Abstract
Objectives
This prospective, parallel‐group, examiner‐blinded, multicentre, randomized, controlled clinical trial aimed to assess the efficacy of an oscillating chitosan brush (OCB) versus titanium curettes (TC) on clinical parameters in the non‐surgical treatment of peri‐implantitis.
Material and Methods
In five dental specialist clinics, 39 patients with one implant with mild to moderate peri‐implantitis, defined as 2–4 mm radiographic reduced bone level, bleeding index (BI) ≥ 2, and probing pocket depth (PPD) ≥ 4 mm were randomly allocated to test and control groups, receiving OCB or TC debridement, respectively. Treatment was performed at baseline and three months. PPD, BI, and Plaque index (PI) were measured at six sites per implant and recorded by five blinded examiners at baseline, one, three, and six month(s). Pus was recorded as present/not present. Changes in PPD and BI were compared between groups and analysed using multilevel partial ordinal and linear regression.
Results
Thirty‐eight patients completed the study. Both groups showed significant reductions in PPD and BI at six months compared with baseline (p < .05). There was no statistically significant difference in PPD and BI changes between the groups. Eradication of peri‐implant disease as defined was observed in 9.5% of cases in the OCB group and 5.9% in the TC group.
Conclusions
Within the limitations of this six‐month multicentre clinical trial, non‐surgical treatment of peri‐implantitis with OCB and TC showed no difference between the interventions. Eradication of disease was not predictable for any of the groups.
Keywords: dental implants, multilevel analysis, peri‐implantitis, randomized controlled trial, single‐blind method
1. INTRODUCTION
The formation of dysbiotic biofilm on dental implants is associated with peri‐implant inflammation and peri‐implant bone loss (Costa et al., 2019). Peri‐implant mucositis presents when mucosa surrounding dental implants shows clinical signs of bleeding on probing (BoP), erythema, swelling, and/or purulent exudate (Berglundh et al., 2018). Peri‐implant mucositis often involves an increase in probing pocket depth (PPD). Inflammation in the peri‐implant mucosa, together with progressive peri‐implant bone loss, is termed peri‐implantitis. Various case definitions have been applied in intervention studies in the absence of an unequivocal grading system (Sanz, Chapple, & Working Group 4 of the VIII European Workshop on Periodontology, 2012).
Peri‐implantitis has become a concern, and data suggest the prevalence to be more than 30% of all patients treated with dental implants and exceeding 20% of all dental implants (Kordbacheh Changi et al., 2019). Even though this is a common complication, to date, there is no treatment protocol showing predictable outcomes.
Several devices for the non‐surgical treatment of peri‐implantitis have been presented in the literature. It has been reported that mechanical debridement is difficult to achieve in the irregularities on the implant surface and is thus ineffective in the treatment of peri‐implantitis (Renvert & Polyzois, 2018).
In an animal study, the outcome of non‐surgical versus surgical treatment of peri‐implantitis was tested with a better outcome for new bone‐to‐implant contact after surgical treatment (Schwarz et al., 2006). Nevertheless, it has been suggested that non‐surgical intervention should be considered prior to surgical treatment, as the results may be beneficial and make surgery more efficient due to reduced inflammation at the surgical site (Schwarz et al., 2015). Improvement of clinical inflammatory parameters has been demonstrated after non‐surgical treatment of peri‐implantitis (Schwarz et al., 2015), but an arrest of peri‐implant bone loss over time is dubious (Berglundh et al., 2018). Recent data suggest that implants treated by surgical means are at risk of recurrent peri‐implantitis (Carcuac et al., 2020). However, complete disease resolution is rare in severe cases (Renvert et al., 2019; Roccuzzo et al., 2020). Regardless of treatment strategy, debridement devices that do not adversely affect the implant surface are preferred (Cha et al., 2019). Moreover, it is important that the instrument used for implant debridement does not leave non‐biocompatible remnants that aggravate bone destruction through foreign‐body reactions (van Velzen et al., 2016).
An oscillating chitosan brush (OCB) aimed at the removal of biofilm in less accessible surfaces around dental implants and teeth is commercially available (Labrida BioClean®, Labrida AS, Oslo, Norway). Chitosan is a natural polysaccharide derived from chitin. It is biocompatible, biodegradable, and has antibacterial activity (Muxika et al., 2017). The effectiveness of this novel instrument for debridement of implant surfaces in the treatment of peri‐implantitis has been demonstrated (Larsen et al., 2017; Wohlfahrt et al., 2017, 2019). In a case study with a six‐month follow‐up, patients treated with an OCB showed a significant reduction in inflammation parameters (Wohlfahrt et al., 2017). The efficacy of the OCB in supportive treatment following peri‐implantitis surgery was questioned in a recent randomized clinical trial (RCT) (Koldsland & Aass, 2020). RCTs that assess the efficacy of OCBs in the non‐surgical treatment of peri‐implantitis are still lacking. Non‐surgical treatment of mucositis and peri‐implantitis using titanium curettes (TC) with and without adjunctive antibiotics has been evaluated in several RCTs (Hallstrom et al., 2012; Renvert et al., 2009; Wohlfahrt et al., 2017). Partial reduction in peri‐implant inflammation was observed for the treatment modalities at six months (Hallstrom et al., 2012; Renvert et al., 2009). Non‐surgical treatment with TC combined with photodynamic therapy or minocycline microspheres showed a statistically significant (p < .05) reduction in peri‐implant inflammation three months after initial treatment (Schär et al., 2013). There is no evidence that one non‐surgical approach is more effective than the other when treating peri‐implant disease (Roccuzzo et al., 2020; Wang et al., 2019).
This multicentre randomized controlled clinical trial aimed to evaluate the following outcomes: PPD, bleeding index (BI), presence of pus, radiographic bone level following non‐surgical mechanical treatment of peri‐implantitis with an OCB (test) and TC (control) at one, three, and six month(s) after initial therapy. In addition, patient‐reported pain during intervention was evaluated at three months.
