Abstract
Aims
To assess any disparities in the initiation of second‐line antidiabetic treatments prescribed among people with type 2 diabetes mellitus (T2DM) in England according to ethnicity and social deprivation level.
Materials and methods
This cross‐sectional study used linked primary (Clinical Practice Research Datalink) and secondary care data (Hospital Episode Statistics), and the Index of Multiple Deprivation (IMD). We included people aged 18 years or older with T2DM who intensified to second‐line oral antidiabetic medication between 2014 and 2020 to investigate disparities in second‐line antidiabetic treatment prescribing (one of sulphonylureas [SUs], dipeptidyl peptidase‐4 [DPP‐4] inhibitors, or sodium‐glucose cotransporter‐2 [SGLT2] inhibitors, in combination with metformin) by ethnicity (White, South Asian, Black, mixed/other) and deprivation level (IMD quintiles). We report prescriptions of the alternative treatments by ethnicity and deprivation level according to predicted percentages derived from multivariable, multinomial logistic regression.
Results
Among 36 023 people, 85% were White, 10% South Asian, 4% Black and 1% mixed/other. After adjustment, the predicted percentages for SGLT2 inhibitor prescribing by ethnicity were 21% (95% confidence interval [CI] 19–23%), 20% (95% CI 18–22%), 19% (95% CI 16–22%) and 17% (95% CI 14–21%) among people with White, South Asian, Black, and mixed/other ethnicity, respectively. After adjustment, the predicted percentages for SGLT2 inhibitor prescribing by deprivation were 22% (95% CI 20–25%) and 19% (95% CI 17–21%) for the least deprived and the most deprived quintile, respectively. When stratifying by prevalent cardiovascular disease (CVD) status, we found lower predicted percentages of people with prevalent CVD prescribed SGLT2 inhibitors compared with people without prevalent CVD across all ethnicity groups and all levels of social deprivation.
Conclusions
Among people with T2DM, there were no substantial differences by ethnicity or deprivation level in the percentage prescribed either SGLT2 inhibitors, DPP‐4 inhibitors or SUs as second‐line antidiabetic treatment.
Keywords: ethnicity, oral antidiabetics, pharmacoepidemiology, socioeconomic deprivation, type 2 diabetes
1. INTRODUCTION
Most healthcare systems report inequities in disease incidence, healthcare delivery and outcomes according to people's socioeconomic status and ethnicity. 1 , 2 For countries with single‐payer systems such as England, national recommendations from agencies like the National Institute for Health and Care Excellence (NICE) encourage access to effective and cost‐effective interventions to maximize clinical benefit while also reducing health inequalities. 3 , 4 , 5 Nonetheless in countries such as England, inequities in using healthcare interventions according to people's socioeconomic characteristics persist for diseases such as cardiovascular disease (CVD), 6 chronic kidney disease (CKD), 7 and type 2 diabetes mellitus (T2DM). 8
There are inequities in T2DM prevalence and outcomes according to ethnicity 8 and deprivation. 9 People of Black and South Asian ethnicity, and people with lower income or lower educational attainment have a higher prevalence of T2DM, worse blood glucose control and earlier onset of macro‐ and microvascular complications compared with people of ethnicities other than Black and South Asian, higher incomes or higher educational attainment. 8 , 9 , 10 , 11 , 12 , 13 Ethnic minorities also tend to experience delays in T2DM treatment intensification when clinically indicated (therapeutic inertia), 12 which may contribute to worse outcomes compared with White people. 14 , 15 , 16 , 17 , 18 Other ethnic and socioeconomic inequities in T2DM treatment that could impact clinical outcomes, such as the type of second‐line antidiabetic treatment prescribed at treatment intensification from metformin monotherapy, are less well understood. Hence, we chose to examine the potential disparities in second‐line antidiabetic treatment prescribing by ethnicity and deprivation status.
For people with T2DM whose glycated haemoglobin (HbA1c) levels are poorly controlled, an important choice is which second‐line oral antidiabetic therapy to prescribe in addition to metformin. 19 Between 2015 and 2021, NICE technology appraisals and clinical guidelines recommended that, for most people with T2DM, several second‐line oral treatment options should be available, including sodium‐glucose cotransporter 2 (SGLT2) inhibitors, dipeptidyl peptidase‐4 (DPP‐4) inhibitors or the lower‐cost option of sulphonylureas (SUs). 19 , 20 Updated NICE guidelines (2022) recommend SGLT2 inhibitors for individuals at high risk of or with prevalent CVD but that, for other eligible patients, any of these three treatments may be suitable. 21 The decision to allow local discretion in the choice of second‐line treatment may reflect the uncertainty over comparative effectiveness and cost‐effectiveness of these three treatment choices, which is partly related to the lack of randomized controlled trials (RCTs) providing head‐to‐head comparisons of these three oral antidiabetic drugs. In contrast, international diabetes guidance and consensus reports recommend SGLT2 inhibitors for people with established atherosclerotic CVD, heart failure and CKD, 22 irrespective of the additional costs of SGLT2 inhibitors compared to SUs, drawing on evidence from placebo‐controlled RCTs showing improved CVD and kidney disease outcomes when prescribing SGLT2 inhibitors.
Previous research has found wide variation in clinical practice in the United Kingdom (UK) in the choice of second‐line oral antidiabetic treatment. 23 , 24 However, no previous study has considered whether disparities exist in prescriptions of this second‐line treatment according to ethnicity and socioeconomic status. We aimed to assess whether ethnic minorities and people with higher deprivation status had a lower probability of being prescribed SGLT2 inhibitors compared with DPP‐4 inhibitors or the lower‐cost SUs, both overall and by prevalent CVD status.
2. METHODS
2.1. Study design and setting
We conducted a cross‐sectional study, nested within the Personalized Medicine for Intensification of Treatment in people with T2DM (PERMIT) cohort study, 25 to investigate disparities in second‐line antidiabetic treatment prescribed to people with T2DM by ethnicity and by deprivation status. Data sources included the Clinical Practice Research Datalink (CPRD) Gold and Aurum datasets (primary care), Hospital Episode Statistics (HES; secondary care), the Index of Multiple Deprivation (IMD), and the Office of National Statistics (ONS) death data.
