Abstract
Background and purpose
Advanced analysis of electroencephalography (EEG) data has become an essential tool in brain research. Based solely on resting state EEG signals, a data‐driven, predictive and explanatory approach is presented to discriminate painful from non‐painful diabetic polyneuropathy (DPN) patients.
Methods
Three minutes long, 64 electrode resting‐state recordings were obtained from 180 DPN patients. The analysis consisted of a mixture of traditional, explanatory and machine learning analyses. First, the 10 functional bivariate connections best differentiating between painful and non‐painful patients in each EEG band were identified and the relevant receiver operating characteristic was calculated. Later, those connections were correlated with selected clinical parameters.
Results
Predictive analysis indicated that theta and beta bands contain most of the information required for discrimination between painful and non‐painful polyneuropathy patients, with area under the receiver operating characteristic curve values of 0.93 for theta and 0.89 for beta bands. Assessing statistical differences between the average magnitude of functional connectivity values and clinical pain parameters revealed that painful DPN patients had significantly higher cortical functional connectivity than non‐painful ones (p = 0.008 for theta and p = 0.001 for alpha bands). Moreover, intra‐band analysis of individual significant functional connections revealed a positive correlation with average reported pain in the previous 3 months in all frequency bands.
Conclusions
Resting state EEG functional connectivity can serve as a highly accurate biomarker for the presence or absence of pain in DPN patients. This highlights the importance of the brain, in addition to the peripheral lesions, in generating the clinical pain picture. This tool can probably be extended to other pain syndromes.
Keywords: biomarkers for pain, functional connectivity, multivariate analysis, painful polyneuropathy, resting state EEG
Significance.
Resting state electroencephalography based biomarkers can accurately classify pain in non‐painful diabetic polyneuropathy patients. This is based on a machine learning analysis of inter‐electrode connectivity. Painful patients show higher connectivity, suggesting that a certain level of ‘cross‐talk’ between brain regions is required to perceive pain.
INTRODUCTION
Objective, easy to obtain and stimulation‐free biomarkers for clinical pain are intensely sought after. In healthy volunteers, experimental pain evokes a widespread increase of cortical activity in most frequency bands [1] and slower peak alpha frequency (PAF) tested over the sensorimotor cortex at rest and during tonic pain stimulation, with an inverse correlation between PAF and experimental pain scores [2]. In line, clinical data show slower PAF and increased alpha/theta activity in chronic [3, 4] and neuropathic pain [5, 6, 7]. Furthermore, abnormal cortical activity is normalized after pain alleviation [8, 9, 10, 11].
Advancing from univariate local cortical activity to multivariate analysis approaches (e.g., machine learning) of dynamic cortical networks has brought some more insights. Fairly good predictions of response to various intensities of experimental thermal pain in healthy participants were reported [12] across all electroencephalography (EEG) bands. In line, applying machine learning analysis on spontaneous frontal delta EEG activity during tonic cold pain stimulus [13] successfully classified the responders versus non‐responders to opioid analgesia in healthy subjects and later efficiently defined the responders to postoperative opioid treatment [14]. In another study [15] accuracy higher than 80% was achieved in identifying patients at risk for central pain after spinal cord injuries.
Further development of EEG analysis is the study of connectivity between brain regions. This is parallel to an established body of research on magnetic resonance imaging (MRI) based pain‐related functional brain connectivity in various diseases and healthy controls (for a review, see [16]). A recent study on resting state EEG connectivity [17] was based on transforming the discrete electrode‐derived data into a continuous map of brain activity, using sLORETA, and then applying voxel based analysis similar to what is done for MRI data. The authors were able to discriminate chronic pain patients from the control subjects, primarily based on prefrontal theta and gamma connectivity, although they achieved a medium accuracy of 57%. They pointed to increased functional connectivity for alpha and beta bands in the sensorimotor cortex during experimental tonic pain. An additional article from that group has reported increased alpha band connectivity between sensorimotor and prefrontal cortices in response to experimental pain in healthy volunteers [18].
This work aims to further advance the methodology in exploring whether resting state EEG based functional brain activity differs between painful and non‐painful polyneuropathy participants. In order to do so, an alternative data‐driven approach is proposed for studying EEG connectivity. First, functional connectivity information is extracted directly from the raw EEG signal, relating each electrode to all others. Second, the signal‐to‐noise ratio is improved by identifying the most discriminative bivariate pairs of functional connections discriminating painful from non‐painful patients. This is done using attribute selection statistical methods, which in turn allow for performing correlation analysis of physiological–clinical relations on these connections.
MATERIALS AND METHODS
Data collection
This is a cross‐sectional study using a two‐group comparison convenience sample. The Investigational Review Board approved the experimental protocol of Rambam Health Care Campus, Haifa, Israel; the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Informed consent was obtained from all participants: National Institutes of Health clinical trial identifier number NCT02402361. This study was a part of a larger multi‐centre study examining pain in neuropathy (for the detailed study protocol, see [19]).
Two hundred patients with confirmed diabetic polyneuropathy (DPN) participated in this study. Patients over 18 years old were recruited through advertising and local diabetes clinics. To be classified as painful diabetic neuropathy, patients had (i) to be diagnosed as DPN based on a prior clinical assessment combined with supportive clinical findings, including either abnormal nerve conduction studies and/or abnormal findings on thermal quantitative sensory testing [20]; (ii) to have positively answered the question ‘Are you bothered at the present time by pain in your feet (continuous or intermittent)?’ [21]; and (iii) to meet the NeupSIG criteria for their pain being definitely neuropathic [22]. To be classified as non‐painful diabetic neuropathy, patients had (i) to be diagnosed as DPN as above and (ii) to have negatively answered the above question. Patients with insufficient mental capacity or proficiency in Hebrew to obtain consent were excluded. Other exclusion criteria included significant neurological or psychiatric disorders and moderate to severe pain from other causes that may confound assessment or pain reporting.