2. MATERIALS AND METHODS
2.1. Study design
A two‐arm parallel‐group, multicentre, randomized, examiner‐blinded, controlled study was designed to test the efficacy of two treatment modalities in the management of mild to moderate peri‐implantitis, defined as PPD ≥ 4 mm, BI score of at least 2, and a peri‐implant radiographic bone level of 2–4 mm measured from the most coronal intraosseous part of the implant (Sanz et al., 2012). The patients were randomly allocated to either the test group and treated with an OCB or the control group treated with TC. One single implant was treated in each patient. When several implants with treatment needs in the same patients were observed, one implant was randomly selected by drawing lots to assure unbiased inclusion.
The null hypothesis was that there would be no difference in the reduction of evaluated parameters between the two groups.
The study was performed in compliance with Good Clinical Practice, and the Declaration of Helsinki (Fortaleza, Brazil, 2013). The study was registered at ClinicalTrials.gov (12/08/2017, NCT03373448) and approved by the Regional Committee for Medical and Health Research Ethics, South‐East Norway (REK sør‐øst 2017/710) and by Swedish Ethical Review Authority, Linköping (EPN 2017/36‐31).
2.2. Sample size assessment and power
The study was designed to have 80% power to detect a PPD change of 1 mm between the test and the control group. With a standard deviation of 0.8 mm (Aljateeli et al., 2014) and an alpha level of 0.05, the appropriate number of patients per group was calculated to be 17.
2.3. Study population
Consecutive patients presenting for supportive dental care or patients referred for treatment of peri‐implantitis between April 2018 and October 2019 were invited to participate in this study. In total, 45 patients were assessed for study eligibility in five dental specialist practices in Norway and Sweden.
Absence of visual plaque around the included implant at baseline was a prerequisite for inclusion. All included patients underwent oral hygiene instructions until this criterion was met. Periodontal and/or endodontic diseases were treated prior to the start of the study.
Inclusion criteria encompassed: (1) Peri‐implantitis as defined above on an implant in function for more than 12 months; (2) age above 18 years; (3) eligible for treatment in an outpatient dental clinic (ASA I and II); (4) full‐mouth plaque scores ≤20% prior to final inclusion; (5) signed informed consent; and (6) consent to complete all follow‐up visits.
Exclusion criteria included: patients/implants registered with (1) peri‐implant bone loss >4 mm; (2) supraconstructions that for technical reasons made it impossible to access the implant for clinical measurements; (3) technical complications which, according to the examiners' judgment, had contributed to the disease state and were not possible to resolve prior to final inclusion; (4) mobile implant; (5) diagnosed active periodontal disease; (6) implants previously treated for peri‐implantitis with grafting materials; (7) receiving medications known to induce mucosal hyperplasia; (8) receiving systemic antibiotics < three months prior to inclusion; (9) acute or chronic medical conditions that constituted an unwarranted risk and that would limit the patients' ability to participate in the study; (10) unwillingness to undergo treatment; (11) advanced, untreated, and uncontrolled peri‐implantitis on neighbouring implants; (12) patients presented with poorly designed prosthetic constructions resulting in non‐balanced traumatic occlusion; (13) ongoing or previous radiotherapy to the head–neck region; (14) ongoing chemotherapy; and (15) ongoing corticosteroid treatment.
2.4. Randomization and allocation concealment
All patients were appointed a patient number and randomly assigned to treatment in blocks of 10. Computer‐generated block randomization was performed by the study administrator (RANDOM.ORG, Randomness and Integrity Services Ltd., Dublin, Ireland). Clinicians who performed treatment were provided with lists consisting of patient numbers and treatment assignments. The examiners were blinded to the treatment allocation.
2.5. Clinical and radiographic outcomes
The patients were clinically examined by five specialists in periodontology. Calibration meeting was held to discuss the study protocol.
Examinations were performed at baseline prior to treatment and at one‐, three‐, and six‐ month(s) post treatment. The evaluated parameters for the included implants were PPD, BI, plaque index (PI), pus, and height of keratinized mucosa (KM). PPD, BI, and PI registrations were performed at six sites per implant (mesiobuccal, buccal, distobuccal, distopalatal, palatal, and mesiopalatal).
Plaque was assessed by running a probe along the implant neck. Plaque was scored using the PI and dichotomized as present or not present (O'Leary et al., 1972). BI was used to classify the degree of inflammation as 0–3, according to Roos‐Jansaker et al. (2007). Degrees were noted as 0 = no bleeding, 1 = isolated minimal bleeding spots, 2 = blood forming a confluent red line on the margin, and 3 = heavy or profound bleeding. PPD was measured in millimetres. Pus was registered as present or not present. Keratinized mucosa was assessed midbuccaly with a periodontal probe.
Clinical examinations were performed using a manual 0.20 N defined force periodontal probe (University of North Carolina, DB764R, AESCULAR B Braun, Germany). The implant‐retained fixed dental prosthesis was in place during the entire study period.
Peri‐apical radiographs were obtained using the long‐cone paralleling technique with digital X‐rays. ImageJ®, image processing and analysis software program was used to measure peri‐implant radiographic bone level (RBL) at baseline (Preus et al., 2015). The RBL was analysed by one blinded examiner as the distance from the implant neck to bone‐to‐implant contact. The size of intraoral phosphor plates and sensors were used to calibrate the radiographs. Baseline and 6‐month RBL were measured three times. The intra‐examiner agreement test resulted in an intraclass correlation coefficient (ICC) of 0.98.
Digital intraoral radiographs were taken at six months to observe potential adverse effects or further bone loss.
PPD, BI, PI, and pus were registered at baseline and one, three and six month(s) following baseline. PPD and BI were registered for the same sites throughout the study. For PPD, the mean of the sites ≥4 mm was calculated for each time point for each implant.
PPD change was used as primary outcome variable. The secondary outcome variables were BI/pus, change in radiographic bone level, and patient‐reported pain during intervention.
A composite outcome of disease eradication was based on frequency analysis of implants with absence of peri‐implant sites with pocket depth PPD ≥ 4 mm, no bleeding (BI 0)/suppuration, and no radiographic bone loss between baseline and 6 months. Inflammation control was defined as BI = 0 at any implant site.
Following treatment at three months, patients were asked to record pain associated with the treatment via a visual analogue scale (VAS). No pain was categorized as 0 and worst possible pain as 10.
