Abstract
The consequences of extremely intense long‐term exercise for brain health remain unknown. We studied the effects of strenuous exercise on brain structure and function, its dose‒response relationship, and mechanisms in a rat model of endurance training. Five‐week‐old male Wistar rats were assigned to moderate (MOD) or intense (INT) exercise or a sedentary (SED) group for 16 weeks. MOD rats showed the highest motivation and learning capacity in operant conditioning experiments; SED and INT presented similar results. In vivo MRI demonstrated enhanced global and regional connectivity efficiency and clustering as well as a higher cerebral blood flow (CBF) in MOD but not INT rats compared with SED. In the cortex, downregulation of oxidative phosphorylation complex IV and AMPK activation denoted mitochondrial dysfunction in INT rats. An imbalance in cortical antioxidant capacity was found between MOD and INT rats. The MOD group showed the lowest hippocampal brain‐derived neurotrophic factor levels. The mRNA and protein levels of inflammatory markers were similar in all groups. In conclusion, strenuous long‐term exercise yields a lesser improvement in learning ability than moderate exercise. Blunting of MOD‐induced improvements in CBF and connectivity efficiency, accompanied by impaired mitochondrial energetics and, possibly, transient local oxidative stress, may underlie the findings in intensively trained rats.
Keywords: brain connectomics, cognition, endurance exercise, mitochondria, physical activity load
The consequences of extremely intense long‐term exercise for brain health remain unknown. We studied the effects of strenuous exercise on brain structure and function in rats that were assigned to moderate (MOD) or intense (INT) exercise or a sedentary (SED) group. INT yields a lesser improvement in learning ability compared to MOD and blunts MOD‐induced improvements in cerebral blood flow and connectivity efficiency.
INTRODUCTION
Empirical data have provided compelling evidence that physical exercise has positive effects on brain health. Human and animal studies have demonstrated that moderate exercise improves cognitive function, enhances synaptic plasticity and neurogenesis, and induces changes in brain volumes and connectivity. 1 Recently, the Physical Activity Guidelines for Americans 2 emphasized emerging evidence supporting the brain health benefits of being physically active and suggested that additional benefits would occur with more physical activity. The promotion of an active lifestyle has been accompanied by a societal trend toward strenuous exercise. In recent years, the number of young and middle‐aged individuals undertaking very high loads of physical activity, such as those required for marathon or triathlon training, has risen exponentially. 3 The consequences of such exercise loads have only recently been addressed. Preclinical 4 , 5 and clinical 6 , 7 studies have suggested that intense physical activity (e.g., regular marathon running 6 or “daily exercise causing sweating and a rapid heartbeat” 7 ) increases the incidence of atrial and ventricular arrhythmias, ischemic heart disease, and stroke. A U‐shaped dose‒response relationship between physical activity load and cardiovascular risk is increasingly being recognized. 3
It remains unexplored whether a nonlinear pattern also holds true for the relationship between exercise load and cognitive function. Recent reports have proposed that chronic 8 strenuous exercise and single high‐intensity exercise bouts 10 might have detrimental cognitive effects in athletes, but the interpretation of these results is complicated by the presence of unobserved and potentially confounding variables, the lack (and, indeed, the unfeasibility) of long‐term causal analyses, and the observational nature of some of this work.
Research in animal models limits the effects of the confounding factors inherent to human retrospective cohort studies and enables a comprehensive analysis at the molecular and cellular levels, particularly focusing on signaling, neurogenesis, and synaptic plasticity processes involved in behavior. Rodent models have confirmed the beneficial effects exerted on learning and memory by several forms of physical activity, including chronic voluntary or forced exercise of a light 11 or moderate 12 , 13 intensity, high‐intensity interval training, 14 and single acute high‐intensity training bouts. 15 However, the consequences of long‐term strenuous exercise mimicking some current forms of human exercise, compared with moderate exercise mirroring a healthy human lifestyle, have not been addressed. Hence, we aimed to study the effects of a long‐term, sustained strenuous exercise load on brain structure and function; compare them to those of moderate exercise; and assess the potential contributing mechanisms in a clinically relevant rodent model of long‐term treadmill training.
MATERIALS AND METHODS
Animals
Male Wistar rats (100–150 g, approximately 5 weeks old; Charles River Laboratories, Germany) were housed at the University of Barcelona animal facility in a controlled environment with 12/12‐h light/dark cycles and provided with food and water ad libitum. All the experimental procedures followed the European Community Directive (2010/63/UE) and Spanish guidelines (RD 53/2013) for the use of experimental animals and were approved by the Ethical Committee of Animal Experimentation of the University of Barcelona and the Generalitat of Catalunya (protocol number 9451). The experimental reporting adheres to the ARRIVE guidelines (Supplementary Appendix). Raw data from the western blot experiments are shown in Figures S1–S3; further data are available from the corresponding author upon reasonable request.
Exercise training protocol
Sixty rats were randomly assigned to one of three experimental groups: a forced moderate exercise (MOD) group (35 cm/s for 45 min), a forced intense exercise (INT) group (60 cm/s for 60 min), or a control group consisting of age‐matched sedentary (SED) animals. Treadmill speeds were selected to impose different exercise intensities based on estimated VO2max; the training speed in the MOD group roughly corresponds to 60% VO2max, while the INT group trained at 85–90% VO2max. 5 , 16 Exercised rats were conditioned to run on a five‐lane treadmill 5 days/week for 16 weeks. A 16‐week training program has been shown to promote deleterious vascular remodeling involving aortic stiffening 17 and is roughly equivalent to a 10‐year period in humans; shorter periods of time (8‐week training programs) did not elicit such deleterious effects. 4 , 5 Overall, we aimed to mimic humans with a healthy lifestyle (MOD group) and heavily trained athletes (INT group) by adapting the exercise intensity and duration.
The treadmill speed and exercise duration were progressively increased during an initial 2‐week adaptation period to familiarize the animals with the training setup. Rats were supervised during all training sessions to ensure proper running; animals that did not properly adapt to the exercise routine or showed signs of pain, suffering, or distress were excluded from the study.
Rats were trained from adolescence (5 weeks old) until adulthood (22–23 weeks old), recapitulating common training practices in humans. At the end of the training protocol, 8–10 rats per group underwent brain magnetic resonance imaging (MRI) and learning and motivation tests. Upon completion, rats were sacrificed with an overdose of 4% to 5% inhaled isoflurane. The brains were perfused with formaldehyde and used for immunohistochemical studies. An additional group of rats (n = 7–12 per group) was euthanized at the end of the exercise protocol (>48 h after the last training session), and brain samples were collected and immediately frozen in liquid nitrogen for molecular biology experiments. All data acquisition and analyses were performed blind to the group assignments.
Magnetic resonance imaging
MRI was conducted on a 7.0‐T BioSpec 70/30 horizontal animal scanner (Bruker BioSpin, Ettlingen, Germany) equipped with an actively shielded gradient system (400 mT/m, 12‐cm inner diameter). The receiver coil was a four‐channel phased‐array surface coil for the rat brain. Animals were placed in the supine position in a Plexiglas holder with a nose cone for the administration of anesthetic gas (1.5% isoflurane in a mixture of 30% O2 and 70% N2).
Localizer scans were used to ensure accurate positioning of the head in the magnetic isocenter. T2‐weighted images were acquired by a rapid acquisition with a relaxation enhancement (RARE) sequence with an effective echo time (TE) of 35.3 ms, a repetition time (TR) of 6000 ms, and a RARE factor of 8. The matrix size was 256 × 256 with an in‐plane voxel size of 0.12 × 0.12 mm2, 40 slices, and a slice thickness of 0.8 mm, resulting in a field of view (FOV) of 30 × 30 × 32 mm3. T1‐weighted images were acquired using a modified‐driven equilibrium Fourier transform protocol with the following parameters: TE, 2 ms; TR, 4000 ms; matrix size, 256 × 256 × 36; voxel size, 0.14 × 0.14 × 0.5 mm3; and FOV, 35 × 35 × 18 mm3.
Diffusion‐weighted images were acquired using a spin‐echo echo‐planar imaging sequence (TE, 24.86 ms; TR, 15,000 ms; and four segments) using 60 gradient directions with a b‐value of 1000 s/mm2 and five volumes without diffusion weight (b‐value = 0 s/mm2). Sixty slices were acquired with a 72 × 72 matrix size and a 0.31 × 0.31 × 0.31 mm3 isometric voxel size, resulting in an FOV of 22.23 × 22.23 × 18.54 mm3.
Cerebral blood flow (CBF) was measured with a pulsed arterial spin labeling (ASL) method using a flow‐sensitive alternating inversion recovery echo‐planar imaging sequence and the following parameters: FOV, 30 × 30 mm2; matrix size, 80 × 80; one slice with a thickness of 1 mm; axial orientation; TE, 18 ms; TR, 12,000 ms; flip angle, 90°; 25 inversion times (i.e., 15, 75, 150, 225, 300, 375, 450, 525, 600, 675, 750, 825, 900, 1000, 1200, 1400, 1600, 1800, 2000, 2500, 3500, 5000, 7000, 9000, and 10,000 ms). Fully automated in‐house software written in MATLAB was used to obtain T1 maps from the ASL data and to compute CBF maps. 18
Connectomics—Brain network analyses
The MRI data were processed to obtain the structural brain network of each rat, and the network organization was evaluated by graph metrics. T1‐weighted and T2‐weighted images were used for tissue segmentation and brain region parcellation, respectively, and the connections between brain regions were estimated based on the diffusion‐weighted images.
Brain parcellation and segmentation were performed on the basis of a rat brain atlas as described elsewhere, 19 and the results obtained from T1‐ and T2‐weighted volumes were registered to the diffusion images. Parcellation resulted in 76 brain regions (first column in Table S1), which were considered the nodes of the brain network.
To characterize the connections between nodes, diffusion‐weighted volumes were preprocessed; the preprocessing steps included eddy current correction using FSL, 20 denoising, 21 and bias correction. 22 The baseline images were averaged and registered to the T2‐weighted images to correct for echo‐planar imaging distortions. Diffusion tensor images were estimated, and fractional anisotropy (FA) was computed using Dipy. 23 Tractography was performed using a deterministic algorithm based on a constrained spherical deconvolution model. 24 The voxels in the white matter mask obtained from anatomical segmentation were considered seed points for streamlines, and an FA < 0.1 was considered the stopping criterion for tractography reconstruction of white matter tracts.
