Abstract
Background
Psychological stress is prevalent among reproductive‐aged men. Assessment of semen quality for epidemiological studies is challenging as data collection is expensive and cumbersome, and studies evaluating the effect of perceived stress on semen quality are inconsistent.
Objective
To examine the association between perceived stress and semen quality.
Material and methods
We analyzed baseline data on 644 men (1,159 semen samples) from two prospective preconception cohort studies during 2015–2021: 592 in Pregnancy Study Online (PRESTO) and 52 in SnartForaeldre.dk (SF). At study entry, men aged ≥21 years (PRESTO) and ≥18 years (SF) trying to conceive without fertility treatment completed a questionnaire on reproductive and medical history, socio‐demographics, lifestyle, and the 10‐item version of the Perceived Stress Scale (PSS; interquartile range [IQR] of scores: 0–40). After enrollment (median weeks: 2.1, IQR: 1.3–3.7), men were invited to perform in‐home semen testing, twice with 7–10 days between tests, using the Trak Male Fertility Testing System. Semen quality was characterized by semen volume, sperm concentration, and total sperm count. We fit generalized estimating equation linear regression models to estimate the percent difference in mean log‐transformed semen parameters by four PSS groups (<10, 10–14, 15–19, ≥20), adjusting for potential confounders.
Results
The median PSS score and IQR was 15 (10–19), and 136 men (21.1%) had a PSS score ≥20. Comparing men with PSS scores ≥20 with <10, the adjusted percent difference was −2.7 (95% CI: −9.8; 5.0) for semen volume, 6.8 (95% CI: ‐10.9; 28.1) for sperm concentration, and 4.3 (95% CI: −13.8; 26.2) for total sperm count.
Conclusion
Our findings indicate that perceived stress is not materially associated with semen volume, sperm concentration, or total sperm count.
Keywords: cross‐sectional study, in‐home semen testing, male fertility, perceived stress, preconception, semen quality
1. INTRODUCTION
The estimated prevalence of infertility is 10–15% 1 and male factors account for about 50 of infertility. 2 A 2017 meta‐analysis including 185 studies and 42,935 men found a global 50–60% decline in sperm counts over the past 40 years. 3 A Danish study assessed temporal trends in semen quality. 4 Based on more than 6,000 draftees representing the general male population, this study reported a high prevalence of low semen quality over the past 20 years that was stable over time.
A national Danish survey from 2017 5 indicated that 23–24% of men aged 16–34 years experienced high levels of perceived stress, as assessed by the 10‐item Perceived Stress Scale (PSS). Similarly, in a survey conducted in 2014 by the American Psychological Association, males of reproductive age reported an average stress score of 5.5 on a scale of one to ten, where one means little or no stress in the past month. 6
Stress can be acute or chronic. While a short period of stress is often a natural response to handle short term stressors, chronic stress can have serious social, psychological or health‐related consequences. Stress may affect semen quality through decreased testosterone levels, modified spermatogenesis and sexual dysfunctions. 7 , 8
A review of lifestyle and male fertility suggests that permanently high levels of glucocorticoids in testes during chronic stress may induce apoptosis of sperm cells, which leads to impaired semen quality. 9 A study of medical students compared stress and nonstress periods and found an inverse association between concentration of nitric oxide and sperm concentration and percentage of rapid progressive motility of spermatozoa, suggesting a potential link between stress and semen quality via the L‐arginine‐nitric oxide pathway. 10
The findings from studies on perceived stress and semen quality have been inconsistent. Two cross‐sectional studies on 1,215 and 1,388 Danish men recruited at the medical examination for military service with a median age of 19 years indicated an inverse association between self‐reported stress and semen quality. 11 , 12 Another Danish study on 418 men enrolled in a prospective cohort study of couples trying to conceive concluded that a man's daily life psychologic stress has no effect on semen quality. 13 A cross‐sectional study of 193 men aged 38–49, whose mothers participated in the American, Child Health and Development Studies, assessed both perceived stress and the occurrence of stressful life events and found an inverse association between perceived stress and semen quality. 14 The above studies were based on traditional laboratory‐based methods to collect and analyze data on semen quality. These methods require face to face contact for participants and transportation to a research laboratory facility, limiting the geographic diversity of study populations.
In this study, we examined the degree of association between perceived stress and in‐home assessed semen quality among men enrolled in two prospective cohort studies of couples trying to conceive without fertility treatment.
2. MATERIAL AND METHODS
2.1. Design and study population
This study used baseline data from two online ongoing prospective cohorts, SnartForaeldre.dk (SF) and Pregnancy Study Online (PRESTO). SF has recruited Danish couples trying to conceive since August 2011, 15 , 16 while PRESTO has recruited North American couples since June 2013. 17 , 18 , 19 To be enrolled in SF or PRESTO men and women first completed a screener to confirm eligibility. Eligible women were 18–49 years (SF) and 21–45 years (PRESTO), while eligible men were ≥18 years (SF) and ≥21 years (PRESTO). Further, men and women had to be in a relationship with a partner of the opposite sex, trying to become pregnant and not use any contraception or receive fertility treatment.
