approach from pathophysiology to management. Mov Disord. 2016;31:1785‐1795. [DOI] [PubMed] [Google Scholar]
- 41. Doherty KM, van de Warrenburg BP, Peralta MC, et al. Postural deformities in Parkinson's disease. Lancet Neurol. 2011;10:538‐549. [DOI] [PubMed] [Google Scholar]
- 42. Fabbri M, Pongmala C, Artusi CA, et al. Video analysis of long‐term effects of levodopa‐carbidopa intestinal gel on gait and posture in advanced Parkinson's disease. Neurol Sci. 2020;41:1927‐1930. [DOI] [PubMed] [Google Scholar]
- 43. Ho AK, Bradshaw JL, Iansek R. For better or worse: the effect of levodopa on speech in Parkinson's disease. Mov Disord. 2008;23:574‐580. [DOI] [PubMed] [Google Scholar]
- 44. Skodda S, Visser W, Schlegel U. Short‐ and long‐term dopaminergic effects on dysarthria in early Parkinson's disease. J Neural Transm (Vienna). 2010;117:197‐205. [DOI] [PubMed] [Google Scholar]
- 45. Skodda S, Flasskamp A, Schlegel U. Instability of syllable repetition in Parkinson's disease–influence of levodopa and deep brain stimulation. Mov Disord. 2011;26:728‐730. [DOI] [PubMed] [Google Scholar]
- 46. Skodda S, Grönheit W, Schlegel U. Intonation and speech rate in Parkinson's disease: general and dynamic aspects and responsiveness to levodopa admission. J Voice. 2011;25:e199‐e20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in an anonymized dataset from the corresponding author upon reasonable request.