2.6. Treatment procedures
The implants in the test group were debrided with an OCB. The brush was soaked in sterile saline for two minutes before it was seated on an oscillating dental handpiece (NSK ER10, TEQ‐Y, Nakanishi International Inc., Tochigi, Japan) as recommended in the instructions for use by the manufacturer. The implants in the control group were debrided utilizing TC (Langer and Langer, Rønvig, Denmark). Infiltration anaesthesia was used if requested by the patient. The included implants were treated for two minutes and irrigated with sterile saline after mechanical debridement for both the test and control groups. Treatment was performed at baseline and repeated at three months after initial therapy, at implants with PPD ≥ 4 mm and BI > 0 by five registered dental hygienists. In cases with multiple implants with treatment need, all implants were treated in the same session with the same assigned treatment.
2.7. Data management and statistical analysis
All clinical recordings and patient data were registered in a web‐based clinical report form (Nettskjema Version 2.0, University of Oslo, Norway). Per‐protocol (PP) analysis was performed on patients who were assessed at all time‐points. Calculations and analyses were performed using Stata Statistical Software, Version 16.1 (StataCorp.2001. Statistical Software: Release 7.0. College Station, TX: Stata Corporation). The significance level was set at α = 0.05.
Characteristics of the patients and the implants in the test and control groups were described using frequencies and percentages for categorical variables and means with standard deviations (SDs) for continuous data.
Data on PPD and BI were obtained at baseline and at one‐, three‐, and six month(s) for one implant in each individual. The included patients attended five different clinics. Therefore, to account for possible dependences of the data within participants who were nested within clinics, three‐level linear and partial ordinal multilevel models with random intercept and random effect of time (level 3) on patients (level 2) and on clinics at level 1 for PPD and BI were performed, respectively. The assumption of proportionality between categories of the ordered variable was violated for some independent variables. A multilevel partial ordinal logistic model using gologit2 was fitted to the BI data. The differences in PPD and BI between the groups at each study time point were obtained from the two‐way interaction of time with the groups. Estimates of ICC, which described the amount of variability in both PPD and BI that could be attributed to differences between patients and clinicians, were obtained.
Consolidation Standards of Reporting Trials (CONSORT) Statement guidelines were followed (Schulz et al., 2010).
3. RESULTS
After verification of inclusion and exclusion criteria, 39 patients gave informed consent and were enroled in the present study. The flow diagram of the study is presented in Figure 1. No adverse events were reported. Complete observations were available for 38 patients. There were no statistically significant baseline differences in patient and implant characteristics between the groups (Table 1). No group differences in PI were detected throughout the study period.
FIGURE 1.
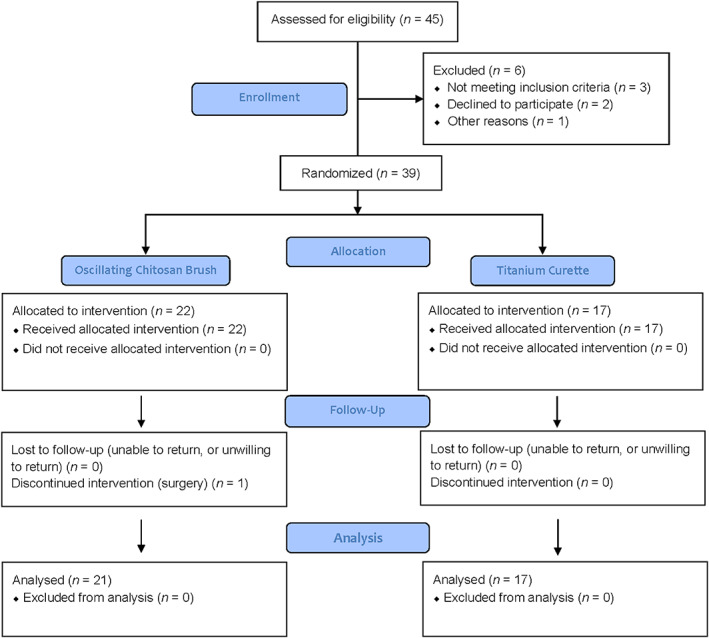
A CONSORT flowchart of enrolment, allocation, follow‐up, and analysis
TABLE 1.
Baseline characteristics of the study patients and implants
| Variable | Total | (%) | Test group | (%) | Control group | (%) |
|---|---|---|---|---|---|---|
| Patients/Implants (n) | 38 | 100 | 21 | 55.3 | 17 | 44.7 |
| Mean age (±SD) | 61.99 | 62.86 (±12.19) | 61.12 (±3.67) | |||
| Gender | ||||||
| Male | 14 | 36.8 | 5 | 23.8 | 9 | 52.9 |
| Female | 24 | 63.2 | 16 | 76.2 | 8 | 47.1 |
| Daily smoker | 5 | 13.2 | 4 | 19.0 | 1 | 5.9 |
| Diabetes | 7 | 18.4 | 4 | 19.0 | 3 | 17.6 |
| Tooth loss due to periodontitis | 11 | 28.2 | 6 | 27.3 | 5 | 29.4 |
| Front | 17 | 44.7 | 8 | 38.1 | 9 | 52.9 |
| Premolar | 18 | 47.4 | 11 | 52.4 | 7 | 41.2 |
| Molar | 3 | 7.9 | 2 | 9.5 | 1 | 5.9 |
| Non‐modified implant surface | 5 | 13.2 | 3 | 14.3 | 2 | 11.8 |
| Screw‐retained | 31 | 81.6 | 16 | 76.2 | 15 | 88.2 |
| Cement‐retained | 6 | 15.9 | 5 | 23.8 | 1 | 5.9 |
| Not reported | 1 | 2.6 | 0 | 0 | 1 | 5.9 |
| Implant‐retained crown | 15 | 39.5 | 10 | 66.7 | 5 | 33.3 |
| Implant‐retained fixed dental prosthesis | 23 | 60.5 | 11 | 47.8 | 12 | 52.2 |
| Keratinized mucosa (mm) | 30 | 78.9 | 2.8 | 90 | 2.5 | 64.7 |
| Implants with suppuration | 22 | 57.9 | 11 | 52.4 | 11 | 64.7 |
| Radiographic bone level (±SD) | 38 | 100 | 2.43 (±0.51) | 55.3 | 2.58 (±0.58) | 44.7 |
| BI ≥ 2 (±SD) | 38 | 100 | 2.33 (±0.48) | 55.3 | 2.24 (±0.44) | 44.7 |
| PPD mean ≥ 4 mm (±SD) | 38 | 100 | 5.3 (±0.16) | 55.3 | 5.5 (±0.29) | 44.7 |
| PPD ≥ 4 mm (±SD) | 38 | 100 | 6.8 (±1.6) | 55.3 | 6.5 (±1.7) | 44.7 |
| PPD ≥ 6 mm (±SD) | 29 | 76.3 | 7.6 (±1.1) | 39.5 | 7.0 (±1.5) | 36.8 |
Abbreviations: BI, bleeding index; PPD, probing pocket depth.