Clinical, psychophysical and psychological assessment
After providing informed consent, the participants underwent (i) a clinical neurological examination scored according to the Toronto Clinical Neuropathy Score (TCNS); (ii) quantitative sensory testing via a modified version of the German Research Network on Neuropathic Pain assessment protocol; (iii) pain modulation profile assessment; (iv) assessment of perceptual and psychological parameters via a battery of questionnaires (this battery of assessments is fully described in [19]); (v) EEG recording at rest and contact heat evoked potentials.
Resting state EEG recording
Electroencephalography was recorded for 3 min at rest in a seated position with eyes closed, and standardized instructions were used. The recording was performed with a 64 ActiCHamp Brain Vision system (Brain Products GmbH). The sampling rate was set to 500 Hz, with a 0.15–100 Hz bandpass filter and a notch filter of 50 Hz to reduce electrical net interference. Each electrode's impedance was lowered to 20 kΩ or less. The reference electrode was placed on the bridge of the participant's nose. An average reference was calculated.
Signal analysis
Our data analysis scheme is based on a commonly used data analysis pipeline consisting of several steps, including (i) data acquisition and pre‐processing, (ii) extraction of functional connectivity information [23], (iii) variable selection [24], (iv) traditional explanatory analysis and multivariate and correlation analysis between clinical and physiological data. These steps are schematically depicted in Figure 1 and explained in further detail below. MATLAB® [25] software was used for all steps of the analysis pipeline.
FIGURE 1.
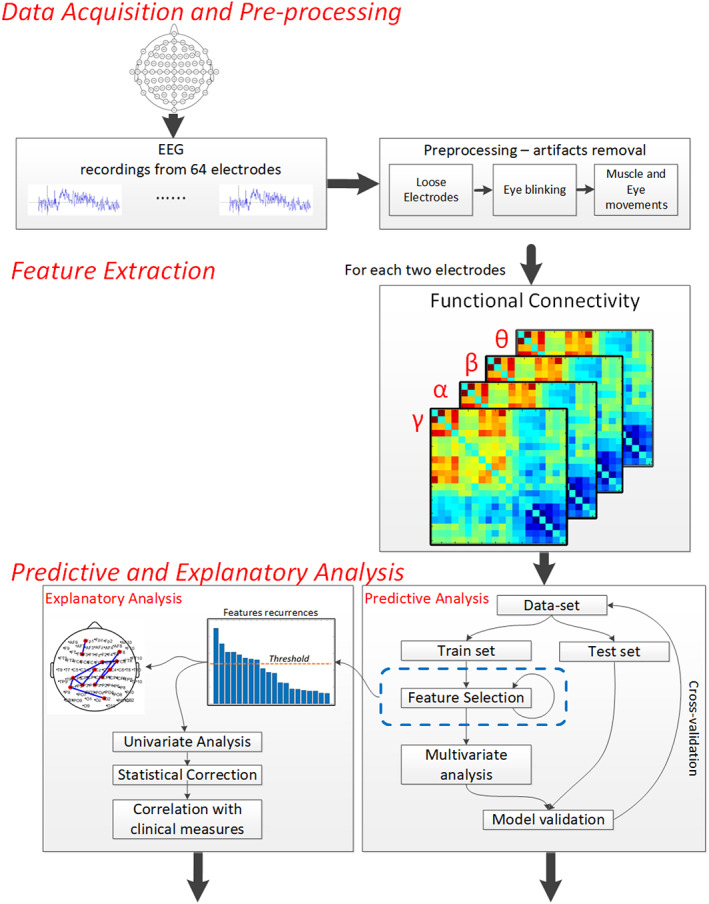
The analysis scheme is composed of three main stages: (i) data acquisition and pre‐processing; (ii) feature extraction, including calculation of functional connectivity values between each possible pair of electrodes for each of the standard frequency bands, using the MSC and applying the ReliefF algorithm to rank the features, aiming to choose the top 10 representing the best informative subset; (iii) predictive analysis—a multivariate analysis and ROC curve representation of the selected functional connections
Data acquisition and pre‐processing
Averaging neighbouring electrodes replaced noisy and detached electrodes [26]. The acquired EEG signal was segmented into 1‐s epochs, and segments with visually detected artifacts were removed. The total number of segments encompassed in the analysis varied from 150 (minimum) to 180. Since the number of noisy epochs was relatively small, artifact rejection (i.e., removal of contaminated epochs from the analysis) was applied instead of artifact correction (e.g., independent component analysis based correction).
Features extraction (i.e., functional connectivity)
During the features extraction process, functional connectivity values between each possible pair of electrodes were calculated separately for each of the standard frequency bands, that is, theta (3.5–7.5 Hz), alpha (7.5–12.5 Hz), beta (12.5–30 Hz) and gamma (30–40 Hz), using the magnitude‐squared coherence estimate (MSC) [23]. In short, EEG coherence is defined as the spectral correlation between two EEG signals. When applied to a windowed signal, this process allows the temporal synchronization in the frequency domain to be evaluated. If two signals are highly correlated, the value of MSC is 1; otherwise MSC is close to 0. The high coherence between EEG signals recorded at different electrodes provides information about the potential cortical interactions. Whilst local functional connectivity (i.e., between close electrode leads) may indicate high fidelity neural communication, global coherence (i.e., between different or distant brain areas) may indicate modulation of one region to another or an interaction between the regions.