SF primarily recruited female participants through e‐Boks, which is a Danish online communication platform used by Danish authorities. No incentives were offered to couples participating in SF. PRESTO primarily recruited women through online media such as Facebook and online ads. In both cohorts, female participants were encouraged to invite their male partners to participate.
At enrollment, both members of the couple were invited to complete a baseline questionnaire on reproductive and medical history, socio‐demographics, lifestyle, and perceived stress. Ten days later, they completed a validated food frequency questionnaire (FFQ). We invited men in both cohorts to perform in‐home semen testing using the Trak Male Fertility Testing System (Trak).
In SF, all men who completed both the baseline questionnaire and the FFQ were invited for in‐home semen testing irrespective of number of months they had tried to conceive. In total, 52 of the 118 (44%) invited men participated in the in‐home semen sub‐study, which started April 2019 and continued through September 2019. Of those, 20 (38%) reported at study entry that they had tried to conceive for <3 months, and 32 (62%) had tried for ≥3 months.
Men enrolled were invited for in‐home semen testing after completing the baseline questionnaire in PRESTO, if their partner reported she had regular menstrual cycles, and they had tried to conceive ≤6 months (October 2015‐September 2021). In total, 592 (45%) of the 1,327 invited men participated in the in‐home semen testing. Men who completed both semen tests were given a $20 electronic gift card for their time and effort.
In total, we included 644 men who provided informed consent and at least one semen sample resulting in 1,159 samples. The first in‐home semen test was performed median 2.1 (interquartile range [IQR]: 1.3‐3.7) weeks after enrollment.
2.2. Assessment of perceived stress
We assessed the level of perceived stress at baseline using the PSS, 20 which measures the extent to which individuals find their lives to be overloaded, unpredictable, and uncontrollable. 20 The PSS comprises 10 questions, each with five response options ranging from 0 (never) to 4 (very often). The total PSS score, with a range 0–40, is the sum of scores from each item. A higher score indicated a higher level of perceived stress.
2.3. Assessment of semen quality
After completion of the male baseline questionnaire (PRESTO) and the FFQ (SF), participants were invited to participate in the in‐home semen testing sub‐study. Participants who provided informed consent for the in‐home semen testing sub‐study were sent a Trak kit with supplies to complete two tests within 7–10 days. The Trak device uses a graduated sample collection cup to measure semen volume and a centrifuge (Trak Engine) and cell separation cartridges (Props) to measure sperm concentration. 21
Trak is a US Food and Drug administration (FDA) 510(k)‐cleared diagnostic device and is commercially available for semi‐quantitative over‐the‐counter measurement of sperm concentration and semen volume. The Trak product was developed at Sandstone Diagnostics, Inc. and acquired by Laboratory Corporation of America (Labcorp) in 2021. Validation studies for the sperm concentration and semen volume assays involved lay user and technician assessed Trak results compared with gold standard laboratory‐based volume assessments and cell‐counting procedures (Hamilton‐Thorne CEROS CASA instruments running version 14.13). 22 , 23 , 24 Sperm concentration validation included N = 239 donors, yielding a strong linear correlation with between Trak results and the standard laboratory‐based method (Pearson coefficient r = 0.99). 22 Semen volume validation included N = 232 donors with r = 0.99. 23
We characterized semen quality using three parameters: semen volume (ml), sperm concentration (million/ml), and total sperm count (million). All participants were instructed to abstain from ejaculation for 2–7 days before testing, and to collect the samples via masturbation and without the use of condoms or lubricants. Further, they were instructed not to let semen testing interfere with their aim of achieving a pregnancy. The test results for sperm concentration were photographed and uploaded to the study websites. In addition, participants reported the semen volume. All de‐identified photos for sperm concentration were optically read and recalibrated by staff at Sandstone Diagnostics, Inc., Pleasanton, CA.
2.4. Assessment of covariates
We obtained information on covariates from the baseline questionnaire, which included age, education, job hours per week, employment status, height and weight, alcohol consumption, smoking, sleep duration, caffeine intake, ever impregnated a partner, diagnosis of depression, anxiety and diabetes, fever within the past 3 months, and intercourse frequency. We used baseline data on height and weight to calculate body mass index (BMI) (kg/m2). When the participants uploaded their test results, they were asked to report abstinence time (i.e., number of days since the most recent ejaculation) for each semen sample.
2.5. Data analysis
We analyzed data from the combined cohorts, as the data collection procedures and the instruments used were almost identical. We also analyzed the cohorts separately (results are presented in Supplementary Tables). We described participant characteristics by medians, IQR, and proportions. Given the lack of a clinical cut‐off for the PSS score, we categorized the score in four categories (<10, 10–14, 15–19, ≥20).