The CPRD is a large, population‐based dataset covering approximately 20% of the UK population and is representative in terms of age, sex and ethnicity. 26 , 27 These data include clinical diagnoses, laboratory test results, and prescribing information recorded in primary care. Linkage of CPRD data to HES data is available for approximately 80% of people in the CPRD registered at general practices in England. HES data include diagnoses and demographic information related to all NHS‐funded hospitalizations. 28 The IMD is commonly used in epidemiological research as a proxy for socioeconomic status in England. It ranks individuals according to deprivation status based on their postcode, and is usually reported in quintiles (1 being the least deprived, 5 being most deprived). 29 ONS mortality data include information on all deaths registered in England and Wales. 30
2.2. Patient and public involvement
One patient and public (PP) representative (P.C.) was involved in this study's design, provided feedback on this manuscript, and is a co‐author. The PERMIT study protocol describes PP contributions to the study design. 25 PP representatives will assist with drafting lay summaries, which we will share on the study website (https://www.lshtm.ac.uk/research/centres-projects-groups/permit) and at study workshops with a wider group of multi‐ethnic PP representatives. We will work with the Centre for Ethnic Health Research, led by co‐author K.K., to make culturally adapted lay summaries.
2.3. Study population
We included people aged 18 years or older with a T2DM diagnosis, in whom incident second‐line oral antidiabetic treatment was prescribed for the first time between January 1, 2014 and March 31, 2020 after first‐line antidiabetic treatment with metformin monotherapy. We used the complete historical general practice (GP) electronic health record to ensure this was the first time each person had a record of being prescribed an SU, a DPP‐4 inhibitor or an SGLT2 inhibitor. The second‐line therapy, an SU, a DPP‐4 inhibitor or an SGLT2 inhibitor, had to have been added on to metformin, and had not replaced it. These three treatments constituted approximately 99% of the second‐line treatments prescribed, therefore, other second‐line antidiabetic treatments were excluded from this study. 23 , 31 Eligible people had to have had a prescription for metformin monotherapy within 60 days prior to the first prescription for second‐line treatment to ensure they were continuous users of metformin monotherapy prior to intensification. Also, to ensure the second‐line treatments were an addition to, rather than a switch from, metformin, the individuals were required to have been prescribed metformin on the same day or within 60 days after the first prescription for the second‐line antidiabetic treatment.
We excluded women with a record of pregnancy within 12 months prior to second‐line treatment initiation since antidiabetic prescribing guidelines are different for this population. 19 We also excluded people whose last recorded estimated glomerular filtration rate (eGFR) was less than 30 ml/min/1.73 m2 since metformin is contraindicated in this group, and SGLT2 inhibitors are not recommended for this group in the United Kingdom for the purpose of lowering blood glucose. 19 , 32
2.4. Definitions of ethnicity and deprivation
We defined ethnicity according to clinical and demographic codes recorded within the CPRD or linked HES data prior to or on the same day as the first‐ever prescription date for one of the three second‐line antidiabetic treatments of interest, that is, the index date. Ethnicity was grouped into 16 categories in primary care and 11 categories in secondary care, which we further re‐grouped as the following: (1) White, (2) South Asian, (3) Black, and (4) Mixed/other (Table S1). We considered this re‐grouping necessary to ensure sufficient sample sizes within each ethnic group, and to follow precedent studies using the same data sources, 12 , 33 , 34 as well as the ethnic groupings used in the 2011 England and Wales census. 35 If the two sources for ethnicity provided different categorizations, then we used ethnicity as defined in the CPRD since these data have been shown to be more reliable than HES inpatient data. 33 If no ethnicity data were available within the CRPD, we categorized ethnicity using HES data. If ethnicity was not recorded in either source, we considered ethnicity as missing and the individual was excluded from the complete case analyses.
We used the small area IMD to define deprivation. The IMD combines seven indices which capture dimensions of deprivation at the Lower‐Layer Super Output Area or neighbourhood level, and ranks each neighbourhood from 1 to 32 844. 29 Neighbourhood rankings were divided into quintiles and used to compare relative levels of deprivation among people in this study living in different neighbourhoods in England.
We also considered how the proportion of patients receiving the alternative second‐line treatments may differ according to calendar time, recognizing that the dissemination and awareness of the safety and efficacy of SGLT2 inhibitors for patients with T2DM increased over the time period, with the publication of important RCT results. 36 , 37 , 38 We considered this hypothesis in grouping calendar time into years 2014, 2015 to 2016, 2017 to 2018, and 2019 to 2020.
2.5. Covariates
We adjusted for several additional variables, derived from data captured before or on the same day as the index date. These were sex, age, duration of time on metformin monotherapy, number of patients registered at the individual's general practice, geographic region, co‐prescriptions for renin‐angiotensin system inhibitors and/or statins, history of proteinuria, history of hypoglycaemia, clinical measures (body mass index [BMI] and HbA1c), smoking status, alcohol intake, and comorbidities at the time of second‐line antidiabetic treatment initiation. Comorbidities included CKD stage (no known CKD, stages 1, 2, 3a, and 3b, assigned using the latest recorded eGFR), cancer (any), blindness, congestive heart failure, previous myocardial infarction (MI), unstable angina, previous stroke, other ischaemic heart disease, and uncontrolled hypertension based on the most recent blood pressure measures recorded in primary care. We defined prevalent CVD as a composite of heart failure, ischaemic heart disease, unstable angina, previous myocardial infarction, or previous stroke.
2.6. Treatment prescribed
Our dependent variable of interest was incident second‐line oral antidiabetic treatment prescribed (SUs, DPP‐4 inhibitors, or SGLT2 inhibitors, in addition to metformin), defined using CPRD prescribing data.