Variable selection
It is well accepted that statistical learning tools are directly affected by both the relevance and the number of features. For example, a high number of irrelevant features (in our case, individual connections) or a high number of features compared to the amount of data can lead to a low signal‐to‐noise ratio. It is a variant of the famous curse of dimensionality [27] or a small‐n large‐p [28] problem. To resolve it, the ReliefF [24] algorithm is applied to rank the features, aiming to choose the top 10 representing the best informative subset. The goal here was two‐fold: identify features capable of successfully classifying patients with painful and non‐painful polyneuropathy; indicate a brain‐wide localization of the origin of differences between the groups. In other words, the method should be sufficiently predictive and explanatory. Note that relevant features (both for explanatory and predictive analysis) were selected using the training data only to avoid double‐dipping, which entails using both training and testing data partitions to select relevant features [29]. In other words, the analysed features were selected from a sub‐sampled dataset and cross‐validated against statistical biases.
Due to the nature of the cross‐validation training iterations leaving substantial data for validation, the feature selection process is non‐deterministic (i.e., each iteration is likely to result in the selection process of slightly different features). To overcome such uncertainty, a histogram methodology was used. The analysis (Figure 1, blue rectangle) was re‐run many times and the frequency (or a recurrence) of each specific functional connection being selected as a discriminating feature was counted, as proposed in [30]. An example of such a run can be seen in Figure 1 (explanatory analysis path) and Figure 2, where the x‐axis indicates selected functional connections (i.e., features) and the y‐axis indicates frequency (i.e., the repeatability of a specific feature through different selections).
FIGURE 2.
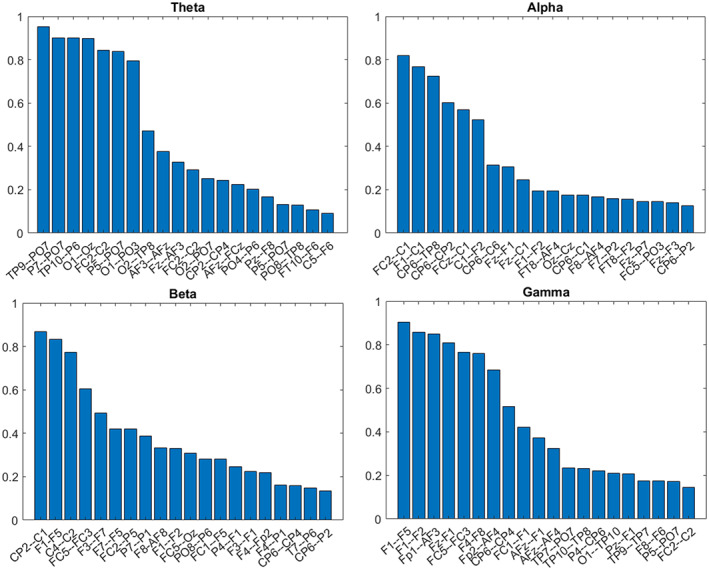
Functional connections differentiating between painful and non‐painful diabetic neuropathy patients. The x‐axis represents the selected functional connections, and the y‐axis describes the recurrence (i.e., histogram) of each connection out of 300 reiterations on a 0–1 range. The higher the score, the higher the likelihood of the specific pair being selected in a replicative study. For example, the leftmost column in the alpha range (FC2–C1) represents an 80% chance of being found significant again in similar studies
Predictive and explanatory analysis
After extraction of the functional connectivity values, the data analysis is provided in two phases: (i) using the multivariate analysis and the receiver operating characteristic (ROC) curve representation, the aim was to show that those indicators (functional connections) can act as a sole biomarker for separating the painful and non‐painful DPN patients; (ii) by a statistical examination of the selected indicators (i.e., features used in the classification decision) and traditional univariate methods, an effort was made to explain the classification outcomes and comprehend their clinical associates.
Predictive, multivariate analysis
In essence, this type of analysis aims to look at the brain from the perspective of a dynamic system or a pattern, as opposed to the traditional EEG based approaches analysing activity in terms of time and frequency domains in each brain area independently. The classification aims to find a multivariate activation pattern between those electrode pairs that can be reliably associated with distinguishing painful and non‐painful polyneuropathy, more specifically a support vector machine as presented in [31] with C‐SVC (Support Vector Classification) kernel type and the Radial Basis Classification Function (i.e., Gaussian kernel). The major advantage of the method lies in its generalization ability on small and imbalanced datasets. Our recently published methodological paper describes more details and extensively discusses the explanatory and predictive methodologies [32].
Explanatory analysis
The connectivity values of the 10 selected functional connections and their average value in each band between painful and non‐painful participants (two‐sample t test for normally distributed parameters and Mood's median test for abnormally distributed parameters) were compared. After correcting each band for multiple comparisons using Bonferroni correction, the connections found significantly different between the two groups were used for correlation analysis with clinical parameters (i.e., average self‐reported pain intensity in the previous 3 months). In order to correct for multiple comparisons, multiplication by the number of analysed variables (10 in our case) was carried out. JMP (SAS Institute) was used for statistical analyses. When applicable, demographic and clinical parameters were compared using the t test or via non‐parametric tests.
RESULTS
Patient characteristics
Two hundred patients with DPN were recruited to the study; 180 of them had an EEG recording at rest adequate for analysis; six patients refused EEG, for five patients EEG was not recorded due to technical malfunction and nine recordings were unusable due to poor quality. Of these 180, 133 patients were classified as having painful diabetic neuropathy and 47 as non‐painful based on the above described classifying question (Table 1). It is noted that the protocol included several questionnaires, and some of the patients were classified as non‐painful based on the classifying question, although they did indicate some level of pain in other questionnaires. The painful patients had expectedly higher scores in the TCNS, probably due to the pain‐related components of this score (Table 1). Both groups had similar diabetes duration and severity of the neuropathy, as reflected by the similar foot sensory thresholds.