Semen quality (semen volume, sperm concentration, and total sperm count) was described by medians and IQR. We calculated total sperm count (million) as sperm concentration (million/ml) × semen volume (ml).
We assessed the number of men with impaired semen quality according to World Health Organization's (WHO) lower reference limit for each semen characteristic, defined as semen volume <1.5 ml, sperm concentration <15 million/ml, and total sperm count <39 million.
We fit a generalized estimating equation (GEE) linear regression model to estimate the percent difference in mean log‐transformed semen parameters by stress category. By using a GEE linear regression model, we accounted for multiple semen samples per participant (up to two samples).
We identified potential confounders based on a directed acyclic graph (Figure 1) and the distribution of covariates across PSS categories. In our primary model (Model I), we adjusted for cohort (SF/PRESTO), abstinence time (continuous), education >15 years (yes/no), sleep duration <7 hours (yes/no), fever within the past 3 months (yes/no), employed (yes/no), caffeine intake (continuous, mg/day), and ever impregnated a partner (yes/no). Because sleep duration may be considered an intermediate variable, we repeated the primary model excluding sleep duration. In a second model, we further adjusted for diagnosis with depression or anxiety (Model II). In addition, we fit a restricted cubic spline regression to evaluate the shape of the association between PSS score and semen quality adjusting for potential confounders. Each spline curve has four knot points at 5th, 35th, 65th, and 95th percentile corresponding to the following PSS scores 5, 12, 17, and 25 (Figure 2).
FIGURE 1.
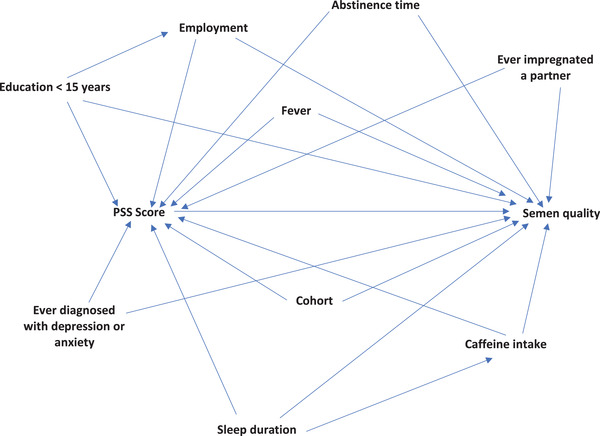
Directed acyclic graph illustrating the association between perceived stress (Perceived Stress Scale [PSS] score) and semen quality
FIGURE 2.
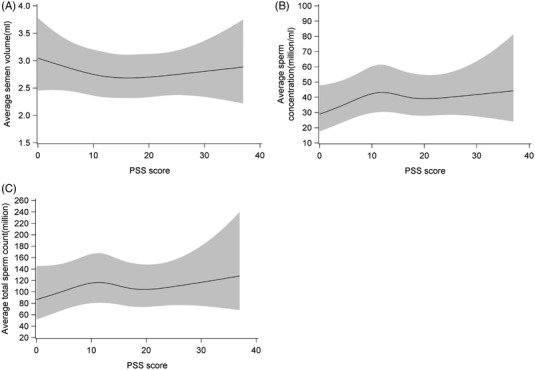
Evaluation of the association between perceived stress (Perceived Stress Scale [PSS] score) and semen quality (semen volume, sperm concentration, and total sperm count) using restricted cubic splines, N = 644. The solid line represents the median estimate, and the grey area represents the 95% CI. The splines are adjusted for cohort, abstinence time, education, employment, caffeine intake, fever, ever impregnated a partner, and sleep duration, and each spline has four knot points at 5, 12, 17 and 25
To evaluate the potential for selection bias, we compared the PSS score of the 642 (before imputation) men participating in the in‐home semen sub‐study with the score in the remaining 3,011 men (SF + PRESTO).
We used multiple imputation to impute missing data on exposure, covariates, and outcome in each cohort, separately. 25 In the PRESTO dataset, we imputed missing values on weight (missing = 1), caffeine intake (missing = 1), ever impregnated a partner (missing = 17), PSS score (missing = 2), and sperm concentration in first sample (missing = 1) using an imputation model that also included age, height, waist, weight at age 17, race/ethnicity, intercourse frequency, previously tried to get pregnant without success, ever impregnated a partner, diagnoses of depression and anxiety, Major Depression Inventory score, total metabolic equivalents of energy expenditure, vigorous and moderate physical activity, sleep duration, use of hot sauna, use of laptop in lap, sitting watching TV/video, sitting other than watching TV/video, biking, fever during the past 3 months, times visited primary care physician in past year, fish oil capsules, alcohol intake, sugar‐sweetened beverage intake, education, hours worked per week, smoking status, marijuana use, semen volume, total sperm count, motility, and abstinence time between tests. Because SF had fewer subjects, we used an imputation model with fewer variables. The model included, PSS score (missing = 3), caffeine intake (missing = 3), sleep duration (missing = 1), employment (missing = 1), depression diagnosis (missing = 2), anxiety diagnoses (missing = 2), ever impregnated a partner, smoking status, age, intercourse frequency, and weight. Education (missing = 1), fever during the past 3 months (missing = 2), and hours worked per week (missing = 15) could not be imputed in SF due to convergence issues. For both cohorts, we generated 20 imputed datasets using PROC MI, analyzed each dataset and subsequently combined the results across the 20 imputed datasets using PROC MIANALYZE.
