Abstract
The cytokine interleukin‐6 (IL‐6) is involved in a diverse set of physiological processes. Traditionally, IL‐6 has been thought of in terms of its inflammatory actions during the acute phase response and in chronic conditions such as rheumatoid arthritis and obesity. However, IL‐6 is also an important signaling molecule during exercise, being acutely released from working muscle fibers with increased exercise duration, intensity, and muscle glycogen depletion. In this context, IL‐6 enables muscle‐organ crosstalk, facilitating a coordinated response to help maintain muscle energy homeostasis, while also having anti‐inflammatory actions. The range of actions of IL‐6 can be explained by its dichotomous signaling pathways. Classical signaling involves IL‐6 binding to a cell‐surface receptor (mbIL‐6R; present on only a small number of cell types) and is the predominant signaling mechanism during exercise. Trans‐signaling involves IL‐6 binding to a soluble version of its receptor (sIL‐6R), with the resulting complex having a much greater half‐life and the ability to signal in all cell types. Trans‐signaling drives the inflammatory actions of IL‐6 and is the predominant pathway in disease. A single nucleotide polymorphism (rs2228145) on the IL‐6R gene can modify the classical/trans‐signaling balance through increasing the levels of sIL‐6R. This SNP has clinical significance, having been linked to inflammatory conditions such as rheumatoid arthritis and type 1 diabetes, as well as to the severity of symptoms experienced with COVID‐19. This review will describe how acute exercise, chronic training and the rs2228145 SNP can modify the IL‐6 signaling pathway and the consequent implications for health and athletic performance.
Keywords: Interleukin‐6, rs2228145, sgp130, sIL‐6R
1. INTRODUCTION
Interleukin‐6 (IL‐6) is a cytokine that mediates a diverse set of physiological and pathophysiological processes. Traditionally, IL‐6 has been associated with both the acute phase of the inflammatory response in contexts such as sepsis 1 and viral infection, 2 as well as conditions with a chronic inflammatory component such as rheumatoid arthritis, type 2 diabetes, and obesity. 3 The inflammatory state that is present in the latter conditions is partly driven by IL‐6 itself, with the cytokine being produced by many cell types including leukocytes and adipocytes. 4 Additionally, contracting skeletal muscle is a source of IL‐6 as a “myokine”; in this capacity, IL‐6 appears to enhance exercise performance, at least in mice models, 5 possibly through promoting fuel mobilization 6 and eliciting training adaptations. 7 However, in an exercise context, IL‐6 signaling has been associated with greater perceived fatigue 8 and has been suggested to play a role in the overtraining syndrome. 9
The diverse and apparently opposing actions of IL‐6 can be explained by its dichotomous signaling pathways. 10 Classical signaling involves blood‐borne IL‐6 binding to a membrane‐bound IL‐6 receptor (mbIL‐6R), and hence only occurs in the few cell types that possess mbIL‐6R, principally hepatocytes, monocyte–macrophages, lymphocytes, and skeletal myocytes. 11 , 12 , 13 This mbIL‐6R is associated with two monomers of the transmembrane glycoprotein gp130, which dimerise upon receptor activation. This dimerization leads to phosphorylation of janus kinase (JAK), the first step in the JAK–STAT signaling pathway, which goes on to mediate signaling responses in target cells. 11 In inflammatory contexts such as sepsis and infection, IL‐6‐mediated responses appear to be triggered by—and involved in the resolution of—inflammatory episodes that have been initiated by release of pro‐inflammatory cytokines such as IL‐1, IL‐18, and TNFα following injury, infection, or in obesity. In contrast, release of IL‐6 as a myokine from contracting myocytes has been suggested to promote fuel mobilization during activity. 14 For example, IL‐6 appears to be a factor in exercise‐mediated activation of AMPK, a cellular energy‐sensing enzyme that increases fatty acid oxidation, glucose transport, and lipolysis within skeletal muscle fibers, liver, and adipose tissue. 15 The ability of myocyte‐derived IL‐6 to interact with mbIL‐6R in non‐muscle cells such as monocyte–macrophages and lymphocytes correlates with (possibly via its impact on JAK–STAT‐mediated gene expression) a range of longer‐term anti‐inflammatory responses in these cell types. 16 Similarly, AMPK's ability to activate transcriptional co‐activators also leads to longer‐term effects such as mitochondrial biogenesis (and hence enhanced aerobic capacity). 17 Thus, the current literature suggests that the classical IL‐6 signaling pathway may contribute to both performance‐related training adaptations and the anti‐inflammatory benefits of exercise. 18