TABLE 1.
Participant characteristics
| Total | Painful DPN | Non‐painful DPN | p value, painful vs. non‐painful | |
|---|---|---|---|---|
| N | 180 | 133 | 47 | |
| Demographic parameters | ||||
| Female (N, %) | 50 (27.7) | 41 (30.8) | 9 (19.5) | 0.1149 |
| Age in years (mean, SD) | 64.5 (10.1) | 63.3 (10.85) | 67.9 (6.93) | 0.0074* |
| Clinical parameters | ||||
| TCNS (mean, SD) | 10.73 (4.36) | 11.223 (4.4) | 9.31 (3.93) | 0.0093* |
| Diabetes duration in years (median, range) | 15 (0–46) | 15 (0.3–46) | 18 (0–44) | 0.4245 |
| Average foot pain rating (on a 0–10 numerical pain rating scale) 24 h prior to testing (mean, SD) | 3.25 (2.88) | 4.47 (2.5) | 0.48 (1.28) | <0.0001* |
| DFNS quantitative sensory testing on the foot | ||||
| Cold detection threshold (mean, SD) | −14.05 (9.5) | −14.72 (9.91) | −12.58 (8.42) | 0.1895 |
| Warm detection threshold (mean, SD) | 13.16 (3.87) | 13.26 (3.93) | 12.99 (3.74) | 0.6861 |
| Vibration detection threshold (mean, SD) | 5.05 (2.18) | 4.97 (2.12) | 5.16 (2.45) | 0.6128 |
Abbreviations: DFNS, German Research Network on Neuropathic Pain; DPN, diabetic polyneuropathy; TCNS, Toronto Clinical Neuropathy Score.
p value < 0.05.
Variable selection
This analysis scheme is based on the 10 functional connections most accurately distinguishing between painful and non‐painful DPN patients (selected by the ReliefF algorithm, see the section Signal analysis). First, the consistency of the selected connections was demonstrated. Figure 2 presents a histogram of the selected functional connections after cross‐validation via 300 iterations, with 20% of the randomly excluded participants at each iteration. It can be seen that there is a monotonous decrease in repeatability with an increasing number of electrode pairs, such that the number of functional connections in the analysis must be limited to prevent excessive noise.
Multivariate, predictive analysis
The classification results based on the 10 selected functional connections are presented in terms of a cross‐validated ROC curve presenting mean and standard deviation confidence intervals for each frequency band (Figure 3).
FIGURE 3.
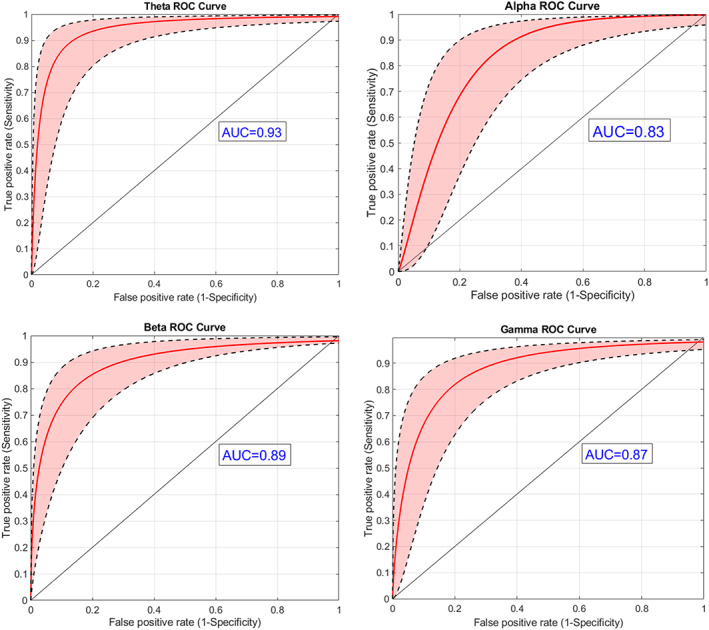
Grouping of painful/non‐painful patients based on brain connectivity: ROC analysis (red line), with standard deviation confidence intervals (black broken lines) based on the 10 selected functional connections for each one of the frequency bands. The black diagonal line indicates an AUC of 0.5. It is evident that the theta and beta bands contain most of the information required for discrimination between painful and non‐painful neuropathy patients as reflected in the ROC curve AUC values: 0.93 for the theta band and 0.895 for the beta band. Nevertheless, the discriminability based on alpha and gamma data was relatively high as well, with AUCs of 0.83 and 0.87, respectively
Explanatory, univariate statistical analysis
Per‐band univariate statistical analysis revealed that painful patients, on average, had higher connectivity values compared to non‐painful patients (Figure 4a). The differences in the averaged connectivity values from the 10 most discriminating functional connections per band were statistically significant with p < 0.05 after the Bonferroni correction in the theta and alpha frequency bands (see Figure 4a).
FIGURE 4.