Subsequently, the FA‐normalized connectome was estimated. A connection between two regions (e.g., A and B) was plotted if there was at least one streamline starting in region A and ending in region B. The average FA in all the streamlines connecting each pair of regions was computed to obtain the FA‐weighted connectome for each rat. Afterward, each FA‐weighted connectome was normalized by its strength (i.e., the sum of all the connection weights in the network), resulting in the FA‐normalized connectome. This enabled FA‐strength‐independent quantification of brain organization. 25
Network organization was described by graph metrics. Global and local efficiency and average clustering coefficients were used to describe whole‐brain network organization, whereas the nodal strength, efficiency, and clustering coefficient of each of the 76 brain regions were computed to evaluate regional connectivity. Local efficiency and clustering coefficients were used to evaluate the segregation of the brain network. Local efficiency was defined as the whole‐brain average of nodal efficiency, which estimates how information can be exchanged in the subnetwork associated with a given region and, therefore, how effectively the network would resist if this node failed. The clustering coefficient quantifies how many neighbors of a node are also neighbors to each other, and its average value over the whole network was calculated to obtain the average clustering coefficient. Global efficiency describes network integration and estimates how information can be transferred between regions in the whole network. Finally, nodal strength is defined as the total weight of all the connections of a region.
Learning and motivation tests
Learning was studied in all groups with operant conditioning experiments. Operant conditioning maintained by food was first described by Skinner and is widely used to assess reward learning, which models human decision‐making. 26 Operant conditioning experiments involve associative causal relationship learning 27 and, implicitly, memory. 28 We used rat operant chambers (Med Associates, Georgia, VT, USA) equipped with two levers, each of which was selected as either an active or inactive lever. Pressing the active lever resulted in a reinforcer (food pellet delivery), while pressing the inactive lever had no consequences. The chambers were housed in sound‐ and light‐attenuated boxes equipped with fans to provide ventilation and ambient noise. A food receptacle equidistant between the two levers permitted the delivery of food pellets when appropriate.
The rats had their food intake partially restricted for 4 days to reduce their body weight to 95% of its previous value. The same food restriction regime was maintained throughout the whole evaluation of food‐maintained operant behavior. Water was available ad libitum during this experimental phase. On day 4 of food restriction, rats were trained in operant chambers to lever‐press for food pellets (45 mg, Dustless Precision Pellets, PHYMEP, France). Self‐administration sessions (1 h daily) were conducted 5 days per week. A light was turned on for 3 s at the beginning of the session and then turned off for the remainder of the session.
First, rats were trained on a fixed ratio 1 (FR1) reinforcement schedule. A 10‐s time‐out period was established after each reinforcement. During this 10‐s period, the cue light was off, and no reward was provided for responses on the active lever. Responses on the active and inactive levers during the trials and during the 10‐s time‐out periods were recorded. The session was terminated after 100 reinforcers were delivered or after 1 h, whichever occurred first. The criteria for successful acquisition were achieved when rats maintained a stable response rate with less than 20% deviation from the mean of the total number of reinforcers earned in three consecutive sessions (i.e., 80% stability), made at least 75% of their responses on the active lever, and earned 100 reinforcers per session. Once the acquisition criteria were met, the reinforcement schedule was changed to a fixed ratio 3 (FR3), in which rats were required to press the active lever three consecutive times to obtain a reinforcer. As with the FR1 schedule, rats were required to meet the criteria of 80% stability, 75% active lever responses, and 100 reinforcers per session in order to move from the FR3 schedule to the progressive ratio (PR) test.
PR tasks are used to analyze motivation for various reinforcers, that is, the willingness of the organism to exert effort in order to obtain a reward. 29 In our work, the number of responses required to earn a food pellet in the PR experiments escalated as follows: 1‐2‐3‐5‐12‐18‐27‐40‐60‐90‐135‐200‐300‐450‐675‐1000. The PR session was performed only once and lasted for 2 h or until rats failed to complete the response requirement for delivery of a reinforcer within 1 h. The breakpoint for the extinction of food‐maintained operant behavior was determined for each animal. After each session, the rats were returned to their cages.
Immunohistochemistry
After euthanasia, rats (n = 8–10/group) were transcardially perfused with 60 ml of 0.9% saline at a flow rate of 26 ml/min followed by 250 ml of 4% paraformaldehyde at a flow rate of 15.3 ml/min with a syringe pump system (NE‐1600, New Era Instruments, Tampa, FL, USA). Brains were cut into three coronal sections of a similar size. The sections were postfixed in the perfusion solution for 24 h and later embedded in paraffin. Four‐micrometer‐thick coronal sections were cut on a microtome (RM2125RT, Leica Biosystems, Germany) and placed on slides. For each rat, three to five consecutive coronal sections at a similar, comparable histological site (3–3.75 mm anterior to bregma) were selected. The slides were stained with hematoxylin and incubated with primary antibodies against the glial fibrillary acidic protein (GFAP) (1:2000; Z0334, Agilent Dako, Santa Clara, CA, USA) and ionized calcium‐binding adaptor molecule 1 (IBA1) (1:400; 019–19741, Fujifilm Wako, Richmond, VA, USA). Images were taken of each sample with an Olympus BX41TF microscope and DP73 camera (Olympus Life Science, Japan). Among all sections from every animal, at least three images from the right and left dentate gyrus of the hippocampus and from the frontal cortex were analyzed. Positive cells (GFAP+ or IBA+) were counted in the total area analyzed (1.4 mm2 for frontal cortex areas and 0.4 mm2 for hippocampus areas). Positive cells were analyzed using ImageJ v1.49 software (National Institutes of Health, Bethesda, MD, USA).
Preparation of plasma and brain samples
In separate groups of rats (n = 7–12 rats/group), blood samples were collected in EDTA tubes by inferior cava puncture during the sacrifice. Plasma was obtained by centrifugation (2000 × g at 4°C for 10 min), and the supernatant was collected and aliquoted. Whole brains were obtained, and the hippocampus (left and right) and frontal cortex (left and right) were dissected, separated, and immediately frozen in liquid nitrogen. The hippocampus plays a crucial role in the encoding of new information and, through its interaction with the frontal pole, is important for memory consolidation and retrieval, 30 as well as learning 31 processes. Samples were micronized in a nitrogen atmosphere. Similar amounts from the left and right areas were mixed and homogenized for different analyses. All samples were kept at −80°C until analyzed.
RNA isolation and real‐time PCR assays
Total RNA was isolated from the frontal cortex and hippocampus samples with TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA, USA) followed by a column‐based isolation method (mirVana miRNA Isolation Kit protocol, Thermo Fisher Scientific). The RNA concentration was measured with a Qubit Fluorometer (Thermo Fisher Scientific). Total RNA was reverse transcribed with a High‐Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Specific mRNA levels were assessed by real‐time reverse transcription polymerase chain reaction (RT‐PCR) using Power up SYBR Green Master Mix and ViiA7 (Applied Biosystems, Thermo Fisher Scientific). The results were normalized to the cyclophilin B (Ppib) housekeeping gene and are shown with the 2−ΔΔCt method. Primer sequences and PCR product lengths are listed in Table S2.
Protein extraction and western blots
Frontal cortex and hippocampus samples were homogenized with an Omni TH homogenizer (Omni International Inc., Kennesaw, GA, USA) in a lysis solution containing RIPA buffer (R0278, Sigma‐Aldrich, Merck, Saint Louis, MO, USA), Halt Protease Inhibitor Cocktail 100x (Thermo Fisher Scientific), and Halt Phosphatase Inhibitor Cocktail 100x (Thermo Fisher Scientific). After 1 h of rotation at 4°C, the samples were centrifuged (10,000 × g at 4°C for 30 min), and the supernatant was collected. The total protein concentration was quantified with a Pierce BCA protein assay (23227, Thermo Fisher Scientific).
Protein extracts (20–30 μg) from the frontal cortex and hippocampus were subjected to electrophoresis on NuPAGE 4–12% Bis‐Tris Gels (NP0336, Life Technologies, Thermo Fisher Scientific) and transferred to nitrocellulose membranes using the iBlot Dry Blotting System (Life Technologies). Membranes were blocked for 1 h with 5% milk/BSA in Tris‐buffered saline with 0.1% Tween 20 (TBST) and incubated overnight at 4°C with primary antibodies against the following: OXPHOS (1:500; ab110413, Abcam, UK), p‐AMPK (1:500; 2535, Cell Signaling Technology, Danvers, MA, USA), and AMPK (1:1000; 5831, Cell Signaling Technology). After being washed with TBST, the membranes were incubated for 1 h at room temperature with HRP‐conjugated goat anti‐rabbit (1:3000; 7074, Cell Signaling Technology) or HRP‐conjugated goat anti‐mouse secondary antibodies (1:3000; A9044, Sigma‐Aldrich). Chemiluminescence signals were visualized by a LAS4000 imaging system (GE Healthcare, Aurora, OH, USA) using the ECL kit Supersignal West Pico PLUS Chemiluminescent Substrate (34577, Life Technologies, Thermo Fisher Scientific). Quantification of band density was performed using Image Lab™ v 6.0 Software (Bio‐Rad, Hercules, CA, USA). Samples were normalized to the full protein load assessed by Ponceau S staining. Original western blot figures are provided in Figures S1–S3.
Protein oxidation detection
An OxyBlot™ Protein Oxidation Detection Kit (S7150, Millipore, Burlington, MA, USA) was used for immunoblotting to detect carbonyl groups introduced into proteins by oxidative reactions. In accordance with the manufacturer's instructions, protein samples were denatured with one volume of 12% SDS and derivatized with two volumes of 1X DNPH (dinitrophenylhydrazine) solution for 15 min. Twenty micrograms of the frontal cortex and hippocampal protein extracts were used for each derivatization reaction. Then, derivatized proteins were subjected to SDS–PAGE and transferred to nitrocellulose membranes as described in the previous section. Immunodetection was performed using the antibodies supplied within the kit: rabbit anti‐DNP (1:150) as the primary antibody and goat anti‐rabbit IgG (1:300) as the secondary antibody.
