Summary
Dysregulation of the alternative complement pathway predisposes individuals to a number of diseases. It can either be evoked by genetic alterations in or by stabilizing antibodies to important pathway components and typically leads to severe diseases such as paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, C3 glomerulopathy, and age‐related macular degeneration. In addition, the alternative pathway may also be involved in many other diseases where its amplifying function for all complement pathways might play a role. To identify specific alternative pathway inhibitors that qualify as therapeutics for these diseases, drug discovery efforts have focused on the two central proteases of the pathway, factor B and factor D. Although drug discovery has been challenging for a number of reasons, potent and selective low‐molecular weight (LMW) oral inhibitors have now been discovered for both proteases and several molecules are in clinical development for multiple complement‐mediated diseases. While the clinical development of these inhibitors initially focuses on diseases with systemic and/or peripheral tissue complement activation, the availability of LMW inhibitors may also open up the prospect of inhibiting complement in the central nervous system where its activation may also play an important role in several neurodegenerative diseases.
Keywords: alternative complement pathway; complement factor B, complement factor D, complement therapeutics, drug discovery, low‐molecular weight inhibitor
1. INTRODUCTION
The complement system, a major arm of the humoral innate immune system, comprises a cascade of sequentially activated proteases directed at the elimination of pathogens. Deposition of complement components on pathogen surfaces, either directly, or via surface‐bound antibodies induces a proteolytic cascade that generates multiple pro‐inflammatory protein fragments with diverse functions: Direct cell lysis, chemoattraction and activation of innate immune effector cells, and opsonization, that is, facilitation of pathogen removal by phagocytic cells. As the complement system is a rapid and amplified pro‐inflammatory effector system, it needs to be tightly controlled to prevent aberrant and destructive activation. Primary failure of these control mechanisms due to mutations of complement regulators, gain‐of function mutations in components of the protease cascade or secondary activation of the complement system due to autoantibodies that stabilize activating complement proteases or neutralize complement regulators or are directed at “self” antigens, all lead to devastating auto‐inflammatory diseases.
2. ACTIVATION AND REGULATION OF THE ALTERNATIVE COMPLEMENT PATHWAY
Depending on the trigger, the complement cascade is activated through three distinct pathways (classical, lectin, and alternative), which all converge on the proteolytic cleavage of the central complement component C3 into C3a and C3b. While the classical and lectin pathways are triggered by immune complexes and specific microbial polysaccharides, respectively, the alternative pathway is constitutively active at a low level through spontaneous “tick‐over” hydrolysis of C3 (C3[H2O]). 1 Properdin stabilizes C3b and C3Bb for local amplification of the alternative pathway.
The activity of the alternative pathway is driven by two trypsin‐like serine proteases, factor D (CFD) and factor B (CFB, Figure 1A). Spontaneous hydrolysis of C3 or its proteolytic cleavage to C3b induces a pronounced conformational change within its thioester domain. This exposes a binding site for factor B and allows formation of the C3bB complex in a Mg2+ dependent manner. This binding step exposes a flexible linker structure within factor B, thus making it vulnerable for cleavage by factor D. 2 Factor D cleavage of factor B releases the Ba fragment and generates the C3 convertase C3bBb, which cleaves further C3 molecules and amplifies the complement response locally: Newly generated C3b can bind covalently to cell surfaces via its reactive thioester group. Since the thioester has a very short half‐life of approximately 60 μs, C3b attachment normally occurs within a few nanometers from the C3 convertase. 3 C3bBb also has a short half‐life of 90 seconds, 4 and binding to properdin stabilizes the complex by a factor of ten. 5 C3b formation can either lead to the localized formation of novel surface‐bound C3 convertases (C3bBb) for cleavage of more C3 into the pro‐inflammatory anaphylatoxin C3a and C3b or, if the local concentration of C3bBb is already high, new C3b may bind to the C3 convertase directly. This results in formation of the C5 convertase (C3bBbC3b) for cleavage of C5 and initiation of the terminal complement pathway, which culminates in formation of the membrane attack complex C5b‐9. Finally, local degradation of C3bBb by factor I (CFI) and its co‐factor factor H (CFH) leads to the formation of iC3b and C3d, which both act as opsonins, that is, facilitators of phagocytosis.
FIGURE 1.
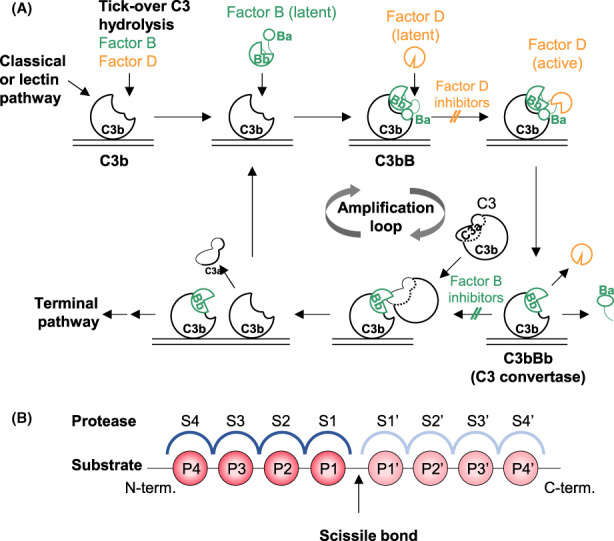
Protease function in the alternative pathway. (A) Proteolytic activation of the alternative complement pathway. For detailed description see text. (B) Schematic representation of the active site of proteases, indicating individual binding pockets of the protease (S, blue) and corresponding substrate amino acids (P, red), N‐terminally (non‐prime, dark colors) or C‐terminally (prime, light colors) of the scissile bond
It is of note that, in addition to their role in initiation of the alternative pathway, C3, factor B and factor D, support rapid amplification of all three complement pathways in the so‐called amplification loop. 6
Although the alternative pathway leads to the proteolytic formation of many immune effector proteins, this review will specifically focus on the two core protease components of the alternative complement pathway, factor B and factor D, and past and current pharmacological attempts to interfere with their function using low molecular weight (LMW) antagonists.
The core components of the alternative pathway, complement factors D and B, are serine proteases. Serine proteases have been targeted by the pharmaceutic industry for many years and several protease inhibitors have been approved for therapeutic use, for example, direct oral anticoagulants (factor Xa and thrombin inhibitors), anti‐diabetic agents (dipeptidyl peptidase 4 inhibitors) and treatments for hereditary angioedema (plasma kallikrein inhibitors). 7 In serine proteases, the active site serine mediates the nucleophilic attack at the peptide bond of the substrate protein via a covalent acyl enzyme intermediate and subsequent hydrolysis by water, and substrate specificity is determined by sub‐pockets in the active site. These pockets are numbered S1, S2, …Sn from the scissile bond towards the N‐terminus of the substrate (non‐primed site) and S1', S2', …Sn' from the scissile bond towards the C‐terminus of the substrate (primed site) as shown in Figure 1B. 8 The substrate residues that each of these pockets accommodate are designated as P1, P2, …Pn on the non‐primed and P1', P2', …Pn' on the primed site. Depending on the size and architecture of the sub‐sites, the specificity of proteases varies greatly. Serine protease inhibitors typically bind into the active site of the protease where substrate recognition and hydrolysis occur, and the sub‐pockets most closely to the scissile bond have most successfully been used to design selective inhibitors for the respective proteases.
3. THE ALTERNATIVE COMPLEMENT PATHWAY IN INFLAMMATORY DISEASES
Activation of the alternative pathway has been implicated in many diseases (Table 1). Diagnostically, complement activation in a disease is inferred, for example, by immunohistochemistry of inflamed target tissues or, for systemic diseases, by ELISA‐based assays for complement fragments or for autoantibodies against complement fragments. The detection of somatic or germ‐line mutations affecting the alternative pathway may provide additional evidence. More recently, the successful clinical use of antibody therapeutics directed against C5 strengthened the evidence for the involvement of complement pathways in some indications. To build mechanistic hypotheses for the specific involvement of the alternative pathway in diseases, researchers have used factor D or factor B deficient mice or rodent models treated with pathway‐specific inhibitors. In addition, translational studies using antagonists of the alternative complement pathway on patient biosamples or recombinant mutant patient proteins does not only provide strong evidence to position clinical candidate compounds in an indication, it also supports the functional dissection of complex pathologies. 9 , 10
TABLE 1.
Diseases associated with activation of the alternative pathway (AP) and therapeutic approaches using small‐molecule inhibitors
| Disease | Rationale for involvement of the alternative pathway | Low‐molecular weight antagonists in Phase 2/3 trials |
|---|---|---|
| ||
| Paroxysmal nocturnal hemoglobinuria (PNH) |
Genetic: Mutations in the X‐linked phosphatidylinositol glycan class A (PIG‐A) gene on some hematopoietic stem cell clones reduces membrane expression the C3bBb regulator CD55 and the C9 regulator CD59 135 Functional: Erythrocytes from conditional PIG‐A−/− mice show sensitivity to complement attack, 136 , 137 PNH patient erythrocytes are susceptible to complement lysis 138 Pharmacological: Clinical efficacy of eculizumab 139 and ravulizumab 140 in PNH |
Danicopan Vemircopan BCX9930 Iptacopan |
| Atypical hemolytic uremic syndrome (aHUS) |
Genetic: Surface dysregulation of the AP by diverse genetic or acquired mechanisms in 26–62% or patients 12 Functional: Mice with hyperfunctional C3 or aHUS‐related factor H mutation show aHUS‐like disease 141 , 142 Pharmacological: Clinical efficacy of eculizumab 143 and ravulizumab 144 |
Iptacopan |
| Age‐related macular degeneration (AMD) and secondary geographic atrophy (GA) |
Genetic: Polymorphisms in factor H (e.g., Y402H), C3 or FB/C2 145 Functional: Cfh−/− mice show disturbed retinal morphology and blood supply, 146 , 147 Pharmacological: Systemic eculizumab did not show clinical benefit in AMD patients 148 |
Danicopan Iptacopan |
|
C3 glomerulopathy (C3G) |
Genetic: Mutations in factor H (similar to aHUS 149 ) or factor H related proteins 150 ; or autoantibodies against factor H, or C3 stabilizing antibodies (nephritic factors, C3nef) 151 Clinical: Glomerular C3 staining and glomerular/sub‐endothelial electron‐dense deposits. Low plasma C3 levels common Functional: Cfh−/− mice 116 and humanized C3 mice lacking C3 regulation by (murine factors 152 ) show C3G‐like disease Pharmacological: Case report for efficacy of eculizumab in C3G 153 , 154 |
Danicopan BCX9930 Iptacopan |
| ||
| IgA nephropathy |
Pathophysiology: Immune complexes of galactose‐deficient (Gd‐)IgA1 and autoantibodies in urine 155 or blood. 156 Deficiency in FHR1 and FHR3 protects 157 , 158 Clinical: Mesangial IgA/C3 deposits 159 ; mesangial C3 deposition, 160 MBL deposition 30 or rare C1q deposition 161 correlates with severity; IgA can activate alternative 27 or lectin 30 pathway Functional: C5a−/− or C3a−/− are protected in a mouse model of IgAN 162 Pharmacological: Case report for partial efficacy of eculizumab 163 |
Vemircopan BCX9930 Iptacopan |
| Lupus nephritis |
Pathophysiology: Multifactorial. Genetic variants of factor H are associated with lupus 164 Clinical: C3 deposition in the kidney without C1q/C4 deposits had worse outcomes in patients, 165 serum factor H levels correlate negatively with disease score/SLEDAI 166 Functional: Factor B‐or Factor D‐deficient MRL/lpr mice are protected, 117 , 120 factor H‐deficient MRL/lpr mice show faster disease progression 167 Antisense oligonucleotides targeting FB improve lupus nephritis in MRL/lpr and NZB/W mice 168 Pharmacological: Case report for efficacy of eculizumab 169 |
Vemircopan Iptacopan |
| Cold agglutinin disease (CAD) |
Pathophysiology: Clonal proliferative disorder of B cells producing cold agglutinin (erythrocyte anti‐I antigen IgM). No known genetic link. Clinical: Monospecific direct antiglobulin test detects C3d on surface of erythrocytes. 170 Alternative pathway as amplification loop. Pharmacological: Clinical efficacy of sutimlimab (anti‐C1s, 171 ) modest efficacy of eculizumab 172 |
Iptacopan |
| (Primary) immune Thrombocytopenia/ (Idiopathic) immune thromboctopenic purpura (ITP) |
Pathophysiology: Autoantibodies against platelet surface glycoproteins. No known genetic link. Clinical: Patients with ITP activate the classical complement pathway (CP) and/or fix complement on platelet surfaces. 173 , 174 Alternative pathway as amplification loop. Pharmacological: Clinical efficacy of sutimlimab (anti‐C1s 175 ) |
Iptacopan |
| Idiopathic/primary membranous nephropathy (iMN) |
Pathophysiology: Associated with anti‐phospholipase A2 receptor antibodies, which bind to the corresponding antigens on podocytes to form immune complexes. Not a genetic disease. Functional: Cfb−/− mice are protected from proteinuria induced by α3NC1 immunization, 176 LNP023 protects in rat passive Heymann nephritis 9 |
BCX9930 Iptacopan |
4. PROTOTYPICAL DISEASES WITH HYPERACTIVE ALTERNATIVE PATHWAY
Over the past decades, genetic evidence or complement‐specific autoantibodies have pointed to the involvement of the alternative pathway in four prototypical diseases: Paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), C3 glomerulopathy (C3G), and age‐related macular degeneration/geographic atrophy (AMD/GA). Complement hyperactivation in these four diseases affects either both the alternative and the terminal pathway (PNH), the alternative pathway on cell surfaces (aHUS, AMD/GA) or in the fluid phase (C3G).