All analyses were performed using SAS statistical software (version 9.4, SAS Institute).
2.6. Ethical approval
The Committee on Health Research Ethics in Central Denmark Region approved the SnartForaeldre.dk/Saedkvalitet study (project number 1‐10‐72‐14‐19). The parent study and the semen testing sub‐study were approved by the Boston University Medical Campus Institutional Review Board (protocol numbers: H‐31848 and H‐34223).
All participants provided informed consent before receiving the Trak Male Fertility Testing System.
3. RESULTS
PRESTO included 592 men, of whom 466 (78.7%) provided both semen samples, while SF enrolled 52 men, of whom 49 (94.2%) contributed two samples. In total, 644 men provided 1,159 semen samples (Table 1). The median (IQR) for semen volume was 4 (3‐5) ml and was 44 (24‐80) million/ml for sperm concentration, while the total sperm count was 165 (90‐288) million. According to the WHO criteria for impaired semen quality, 7 (1.1%) men had semen volume <1.5 ml, 67 (10.4%) men had sperm concentration <15 million/ml, and 48 (7.5%) men had total sperm count <39 million (Supplementary Table S1).
TABLE 1.
Descriptive statistics of the study population, SF and PRESTO cohorts, 2015–2021
| NumberA | Median (IQR) | 10th percentile | 90th percentile | |
|---|---|---|---|---|
| PSS score | 644 | 15 (10–19) | 7 | 23 |
| Semen volume (ml) | 1159 | 4 (3–5) | 2 | 6 |
| Sperm concentration (million/ml) | 1159 | 44 (24–80) | 13 | 136 |
| Total sperm count (million) | 1159 | 165 (90–288) | 42.1 | 468 |
Abbreviations: IQR=interquartile range; PRESTO=Pregnancy Study Online; PSS=Perceived Stress Scale; SF=SnartForaeldre.dk;.
Number for PSS score is based on men, while the number for semen parameters is based on semen samples.
For the pooled cohorts, the median PSS score was 15 (IQR: 10–19), and 136 men (21.1%) had a PSS score ≥20. In comparison, the median PSS score for SF men participating in the semen study was 10 (IQR: 6–15) versus 11 (IQR: 8–16) for non‐participants. Similarly, for PRESTO, the mean PSS score was 15 (IQR: 11–19) for participants versus 14 (IQR: 11–19) for non‐participants.
The distribution of age, abstinence time, hours worked weekly, and alcohol intake were similar across PSS groups (Table 2 and Supplementary Table S3a and S3b). Men with PSS scores ≥20 were more likely to have intercourse ≥4 times a week than men with PSS scores <10. Enrolled men tended to be overweight, and men in the highest exposure group had a BMI close to 28. Men with PSS scores ≥20 were more likely to sleep <7 hours/night, consume more caffeine and have an education <15 years compared with those who had a PSS score <10. Further, diagnoses of depression or anxiety were more frequent among men with higher PSS scores (Table 2). Overall, nine (1.4%) men reported they previously had had a semen analysis. When comparing PSS scores ≥20 with <10, the adjusted percent differences with confidence intervals (CI) were −2.7 (95% CI: −9.8; 5.0) for semen volume, 6.8 (95% CI: −10.9; 28.1) for sperm concentration and 4.3 (95% CI: −13.8; 26.2) for total sperm count (Model I, Table 3). The estimates were similar after additional adjustment for diagnoses of depression or anxiety (Model II, Table 3) and also after excluding sleep duration from the model (data not shown). Stratified by cohorts, the adjusted percent differences when comparing PSS scores ≥20 with <10 for SF were −17.9 (−42.0; 16.1) for semen volume, −23.4 (−78.7; 175.6) for sperm concentration and −35.6 (−85.0; 176.5) for total sperm count. The adjusted percent differences for PRESTO were −3.0 (−10.4; 5.1) for semen volume, 6.9 (−10.7; 28.0) for sperm concentration and 3.7 (−13.9; 25.0) for total sperm count. The restricted cubic splines did not indicate a meaningful association between PSS score and semen quality (Figure 2).
TABLE 2.