2.7. Analysis
We described baseline characteristics of the study population stratified by ethnicity and IMD. We then built mixed‐effect multivariable, multinomial logistic regression models which compared the odds of initiating SGLT2 inhibitors and DPP‐4 inhibitors versus SUs (reference outcome), as well as, in a separate model, SGLT2 inhibitors versus DPP‐4 inhibitors (reference outcome), first adjusting for just age and sex. In the final adjusted model, we adjusted for all covariates, as well as mutual adjustment for ethnicity and deprivation (fixed effects) and clustering at the Clinical Commissioning Group (CCG) level (random effect). Because odds ratios can be misleading, particularly when the outcome is common, 39 we calculated and plotted predicted percentages from the adjusted model using recycled predictions. 40 These percentages refer to people prescribed each second‐line antidiabetic treatment stratified by ethnicity, and separately by deprivation, while still adjusting for all measured covariates, and accounting for clustering at the CCG level. We obtained P values from Wald tests comparing the predicted percentage of being prescribed one of SUs, DPP‐4 inhibitors or SGLT2 inhibitors by each non‐White ethnic group versus White ethnic group and by deprivation Quintiles 2 to 5 versus deprivation Quintile 1. We also performed joint tests to test whether predicted percentages for each ethnic group or for each deprivation quintile were equal for each second‐line antidiabetic treatment. These percentages and P values were used to support our final conclusions on disparities in second‐line antidiabetic treatment prescribing by ethnicity or by deprivation. 39 We then stratified the adjusted predicted percentages by prevalent CVD status at baseline to determine if there were differences in prescribing by ethnicity or by deprivation quintile according to prevalent CVD status.
In the secondary analyses, we compared the change in odds ratios between the fixed‐effect model (model including ethnicity, deprivation, and all covariates) and the mixed‐effect model (the fixed effect model plus accounting for CCG clustering as a random effect). We also compared the final adjusted mixed‐effect model with and without adjustment for deprivation to observe any changes in ethnic disparities in second‐line antidiabetic treatment prescribed when adding this variable to the multivariable model, since deprivation could be a mediator between ethnicity and second‐line antidiabetic treatment prescribed. Because awareness of the cardio‐ and kidney‐protective effects of SGLT2 inhibitors versus placebo have increased over time, we considered year of second‐line oral antidiabetic treatment initiation as the independent variable of interest, repeating the main analysis to observe any differences in second‐line antidiabetic treatment prescribed over time, overall and stratified by prevalent CVD status. Finally, we investigated whether there were interactions between (1) ethnicity and IMD, (2) ethnicity and calendar time, and (3) deprivation and calendar time, informed by the results of likelihood ratio tests on the final adjusted multinomial models, and by joint tests on whether predicted percentages for interaction terms were equal for each of the three second‐line treatments prescribed.
Data management and analyses were performed using Stata 17.
3. RESULTS
3.1. Baseline characteristics
The study population included 36 023 people with complete data on all variables of interest who initiated second‐line oral antidiabetic treatment during the study period with linked secondary care data (Figure 1). Eighty‐four percent of the cohort were White, 10% were South Asian, 4% were Black, and 1% were Mixed/other ethnicity. We excluded 6150 people with missing data for at least one variable, including 348 with missing ethnicity data and 20 with missing IMD data (Table S4).
FIGURE 1.
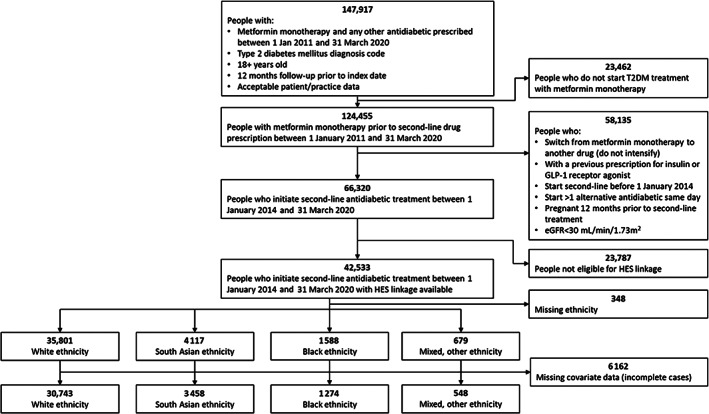
Flow diagram illustrating selection of study population diagnosed with type 2 diabetes mellitus (T2DM) and initiating second‐line antidiabetic treatment. eGFR, estimated glomerular filtration rate; HES, Hospital Episodes Statistics.
Overall, 41% of the cohort was female, with a mean age of 59 years (Table 1). People of White ethnicity were more likely to be male (60%) and older (mean age 60.1 years) compared with people of South Asian ethnicity (53% male, mean age 52.6 years), Black ethnicity (49% male, mean age 55.2 years), and people of Mixed/other ethnicity (58% male, mean age 55.4 years). People of South Asian and Black ethnicities were over‐represented in the lowest IMD quintile (34% and 46%, respectively). Recorded CVD prevalence was 24% overall and was lower in people of Black (15%) compared to White ethnicity (23%). Mean BMI was highest in people of White ethnicity (33.8 kg/m2) and lowest in people with South Asian ethnicity (30.2 kg/m2).
TABLE 1.