Total antioxidant capacity assay
An Oxiselect total antioxidant capacity kit (STA‐360, Cell Biolabs, San Diego, CA, USA) was used to measure antioxidant capacity in frontal cortex and hippocampus samples according to the manufacturer's instructions. Ten to 15 mg of tissue were homogenized in cold PBS following centrifugation at 10,000 × g for 10 min at 4°C, and the supernatant was collected. Absorbances were read by spectrophotometry, and values were correlated with uric acid standards. The results are expressed relative to the protein concentration quantified by the Pierce BCA protein assay method (23227, Thermo Fisher Scientific). The intra‐assay coefficient of variance for the total antioxidant capacity ELISA was 1.91%.
Brain‐derived neurotrophic factor measurement
Levels of the brain‐derived neurotrophic factor (BDNF) protein were quantified in plasma samples and protein extracts from the frontal cortex and hippocampus with a commercially available ELISA kit (CYT306, Millipore) according to the manufacturer's instructions. The intra‐assay coefficient of variance was 4.65%.
Statistical analyses
Continuous variables are summarized or represented as the mean ± standard error of the mean or boxplots. The normality of the residuals was assessed by visual inspection of the Q–Q plots. Comparisons between groups were performed by one‐way ANOVA followed by Fisher's least significant differences (LSD) test in the case of a significant omnibus test. Because of the large number of post hoc comparisons, the analysis of regional network metrics was corrected for multiple comparisons using the false discovery rate. When normality could not be demonstrated, the nonparametric Kruskal‒Wallis test was used. The statistical test used in every analysis and, in the case of ANOVA, the number of degrees of freedom, and the F‐statistic are shown in Table S3. A two‐tailed p‐value < 0.05 was considered statistically significant. Analyses were carried out with GraphPad Prism v6.0 (GraphPad Software, San Diego, CA, USA) and R v3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Long‐term moderate, but not intense, exercise improves learning
Reward learning and motivation were assessed in all groups after the 16‐week training protocol. Learning ability was studied in the food‐maintained operant response experiments, and the results are summarized in Figure 1. Compared with SED rats, MOD but not INT rats reached the FR1 criteria in a significantly reduced time (Figure 1A), demonstrating enhanced learning capacity. No significant differences were found among the groups in latency to meet the FR3 criteria (Figure 1B). The motivation was analyzed after the learning tests in a PR test. MOD rats showed a higher breakpoint than INT rats (Figure 1C). Overall, our data showed enhanced learning ability as a result of moderate‐ but not intense‐load exercise compared with sedentarism; strenuous exercise decreased the enhancement of motivation caused by moderate exercise.
FIGURE 1.
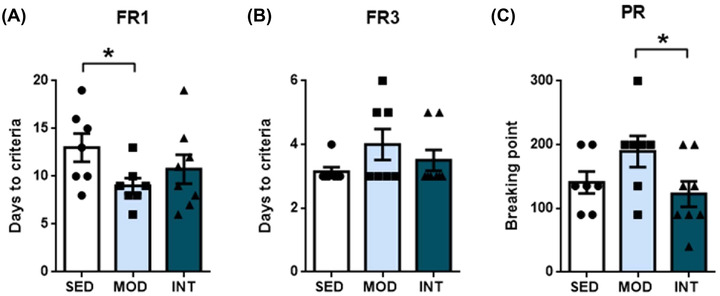
Learning and motivation assessment. The results of the fixed ratio 1 (FR1) learning test (A), fixed ratio 3 (FR3) learning test (B), and progressive ratio (PR) motivation test (C) in sedentary (SED, n = 7), moderate (MOD, n = 7), and intense (INT, n = 8) exercise‐trained rats. The omnibus test for FR1 and PR showed p < 0.05; *p < 0.05.
Brain connectome efficiency increases after moderate exercise only
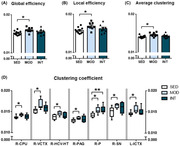
To study whether structural remodeling underlies exercise‐induced changes in reward learning and motivation function, brain MRI data were obtained. The main results of the connectome analyses are plotted in Figure 2. Compared with the sedentary group, the moderate exercise group showed increased global (Figure 2A) and local (Figure 2B) efficiency of the FA‐normalized connectome. Similarly, the MOD group showed a higher average clustering coefficient (Figure 2C) than the SED group. Regarding global and local efficiency and the average clustering coefficient, INT rats showed values comparable to those of the SED group, suggesting that moderate exercise resulted in the most efficient network organization.
FIGURE 2.
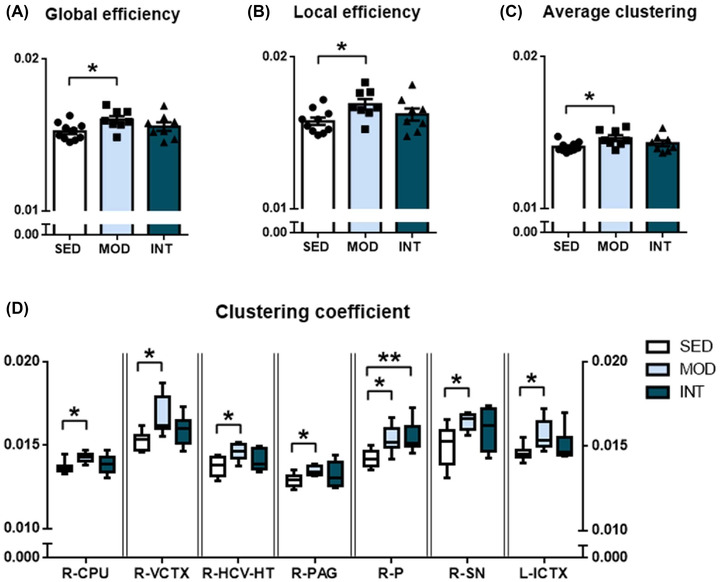
Global and regional network metrics of the FA‐normalized structural connectome. Global (A) and local (B) efficiency and average clustering coefficient (C) in sedentary (SED, n = 10), moderate (MOD, n = 8), and intense (INT, n = 8) exercise‐trained rats. Regions with statistically significant differences in clustering coefficients between the experimental groups are plotted (D) and include the right caudate–putamen (R‐CPU), right visual cortex (R‐VCTX), right ventral hippocampus and hypothalamus (R‐HCV‐HT), right periaqueductal gray (R‐PAG), right pons (R‐P), right substantia nigra (R‐SN), and left insular cortex (L‐ICTX). The omnibus test for global and local efficiency, R‐CPU, R‐VCTX, R‐HCV‐HT, R‐PAG, R‐P, R‐SN, and L‐ICTX showed p < 0.05; *p < 0.05; **p < 0.01.
We subsequently analyzed the clustering coefficient of each of the 76 regions identified as network nodes. All results are provided in Table S1, and those regions with significant changes after multiple comparison adjustments are shown in Figure 2D. Most changes were promoted by moderate exercise; these changes were located in the right hemisphere (i.e., the right caudate–putamen, visual cortex, ventral hippocampus and hypothalamus, periaqueductal gray, pons, and substantia nigra) and only one region of the left hemisphere (the insular cortex). Intense exercise resulted in an enhanced clustering coefficient only in the right pons region.
Cerebral perfusion differs between long‐term moderate and intense exercise
Long‐term moderate exercise training was associated with a significant increase in whole‐brain CBF (Figure 3A), but rats that had performed intense exercise showed whole‐brain CBF that was not significantly different from that of SED rats. Improved perfusion in MOD rats was evident in the right and left cortex and left hippocampus, while the INT group presented higher blood flow than the SED group only in the left cortex (Figure 3B).
FIGURE 3.
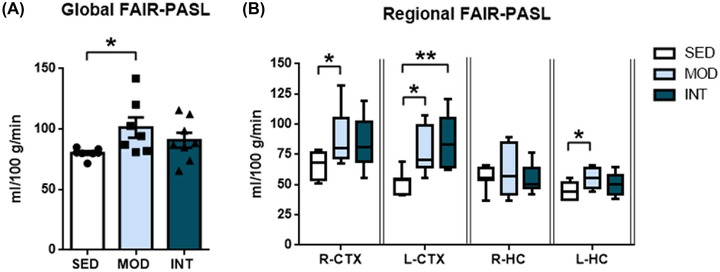
Cerebral blood flow. Global FAIR‐PASL (A) and regional FAIR‐PASL (B) in sedentary (SED, n = 7), moderate (MOD, n = 7), and intense (INT, n = 8) exercise‐trained rats. Data are shown for the right (R‐CTX) and left (L‐CTX) cortices and the right (R‐HC) and left (L‐HC) hippocampi. The omnibus test for global and regional FAIR‐PASL showed p < 0.05; *p < 0.05; **p < 0.01.
Adaptation of neurotrophic factors to long‐term exercise
Protein levels of the neurotrophic factor BDNF were quantified in plasma and brain homogenates. BDNF concentrations in plasma were similar across groups (Figure 4A). BDNF levels in frontal cortex samples were not modified by either moderate or intense exercise (Figure 4B). However, in the hippocampus, a significant decrease in BDNF protein levels was induced in the moderate long‐term exercise group compared with both the SED and INT groups (Figure 4C). Similar mRNA levels of Bdnf in all groups (Figure 4D,E) suggested posttranscriptional modifications as the origin of the total protein changes among the three groups. Moreover, the mRNA levels of BDNF receptor tyrosine receptor kinase B (Trkb), vascular endothelial growth factor (Vegf), and insulin‐like growth factor‐1 (Igf1) (Figure 4D,E) were comparable in the cortex and hippocampus of SED, MOD, and INT rats.
FIGURE 4.
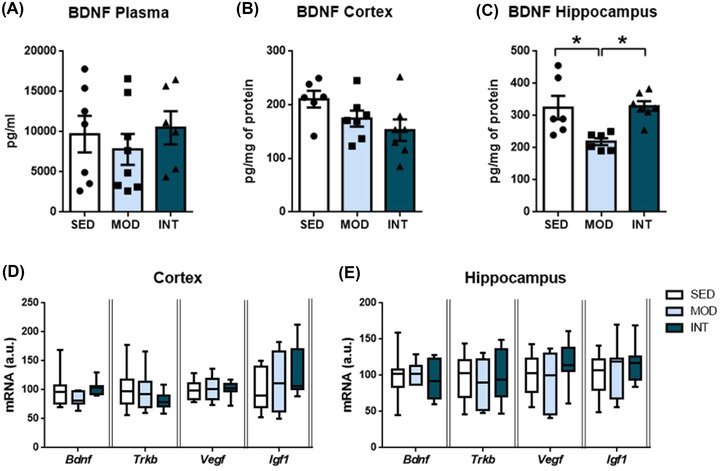
Neurotrophic factor analysis. Quantification of BDNF levels in the plasma (A), cortex (B), and hippocampus (C) of sedentary (SED), moderate (MOD), and intense (INT) exercise‐trained rats (n = 6–8/group). Bdnf, Trkb, Vegf, and Igf1 mRNA levels in the frontal cortex (D) and hippocampus (E) of the same experimental groups (n = 7–12/group). A comprehensive description of sample sizes is provided in Table S4. The omnibus test for hippocampal BDNF levels showed p < 0.05; *p < 0.05. Abbreviations: AMPK, AMP‐activated protein kinase; Bdnf, brain‐derived neurotrophic factor; Igf1, insulin‐like growth factor‐1; OXPHOS, oxidative phosphorylation proteins; p‐AMPK, phosphorylated AMP‐activated protein kinase; Trkb, tyrosine receptor kinase B; Vegf, vascular endothelial growth factor.