3.1. Changes in PPD between groups
Table 2 show the changes in PPD between the groups at each time point. Figure 2 shows the changes in PPD from baseline for the groups at each study time point. There were no statistically significant differences in PPD changes between the groups at baseline or after one, three, and six month(s).
TABLE 2.
Changes in mean PPD and BI between the groups at each time point obtained from partial ordinal and linear multilevel regression model with clinic and patient random effects
| Baseline | 1 month | 3 months | 6 months | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p‐value | β (95% CI) | p‐value | β (95% CI) | p‐value | β (95% CI) | p‐value | |
| Group (ref: TC) | ||||||||
| PPD Test group | 0.4 (−1.01, 0.2) | .22 | −0.3 (−0.9, 0.4) | .40 | −0.1 (−0.6, 0.7) | .88 | −0.1 (−0.7, 0.5) | .74 |
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | OR (95% CI) | p‐value | OR (95% CI) | p‐value | |
|---|---|---|---|---|---|---|---|---|
| Between the groups (ref: Control) | ||||||||
| Profuse versus no bleeding (BI 3 vs BI 0) | 0.8 (0.2, 3.6) | .77 | 4.3 (0.5, 35.0) | .17 | 0.4 (0.1, 1.6) | .21 | 0.8 (0.1, 12.9) | .89 |
| Line versus no bleeding (BI 2 vs BI 0) | 2.5 (0.6, 10.4) | .20 | 1.5 (0.6, 4.2) | .43 | 1.4 (0.5, 4.1) | .49 | 0.8 (0.3, 2.4) | .72 |
| Spot versus no bleeding (BI 1 vs BI 0) | 0.7 (0.3, 2.1) | .53 | 1.3 (0.5, 3.3) | .62 | 0.3 (0.1, 1.0) | .06 | 1.2 (0.5, 3.4) | .68 |
Abbreviations: BI, bleeding index; CI, confidence interval; PPD, probing pocket depth; TC, titanium curettes.
FIGURE 2.
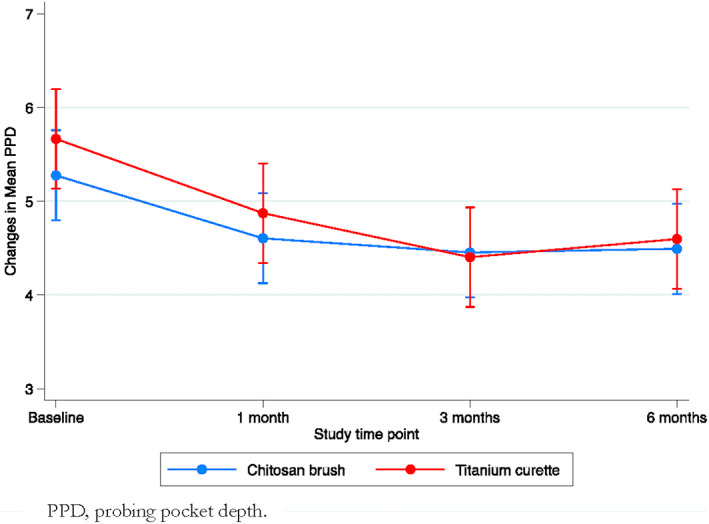
Changes in mean PPD between and within the groups
3.2. Changes in BI and presence of pus between groups
There were no statistically significant differences in BI between the groups at any time point (Table 2). At six months, pus was registered at 33.3% and 64.7% of the implants in the test and control group, respectively (p > .05). Figure 3 shows the probability of BI 0–3 between and within both groups.
FIGURE 3.
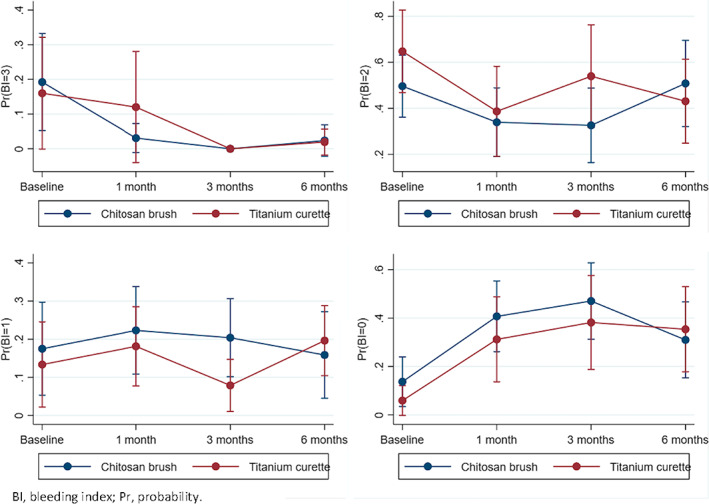
The probability of BI 0–3 between and within the groups at each time point
3.3. Radiographic bone level
At six months, the mean radiographic bone level was 2.5 mm (±0.5) for the test group and 2.6 mm (±0.7) for the control group. The between group RBL change from baseline to six months was not statistically significant.
3.4. Composite outcome
At six months, two implants in the test group (9.5%) and one implant in the control group (5.9%) presented disease eradication with PPD < 4 mm, no bleeding (BI 0), and no change in radiographic bone level compared with baseline. The difference between the groups was not statistically significant.
3.5. Keratinized mucosa
The mean KM at baseline is presented in Table 1. At baseline, 36.8% (n = 7) implants in the test group and 54.5% (n = 6) implants in the control group had KM ≤ 2 mm.