Baseline characteristics of 644 men according to Perceived Stress Scale score 1
| Perceived Stress Scale score | ||||
|---|---|---|---|---|
| <10 | 10‐14 | 15‐19 | ≥20 | |
| Number of participants, (%) | 134 (20.8) | 179 (27.8) | 195 (30.3) | 136 (21.1) |
| Number of SF participants, (%) | 26 (50.0) | 11 (21.2) | 11 (21.2) | 4 (7.7) |
| Number of PRESTO participants, (%) | 108 (18.2) | 168 (28.4) | 184 (31.1) | 132 (22.3) |
| Number of semen samples | 245 | 324 | 349 | 241 |
| Age, years, median (IQR) | 31.0 (29.0; 35.0) | 31.0 (29.0; 35.0) | 31.0 (28.0; 35.0) | 32.0 (28.0; 35.0) |
| Intercourse frequency, % | ||||
| ≤3 times/month | 14.2 | 21.2 | 22.1 | 15.4 |
| 1 time/week | 50.0 | 45.8 | 45.6 | 35.3 |
| 2–3 times/week | 9.7 | 10.6 | 6.2 | 11.8 |
| ≥4 times/week | 26.1 | 22.3 | 26.2 | 37.5 |
| Abstinence time, days, median (IQR) | 3.5 (3.0; 4.5) | 3.5 (3.0; 4.5) | 3.5 (3.0; 4.5) | 3.0 (3.0; 5.0) |
| BMI kg/m2, median (IQR) | 26.6 (23.8; 29.8) | 26.6 (23.9; 30.0) | 26.6 (23.8; 31.0) | 27.9 (24.3; 32.6) |
| Education >15 years, % | 82.1 | 76.0 | 81.0 | 66.9 |
| Employed or student, % | 96.2 | 96.6 | 93.4 | 89.7 |
| Hours worked per week, median (IQR) | 40.0 (40.0; 45.0) | 40.0 (40.0; 45.0) | 40.0 (40.0; 45.0) | 40.0 (40.0; 50.0) |
| Smoking, % | 7.5 | 7.8 | 7.7 | 11.8 |
| Current caffeine intake, mg/day median (IQR) | 145.4 (38.6; 272.9) | 125.9 (44.4; 264.3) | 137.3 (40.0; 250.7) | 183.9 (87.9; 290.7) |
| Current alcohol intake, drinks/week median (IQR) | 3.0 (1.0; 7.0) | 4.0 (1.0; 7.0) | 4.0 (1.0; 8.2) | 3.0 (0.8; 8.0) |
| Sleep duration <7 hours/night, % | 16.4 | 31.3 | 24.6 | 35.3 |
| Ever impregnated a partner, % | 29.9 | 36.9 | 42.1 | 50.0 |
| Ever diagnosed with depression, % | 6.0 | 11.2 | 16.4 | 30.1 |
| Ever diagnosed with anxiety, % | 4.5 | 6.1 | 11.3 | 21.3 |
| Ever diagnosed with diabetes, % | 2.2 | 3.4 | 3.1 | 2.9 |
| Fever during the past 3 months, % | 9.7 | 11.7 | 11.3 | 14.0 |
Abbreviations: BMI=body mass index; IQR=interquartile range; PRESTO=Pregnancy Study Online; SF=SnartForaeldre.dk.
1Baseline characteristics are based on the first dataset resulting from multiple imputation.
TABLE 3.
Percent difference in semen parameter by Perceived Stress Scale score
| Unadjusted (95% CI)A, 1 | Adjusted (95% CI) B, 2 Model I | Adjusted (95% CI) C, 3 Model II | |
|---|---|---|---|
| Semen volume, ml | |||
| PSS <10 | Reference | Reference | Reference |
| PSS 10–14 | −3.5 (−10.1; 3.5) | −2.0 (−8.6; 5.1) | −2.0 (−8.6; 5.1) |
| PSS 15–19 | −5.0 (−11.4; 1.9) | −4.2 (−10.6; 2.6) | −4.4 (−10.8; 2.5) |
| PSS ≥20 | −4.7 (−11.6; 2.8) | −2.7 (−9.8; 5.0) | −3.0 (−10.2; 4.8) |
| Sperm concentration, million/ml | |||
| PSS <10 | Reference | Reference | Reference |
| PSS 10–14 | 4.7 (−11.5; 23.9) | 5.9 (−10.3; 25.0) | 5.9 (−10.3; 25.0) |
| PSS 15–19 | 6.5 (−9.7; 25.6) | 6.5 (−9.6; 25.4) | 6.5 (−9.7; 25.5) |
| PSS ≥20 | 7.9 (−9.9; 29.2) | 6.8 (−10.9; 28.1) | 6.8 (−11.2; 28.4) |
| Total sperm count, million | |||
| PSS <10 | Reference | Reference | Reference |
| PSS 10–14 | 1.4 (−15.2; 21.3) | 4.2 (−12.5; 24.1) | 4.2 (−12.5; 24.1) |
| PSS 15–19 | 1.6 (−14.8; 21.2) | 2.3 (−13.8; 21.5) | 2.2 (−14.0; 21.4) |
| PSS ≥20 | 3.2 (−14.8; 25.1) | 4.3 (−13.8; 26.2) | 4.0 (−14.3; 26.2) |
Note: The analyses are based on A: 1,159 samples, B: 1,157 samples, and C: 1,157 samples.