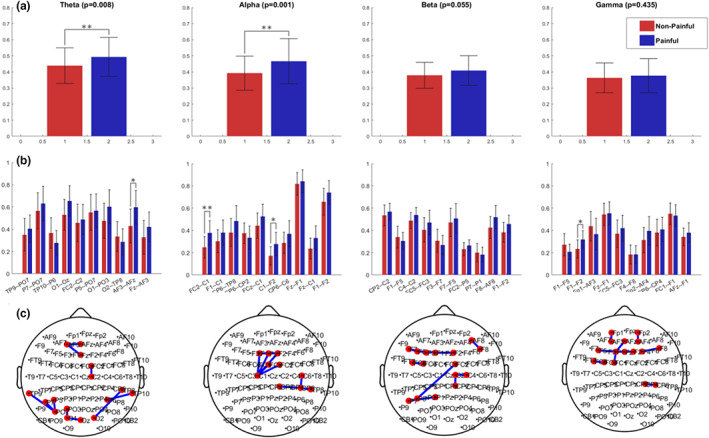
(a) Average connectivity values of the 10 most differentiating connections for the painful (red) and non‐painful (blue) DPN patients; (b) within‐band particular functional connections average values; (c) the cortical locations of those functional connections. Asterisks indicate significant connections after Bonferroni correction (*p < 0.05; **p < 0.005)
Intra‐band analysis of the 10 most discriminating pairs in each band showed significantly higher connectivity in painful patients in the following electrode pairs: AF3–AFZ in the theta band, FC2–C1 and C1–F2 in the alpha band, and between F1 and F2 in the gamma band (Figure 4b). In addition, these pairs appeared to have a positive correlation to the average reported pain in the previous 3 months in all frequency bands (see Figure 5).
FIGURE 5.
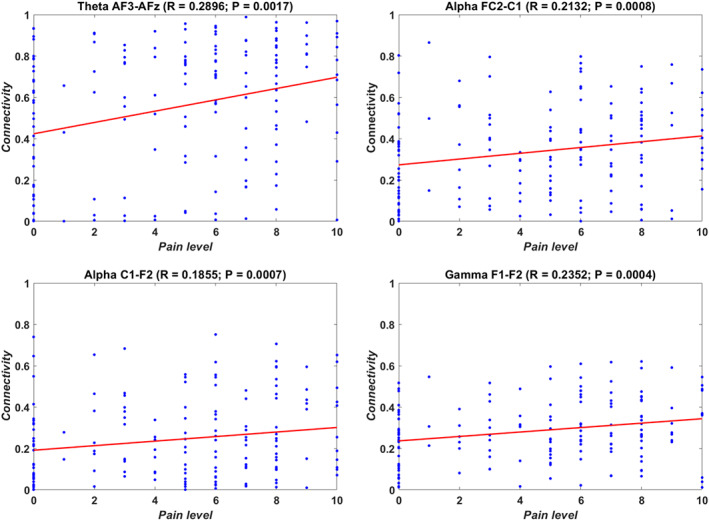
Correlations between connectivity values of selected electrode couples and average self‐reported pain intensity in the previous 3 months on a numerical pain rating scale
DISCUSSION
This study presents a data‐driven, explanatory and predictive, multivariate approach to EEG connectivity analysis to discriminate painful from non‐painful patients in DPN. Machine learning on resting state EEG recordings was applied to achieve a remarkably high discriminatory capability between those with and those without neuropathic pain.
Electroencephalography functional connectivity is a relatively new approach in pain research, shifting from searching for locally activated areas of the cortex towards identifying functional networks across the whole brain surface. With the advent of our computational abilities and the interest in central pain processing, advanced EEG analysis has become an important research tool in recent years. The field has advanced from simple frequency domain analyses such as peak alpha frequency and power to a multivariate approach. This is a step forward for EEG analyses, parallel to the many MRI based brain connectivity studies.
Generally, the connectivity values were higher amongst painful DPN patients than non‐painful ones, in line with previous EEG studies [17], suggesting that a certain level of ‘cross‐talk’ between brain regions is probably required to perceive pain. This is compatible with the fact that many brain regions are activated by pain and there is an apparent expectation for communication amongst them. In line, there is a likelihood of lower expression of clinical pain in the setup of a less ‘internally connected’ brain.
The most impressive classification results were observed in theta and beta bands. Our main finding is the outstanding ability of the connectivity between the 10 best discriminating pairs of electrodes to discriminate between painful and non‐painful DPN. It is conventional to rate the coefficients of the area under the curve (AUC) between 0.6 and 0.7 as ‘poor test’, 0.7 and 0.8 as ‘fair test’, 0.8 and 0.9 as ‘good test’ and 0.9 and 1 as ‘excellent test’ (AUC of 1 considered as ‘perfect test’) [33]. In our case, a discriminatory capability of AUC = 0.93 for the theta band and AUC = 0.89 for the beta band is an excellent level of discriminatory capability. In practical terms, it indicates that an individual patient's classification as painful or non‐painful based solely on their resting state EEG will be correct in 93% of cases.
The differences in brain activity between these two groups suggest that the brain construct contributes to the generation of the clinical pain picture beyond and in addition to the pain generators in the peripheral nerve itself. Causative relations cannot be explored in a cross‐sectional study; the altered brain function in the painful group could be either a consequence of the chronic peripherally generated pain or the cause of pain levels due to the peripheral activity. It is noted that most of the connectivity pairs represent local short‐distance functional connections, mainly within the posterior (centro‐parietal) ‘sensing’ brain regions and frontal (including the mid‐frontal) ‘controlling’ brain regions. Our findings on the increased frontal theta/alpha connectivity in chronic neuropathic pain patients align with those of chronic low back pain patients [17] and fibromyalgia [34, 35]. Furthermore, similar to the connectivity patterns in our study, these studies emphasized the predominance of short‐distance association U‐fibres at theta and alpha frequencies indicating the importance of ‘cross‐talk’ between neighbour brain areas in the expression and regulation of pain. Finally, the described connectivity topography findings are supported by reports from animal chronic pain models on the broadband increase in oscillations from theta to beta frequencies in the primary somatosensory and medial prefrontal cortices (summarized in [36]).