Mitochondrial energetics and oxidative stress are impaired in the frontal cortex after intense exercise
Mitochondria are key players in cellular bioenergetics, and their dysregulation is an early marker of cognitive impairment. In our study, the expression of mitochondrial oxidative phosphorylation proteins (OXPHOS) was determined by western blot (results and representative blots in Figure 5A–D). Figure 5A shows the reduced expression of OXPHOS complex IV in the frontal cortex of the INT group compared with the SED group, while no differences were found in the hippocampus (Figure 5C), suggesting that long‐term intense exercise results in diminished ATP synthesis in the frontal cortex. To further test this hypothesis, activation of AMP‐activated protein kinase (AMPK) protein was assessed (Figure 5E–J). Phosphorylated AMPK protein levels were elevated in the frontal cortex (Figure 5E) but not in the hippocampus (Figure 5H) of intensively trained rats. Total AMPK levels were similar in all groups and regions.
FIGURE 5.
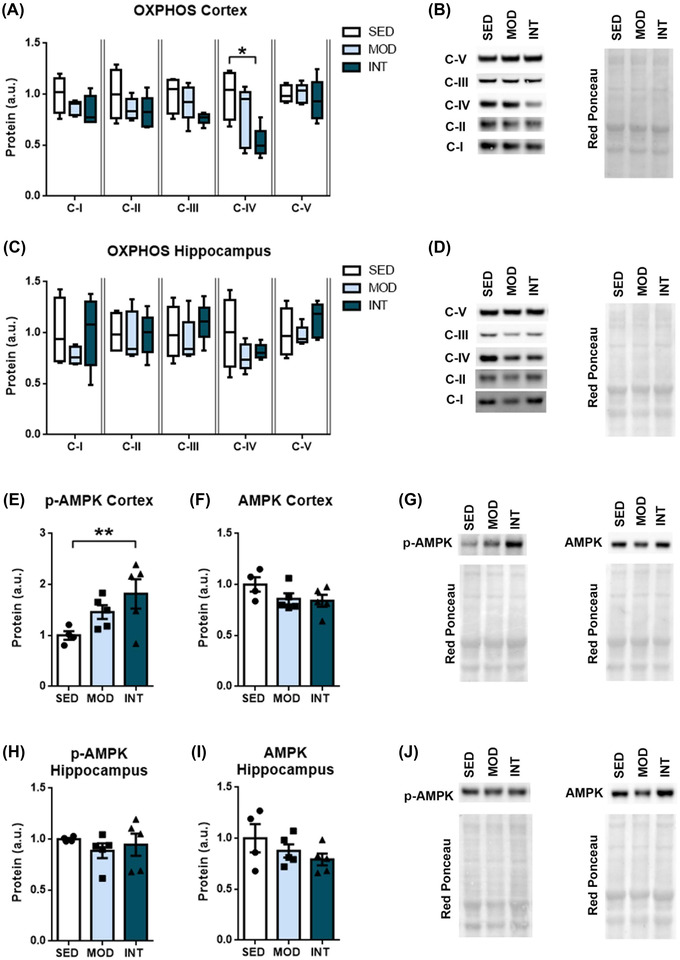
Mitochondrial bioenergetics. Relative protein levels of OXPHOS subunits in the frontal cortex (A) and hippocampus (C) in sedentary (SED, n = 4), moderate (MOD, n = 5), and intense (INT, n = 5) exercise‐trained rats. Relative phosphorylated and total AMPK protein levels in the frontal cortex (E, F) and hippocampus (H, I) of the same experimental groups. Cropped representative bands and red Ponceau staining corresponding to each experimental group are shown (B, D, G, J). Different exposure times were used for different complexes to optimize the quantifiability of the results. The omnibus test for OXPHOS complex IV and p‐AMPK protein levels showed p < 0.05; *p < 0.05; **p < 0.01. Abbreviations: AMPK, AMP‐activated protein kinase; C‐I, complex I; C‐II, complex II; C‐III, complex III, C‐IV, complex IV; C‐V, complex V; OXPHOS, oxidative phosphorylation proteins; p‐AMPK, phosphorylated AMP‐activated protein kinase.
We next analyzed protein carbonylation to estimate oxidative stress. Exercise training did not alter protein carbonylation (Figure 6A–D) or the mRNA levels of the antioxidant enzymes catalase (Cat), glutathione peroxidase (Gpx1), and superoxide dismutase (Sod2) in the frontal cortex or hippocampus (Figure 6E,F). The total antioxidant capacity was similar among the groups in the hippocampus (Figure 6H). Conversely, in the frontal cortex, the MOD group showed a reduction in total antioxidant capacity compared with both the SED and INT groups, while the INT group demonstrated the highest total antioxidant capacity (Figure 6G).
FIGURE 6.
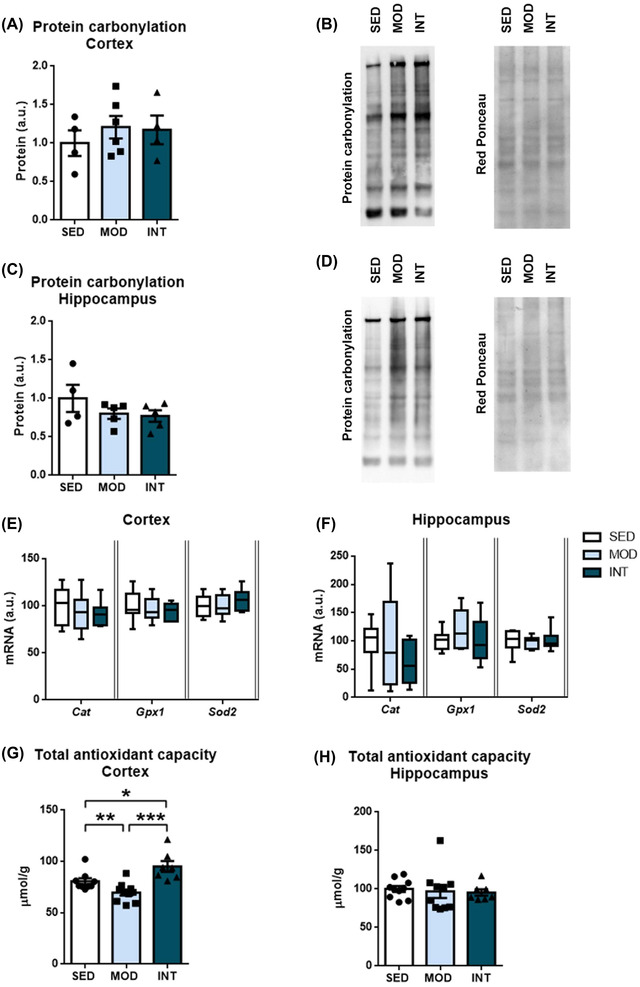
Oxidative stress and antioxidant response analysis. Relative carbonylated protein levels in the frontal cortex (A) and hippocampus (C) in sedentary (SED), moderate (MOD), and intense (INT) exercise‐trained rats (n = 4–6/group). Representative bands and Ponceau staining correspond to each experimental group (B, D). mRNA levels of antioxidant enzymes in the frontal cortex (E) and hippocampus (F) of the experimental groups (n = 7–12/group). Total antioxidant capacity was assessed in the frontal cortex (G) and hippocampus (H) (n = 7–10/group). A comprehensive description of sample sizes is provided in Table S4. The omnibus test for total antioxidant capacity in frontal cortex samples showed p < 0.05; *p < 0.05; **p < 0.01. Abbreviations: Cat, catalase; Gpx1, glutathione peroxidase; Sod2, superoxide dismutase.
Prolonged exercise training does not enhance neuroinflammation
The immunohistochemical expression of GFAP and IBA1, reflecting astroglial and microglial activation, respectively, was determined to assess neuroinflammation. No expression changes were detected among the groups in the frontal cortex or hippocampus (Figure 7A–F). Similarly, no significant exercise‐induced differences were found in the mRNA levels of proinflammatory tumor necrosis factor α (Tnfa) and adhesion G protein‐coupled receptor E1 (Adgre1) (Figure 7G,H).
FIGURE 7.
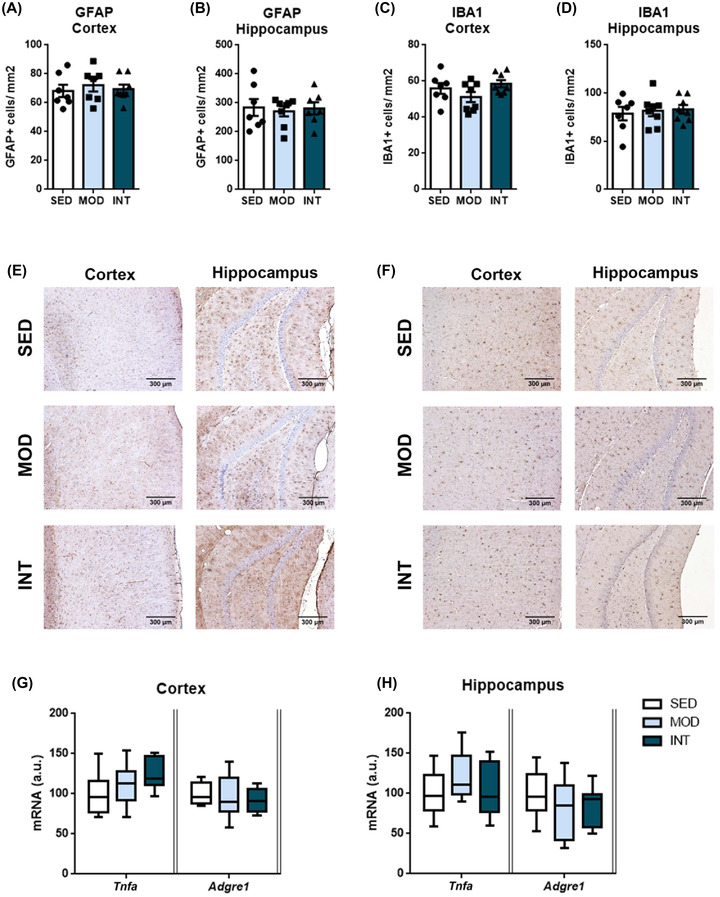
Neuroinflammation analysis. Quantification of GFAP‐ and IBA1‐immunopositive cells in the frontal cortex (A, C) and dentate gyrus of the hippocampus (B, D) in sedentary (SED), moderate (MOD), and intense (INT) exercise‐trained rats (n = 7–8/group). Representative images of GFAP (E) and IBA1 (F) immunostaining are shown. Tumor necrosis factor α (Tnfa) and adhesion G protein‐coupled receptor E1 (Adgre1) mRNA levels in the frontal cortex (G) and hippocampus (H) of the same experimental groups (n = 7–12/group). A comprehensive description of sample sizes is provided in Table S4. Abbreviations: GFAP, glial fibrillary acidic protein; IBA1, ionized calcium‐binding adaptor molecule 1.