The underlying cause of PNH are somatic mutations in the PIG‐A gene in hematopoietic stem cells, resulting in a decreased or absent expression of GPI‐anchored proteins on the cell surface, including complement regulators CD55 and CD59, which limit C3bBb and C5b‐9 formation, respectively. Mature erythrocytes lack surface expression of an additional complement regulator, CD46. 11 As a consequence, CD55/CD59 deficient erythrocytes are particularly vulnerable to unbridled activation of C3bBb and attack of the terminal pathway, leading to episodic complement‐mediated hemolysis.
More diverse mutations in complement‐regulatory genes or antibodies against the alternative pathway regulatory protein factor H have been found in patients suffering from atypical hemolytic uremic syndrome (aHUS), a systemic thrombotic microangiopathy that can lead to stroke and renal failure. 12 , 13 Most of these mutations and autoantibodies lead to reduced binding of factor H to cell surfaces, thereby limiting local complement regulation, while in many of these patients, there are no detectable systemic signs of complement consumption. In contrast, C3 glomerulopathy (C3G) is characterized by alternative pathway dysregulation in the fluid phase, either caused by mutations in complement‐regulatory proteins or factor H neutralizing autoantibodies or autoantibodies stabilizing C3 or factor B. C3G constitutes a more heterogeneous disease entity including dense deposit disease (DDD) and C3 glomerulonephritis (C3GN), which are distinguishable based on electron microscopical features. Despite complement dysregulation in the fluid phase, deposition of C3 fragments but not of immunoglobulins is typically found in the kidney of these patients, pointing to additional local complement activation. It is of note in this context that membranoproliferative glomerulonephritis (MPGN) does not refer to a disease entity, but to a histological pattern of hypercellularity and thickening of the renal glomerular basement membrane that is observed, amongst other, in C3G.
Another disease with mutations leading to increased alternative pathway activation is age‐related macular degeneration (AMD), a pathology that manifests in the retina and may progress to local retinal degeneration called geographic atrophy (GA) and visual function loss. These patients show deposition of complement cleavage fragments around subretinal drusen, that is, accumulations of lipids and proteins, 14 , 15 and commonly have loss‐of‐function mutations of factor H or, in rare cases, mutations in C3 or factor B. 16
The alternative pathway is a potent effector arm of anti‐infectious immunity, and elevated alternative pathway activity confers a survival advantage in patients with acute respiratory failure, possibly due to reduced risk of bacteremia. 17 Some viruses and bacteria have evolved strategies to evade complement attack, 18 , 19 , 20 and the evolutionary “arms race” with pathogens could be a driver for activating mutations of the alternative pathway, yet in some cases they may be pathogenic themselves.
All four pathologies with direct evidence of hyperactivated alternative pathway activity offer defined entry points for clinical trials for specific antagonists of the alternative complement pathway, with clear clinical readouts and the potential to interfere pharmacologically with the root cause of disease. As the key component of the alternative pathway, the C3 convertase (C3bBb), is predominantly activated locally on cell surfaces, alternative pathway‐driven diseases often manifest locally. However, several of these diseases also display signs of systemic pathway activation, for example, through the production of soluble complement activation and inactivation products or, particularly in C3G, through the generation of systemic C3 convertase. 21 Therefore, effective treatment of these diseases will require systemically and locally active compounds for alternative pathway inhibition.
5. AUTOIMMUNE DISEASES WITH HYPERACTIVATION OF THE ALTERNATIVE PATHWAY
The alternative pathway has also been invoked as a critical contributor to autoimmune diseases, downstream of autoantibody formation. An autoimmune etiology is observed in some patients suffering from prototypic diseases with alternative pathway activation where autoantibodies enhance alternative pathway activity directly. Examples are neutralizing antibodies to the complement‐regulatory factor H, which are observed in 3% of patients with C3G, and in 11% of patients with aHUS. 22 Other alternative pathway‐enhancing autoantibodies are the so‐called nephritic factors, C3nef and C5nef, which are frequently detected in C3G patients. 23 Nephritic factors bind to neo‐epitopes of the assembled C3 and C5 convertases, respectively, thus stabilizing these protease complexes and enhancing overall pathway activity. Of note, anti‐C3 autoantibodies that enhance alternative pathway activity have also been described for a subset of patients with systemic lupus erythematosis. 24 , 25 , 26
A second class of autoantibodies trigger activation of the alternative pathway indirectly by different mechanisms. One example is the local deposition of immune complexes containing galactose‐deficient IgA1 complexes and IgG autoantibodies in the kidneys of IgA nephropathy (IgAN) patients. IgA aggregates in serum 27 and surface‐bound IgA1 28 can lead to alternative pathway activation, which may contribute to the pathology of IgA nephropathy. 27 , 29 The lectin pathway may contribute to the disease in a subset of IgAN patients as well. 30
Another example of how alternative pathway proteases are involved in disease is via the amplification loop secondary to classical or lectin pathway activation. Autoantibody‐driven diseases where the classical pathway is hypothesized to play a crucial part are idiopathic thrombocytopenia (ITP) or cold agglutinin disease (CAD) where autoantibodies bind to platelets or erythrocytes, respectively. In vitro data suggest that the amplification can contribute up to 80% to C5b‐9 generation after classical pathway activation. 31
The functional connection of autoantibodies to alternative pathway activation is yet more complex in ANCA vasculitis and systemic lupus erythematosus (SLE), as neutrophils play a central role in both diseases. In ANCA vasculitis, preclinical data suggest that autoantibodies to neutrophil myeloperoxidase (MPO) or proteinase 3 (PR3) trigger neutrophil release of microparticles, which in turn activate the alternative pathway. 32 , 33 In SLE patients, formation of neutrophil extracellular traps (NETs), 34 , 35 and their persistence may activate the classical and/or alternative pathway. 36 , 37 However, not all lupus patients show enhanced formation of NETs, 35 and some may even express anti‐MPO autoantibodies, as observed for ANCA vasculitis patients. 38 Finally, lupus nephritis patients show “full house” nephropathy, that is, local deposition of IgM, IgA, IgG, C1q, and C3, demonstrating that multiple complement activating mechanisms may be at play. It will be interesting to analyze whether prevalence of a certain isotype of autoantibodies correlates to classical or alternative complement pathway activation. This all points to the extreme heterogeneity of SLE and to the need of using biomarkers to identify SLE patients that will benefit most from alternative pathway inhibitors. Alternatively, SLE may offer the opportunity to rationally combine alternative pathway inhibitors with other disease‐modifying drugs. For example, hydroxychloroquine, the standard of care treatment in SLE, requires 6 months to reach efficacious steady‐state exposures, 39 while small molecule antagonists of the alternative pathway can have a rapid onset of action 40 and may thus offer more immediate relief to patients.
Yet another field in which antagonists of the alternative pathway could be considered are acute infections where uncontrolled complement activation may contribute to immunopathology. For example, factor D inhibition has been proposed as a treatment for COVID‐19, as SARS‐CoV‐2 activates the alternative pathway through competition with factor H for cell surface binding. 41 , 42 However, these considerations require a careful risk–benefit assessment, as controlled complement activation contributes to pathogen clearance as well. Therefore, complement inhibition during acute infectious immunopathologies should ideally be combined with antibiotic treatment and of short duration. This requirement for short serum half‐life makes small molecules the best modality for this purpose in an intensive care setting.
6. TARGET TISSUE PREDILECTION OF ALTERNATIVE PATHWAY‐MEDIATED DISEASES
An intriguing observation from genetic and autoantibody‐associated alternative pathway‐mediated diseases is that the strongest end‐organ damage often manifests in the kidney and the eye. Interestingly, kidney diseases such as aHUS and C3G may also show ocular symptoms 43 , 44 , 45 and ocular diseases such as AMD may be associated with decreased renal function. 46
A plausible reason for this organ predilection is the specific tissue microenvironment. In the kidney and the eye, blood vessels have a fenestrated structure. This enables plasma components to permeate the surrounding tissue, while erythrocytes cannot pass through the vessel walls. Consequently, ocular and renal tissue is bathed in plasma without the local presence of CR1 on erythrocytes which could confer protection against local complement attack. Glomerular microvascular endothelial cells also express lower levels of the complement regulators (e.g., CD46, CD55, thrombomodulin, CFI and CFH), thus facilitating local complement activation. 47 In addition, loss of heparan sulfate proteoglycans in the glomerular basement membrane (GBM), which is observed in several kidney diseases including membranous nephropathy and lupus nephritis, reduces factor H binding and local regulation of alternative pathway activity. 48 Finally, reduced glomerular filtration leads to increased levels of complement factor D in plasma and may drive a systemic alternative pathway‐driven inflammatory feed‐forward loop. 49
In the eye, photo‐oxidation products of the retinal pigment epithelium (RPE) can lead to alternative pathway activation 50 and oxidized photoreceptor outer segments lead to downregulation of complement regulators by RPE cells. 51 Moreover, at least in rodents, complement factor B is upregulated in the aged RPE, and the alternative pathway is continuously active in the anterior chamber of the eye, 52 , 53
Overall, there are multiple opportunities to benefit patients with antagonists of the alternative pathway, and Figure 2 illustrates some potential and on‐going clinical development spaces for these compounds. Some of the diseases are genetic diseases where alternative pathway inhibition is expected to deliver transformative efficacy. Others are more heterogeneous, complex diseases, in which ongoing clinical trials will tell which subset of patients may benefit from these treatments.
FIGURE 2.
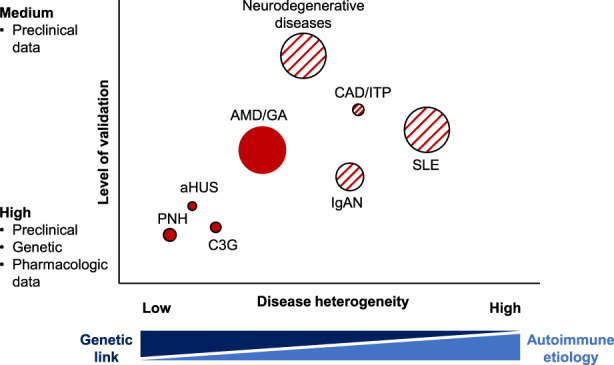
Potential indications for antagonists of the alternative complement pathway. Clinical development of factor D and factor B antagonists has been pursued in diseases of low heterogeneity and a high level of preclinical and pharmacological validation. Area of circles represents prevalence of the diseases (PNH 0.1/100.000, C3G: 0.2/100.000, aHUS: 0.3/100′000, dry AMD: 440/100′000, CAD 1.6/100′000, ITP: 9.5/100′000, IgAN: 2.5/100′000; ANCA vasculitis: 5–18/100′000; SLE: 20–150/100′000) and hatched circles indicate that clinical responses to alternative pathway antagonists may vary between patient subgroups
These therapeutic prospects have triggered numerous alternative pathway‐directed drug discovery efforts over the past 15 years. In most cases, the focus of these has been on low‐molecular weight (LMW) inhibitors of the pathway‐specific proteases factor D and B for three main reasons. First, as outlined above, effective treatment of many of these diseases requires inhibition both in inflamed tissues and systemically, and LMW inhibitors have a higher potential for tissue penetration than neutralizing antibodies. Second, low molecular weight antagonists might have a safety advantage with respect to infections. Pharmacological blockade of C5 with eculizumab leads to an increased risk of infection with encapsulated bacteria 54 and the alternative pathway may play a role in anti‐bacterial protection as well. 55 In a situation of enhanced infectious risk, the use of a LMW inhibitor with a short serum half‐life has a general advantage over biologics, as they are washed out more quickly to establish full immunocompetence should an infection occur. And finally, LMW inhibitors have only a marginal effect on the metabolic stability of their target proteins, while antibodies often significantly increase the in vivo half‐life and steady‐state levels of their targets. 56 , 57 This effect would be most dramatic for a protein like factor D which is cleared through the kidney with a plasma half‐life of about 1 h 58 (see Table 3). Constant high production of factor D would rapidly outcompete systemic concentrations of a neutralizing antibody, such that constant antibody infusion would be required to reach efficacious exposures. One approach to avoid this mechanism could be local treatment, as was tried for a neutralizing factor D antibody, lampaliumab, which was administered intravitreally for local treatment of AMD, yet not further developed thus far. 59 Similarly, factor B is present in blood at a rather high concentration of 2–3 μM, and while its production rate is much slower (1.6% per h), 60 efficient neutralization may still require unrealistically high steady‐state drug levels.