Most previous studies applying machine learning classification to EEG data have been done on healthy controls, on spectral/temporal data or both, not taking into account the functional interactions, for example information transfer between brain areas which is believed to play an essential role in generating the clinical pain picture. One exception is a recent clinical study on connectivity data [17] distinguishing between chronic pain patients and healthy controls. Ta Dinh and colleagues [17] reached a relatively low average classification rate of 57%. It is believed that this might have resulted from (i) high cohort heterogeneity and (ii) the high ratio between the initial number of variables to sample size to levels that makes it difficult to compensate even by feature selection methods. In their study, spectral data did not differ between chronic pain patients and healthy control groups, whereas connectivity features did; in line with our findings, they reported significantly increased connectivity at theta and gamma frequencies in frontal brain areas. These findings support the hypothesis that pain processing is reflected in functional connectivity and not only in local activity. To overcome these challenges, a larger sample size of patients was used with the same diagnosis and the number of functional connections used as parameters for analysis was limited to 10.
Unlike our resting state EEG based study, other researchers applied machine learning on pain evoked potentials with promising results [37, 38, 39]. The analysis is based on experimentally induced pain perception data in these studies, not on ongoing clinical pain [12]. Our study substantially expands the concept, as a stimulus‐free state highlighting clinical pain rather than stimulus‐evoked pain was studied.
Significantly higher connectivity in painful patients was demonstrated in the following electrode pairs: AF3–AFZ in the theta band, FC2–C1 and C1–F2 in the alpha band, and between F1 and F2 in the gamma band. These findings align with resting state functional MRI connectivity studies that investigated pain processing in patients with chronic pain [40]. Several studies identified alterations in default mode network connectivity, a network of brain regions including the precuneus, posterior cingulate cortex, medial prefrontal cortex and angular gyrus [41, 42]. Higher connectivity between areas of this network suggests a hyperactive state in chronic pain. To the best of our knowledge, no resting state functional MRI connectivity studies have investigated the differences between painful and non‐painful DPN patients.
An effort was made to establish clinical–physiological correlations for our study's EEG connectivity data by exploring the connectivity values of the significant electrode pairs versus clinical parameters. It was found that connectivity between some differentiating electrode pairs in most frequency bands is correlated to the severity of clinical pain. A possible explanation could be that two forces drive this increased connectivity: on the one hand, the presence of peripheral sensory loss might be compensated by better communication between brain sites; on the other, higher pain levels might also cause increased connectivity. A longitudinal study exploring the relationship between the severity of the neuropathy and levels of brain connectivity might resolve the question of the variation in pain expression in patients with similar levels of neuropathy severity. Suppose the development of pain in previously non‐painful neuropathy patients is preceded by high brain connectivity. In that case, it will tell whether brain connectivity is a co‐generator or a consequence of the neuropathy‐induced pain.
Scalp versus source analysis
In recent years, source analysis has gained increasing attention due to its ability to reliably represent sources, that is, anatomical locations of the scalp recorded signals. This is mainly due to better handling of scalp conduction noise problem, as happens when more than one electrode channel can pick up the activity of the underlying source. Whilst for some signal analysis methods this phenomenon might be considered noise, the primary focus of this work is on the detection of (i) a reliable, (ii) objective and (iii) easy to apply biomarker. Thus, an ‘information‐wise’ point of view was adopted: this noise can be thought of as additional (and valuable) information. In this sense, on the one hand, the proposed method has limited accuracy on sources of activity. However, on the other hand, it can lower the number of required electrodes in its clinical application. This contrasts with the source analysis methods that always require the same high number of electrodes, usually not available in clinical use.
Limitations of this study
Several limitations of this study need to be pointed out. First, source localization algorithms were not incorporated in our analysis scheme (see the above discussion regarding the limitations of the coherence estimate); thus, our ability to relate brain regions to electrode sites is possibly limited. This study shows that although pain‐relevant information, that is, the signal‐to‐noise ratio, is clearly identified (see the t statistic presented in Figure 4), the automatically selected electrode pairs might be different (see Figure 2) in similar studies. Much larger studies are required to establish the definite functional connections responsible for discrimination between painful and non‐painful polyneuropathy patients. Future studies should explore a more accurate localization of the sources for which connectivity is studied. Secondly, patients had neuropathic pain, so it is unclear whether our results are unique to neuropathic pain or, most likely, can be generalized to all classes of pain. Lastly, in our cohort, non‐painful participants were older than the painful ones, which might confound connectivity differences between the groups. However, the average age difference between the groups is only 4 years which is unlikely to explain connectivity differences. Indeed, after balancing the age groups by excluding several young participants from the painful group (see Figure S1), the age feature did not contribute to the classification analysis (see Figure S2).
In conclusion, a new EEG based biomarker for clinical pain with high accuracy in discriminating painful from non‐painful diabetic neuropathy is reported. It is believed that our approach is expandable to other pain syndromes, providing a strong and objective tool for pain research.
AUTHOR CONTRIBUTIONS
Leah Shafran Topaz: investigation, data curation, formal analysis, writing—original draft, writing—review and editing. Alex Frid: methodology, software, formal analysis, visualization, writing—original draft. Yelena Granovsky: funding acquisition, investigation, supervision, writing—original draft. Rabab Zubidat: investigation, project administration, data curation. Shoshana Crystal: investigation, data curation. Chen Buxbaum: investigation, writing—reviewing and editing. Noam Bosak: investigation, writing—reviewing and editing. Rafi Hadad: investigation, writing—reviewing and editing. Erel Domany: investigation, writing—reviewing and editing. Tayir Alon: investigation, writing—reviewing and editing. Lian Meir Yalon: investigation, data curation. Merav Shor: project administration, investigation. Mogher Khamaisi: resources, writing—reviewing and editing. Irit Hochberg: resources, writing—reviewing and editing. Nataliya Yarovinsky: resources, writing—reviewing and editing. Zeev Volkovich: software, formal analysis. David L. Bennett: conceptualization, writing—reviewing and editing. David Yarnitsky: conceptualization, methodology, funding acquisition, writing—reviewing and editing.