Abbreviation: PSS=Perceived Stress Scale.
Adjusted for cohort.
2Adjusted for cohort, abstinence time, employment, education, caffeine intake, sleep duration, fever, ever impregnated a partner.
3Adjusted for cohort, abstinence time, employment, education, caffeine intake, sleep duration, fever, ever impregnated a partner and diagnosed with depression or anxiety.
4. DISCUSSION
In this study nested within a preconception cohort study of couples planning a pregnancy, we did not find a meaningful association between male perceived stress score and three semen parameters: semen volume, sperm concentration, and total sperm count.
Our findings are consistent with those reported in a Danish prospective cohort of couples trying to conceive, where they reported no association between self‐reported male stress and semen quality. 13 In contrast, Nordkap et al. (2016 and 2020) and Janevic et al. reported inverse associations between perceived stress and several semen parameters. 11 , 12 , 14 Nordkap et al. (2016) measured stress by The Copenhagen Psychosocial Questionnaire, a four‐item scale, 11 whereas Hjollund et al. and Janevic et al., used two comprehensive scales, the 12 item General Health Questionnaire, and the 10 item PSS. 13 , 14 Nordkap et al. (2020) used three different measures of stress, a 14 item Stress Symptoms Scale, PSS, and the occurrence of stressful life events. 12 In that study, Nordkap et al. (2020) found a linear association between PSS and semen parameters, but no dose‐response association for the two other stress scales. Thus, the differences in instruments may explain the inconsistent findings across studies.
The studies by Nordkap et al. (2016 and 2020) enrolled Danish men aged 19, most of whom we assume to be unaware of their semen quality, while Janevic et al. included men aged 38–49 years, many of whom had previously fathered a child and were aware of their fertility. 11 , 12 , 14 In comparison, in our study, the median age at enrollment was 32.0 years (IQR: 28.8–35.3), and 40% had previously impregnated a partner. To the extent that age and awareness of own fertility modify the association between perceived stress and semen quality, these differences across studies could also have influenced the results. 9
Our study and Janevic et al. used comparable approaches to collect data on semen quality. Men in both studies were asked to collect two semen samples within a similar time period (our study: within 7–10 days and Janevic et al.: two weeks apart). Likewise, abstinence time was similar 2–7 days (PRESTO/SF) and 2–5 days (Janevic et al.). However, differences in assessment of semen quality may contribute to the inconsistent findings, 9 as we used Trak to assess semen quality while Nordkap et al., Janevic et al., and Hjollund et al. used traditional laboratory‐based methods to analyze semen samples.
We used the PSS to operationalize perceived stress. The PSS measures the extent to which individuals find their lives to be overloaded, unpredictable, and uncontrollable. 20 Among respondents with at least a junior high school education, validation studies show that the PSS can capture stress experienced during the past two months. 20 , 26 , 27 Further, assessment of reliability via Cronbach alpha and test‐retest demonstrated high internal consistency and high correlations when the test‐retest was completed within a short time period. 20 PSS is a subjective measurement of stress, but stress can also be measured by reporting stressful life events, which has been done in a study by Janevic et al. and by Nordkap et al. (2020). 12 , 14 However, a measure of stressful life events does not account for individual differences in stress reactions or coping strategies. Our study did not use biomarkers of physiological stress using tissue samples and salivary measures of cortisol or alpha‐amylase. Hence, our estimates may also reflect differences in the physiological stress response between participants reporting the same PSS score.
Using traditional laboratory‐based methods to collect and analyze data on semen quality in large cohorts is expensive and cumbersome. The Trak Male Fertility Testing System is an FDA approved and validated test kit for in‐home assessment of sperm concentration and semen volume. 22 It is a convenient and feasible device to assess semen quality, 21 as it does not require face‐to‐face appointments. As the participants receive Trak by mail, recruitment is not limited to specific geographic areas. Thus, we recruited participants distributed across Denmark and North America. Trak has demonstrated adequate reproducibility and detection range for semen volume and sperm concentration compared with WHO cut‐off values. 21 , 22 , 23 , 24 We did not collect data on sperm motility, DNA fragmentation, or sperm morphology, which are other potentially important markers of semen quality.
Although we pooled data across cohorts, some of our associations lacked precision, and the estimates for the cohort‐specific associations were too imprecise for solid interpretation.
We adjusted the pooled analyses for several potential confounders. These include fever within the past 3 months, and ever being diagnosed with depression or anxiety; the factors that were most prevalent among men with a high PSS score. None of these factors changed the point estimates considerably. However, our data may not reflect fever episodes, or depression and anxiety at the time points most pertinent to spermatogenesis.