TABLE 3.
Properties of factor B and factor D relevant to drug discovery
| Factor B | Factor D | |
|---|---|---|
| Serum concentration in humans |
200–400 μg/mL (2–3 μM) Serum levels can increase by 50% during infection 95 |
2 μg/mL, i.e., 80 nM Serum levels increase by >10 fold in situations of kidney impairment 58 |
| Half‐life | Low clearance: Plasma half‐life 30–70 h. Fractional metabolic rate (FMR) 1.6%/h in healthy individuals, increased in patients 60 , 177 | Very high clearance, plasma half‐life approximately 1 h. Fractional metabolic rate (FMR) 59.6%/h 58 |
| Major site of production | Hepatocytes, may be expressed by multiple cell types at sites of inflammation | Adipocytes, myeloid cells, hepatocytes |
| Observations in ko mice | Normal, healthy 115 | Spontaneous glomerulonephritis 123 |
| Alternative pathway activation by cobra venom factor is fully inhibited 178 | Reduced, but residual alternative pathway activation by cobra venom factor 178 |
7. COMPLEMENT FACTOR D AS A DRUG TARGET
7.1. Key aspects of factor D biology relevant for drug discovery
Complement factor D, also known as adipsin, is a 24 kDa trypsin‐like S1 serine protease of 228 amino acids. It has a high turnover rate (Table 3) and is excreted through the kidney. 58 In healthy individuals, factor D has the lowest steady‐state serum concentration of all central complement proteins (approximately 2 μg/mL or 80 nM), 61 yet its serum levels can increase 10‐fold or more in renally impaired patients. 49 , 58 , 62 , 63 , 64
Factor D is synthesized by adipocytes as a pro‐enzyme and processed in plasma to its latent mature form by mannose‐associated serum protease 3 (MASP‐3). 65 , 66 Patients suffering from MASP‐1/3 deficiency (Malpuech–Michels–Mingarelli–Carnevale disease) still express low levels of mature latent factor D suggesting that a MASP‐3 independent mechanism for pro‐factor D cleavage may exist. 66 , 67 Latent factor D is characterized by an atypical active site architecture: The catalytic triad is in an inactive conformation, a self‐inhibitory loop (amino acids 214–218) restricts access to the non‐primed substrate recognition sites, and an Arg218‐Asp189 salt bridge is at the bottom of the S1 pocket. 68 Latent factor D acquires its full proteolytic activity by a conformational change which is induced upon binding to its surface‐bound endogenous substrate, C3bB. This triggers factor B cleavage to yield the C3 convertase C3bBb. 69 Initially, factor D was thought to be the rate‐limiting protease of the alternative pathway, based on low plasma concentrations and studies with reconstituted factor D deficient plasma. 70 , 71 However, more recently it has become clear that in diseases with increased alternative pathway activity, such as C3G, very low levels of factor D (~1% of plasma levels) are sufficient to activate the alternative pathway, especially if the C3 convertase is stabilized. This also suggested that >90% of enzyme inhibition may be required to fully shut down the alternative complement pathway. 72 Beyond its function in innate immunity, factor D lowers blood glucose 73 and promotes adipogenesis via generation of C3a, 74 and recent data suggests that factor D may play a role in aging of dermal fibroblasts as well. 75
7.2. Factor D inhibitors
Recombinant factor D does not recognize peptide substrates due to the self‐inhibitory loop which restricts access to the non‐primed site, but it possesses weak catalytic activity against synthetic lysine‐derived thioesters that only require binding into the S1 pocket. 76 Since the short half‐life of the C3b‐factor B complex limits its use as substrate for biochemical in vitro assays and inhibitor profiling, factor B bound to cobra venom factor (CVF) was used as a stable surrogate of the endogenous C3bB substrate. 77 This biochemical assay system was used to discover the first covalent prototypical inhibitors of factor D (Figure 3A). These inhibitors are hydrolyzed by the enzyme and stay covalently attached to the hydroxyl group of the catalytic serine, that is, they are “suicide” inhibitors. 3,4‐dichloroisocoumarin ( 1 ) 78 and 6‐amidino‐2‐naphthyl 4‐guanidino benzoate (FUT‐175) 77 are examples of such inhibitors (Figure 3A ).
FIGURE 3.
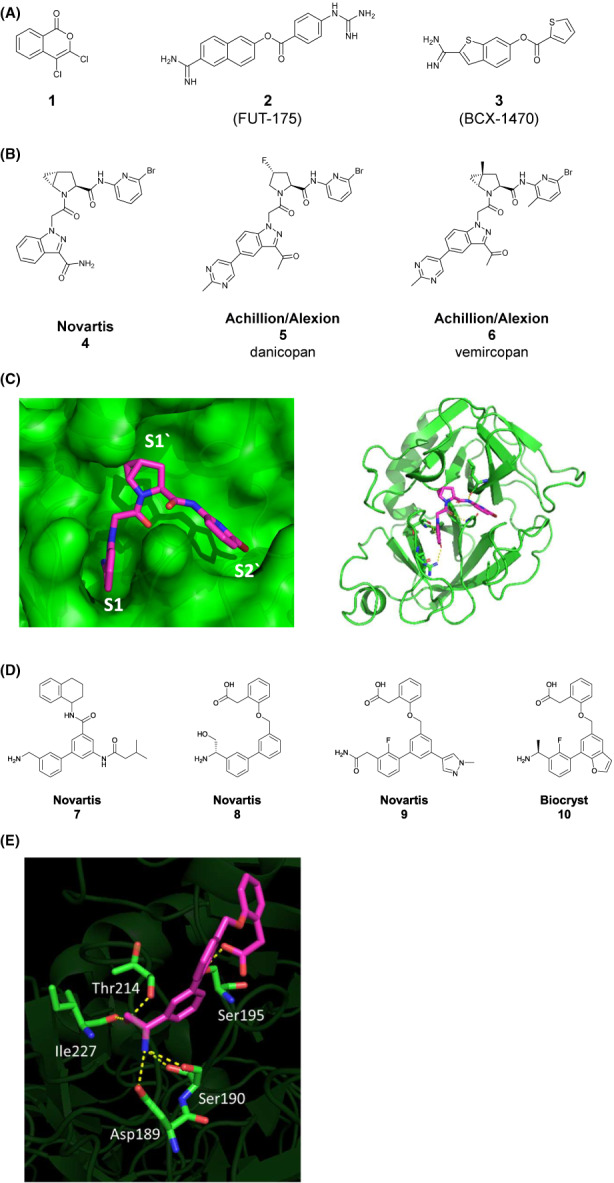
Low molecular weight inhibitors of factor D. (A) Prototypic covalent factor D inhibitors. (1) 3,4‐Dichloroisocoumarin, 78 (2) 6‐Amidino‐2‐naphthyl 4‐guanidino benzoate (FUT‐175). 77 (3) BCX‐1470 (Biocryst). 80 (B) Proline‐based factor D inhibitors. (4) from Novartis 81 ; (5) danicopan 83 and (6) vemircopan 86 from Achillion/Alexion. (C) Crystal structure of the complex between human factor D (green) and compound (4) (magenta, PDB code 5NB7) expanding from the S1 pocket into the S1 and S2 prime site. (D) Representative examples from benzylamine and benzylamide‐based factor D inhibitors extracted from publications and patents. Compounds (7), 87 (8), 89 (9) 90 from Novartis and (10) 91 from Biocryst. (E) Crystal structure of the complex between human factor D (green) and compound (8) 88 (magenta, PDB code 6QMR) with extended H‐bond network of aminopropanol motif of ligand to Asp189, Ser190, Ile227 and Thr214 in S1 pocket
The first factor D inhibitor that entered clinical development was BCX‐1470 (Biocryst, Figure 3A). It is structurally related to the first prototypical inhibitors and has an IC50 value of 93 nM in the above biochemical assay. 79 It entered Phase 1 clinical trials as an i.v. injectable compound. Of note, BCX‐1470 lacks factor D specificity and shows activity towards multiple other serine proteases, including C1s (IC50: 1.6 nM), thrombin (IC50: 42 nM), factor Xa (IC50: 3.2 nM), plasma kallikrein (IC50: 1.4 nM), and factor XIIa (IC50: 6.0 nM) 80 and its clinical development was discontinued, potentially due to safety concerns based on the strong off‐target activities.
To discover the next generation of more selective, orally bioavailable inhibitors of factor D, scientists at Novartis focused on non‐covalent inhibition principles and followed multiple hit‐finding strategies. However, two high‐throughput screens, one based on the above described thioesterolysis assay and the other on formation of the membrane attack complex (MAC, C5b‐9), each with >1 million compounds, failed to deliver validated hits. The Novartis laboratories eventually derived the first non‐covalent factor D inhibitor using a structure‐based design approach in combination with in‐silico docking of chemical fragments. This approach was inspired by the co‐crystal structure of kallikrein 7, a structurally related serine protease, with a proline‐based inhibitor that binds into the S1 pocket of kallikrein 7 and extends towards the primed site, a region which is open and accessible in the latent form of factor D. The initial hit was further optimized, also with the help of additional fragment‐based screening and resulted in the discovery of the first reported non‐covalent, potent, and orally bioavailable factor D inhibitor ( 4 ). 81 The inhibitor has an IC50 value of 30 nM and is highly selective against all 121 tested proteases, enzymes, receptors, and ion channels (Figure 3B).
The co‐crystal structure of compound 4 bound to factor D shows the azaindole moiety positioned in the S1 pocket (Figure 3C). Its carboxamide group is interacting with the salt bridge at the bottom of the S1 pocket through extensive hydrogen bonding. The central proline‐based moiety fills the S1 prime and the bromo pyridine moiety the S2 prime pocket, demonstrating the success of the initial design principles. 82
Since the self‐inhibitory loop in rodent factor D adopts a somewhat different conformation compared with the human protease, the rodent S1 pocket is significantly smaller and hinders binding of compound 4 to rodent factor D. Therefore, knock‐in mice for human factor D were used for in vivo pharmacology studies. In these mice, compound 4 fully blocked LPS‐induced activation of the alternative complement pathway at 30 mg/kg p.o. and caused sustained inhibition of Ba generation in plasma. 82
The first oral, clinical stage inhibitor of factor D, ACH‐4471 (5) (also known as ACH‐0144471, ALXN2040 or danicopan) was reported by Achillion. Danicopan shares common substructure motifs with compound 4 consisting of a proline core coupled to an amino bromo pyridine and an azaindole moiety (Figure 3B). It inhibits factor D proteolytic activity with an IC50 value of 15 nM and alternative pathway activation in vitro with an IC50 value of 23 nM. 83
In healthy volunteers, danicopan showed rapid and complete inhibition of alternative pathway activity for at least 16 hours after a single oral dose of 1200 mg. 83 However, multiple dosing was associated with elevations of liver enzymes in individual subjects at doses at and above 500 mg twice daily and limited the maximal dosing for further clinical studies to 200 mg three times per day. Two phase 2 studies in PNH patients were conducted with this compound (NCT03053102; NCT03472885, Table 2). In treatment‐naive patients, danicopan monotherapy significantly reduced mean LDH levels, a measure of erythrocyte lysis, from 5.7 times the upper level of normal (ULN) to 1.8 times ULN and increased mean hemoglobin levels by 1.7 g/dL at day 84. 84 Transfusions were reduced from 12 units during the 84 days preceding treatment with danicopan to 9 units during the 84 treatment days, suggesting pronounced, but incomplete alternative pathway inhibition at the doses tested. When given on top of eculizumab, a neutralizing anti‐C5 antibody and current standard of care for PNH, danicopan increased mean hemoglobin levels by 2.4 g/dL and reduced transfusion requirement from 31 transfusions during the 24 weeks pretreatment period (n = 10 patients; eculizumab only) to 1 transfusion during the 24‐week treatment period. 85 Based on this result, the compound is currently in Phase 3 clinical trials in PNH patients as an add‐on therapy to C5 antibodies (eculizumab or ravulizumab). In addition, it is in a Phase 2 trial in Geographic Atrophy secondary to AMD. Danicopan was also tested in patients with C3G or different types of immune complex‐mediated membranoproliferative glomerulonephritides (NCT03369236 and NCT03124368), but development in C3G has been discontinued.
