The emergence of highly immune-escape Omicron variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to concerns about the efficacy of vaccines and therapeutic monoclonal antibodies. Omicron subvariants BA.4.6, BF.7, BQ.1.1, XBB, and XBB.1 harbor the spike protein R346T substitution which contributes to evasion of class III anti-spike monoclonal antibody recognition while XBB, and XBB.1.1 subvariants harbor the F486S substitution which reduces binding for class I and II monoclonal antibodies.1 BQ.1, BQ.1.1, XBB, and XBB.1 are increasing rapidly in the United States, India, Europe, and other parts of the world, whereas BF.7 is one of the dominant strains currently circulating in China. Due to humoral immune imprinting, a phenomenon in which initial exposure to the original strain of SARS-CoV-2, by infection or vaccination, limits a person's future immune response against variants, the bivalent vaccine booster and hybrid immunity may not provide sufficient protection against emerging Omicron subvariants.2, 3, 4, 5, 6 We have previously shown that an mRNA vaccine booster in individuals vaccinated with two doses of inactivated vaccine significantly increased the level of plasma-neutralizing antibodies against Omicron BA.1.7 Whether this vaccination strategy retains neutralizing activity against the emerging Omicron subvariants remains unknown.
We used enzyme-linked immunosorbent assay and lentivirus-based pseudovirus neutralizing assay (see the Supplementary Methods section in the Supplementary Appendix),8,9 to evaluate the levels of binding and neutralizing antibodies in 77 plasma samples collected from 67 healthy volunteers within 1–5 months after vaccination or infection (Tables S1 and S2). The participants were grouped based on their vaccination and infection history: three doses of inactivated vaccine (CoronaVac [Sinovac] or BBIBP-CorV [Sinopharm]); three doses of monovalent mRNA vaccine (BNT162b2 [Pfizer–BioNTech] or mRNA-1273 [Moderna]); two doses of inactivated vaccine followed by an mRNA vaccine booster; three doses of inactivated vaccine followed by one or two doses of mRNA vaccine booster; infected during the G614 wave and sampled after one or two subsequent doses of mRNA vaccine; and breakthrough infection during the Omicron BA.1 wave.
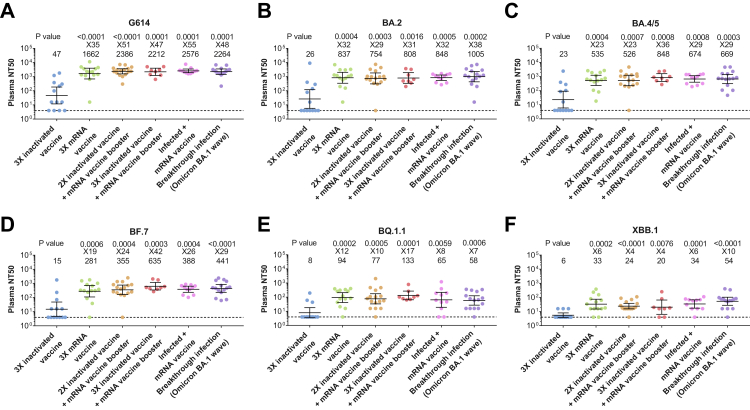
In all six groups, the level of receptor-binding domain (RBD)-specific IgG and the neutralizing activity were lower against all Omicron subvariants tested than the original G614 strain, with the lowest level measured against the XBB.1 subvariant (Fig. 1, and Figs. S1 and S2). The level of specific IgG and the geometric mean half-maximal neutralizing titers (NT50) against Omicron subvariants in the heterologous vaccination groups were significantly higher than that in individuals receiving three doses of a homologous inactivated vaccine, reaching a level similar to those who received three doses of homologous mRNA-vaccine or a boost of mRNA vaccine after infection or experienced breakthrough infection (Fig. 1 and Fig. S1). The geometric mean NT50 of heterologous vaccination groups were 47- to 51-folds higher against G614 and 4- to 42-folds higher against Omicron subvariants than that of three doses of a homologous inactivated vaccine group (Fig. 1). Although the geometric mean NT50 of each vaccinated/infected group against BF.7, BQ.1.1, and XBB.1 were 3.1- to 108.6-fold lower compared to that against G614, the geometric mean NT50 against these Omicron subvariants were 20–635 in the heterologous vaccination groups as well as homologous mRNA vaccine group, while those in three doses of a homologous inactivated vaccine group were below the limit of detection (Fig. S2B) and may account for the surge of COVID-19 cases in China where inactivated vaccines are mainly used.
Fig. 1.