Baseline characteristics of study population (at time of second‐line treatment initiation)
| Total | White | South Asian | Black | Mixed/Other | |
|---|---|---|---|---|---|
| 36 023 (100%) | 30 743 (85%) | 3458 (10%) | 1274 (4%) | 548 (1%) | |
| Female, n (%) | 14 643 (41) | 12 149 (40) | 1616 (47) | 646 (51) | 232 (42) |
| Age at baseline, n (%) | |||||
| 18‐49 years | 7734 (21) | 5711 (19) | 1450 (42) | 398 (31) | 175 (32) |
| 50–59 years | 11 128 (31) | 9396 (31) | 1059 (31) | 483 (38) | 190 (35) |
| 60‐69 years | 9622 (27) | 8613 (28) | 662 (19) | 236 (19) | 111 (20) |
| 70+ years | 7539 (21) | 7023 (23) | 287 (8) | 157 (12) | 72 (13) |
| Year of second‐line treatment initiation, n (%) | |||||
| 2014 | 4092 (11) | 3584 (12) | 318 (9) | 139 (11) | 51 (9) |
| 2015‐2016 | 10 910 (30) | 9467 (31) | 915 (26) | 364 (29) | 164 (30) |
| 2017‐2018 | 12 221 (34) | 10 379 (34) | 1186 (34) | 459 (36) | 197 (36) |
| 2019‐2020 | 8800 (24) | 7313 (24) | 1039 (30) | 312 (24) | 136 (25) |
| Years on first‐line (metformin monotherapy), n (%) | |||||
| <0.5 | 6676 (19) | 5710 (19) | 539 (16) | 320 (25) | 107 (20) |
| 0.5‐0.99 | 3654 (10) | 3183 (10) | 288 (8) | 123 (10) | 60 (11) |
| ≥1 | 25 693 (71) | 21 850 (71) | 2631 (76) | 831 (65) | 381 (70) |
| Median (IQR) no. of patients registered at the person's GP | 10 295 (6981‐14254) | 10 471 (7184‐14275) | 8793 (5213‐12861) | 10 357 (6484‐15253) | 10 674 (7377‐15196) |
| Hospitalization (any) within 1 year prior to second‐line treatment initiation, n (%) | 10 216 (28) | 8818 (29) | 873 (25) | 361 (28) | 164 (30) |
| IMD quintile, n (%) | |||||
| 1 (least deprived) | 5739 (16) | 5322 (17) | 271 (8) | 45 (4) | 101 (18) |
| 2 | 6484 (18) | 5922 (19) | 419 (12) | 77 (6) | 66 (12) |
| 3 | 6915 (19) | 6075 (20) | 601 (17) | 153 (12) | 86 (16) |
| 4 | 8020 (22) | 6473 (21) | 995 (29) | 416 (33) | 136 (25) |
| 5 (most deprived) | 8865 (25) | 6951 (23) | 1172 (34) | 583 (46) | 159 (29) |
| HbA1c at baseline, n (%) | 9.1 | 8 | 9 | 9.1 | |
| <53 mmol/mol (7%) | 1318 (4) | 1070 (3) | 139 (4) | 84 (7) | 25 (5) |
| 53‐74 mmol/mol | 19 443 (54) | 16 548 (54) | 2057 (59) | 541 (42) | 297 (54) |
| 75+ mmol/mol (9%) | 15 262 (42) | 13 125 (43) | 1262 (36) | 649 (51) | 226 (41) |
| Uncontrolled hypertension, based on last recorded blood pressure, n (%) | |||||
| Normotensive | 9749 (27) | 8073 (26) | 1155 (33) | 351 (28) | 170 (31) |
| Hypertensive | 26 274 (73) | 22 670 (74) | 2303 (67) | 923 (72) | 378 (69) |
| Mean (SD) BMI, kg/m 2 | 33.4 (7.0) | 33.8 (7.1) | 30.2 (5.8) | 32.2 (6.9) | 31.3 (6.8) |
| Smoking status, n (%) | |||||
| Non‐smoker | 7371 (20) | 5691 (19) | 1145 (33) | 389 (31) | 146 (27) |
| Current smoker | 9874 (27) | 8608 (28) | 804 (23) | 317 (25) | 145 (26) |
| Ex‐smoker | 18 778 (52) | 16 444 (53) | 1509 (44) | 568 (45) | 257 (47) |
| Alcohol status, n (%) | |||||
| Non‐drinker | 3846 (11) | 2276 (7) | 1199 (35) | 239 (19) | 132 (24) |
| Current drinker | 22 082 (61) | 20 136 (65) | 1112 (32) | 583 (46) | 251 (46) |
| Ex‐drinker | 10 095 (28) | 8331 (27) | 1147 (33) | 452 (35) | 165 (30) |
| Co‐prescriptions, n (%) | |||||
| RAS inhibitors | 17 949 (50) | 15 700 (51) | 1450 (42) | 535 (42) | 264 (48) |
| Statins | 24 907 (69) | 21 472 (70) | 2376 (69) | 713 (56) | 346 (63) |
| Cancer (any) | 4048 (11) | 3794 (12) | 129 (4) | 91 (7) | 34 (6) |
| Macrovascular comorbidities, n (%) | |||||
| CVD composite a | 8466 (24) | 7589 (25) | 600 (17) | 191 (15) | 86 (16) |
| Amputation | 283 (1) | 270 (1) | 8 (0) | <5(0) | <5 (0) |
| Heart failure | 2110 (6) | 1936 (6) | 109 (3) | 51 (4) | 14 (3) |
| Myocardial infarction | 2521 (7) | 2288 (7) | 174 (5) | 37 (3) | 22 (4) |
| Stroke | 1640 (5) | 1470 (5) | 103 (3) | 54 (4) | 13 (2) |
| Ischaemic heart disease | 6823 (19) | 6117 (20) | 495 (14) | 134 (11) | 77 (14) |
| Unstable angina | 1175 (3) | 1049 (3) | 83 (2) | 29 (2) | 14 (3) |
| Microvascular comorbidities, n (%) | |||||
| eGFR at baseline category (mL/min/1.73 m2) | |||||
| No known CKD (eGFR missing) | 675 (2) | 612 (2) | 31 (1) | 11 (1) | 21 (4) |
| 90+ (Stage 1) | 21 391 (59) | 17 676 (57) | 2694 (78) | 640 (50) | 381 (70) |
| 60‐89 (Stage 2) | 11 913 (33) | 10 608 (35) | 648 (19) | 539 (42) | 118 (22) |
| 45‐59 (Stage 3a) | 1585 (4) | 1432 (5) | 65 (2) | 66 (5) | 22 (4) |
| 30‐44 (Stage 3b) | 459 (1) | 415 (1) | 20 (1) | 18 (1) | 6 (1) |
| Blindness | 486 (1) | 432 (1) | 33 (1) | 13 (1) | 8 (1) |
| Hypoglycaemia | 320 (1) | 272 (1) | 23 (1) | 16 (1) | 9 (2) |
| Proteinuria | 2586 (7) | 2202 (7) | 260 (8) | 80 (6) | 44 (8) |
CVD composite: heart failure, ischaemic heart disease, myocardial infarction, stroke, unstable angina.
Abbreviations: BMI, body mass index; CPRD, Clinical Practice Research Datalink; CVD, cardiovascular; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; GP, general practice; HES, Hospital Episode Statistics; IMD, Index of Multiple Deprivation; IQR, interquartile range; RAS, renin‐angiotensin system; SGLT2, sodium‐glucose cotransporter 2; SD, standard deviation; SU, sulphonylureas; UK, United Kingdom.