TABLE 2.
Phase 2 and Phase 3 clinical trials with low molecular weight antagonists of Factor D or Factor B. Source: clinicaltrials.gov
| Compound | Indication | Phase | Comparator/combination | Identifier (status, 07/22) |
|---|---|---|---|---|
| A. Factor D inhibitors | ||||
|
Danicopan (Achillion/Alexion) |
PNH | 3 | C5 inhibitor co‐administration |
NCT04469465 (ALPHA, recruiting), NCT05389449 (not yet recruiting) NCT03472885 (active, not recruiting) |
| 2 | None |
NCT03053102 (completed) NCT03181633 (active, not recruiting) |
||
| C3G | 2 | None | NCT03369236 (completed) | |
| C3G or IC‐MPGN | 2 | None |
NCT03124368 (completed) NCT03459443 (completed) |
|
| Geographic Atrophy secondary to AMD | 2 | none | NCT05019521 (recruiting) | |
| COVID‐19 | 2 | Remdisivir | NCT04988035 (ACTIV‐5, BET‐C, completed) | |
| Vemircopan (Achillion/Alexion) | PNH | 2 | None | NCT04170023 (recruiting) |
| Myasthenia Gravis | 2 | None | NCT05218096 (recruiting) | |
| Proliferative Lupus Nephritis and IgAN | 2 | None | NCT05097989 (recruiting) | |
| BCX9930 (BioCryst) |
PNH |
2 | None |
NCT05116774 (REDEEM‐1; active, not recruiting) NCT05116787 (REDEEM‐2; active, not recruiting) NCT04702568 (active, not recruiting) NCT04330534 (completed) |
| C3G, IgAN and MN | 2 | None | NCT05162066 (RENEW; active, not recruiting) | |
| B. Factor B inhibitors | ||||
| Iptacopan (Novartis) | PNH | 3 | Anti C5 antibody head‐to‐head | NCT04558918 (APPLY‐PNH; active, not recruiting) |
| None |
(APPOINT‐PNH, recruiting) NCT04747613 (recruiting) |
|||
| 2 | C5 inhibitor co‐administration |
NCT03439839 (completed) |
||
| None |
NCT03896152 (completed) |
|||
| aHUS | 3 | None | NCT04889430 (APPELHUS; recruiting) | |
|
C3G |
3 | None | NCT04817618 (APPEAR‐C3G; recruiting) | |
| 2 | None |
NCT03955445 (recruiting) NCT03832114 (completed) |
||
|
IgAN |
3 | None |
NCT04578834 (APPLAUSE‐IgAN; recruiting) NCT04557462 (recruiting) |
|
| 2 | None | NCT03373461 (completed) | ||
| Autoimmune Benign Hematological Disorders (ITP and CAD) | 2 | None | NCT05086744 (recruiting) | |
| Lupus Nephritis | 2 | None | NCT05268289 (recruiting) | |
| AMD | 2 | None | NCT05230537 (recruiting) | |
| Idiopathic MN | 2 | None | NCT04154787 (recruiting) | |
Achillion (acquired by Alexion and subsequently by AstraZeneca) is also developing a second oral factor D inhibitor molecule based on the same chemical scaffold, ACH‐0145228 (6) (also known as ALXN2050 or vemircopan) (Figure 3B ). This compound appears to be more efficacious than danicopan, as it blocks alternative pathway activity by >85% at lower clinical doses (120 mg twice daily 86 ) and provides a plasma Ctrough level of at least about 65 ng/mL. It may qualify for monotherapy in PNH and other indications and is currently in Phase 2 clinical trials for PNH, myasthenia gravis, lupus nephritis, and IgAN (Table 2).
An additional inhibitor series discovered by Novartis scientists are benzylamines. This series originated from a chemical fragment‐based drug discovery approach starting from low affinity hits with millimolar binding affinity and resulted in the lead compound (7), an inhibitor with an IC50 value of 3.4 μM. 87 In continuation of the drug discovery program, more potent inhibitors derived from the same scaffold were later reported by Novartis, 88 including compound (8) 89 (IC50: 12 nM) and the non‐basic analog ( 9 ) (IC50: 23 nM). 90 (Figure 3D). Consistent with the design rationale, the hydroxyl methyl group in 8, when crystallized with factor D, occupied the small pocket created in the active conformation of factor D making H‐bonding interactions with Thr214 and Ile227 (Figure 3E). The hydroxyl methyl group leads to increased selectivity against related S1 proteases, for example fXIa (IC50 7.7 μM). In human factor D knock‐in mice, compound 8 fully blocked LPS‐induced activation of the alternative complement pathway at 10 mg/kg p.o. and caused sustained inhibition of Ba generation in plasma. 88
More recently, another clinical stage factor D inhibitor, BCX9930, was discovered by Biocryst. While the structure of this compound has not been published, the company disclosed a series of benzylamine derived inhibitors in several patent applications. Compound 10 is a representative structure highlighted in several applications, including in the latest filing, 91 which also includes Phase 1 data, potentially referring to testing BCX9930 in healthy volunteers. The IC50 obtained by PK/PD modeling in a Phase I study (21.7 and 40.7 nM) 92 was very similar to its in vitro potency in a biochemical assay (28.1 nM) and a cellular assay measuring C3 fragment deposition on PNH erythrocytes (39.3 nM). 93
The compound was generally well tolerated and AP suppression >98% was observed at steady state for MAD doses ≥200 mg twice daily. Of note, in the single cohort of healthy volunteers treated for an extended period of time (50 mg twice daily, 14 days), a substantial fraction of individuals (5/12) did not complete the study for unknown reasons. 91 The Phase 1 study included a cohort of PNH patients which was also treated for 2 weeks with 50 mg twice daily, followed by treatment with 100 mg twice daily for two additional weeks. Treatment resulted in a rapid decline of LDH and an increase in hemoglobin levels, both of which were further improved after increasing the dose to 100 mg twice daily. Further doubling of the dose to 200 mg twice daily in the extension phase of the study lead to a further decline in LDH levels. 94 Data for another cohort with higher exposures (200 mg twice daily, followed by 400 mg twice daily after 14 days) has not been published yet. BCX9930 has progressed into phase 2/3 clinical trials (Table 2) at a dose of 500 mg twice daily for PNH (NCT05116774 and NCT05116787) and a basket trial in C3G, IgAN and primary membranous nephropathy (NCT05162066), but after a transient partial clinical hold due to increased serum creatinine levels, potentially pointing to kidney related adverse findings, the company is now exploring efficacy with a reduced dose of 400 mg twice daily.
8. COMPLEMENT FACTOR B
8.1. Key aspects of factor B biology
Factor B is a single‐chain glycoprotein of ~93 kDa of 764 amino acids that is synthesized mainly in the liver, but also in other organs. Factor B levels in serum are high, ranging from 200 to 400 μg/mL (2–4 μM). They might increase by about 50% upon infection, 95 therefore FB belongs to the acute phase proteins.
Factor B has two separate contact sites to C3b, and it consists of five distinct domains and a linker. The N‐terminal Ba part of factor B (~30 kDa) is formed by three N‐terminal CCP or SCR domains (complement control protein modules or short consensus repeats) of approximately 60 amino acid residues each. This part of factor B is required for the initial Mg2+‐independent binding of factor B to C3b or C3(H2O). 96 It is linked to the Bb part of factor B via a 45 amino acid linker peptide that contains the scissile bond (Arg234‐Lys 235) and is cleaved by factor D during activation. The Bb part consists of a von Willebrand factor type A domain (VWA) and the serine protease domain with its catalytic machinery. Initial binding to C3b via the CCP domains induces a Mg2+ ion–dependent adhesion site (MIDAS) in the VWA domain, 97 thus allowing for binding of the Bb part to C3b. This induces another conformational change in factor B which exposes the linker region and its scissile bond. Cleavage by factor D liberates the Ba fragment and generates the proteolytically active and C3b‐bound Bb portion. 2 , 98 , 99 , 100 The open, that is, factor D cleavable form of factor B can also be generated at a low frequency by its binding to C3(H2O), and this may allow low‐level “tick over” alternative pathway activation with limited involvement of factor D. 64 In this context, it might be interesting to note that kallikrein has been suggested as an alternative protease cleaving factor B (and C3). 101
Complement factor B is highly conserved across species. As a consequence, and different to factor D, factor B inhibitors generally inhibit the protease from many different species, including rodent, dog, and monkey, and this facilitates preclinical testing of factor B inhibitors in in vivo models.
8.2. Factor B inhibitors
Less research has been carried out to discover factor B inhibitors than for factor D inhibitors, likely due to the concern that the high serum levels of factor B would make it hard to reach systemic inhibitor exposures that fully block its enzymatic activity without compound‐related side effects. Due to its unspecific and reactive nature, the factor D inhibitor FUT‐175 (2) has also been found to inhibit factor B, 102 although there is little structural similarity between the two active sites. Also, covalent peptide aldehyde inhibitors of factor B are known, 103 but none of these inhibitors qualified for clinical development due to their unfavorable overall biopharmaceutical properties.
To identify potent and selective factor B inhibitors with potential for clinical development, Novartis scientists screened a chemically diverse compound collection (2.5 × 105 compounds) using a proteolytic assay that employed a cobra venom factor (CVF):Bb complex as stable surrogate of the C3 convertase. 104 This screen identified the naphthalene‐dihydro‐imidazole amine compound 11 which inhibits factor B with an IC50 value of 7 μM 9 , 105 (Figure 4A). This compound was subsequently optimized for potency and better drug‐like properties. Key steps were the replacement of the naphthyl by an indole ring which fills the S1 pocket more efficiently, the replacement of the dihydro‐imidazole amine by piperidine, and the addition of a benzoic acid moiety in position 2 of the piperidine ring which forms an important hydrogen bond network and significantly adds to potency. The final molecule, LNP023/iptacopan, (12), is a highly potent factor B inhibitor (IC50 value of 0.01 μM 9 ). The co‐crystal structure of iptacopan bound to the catalytic domain of human factor B shows the indole ring binding into the S1 pocket and orienting the rest of the molecule to further fill the non‐primed site. The central piperidine moiety features a key hydrogen bond to Gly216 (Figure 4B).
FIGURE 4.
