Abstract
Based on the Global Cancer Update Programme, formally known as the World Cancer Research Fund/American Institute for Cancer Research Continuous Update Project, we performed systematic reviews and meta‐analyses to investigate the association of postdiagnosis body fatness, physical activity and dietary factors with breast cancer prognosis. We searched PubMed and Embase for randomised controlled trials and longitudinal observational studies from inception to 31 October 2021. We calculated summary relative risks (RRs) and 95% confidence intervals (CIs) using random‐effects meta‐analyses. An independent Expert Panel graded the quality of evidence according to predefined criteria. The evidence on postdiagnosis body fatness and higher all‐cause mortality (RR per 5 kg/m2 in body mass index: 1.07, 95% CI: 1.05‐1.10), breast cancer‐specific mortality (RR: 1.10, 95% CI: 1.06‐1.14) and second primary breast cancer (RR: 1.14, 95% CI: 1.04‐1.26) was graded as strong (likelihood of causality: probable). The evidence for body fatness and breast cancer recurrence and other nonbreast cancer‐related mortality was graded as limited (likelihood of causality: limited‐suggestive). The evidence on recreational physical activity and lower risk of all‐cause (RR per 10 metabolic equivalent of task‐hour/week: 0.85, 95% CI: 0.78‐0.92) and breast cancer‐specific mortality (RR: 0.86, 95% CI: 0.77‐0.96) was judged as limited‐suggestive. Data on dietary factors was limited, and no conclusions could be reached except for healthy dietary patterns, isoflavone and dietary fibre intake and serum 25(OH)D concentrations that were graded with limited‐suggestive evidence for lower risk of the examined outcomes. Our results encourage the development of lifestyle recommendations for breast cancer patients to avoid obesity and be physically active.
Keywords: body fatness, breast cancer survival, diet, evidence grading, physical activity
What's new?
A better understanding of the association of modifiable lifestyle factors with outcomes after breast cancer diagnosis can inform the development of tailored prevention strategies for breast cancer survivors. We performed systematic reviews, meta‐analyses and independent quality of evidence grading to investigate the association of postdiagnosis body fatness, physical activity and dietary factors with breast cancer prognosis. The results support the development of lifestyle recommendations for breast cancer patients to avoid obesity and be physically active.
Abbreviations
- 25(OH)D
25‐hydroxy‐vitamin D
- AICR
American Institute for Cancer Research
- BMI
body mass index
- CIs
confidence intervals
- CUP Global
Global Cancer Update Programme
- CUP
Continuous Update Project
- HC
hip circumference
- MA
meta‐analysis
- MET
metabolic equivalent of task
- RCTs
randomised controlled trials
- RR
relative risk
- WC
waist circumference
- WCRF
World Cancer Research Fund
- WHR
waist to hip circumference ratio
1. INTRODUCTION
With the improvement in breast cancer survival rates, 1 there is an urgent need to understand the relationship between modifiable risk factors such as body fatness, physical activity and diet assessed after breast cancer diagnosis with subsequent outcomes to develop evidence‐based recommendations for breast cancer survivors. To date, although there is a breadth of knowledge on the relationship between modifiable lifestyle factors and breast cancer incidence, 2 , 3 , 4 , 5 less is known about how they might influence outcomes after breast cancer diagnosis.
The Third Expert Report from the World Cancer Research Fund (WCRF)/American Institute for Cancer Research (AICR), with evidence collected up to 30 June 2012, 2 identified that having a healthy body weight, being physically active and following a diet rich in fibre and soy after breast cancer diagnosis were associated with better overall survival. However, because of limitations in the design or conduct of the studies available for inclusion at that time, this evidence was judged as limited‐suggestive and specific recommendations were not developed. In recent years, new evidence from more than 100 observational studies and randomised controlled trials (RCTs) has been published in this field. 6 , 7 , 8 , 9
We performed parallel systematic reviews and meta‐analyses in three areas to update the evidence on body fatness, physical activity and diet with outcomes after breast cancer diagnosis. An independent Expert Panel graded the quality of the evidence. This article presents the summary of the evidence grading and more details on the rationale, methods and findings are provided in the accompanied systematic review articles. 10 , 11 , 12
2. METHODS
The systematic reviews were conducted as part of the on‐going Global Cancer Update Programme (CUP Global), formally known as the WCRF/AICR Continuous Update Project (CUP). 13 The peer‐reviewed protocol is available online. 14
2.1. Structure of evidence synthesis team
This work involved three teams:
The CUP Global Secretariat was responsible for coordinating the project, drafting the research questions, facilitating the Panel meeting and summarising its main outputs.
The CUP Global research team at Imperial College London drafted the systematic review protocols based on the specification developed for the WCRF/AICR Second Expert Report, 15 completed the literature search and review for eligibility, extracted data in the CUP Global database, conducted data analysis, produced narrative and tabular summaries of the results and drafted articles.