After stratifying by IMD quintile, we found that people in the most deprived quintile were over‐represented (25%) and people in the least deprived quintile were under‐represented (16%; Table S2). People in the most deprived quintile were younger (mean age 56.6 years) compared with the least deprived quintile (mean age 61.8 years). The most deprived quintile included a higher proportion of South Asian and Black people (13% and 7%, respectively) compared with the least deprived quintile (5% and 1%, respectively).
People with missing covariate information were similar according to sex, age, IMD and prevalent CVD versus those with fully observed covariate information (Tables S3,S4).
3.2. Ethnicity and second‐line antidiabetic treatment choice
In people of Black ethnicity, the most common second‐line treatment during the study period was SUs (593 [46%]), whereas for all other ethnic groups DPP‐4 inhibitors was the most commonly prescribed second‐line treatment (13 398 [43%], 1530 [44%], and 249 [45%], among people of White, South Asian, and Mixed/other ethnic groups, respectively; Table 2). SGLT2 inhibitors were the least common second‐line treatment across all ethnic groups, ranging from 14% prescribed, among people of Black ethnicity, to 21%, among people of White ethnicity (Table 2).
TABLE 2.
Crude proportions of second‐line treatment prescribed by ethnicity or by deprivation
| Second‐line antidiabetic treatment prescribed | |||
|---|---|---|---|
| Variable of interest | Metformin‐SUs | Metformin‐DPP‐4 inhibitors | Metformin‐SGLT2 inhibitors |
| Ethnicity, n (row %) | |||
| White | 11 584 (37) | 13 398 (43) | 6455 (21) |
| South Asian | 1316 (37) | 1530 (44) | 667 (19) |
| Black | 593 (46) | 524 (40) | 179 (14) |
| Mixed/other | 216 (39) | 249 (45) | 93 (17) |
| IMD quintile, n (row %) | |||
| 1 (least deprived) | 2155 (37) | 2483 (42) | 1264 (21) |
| 2 | 2432 (37) | 2833 (43) | 1388 (21) |
| 3 | 2654 (37) | 2972 (42) | 1476 (21) |
| 4 | 3112 (38) | 3975 (44) | 1628 (20) |
| 5 (most deprived) | 3356 (37) | 3975 (44) | 1638 (18) |
Abbreviations: DPP‐4, dipeptidyl peptidase‐4; IMD, Index of Multiple Deprivation; SGLT2, sodium‐glucose cotransporter 2; SU, sulphonylurea.
There was some evidence that adjusted predicted percentages for being prescribed SGLT2 inhibitors were greatest for White people (21% [95% CI 19–23%]) compared with South Asian people (20% [95% CI 18–22%]), Black people (19% [95% CI 16–22%]) and Mixed/other people (17% [95% CI 14–21%]; P = 0.003 [Figure 2, Table S5]). There was no evidence of differences in adjusted predicted percentages for being prescribed DPP‐4 inhibitors or SUs according to ethnicity (Figure 2, Table S5). The results from the multinomial, multivariable logistic regression model used to calculate these adjusted predicted percentages are described in Table S6.
FIGURE 2.
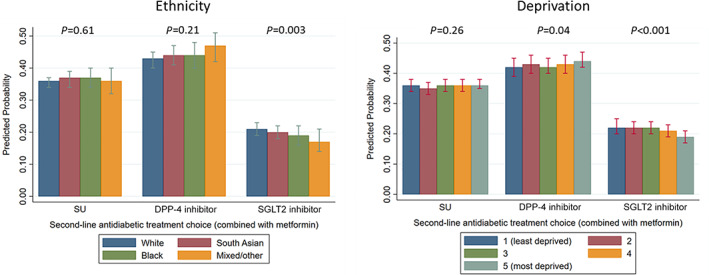
Adjusted predicted percentages of second‐line antidiabetic treatment prescribed, according to ethnicity or deprivation. P values are generated from joint tests comparing the adjusted predicted percentages for being prescribed a particular second‐line antidiabetic drug (a sulphonylurea [SU], a dipeptidyl peptidase‐4 [DPP‐4] inhibitor or a sodium‐glucose cotransporter‐2 [SGLT2] inhibitor) across ethnic groups or across deprivation levels. Predicted percentages are mutually adjusted for deprivation (ethnicity estimates) and ethnicity (deprivation estimates), as well as the number of patients registered at the patient's general practice, years on first‐line treatment category, age category, sex, last glycated haemoglobin value prior to second‐line treatment initiation category, body mass index, prevalent heart failure, ischaemic heart disease, myocardial infarction, stroke, unstable angina, renin‐angiotensin system inhibitors and/or statin co‐prescription, chronic kidney disease category, blood pressure category, history of proteinuria, blindness, cancer (any), hospitalization (any) in past year, smoking status, alcohol status, region, all as fixed effects, and Clinical Commissioning Group‐clustering as a random effect.
3.3. Social deprivation and second‐line antidiabetic treatment choice
The crude proportion of people prescribed each second‐line antidiabetic treatment option across deprivation quintiles are presented in Table 2.
There was some evidence of a small difference in adjusted predicted percentages of people prescribed SGLT2 inhibitors according to deprivation: 19% (95% CI 17–21%) were prescribed SGLT2 inhibitors in the most deprived quintile, and 22% (95% CI 20–24%) were prescribed SGLT2 inhibitors in the least deprived quintile (P < 0.001; Figure 2, Table S5). Conversely, there was some evidence that people in the most deprived quintile had a small increase in the adjusted predicted percent of being prescribed DPP‐4 inhibitors 44% (95% CI 42–47%) versus 42% (95% CI 39–45%) of people in the least deprived quintile (P = 0.04). There was no evidence of any differences in the adjusted predicted percentages of people prescribed SUs according to deprivation (P = 0.26).