DISCUSSION
The present study showed that long‐term moderate‐ but not intense‐load exercise induced beneficial effects on reward‐related learning (and, implicitly, memory), as well as structural adaptation in juvenile to adult rats, resulting in an inverted U‐shaped relationship between physical exercise and brain health. Our main findings were as follows: (1) moderate but not intense exercise training increased learning capacity, while motivation was decreased in the intense exercise group compared with moderately trained rats; (2) moderate exercise increased global and regional brain network efficiency and clustering and promoted an increase in CBF, while these effects were not evident with intense exercise; (3) intense exercise impaired mitochondrial bioenergetics, particularly in the cortex; and (4) moderate exercise was associated with reduced hippocampal BDNF levels and frontal cortex antioxidant capacity.
Effects of high‐intensity exercise on brain health
The evidence to date has substantiated the concept that moderate physical activity promotes cognitive improvements, but the consequences of very high exercise loads are still under debate. Some studies performed in active middle‐aged 32 and older 33 master athletes with a long history of sport participation showed improved neurocognitive performance in comparison with nonathletes, but conflicting data have been recently reported. For example, neurodegenerative diseases at death are more prevalent in professional soccer players than among matched controls. 8 Although repetitive concussion has been claimed to underlie these findings, no differences were found between strikers and goalkeepers, the latter of whom are presumed to be relatively protected from repetitive head trauma.
Establishing a causal link between strenuous training in humans and its effects on the brain is hindered, at least partially, by confounding variables and biases that are seldom considered or cannot be adjusted for. Conversely, while caution is still needed, animal models enable researchers to assess the effects of exercise while minimizing the impact of some of these biases and confounding factors. 34 In our model, chronic moderate exercise increased the learning capacity of animals compared with sedentary controls, but this was not evident in intensively trained animals. Our results are consistent with previous work suggesting that exercise duration and intensity might drive the brain health benefits of exercise in a nonlinear, inverted U‐shaped relationship. 12 , 35
Animal models also allow exploration of pathophysiological insights and have shown that exercise might promote cognitive improvements through a complex interplay of improved CBF, 36 neuroplasticity, 13 and oxidative stress protection, 37 among others. 1
Exercise load determines neuroplasticity
Regular moderate exercise is a potent trigger for structural and functional brain remodeling. Short‐term exercise has been associated with enlargement of the hippocampus 38 and areas controlling exercise‐specific tasks. 39 Enhanced structural connectivity among neurons may improve the efficiency of brain functioning. Moderate aerobic exercise, defined by an intensity of 60% of VOmax or a score of 11–16 on the Borg scale, improved structural neuronal connectivity in patients with multiple sclerosis 40 and mild traumatic brain injury. 41 Our results are consistent with these reports and demonstrated improved connectivity efficiency and an increase in the average and regional clustering coefficients in moderately trained rats compared with sedentary healthy rats. These results indicated increased integration and segregation in the organization of the brain network as a result of moderate exercise, which may boost the information flow and thus enhance reward‐related learning performance and, possibly, memory.
Conversely, the long‐term effects of very high‐intensity endurance exercise on brain structure have seldom been studied, and, to our knowledge, no data are available on the effects of long‐term, very high‐intensity endurance exercise on structural brain connectivity. In the present study, we showed that, unlike moderate exercise, high‐intensity exercise did not enhance structural brain connectivity, which might explain the lack of learning improvement in heavily trained healthy rats.
Preclinical research supports that the neurotrophic factors BDNF, 42 VEGF, 36 IGF1, 43 and growth hormone 12 are critical for improved cognitive performance after exercise. In contrast to some previous findings, we observed reduced frontal cortex BDNF levels along with improved brain function in our model after long‐term moderate exercise. Our results are in line with previous human observational work associating long periods (7 years on average) of regular, chronic exercise with enhanced memory function despite reduced BDNF serum levels. 44 , 45 The reasons for such a decrease in BDNF levels remain elusive, but it is likely that decreased BDNF levels result from long‐term adaptation to chronic physical activity in contrast to the increased levels occurring after single bouts of exercise or short‐ to medium‐term moderate exercise programs. 12 , 45 This is supported by data showing that BDNF plasma levels peaked early (35‐day training) in a mouse model of long‐term voluntary exercise and subsequently (42‐ and 49‐day training) dropped. 12 Exercise duration is not the only variable that can modulate BDNF levels: strenuous exercise, such as running a marathon, has been recently shown to transiently decrease plasma BDNF levels; 46 whether this mirrors changes in brain synthesis remains unknown. Altogether, it has been suggested that the effects of exercise on neurotrophic factors might be fast and transient compared to long‐lasting structural vascular and neuronal remodeling. 47 , 48 Indeed, the absence of long‐term exercise‐induced changes in Bdnf, Igf1, and Vegf mRNA levels in any region serves as further evidence that chronic adaptation to neurotrophic factors may not replicate acute or short‐term changes and emphasizes the complex interaction among exercise load, training duration, and neurotrophic factors. Notably, in our work, hippocampal BDNF levels were similar in sedentary and intensively trained rats, further demonstrating clear differences between long‐term moderate and intense exercise.
Role of vascular remodeling and CBF in cognitive performance
Improved CBF has been reported to mediate the beneficial effects of exercise on cognitive function. 49 Voluntary physical exercise of a moderate intensity improves angiogenesis and increases CBF in animal models. 50 Our results corroborate previous data by showing that moderate exercise training increases global CBF as well as cortical and left hippocampal blood flow in healthy rats. However, compared with sedentary animals, animals experiencing high exercise loads failed to show significantly increased global CBF, thereby indicating that chronic high‐intensity exercise blunts the increased CBF perfusion quantified in moderately trained rats. The physiopathology behind the reduced benefit of very intense exercise in terms of CBF was not addressed in this study. However, our previous work found that while moderate exercise improved endothelial function, intense training promoted deleterious aortic and carotid remodeling involving tunica media fibrosis and stiffening, 17 potentially inducing neurovascular uncoupling and brain dysfunction. 51 Further supporting the link between deleterious vascular remodeling and the cognitive effects of strenuous exercise, emerging clinical and experimental data have demonstrated that arterial stiffness may impact cognitive performance. 52 , 53 Overall, vascular dysfunction and subsequent changes in CBF may contribute to the blunting of exercise‐induced learning benefits with increased loads of physical activity.
Mitochondrial function and oxidative stress as early markers of neuronal function
Owing to the high metabolic and energetic demands of the brain, mitochondrial function plays an especially important role in maintaining brain homeostasis and neuronal function. Given the brain's high energy consumption and narrow reserve, a continuous and stable energy supply is critical. Neurons are extremely dependent on OXPHOS to fulfill their energetic requirements, and reduced levels of mitochondrial IV complex (cytochrome c oxidase) are common in numerous neurodegenerative disorders; compromised mitochondrial ATP synthesis occurs early in brain aging 54 and cognitive impairment, 55 and ATP levels are reduced in the brain and peripheral cells of Alzheimer's disease patients. 56 In animal models, moderate exercise increases OXPHOS complex synthesis, 37 enhances mitochondrial energetic efficiency, 58 and improves brain mitochondrial bioenergetic function through mitochondrial enzyme induction. 57 Our experiments showed a significant reduction in OXPHOS complex IV in the frontal cortex of the INT group. This decrease resulted in reduced mitochondrial oxidative phosphorylation, decreased ATP production, and AMPK upregulation (also in the frontal cortex; Figure 5); this energy sensor is upregulated when the AMP/ATP ratio is heightened.
Mitochondrial respiratory inefficiency in the cortex and an imbalance between oxygen supply and demand may result in reactive oxygen species (ROS) generation and oxidative stress. Although our findings reflect a complex interplay of potentially involved factors, three important facts should be considered in the interpretation of our results. First, our data point to a decreased oxidative phosphorylation capacity and blunted ATP production in the INT group, posing a risk of oxidative phosphorylation uncoupling and ROS formation in highly sensitive neuronal cells. Reduced mitochondrial function after strenuous exercise is not found exclusively in the brain; in the muscle, low to moderate levels of exercise improve mitochondrial function, while high‐intensity exercise decreases its functioning. 59 Second, no changes in protein carbonylation were observed despite the unequal total antioxidant capacity among the groups. Third, our samples were collected far away from the acute exercise period (>48 h after the last training bout) and, therefore, may have failed to capture acute changes induced by exercise.
Altogether, we hypothesize that very intense exercise bouts promote transient systemic and local oxidative stress, which may produce acute deleterious neuronal effects. Indeed, ROS are often involved in neurodegeneration. 60 Repetitive transient oxidative stress in the INT group may, in the long term, induce the synthesis of antioxidant agents. Conversely, moderate exercise has well‐known systemic, muscular, and cardiovascular antioxidant effects in the absence of an overt postexercise pro‐oxidant cascade, which may decrease the need for local antioxidant synthesis. Dedicated studies are warranted to test these specific hypotheses.