The CUP Global Expert Panel comprised independent experts in nutrition, physical activity, body fatness, cancer biology, epidemiology (especially cancer or nutritional epidemiology), oncology, cellular and other mechanisms of cancer development and progression, genetic and epigenetic aspects of cancer susceptibility and of tumour behaviour, gene‐nutrient interactions, public health, cancer survivors and a public representative. The Panel reviewed the research questions and protocols, interpreted the systematic literature reviews and graded the quality of the evidence. The quality of evidence was graded during 2‐day online Panel meetings in November 2020 and May 2022.
2.2. Methods for systematic review
We searched PubMed and Embase for relevant publications from inception to 31 October 2021. The reference lists of identified articles were screened for additional publications. Eligible studies were RCTs, longitudinal observational studies and pooled analyses; with at least 100 women diagnosed with first primary breast cancer during adulthood; with at least 6 months study period (for RCTs) (intervention and/or follow‐up); that reported results on body fatness (ie, body mass index [BMI], waist [WC] or hip [HC] circumference, waist to hip circumference ratio [WHR] and changes in weight or BMI), physical activity (any type) and diet (ie, foods, food components, nutrients, dietary patterns and supplements) in relation to all‐cause mortality, breast and nonbreast cancer‐specific mortality, breast cancer recurrence and second primary cancers. Study and participants' characteristics and results were extracted from each included publication into the CUP Global database. The quality of individual studies was not graded using a specific tool. Instead, relevant study characteristics that could be used to explore potential sources of bias were included in the CUP Global database after identifying the most likely influential sources of bias in cancer survival studies. 16 , 17 In the Expert Panel meeting, whether the studies had serious quality issues were discussed when judging the evidence.
2.3. Methods for meta‐analysis
We calculated (or updated if reviewed previously) summary relative risk (RR) estimates and their 95% confidence intervals (CIs) using the inverse variance DerSimonian‐Laird random‐effects model, 18 when at least three (additional) studies were identified since our previous review that contained evidence up to 30 June 2012, 2 otherwise the studies were descriptively synthesised. For evidence that was judged as limited‐suggestive or above in the previous systematic review or was related to the WCRF/AICR Cancer Prevention Recommendations, the accumulated evidence was summarised in an updated meta‐analysis regardless of the number of studies identified during the update. Multivariable adjusted estimates were selected for the meta‐analyses. Linear dose‐response meta‐analyses were conducted using the generalised weighted least‐squares regression model. 19 Nonlinear meta‐analyses were conducted using restricted cubic splines when five or more studies, each with data for at least three exposure levels, were available. 20 Between‐study heterogeneity was assessed by Cochran's Q test and I 2 statistic. 21 Predefined subgroup meta‐analyses and random‐effects meta‐regression analyses were conducted to explore potential sources of heterogeneity. 22 The Egger's regression asymmetry test and visual inspection of the funnel plots were conducted to examine small study effects such as publication bias, when there were more than 10 studies. 23
2.4. Grading the quality of evidence
An independent CUP Global Expert Panel graded the quality of the evidence as strong (subgrades evaluating likelihood of causality: convincing or probable or substantial effect on risk unlikely) or limited (subgrades evaluating likelihood of causality: limited suggestive or limited—no conclusion) according to predefined criteria listed in Table 1, which evaluate the quantity, consistency, magnitude and precision of the summary estimates, existence of a dose‐response, study design and risk of bias, generalisability and mechanistic plausibility of the results.
TABLE 1.
Grading criteria for evidence on diet, nutrition, physical activity and survival in women with breast cancer
| Evidence grades | Grading criteria for evidence on diet, nutrition, physical activity and survival in women with breast cancer | Het | PB | Mec |
|---|---|---|---|---|
| Strong evidence | ||||
| Convincing | Evidence of an effect from a meta‐analysis of RCTs or at least two well‐designed independent RCTs | No | No | Desirable |
| Probable | Evidence of an effect from a meta‐analysis of RCTs or two well‐designed RCTs | Some | No | Desirable |
| OR Evidence of an effect from one well‐designed RCT and one well‐designed cohort study | No | No | Required | |
| OR Evidence from at least one well‐designed pooled analysis of follow‐up studies | No | No | Required | |
| OR Evidence from at least two independent well‐designed follow‐up studies | No | No | Required | |
| Limited evidence | ||||
| Limited suggestive | Evidence from a meta‐analysis of RCTs or at least two well‐designed RCTs but the confidence interval may include the null | Some | No | Not required |
| OR Evidence from one well‐designed RCT but the confidence interval may include the null | No | No | Required | |
| OR Evidence of an effect from a pooled analysis of follow‐up studies | Some | No | Not required | |
| OR Evidence from a pooled analysis of follow‐up studies but the confidence interval may include the null | Some | No | Required | |
| OR Evidence of an effect from at least one follow‐up study | No | No | Required | |
| OR Evidence of an effect from at least two follow‐up studies | No | No | Not required | |
| OR Evidence from at least two follow‐up studies but the confidence interval may include the null | Some | No | Required | |
| Limited—no conclusion | Any of the following reasons:
|
— | — | — |
| Strong evidence | ||||
| Substantial effect on risk unlikely |
Evidence of the absence of an effect (a summary estimate close to 1.0) from any of the following: a. A meta‐analysis of RCTs b. At least two well‐designed independent RCTs c. A well‐designed pooled analysis of follow‐up studies d. At least two well‐designed follow‐up studies
|
No | — | Absence |
Note: Special upgrading factors: (a) Presence of a plausible biological gradient (‘dose response’) in the association. Such a gradient need not be linear or even in the same direction across the different levels of exposure, so long as this can be explained plausibly. (b) A particularly large summary effect size (a relative risk of 2.0 or more, or 0.5 or less, depending on the unit of exposure), after appropriate control for confounders. (c) Evidence from appropriately controlled experiments demonstrating one or more plausible and specific mechanisms. (d) All plausible known residual confounders or biases including reverse causation would reduce a demonstrated effect, or suggest a spurious effect when results show no effect. Special considerations important for evidence for breast cancer survivors including the following potential confounding variables—the type of tumour, type of treatment, amount of treatment received and the dissemination of the disease.