We observed a lower mean PSS score among Danish men compared with North American men despite using the same approach to measure perceived stress. This might reflect that Danish and North American men perceive their daily stress levels differently, or Danish men have a lower absolute level of stress. If a threshold value exists for stress to impact semen quality, we have limited ability to identify an association between high levels of stress and semen quality among Danish men as only four (8%) reported a PSS score ≥20. However, as Danish men only constituted 8% of the total study population, we do not expect this to explain the overall the null finding.
Both SF and PRESTO enroll couples with fertility ranging from highly fertile to infertile. Overall, 40% of the participants had previously fathered a child, thus demonstrating their ability to impregnate a partner. Unfortunately, due to small numbers we were unable to analyze data in sub‐groups of men, who previously fathered a child and men who did not. We did not enroll couples with unintended pregnancies, who are more likely to have higher fertility. Hence, men in our study may have lower semen quality than men in the general population. Nevertheless, only 1.4% of the men reported a previous semen analysis, thus most men were unaware of their semen quality at study entry. We could not determine if stressed men were more or less likely to participate in the parent SF and PRESTO cohorts. However, our subanalysis demonstrated a similar PSS score for participants and non‐participants in the in‐home semen study. Thus, we have no indication that selection bias is a major problem in the semen study.
In conclusion, our findings did not indicate a meaningful association between perceived stress and semen quality.
AUTHOR CONTRIBUTIONS
Katrine H. Lund, Ellen M. Mikkelsen, Elizabeth E. Hatch, Lauren A. Wise, Anne Sofie D. Laursen, Gunnar Toft, Greg J. Sommer, Amelia K. Wesselink, Michael L. Eisenberg, Henrik Toft Sørensen, and Kenneth J. Rothman designed the study. Katrine H. Lund wrote the first and successive drafts of the paper. Katrine H. Lund, Bjarke H Jacobsen, Tanran R. Wang, and Therese K. Grønborg carried out the statistical analysis. Greg J. Sommer developed the semen testing kit that was used for primary data collection. All authors contributed to the interpretation of results, reviewed, and approved the final manuscript.
CONFLICT OF INTEREST
PRESTO has received in‐kind donations from Sandstone Diagnostics (for semen kits), Swiss Precision Diagnostics (home pregnancy tests), Kindara.com (fertility app), and FertilityFriend.com (fertility app). Dr. Lauren Wise serves as a fibroid consultant for AbbVie, Inc.
Supporting information
Supp information
ACKNOWLEDGMENTS
The authors thank Tina Christensen for her support with data collection and media contacts in SF, and Benjamin R. Johannesen and Katrine Eriksen, who have helped with data cleaning and provided guidance for the statistical analyses. We also acknowledge the assistance of Michael Bairos, Alina Chaiyasarikul, Jessica Levinson, Martha Koenig, and Andrea Kuriyama. The study was supported by the National Institute of Child Health and Human Development (R01‐HD086742), (R01‐HD060690), and R21‐HD094322 and R01‐HD105863.
Lund KH, Laursen ASD, Grønborg TK, et al. Perceived stress and semen quality. Andrology. 2023;11:45–53. 10.1111/andr.13301
REFERENCES
- 1. Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324‐1331. https://doi.org10.1016/j.fertnstert.2012.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Irvine DS. Epidemiology and aetiology of male infertility. 1998;13:33‐44. [DOI] [PubMed] [Google Scholar]
- 3. Levine H, Jorgensen N, Martino‐Andrade A, et al. Temporal trends in sperm count: a systematic review and meta‐regression analysis. Hum Reprod Update. 2017;23(6):646‐659. https://doi.org10.1093/humupd/dmx022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Priskorn L, Nordkap L, Bang AK, et al. Average sperm count remains unchanged despite reduction in maternal smoking: results from a large cross‐sectional study with annual investigations over 21 years. Hum Reprod. 2018;33(6):998‐1008. https://doi.org10.1093/humrep/dey090 [DOI] [PubMed] [Google Scholar]
- 5. Danish Health Authority . Danskernes Sundhed ‐ Den Nationale Sundhedsprofil 2017. Danish Health Authority; 2018. [Google Scholar]
- 6. Anderson NBBC, Breckler SJ, Nordal KC, et al. Stress in America ‐ Paying With Our Health. American Psychological Association; 2015. [Google Scholar]
- 7. Nargund VH. Effects of psychological stress on male fertility. Nat Rev Urol. 2015;12(7):373‐382. https://doi.org10.1038/nrurol.2015.112 [DOI] [PubMed] [Google Scholar]