3.6. Withdrawal
One implant in the OCB‐group showed progression of peri‐implant disease at the 3 months screening and was excluded from the study for surgical intervention.
3.7. Intraclass correlation coefficient
Estimates of ICC for both the variance component and adjusted models are presented in Table S1. For PPD, 7.7% of the variability was explained by the differences between the clinics, while 51.2% of the variability was nested in patients. For BI, 10.3% of the variability was explained by the differences between the clinics, while patients nested in clinics explained 22.2% of the variability.
3.8. Visual analogue scale for pain
VAS information for pain during treatment was recorded for 22 patients (OCB (n) = 14, TC (n) = 8), leading to a response rate of 57.9%. Seven patients received anaesthesia before treatment and were therefore not asked to fill out the VAS form (OCB (n) = 4, TC (n) = 3). No data were reported for nine patients because screeners did not collect VAS forms (OCB (n) = 4, TC (n) = 6). The mean VAS scores (±SD) for the test and control groups were 2.9 (±1.93) and 3.4 (±2.09), respectively. There was no statistically significant difference in VAS between the groups (p > .05).
4. DISCUSSION
In the present prospective intervention study comparing non‐surgical treatment with OCB and TC showed no significant difference between the treatment groups. However, both interventions significantly reduced the investigated inflammation parameters at implants affected by peri‐implantitis. This might be explained by the sample size. A larger sample might have resulted in a different outcome. The included patients underwent an initial hygiene phase and two active treatments: at baseline and three months. PPD and the BI score significantly reduced between baseline and six months in both groups. In a pilot RCT assessing treatment of peri‐implant mucositis with an OCB and TC, a similar decrease in inflammation parameters was reported (Wohlfahrt et al., 2019). In contrast to the study by Wohlfahrt et al. (2019) and the present study, Koldsland and Aass (2020) showed that supportive treatment with OCB or TC failed to further reduce PPD and BoP among patients in maintenance following recent surgical treatment of peri‐implantitis. Both studies were RCTs comparing treatment with an OCB and TC, but the included patients had different disease entities and treatment strategies. A possible explanation for the different outcomes in the study performed by Wohlfahrt et al. (2019), Koldsland and Aass (2020), and the present study may be the treatment of mucositis, post‐surgical maintenance, and treatment of peri‐implantitis, respectively. Moreover, supportive treatment after peri‐implantitis surgery involves debridement of a complex intraorally exposed implant surface. The moderately rough implant surface may complicate home care maintenance and professional removal of biofilm, which was indicated by increasing plaque scores throughout the study period compared with baseline (Koldsland & Aass, 2020).
The results of the present study agree with the findings from a RCT where non‐surgical, mechanical treatment with TC and an ultrasonic device was compared (Renvert et al., 2009). The studies are also comparable in terms of the number of included patients, follow‐up period, and a hand instrument that was compared with a machine‐driven device. In contrast to the present study, Renvert et al. (2009) included patients with high plaque scores at baseline. The plaque scores diminished significantly at the end of the study (from 73% to 53%). Significantly reduced BoP score within the groups and no difference between the groups was reported from baseline to six months, as in the present study. A longer follow‐up period and frequently repeated treatment may be required to demonstrate significant differences between non‐surgical mechanical treatment modalities (Bertoldi et al., 2017). Mechanical debridement alone may be inadequate in the absence of an ideal treatment frequency (Karring et al., 2005).
To date, some RCTs have compared different non‐surgical modalities for the treatment of peri‐implantitis (Faggion et al., 2014). The studies vary with regard to disease severity among the included patients and what is considered a successful endpoint (Faggion et al., 2014). In the present multicentre study, radiographs and PPD registration from the time of first prosthetic loading were not mandatory to be included. The reduced bone level was measured on digital radiographs taken at the time of study recruitment. An assumed initial bone level was used as a reference for the bone loss measurements. In a consensus report, a minimum of 2 mm of assumed bone loss in addition to inflammation was suggested as criteria for peri‐implantitis for clinical studies when the baseline bone level is not known (Sanz et al., 2012). Thus, cases with 2–4 mm assumed bone level reduction were included in the current trial. Renvert et al. included implants with 1.5 mm mean bone loss (Renvert et al., 2009). Furthermore, the peri‐implant tissues of the selected implants in the present study demonstrated. In a recent consensus report, RBLs ≥ 3 mm apical to the most intraosseous part of the implant, along with BoP and PPD ≥ 6 mm, were suggested as case definitions of peri‐implantitis when data from the time of prosthetic loading are unavailable (Berglundh et al., 2018). In the present study, PPD ≥ 4 mm at the time of study enrolment was decided as an inclusion criterion and later used as a reference for changes in PPD throughout the study. Nevertheless, 76.3% of all implants in the present study had PPD ≥ 6 mm and could be classified as peri‐implantitis according to Berglundh et al. (2018).
In the present study, patients were recruited from specialist clinics in Norway and Sweden. The multicentre study design is considered to have several benefits: the participation of a compound group of investigators, the participants being included and treated by different centres may improve the validity of the results, and the main advantage is the recruitment of patients from a wider population. To achieve the benefits of a multicentre study, it is an absolute prerequisite that the data are collected in the same reliable way throughout the project period. Calibration of operators can be challenging because of the number of operators and geographical distance. In the present study, calibration meeting was held to assure study quality. However, practical calibration training was not performed, and ICC was not calculated.
BI, pus, and PPD are surrogate parameters for inflammation, and reduction in these parameters may indicate a positive but potentially transient outcome of an intervention and may not be an indication of permanent resolution of peri‐implantitis (Faggion et al., 2014). This emphasizes the importance of regular evaluation of treatment and preventive maintenance therapy to control peri‐implant disease. Further investigations may focus on how long it is possible to keep the implant free of inflammation.
The efficacy of non‐surgical treatment with TCs, air‐polishing with glycine‐based powder, ultrasonic curettes, or photodynamic therapy has been assessed and reported in a range of studies, but it remains inconclusive as to what treatment the exhibits superior outcome of peri‐implant inflammation (2019). Several of these studies emphasize the importance of non‐surgical intervention as the preferred treatment (Faggion et al., 2014). Although no golden standard for treatment of peri‐implantitis has been defined (Graziani et al., 2012), TC was chosen as the control treatment in the present study based on what seems to be a common method among many implant clinics and in the literature. For non‐surgical treatment, data suggest that greater PPD reduction can be achieved when a combination of therapies is applied (Faggion et al., 2014). In the present study, mechanical debridement alone was performed.