Abbreviations: Het, substantial unexplained heterogeneity or some unexplained heterogeneity; PB, publication bias; Mec, strong and plausible mechanistic evidence is required, desirable but not required, not required or absent.
3. RESULTS
Table 2 and Figure 1 show the summary findings and the judgement of the CUP Global Expert Panel.
TABLE 2.
Evidence grades and main findings from the systematic literature reviews on postdiagnosis diet, nutrition, physical activity and survival in women with breast cancer
| All‐cause mortality | BrCA mortality | BrCA recurrence | Second primary BrCA | Non‐BrCA mortality | CVD mortality | |
|---|---|---|---|---|---|---|
| Exposure |
RR (95% CI) Studies I 2 |
RR (95% CI) Studies I 2 |
RR (95% CI) Studies I 2 |
RR (95% CI) Studies I 2 |
RR (95% CI) Studies I 2 |
RR (95% CI) Studies I 2 |
| Strong evidence (Convincing) | — | — | — | — | — | — |
| Strong evidence (Probable) | ||||||
| BMI (per 5 kg/m2) |
1.07 (1.05‐1.10) 64 studies I 2 = 56% |
1.10 (1.06‐1.14) 39 studies I 2 = 60% |
— |
1.14 (1.04‐1.26) 11 studies I 2 = 66% |
— | — |
| Waist circumference (per 10 cm) |
1.18 (1.07‐1.31) 5 studies I 2 = 55% |
1.12 (1.03‐1.22) 3 studies I 2 = 0% |
— | — | — | — |
| Waist‐hip‐ratio (per 0.1 unit) |
1.30 (1.20‐1.40) 8 studies I 2 = 0% |
1.21 (1.08‐1.35) 6 studies I 2 = 6% |
— | — | — | — |
| Limited evidence (Limited suggestive) | ||||||
| BMI (per 5 kg/m2) | — | — |
1.05 (1.03‐1.08) 63 studies I 2 = 54% |
— |
1.06 (0.94‐1.19) 10 studies I 2 = 78% |
1.16 (0.96‐1.41) 2 studies I 2 = 0% |
| Waist circumference | — | — | No MA a | — | — | — |
| Waist‐hip‐ratio (per 0.1 unit) | — | — | No MA a | — | — | — |
| Recreational physical activity (per 10 MET‐h/week) |
0.85 (0.78‐0.92) 12 studies I 2 = 87% |
0.86 (0.77‐0.96) 11 studies I 2 = 65% |
0.97 (0.91‐1.05) 6 studies I 2 = 68% |
— | — | — |
| Predefined healthy dietary and lifestyle patterns | No MA b | — | — | — | No MA b | — |
| Soy foods (isoflavones per 2 mg/day) |
0.96 (0.92‐1.02) 5 studies I 2 = 66% |
0.83 (0.64‐1.07) 3 studies (pooled analysis) |
0.75 (0.61‐0.92) 3 studies (pooled analysis) |
— | — | — |
| Dietary fibre (per 10 g/day) |
0.87 (0.80‐0.94) 4 studies I 2 = 0% |
— | — | — | — | — |
| 25(OH)D (per 10 nmol/L) |
0.93 (0.89‐0.97) 6 studies I 2 = 63% |
0.94 (0.90‐0.99) 5 studies I 2 = 24% |
— | — | — | — |
| Limited evidence (Limited—no conclusion) | ||||||
| Weight/BMI change | No MA a | |||||
| Other diet b | No MA a | |||||
Abbreviations: 25(OH)D, 25‐hydroxy vitamin D concentrations; BMI, body mass index; BrCA, breast cancer; CI, confidence interval; CVD, cardiovascular disease; MA, meta‐analysis; MET, metabolic equivalent of task; RR, summary relative risk.
Studies were descriptively synthesised, as the data was not sufficient for conducting meta‐analysis.