3.4. Second‐line antidiabetic treatment prescribed among people with prevalent CVD
When stratifying by prevalent CVD status (n = 8466 with prevalent CVD, n = 27 557 without prevalent CVD), adjusted predicted percentages showed no substantial differences in SU prescribing across ethnicities and across deprivation quintiles. However, adjusted predicted percentages showed evidence of a slightly higher proportion of people prescribed DPP‐4 inhibitors with prevalent CVD versus no CVD across all ethnicities. Conversely, adjusted predicted percentages showed evidence of slightly less SGLT2 inhibitor prescribing among people with prevalent CVD versus no CVD across all ethnicities (Table S7).
3.5. Secondary analyses
Results were similar in the fixed‐effect and mixed‐effect models (Table S6), and we did not observe any substantial mediation by deprivation level on the association between ethnicity and second‐line treatment prescribed (Table S8).
Second‐line antidiabetic treatments prescribed changed over time (Tables S9–S11, Figure S1). Briefly, SU prescribing decreased substantially over time (adjusted predicted percent 60% [95% CI 57–62%] in 2014 versus 23% [95% CI 21–24%] in 2019–2020), while prescribing of DPP‐4 inhibitors and SGLT2 inhibitors increased over the same time periods (DPP‐4 inhibitors: 34% [95% CI 31–36%] in 2014 versus 43% [95% CI 40–46%] in 2019–2020; and SGLT2 inhibitors: 7% [95% CI 5–8%] in 2014 versus 34% [95% CI 32–37%] in 2019–2020; Table S10). Among people with and without prevalent CVD, those with prevalent CVD had consistently lower probabilities of being prescribed SGLT2 inhibitors compared with those without prevalent CVD across time periods (Table S10).
There was no evidence of an interaction effect between ethnicity and IMD on second‐line antidiabetic treatment prescribed on the odds scale (P = 0.45). On the adjusted predicted percent scale, there was some evidence that the percent prescribed SGLT2 inhibitors among people of White ethnicity and Mixed/other ethnicity decreased with increasing deprivation (P < 0.001 and P = 0.04). There was no evidence of an interaction between calendar time and ethnicity (P = 0.11), nor between calendar time and deprivation quintile (P = 0.66).
4. DISCUSSION
We found statistically significant, but small absolute differences in SU, DPP‐4 inhibitor and SGLT2 inhibitor prescribing as second‐line antidiabetic treatment, in combination with metformin, according to ethnicity and deprivation level, after accounting for several covariates and clustering at the CCG level in England. There was some evidence that across ethnic groups and levels of deprivation, people with prevalent CVD had a lower probability of being prescribed SGLT2 inhibitors compared with those without prevalent CVD.
It is reassuring that we did not observe substantial ethnic differences in second‐line antidiabetic treatment prescribing, as previous research has described many other ethnic disparities related to T2DM. In the UK, ethnic minorities with T2DM had longer delays in intensification to second‐line treatment than White people with T2DM, and experienced greater treatment inertia following identification of uncontrolled HbA1c. 12 In the United States, which does not have a universal healthcare system, ethnic minorities are less likely to be prescribed SGLT2 inhibitors at any time after T2DM diagnosis compared to White people, even after adjusting for deprivation level. 41
This cross‐sectional study used one of the largest primary care datasets in the world. 26 , 27 While our results suggest statistical evidence of a lower percentage of ethnic minorities and people from more deprived areas in England being prescribed SGLT2 inhibitors compared with people of White ethnicity, these differences were not substantial and unlikely to represent major disparities in T2DM care. Factors such as willingness to try newer treatments on the part of both the healthcare team and the patient are unmeasured, and could have contributed to the small differences in second‐line antidiabetic treatments prescribed that we observed in this study.
It is, however, concerning that people with prevalent CVD had a lower probability of receiving SGLT2 inhibitors versus those without, since trials comparing SGLT2 inhibitors versus placebo have shown substantial improvements in diabetic‐related outcomes among those with atherosclerotic CVD, heart failure, and kidney disease. 38 , 42 , 43 However, national and international guidance/guidelines recommending SGLT2 inhibitors among those with prevalent CVD were only updated towards the end of our study period. 22 , 44 We hope future research shows increased SGLT2 inhibitor prescribing in those with prevalent CVD after 2020.
This study has some limitations. We did not include people with missing documentation of ethnicity; however, only 348 people (0.8%) in the total sample had missing ethnicity data, which limited our ability to include these people as a separate group in our analyses. Further, some exposure misclassification may exist in our study, since deprivation was measured at the small area/neighbourhood level and patient‐level deprivation status may differ from this measure. There is also likely to be residual confounding according to other clinical characteristics such as history of alcohol misuse, pancreatic disease, urinary tract infections, mycotic urinary infections, and unobserved factors such as prescriber characteristics and patient frailty status. Our data came from electronic health records, which are not designed primarily for research and thus some degree of misclassification is expected for covariates, particularly those which are not necessarily recorded on the same day as second‐line treatment initiation (eg, HbA1c, BMI, eGFR, blood pressure). Finally, we were limited to prescribing data from primary care to define second‐line antidiabetic treatment choice. We were unable to use dispensing data from pharmacies since these data are not available for linkage to CPRD data, nor were we able to determine if treatment initiation occurred during an inpatient stay (secondary care), where prescription of newer drug classes may be relatively more likely. We adjusted for any hospitalization in the past year to try and account for this. However, even if a prescription was initiated or recommended by specialist care, primary care would probably continue prescriptions of these therapies.
In conclusion, we found statistically significant, but small differences in second‐line oral antidiabetic treatment prescribing by ethnicity and social deprivation status in England. These differences are unlikely to be clinically important. We consider it encouraging that, after accounting for various clinical characteristics and variation at the CCG level, ethnic minorities and people from more deprived backgrounds did not have substantially lower probabilities of being prescribed SGLT2 inhibitors compared with DPP‐4 inhibitors and SUs. Future work should investigate other factors at the individual and local CCG level which may drive treatment choice to understand how these treatments are used in routine care, and to highlight the need for future research to directly evaluate the comparative effectiveness of these three second‐line antidiabetic treatment choices to optimize oral antidiabetic treatment prescribing.