In an in vitro study comparing instrumentation with an OCB, TC, and Er:YAG laser, shallow alterations of the implant surface for the TC group were observed when debridement was performed for three minutes (Larsen et al., 2017). In vivo, the release of titanium particles from the damaged/scratched implant surface to the peri‐implant tissues may trigger the immune system by a foreign‐body reaction and result in osteolysis of the peri‐implant bone (Purdue et al., 2007). Furthermore, an altered implant surface may affect the recolonization of microbial biofilm and the proliferation of soft and hard tissue cells (Cao et al., 2018).
In the present study, the control group had a higher incidence of implants with KM ≤ 2 mm than the test group. Although more brushing discomfort and plaque accumulation is reported for implants with KM ≤ 2 mm (Souza et al., 2016), lower height of KM is not clearly associated with higher risk of peri‐implantitis (Schwarz et al., 2018). And thus, it might be speculated that insufficient KM might affect the outcome of treatment. In the present study, the PI remained equally low in both groups.
Peri‐implant health is characterized by the absence of all inflammation signs (Berglundh et al., 2018). In the present study, pus was registered at 52.4% and 64.7% of the implants at baseline in the test and control groups, respectively. At six months, the pus score decreased to 33.3% for the test group. No change in the presence of pus was registered from baseline to six months for the control group. In a recent RCT, pus scores varied from 19% to 37% at baseline and decreased to 0% to 15% at six months (Merli et al., 2020). The study patients were allocated to four intervention groups and treated either non‐surgically alone or in combination with chemical agents (Merli et al., 2020). No pus was registered at six months when implants were treated with two chemical agents in addition to mechanical debridement (Merli et al., 2020). Contrary to our study, the implant‐retained restorations were removed before the mechanical debridement (Merli et al., 2020). In addition, the study patients were instructed to rinse with a 0.12% chlorhexidine solution twice per day for the first 15 days (2020).
In the present study, no statistically significant difference between treatments with an OCB or TC was detected. Positive changes in PPD and BI were registered, but this may be a short, transient stage. BI results should be interpreted with caution due to the large confidence intervals. A decrease in the presence of pus was observed in the test group. The presence of pus in both groups indicates active disease and the need for further intervention. Studies with longer follow‐up with an assessment of radiographic bone loss and a larger sample size may be interesting to pursue.
The current study, while limited in size has sought to assess the efficacy of an oscillating chitosan brush (OCB) versus titanium curettes (TC). A major limitation in estimating the sample size of the current clustered study was the unavailability from literature of statistical measures such as the intra‐cluster correlation (ICC), hence we relied on a reasonable educated guess. However, the richness of the data generated in this study can be used in formulating hypotheses of much bigger studies.
Within the limitations of this six‐month multicentre clinical trial, it can be concluded that non‐surgical treatment of peri‐implantitis with OCB or TC demonstrated equal efficacy and no difference between the interventions. Eradication of disease was not predictable for any of the groups.
AUTHOR CONTRIBUTIONS
Sadia N. Khan: Conceptualization (lead); data curation (lead); formal analysis (equal); investigation (supporting); project administration (lead); software (lead); supervision (equal); writing – original draft (lead); writing – review and editing (lead). Odd Carsten Koldsland: Conceptualization (lead); methodology (lead); supervision (lead); writing – review and editing (lead). Ann‐Marie Roos‐Jansåker: Conceptualization (equal); investigation (lead); methodology (equal); validation (equal); writing – review and editing (equal). Johan Caspar Wohlfahrt: Conceptualization (lead); methodology (lead); writing – review and editing (equal). Anders Verket: Investigation (lead); writing – review and editing (equal). Carl Hjortsjö: Methodology (lead); project administration (lead); supervision (lead); validation (lead); writing – review and editing (lead).
FUNDING INFORMATION
The study was funded by the Research Council of Norway and university grants.
CONFLICT OF INTEREST
Caspar Wohlfahrt is the inventor and patentholder of the chitosan brush (Labrida Bioclean®, Labrida AS Oslo, Norway). Ann Marie Roos‐Jansåker and Caspar Wohlfahrt are shareholders in Labrida AS. Drs Khan, Koldsland, Verket, Mdala, Magnusson, Salvesen and Hjortsjö report no conflicts of interest related to this study.
ETHICAL APPROVAL
The study was approved by the Regional Committee for Medical and Health Research Ethics, South‐East Norway (REK sør‐øst 2017/710) and by Swedish Ethical Review Authority, Linköping (EPN 2017/36‐31).
PATIENT CONSENT STATEMENT
Informed consent was signed by the study patients prior to study start.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
CLINICAL TRIALS REGISTRATION
The study was registered at ClinicalTrials.gov (12/08/2017, NCT03373448).
Supporting information
Table S1
ACKNOWLEDGMENTS
We acknowledge and thank the dental hygienists Åsa Thelin, Dagrun Ernø, Ane‐Mari Igland Naustdal, Monica Palmér Tvedt, Sofia Hald, Menaka Seevaratnam, and Helena Olsson for their participation in the study, head engineer Gerald Torgersen for blinding, calibrating the digital radiographs, and Anne Christophersen for administration and moderating the study.
Khan, S. N. , Koldsland, O. C. , Roos‐Jansåker, A.‐M. , Wohlfahrt, J. C. , Verket, A. , Mdala, I. , Magnusson, A. , Salvesen, E. , & Hjortsjö, C. (2022). Non‐surgical treatment of mild to moderate peri‐implantitis using an oscillating chitosan brush or a titanium curette—A randomized multicentre controlled clinical trial. Clinical Oral Implants Research, 33, 1254–1264. 10.1111/clr.14007
DATA AVAILABILITY STATEMENT
Data are stored at a central university facility and are available upon request from the corresponding author, S.K.