Other diet comprises low/high fat dietary patterns, healthy dietary patterns (for breast cancer‐specific mortality and recurrence), alcoholic drinks, fruit and vegetables, dietary fibre (for breast cancer‐specific mortality and recurrence), wholegrains, red and processed meat, fish, eggs, milk and dairy products, nutrients (fats, carbohydrate, animal protein, plant protein), supplements, vitamin D (blood levels on recurrence).
FIGURE 1.
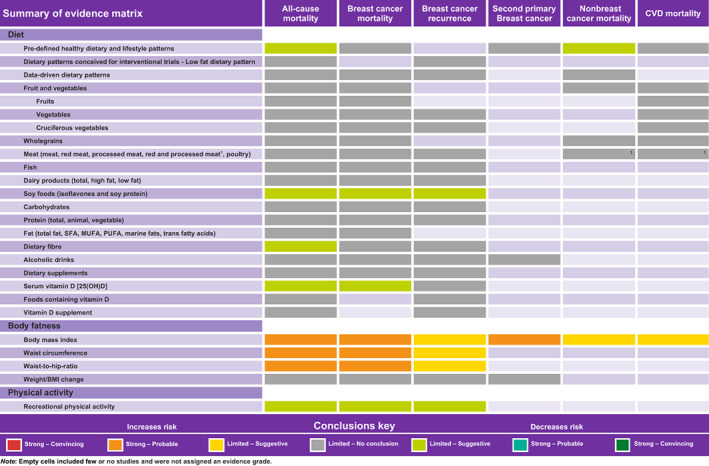
Summary quality of evidence matrix from the systematic literature reviews on postdiagnosis diet, nutrition, physical activity and survival in women with breast cancer [Color figure can be viewed at wileyonlinelibrary.com]
3.1. Evidence summary for postdiagnosis body fatness and breast cancer prognosis
We included 225 studies from longitudinal observational studies in the systematic review, which comprised more than 456 000 women with breast cancer, of whom more than 36 000 died of any cause and approximately 21 000 died of breast cancer. 10 One relevant weight loss RCT was identified. 24
The evidence on postdiagnosis body fatness (BMI, WC and WHR) and higher risk of all‐cause mortality, breast cancer‐specific mortality and second primary breast cancer (BMI only) was substantial, consistent across different study designs and populations and showed evidence of a dose‐response relationship, which was unlikely to be caused by chance or bias. The quality of this evidence was graded as strong (subgrade: probable). It did not reach the convincing subgrade due to sparseness of RCT evidence. Higher postdiagnosis BMI was associated with a higher risk of all‐cause mortality (64 studies, summary RR per 5 kg/m2: 1.07, 95% CI: 1.05‐1.10, I 2: 56%), breast cancer‐specific mortality (39 studies, RR: 1.10, 95% CI: 1.06‐1.14, I 2: 60%) and second primary breast cancer (11 studies, RR: 1.14, 95% CI: 1.04‐1.26, I 2: 66%). Findings for WC and WHR had the same inference to the BMI findings, but fewer studies used these indices (Table 2, Figure 1). There was evidence for nonlinear relationships between BMI and all‐cause (J‐shaped) and breast cancer‐specific mortality. When the meta‐analyses were performed in subgroups according to menopausal status, cancer subtype by hormone receptor status, geographic location, study design (prospective vs retrospective cohorts vs follow‐up of RCTs), length of follow‐up, number of events, timing of exposure assessment, treatment period and number of adjustments, the results were similar. 10 The only notable difference was the inverse association observed for BMI and breast cancer recurrence in participants with metastatic disease at recruitment compared to the positive associations observed in earlier stages (P heterogeneity: 0.003).
The evidence for body fatness (BMI, WC and WHR) and breast cancer recurrence was limited in quality (primarily outcome measurement error and reverse causation bias) but suggestive of a positive dose‐response relationship (63 studies, RR for BMI, 1.05, 95% CI: 1.03‐1.08, I 2: 54%). The evidence suggesting a higher risk of nonbreast cancer‐related mortality (10 studies, BMI only, RR: 1.06, 95% CI: 0.94‐1.19, I 2: 78%) and cardiovascular mortality (2 publications, BMI only, RR: 1.16, 95% CI: 0.96‐1.41, I 2: 0%) with greater body fatness was also limited suggestive (Table 2, Figure 1). The evidence on weight or BMI change and any outcome was limited and inconsistent and no conclusions could be made. The weight loss RCT suggested improved survival in the lifestyle intervention vs the education control group, but the CIs were wide. 24
3.2. Evidence summary for physical activity and outcomes after breast cancer
We included 20 longitudinal observational studies in the systematic review. 11 Most of these articles published data on recreational physical activity; thus, studies that evaluated total physical activity, nonrecreational physical activity, physical activity changes and sedentary behaviour were descriptively synthesised. There were also three exploratory RCTs from two publications that met our inclusion criteria, 25 , 26 and their results suggested a beneficial effect of physical activity interventions on outcomes after breast cancer, but these findings were limited by small numbers of events and wide CIs.