Plasma neutralization activity against G614 and Omicron subvariants. Plasma neutralization activity against the G614 strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (panel A) and the Omicron subvariants BA.2 (panel B), BA.4/5 (panel C), BF.7 (panel D), BQ.1.1 (panel E), and XBB.1 (panel F) in participants who received three doses (3X) of inactivated vaccine (n = 13), three doses of mRNA vaccine (n = 14), two doses (2X) of inactivated vaccine followed by an mRNA vaccine booster (n = 16), three doses of inactivated vaccine followed by one or two doses of mRNA vaccine booster (n = 5; 8 samples), one or two doses of mRNA vaccine after infection (G614 wave) (Infected plus mRNA vaccine, n = 9; 11 samples) or had experienced breakthrough infection during the Omicron BA.1 wave (Breakthrough infection, n = 15). The geometric mean half-maximal neutralizing titers (NT50) and the ratio of the geometric mean NT50 of each group versus the group who received three doses of inactivated vaccine are shown on the top of each panel. Symbols represent individual samples and horizontal black lines indicate the geometric mean NT50. Whiskers indicate the 95% confidence intervals. A two-sided Mann–Whitney U test was used to compare the neutralizing activity between three doses of inactivated vaccine group and other groups in each panel. The dashed line indicates the limit of detection of the assay (NT50 of 4).
Thus, among all omicron subvariants tested, the lowest neutralization activity elicited by vaccines and breakthrough infection (BTI) was against BQ.1.1, and XBB.1. Our results suggest that heterologous vaccination involving parental mRNA vaccine as a booster or second booster in individuals who received two/three doses of inactivated vaccines strongly augments the neutralizing activity and may still be effective against emerging Omicron subvariants. Limitations of this study include the small cohort size, particularly for the group receiving three doses of inactivated vaccine plus mRNA vaccine booster, and comparison of the data performed using cross-sectional analysis. Furthermore, the clinical efficacy of the heterologous inactive/mRNA vaccines against the emerging Omicron subvariants should be further investigated. Nevertheless, a heterologous boosting strategy with mRNA-based vaccines should be considered in populations where inactivated vaccines were primarily used.
Contributors
F.Z., L.H., H.M., and Q.P.H conceived and designed the study. F.Z., R.S., H.A., L.D., Y.W., S.V., F.B., M.S., N.R., Z.C., C.G., A.C., J.A., M.K-B., Y.X., Y.C., M.H., D.F.R., and X.S.X. were involved in collection, processing, and preparation of samples for immunologic assays. F.Z. and H.M. were involved in interpretation of raw data and writing the manuscript. F.Z. performed computational analysis and image preparation. L.H., H.M., and Q.P.H. designed the combined laboratory protocols and supervised the entirety of the project. All authors reviewed and approved the manuscript.
Declaration of interests
X.S.X. and Y.C. are the inventors of the provisional patent applications for BD series neutralizing antibodies and are founders of Singlomics Biopharmaceuticals. All other authors declare no competing interests.
Acknowledgments
This work was supported by The European Union's Horizon 2020 research and innovation program (ATAC, 101003650, D.F.R., M.H., L.H., H.M., Q.P.H), the Center for Innovative Medicine at the Karolinska Institutet (FoUI-963219, Q.P.H), the Swedish Research Council (2019-01302, 2020-06116, Q.P.H), the Knut and Alice Wallenberg Foundation (KAW2020.0102, L.H., Q.P.H), and the Magnus Bergvalls Stiftelse (2022-111, F. Z). The funders played no roles in study design, data collection, data analysis, interpretation, or writing of the report.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100762.
Appendix A. Supplementary data
References
- 1.Qu P., Evans J.P., Faraone J.N., et al. Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. Cell Host Microbe. 2023;31:9–17. doi: 10.1016/j.chom.2022.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurhade C., Zou J., Xia H., et al. Low neutralization of SARS-CoV-2 Omicron BA. 2.75. 2, BQ. 1.1, and XBB. 1 by parental mRNA vaccine or a BA. 5-bivalent booster. Nat Med. 2022 doi: 10.1038/s41591-022-02162-x. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y., Jian F., Wang J., et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature. 2022 doi: 10.1038/s41586-022-05644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q., Bowen A., Valdez R., et al. Antibody response to Omicron BA.4–BA.5 bivalent booster. N Engl J Med. 2023 doi: 10.1056/NEJMc2213907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcotte H., Hammarström L., Pan-Hammarström Q. Limited cross-variant neutralization after primary Omicron infection: consideration for a variant-containing booster. Signal Transduct Target Ther. 2022;7(1):294. doi: 10.1038/s41392-022-01146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer-Babajew D., Wang Z., Muecksch F., et al. Antibody feedback regulates immune memory after SARS-CoV-2 mRNA vaccination. Nature. 2023;613:735–742. doi: 10.1038/s41586-022-05609-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo F., Abolhassani H., Du L., et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat Commun. 2022;13:2670. doi: 10.1038/s41467-022-30340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherina N., Piralla A., Du L., et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6–8 months after the infection. Med. 2021;2(3):281–295.e4. doi: 10.1016/j.medj.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuo F., Marcotte H., Hammarström L., Pan-Hammarström Q. Mucosal IgA against SARS-CoV-2 Omicron infection. N Engl J Med. 2022;387:e55. doi: 10.1056/nejmc2213153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.