Cellular responses can also be induced via IL‐6 trans‐signaling. This involves a soluble fragment of the IL‐6 receptor (sIL‐6R) which can bind to free IL‐6 within plasma and other extracellular fluids to form a biologically active complex. 19 The formation of this IL‐6/sIL‐6R complex potentiates the actions of IL‐6, by extending its half‐life from minutes to hours and thus enabling IL‐6 to signal distant from its site of production. 20 The production of sIL‐6R is primarily achieved through the actions of the ADAM10 and ADAM17 metalloproteases, which can cleave mbIL‐6R, with the biologically active extracellular domain being shed into the circulation as sIL‐6R. 19 This process has been well‐documented in monocyte–macrophages and T cells 21 , 22 but it remains unclear whether this process also occurs in other cell types that express mbIL‐6R. A secondary source of sIL‐6R comes from alternative splicing of the mRNA that codes for the receptor, 23 in which the expressed receptor lacks a transmembrane domain and consequently is released into the plasma rather than being anchored in the plasma membrane. However, this process contributes only a low proportion (<1%) of sIL‐6R generation. 23
The IL‐6/sIL‐6R complex can bind directly to gp130, without the need for target cells to possess mbIL‐6R. As gp130 can be found on the plasma membrane of almost all cell types, this vastly increases the variety of target cells that are responsive to IL‐6/sIL‐6R (compared to those that are responsive to IL‐6 alone), thus broadening the cytokine's actions. In the downstream signal transduction of trans‐signaling, gp130 dimerization leads to activation of the JAK–STAT cascade and subsequent modification of gene expression similar to that seen in classical signaling. However, the different nature of the target cells in question, along with their different repertoires of JAK–STAT target genes, means that trans‐signaling results in pro‐inflammatory actions such as promotion of leukocyte infiltration, inhibition of T‐cell apoptosis, and impairment of the immunosuppressive actions of regulatory T cells, 10 , 24 rather than the predominately anti‐inflammatory effects evoked during classical signaling.
Several factors are known to influence the balance between the anti‐inflammatory, classical signaling and pro‐inflammatory, trans‐signaling actions of IL‐6. Higher plasma levels of free sIL‐6R would be expected to promote greater rates of trans‐signaling due to the potential to form more IL‐6/sIL‐6R complexes. However, inhibition of IL‐6 trans‐signaling occurs naturally in vivo, with a soluble version of gp130 (sgp130) able to bind to IL‐6/sIL‐6R to produce an inactive complex that is unable to take part in IL‐6 signaling. Thus, sgp130 has been suggested to act as part of a buffer system, preventing an excessive degree of pro‐inflammatory trans‐signaling. 25 , 26
Genetics can also affect the classical/trans‐signaling balance. For example, the single nucleotide polymorphism (SNP) rs2228145 within the IL‐6R gene leads to substitution of Aspartic acid with Alanine in position 358 of the encoded protein, which in turn favors the sIL‐6R shedding process described above. 27 Thus, two variants are associated with this SNP, the major Asp358 variant (A allele) and the minor Ala358 variant (C allele), with the “CC” genotype being associated with approximate doubling of the plasma concentration of sIL‐6R compared to the AA variant. 28 Studies have linked this SNP with a number of conditions including non‐alcoholic steatohepatitis, 29 rheumatoid arthritis, 30 and the adult onset of type 1 diabetes mellitus. 31 Most recently, rs2228145 genotype was found to be associated with the likelihood of being hospitalized following COVID‐19 infection. 32 As will be described in more detail below, it appears that the mechanism behind these SNP/disease associations may be attributed to variations in circulating levels of sIL‐6R and/or variations in retention of mbIL‐6R molecules on the plasma membrane. 26 , 33
Currently, it is unknown whether the rs2228145 SNP has an impact on exercise. However, we have recently published data indicating an association between self‐selected physical activity levels and rs2228145 genotype, with the AA genotype being associated with greater activity levels, leading to the proposal that increased levels of sIL‐6R may disrupt the classical/trans‐signaling balance in individuals bearing the C allele. 34 This is supported by the fact that, with plasma sIL‐6R being in excess of IL‐6 at rest, increased production of IL‐6 as a myokine results in increased formation of the IL‐6/sIL‐6R complex following exercise. 35 , 36 Thus, the higher sIL‐6R levels that accompany the CC genotype may lead to increased trans‐signaling (and reduced classical signaling) following exercise; this could result in reduced exercise tolerance and lower self‐selected physical activity levels. 34
Thus, as shown in Table 1, different levels of IL‐6, sIL‐6R, and sgp130 are seen in different contexts, including exercise. Given the roles of IL‐6, sIL‐6R, and sgp130 in triggering the downstream signaling processes outlined above, it seems reasonable to conclude that the IL‐6 signaling axis plays a significant role in many aspects of health, disease, and exercise performance. Therefore, this review will consolidate our current understanding of how exercise can modulate IL‐6 signaling, with a focus being placed on the actions of IL‐6, sIL‐6R, sgp130, and the rs2228145 SNP during both acute exercise and long‐term training. We will also explore how knowledge of the IL‐6 signaling pathway could be useful for determining the success of a training program, and in the personalization of exercise prescription.