Recreational physical activity was associated with a lower risk of all‐cause (12 studies, summary RR per 10 metabolic equivalent of task [MET]‐hour/week: 0.85, 95% CI: 0.78‐0.92, I 2: 87%) and breast cancer‐specific mortality (11 studies, RR: 0.86, 95% CI: 0.77‐0.96, I 2: 65%), but the evidence was judged as limited suggestive due to limitations in the methodological quality of the included studies (primarily exposure measurement error and reverse causation bias). There was evidence for a nonlinear association, where the risk of all‐cause and breast cancer‐specific mortality decreased linearly by up to ~47% with increasing physical activity levels up to around 20 MET‐hour/week without further risk reduction at higher levels. In subgroup meta‐analyses, the results were not different according to menopausal status, cancer subtype by hormone receptor status, BMI, different physical activity intensities and timing of exposure assessment, and none of these factors explained fully the between‐study heterogeneity.
The evidence on recreational physical activity and risk of breast cancer recurrence was judged as limited and no conclusion could be drawn (6 studies, RR: 0.97, 95% CI: 0.91‐1.05, I 2: 68%).
3.3. Evidence summary for diet and outcomes after breast cancer
We included 108 publications from RCTs and longitudinal observational studies in the systematic review, 12 which comprised more than 151 000 women with breast cancer, of whom more than 14 900 died of any cause and 5900 died of breast cancer. Meta‐analyses were possible only for intakes of fruits, dairy, isoflavone (from soy foods), carbohydrates, proteins, fats, dietary fibre, alcohol and serum 25‐hydroxy‐vitamin D (25(OH)D) concentrations. Few RCTs were identified only for dietary patterns, which were descriptively synthesised.
The evidence was graded as limited‐suggestive for healthy dietary and lifestyle patterns and lower risk of all‐cause mortality and mortality from other causes of death. Twelve observational studies investigated high vs low categories of 18 predefined healthy dietary and lifestyle patterns, and results were generally consistent in the direction of an inverse association with all‐cause mortality (RRs ranged from 0.32 to 1.03; in 8 out of 17 unique patterns, 95% CIs did not include 1) and mortality from other causes of death (4 studies, 10 patterns, RRs ranged from 0.44 to 0.95; in 7 out of 10 patterns, 95% CIs did not include 1).
The evidence was graded as limited‐suggestive for soy food intake and lower risk of all‐cause mortality, breast cancer‐specific mortality and recurrence. A 2 mg/day higher isoflavone intake from soy foods yielded a 4% lower all‐cause mortality risk but with a narrow CI crossing the null value (5 studies, RR: 0.96, 95% CI: 0.92‐1.02, I 2: 66%). No association was observed between isoflavone intake and breast cancer‐specific mortality (3 studies, RR for high vs low: 0.83, 95% CI: 0.64‐1.07), whereas an inverse association was observed for breast cancer recurrence (RR ≥10.0 vs <4.0 mg/day: 0.75, 95% CI: 0.61‐0.92) in a pooled analysis of two US cohorts and one Chinese cohort. 27 In addition, soy protein intake was inversely associated with all‐cause mortality in two Chinese studies, 28 , 29 and a lower risk was also observed for breast cancer‐specific mortality and recurrence combined in one study. 28
The evidence was judged as limited‐suggestive for dietary fibre intake and lower risk of all‐cause mortality (4 studies, RR per 10 g/day: 0.87, 95% CI: 0.80‐0.94, I 2: 0%) and for serum 25(OH)D concentrations with all‐cause (6 studies, RR per 10 nmol/L: 0.93, 95% CI: 0.89‐0.97, I 2: 63%) and breast cancer‐specific mortality (5 studies, RR: 0.94, 95% CI: 0.90‐0.99, I 2: 24%).
The evidence on the remaining associations (ie, low/high fat dietary patterns, healthy dietary patterns [for breast cancer‐specific mortality and recurrence], alcoholic drinks, fruit and vegetables, dietary fibre [for breast cancer‐specific mortality and recurrence], wholegrains, red and processed meat, fish, eggs, milk and dairy products, nutrients [fats, carbohydrate, animal protein, plant protein], supplements, vitamin D [blood levels on recurrence]) was limited and sparse, thus they were graded as limited and no conclusion could be made.
4. DISCUSSION
As part of CUP Global, we conducted systematic reviews and meta‐analyses in three areas and graded the quality of the evidence for the association of body fatness, physical activity and dietary factors with outcomes among breast cancer survivors. A better understanding of the association of modifiable lifestyle factors with outcomes after breast cancer diagnosis can inform the development of tailored prevention strategies for breast cancer survivors.