REFERENCES
- Aljateeli, M. , Koticha, T. , Bashutski, J. , Sugai, J. V. , Braun, T. M. , Giannobile, W. V. , & Wang, H. L. (2014). Surgical periodontal therapy with and without initial scaling and root planing in the management of chronic periodontitis: A randomized clinical trial. Journal of Clinical Periodontology, 41(7), 693–700. 10.1111/jcpe.12259 [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Armitage, G. , Araujo, M. G. , Avila‐Ortiz, G. , Blanco, J. , Camargo, P. M. , Chen, S. , Cochran, D. , Derks, J. , Figuero, E. , Hämmerle, C. H. F. , Heitz‐Mayfield, L. J. A. , Huynh‐Ba, G. , Iacono, V. , Koo, K. T. , Lambert, F. , McCauley, L. , Quirynen, M. , Renvert, S. , … Zitzmann, N. (2018). Peri‐implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. Journal of Periodontology, 89 Suppl 1(Suppl 1), S313–S318. 10.1002/JPER.17-0739 [DOI] [PubMed] [Google Scholar]
- Bertoldi, C. , Lusuardi, D. , Battarra, F. , Sassatelli, P. , Spinato, S. , & Zaffe, D. (2017). The maintenance of inserted titanium implants: In‐vitro evaluation of exposed surfaces cleaned with three different instruments. Clinical Oral Implants Research, 28(1), 57–63. 10.1111/clr.12759 [DOI] [PubMed] [Google Scholar]
- Cao, J. , Wang, T. , Pu, Y. , Tang, Z. , & Meng, H. (2018). Influence on proliferation and adhesion of human gingival fibroblasts from different titanium surface decontamination treatments: An in vitro study. Archives of Oral Biology, 87, 204–210. 10.1016/j.archoralbio.2017.12.013 [DOI] [PubMed] [Google Scholar]
- Carcuac, O. , Derks, J. , Abrahamsson, I. , Wennstrom, J. L. , & Berglundh, T. (2020). Risk for recurrence of disease following surgical therapy of peri‐implantitis—A prospective longitudinal study. Clinical Oral Implants Research, 31(11), 1072–1077. 10.1111/clr.13653 [DOI] [PubMed] [Google Scholar]
- Cha, J. K. , Paeng, K. , Jung, U. W. , Choi, S. H. , Sanz, M. , & Sanz‐Martin, I. (2019). The effect of five mechanical instrumentation protocols on implant surface topography and roughness: A scanning electron microscope and confocal laser scanning microscope analysis. Clinical Oral Implants Research, 30(6), 578–587. 10.1111/clr.13446 [DOI] [PubMed] [Google Scholar]
- Costa, F. O. , Ferreira, S. D. , Cortelli, J. R. , Lima, R. P. E. , Cortelli, S. C. , & Cota, L. O. M. (2019). Microbiological profile associated with peri‐implant diseases in individuals with and without preventive maintenance therapy: A 5‐year follow‐up. Clinical Oral Investigations, 23(8), 3161–3171. 10.1007/s00784-018-2737-y [DOI] [PubMed] [Google Scholar]
- Faggion, C. M., Jr. , Listl, S. , Fruhauf, N. , Chang, H. J. , & Tu, Y. K. (2014). A systematic review and Bayesian network meta‐analysis of randomized clinical trials on non‐surgical treatments for peri‐implantitis. Journal of Clinical Periodontology, 41(10), 1015–1025. 10.1111/jcpe.12292 [DOI] [PubMed] [Google Scholar]
- Graziani, F. , Figuero, E. , & Herrera, D. (2012). Systematic review of quality of reporting, outcome measurements and methods to study efficacy of preventive and therapeutic approaches to peri‐implant diseases. Journal of Clinical Periodontology, 39(Suppl 12), 224–244. 10.1111/j.1600-051X.2011.01832.x [DOI] [PubMed] [Google Scholar]
- Hallstrom, H. , Persson, G. R. , Lindgren, S. , Olofsson, M. , & Renvert, S. (2012). Systemic antibiotics and debridement of peri‐implant mucositis. A randomized clinical trial. Journal of Clinical Periodontology, 39(6), 574–581. 10.1111/j.1600-051X.2012.01884.x [DOI] [PubMed] [Google Scholar]
- Karring, E. S. , Stavropoulos, A. , Ellegaard, B. , & Karring, T. (2005). Treatment of peri‐implantitis by the vector system. Clinical Oral Implants Research, 16(3), 288–293. 10.1111/j.1600-0501.2005.01141.x [DOI] [PubMed] [Google Scholar]
- Koldsland, O. C. , & Aass, A. M. (2020). Supportive treatment following peri‐implantitis surgery: An RCT using titanium curettes or chitosan brushes. Journal of Clinical Periodontology, 47(10), 1259–1267. 10.1111/jcpe.13357 [DOI] [PubMed] [Google Scholar]
- Kordbacheh Changi, K. , Finkelstein, J. , & Papapanou, P. N. (2019). Peri‐implantitis prevalence, incidence rate, and risk factors: A study of electronic health records at a U.S. dental school. Clinical Oral Implants Research, 30(4), 306–314. 10.1111/clr.13416 [DOI] [PubMed] [Google Scholar]
- Larsen, O. I. , Enersen, M. , Kristoffersen, A. K. , Wennerberg, A. , Bunaes, D. F. , Lie, S. A. , & Leknes, K. N. (2017). Antimicrobial effects of three different treatment modalities on dental implant surfaces. The Journal of Oral Implantology, 43(6), 429–436. 10.1563/aaid-joi-D-16-00147 [DOI] [PubMed] [Google Scholar]
- Merli, M. , Bernardelli, F. , Giulianelli, E. , Carinci, F. , Mariotti, G. , Merli, M. , Pini‐Prato, G. , & Nieri, M. (2020). Short‐term comparison of two non‐surgical treatment modalities of peri‐implantitis: Clinical and microbiological outcomes in a two‐factorial randomized controlled trial. Journal of Clinical Periodontology, 47(10), 1268–1280. 10.1111/jcpe.13345 [DOI] [PubMed] [Google Scholar]
- Muxika, A. , Etxabide, A. , Uranga, J. , Guerrero, P. , & de la Caba, K. (2017). Chitosan as a bioactive polymer: Processing, properties and applications. International Journal of Biological Macromolecules, 105(Pt 2), 1358–1368. 10.1016/j.ijbiomac.2017.07.087 [DOI] [PubMed] [Google Scholar]