- 8. Byun JS, Lyu SW, Seok HH, Kim WJ, Shim SH, Bak CW. Sexual dysfunctions induced by stress of timed intercourse and medical treatment. BJU Int. 2013;111:E227‐E234. https://doi.org10.1111/j.1464‐410X.2012.11577.x [DOI] [PubMed] [Google Scholar]
- 9. Ilacqua A, Izzo G, Emerenziani GP, Baldari C, Aversa A. Lifestyle and fertility: the influence of stress and quality of life on male fertility. Reprod Biol Endocrinol. 2018;16(1):115. https://doi.org10.1186/s12958‐018‐0436‐9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eskiocak S, Gozen AS, Taskiran A, Kilic AS, Eskiocak M, Gulen S. Effect of psychological stress on the L‐arginine‐nitric oxide pathway and semen quality. Braz J Med Biol Res. 2006;39(5):581‐588. 10.1590/s0100-879x2006000500003 [DOI] [PubMed] [Google Scholar]
- 11. Nordkap L, Jensen TK, Hansen AM, et al. Psychological stress and testicular function: a cross‐sectional study of 1,215 Danish men. Fertil Steril. 2016;105(1):174‐187. https://doi.org10.1016/j.fertnstert.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 12. Nordkap L, Priskorn L, Bräuner EV, et al. Impact of psychological stress measured in three different scales on testis function: a cross‐sectional study of 1362 young men. Andrology. 2020;8(6):1674‐1686. https://doi.org10.1111/andr.12835 [DOI] [PubMed] [Google Scholar]
- 13. Hjollund NH, Bonde JP, Henriksen TB, Giwercman A, Olsen J. Reproductive effects of male psychologic stress. Epidemiology (Cambridge, Mass). 2004;15(1):21‐27. https://doi.org10.1097/01.ede.0000100289.82156.8b [DOI] [PubMed] [Google Scholar]
- 14. Janevic T, Kahn LG, Landsbergis P, et al. Effects of work and life stress on semen quality. Fertil Steril. 2014;102(2):530‐538. https://doi.org10.1016/j.fertnstert.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sørensen HT. Cohort profile: the Danish web‐based pregnancy planning study–‘snart‐gravid. Int J Epidemiol. 2009;38(4):938‐943. https://doi.org10.1093/ije/dyn191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huybrechts KF, Mikkelsen EM, Christensen T, et al. A successful implementation of e‐epidemiology: the Danish pregnancy planning study ‘Snart‐Gravid. Eur J Epidemiol. 2010;25(5):297‐304. https://doi.org10.1007/s10654‐010‐9431‐y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Christensen T, Riis AH, Hatch EE, et al. Costs and efficiency of online and offline recruitment methods: a web‐based cohort study. J Med Internet Res. 2017;19(3):e58. https://doi.org10.2196/jmir.6716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hatch EE, Hahn KA, Wise LA, et al. Evaluation of selection bias in an internet‐based study of pregnancy planners. Epidemiology (Cambridge, Mass). 2016;27(1):98‐104. https://doi.org10.1097/ede.0000000000000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wise LA, Rothman KJ, Mikkelsen EM, et al. Design and conduct of an internet‐based preconception cohort study in North America: pregnancy study online. Paediatr Perinat Epidemiol. 2015;29(4):360‐371. https://doi.org10.1111/ppe.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385‐396. [PubMed] [Google Scholar]
- 21. Sommer GJ, Wang TR, Epperson JG, et al. At‐home sperm testing for epidemiologic studies: evaluation of the Trak male fertility testing system in an internet‐based preconception cohort. Paediatr Perinat Epidemiol. 2019;34(5):504‐512. https://doi.org10.1111/ppe.12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schaff UY, Fredriksen LL, Epperson JG, et al. Novel centrifugal technology for measuring sperm concentration in the home. Fertil Steril. 2017;107(2):358‐364. https://doi.org10.1016/j.fertnstert.2016.10.025 [DOI] [PubMed] [Google Scholar]
- 23. Fredriksen LLEJ, Hong K, Lacovetti G, Doig I, Sommer G, Schaff U. Design and validation of the trak® volume cup ‐ a dual purpose semen collection and volume measurement device for diagnosing hypospermia. Fertil Steril. 2018;110(4):e306. 10.1016/j.fertnstert.2018.07.777 [DOI] [Google Scholar]
- 24. WHO . WHO Laboratory Manual for the Examination and Processing of Human Semen Fifth Edition Ed . World Health Organization; The UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), WHO Department of Reproductive Health and Research (RHR; ); 2010. [Google Scholar]
- 25. Pedersen AB, Mikkelsen EM, Cronin‐Fenton D, et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. 2017;9:157‐166. https://doi.org10.2147/clep.S129785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee EH. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res (Korean Soc Nurs Sci). 2012;6(4):121‐127. https://doi.org10.1016/j.anr.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 27. Cohen S, Williamson G, Spacapam S, Oskamp S. Perceived stress in a probability sample of the United States. Soc Psychol Heal Claremont Symp Appl Soc Psychol. 1988;3:31‐67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp information