The evidence on postdiagnosis body fatness and higher risk of all‐cause mortality, breast cancer‐specific mortality and second primary cancer was graded as strong (subgrade: probable). There was evidence for J‐shaped relationships between BMI and all‐cause and breast cancer‐specific mortality, suggesting favourable survival in women of high‐normal to low‐overweight BMI. The evidence for body fatness and breast cancer recurrence was limited in quality, but suggestive of a positive dose‐response relationship. The evidence for the association of recreational physical activity and lower risk of all‐cause and breast cancer‐specific mortality was also rated as limited‐suggestive. The association of recreational physical activity with the risk of all‐cause and breast cancer‐specific mortality decreased linearly with increasing physical activity levels up to around 20 MET‐hour/week without further risk reduction after that, suggesting that even low physical activity might provide survival benefits. Data on dietary factors and outcomes after breast cancer diagnosis was limited and inconsistent, and no firm conclusions could be reached except for healthy dietary and lifestyle patterns, soy foods and dietary fibre intake and serum 25(OH)D concentrations that received limited‐suggestive evidence gradings for a lower risk of the examined outcomes.
This programme of work has several strengths. We conducted a comprehensive up to date systematic literature search for body fatness, physical activity and dietary factors in relation to all‐cause and cause‐specific mortality and recurrence after a breast cancer diagnosis. We performed both linear and nonlinear dose‐response meta‐analyses to avoid the limitations of categorical meta‐analyses and to enable more precise inference and potential recommendations regarding the effective dose of the modifiable lifestyle exposures. In addition, an independent Expert Panel graded the quality and uncertainty of the evidence according to the predefined WCRF/AICR evidence grading criteria (Table 1).
The evidence also has limitations. Very few RCTs of lifestyle interventions in breast cancer patients have examined survival outcomes, and in general RCTs in this area are limited by small sample size, short duration of follow‐up and poor adherence to lifestyle interventions. 30 Our findings are susceptible to potential bias derived from observational studies. 16 An important limitation is the possibility of reverse causality, as patients may tend to adjust their lifestyles according to the disease severity and prognosis, but most cohort studies excluded cancer patients who experienced a recurrent event close to the lifestyle assessment. Another limitation is residual confounding by cancer treatment, disease progression and precancer exposures that not all studies were able to adequately adjust for. We can also not exclude the possibility of selection bias, because inclusion of participants in the studies depends on survival time after disease diagnosis. In addition, all studies measured physical activity levels and dietary intakes using self‐reported data, and most studies assessed the lifestyle exposures at one point in time introducing potential measurement error. Considering the potential methodological limitations of the observational cancer survival research, the independent Expert Panel graded the quality of the evidence conservatively.
The present review supports the advice for women who have completed primary treatment for breast cancer to follow the WCRF/AICR Cancer Prevention Recommendations to have a healthy weight, be physically active and eat a healthy diet if it fits with the specific medical advice given by their cancer management team. 2 The results of the current work further support the development of lifestyle recommendations for breast cancer patients to avoid obesity and be physically active. This in line with the recently released American Cancer Society Guideline on Diet and Activity for Cancer Survivors, 31 and the World Health Organisation guidelines on physical activity and sedentary behaviour. 32 This is further supported by the recommendation from the American College of Sports Medicine, which reviewed RCT evidence in 2018 and concluded that exercise training is safe for cancer survivors and facilitates improvements in health‐related quality of life, anxiety, depressive symptoms, fatigue and physical functioning, 33 and the recommendation from the Physical Activity Guidelines for Americans in 2018 that reported regular physical activity to be associated with lower risk of all‐cause and breast cancer‐specific mortality in women diagnosed with breast cancer. 34 A difference of the present CUP Global review was that the whole systematic review and meta‐analysis process was conducted by CUP Global team members for several nutrition‐related exposures and survivorship outcomes following a detailed and standardised approach, and then the quality of the evidence was graded by an independent Expert Panel. In contrast, the significant work and subsequent recommendations of most other organisations focused on physical activity and were based on existing studies and/or systematic reviews. Additional studies are needed to determine associations in important subgroups according to breast cancer subtype, survivorship phase, socioeconomic status, race/ethnicity and duration, frequency and type of lifestyle exposure. Additional research is also needed to determine the most effective strategies to increase uptake and adherence to WCRF/AICR Cancer Prevention Recommendations in breast cancer patients and survivors. Evidence‐informed policy recommendations should also be developed to help governments and policymakers take effective action to reduce preventable cases and deaths of cancer. 35
In conclusion, our systematic reviews and meta‐analyses provide strong evidence that higher postdiagnosis body fatness increases mortality among breast cancer survivors and limited but suggestive evidence that higher levels of physical activity lower mortality rates. Our results encourage the development of lifestyle recommendations for breast cancer patients to avoid obesity and be physically active if able and fit with their specific medical advice.