FUNDING INFORMATION
Study funded by European Union's Horizon 2020 research and innovation programme no. 633491 (DOLORisk).
CONFLICT OF INTEREST
No authors declare a conflict of interest related to this work.
Supporting information
Figure S1
Figure S2
ACKNOWLEDGEMENTS
The collected data are available to all members of the DOLORisk consortium and can be received by contacting the consortium.
Topaz LS, Frid A, Granovsky Y, et al. Electroencephalography functional connectivity—A biomarker for painful polyneuropathy. Eur J Neurol. 2023;30:204‐214. doi: 10.1111/ene.15575
Trial registration: NIH clinical trial identifier number NCT02402361.
DATA AVAILABILITY STATEMENT
The collected data is available to all members of the DOLORisk consortium and can be received by contacting the consortium.
REFERENCES
- 1. Hansen TM, Mark EB, Olesen SS, Gram M, Frøkjær JB, Drewes AM. Characterization of cortical source generators based on electroencephalography during tonic pain. J Pain Res. 2017;10:1401‐1409. doi: 10.2147/JPR.S132909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Furman AJ, Meeker TJ, Rietschel JC, et al. Cerebral peak alpha frequency predicts individual differences in pain sensitivity. Neuroimage. 2018;167:203‐210. doi: 10.1016/J.NEUROIMAGE.2017.11.042 [DOI] [PubMed] [Google Scholar]
- 3. Pinheiro ES, de Queirós FC, Montoya P, et al. Electroencephalographic patterns in chronic pain: a systematic review of the literature. Schalk G, ed. PLoS ONE. 2016;11(2):e0149085. doi: 10.1371/journal.pone.0149085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ploner M, May ES. Electroencephalography and magnetoencephalography in pain research—current state and future perspectives. Pain. 2018;159(2):206‐211. doi: 10.1097/j.pain.0000000000001087 [DOI] [PubMed] [Google Scholar]
- 5. Boord P, Siddall PJ, Tran Y, Herbert D, Middleton J, Craig A. Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord. 2008;46(2):118‐123. doi: 10.1038/SJ.SC.3102077 [DOI] [PubMed] [Google Scholar]
- 6. van den Broeke EN, Wilder‐Smith OHG, van Goor H, Vissers KCP, van Rijn CM. Patients with persistent pain after breast cancer treatment show enhanced alpha activity in spontaneous EEG. Pain Med. 2013;14(12):1893‐1899. doi: 10.1111/pme.12216 [DOI] [PubMed] [Google Scholar]
- 7. Vuckovic A, Hasan MA, Fraser M, Conway BA, Nasseroleslami B, Allan DB. Dynamic oscillatory signatures of central neuropathic pain in spinal cord injury. J Pain. 2014;15(6):645‐655. doi: 10.1016/J.JPAIN.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang N, Li G, Wei J, Wei B, Zhu FF, Hu Y. Transcranial direct current stimulation of the primary motor cortex on postoperative pain and spontaneous oscillatory electroencephalographic activity following lumbar spine surgery: a pilot study. Restor Neurol Neurosci. 2018;36(5):605‐620. doi: 10.3233/RNN-180816 [DOI] [PubMed] [Google Scholar]
- 9. Navid MS, Lelic D, Niazi IK, et al. The effects of chiropractic spinal manipulation on central processing of tonic pain—a pilot study using standardized low‐resolution brain electromagnetic tomography (sLORETA). Sci Rep. 2019;9(1):6925. doi: 10.1038/S41598-019-42984-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129(1):55‐64. doi: 10.1093/brain/awh631 [DOI] [PubMed] [Google Scholar]
- 11. Vučković A, Altaleb MKH, Fraser M, McGeady C, Purcell M. EEG correlates of self‐managed neurofeedback treatment of central neuropathic pain in chronic spinal cord injury. Front Neurosci. 2019;13:762. doi: 10.3389/fnins.2019.00762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vijayakumar V, Case M, Shirinpour S, He B. Quantifying and characterizing tonic thermal pain across subjects from EEG data using random forest models. IEEE Trans Biomed Eng. 2017;64(12):2988‐2996. doi: 10.1109/TBME.2017.2756870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gram M, Graversen C, Olesen AE, Drewes AM. Machine learning on encephalographic activity may predict opioid analgesia. Eur J Pain. 2015;19(10):1552‐1561. doi: 10.1002/EJP.734 [DOI] [PubMed] [Google Scholar]
- 14. Gram M, Erlenwein J, Petzke F, et al. Prediction of postoperative opioid analgesia using clinical‐experimental parameters and electroencephalography. Eur J Pain. 2017;21(2):264‐277. doi: 10.1002/EJP.921 [DOI] [PubMed] [Google Scholar]
- 15. Vuckovic A, Gallardo VJF, Jarjees M, Fraser M, Purcell M. Prediction of central neuropathic pain in spinal cord injury based on EEG classifier. Clin Neurophysiol. 2018;129(8):1605‐1617. doi: 10.1016/J.CLINPH.2018.04.750 [DOI] [PubMed] [Google Scholar]