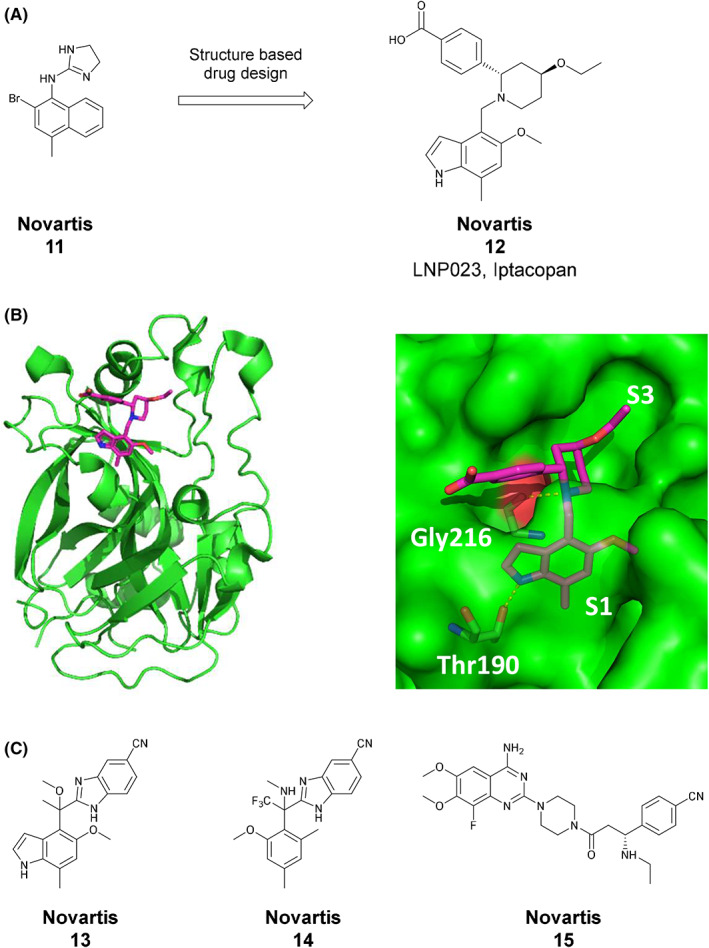
Low molecular weight inhibitors of factor B. (A) Iptacopan (12) was developed from a fragment‐like high‐throughput screening hit (11), by optimizing interactions in the S1 and in the extended S3 pocket. 9 , 105 (B) Crystal structure of the complex between human factor B (green) and iptacopan (magenta, PDB code 6RAV). The indole moiety is binding into the S1 pocket and the piperidine core into the S3 pocket; Shown are key hydrogen bonds to Thr190 and Gly216, respectively. (C) Representative examples of factor B inhibitors with different structural motifs compared to iptacopan extracted from Novartis patents: (13), 112 (14) 113 and (15) 114
Iptacopan is a highly selective inhibitor of factor B, showing no inhibition of factor D, no inhibition of classical or lectin complement pathway activity and no significant inhibition in a broad assay panel of receptors, ion channels, kinases, and proteases. Further profiling demonstrated the compound to potently inhibit alternative pathway‐induced C5b‐9 formation in 50% human serum (IC50 value of 0.13 μM) and to be highly efficacious in a number of mouse models, including LPS‐induced alternative complement pathway activation, KxB/N arthritis and passive Heyman nephritis, a model for membranous nephropathy. 9 Moreover, using erythrocytes from PNH patients, iptacopan was able to block C3 deposition and prevent their complement‐mediated lysis with an IC50 value of 0.4 μM.
Novartis advanced iptacopan into clinical development (Table 2) for the following reasons. (i) Despite the high factor B serum levels, the compound fully blocks factor B activity in vivo due to its very high affinity for factor B in combination with the long half‐life (30 to 70 h) of the protease. 60 , 106 , 107 This means that only a small amount of newly synthesized factor B needs to be inhibited by free compound at steady state. (ii) Iptacopan has a favorable overall efficacy and safety profile. (iii) The compound is able to block C3 convertase and, as a consequence, downstream C5 convertase activity, even in settings where factor B might be activated by other proteases than factor D. 101 , 108 (iv) In alternative pathway‐dependent nephropathies like C3G and IgAN, factor B levels are independent of renal function, while factor D levels substantially increase with declining kidney function. 58 As a consequence, inhibitors targeting factor D might require an adapted higher dosing regimen in renally impaired patients.
Iptacopan was shown to be safe and well tolerated in Phase 1 clinical trials. Single doses of up to 400 mg and multiple doses up to 200 mg twice daily for 2 weeks did not show any serious adverse effects. Strong alternative pathway inhibition at steady state was achieved at 200 mg twice daily (unpublished), and this dose was selected for the first Phase 2 trial treating PNH patients (n = 10) with incomplete response to eculizumab (NCT03439839). In this open label trial, iptacopan reduced LDH levels from 539 IU/L to 235 U/L and improved hemoglobin concentrations from 97.7 g/L to 129.5 g/L at week 13. In addition, all patients were transfusion free (from a baseline of a mean of 14.3 transfusions per patient in the year prior to study entry) and hematological benefits were maintained also throughout the study extension, including for seven patients who stopped concomitant standard‐of‐care treatment and continued on iptacopan as monotherapy. 109 Iptacopan was subsequently tested in treatment‐naive PNH patients (NCT03896152) with similar results, that is, normalization of hematologic parameters and no requirement for blood transfusions at doses of 200 mg twice daily in almost all patients. 40 Moreover, iptacopan was well tolerated and no thromboembolic event or break‐through hemolysis was observed.
In C3G, a 12‐week treatment with iptacopan (200 mg twice daily) resulted in a 45% reduction in proteinuria (urine protein creatinine ratio, UPCR), normalization of C3 levels and a stabilization of eGFR as well as a significant reduction in C3 deposition in the kidney in patients with recurrent disease after kidney transplantation 110 (NCT03832114). In addition, treatment of IgA nephropathy patients for 6 months with iptacopan (200 mg twice daily) reduced UPCR by up to 40% from baseline and ≥ 28% versus placebo 111 (NCT03373461).
Iptacopan is currently in Phase 3 clinical trials for PNH, C3G, IgAN, and aHUS, respectively. Phase 2 studies in idiotypic membranous nephropathy, cold agglutinin disease, immune thrombocytopenic purpura (ITP), lupus nephritis, and intermediate AMD are ongoing.
In order to expand the chemical space for factor B inhibitors further, Novartis scientists have invested in additional and extensive hit finding activities which led to the discovery of a number of potent factor B inhibitors with different chemical structures. Representative examples are compounds 13, 112 14, 113 and 15, 114 which inhibit factor B with IC50 values of 0.003, 0.023, and 0.001 μM, respectively (Figure 4C). However, none of those has been reported to advance into clinical trials.
9. CONCLUSIONS AND PERSPECTIVES
Today, factor D and factor B have been established as promising targets to treat diseases with a strong evidence of alternative pathway dysregulation, for example, PNH, C3G, aHUS, and AMD. However, drug discovery for both targets has been challenging initially. Both proteases are latent enzymes with little enzymatic activity outside of their natural context and only binding of their natural substrates induces their fully active conformation. Targeting factor B may have also been considered risky in light of its high abundance in blood. However, the screening efforts for factor B inhibitors resulted in several diverse starting points which could be optimized to potent, selective, and orally bioavailable inhibitors that lead to full inhibition at steady state at low free compound exposures. Their high intrinsic binding affinity to the target compensates for the high factor B blood levels. Identifying specific factor D inhibitors proved even harder as the self‐inhibitory loop prevents access to the non‐primed site regions beyond the S1 pocket resulting in a rather narrow binding site for potential inhibitors. Repeated high throughput screening of a large compound collection using either a direct enzyme or a pathway readout remained unsuccessful and provided no validated hits. Key success factors to identify potent, selective, and orally bioavailable factor D inhibitors was a mix of fragment‐based drug discovery with an in‐silico target family approach starting from a kallikrein 7 inhibitor (with no binding affinity to factor D). When assessing the full preclinical profile of the Novartis factor D and factor B inhibitors, it was decided to pursue the factor B inhibitor iptacopan in light of its superior overall profile including pharmacokinetic and preclinical safety data. Other companies have discovered factor D inhibitors which also qualified for clinical development and, today, inhibitors for both targets are in clinical trials for a number of alternative pathway‐related diseases including PNH, C3G, IgAN, and AMD.
Despite early clinical data indicating that both factor B and factor D inhibitors can fully block the alternative complement pathway in humans at steady state, it may be worth noting that factor B and factor D deficient mice have somewhat different phenotypes. Factor B deficient mice are healthy and fertile, 115 and protected in many disease models, including factor H deficiency‐induced glomerulonephritis, 116 lupus nephritis, 117 KxB/N arthritis, 118 and collagen‐induced arthritis. 119 Young factor D deficient mice are also healthy and fertile. They were tested in fewer disease models, but also showed protection in lupus nephritis 120 and liver inflammation. 121 , 122 In contrast, aged factor D deficient mice develop spontaneous immune complex glomerulonephritis for unknown reasons, 123 and factor H deficiency‐induced glomerulonephritis is not rescued by genetically removing factor D. 64 However, the latter finding, which is thought to reflect factor D independent C3(H2O)‐mediated alternative pathway activation, might only be clinically relevant in very rare settings of almost complete factor H deficiency. So far, no fundamental difference has been observed for the efficacy of factor B and factor D inhibition in the clinic, based on relatively small patient numbers and no head‐to‐head comparison between inhibitors. With increasing patient numbers, broadening of the indication space, and expansion of treatments duration, it will be interesting to learn if inhibition of the alternative pathway at different nodes will result in a clinical difference.
Clinical development of LMW inhibitors for the alternative pathway is most advanced in prototypic diseases with strong evidence for a hyperactivated alternative pathway and high unmet medical need. In parallel, companies have initiated clinical trials to explore the use of these compounds for patients or patient subsets suffering from other autoimmune diseases, in which secondary pathological activation of the alternative pathway may occur. Autoantibody‐driven diseases like immune thrombocytopenia and cold agglutinin disease are particularly noteworthy in this context. Here, autoantibodies activate the complement cascade through the classical pathway, and the alternative pathway might be a key driver of disease via the amplification loop. Positive results of these clinical trials might substantially open the indication space for LMW alternative pathway inhibitors in many other autoantibody‐driven diseases.
Humans with genetic deficiencies of activating components of almost all complement pathways carry an increased risk of infection with encapsulated bacteria. 55 Such an increased infection risk has also been observed upon pharmacologic inhibition of the terminal pathway with anti‐C5 antibodies. 55 This shows that bacterial infections are an important safety concern for patients taking complement inhibitors. To date, only two individuals with factor B deficiency 124 , 125 and 13 individuals with factor D deficiency have been identified, 70 , 126 , 127 , 128 , 129 , 130 , 131 who were discovered based on a history of infections with encapsulated bacteria, (for example, Neisseria meningitidis, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pyogenes) in at least one family member. While 10 of the 13 individuals with factor D deficiency had a history of infections, this was not the case for the other three. 129 Therefore, it has been argued that individuals with alternative pathway deficiency might still be protected to some degree by an intact classical and lectin pathway even in the absence of the amplification loop. In addition, several studies were performed to determine if vaccination can mitigate the potential infection risk upon alternative pathway inhibition. Indeed, danicopan inhibited meningococcal killing in the serum of unvaccinated, but not in that of most vaccinated individuals, whereas the anti‐C5 antibody eculizumab blocked meningococcal killing independent of vaccination status. 54 In a separate study, supra‐efficacious concentrations of the factor B inhibitor iptacopan or a factor D inhibitor did not inhibit meningococcal killing by serum of vaccinated individuals, while inhibition at the level of C3 or C5 did. 132 Similarly, killing of pneumococci was strongly dependent on the alternative pathway only in naive, but not in vaccinated individuals. 133 These data imply that vaccination‐induced protective antibody titers can bypass the requirement of the alternative pathway to inhibit the growth of encapsulated bacteria. Nevertheless, continuous attention should be given to bacterial strains for which no vaccination is available, and to individuals that mounted insufficient antibody titers upon vaccination.
The discovery of LMW factor B and factor D inhibitors has opened the way to selective and potent inhibition of the alternative complement pathway. Based on initial clinical data, expectations are high for their therapeutic efficacy in prototypic diseases of alternative pathway dysregulation and for diseases where alternative pathway proteases drive disease through the amplification loop. For the latter, it will be instructive to see how efficacious these inhibitors are compared with neutralizing antibodies or other biologics directed against the classical, lectin, and terminal complement pathways. In addition, drug discovery efforts for inhibitors of the alternative pathway have strongly focused on systemic complement‐mediated diseases so far, while it has become evident that pathologic complement activation might also play an important role in a number of neurodegenerative diseases, including Alzheimer's disease, Huntington's disease, and multiple sclerosis. 134 Better understanding of the involvement of the alternative pathway in these diseases and new clinical trials in these indications have the potential to significantly broaden the therapeutic scope of complement inhibitors. However, since most available complement inhibitors are antibody‐, protein‐, or peptide‐based, their ability to cross the blood–brain barrier is rather limited, and there is an urgent need for LMW complement inhibitors with good brain tissue exposure. This shows that the clinical development of alternative pathway LMW antagonists is at an exciting inflection point, and the use of these compounds in translational assays on patient biosamples opens the opportunity for better understanding of diseases that may become future clinical indications. Today, feasibility for drug discovery for LMW antagonists of the alternative pathway has been firmly established. The coming years will widen the path and tell us which patients benefit from their clinical use.
CONFLICT OF INTEREST
All authors are employees of Novartis Pharma AG.