The hippocampus and frontal cortex are the main cognition‐related brain areas. 30 Notably, we observed the greatest structural remodeling in the frontal cortex rather than in the hippocampus. Inherent regional differences in mitochondrial bioenergetics 61 and cerebrovascular response to hypoxia 62 could underlie selective regional susceptibility to brain impairments, as occurs in accelerated brain senescence processes. 63
Moderate and intense exercise bouts elicit different hemodynamic and systemic responses: Basis for chronic remodeling
The divergent consequences of moderate and very intense exercise in terms of brain structure, blood flow, and energetics may have resulted from the different physiological responses to exercise bouts as their intensity increased. Indeed, strenuous exercise has been associated with characteristic hemodynamic, metabolic, and inflammatory patterns.
Cardiovascular hemodynamic load dramatically increases with strenuous exercise bouts, challenging CBF regulation. CBF usually increases after short‐term and moderate exercise but decreases as exercise time and intensity increase, thereby attenuating dynamic cerebral autoregulation. 64 Whether a decrease in CBF due to strenuous exercise results in brain oxygenation abnormalities remains disputed, 65 with some reports indicating that oxygen delivery to the mitochondria might be compromised. 66 These acute effects on CBF may be augmented by the stiffening of large arteries 67 that is suggested to occur after long‐term strenuous training. 17
Strenuous exercise bouts are associated with a plethora of metabolic changes, including ionic and hormonal abnormalities, dehydration, renal failure, and energy depletion, which may locally impact the brain. Triathlon participants suffer from a large but transient reduction in brain volume averaging 6%, which might reflect profound disturbances in neuronal metabolism. 68 Overall, both CBF and metabolic abnormalities may underlie the well‐demonstrated decrease in cognitive performance and increase in mental fatigue after intense exercise, 9 , 69 potentially mediated by reduced activation of the prefrontal cortex. 9
Finally, exercise bouts promote an inflammatory cascade whose intensity depends on the training load. Previous work suggested acute exercise‐induced brain inflammation, 70 but we could not confirm the occurrence of chronic neuroinflammation as a consequence of high‐intensity exercise.
Limitations
Some limitations of our work should be acknowledged. First, although animal models are an invaluable tool for translational research, caution is needed when extending the results of animal studies to humans. Our training protocol for the rat model began during adolescence and continued into adulthood; adolescence is a highly sensitive stage of development for neuronal and behavioral changes, and exercise has been demonstrated to have the greatest beneficial effects if initiated during adolescence. 71 Accordingly, regular training initiated during adulthood may yield different conclusions. Second, our study was performed on healthy young male rats subjected to treadmill endurance training. Further studies are needed to clarify the potential contribution of sex, comorbidity burden, and response to other types of exercise. Finally, the results may have been confounded by stress or the development of overtraining. Maximum efforts were made to minimize stress responses due to treadmill exercise, and training was under continuous surveillance by a researcher. Indeed, we have previously reported no significant stress‐related effects in this rat model of intense exercise. 4 On the other hand, overtraining is characterized by fatigue and a decrease in physical performance and might be accompanied by worsened executive function, 72 , 73 which could partially explain our findings. However, we found no evidence of decreased physical performance, reward learning, or motivation in our intensively trained rats compared to healthy sedentary rats.
CONCLUSIONS
In conclusion, the present study demonstrates that long‐term moderate exercise induces improvements in brain structure and learning, while very high‐intensity exercise does not. Improved CBF and enhanced structural connectivity in moderately trained rats underlie improvements in reward learning. There were no improvements in learning ability, global connectivity efficiency, or CBF after intensive training, which was associated with impaired mitochondrial energetics in the frontal cortex. Our results support the existence of an inverted U‐shaped relationship between exercise load and brain health.
AUTHOR CONTRIBUTIONS
All authors significantly contributed to this manuscript and the research presented herein. GSangüesa, M.B., C.R., L.M., and E.G. defined the objectives and designed the project. M.B., E.G., and L.M. obtained funding. E.M.M., GSoria, C.R., L.S.R., E.S., E.M.‐H., S.A., and S.L. designed the methodological approach to reach the objectives. GSangüesa, M.B., GSoria, E.M.M., A.A., C.R., and L.S.R. performed the experiments and collected the data. GSangüesa, E.M., and E.G. performed the statistical analyses. GSangüesa, M.B., E.S., E.M.H., S.A., S.L., L.M., and E.G. interpreted the results. GSangüesa wrote the first draft of the manuscript. A.M.R. contributed to the revision of the manuscript and the exercise intensity estimates. All authors read and edited the manuscript and provided scientific content for it; E.G. prepared the final version of the manuscript.
COMPETING INTERESTS
The authors declare no competing interests relevant to the topic of this manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/nyas.14912.
Supporting information
Figure S1. Full‐length blots of OXPHOS protein levels and red Ponceau staining in the frontal cortex (A, B) and hippocampus (C, D) samples. Because of the high hydrophobicity of C‐IV, it commonly migrates differently, within the 30–40 kD range, as stated, shown, and extensively reported in the antibody datasheet.
Figure S2. Full‐length blots of p‐AMPK and AMPK protein levels and red Ponceau staining in the frontal cortex (A–D) and hippocampus (E–H) samples.
Figure S3. Full‐length blots of protein carbonylation and its red Ponceau stain in the frontal cortex (A, B) and hippocampus (C, D) samples.
Table S1: Regional clustering coefficients. The clustering coefficient (mean±SEM) of the 76 regions of the structural FA‐normalized connectome was analyzed in the sedentary and moderate and intense exercise groups. *p<0.05; **p<0.01 versus sedentary group.
Table S2: Primers used for RT‐PCR.
Table S3: Statistical analyses used for all comparisons. In the case of ANOVA, the number of degrees of freedom and the F‐statistic are reported.
Table S4: Sample size for all experiments in Figures 4, 6, and 7.
ACKNOWLEDGMENTS
We thank Nadia Castillo for providing excellent technical assistance. We are indebted to the Neurological Tissue Bank of the HCB‐IDIBAPS Biobank for sample and data procurement. We are also indebted to the Experimental MRI 7T Unit of the IDIBAPS and to the CERCA Programme (Generalitat de Catalunya). This work was supported by grants from the Instituto de Salud Carlos III (PI16/00703, PI19/00443, PI22/00953, CD20/00055, and FI20/00080) and co‐funded by the European Union, CERCA Programme/AGAUR 2017 SGR 1548/Generalitat de Catalunya, Sociedad Española de Cardiología, and CIBERCV (CB16/11/00354).
Sangüesa, G. , Batlle, M. , Muñoz‐Moreno, E. , Soria, G. , Alcarraz, A. , Rubies, C. , Sitjà‐Roqueta, L. , Solana, E. , Martínez‐Heras, E. , Meza‐Ramos, A. , Amaro, S. , Llufriu, S. , Mont, L. , & Guasch, E. (2022). Intense long‐term training impairs brain health compared with moderate exercise: Experimental evidence and mechanisms. Ann NY Acad Sci., 1518, 282–298. 10.1111/nyas.14912
REFERENCES
- 1. Cabral, D. F. , Rice, J. , Morris, T. P. , Rundek, T. , Pascual‐Leone, A. , & Gomes‐Osman, J. (2019). Exercise for brain health: An investigation into the underlying mechanisms guided by dose. Neurotherapeutics, 16, 580–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piercy, K. L. , Troiano, R. P. , Ballard, R. M. , Carlson, S. A. , Fulton, J. E. , Galuska, D. A. , George, S. M. , & Olson, R. D. (2018). The physical activity guidelines for Americans. JAMA, 320, 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guasch, E. , & Mont, L.‐S. (2017). Diagnosis, pathophysiology, and management of exercise‐induced arrhythmias. Nature Reviews Cardiology, 14, 88–101. [DOI] [PubMed] [Google Scholar]
- 4. Guasch, E. , Benito, B. , Qi, X. , Cifelli, C. , Naud, P. , Shi, Y. , Mighiu, A. , Tardif, J.‐C. , Tadevosyan, A. , Chen, Y. , Gillis, M.‐A. , Iwasaki, Y.‐K. , Dobrev, D. , Mont, L. , Heximer, S. , & Nattel, S. (2013). Atrial fibrillation promotion by endurance exercise: Demonstration and mechanistic exploration in an animal model. Journal of the American College of Cardiology, 62, 68–77. [DOI] [PubMed] [Google Scholar]
- 5. Sanz‐De La Garza, M. , Rubies, C. , Batlle, M. , Bijnens, B. H. , Mont, L. , Sitges, M. , & Guasch, E. (2017). Severity of structural and functional right ventricular remodeling depends on training load in an experimental model of endurance exercise. American Journal of Physiology‐Heart and Circulatory Physiology, 313, H459–H468. [DOI] [PubMed] [Google Scholar]
- 6. Merghani, A. , Maestrini, V. , Rosmini, S. , Cox, A. T. , Dhutia, H. , Bastiaenan, R. , David, S. , Yeo, T. J. , Narain, R. , Malhotra, A. , Papadakis, M. , Wilson, M. G. , Tome, M. , Alfakih, K. , Moon, J. C. , & Sharma, S. (2017). Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation, 136, 126–137. [DOI] [PubMed] [Google Scholar]
- 7. Armstrong, M. E. G. , Green, J. , Reeves, G. K. , Beral, V. , & Cairns, B. J. (2015). Frequent physical activity may not reduce vascular disease risk as much as moderate activity: Large prospective study of women in the United Kingdom. Circulation, 131, 721–729. [DOI] [PubMed] [Google Scholar]