AUTHOR CONTRIBUTIONS
Patrick Bidulka, David Lugo‐Palacios, Stephen O'Neill, Anirban Basu, Kamlesh Khunti and Richard Grieve conceived and designed the study. Patrick Bidulka and Stephen O'Neill conducted the data management and analyses. Patrick Bidulka wrote the first draft of the manuscript. All authors (Patrick Bidulka, Rohini Mathur, David Lugo‐Palacios, Stephen O'Neill, Anirban Basu, Richard Silverwood, Paul Charlton, Andrew Briggs, Liam Smeeth, Amanda Adler, Ian Douglas, Kamlesh Khunti and Richard Grieve) reviewed and commented on the manuscript, and approved the final version for submission.
CONFLICT OF INTERESTS
All authors have completed an ICJME form. Patrick Bidulka, Stephen O'Neill, Anirban Basu, Richard Silverwood and Liam Smeeth have nothing to declare. Kamlesh Khunti has acted as a consultant, speaker or received grants for investigator‐initiated studies for Astra Zeneca, Novartis, Novo Nordisk, Sanofi‐Aventis, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin‐Chemie AG / Menarini Group, Janssen and Napp. Rohini Mathur has received consulting fees from AMGEN. Paul Charlton sat on an NIHR HTA Commissioning Committee member until September 2021. Anirban Basu is an economic advisor on the DiRECT trial, with ongoing responsibility for economic analysis during the long‐term follow‐up phase, and has also acted as consultant to GlaxoSmithKline, Merck, Novo Nordisk and Boehringer Ingelheim in relation to their diabetes products. Amanda Adler receives salary from the NIHR BRC via the Oxford Centre for Diabetes, Endocrinology and Metabolism. Ian Douglas holds an unrestricted research grant from GSK and holds shares in GSK. Richard Grieve sits on the NIHR commissioning committee.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14874.
ETHICS APPROVAL
This research was approved by the London School of Hygiene & Tropical Medicine ethics committee (reference 21 395) and the Independent Scientific Advisory Committee (reference 20_064).
TRANSPARENCY STATEMENT
Patrick Bidulka, as corresponding author, confirms that the manuscript is an honest, accurate, and transparent account of the study being reported and that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. The study was approved by the Independent Scientific Advisory Committee (approval number: 20_064). Kamlesh Khunti is supported by the National Institute for Health Research (NIHR; grant number NIHR128490) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre (BRC). Rohini Mathur is supported by a Sir Henry Wellcome Postdoctoral Fellowship (201375/Z/16/Z). Patrick Bidulka and Richard Grieve are joint guarantors and take full responsibility for this work as a whole, including the study design, access to data, and the decision to submit and publish this manuscript. This work was supported by National Institute for Health and Care Research (NIHR) grant number NIHR128490.
Bidulka P, Mathur R, Lugo‐Palacios DG, et al. Ethnic and socioeconomic disparities in initiation of second‐line antidiabetic treatment for people with type 2 diabetes in England: A cross‐sectional study. Diabetes Obes Metab. 2023;25(1):282‐292. doi: 10.1111/dom.14874
Funding information National Institute for Health and Care Research (NIHR), Grant/Award Number: NIHR128490
DATA AVAILABILITY STATEMENT
Due to data‐sharing restrictions, we cannot share the data used in this study directly. However, researchers may apply to use CPRD data linked with other health datasets. Please see the CPRD website for further instruction https://cprd.com/. Codelists to create exposure, outcome, and covariates will be published on LSHTM DataCompass https://datacompass.lshtm.ac.uk/.
REFERENCES
- 1. Braveman P, Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public Health Rep. 2014;129(Suppl 2):19‐31. doi: 10.1177/00333549141291S206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mackenbach JP, Stirbu I, Roskam A‐JR, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med 2008. 2008;358(23):2468‐2481. doi: 10.1056/NEJMsa0707519 [DOI] [PubMed] [Google Scholar]
- 3. NICE: 20 years of evidence‐based decision making . Web. National Institute for Health and Care Excellence (NICE). Accessed September 10, 2021. https://indepth.nice.org.uk/20-years-of-NICE/index.html
- 4. NHS body 'to end postcode prescribing' . Web. BBC News. Accessed September 10, 2021. http://news.bbc.co.uk/1/hi/health/271522.stm
- 5. Our principles : The principles that guide the development of NICE guidance and standards. Web. National Institute for Health and Care Excellence. https://www.nice.org.uk/about/who-we-are/our-principles [PubMed]
- 6. Scarborough PB, Bhatnagar P, Kaur A, Smolina K, Wickramasinghe K, Rayner M. Ethnic differences in Cardiovascular Disease 2010 . 2010. https://www.bhf.org.uk/informationsupport/publications/statistics/ethnic-differences-in-cardiovascular-disease-2010#
- 7. Caskey F, Gavin D. Kidney Health Inequalities in the UK: an agenda for change . 2018. https://kidneyresearchuk.org/wp-content/uploads/2019/09/Health_Inequalities_lay_report_FINAL_WEB_20190311.pdf
- 8. Health Survey for England 2004: The Health of Minority Ethnic Groups . 2005. https://files.digital.nhs.uk/publicationimport/pub01xxx/pub01209/heal-surv-hea-eth-min-hea-tab-eng-2004-rep.pdf
- 9. Ng Fat L. Health survey for England 2019 adults. Health. 2020;9‐13:12‐13. https://files.digital.nhs.uk/23/6B5DEA/HSE19-Adult-health-rep.pdf [Google Scholar]
- 10. Mathur R, Bhaskaran K, Edwards E, et al. Population trends in the 10‐year incidence and prevalence of diabetic retinopathy in the UK: a cohort study in the Clinical Practice Research Datalink 2004–2014. BMJ Open. 2017;7(2):e014444. doi: 10.1136/bmjopen-2016-014444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tillin T, Hughes AD, Mayet J, et al. The relationship between metabolic risk factors and incident cardiovascular disease in Europeans, South Asians, and African Caribbeans: SABRE (Southall and Brent revisited)—a prospective population‐based study. J Am Coll Cardiol 2013;61(17):1777‐1786. doi: 10.1016/j.jacc.2012.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mathur R, Farmer RE, Eastwood SV, Chaturvedi N, Douglas I, Smeeth L. Ethnic disparities in initiation and intensification of diabetes treatment in adults with type 2 diabetes in the UK, 1990–2017: a cohort study. PLoS Med. 2020;17(5):e1003106. doi: 10.1371/journal.pmed.1003106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bachmann MO, Eachus J, Hopper CD, et al. Socio‐economic inequalities in diabetes complications, control, attitudes and health service use: a cross‐sectional study. Diabet Med. 2003;20(11):921‐929. 10.1046/j.1464-5491.2003.01050.x [DOI] [PubMed] [Google Scholar]