Schubart A, Flohr S, Junt T, Eder J. Low‐molecular weight inhibitors of the alternative complement pathway. Immunol Rev. 2023;313:339‐357. doi: 10.1111/imr.13143
This article is part of a series of reviews covering The Alternative Pathway or Amplification Loop of Complement appearing in Volume 313 of Immunological Reviews.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
REFERENCES
- 1. Pangburn MK, Schreiber RD, Muller‐Eberhard HJ. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b‐like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981;154(3):856‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torreira E, Tortajada A, Montes T, Rodriguez de Cordoba S, Llorca O. 3D structure of the C3bB complex provides insights into the activation and regulation of the complement alternative pathway convertase. Proc Natl Acad Sci U S A. 2009;106(3):882‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sim RB, Twose TM, Paterson DS, Sim E. The covalent‐binding reaction of complement component C3. Biochem J. 1981;193(1):115‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pangburn MK, Muller‐Eberhard HJ. The C3 convertase of the alternative pathway of human complement. Enzymic properties of the bimolecular proteinase. Biochem J. 1986;235(3):723‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fearon DT, Austen KF. Properdin: initiation of alternative complement pathway. Proc Natl Acad Sci U S A. 1975;72(8):3220‐3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrison RA. The properdin pathway: an "alternative activation pathway" or a "critical amplification loop" for C3 and C5 activation? Semin Immunopathol. 2018;40(1):15‐35. [DOI] [PubMed] [Google Scholar]
- 7. Sedrani R, Hommel U, Eder J. Protease‐directed drug discovery. Gene family targeted molecular design. John Wiley & Sons; 2009:159. [Google Scholar]
- 8. Schechter I, Berger A. On the size of the active site in proteases I. Papain. Biochem Biophys Res Commun. 1967;27(2):157‐162. [DOI] [PubMed] [Google Scholar]
- 9. Schubart A, Anderson K, Mainolfi N, et al. Small‐molecule factor B inhibitor for the treatment of complement‐mediated diseases. Proc Natl Acad Sci U S A. 2019;116(16):7926‐7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aradottir SS, Kristoffersson AC, Roumenina LT, et al. Factor D inhibition blocks complement activation induced by mutant factor B associated with atypical hemolytic uremic syndrome and membranoproliferative glomerulonephritis. Front Immunol. 2021;12:690821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thielen AJF, Zeerleder S, Wouters D. Consequences of dysregulated complement regulators on red blood cells. Blood Rev. 2018;32(4):280‐288. [DOI] [PubMed] [Google Scholar]
- 12. Fakhouri F, Fremeaux‐Bacchi V. Thrombotic microangiopathy in aHUS and beyond: clinical clues from complement genetics. Nat Rev Nephrol. 2021;17(8):543‐553. [DOI] [PubMed] [Google Scholar]
- 13. Nester CM, Barbour T, de Cordoba SR, et al. Atypical aHUS: state of the art. Mol Immunol. 2015;67(1):31‐42. [DOI] [PubMed] [Google Scholar]
- 14. Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73(6):887‐896. [DOI] [PubMed] [Google Scholar]
- 15. Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103(7):2328‐2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age‐related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bain W, Li H, van der Geest R, et al. Increased alternative complement pathway function and improved survival during critical illness. Am J Respir Crit Care Med. 2020;202(2):230‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tam JC, Bidgood SR, McEwan WA, James LC. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345(6201):1256070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kisselev AF, Mentele R, von der Helm K. Cleavage of the complement system C3 component by HIV‐1 proteinase. Biol Chem. 1997;378(5):439‐442. [PubMed] [Google Scholar]
- 20. Hovingh ES, van den Broek B, Jongerius I. Hijacking complement regulatory proteins for bacterial immune evasion. Front Microbiol. 2016;7:2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith RJH, Appel GB, Blom AM, et al. C3 glomerulopathy ‐ understanding a rare complement‐driven renal disease. Nat Rev Nephrol. 2019;15(3):129‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Ghiringhelli Borsa N, Shao D, et al. Factor H autoantibodies and complement‐mediated diseases. Front Immunol. 2020;11:607211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corvillo F, Okroj M, Nozal P, Melgosa M, Sanchez‐Corral P, Lopez‐Trascasa M. Nephritic factors: an overview of classification, diagnostic tools and clinical associations. Front Immunol. 2019;10:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vasilev VV, Noe R, Dragon‐Durey MA, et al. Functional characterization of autoantibodies against complement component C3 in patients with lupus nephritis. J Biol Chem. 2015;290(42):25343‐25355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Birmingham DJ, Bitter JE, Ndukwe EG, et al. Relationship of circulating anti‐C3b and anti‐C1q IgG to lupus nephritis and its flare. Clin J Am Soc Nephrol. 2016;11(1):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tao J, Song D, Liu XL, Yu F, Zhao MH. Circulating anti‐C3b IgG in lupus nephritis: a large cohort study. Clin Immunol. 2020;217:108514. [DOI] [PubMed] [Google Scholar]
- 27. Hiemstra PS, Gorter A, Stuurman ME, Van Es LA, Daha MR. Activation of the alternative pathway of complement by human serum IgA. Eur J Immunol. 1987;17(3):321‐326. [DOI] [PubMed] [Google Scholar]
- 28. Russell MW, Mansa B. Complement‐fixing properties of human IgA antibodies. Alternative pathway complement activation by plastic‐bound, but not specific antigen‐bound, IgA. Scand J Immunol. 1989;30(2):175‐183. [DOI] [PubMed] [Google Scholar]
- 29. Hiemstra PS, Biewenga J, Gorter A, et al. Activation of complement by human serum IgA, secretory IgA and IgA1 fragments. Mol Immunol. 1988;25(6):527‐533. [DOI] [PubMed] [Google Scholar]
- 30. Roos A, Bouwman LH, van Gijlswijk‐Janssen DJ, Faber‐Krol MC, Stahl GL, Daha MR. Human IgA activates the complement system via the mannan‐binding lectin pathway. J Immunol. 2001;167(5):2861‐2868. [DOI] [PubMed] [Google Scholar]
- 31. Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138(3):439‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Camous L, Roumenina L, Bigot S, et al. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood. 2011;117(4):1340‐1349. [DOI] [PubMed] [Google Scholar]
- 33. Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti‐neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170(1):52‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187(1):538‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moore S, Juo HH, Nielsen CT, Tyden H, Bengtsson AA, Lood C. Role of neutrophil extracellular traps regarding patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus. J Rheumatol. 2020;47(11):1652‐1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leffler J, Martin M, Gullstrand B, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188(7):3522‐3531. [DOI] [PubMed] [Google Scholar]
- 37. Wang H, Wang C, Zhao MH, Chen M. Neutrophil extracellular traps can activate alternative complement pathways. Clin Exp Immunol. 2015;181(3):518‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sen D, Isenberg DA. Antineutrophil cytoplasmic autoantibodies in systemic lupus erythematosus. Lupus. 2003;12(9):651‐658. [DOI] [PubMed] [Google Scholar]
- 39. Tett SE, Cutler DJ, Day RO, Brown KF. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989;27(6):771‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jang JH, Wong Lee Lee L, Ko BS, et al. Iptacopan monotherapy in patients with paroxysmal nocturnal hemoglobinuria: a 2‐cohort open‐label proof‐of‐concept study. Blood Adv. 2022;6(15):4450‐4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS‐CoV‐2 spike proteins is blocked by factor D inhibition. Blood. 2020;136(18):2080‐2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leatherdale A, Stukas S, Lei V, et al. Persistently elevated complement alternative pathway biomarkers in COVID‐19 correlate with hypoxemia and predict in‐hospital mortality. Med Microbiol Immunol. 2022;211(1):37‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sturm V, Menke MN, Landau K, Laube GF, Neuhaus TJ. Ocular involvement in paediatric haemolytic uraemic syndrome. Acta Ophthalmol. 2010;88(7):804‐807. [DOI] [PubMed] [Google Scholar]
- 44. Heiderscheit AK, Hauer JJ, Smith RJH. C3 glomerulopathy: understanding an ultra‐rare complement‐mediated renal disease. Am J Med Genet C Semin Med Genet. 2022. doi: 10.1002/ajmg.c.31986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaartinen K, Safa A, Kotha S, Ratti G, Meri S. Complement dysregulation in glomerulonephritis. Semin Immunol. 2019;45:101331. [DOI] [PubMed] [Google Scholar]
- 46. Leisy HB, Rastogi A, Guevara G, Ahmad M, Smith RT. The association of geographic atrophy and decreased renal function in patients with age‐related macular degeneration. Eye (Lond). 2017;31(1):62‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sartain SE, Turner NA, Moake JL. Brain microvascular endothelial cells exhibit lower activation of the alternative complement pathway than glomerular microvascular endothelial cells. J Biol Chem. 2018;293(19):7195‐7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Borza DB. Glomerular basement membrane heparan sulfate in health and disease: a regulator of local complement activation. Matrix Biol. 2017;57‐58:299‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Volanakis JE, Barnum SR, Giddens M, Galla JH. Renal filtration and catabolism of complement protein D. N Engl J Med. 1985;312(7):395‐399. [DOI] [PubMed] [Google Scholar]
- 50. Zhou J, Jang YP, Kim SR, Sparrow JR. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2006;103(44):16182‐16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen M, Forrester JV, Xu H. Synthesis of complement factor H by retinal pigment epithelial cells is down‐regulated by oxidized photoreceptor outer segments. Exp Eye Res. 2007;84(4):635‐645. [DOI] [PubMed] [Google Scholar]
- 52. Chen M, Muckersie E, Robertson M, Forrester JV, Xu H. Up‐regulation of complement factor B in retinal pigment epithelial cells is accompanied by complement activation in the aged retina. Exp Eye Res. 2008;87(6):543‐550. [DOI] [PubMed] [Google Scholar]
- 53. Sohn JH, Bora PS, Jha P, Tezel TH, Kaplan HJ, Bora NS. Complement, innate immunity and ocular disease. Chem Immunol Allergy. 2007;92:105‐114. [DOI] [PubMed] [Google Scholar]
- 54. Konar M, Granoff DM. Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults. Blood. 2017;130(7):891‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schroder‐Braunstein J, Kirschfink M. Complement deficiencies and dysregulation: pathophysiological consequences, modern analysis, and clinical management. Mol Immunol. 2019;114:299‐311. [DOI] [PubMed] [Google Scholar]
- 56. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548‐558. [DOI] [PubMed] [Google Scholar]
- 57. Bruin G, Loesche C, Nyirady J, Sander O. Population pharmacokinetic modeling of secukinumab in patients with moderate to severe psoriasis. J Clin Pharmacol. 2017;57(7):876‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pascual M, Steiger G, Estreicher J, Macon K, Volanakis JE, Schifferli JA. Metabolism of complement factor D in renal failure. Kidney Int. 1988;34(4):529‐536. [DOI] [PubMed] [Google Scholar]
- 59. Loyet KM, Hass PE, Sandoval WN, et al. In vivo stability profiles of anti‐factor D molecules support long‐acting delivery approaches. Mol Pharm. 2019;16(1):86‐95. [DOI] [PubMed] [Google Scholar]
- 60. Krick EH, De Heer DH, Kaplan RA, Arroyave CM, Vaughan JH. Metabolism of factor B of serum complement in rheumatoid arthritis. Clin Exp Immunol. 1978;34(1):1‐9. [PMC free article] [PubMed] [Google Scholar]
- 61. Volanakis JE, Narayana SV. Complement factor D, a novel serine protease. Protein Sci. 1996;5(4):553‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Snauwaert E, Van Biesen W, Raes A, et al. Accumulation of uraemic toxins is reflected only partially by estimated GFR in paediatric patients with chronic kidney disease. Pediatr Nephrol. 2018;33(2):315‐323. [DOI] [PubMed] [Google Scholar]
- 63. Kobayakawa H, Miyata T, Inagi R, Shinzato T, Maeda K. Effects of excess factor D on early‐ and late‐phase activation of the complement cascade. Nihon Jinzo Gakkai Shi. 1992;34(1):103‐106. [PubMed] [Google Scholar]
- 64. Zhang Y, Keenan A, Dai DF, et al. C3(H2O) prevents rescue of complement‐mediated C3 glomerulopathy in Cfh−/− Cfd−/− mice. JCI . Insight. 2020;5(9). doi: 10.1172/jci.insight.135758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dobo J, Szakacs D, Oroszlan G, et al. MASP‐3 is the exclusive pro‐factor D activator in resting blood: the lectin and the alternative complement pathways are fundamentally linked. Sci Rep. 2016;6:31877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Degn SE, Jensenius JC, Thiel S. The pro‐factor D cleaving activity of MASP‐1/−3 is not required for alternative pathway function. J Immunol. 2014;192(12):5447‐5448. [DOI] [PubMed] [Google Scholar]
- 67. Pihl R, Jensen L, Hansen AG, et al. Analysis of factor D isoforms in malpuech‐michels‐mingarelli‐carnevale patients highlights the role of MASP‐3 as a maturase in the alternative pathway of complement. J Immunol. 2017;199(6):2158‐2170. doi: 10.4049/jimmunol.1700518 [DOI] [PubMed] [Google Scholar]
- 68. Jing H, Babu YS, Moore D, et al. Structures of native and complexed complement factor D: implications of the atypical His57 conformation and self‐inhibitory loop in the regulation of specific serine protease activity. J Mol Biol. 1998;282(5):1061‐1081. [DOI] [PubMed] [Google Scholar]
- 69. Forneris F, Ricklin D, Wu J, et al. Structures of C3b in complex with factors B and D give insight into complement convertase formation. Science. 2010;330(6012):1816‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Langereis JD, van der Molen RG, de Kat AC, et al. Complement factor D haplodeficiency is associated with a reduced complement activation speed and diminished bacterial killing. Clin Transl Immunol. 2021;10(4):e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lesavre PH, Muller‐Eberhard HJ. Mechanism of action of factor D of the alternative complement pathway. J Exp Med. 1978;148(6):1498‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wu X, Hutson I, Akk AM, et al. Contribution of adipose‐derived factor D/Adipsin to complement alternative pathway activation: lessons from lipodystrophy. J Immunol. 2018;200(8):2786‐2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lo JC, Ljubicic S, Leibiger B, et al. Adipsin is an adipokine that improves beta cell function in diabetes. Cell. 2014;158(1):41‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Song NJ, Kim S, Jang BH, et al. Small molecule‐induced complement factor D (adipsin) promotes lipid accumulation and adipocyte differentiation. PLoS One. 2016;11(9):e0162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ezure T, Sugahara M, Amano S. Senescent dermal fibroblasts negatively influence fibroblast extracellular matrix‐related gene expression partly via secretion of complement factor D. Biofactors. 2019;45(4):556‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kam CM, McRae BJ, Harper JW, Niemann MA, Volanakis JE, Powers JC. Human complement proteins D, C2, and B. Active site mapping with peptide thioester substrates. J Biol Chem. 1987;262(8):3444‐3451. [PubMed] [Google Scholar]
- 77. Ikari N, Sakai Y, Hitomi Y, Fujii S. New synthetic inhibitor to the alternative complement pathway. Immunology. 1983;49(4):685‐691. [PMC free article] [PubMed] [Google Scholar]
- 78. Cole LB, Kilpatrick JM, Chu N, Babu YS. Structure of 3,4‐dichloroisocoumarin‐inhibited factor D. Acta Crystallogr D Biol Crystallogr. 1998;54(Pt 5):711‐717. [DOI] [PubMed] [Google Scholar]
- 79. Morikis D, Lambris JD. Structural aspects and design of low‐molecular‐mass complement inhibitors. Biochem Soc Trans. 2002;30(Pt 6):1026‐1036. [DOI] [PubMed] [Google Scholar]
- 80. Babu YS, Bennett, J. C. , Niwas, S. , et al. Novel compounds useful in the complement, coagulat and kallikrein pathways and method for their preparation. Dec 10, 1998.