- 8. Mackay, D. F. , Russell, E. R. , Stewart, K. , MacLean, J. , Pell, J. , & Stewart, W. (2019). Neurodegenerative disease mortality among former professional soccer players. New England Journal of Medicine, 381, 1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blain, B. , Schmit, C. , Aubry, A. , Hausswirth, C. , Le Meur, Y. , & Pessiglione, M. (2019). Neuro‐computational impact of physical training overload on economic decision‐making. Current Biology, 29, 3289–3297. [DOI] [PubMed] [Google Scholar]
- 10. Mekari, S. , Fraser, S. , Bosquet, L. , Bonnéry, C. , Labelle, V. , Pouliot, P. , Lesage, F. , & Bherer, L. (2015). The relationship between exercise intensity, cerebral oxygenation and cognitive performance in young adults. European Journal of Applied Physiology, 115, 2189–2197. [DOI] [PubMed] [Google Scholar]
- 11. Cetinkaya, C. , Sisman, A. R. , Kiray, M. , Camsari, U. M. , Gencoglu, C. , Baykara, B. , Aksu, I. , & Uysal, N. (2013). Positive effects of aerobic exercise on learning and memory functioning, which correlate with hippocampal IGF‐1 increase in adolescent rats. Neuroscience Letters, 549, 177–181. [DOI] [PubMed] [Google Scholar]
- 12. Blackmore, D. G. , Steyn, F. J. , Carlisle, A. , O'Keeffe, I. , Vien, K.‐Y. , Zhou, X. , Leiter, O. , Jhaveri, D. , Vukovic, J. , Waters, M. J. , & Bartlett, P. F. (2021). An exercise “sweet spot” reverses cognitive deficits of aging by growth‐hormone‐induced neurogenesis. iScience, 24, 103275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar, A. , Rani, A. , Tchigranova, O. , Lee, W.‐H. , & Foster, T. C. (2012). Influence of late‐life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiology of Aging, 33, 828.e1–828.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vasconcelos‐Filho, F. S. L. , Da Rocha‐E‐Silva, R. C. , Martins, J. E. R. , Godinho, W. D. N. , Da Costa, V. V. , Ribeiro, J. K. C. , Da Silva, C. A. , Ceccatto, V. M. , Soares, P. M. , & Evangelista, J. S. A. M. (2021). Neuroprotector effect of daily 8‐minutes of high‐intensity interval training in rat Aβ1‐42 Alzheimer disease model. Current Alzheimer Research, 17, 1320–1333. [DOI] [PubMed] [Google Scholar]
- 15. Hayes, K. , Sprague, S. , Guo, M. , Davis, W. , Friedman, A. , Kumar, A. , Jimenez, D. F. , & Ding, Y. (2008). Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathologica, 115, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shepherd, R. E. , & Gollnick, P. D. (1976). Oxygen uptake of rats at different work intensities. Pflügers Archiv: European Journal of Physiology, 362, 219–222. [DOI] [PubMed] [Google Scholar]
- 17. Rubies, C. , Batlle, M. , la Garza, M. S. , Dantas, A. P. , Jorba, I. , Fernandez, G. , Sangüesa, G. , Abuli, M. , Brugada, J. , Sitges, M. , Navajas, D. , Mont, L. , & Guasch, E. (2022). Long‐term strenuous exercise promotes vascular injury by selectively damaging the tunica media: Experimental evidence. JACC: Basic to Translational Science, 7, 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kober, F. , Duhamel, G. , & Cozzone, P. J. (2008). Experimental comparison of four FAIR arterial spin labeling techniques for quantification of mouse cerebral blood flow at 4.7 T. NMR in Biomedicine, 21, 781–792. [DOI] [PubMed] [Google Scholar]
- 19. Muñoz‐Moreno, E. , Tudela, R. , López‐Gil, X. , & Soria, G. (2018). Early brain connectivity alterations and cognitive impairment in a rat model of Alzheimer's disease. Alzheimer's Research & Therapy, 10, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. J. , Woolrich, M. W. , & Smith, S. M. (2012). Review FSL. Neuroimage, 62, 782–790. [DOI] [PubMed] [Google Scholar]
- 21. Coupe, P. , Yger, P. , Prima, S. , Hellier, P. , Kervrann, C. , & Barillot, C. (2008). An optimized blockwise nonlocal means denoising filter for 3‐D magnetic resonance images. IEEE Transactions on Medical Imaging, 27, 425–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tustison, N. J. , Avants, B. B. , Cook, P. A. , Yuanjie Zheng, Egan, A. , Yushkevich, P. A. , & Gee, J. C. (2010). N4ITK: Improved N3 bias correction. IEEE Transactions on Medical Imaging, 29, 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garyfallidis, E. , Brett, M. , Amirbekian, B. , Rokem, A. , Van Der Walt, S. , Descoteaux, M. , & Nimmo‐Smith, I. (2014). Dipy, a library for the analysis of diffusion MRI data. Frontiers in Neuroinformatics, 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tournier, J.‐D. , Calamante, F. , & Connelly, A. (2007). Robust determination of the fibre orientation distribution in diffusion MRI: Non‐negativity constrained super‐resolved spherical deconvolution. Neuroimage, 35, 1459–1472. [DOI] [PubMed] [Google Scholar]
- 25. Muñoz‐Moreno, E. , Fischi‐Gomez, E. , Batalle, D. , Borradori‐Tolsa, C. , Eixarch, E. , Thiran, J.‐P. , Gratacós, E. , & Hüppi, P. S. (2016). Structural brain network reorganization and social cognition related to adverse perinatal condition from infancy to early adolescence. Frontiers in Neuroscience, 10, 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sweatt, J. D. (2016). Neural plasticity and behavior – Sixty years of conceptual advances. Journal of Neurochemistry, 139, 179–199. [DOI] [PubMed] [Google Scholar]
- 27. Gourley, S. L. , Lee, A. S. , Howell, J. L. , Pittenger, C. , & Taylor, J. R. (2010). Dissociable regulation of instrumental action within mouse prefrontal cortex. European Journal of Neuroscience, 32, 1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harb, M. R. , Sousa, N. , Zihl, J. , & Almeida, OF. (2014). Reward components of feeding behavior are preserved during mouse aging. Frontiers in Aging Neuroscience, 6, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson, A. R. , Christensen, B. A. , Kelly, S. J. , & Calipari, E. S. (2022). The influence of reinforcement schedule on experience‐dependent changes in motivation. Journal of the Experimental Analysis of Behavior, 117, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Preston, A. R. , & Eichenbaum, H. (2013). Interplay of hippocampus and prefrontal cortex in memory. Current Biology, 23, R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gould, E. , Beylin, A. , Tanapat, P. , Reeves, A. , & Shors, T. J. (1999). Learning enhances adult neurogenesis. Nature Neuroscience, 2, 260–265. [DOI] [PubMed] [Google Scholar]
- 32. Zhao, E. , Tranovich, M. J. , Deangelo, R. , Kontos, A. P. , & Wright, V. J. (2016). Chronic exercise preserves brain function in masters athletes when compared to sedentary counterparts. Physician and Sportsmedicine, 44, 8–13. [DOI] [PubMed] [Google Scholar]
- 33. Tseng, B. Y. , Uh, J. , Rossetti, H. C. , Cullum, C. M. , Diaz‐Arrastia, R. F. , Levine, B. D. , Lu, H. , & Zhang, R. (2013). Masters athletes exhibit larger regional brain volume and better cognitive performance than sedentary older adults. Journal of Magnetic Resonance Imaging, 38, 1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Voss, M. W. , Vivar, C. , Kramer, A. F. , & Van Praag, H. (2013). Bridging animal and human models of exercise‐induced brain plasticity. Trends in Cognitive Sciences, 17, 525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu, Y. , Deng, F. , Wang, J. , Liu, Y. , Zhou, W. , Qu, L. , & Cheng, M. (2020). Intensity‐dependent effects of consecutive treadmill exercise on spatial learning and memory through the p‐CREB/BDNF/NMDAR signaling in hippocampus. Behavioural Brain Research, 386, 112599. [DOI] [PubMed] [Google Scholar]
- 36. Fabel, K. , Fabel, K. , Tam, B. , Kaufer, D. , Baiker, A. , Simmons, N. , Kuo, C. J. , & Palmer, T. D. (2003). VEGF is necessary for exercise‐induced adult hippocampal neurogenesis. European Journal of Neuroscience, 18, 2803–2812. [DOI] [PubMed] [Google Scholar]
- 37. Bayod, S. , Guzmán‐Brambila, C. , Sanchez‐Roige, S. , Lalanza, J. F. , Kaliman, P. , Ortuño‐Sahagun, D. , Escorihuela, R. M. , & Pallàs, M. (2014). Voluntary exercise promotes beneficial anti‐aging mechanisms in SAMP8 female brain. Journal of Molecular Neuroscience, 55, 525–532. [DOI] [PubMed] [Google Scholar]
- 38. Erickson, K. I. , Voss, M. W. , Prakash, R. S. , Basak, C. , Szabo, A. , Chaddock, L. , Kim, J. S. , Heo, S. , Alves, H. , White, S. M. , Wojcicki, T. R. , Mailey, E. , Vieira, V. J. , Martin, S. A. , Pence, B. D. , Woods, J. A. , Mcauley, E. , & Kramer, A. F. (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America, 108, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sumiyoshi, A. , Taki, Y. , Nonaka, H. , Takeuchi, H. , & Kawashima, R. (2014). Regional gray matter volume increases following 7days of voluntary wheel running exercise: A longitudinal VBM study in rats. Neuroimage, 98, 82–90. [DOI] [PubMed] [Google Scholar]
- 40. Stellmann, J.‐P. , Maarouf, A. , Schulz, K.‐H. , Baquet, L. , Pöttgen, J. , Patra, S. , Penner, I.‐K. , Gellißen, S. , Ketels, G. , Besson, P. , Ranjeva, J.‐P. , Guye, M. , Nolte, G. , Engel, A. K. , Audoin, B. , Heesen, C. , & Gold, S. M. (2020). Aerobic exercise induces functional and structural reorganization of CNS networks in multiple sclerosis: A randomized controlled trial. Frontiers in Human Neuroscience, 14, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yuan, W. , Wade, S. L. , Quatman‐Yates, C. , Hugentobler, J. A. , Gubanich, P. J. , & Kurowski, B. G. (2017). Structural connectivity related to persistent symptoms after mild TBI in adolescents and response to aerobic training: Preliminary investigation. Journal of Head Trauma Rehabilitation, 32, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vaynman, S. , Ying, Z. , & Gomez‐Pinilla, F. (2004). Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience, 20, 2580–2590. [DOI] [PubMed] [Google Scholar]