- 16. Necka EA, Lee I‐S, Kucyi A, Cheng JC, Yu Q, Atlas LY. Applications of dynamic functional connectivity to pain and its modulation. Pain Rep. 2019;4(4):e752. doi: 10.1097/PR9.0000000000000752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ta Dinh S, Nickel MM, Tiemann L, et al. Brain dysfunction in chronic pain patients assessed by resting‐state electroencephalography. Pain. 2019;160(12):2751‐2765. doi: 10.1097/J.PAIN.0000000000001666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nickel MM, Ta Dinh S, May ES, et al. Neural oscillations and connectivity characterizing the state of tonic experimental pain in humans. Hum Brain Mapp. 2020;41(1):17‐29. doi: 10.1002/HBM.24784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pascal MMV, Themistocleous AC, Baron R, et al. DOLORisk: study protocol for a multi‐centre observational study to understand the risk factors and determinants of neuropathic pain. Wellcome Open Res. 2019;3:63. doi: 10.12688/wellcomeopenres.14576.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285‐2293. doi: 10.2337/dc10-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Granovsky Y, Shafran Topaz L, Laycock H, et al. Conditioned pain modulation is more efficient in patients with painful diabetic polyneuropathy than those with non‐painful diabetic polyneuropathy. Pain. 2022;163(5):827‐833. doi: 10.1097/J.PAIN.0000000000002434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599‐1606. doi: 10.1097/j.pain.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carter GC, Knapp CH, Nuttall AH. Estimation of the magnitude‐squared coherence function via overlapped fast Fourier transform processing. IEEE Trans Audio Electroacoust. 1973;21(4):337‐344. doi: 10.1109/TAU.1973.1162496 [DOI] [Google Scholar]
- 24. Kira K, Rendell LA. A practical approach to feature selection. Mach Learn Proc 1992. Published online January 1;1992:249–256. doi: 10.1016/B978-1-55860-247-2.50037-1 [DOI]
- 25. MATLAB . Version 7.10.0 (R2010a). The MathWorks Inc.; 2010. [Google Scholar]
- 26. Lau WS, Du L, Yu DQ, et al. Overview of image smoothing algorithms. J Phys Conf Ser. 2021;1883(1):12024. doi: 10.1088/1742-6596/1883/1/012024 [DOI] [Google Scholar]
- 27. Bellman R. Adaptive control processes: A Guided Tour. Princeton University Press; 1961. [Google Scholar]
- 28. Fort G, Lambert‐Lacroix S. Classification using partial least squares with penalized logistic regression. Bioinformatics. 2005;21(7):1104‐1111. doi: 10.1093/BIOINFORMATICS/BTI114 [DOI] [PubMed] [Google Scholar]
- 29. Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12(5):535‐540. doi: 10.1038/NN.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frid A, Manevitz LM, Nawa NE. Classifying the valence of autobiographical memories from fMRI data. Ann Math Artif Intell. 2020;88(11):1261‐1274. doi: 10.1007/S10472-020-09705-3 [DOI] [Google Scholar]
- 31. Chang C‐C, Lin C‐J. LIBSVM: A Library for Support Vector Machines. Accessed May 6, 2022. www.csie.ntu.edu.tw/
- 32. Frid A, Shor M, Shifrin A, Yarnitsky D, Granovsky Y. A biomarker for discriminating between migraine with and without aura: machine learning on functional connectivity on resting‐state EEGs. Ann Biomed Eng. 2020;48(1):403‐412. doi: 10.1007/S10439-019-02357-3 [DOI] [PubMed] [Google Scholar]
- 33. Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression, 3rd ed. Wiley Ser Probab Stat; 1989:528. Accessed May 6, 2022. https://www.wiley.com/en‐gb/Applied+Logistic+Regression%2C+3rd+Edition‐p‐9780470582473 [Google Scholar]
- 34. Vanneste S, Ost J, Van Havenbergh T, De Ridder D. Resting state electrical brain activity and connectivity in fibromyalgia. PLoS ONE. 2017;12(6):e0178516. doi: 10.1371/journal.pone.0178516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martín‐Brufau R, Gómez MN, Sanchez‐Sanchez‐rojas L, Nombela C. Fibromyalgia detection based on EEG connectivity patterns. J Clin Med. 2021;10(15):3277. doi: 10.3390/JCM10153277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ploner M, Sorg C, Gross J. Brain rhythms of pain. Trends Cogn Sci. 2017;21(2):100‐110. doi: 10.1016/j.tics.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang G, Xiao P, Hung YS, Zhang ZG, Hu L. A novel approach to predict subjective pain perception from single‐trial laser‐evoked potentials. Neuroimage. 2013;81:283‐293. doi: 10.1016/j.neuroimage.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 38. Misra G, Wang W‐E, Archer DB, Roy A, Coombes SA. Automated classification of pain perception using high‐density electroencephalography data. J Neurophysiol. 2017;117(2):786‐795. doi: 10.1152/jn.00650.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schulz E, Zherdin A, Tiemann L, Plant C, Ploner M. Decoding an individual's sensitivity to pain from the multivariate analysis of EEG data. Cereb Cortex. 2012;22(5):1118‐1123. doi: 10.1093/cercor/bhr186 [DOI] [PubMed] [Google Scholar]
- 40. Martucci KT, MacKey SC. Neuroimaging of pain: human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology. 2018;128(6):1241‐1254. doi: 10.1097/ALN.0000000000002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kucyi A, Moayedi M, Weissman‐Fogel I, et al. Enhanced medial prefrontal‐default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci. 2014;34(11):3969‐3975. doi: 10.1523/JNEUROSCI.5055-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martucci KT, Shirer WR, Bagarinao E, et al. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network. A resting‐state study from the MAPP research network. Pain. 2015;156(9):1755‐1764. doi: 10.1097/J.PAIN.0000000000000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Data Availability Statement
The collected data is available to all members of the DOLORisk consortium and can be received by contacting the consortium.