- O'Leary, T. J. , Drake, R. B. , & Naylor, J. E. (1972). The plaque control record. Journal of Periodontology, 43(1), 38. 10.1902/jop.1972.43.1.38 [DOI] [PubMed] [Google Scholar]
- Preus, H. R. , Torgersen, G. R. , Koldsland, O. C. , Hansen, B. F. , Aass, A. M. , Larheim, T. A. , & Sandvik, L. (2015). A new digital tool for radiographic bone level measurements in longitudinal studies. BMC Oral Health, 15, 107. 10.1186/s12903-015-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdue, P. E. , Koulouvaris, P. , Potter, H. G. , Nestor, B. J. , & Sculco, T. P. (2007). The cellular and molecular biology of periprosthetic osteolysis. Clinical Orthopaedics and Related Research, 454, 251–261. 10.1097/01.blo.0000238813.95035.1b [DOI] [PubMed] [Google Scholar]
- Renvert, S. , Hirooka, H. , Polyzois, I. , Kelekis‐Cholakis, A. , Wang, H. L. , & Working, G. (2019). Diagnosis and non‐surgical treatment of peri‐implant diseases and maintenance care of patients with dental implants ‐ consensus report of working group 3. International Dental Journal, 69(Suppl 2), 12–17. 10.1111/idj.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renvert, S. , & Polyzois, I. (2018). Treatment of pathologic peri‐implant pockets. Periodontology 2000, 76(1), 180–190. 10.1111/prd.12149 [DOI] [PubMed] [Google Scholar]
- Renvert, S. , Samuelsson, E. , Lindahl, C. , & Persson, G. R. (2009). Mechanical non‐surgical treatment of peri‐implantitis: A double‐blind randomized longitudinal clinical study. I: Clinical results. Journal of Clinical Periodontology, 36(7), 604–609. 10.1111/j.1600-051X.2009.01421.x [DOI] [PubMed] [Google Scholar]
- Roccuzzo, A. , De Ry, S. P. , Sculean, A. , Roccuzzo, M. , & Salvi, G. E. (2020). Current approaches for the non‐surgical management of peri‐implant diseases. Current Oral Health Reports, 7(3), 274–282. 10.1007/s40496-020-00279-x [DOI] [Google Scholar]
- Roos‐Jansaker, A. M. , Renvert, H. , Lindahl, C. , & Renvert, S. (2007). Surgical treatment of peri‐implantitis using a bone substitute with or without a resorbable membrane: A prospective cohort study. Journal of Clinical Periodontology, 34(7), 625–632. 10.1111/j.1600-051X.2007.01102.x [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Chapple, I. L. , & Working Group 4 of the VIII European Workshop on Periodontology . (2012). Clinical research on peri‐implant diseases: Consensus report of Working group 4. Journal of Clinical Periodontology, 39(Suppl 12), 202–206. 10.1111/j.1600-051X.2011.01837.x [DOI] [PubMed] [Google Scholar]
- Schär, D. , Ramseier, C. A. , Eick, S. , Arweiler, N. B. , Sculean, A. , & Salvi, G. E. (2013). Anti‐infective therapy of peri‐implantitis with adjunctive local drug delivery or photodynamic therapy: Six‐month outcomes of a prospective randomized clinical trial. Clinical Oral Implants Research, 24(1), 104–110. 10.1111/j.1600-0501.2012.02494.x [DOI] [PubMed] [Google Scholar]
- Schulz, K. F. , Altman, D. G. , Moher, D. , & CONSORT Group (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ, 340, c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, F. , Becker, K. , & Renvert, S. (2015). Efficacy of air polishing for the non‐surgical treatment of peri‐implant diseases: A systematic review. Journal of Clinical Periodontology, 42(10), 951–959. 10.1111/jcpe.12454 [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Derks, J. , Monje, A. , & Wang, H. L. (2018). Peri‐implantitis. Journal of Periodontology, 89(Suppl 1), S267–S290. 10.1002/JPER.16-0350 [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Jepsen, S. , Herten, M. , Sager, M. , Rothamel, D. , & Becker, J. (2006). Influence of different treatment approaches on non‐submerged and submerged healing of ligature induced peri‐implantitis lesions: An experimental study in dogs. Journal of Clinical Periodontology, 33(8), 584–595. 10.1111/j.1600-051X.2006.00956.x [DOI] [PubMed] [Google Scholar]
- Souza, A. B. , Tormena, M. , Matarazzo, F. , & Araujo, M. G. (2016). The influence of peri‐implant keratinized mucosa on brushing discomfort and peri‐implant tissue health. Clinical Oral Implants Research, 27(6), 650–655. 10.1111/clr.12703 [DOI] [PubMed] [Google Scholar]
- van Velzen, F. J. , Lang, N. P. , Schulten, E. A. , & Ten Bruggenkate, C. M. (2016). Dental floss as a possible risk for the development of peri‐implant disease: An observational study of 10 cases. Clinical Oral Implants Research, 27(5), 618–621. 10.1111/clr.12650 [DOI] [PubMed] [Google Scholar]
- Wang, C. W. , Renvert, S. , & Wang, H. L. (2019). Nonsurgical treatment of periimplantitis. Implant Dentistry, 28(2), 155–160. 10.1097/ID.0000000000000846 [DOI] [PubMed] [Google Scholar]
- Wohlfahrt, J. C. , Aass, A. M. , & Koldsland, O. C. (2019). Treatment of peri‐implant mucositis with a chitosan brush‐a pilot randomized clinical trial. International Journal of Dental Hygiene, 17(2), 170–176. 10.1111/idh.12381 [DOI] [PubMed] [Google Scholar]
- Wohlfahrt, J. C. , Evensen, B. J. , Zeza, B. , Jansson, H. , Pilloni, A. , Roos‐Jansåker, A. M. , di Tanna, G. L. , Aass, A. M. , Klepp, M. , & Koldsland, O. C. (2017). A novel non‐surgical method for mild peri‐implantitis—A multicenter consecutive case series. International Journal of Implant Dentistry, 3(1), 38. 10.1186/s40729-017-0098-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data are stored at a central university facility and are available upon request from the corresponding author, S.K.