- 14. Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427‐437. 10.1111/dom.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khunti K, Seidu S. Therapeutic inertia and the legacy of dysglycemia on the microvascular and macrovascular complications of diabetes. Diabetes Care. 2019;42(3):349‐351. doi: 10.2337/dci18-0030 [DOI] [PubMed] [Google Scholar]
- 16. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with Type 2 diabetes. Diabetes Care. 2013;36(11):3411‐3417. doi: 10.2337/dc13-0331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khunti S, Khunti K, Seidu S. Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia. Ther Adv Endocrinol Metab. 2019;10:2042018819844694. doi: 10.1177/2042018819844694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schernthaner G, Shehadeh N, Ametov AS, et al. Worldwide inertia to the use of cardiorenal protective glucose‐lowering drugs (SGLT2i and GLP‐1 RA) in high‐risk patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19(1):185. doi: 10.1186/s12933-020-01154-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NG28: Type 2 diabetes in adults: management. NICE. https://www.nice.org.uk/guidance/ng28
- 20. Canagliflozin in combination therapy for treating type 2 diabetes : Technology appraisal guidance TA315. Web. NICE. Updated June 25, 2014. https://www.nice.org.uk/guidance/ta315/chapter/2-The-technology
- 21. NICE guideline [NG28] : Type 2 diabetes in adults: management. Web. NICE. Accessed March 3, 2022, 2022. https://www.nice.org.uk/guidance/ng28/chapter/Recommendations#reviewing-drug-treatments
- 22. Buse JB, Wexler DJ, Tsapas A, et al. Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabet care. 2019, 2020;43(2):487‐493. doi: 10.2337/dci19-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilkinson S, Douglas I, Stirnadel‐Farrant H, et al. Changing use of antidiabetic drugs in the UK: trends in prescribing 2000–2017. BMJ Open. 2018;8(7):e022768. doi: 10.1136/bmjopen-2018-022768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilkinson S, Douglas IJ, Williamson E, et al. Factors associated with choice of intensification treatment for type 2 diabetes after metformin monotherapy: a cohort study in UK primary care. Clin Epidemiol. 2018;10:1639‐1648. doi: 10.2147/CLEP.S176142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bidulka P, O'Neill S, Basu A, et al. Protocol for an observational cohort study investigating personalised medicine for intensification of treatment in people with type 2 diabetes mellitus: the PERMIT study. BMJ Open. 2021;11:e046912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, Smeeth L Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol Jun 2015;44(3):827‐36. doi: 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wolf A, Dedman D, Campbell J, et al. Data resource profile: clinical practice research datalink (CPRD) aurum. Int J Epidemiol. 2019;48(6):1740‐1740g. doi: 10.1093/ije/dyz034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herbert A, Wijlaars L, Zylbersztejn A, Cromwell D, Hardelid P. Data resource profile: hospital episode statistics admitted patient care (HES APC). Int J Epidemiol. 2017;46(4):1093‐1093i. doi: 10.1093/ije/dyx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The English Indices of Deprivation 2015 (GOV.UK) (2015).
- 30. Linked HES‐ONS mortality data . Web. NHS Digital. Accessed March 4, 2022. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/linked-hes-ons-mortality-data#ons-mortality-data
- 31. Wilding J, Godec T, Khunti K, et al. Changes in HbA1c and weight, and treatment persistence, over the 18 months following initiation of second‐line therapy in patients with type 2 diabetes: results from the United Kingdom clinical practice research datalink. BMC Med. 2018;16(1):116. doi: 10.1186/s12916-018-1085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. TA390: Canagliflozing, dapagliflozin and empagliflozin as monotherapies for treating type 2 diabetes. NICE.
- 33. Mathur R, Bhaskaran K, Chaturvedi N, et al. Completeness and usability of ethnicity data in UK‐based primary care and hospital databases. J Public Health. 2014;36(4):684‐692. doi: 10.1093/pubmed/fdt116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eastwood SV, Mathur R, Sattar N, Smeeth L, Bhaskaran K, Chaturvedi N. Ethnic differences in guideline‐indicated statin initiation for people with type 2 diabetes in UK primary care, 2006–2019: a cohort study. PLoS Med. 2021;18(6):e1003672. doi: 10.1371/journal.pmed.1003672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. 2011 Census Variables and Classifications: 2014 . 2014. Accessed May 3, 2022. https://www.ons.gov.uk/census/2011census/2011censusdata/2011censususerguide/variablesandclassifications
- 36. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 37. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 38. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 39. Norton EC, Dowd BE, Maciejewski ML. Odds ratios—current best practice and use. JAMA. 2018;320(1):84‐85. doi: 10.1001/jama.2018.6971 [DOI] [PubMed] [Google Scholar]
- 40. Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962‐970. doi: 10.1093/ije/dyu029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eberly LA, Yang L, Eneanya ND, et al. Association of Race/ethnicity, gender, and socioeconomic status with sodium‐glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open. 2021;4(4):e216139. doi: 10.1001/jamanetworkopen.2021.6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 43. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995‐2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 44. Guideline update : Type 2 diabetes in adults: management; Draft for consultation, September 2021. Web. National Institute of Health and Care Excellence. Accessed September 9, 2021. https://www.nice.org.uk/guidance/GID-NG10246/documents/draft-guideline
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
Due to data‐sharing restrictions, we cannot share the data used in this study directly. However, researchers may apply to use CPRD data linked with other health datasets. Please see the CPRD website for further instruction https://cprd.com/. Codelists to create exposure, outcome, and covariates will be published on LSHTM DataCompass https://datacompass.lshtm.ac.uk/.