- 81. Maibaum J, Liao SM, Vulpetti A, et al. Small‐molecule factor D inhibitors targeting the alternative complement pathway. Nat Chem Biol. 2016;12(12):1105‐1110. [DOI] [PubMed] [Google Scholar]
- 82. Lorthiois E, Anderson K, Vulpetti A, et al. Discovery of highly potent and selective small‐molecule reversible factor D Inhibitors demonstrating alternative complement pathway inhibition in vivo. J Med Chem. 2017;60(13):5717‐5735. [DOI] [PubMed] [Google Scholar]
- 83. Wiles JA, Galvan MD, Podos SD, Geffner M, Huang M. Discovery and development of the oral complement factor D inhibitor danicopan (ACH‐4471). Curr Med Chem. 2020;27(25):4165‐4180. [DOI] [PubMed] [Google Scholar]
- 84. Risitano AM, Kulasekararaj AG, Lee JW, et al. Danicopan: an oral complement factor D inhibitor for paroxysmal nocturnal hemoglobinuria. Haematologica. 2021;106(12):3188‐3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kulasekararaj AG, Risitano AM, Maciejewski JP, et al. Phase 2 study of danicopan in patients with paroxysmal nocturnal hemoglobinuria with an inadequate response to eculizumab. Blood. 2021;138(20):1928‐1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Huang M, Podos, S.D. , Inventor; Achillion Pharmaceuticals, Inc., assignee, assignee . Targeted dosing for the treatment of complement mediated disorders. June 25, 2020.
- 87. Vulpetti A, Ostermann N, Randl S, et al. Discovery and design of first benzylamine‐based ligands binding to an unlocked conformation of the complement factor D. ACS Med Chem Lett. 2018;9(5):490‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Karki RG, Powers J, Mainolfi N, et al. Design, synthesis, and preclinical characterization of selective factor d inhibitors targeting the alternative complement pathway. J Med Chem. 2019;62(9):4656‐4668. [DOI] [PubMed] [Google Scholar]
- 89. Belanger D, Flohr, S ., Gelin, C.F. , et al. Aminomethyl‐biaryl dreivatives as complement factor D inhibitors and uses thereof. Jan 22, 2015.
- 90. Belanger D, Flohr, S ., Gelin, C.F. , et al. Amidomethyl‐biaryl derivatives complement factor D inhibitors and uses thereof. June 9, 2016. [Google Scholar]
- 91. Babu YS, Sheridan, W.P. , Inventor; Biocryst Pharmaceuticals, Inc., assignee, assignee . Dosing regimens for oral complement factor D inhibitors. Feb 04, 2021.
- 92. Davidson M, Mathis A, Mair S, et al. BCX9930, an oral factor D inhibitor, for the potential treatment of alternative pathway mediated diseases: interim results of a phase 1 study in healthy subjects. Blood. 2020;136(Supplement 1):15‐16. [Google Scholar]
- 93. Chen X, Cheng X, Liu Q, et al. Preclinical characterization of BCX9930, a potent oral complement factor D inhibitor, targeting alternative pathway‐mediated diseases including paroxysmal nocturnal hemoglobinuria (PNH). Blood. 2020;136(Supplement 1):32. [Google Scholar]
- 94. Kulasekararaj A, Le Roux Malherbe J, McDonald A, et al. BCX9930, a potent, selective, oral factor D inhibitor, demonstrates proof‐of‐concept as monotherapy in patients with paroxysmal nocturnal hemoglobinuria (PNH). Blood. 2020;136(Supplement 1):14‐15. [Google Scholar]
- 95. Vitale G, Mansueto S, Gambino G, et al. The acute phase response in Sicilian patients with boutonneuse fever admitted to hospitals in Palermo, 1992‐1997. J Infect. 2001;42(1):33‐39. [DOI] [PubMed] [Google Scholar]
- 96. Pryzdial EL, Isenman DE. Alternative complement pathway activation fragment Ba binds to C3b. Evidence that formation of the factor B‐C3b complex involves two discrete points of contact. J Biol Chem. 1987;262(4):1519‐1525. [PubMed] [Google Scholar]
- 97. Hourcade DE, Mitchell LM. Access to the complement factor B scissile bond is facilitated by association of factor B with C3b protein. J Biol Chem. 2011;286(41):35725‐35732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Janssen BJ, Christodoulidou A, McCarthy A, Lambris JD, Gros P. Structure of C3b reveals conformational changes that underlie complement activity. Nature. 2006;444(7116):213‐216. [DOI] [PubMed] [Google Scholar]
- 99. Ponnuraj K, Xu Y, Macon K, Moore D, Volanakis JE, Narayana SV. Structural analysis of engineered Bb fragment of complement factor B: insights into the activation mechanism of the alternative pathway C3‐convertase. Mol Cell. 2004;14(1):17‐28. [DOI] [PubMed] [Google Scholar]
- 100. Gros P, Milder FJ, Janssen BJ. Complement driven by conformational changes. Nat Rev Immunol. 2008;8(1):48‐58. [DOI] [PubMed] [Google Scholar]
- 101. Irmscher S, Doring N, Halder LD, et al. Kallikrein cleaves C3 and activates complement. J Innate Immun. 2018;10(2):94‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Inagi R, Miyata T, Maeda K, Sugiyama S, Miyama A, Nakashima I. FUT‐175 as a potent inhibitor of C5/C3 convertase activity for production of C5a and C3a. Immunol Lett. 1991;27(1):49‐52. [DOI] [PubMed] [Google Scholar]
- 103. Ruiz‐Gomez G, Lim J, Halili MA, et al. Structure‐activity relationships for substrate‐based inhibitors of human complement factor B. J Med Chem. 2009;52(19):6042‐6052. [DOI] [PubMed] [Google Scholar]
- 104. Smith CA, Vogel CW, Muller‐Eberhard HJ. Ultrastructure of cobra venom factor‐dependent C3/C5 convertase and its zymogen, factor B of human complement. J Biol Chem. 1982;257(17):9879‐9882. [PubMed] [Google Scholar]
- 105. Mainolfi N, Ehara T, Karki RG, et al. Discovery of 4‐((2S,4S)‐4‐Ethoxy‐1‐([5‐methoxy‐7‐methyl‐1H‐indol‐4‐yl]methyl)piperidin‐2‐yl)benzoic acid (LNP023), a factor B inhibitor specifically designed to be applicable to treating a diverse array of complement mediated diseases. J Med Chem. 2020;63(11):5697‐5722. [DOI] [PubMed] [Google Scholar]
- 106. Schnabolk G, Coughlin B, Joseph K, et al. Local production of the alternative pathway component factor B is sufficient to promote laser‐induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2015;56(3):1850‐1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bansal L, Nichols EM, Howsmon DP, et al. Mathematical modeling of complement pathway dynamics for target validation and selection of drug modalities for complement therapies. Front Pharmacol. 2022;13:855743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. DiScipio RG. The activation of the alternative pathway C3 convertase by human plasma kallikrein. Immunology. 1982;45(3):587‐595. [PMC free article] [PubMed] [Google Scholar]
- 109. Risitano AM, Roth A, Soret J, et al. Addition of iptacopan, an oral factor B inhibitor, to eculizumab in patients with paroxysmal nocturnal haemoglobinuria and active haemolysis: an open‐label, single‐arm, phase 2, proof‐of‐concept trial. Lancet Haematol. 2021;8(5):e344‐e354. [DOI] [PubMed] [Google Scholar]
- 110. Wong E.K., Nester CM Cavero Escribano T., et al. PO2536: iptacopan, a novel oral complement factor B (FB) inhibitor, significantly reduces proteinuria and C3 deposit scores in native and transplanted kidneys C3 glomerulopathy (C3G) patients. American Society of Nephrology (ASN) 2021 Annual Meeting; 2021. [Google Scholar]
- 111. Barratt J, Rovin B, Zhang H, et al. POS‐546 efficacy and safety of iptacopan in IgA nephropathy: results of a randomized double‐blind placebo‐controlled phase 2 study at 6 months. Kidney Int Rep. 2022;7(2):S236. [Google Scholar]
- 112. Adams CM, Babu, C ., Ding, J ., et al. Complement pathway modulators and uses thereof. Nov 7, 2013.
- 113. Adams C, Ehara, T. , Jendza, K. , et al. 2‐benzyl‐benzimidazole complement factor B inhibitors and uses thereof May 7, 2015.
- 114. Dechantsreiter MA, Grob, J.E. , MacSweeney, A ., et al. Complement pathway modulators and uses thereof Dec 27, 2013.