AUTHOR CONTRIBUTIONS
Konstantinos K. Tsilidis and Doris S. M. Chan are co‐principal investigator of CUP Global at Imperial College London. Konstantinos K. Tsilidis was part of the Expert Panel but was not involved with judging the evidence after becoming a co‐principal investigator of CUP Global. Teresa Norat and Doris S. M. Chan wrote the protocol based on the advice from the Protocol Expertise Group and implemented the study with Konstantinos K. Tsilidis. Doris S. M. Chan and Neesha Nanu did the literature search. Leila Abar, Katia Balducci, Nerea Becerra‐Tomas, Margarita Cariolou, Neesha Nanu and Rita Vieira did the study selections and data extraction. Leila Abar, Katia Balducci, Nerea Becerra‐Tomas, Margarita Cariolou, Doris S. M. Chan and Rita Vieira checked, analysed and interpreted the data. Dagfinn Aune, Georgios Markozannes and Nerea Becerra‐Tomás were CUP Global team members who revised the article. Darren C. Greenwood was statistical adviser. Anne McTiernan, Steven K. Clinton, Edward L. Giovannucci, Ellen Kampman, Alan A. Jackson, Konstantinos K. Tsilidis, Marc J. Gunter and Vivien Lund (lay member) were the Expert Panel members who provided judgements on the evidence and advised on the interpretation of the review. Elio Riboli and Amanda J. Cross were Expert Panel observers. Kate Allen, Nigel T. Brockton, Helen Croker, Daphne Katsikioti, Deirdre McGinley‐Gieser, Panagiota Mitrou and Martin Wiseman were the CUP Global Secretariat members who provided overall coordination for the work and convened and facilitated discussions with the Expert Panel. Konstantinos K. Tsilidis drafted the original article. All authors reviewed and provided comments on the article. Doris S. M. Chan is the guarantor and has full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. The work reported in the article has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
This work was funded by the World Cancer Research Fund network of charities (American Institute for Cancer Research (AICR); World Cancer Research Fund (WCRF); Wereld Kanker Onderzoek Fonds (WKOF)) (CUP GLOBAL Special Grant 2018). Konstantinos K. Tsilidis, Doris S. M. Chan, Rita Vieira, Dagfinn Aune, Katia Balducci, Margarita Cariolou, Georgios Markozannes and Nerea Becerra‐Tomás are supported by the World Cancer Research Fund network of charities. Leila Abar and Neesha Nanu were previously supported by the World Cancer Research Fund network of charities. Teresa Norat was supported by the World Cancer Research Fund network of charities as principal investigator of the WCRF/AICR Continuous Update Project (CUP) and by WCRF International as the CUP Global scientific advisor. Dr. McTiernan was supported by grants from the Breast Cancer Research Foundation (BCRF‐19‐107/BCRF‐20‐107/BCRF‐21‐107). The funders of our study had no role in the decisions about the design and conduct of the study; collection, management, analysis or interpretation of the data; or the preparation, review or approval of the article. The process used was based on the method developed by WCRF International's Methodology Task Force for the WCRF/AICR Second Expert Report. The CUP Global Secretariat, led by WCRF International, provided overall coordination for the work and convened and facilitated discussions with the Expert Panel who provided judgements on the evidence. The views expressed in this review are the opinions of the authors. They may differ from those in future updates of the evidence related to food, nutrition, physical activity and cancer incidence and survival.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
We thank Teresa Norat for leading the WCRF/AICR Continuous Update Project (CUP) as principal investigator from 2007 to 2020. We thank the Protocol Expertise Group: Annie Anderson (University of Dundee), Steven Clinton (The Ohio State University), Ellen Copson (Southampton University), Wendy Demark‐Wahnefried (UAB Comprehensive Cancer Center, Birmingham, AL), John Mathers (Newcastle University), Anne McTiernan (Fred Hutchinson Cancer Research Center), Andrew Renehan (University of Manchester), Lesley Turner (patient representative), Franzel van Duijnhoven (Wageningen University) and Galina Velikova (University of Leeds), for their expert opinion on the review protocol. We thank the CUP Global team members at Imperial College London: Sonia Chemlal, Jakub Sobiecki, Britta Talumaa and Victoria White, for their contribution to the literature search and data extraction; and database managers: Rui Vieira, Christophe Stevens, Yusuf O. Anifowoshe and Lam Teng for implementing and updating the CUP Global database. We also acknowledge the input of Isobel Bandurek and Susannah Brown as past CUP Secretariat members.
Tsilidis KK, Cariolou M, Becerra‐Tomás N, et al. Postdiagnosis body fatness, recreational physical activity, dietary factors and breast cancer prognosis: Global Cancer Update Programme (CUP Global) summary of evidence grading. Int J Cancer. 2023;152(4):635‐644. doi: 10.1002/ijc.34320
Funding information American Institute for Cancer Research, Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018; Breast Cancer Research Foundation, Grant/Award Number: BCRF‐19‐107/BCRF‐20‐107/BCRF‐21‐107; Wereld Kanker Onderzoek Fonds, Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018; World Cancer Research Fund, Grant/Award Number: CUPGLOBAL‐SpecialGrant‐2018
DATA AVAILABILITY STATEMENT
Only publicly available data were used in our study. Data sources and handling of these data are described in the Materials and Methods section. Further details are available from the corresponding author upon request.
REFERENCES
- 1. American Cancer Society . Cancer Facts & Figures 2021. Atlanta: American Cancer Society; 2021. [Google Scholar]
- 2. World Cancer Research Fund/American Institute for Cancer Research . Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report; 2018.