- 43. Ding, Q. , Vaynman, S. , Akhavan, M. , Ying, Z. , & Gomez‐Pinilla, F. (2006). Insulin‐like growth factor I interfaces with brain‐derived neurotrophic factor‐mediated synaptic plasticity to modulate aspects of exercise‐induced cognitive function. Neuroscience, 140, 823–833. [DOI] [PubMed] [Google Scholar]
- 44. Nofuji, Y. , Suwa, M. , Moriyama, Y. , Nakano, H. , Ichimiya, A. , Nishichi, R. , Sasaki, H. , Radak, Z. , & Kumagai, S. (2008). Decreased serum brain‐derived neurotrophic factor in trained men. Neuroscience Letters, 437, 29–32. [DOI] [PubMed] [Google Scholar]
- 45. De la, R. A. , Solana, E. , Corpas, R. , Bartrés‐Faz, D. , Pallàs, M. , Vina, J. , Sanfeliu, C. , & Gomez‐Cabrera, MC. (2019). Long‐term exercise training improves memory in middle‐aged men and modulates peripheral levels of BDNF and Cathepsin B. Science Reports, 9, 3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roeh, A. , Holdenrieder, S. , Schoenfeld, J. , Haeckert, J. , Halle, M. , Falkai, P. , Scherr, J. , & Hasan, A. (2021). Decreased serum brain‐derived neurotrophic factor concentrations 72 hours following marathon running. Frontiers in Physiology, 12, 668454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Knaepen, K. , Goekint, M. , Heyman, E. M. , & Meeusen, R. (2010). Neuroplasticity – Exercise‐induced response of peripheral brain‐derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Medicine, 40, 765–801. [DOI] [PubMed] [Google Scholar]
- 48. Maass, A. , Düzel, S. , Brigadski, T. , Goerke, M. , Becke, A. , Sobieray, U. , Neumann, K. , Lövdén, M. , Lindenberger, U. , Bäckman, L. , Braun‐Dullaeus, R. , Ahrens, D. , Heinze, H.‐J. , Müller, N. G. , Lessmann, V. , Sendtner, M. , & Düzel, E. (2016). Relationships of peripheral IGF‐1, VEGF and BDNF levels to exercise‐related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage, 131, 142–154. [DOI] [PubMed] [Google Scholar]
- 49. Murrell, C. J. , Cotter, J. D. , Thomas, K. N. , Lucas, S. J. E. , Williams, M. J. A. , & Ainslie, P. N. (2013). Cerebral blood flow and cerebrovascular reactivity at rest and during sub‐maximal exercise: Effect of age and 12‐week exercise training. Age, 35, 905–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gertz, K. , Priller, J. , Kronenberg, G. , Fink, K. B. , Winter, B. , Schröck, H. , Ji, S. , Milosevic, M. , Harms, C. , Böhm, M. , Dirnagl, U. , Laufs, U. , & Endres, M. (2006). Physical activity improves long‐term stroke outcome via endothelial nitric oxide synthase‐dependent augmentation of neovascularization and cerebral blood flow. Circulation Research, 99, 1132–1140. [DOI] [PubMed] [Google Scholar]
- 51. Ogoh, S. (2017). Relationship between cognitive function and regulation of cerebral blood flow. Journal of Physiological Sciences, 67, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Badji, A. , Sabra, D. , Bherer, L. , Cohen‐Adad, J. , Girouard, H. , & Gauthier, C. J. (2019). Arterial stiffness and brain integrity: A review of MRI findings. Ageing Research Reviews, 53, 100907. [DOI] [PubMed] [Google Scholar]
- 53. Soria, G. , Tudela, R. , Márquez‐Martín, A. , Camón, L. , Batalle, D. , Muñoz‐Moreno, E. , Eixarch, E. , Puig, J. , Pedraza, S. , Vila, E. , Prats‐Galino, A. , & Planas, A. M. (2013). The ins and outs of the BCCAo model for chronic hypoperfusion: A multimodal and longitudinal MRI approach. PLoS One, 8, e74631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Desler, C. , Hansen, T. L. , Frederiksen, J. B. , Marcker, M. L. , Singh, K. K. , & Juel Rasmussen, L. (2012). Is there a link between mitochondrial reserve respiratory capacity and aging? Journal of Aging Research, 2012, 192503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moreira, P. I. , Carvalho, C. , Zhu, X. , Smith, M. A. , & Perry, G. (2010). Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochimica et Biophysica Acta (BBA) ‐ Molecular Basis of Disease, 1802, 2–10. [DOI] [PubMed] [Google Scholar]
- 56. Cardoso, S. M. , Proença, M. T. , Santos, S. , Santana, I. , & Oliveira, C. R. (2004). Cytochrome c oxidase is decreased in Alzheimer's disease platelets. Neurobiology of Aging, 25, 105–110. [DOI] [PubMed] [Google Scholar]
- 57. Kirchner, L. , Chen, W.‐Q. , Afjehi‐Sadat, L. , Viidik, A. , Skalicky, M. , Höger, H. , & Lubec, G. (2008). Hippocampal metabolic proteins are modulated in voluntary and treadmill exercise rats. Experimental Neurology, 212, 145–151. [DOI] [PubMed] [Google Scholar]
- 58. Navarro, A. , Gomez, C. , López‐Cepero, J. M. , & Boveris, A. (2004). Beneficial effects of moderate exercise on mice aging: Survival, behavior, oxidative stress, and mitochondrial electron transfer. American Journal of Physiology ‐ Regulatory, Integrative and Comparative Physiology, 286, R505–R511. [DOI] [PubMed] [Google Scholar]
- 59. Flockhart, M. , Nilsson, L. C. , Tais, S. , Ekblom, B. , Apró, W. , & Larsen, F. J. (2021). Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metabolism, 33, 957–970.e6.e6. [DOI] [PubMed] [Google Scholar]
- 60. Lin, M. T. , & Beal, M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature, 443, 787–795. [DOI] [PubMed] [Google Scholar]
- 61. Andersen, J. V. , Jakobsen, E. , Waagepetersen, H. S. , & Aldana, B. I. (2019). Distinct differences in rates of oxygen consumption and ATP synthesis of regionally isolated non‐synaptic mouse brain mitochondria. Journal of Neuroscience Research, 97, 961–974. [DOI] [PubMed] [Google Scholar]
- 62. Kannurpatti, S. S. , Biswal, B. B. , & Hudetz, A. G. (2003). Regional dynamics of the fMRI‐BOLD signal response to hypoxia‐hypercapnia in the rat brain. Journal of Magnetic Resonance Imaging, 17, 641–647. [DOI] [PubMed] [Google Scholar]
- 63. Blazer, D. G. , Yaffe, K. , & Karlawish, J. (2015). Cognitive aging: A report from the Institute of Medicine. JAMA, 313, 2121–2122. [DOI] [PubMed] [Google Scholar]
- 64. Whitaker, A. A. , Alwatban, M. , Freemyer, A. , Perales‐Puchalt, J. , & Billinger, S. A. (2020). Effects of high intensity interval exercise on cerebrovascular function: A systematic review. PLoS One, 15, e0241248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coetsee, C. , & Terblanche, E. (2017). Cerebral oxygenation during cortical activation: The differential influence of three exercise training modalities. A randomized controlled trial. European Journal of Applied Physiology, 117, 1617–1627. [DOI] [PubMed] [Google Scholar]
- 66. Rasmussen, P. , Nielsen, J. , Overgaard, M. , Krogh‐Madsen, R. , Gjedde, A. , Secher, N. H. , & Petersen, N. C. (2010). Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. Journal of Physiology, 588, 1985–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jefferson, A. L. , Cambronero, F. E. , Liu, D. , Moore, E. E. , Neal, J. E. , Terry, J. G. , Nair, S. , Pechman, K. R. , Rane, S. , Davis, L. T. , Gifford, K. A. , Hohman, T. J. , Bell, S. P. , Wang, T. J. , Beckman, J. A. , & Carr, J. J. (2018). Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation, 138, 1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Freund, W. , Faust, S. , Gaser, C. , Grön, G. , Birklein, F. , Wunderlich, A. P. , Müller, M. , Billich, C. , & Schütz, U. H. (2014). Regionally accentuated reversible brain grey matter reduction in ultra marathon runners detected by voxel‐based morphometry. BMC Sports Science, Medicine and Rehabilitation, 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Komiyama, T. , Tanoue, Y. , Sudo, M. , Costello, J. T. , Uehara, Y. , Higaki, Y. , & Ando, S. (2020). Cognitive impairment during high‐intensity exercise: Influence of cerebral blood flow. Medicine and Science in Sports and Exercise, 52, 561–568. [DOI] [PubMed] [Google Scholar]
- 70. Sun, L.‐N. , Li, X.‐L. , Wang, F. , Zhang, J. , Wang, D.‐D. , Yuan, L. , Wu, M.‐N. , Wang, Z.‐J. , & Qi, J.‐S. (2017). High‐intensity treadmill running impairs cognitive behavior and hippocampal synaptic plasticity of rats via activation of inflammatory response. Journal of Neuroscience Research, 95, 1611–1620. [DOI] [PubMed] [Google Scholar]
- 71. O'leary, J. D. , Hoban, A. E. , Murphy, A. , O'leary, O. F. , Cryan, J. F. , & Nolan, Y. M. (2019). Differential effects of adolescent and adult‐initiated exercise on cognition and hippocampal neurogenesis. Hippocampus, 29, 352–365. [DOI] [PubMed] [Google Scholar]
- 72. Dupuy, O. , Renaud, M. , Bherer, L. , & Bosquet, L. (2010). Effect of functional overreaching on executive functions. International Journal of Sports Medicine, 31, 617–623. [DOI] [PubMed] [Google Scholar]
- 73. Hynynen, E. , Uusitalo, A. , Konttinen, N. , & Rusko, H. (2008). Cardiac autonomic responses to standing up and cognitive task in overtrained athletes. International Journal of Sports Medicine, 29, 552–558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Full‐length blots of OXPHOS protein levels and red Ponceau staining in the frontal cortex (A, B) and hippocampus (C, D) samples. Because of the high hydrophobicity of C‐IV, it commonly migrates differently, within the 30–40 kD range, as stated, shown, and extensively reported in the antibody datasheet.
Figure S2. Full‐length blots of p‐AMPK and AMPK protein levels and red Ponceau staining in the frontal cortex (A–D) and hippocampus (E–H) samples.
Figure S3. Full‐length blots of protein carbonylation and its red Ponceau stain in the frontal cortex (A, B) and hippocampus (C, D) samples.
Table S1: Regional clustering coefficients. The clustering coefficient (mean±SEM) of the 76 regions of the structural FA‐normalized connectome was analyzed in the sedentary and moderate and intense exercise groups. *p<0.05; **p<0.01 versus sedentary group.
Table S2: Primers used for RT‐PCR.
Table S3: Statistical analyses used for all comparisons. In the case of ANOVA, the number of degrees of freedom and the F‐statistic are reported.
Table S4: Sample size for all experiments in Figures 4, 6, and 7.