- 115. Pekna M, Hietala MA, Landin A, et al. Mice deficient for the complement factor B develop and reproduce normally. Scand J Immunol. 1998;47(4):375‐380. [DOI] [PubMed] [Google Scholar]
- 116. Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31(4):424‐428. [DOI] [PubMed] [Google Scholar]
- 117. Watanabe H, Garnier G, Circolo A, et al. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol. 2000;164(2):786‐794. [DOI] [PubMed] [Google Scholar]
- 118. Ji H, Ohmura K, Mahmood U, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157‐168. [DOI] [PubMed] [Google Scholar]
- 119. Banda NK, Takahashi K, Wood AK, Holers VM, Arend WP. Pathogenic complement activation in collagen antibody‐induced arthritis in mice requires amplification by the alternative pathway. J Immunol. 2007;179(6):4101‐4109. [DOI] [PubMed] [Google Scholar]
- 120. Elliott MK, Jarmi T, Ruiz P, Xu Y, Holers VM, Gilkeson GS. Effects of complement factor D deficiency on the renal disease of MRL/lpr mice. Kidney Int. 2004;65(1):129‐138. [DOI] [PubMed] [Google Scholar]
- 121. Tsuru H, Osaka M, Hiraoka Y, Yoshida M. HFD‐induced hepatic lipid accumulation and inflammation are decreased in Factor D deficient mouse. Sci Rep. 2020;10(1):17593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. McCullough RL, McMullen MR, Sheehan MM, et al. Complement Factor D protects mice from ethanol‐induced inflammation and liver injury. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G66‐G79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Abrera‐Abeleda MA, Xu Y, Pickering MC, Smith RJ, Sethi S. Mesangial immune complex glomerulonephritis due to complement factor D deficiency. Kidney Int. 2007;71(11):1142‐1147. [DOI] [PubMed] [Google Scholar]
- 124. Slade C, Bosco J, Unglik G, Bleasel K, Nagel M, Winship I. Deficiency in complement factor B. N Engl J Med. 2013;369(17):1667‐1669. [DOI] [PubMed] [Google Scholar]
- 125. Gauthier A, Wagner E, Thibeault R, Lavoie A. A novel case of complement factor B deficiency. J Clin Immunol. 2021;41(1):277‐279. [DOI] [PubMed] [Google Scholar]
- 126. Kluin‐Nelemans HC, van Velzen‐Blad H, van Helden HP, Daha MR. Functional deficiency of complement factor D in a monozygous twin. Clin Exp Immunol. 1984;58(3):724‐730. [PMC free article] [PubMed] [Google Scholar]
- 127. Hiemstra PS, Langeler E, Compier B, et al. Complete and partial deficiencies of complement factor D in a Dutch family. J Clin Invest. 1989;84(6):1957‐1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Weiss SJ, Ahmed AE, Bonagura VR. Complement factor D deficiency in an infant first seen with pneumococcal neonatal sepsis. J Allergy Clin Immunol. 1998;102(6 Pt 1):1043‐1044. [DOI] [PubMed] [Google Scholar]
- 129. Biesma DH, Hannema AJ, van Velzen‐Blad H, et al. A family with complement factor D deficiency. J Clin Invest. 2001;108(2):233‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sprong T, Roos D, Weemaes C, et al. Deficient alternative complement pathway activation due to factor D deficiency by 2 novel mutations in the complement factor D gene in a family with meningococcal infections. Blood. 2006;107(12):4865‐4870. [DOI] [PubMed] [Google Scholar]
- 131. Sng CCT, O'Byrne S, Prigozhin DM, et al. A type III complement factor D deficiency: structural insights for inhibition of the alternative pathway. J Allergy Clin Immunol. 2018;142(1):311‐314 e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ispasanie E, Muri L, Schubart A, et al. Alternative complement pathway inhibition does not abrogate meningococcal killing by serum of vaccinated individuals. Front Immunol. 2021;12:747594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Muri L, Ispasanie E, Schubart A, et al. Alternative complement pathway inhibition abrogates pneumococcal opsonophagocytosis in vaccine‐naive, but not in vaccinated individuals. Front Immunol. 2021;12:732146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zelek WM, Morgan BP. Targeting complement in neurodegeneration: challenges, risks, and strategies. Trends Pharmacol Sci. 2022;43(8):615‐628. [DOI] [PubMed] [Google Scholar]
- 135. Hill A, DeZern AE, Kinoshita T, Brodsky RA. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers. 2017;3:17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jasinski M, Keller P, Fujiwara Y, Orkin SH, Bessler M. GATA1‐Cre mediates Piga gene inactivation in the erythroid/megakaryocytic lineage and leads to circulating red cells with a partial deficiency in glycosyl phosphatidylinositol‐linked proteins (paroxysmal nocturnal hemoglobinuria type II cells). Blood. 2001;98(7):2248‐2255. [DOI] [PubMed] [Google Scholar]
- 137. Chen Y, Rong F. Advances in the creation of animal models of paroxysmal nocturnal hemoglobinuria. Hematology. 2021;26(1):491‐496. [DOI] [PubMed] [Google Scholar]
- 138. Pangburn MK, Schreiber RD, Muller‐Eberhard HJ. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983;80(17):5430‐5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233‐1243. [DOI] [PubMed] [Google Scholar]
- 140. Kulasekararaj AG, Griffin M, Langemeijer S, et al. Long‐term safety and efficacy of ravulizumab in patients with paroxysmal nocturnal hemoglobinuria: 2‐year results from two pivotal phase 3 studies. Eur J Haematol. 2022;109:205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Smith‐Jackson K, Yang Y, Denton H, et al. Hyperfunctional complement C3 promotes C5‐dependent atypical hemolytic uremic syndrome in mice. J Clin Invest. 2019;129(3):1061‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ueda Y, Miwa T, Ito D, et al. Differential contribution of C5aR and C5b‐9 pathways to renal thrombic microangiopathy and macrovascular thrombosis in mice carrying an atypical hemolytic syndrome‐related factor H mutation. Kidney Int. 2019;96(1):67‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Fakhouri F, Hourmant M, Campistol JM, et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single‐arm, open‐label trial. Am J Kidney Dis. 2016;68(1):84‐93. [DOI] [PubMed] [Google Scholar]
- 144. Rondeau E, Scully M, Ariceta G, et al. The long‐acting C5 inhibitor, Ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naive to complement inhibitor treatment. Kidney Int. 2020;97(6):1287‐1296. [DOI] [PubMed] [Google Scholar]
- 145. Fleckenstein M, Keenan TDL, Guymer RH, et al. Age‐related macular degeneration. Nat Rev Dis Primers. 2021;7(1):31. [DOI] [PubMed] [Google Scholar]
- 146. Coffey PJ, Gias C, McDermott CJ, et al. Complement factor H deficiency in aged mice causes retinal abnormalities and visual dysfunction. Proc Natl Acad Sci U S A. 2007;104(42):16651‐16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lundh von Leithner P, Kam JH, Bainbridge J, et al. Complement factor h is critical in the maintenance of retinal perfusion. Am J Pathol. 2009;175(1):412‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age‐related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121(3):693‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Servais A, Fremeaux‐Bacchi V, Lequintrec M, et al. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J Med Genet. 2007;44(3):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Marquez‐Tirado B, Gutierrez‐Tenorio J, Tortajada A, et al. Factor H‐related protein 1 drives disease susceptibility and prognosis in C3 glomerulopathy. J Am Soc Nephrol. 2022;33(6):1137‐1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Fakhouri F, Fremeaux‐Bacchi V, Noel LH, Cook HT, Pickering MC. C3 glomerulopathy: a new classification. Nat Rev Nephrol. 2010;6(8):494‐499. [DOI] [PubMed] [Google Scholar]
- 152. Devalaraja‐Narashimha K, Meagher K, Luo Y, et al. Humanized C3 mouse: a novel accelerated model of C3 glomerulopathy. J Am Soc Nephrol. 2021;32(1):99‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Welte T, Arnold F, Kappes J, et al. Treating C3 glomerulopathy with eculizumab. BMC Nephrol. 2018;19(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Rousset‐Rouviere C, Cailliez M, Garaix F, Bruno D, Laurent D, Tsimaratos M. Rituximab fails where eculizumab restores renal function in C3nef‐related DDD. Pediatr Nephrol. 2014;29(6):1107‐1111. [DOI] [PubMed] [Google Scholar]
- 155. Matousovic K, Novak J, Yanagihara T, et al. IgA‐containing immune complexes in the urine of IgA nephropathy patients. Nephrol Dial Transplant. 2006;21(9):2478‐2484. [DOI] [PubMed] [Google Scholar]
- 156. Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose‐deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104(1):73‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gharavi AG, Kiryluk K, Choi M, et al. Genome‐wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43(4):321‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kiryluk K, Li Y, Sanna‐Cherchi S, et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8(6):e1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lai KN, Tang SC, Schena FP, et al. IgA nephropathy. Nat Rev Dis Primers. 2016;2:16001. [DOI] [PubMed] [Google Scholar]
- 160. Kim SJ, Koo HM, Lim BJ, et al. Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS One. 2012;7(7):e40495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lee HJ, Choi SY, Jeong KH, et al. Association of C1q deposition with renal outcomes in IgA nephropathy. Clin Nephrol. 2013;80(2):98‐104. [DOI] [PubMed] [Google Scholar]
- 162. Zhang Y, Yan X, Zhao T, et al. Targeting C3a/C5a receptors inhibits human mesangial cell proliferation and alleviates immunoglobulin A nephropathy in mice. Clin Exp Immunol. 2017;189(1):60‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ring T, Pedersen BB, Salkus G, Goodship TH. Use of eculizumab in crescentic IgA nephropathy: proof of principle and conundrum? Clin Kidney J. 2015;8(5):489‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhao J, Wu H, Khosravi M, et al. Association of genetic variants in complement factor H and factor H‐related genes with systemic lupus erythematosus susceptibility. PLoS Genet. 2011;7(5):e1002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kim H, Kim T, Kim M, et al. Activation of the alternative complement pathway predicts renal outcome in patients with lupus nephritis. Lupus. 2020;29(8):862‐871. [DOI] [PubMed] [Google Scholar]
- 166. Wang FM, Yu F, Tan Y, Song D, Zhao MH. Serum complement factor H is associated with clinical and pathological activities of patients with lupus nephritis. Rheumatology (Oxford). 2012;51(12):2269‐2277. [DOI] [PubMed] [Google Scholar]
- 167. Bao L, Haas M, Quigg RJ. Complement factor H deficiency accelerates development of lupus nephritis. J Am Soc Nephrol. 2011;22(2):285‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Grossman TR, Hettrick LA, Johnson RB, et al. Inhibition of the alternative complement pathway by antisense oligonucleotides targeting complement factor B improves lupus nephritis in mice. Immunobiology. 2016;221(6):701‐708. [DOI] [PubMed] [Google Scholar]
- 169. Coppo R, Peruzzi L, Amore A, et al. Dramatic effects of eculizumab in a child with diffuse proliferative lupus nephritis resistant to conventional therapy. Pediatr Nephrol. 2015;30(1):167‐172. [DOI] [PubMed] [Google Scholar]
- 170. Berentsen S. Complement activation and inhibition in autoimmune hemolytic anemia: focus on cold agglutinin disease. Semin Hematol. 2018;55(3):141‐149. [DOI] [PubMed] [Google Scholar]
- 171. Roth A, Barcellini W, D'Sa S, et al. Sutimlimab in cold agglutinin disease. N Engl J Med. 2021;384(14):1323‐1334. [DOI] [PubMed] [Google Scholar]
- 172. Roth A, Bommer M, Huttmann A, et al. Eculizumab in cold agglutinin disease (DECADE): an open‐label, prospective, bicentric, nonrandomized phase 2 trial. Blood Adv. 2018;2(19):2543‐2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Peerschke EI, Andemariam B, Yin W, Bussel JB. Complement activation on platelets correlates with a decrease in circulating immature platelets in patients with immune thrombocytopenic purpura. Br J Haematol. 2010;148(4):638‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Najaoui A, Bakchoul T, Stoy J, et al. Autoantibody‐mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP). Eur J Haematol. 2012;88(2):167‐174. [DOI] [PubMed] [Google Scholar]
- 175. Broome CM, Röth A, Kuter DJ, et al. Long‐term safety and efficacy of sutimlimab in patients with chronic immune thrombocytopenia. Blood. 2020;136(Supplement 1):14‐15. [Google Scholar]
- 176. Luo W, Olaru F, Miner JH, et al. Alternative pathway is essential for glomerular complement activation and proteinuria in a mouse model of membranous nephropathy. Front Immunol. 2018;9:1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Kolb WP, Morrow PR, Tamerius JD. Ba and Bb fragments of factor B activation: fragment production, biological activities, neoepitope expression and quantitation in clinical samples. Complement Inflamm. 1989;6(3):175‐204. [DOI] [PubMed] [Google Scholar]
- 178. Xu Y, Ma M, Ippolito GC, Schroeder HW Jr, Carroll MC, Volanakis JE. Complement activation in factor D‐deficient mice. Proc Natl Acad Sci U S A. 2001;98(25):14577‐14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study