- 3. Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papadimitriou N, Markozannes G, Kanellopoulou A, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12:4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rezende LFM, Sa TH, Markozannes G, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2018;52:826‐833. [DOI] [PubMed] [Google Scholar]
- 6. Friedenreich CM, Stone CR, Cheung WY, Hayes SC. Physical activity and mortality in cancer survivors: a systematic review and meta‐analysis. JNCI Cancer Spectr. 2020;4:pkz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geidl W, Schlesinger S, Mino E, Miranda L, Pfeifer K. Dose‐response relationship between physical activity and mortality in adults with noncommunicable diseases: a systematic review and meta‐analysis of prospective observational studies. Int J Behav Nutr Phys Act. 2020;17:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lohmann AE, Soldera SV, Pimentel I, et al. Association of obesity with breast cancer outcome in relation to cancer subtypes: a meta‐analysis. J Natl Cancer Inst. 2021;113:1465‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwedhelm C, Boeing H, Hoffmann G, Aleksandrova K, Schwingshackl L. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta‐analysis of cohort studies. Nutr Rev. 2016;74:737‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan DSM, Vieira R, Abar L, et al. Post‐diagnosis body fatness, weight change and breast cancer prognosis: global cancer update programme systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):572‐599. 10.1002/ijc.34322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cariolou M, Abar L, Aune D, et al. Post‐diagnosis recreational physical activity and breast cancer prognosis: global cancer update programme systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):600‐615. 10.1002/ijc.34324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Becerra‐Tomás N, Balducci K, Abar L, et al. Post‐diagnosis dietary factors, supplement use and breast cancer prognosis: global cancer update Programme systematic literature review and meta‐analysis. Int J Cancer. 2023;152(4):616‐634. 10.1002/ijc.34321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Global Cancer Update Programme (CUP Global). 2022. https://www.wcrf.org/diet-activity-and-cancer/global-cancer-update-programme/about-the-global-cancer-update-programme/. Accessed September 2022.
- 14. Imperial College London CUP Global Team . Cancer update programme on diet and cancer: protocol for the data collection and systematic literature reviews on the role of diet, nutrition and physical activity on outcomes after diagnosis of breast cancer. Version 3; 2019. https://www.imperial.ac.uk/school‐public‐health/epidemiology‐and‐biostatistics/research/cancer‐and‐nutritional‐epidemiology/global‐cancer‐update‐programme/. Accessed October 2021.
- 15. World Cancer Research Fund/American Institute for Cancer Research . Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 16. Chubak J, Boudreau DM, Wirtz HS, McKnight B, Weiss NS. Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst. 2013;105:1456‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Savitz DA, Wellenius GA, Trikalinos TA. The problem with mechanistic risk of bias assessments in evidence synthesis of observational studies and a practical alternative: assessing the impact of specific sources of potential bias. Am J Epidemiol. 2019;188:1581‐1585. [DOI] [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 19. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135:1301‐1309. [DOI] [PubMed] [Google Scholar]
- 20. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 22. Stram DO. Meta‐analysis of published data using a linear mixed‐effects model. Biometrics. 1996;52:536‐544. [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goodwin PJ, Segal RJ, Vallis M, et al. The LISA randomized trial of a weight loss intervention in postmenopausal breast cancer. npj Breast Cancer. 2020;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Courneya KS, Segal RJ, McKenzie DC, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc. 2014;46:1744‐1751. [DOI] [PubMed] [Google Scholar]
- 26. Hayes SC, Steele ML, Spence RR, et al. Exercise following breast cancer: exploratory survival analyses of two randomised, controlled trials. Breast Cancer Res Treat. 2018;167:505‐514. [DOI] [PubMed] [Google Scholar]
- 27. Nechuta SJ, Caan BJ, Chen WY, et al. Soy food intake after diagnosis of breast cancer and survival: an in‐depth analysis of combined evidence from cohort studies of US and Chinese women. Am J Clin Nutr. 2012;96:123‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shu XO, Zheng Y, Cai H, et al. Soy food intake and breast cancer survival. JAMA. 2009;302:2437‐2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang YF, Kang HB, Li BL, Zhang RM. Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pac J Cancer Prev. 2012;13:479‐482. [DOI] [PubMed] [Google Scholar]
- 30. Pennington KP, McTiernan A. The role of physical activity in breast and gynecologic cancer survivorship. Gynecol Oncol. 2018;149:198‐204. [DOI] [PubMed] [Google Scholar]
- 31. Rock CL, Thomson CA, Sullivan KR, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72:230‐262. [DOI] [PubMed] [Google Scholar]
- 32. WHO guidelines on physical activity and sedentary behaviour. https://www.who.int/publications/i/item/9789240015128. Accessed 11 August 2021.
- 33. Campbell KL, Winters‐Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51:2375‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. U.S. Department of Health and Human Services . Physical Activity Guidelines for Americans. 2nd ed. Washington, DC: U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 35. World Cancer Research Fund Policy Framework. https://www.wcrf.org/policy/. Accessed September 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Only publicly available data were used in our study. Data sources and handling of these data are described in the Materials and Methods section. Further details are available from the corresponding author upon request.