TABLE 1.
Typical plasma concentrations of IL‐6, sIL‐6R, and sgp130 reported in health, type 2 diabetes/cardiovascular disease, and exercise
| IL‐6 | sIL‐6R | sgp130 | |
|---|---|---|---|
| Resting healthy | 1–5 pg/mlA,B,D | 20–30 ng/mlA,B | 250–500 ng/mlA,B |
| Resting type2 diabetes mellitus | 5–10 pg/mlD | 15–40 ng/mlC | 100–300 ng/mlC |
| Resting Atherosclerosis | 5–24 pg/mlD,E | 40 ng/mlD,E | 200–300 ng/mlD,E |
| Post‐exercise healthy | 5–100 pg/mlA,B | 25–30 ng/mlA,B | 250–500 ng/mlA,B |
1.1. IL‐6 and acute exercise
During exercise, skeletal muscle can acutely elevate plasma IL‐6 concentrations by greater than 100‐fold (Table 1). 39 , 40 An even greater increase in IL‐6 is thought to occur within the skeletal muscle fibers themselves as well as in the surrounding interstitial fluid, 41 which is likely to reflect the autocrine and paracrine activities of IL‐6. Although not a universal finding, 41 some evidence suggests that it is specifically the type I muscle fibers that are the predominant source of plasma IL‐6. 42 , 43 , 44 , 45 As type I muscle fibers are heavily recruited even at low percentages of maximal force production, this suggests that the IL‐6 response may be greater in long‐duration moderate‐intensity exercise compared to resistance training. 14 Small, additional amounts of plasma IL‐6 may derive from the responses of tendinous tissue to the mechanical stress involved in exercise, but this has been reported to account for only a small proportion of total exercise‐associated IL‐6 production. 46 , 47 Lauritzen et al. (2013) found that IL‐6 is stored in vesicles within skeletal muscle fibers, which are depleted through trafficking to the cell surface during exercise. Exercise also upregulates the transcription of IL‐6 mRNA within myocytes, 48 which leads to the replenishment of these vesicular IL‐6 stores.
1.2. IL‐6 and exercise duration
Plasma IL‐6 concentrations peak upon, or shortly after, exercise cessation. Repeat measures taken within an exercise bout have demonstrated that IL‐6 production displays a non‐linear relationship with time. 39 , 49 Instead, plasma IL‐6 appears to rise almost exponentially as a function of exercise duration. It is unsurprising, then, that exercise duration is the primary determinant of the size of the IL‐6 peak. Fischer (2006) found duration could explain over 50% of the variance in post‐exercise values. In support of this, the greatest reported increase in plasma IL‐6 (8000‐fold) was found after a 246 km foot race, 40 while more than a 10‐fold increase is rarely observed in exercise that lasts for less than one hour. 14
1.3. IL‐6 and exercise intensity
Despite exercise duration being the main determinant of the IL‐6 response, exercise intensity is also a contributing factor. Ostrowski et al. (2000) found that peak IL‐6 concentration after a marathon race was positively correlated with running intensity. 50 Similarly, our research group has reported that increasing exercise intensity amplifies the plasma IL‐6 response in duration‐matched cycling bouts. 51 Specifically, this study found that 35 minutes of low‐intensity cycling (mean %VO2max = 50.4 ± 4.6%) led to 1.4 ± 0.1‐fold increases in plasma IL‐6, while high‐intensity bouts (mean VO2max = 69.2 ± 2.1%) of the same duration led to a significantly greater 2.7 ± 0.6‐fold increase (p = 0.04). The increase in IL‐6 was correlated with exercise intensity (%VO2max, %HRmax, lactate, RER). It would also appear that a minimum exercise intensity may be required to induce an IL‐6 response, at least in short‐duration activities. Markovitch et al. (2008) saw no increases in plasma IL‐6, or any anti‐inflammatory responses, to a single 30‐minute walk at 50% VO2max in sedentary middle‐aged men, 52 despite training programs using similar intensities giving many health benefits. 53 However, 5 hours at 40% VO2max (albeit in a different mode of activity: one‐legged dynamic knee extension) has been shown to produce a 19‐fold increase in plasma IL‐6. 39 Taken together, it can be concluded that the interaction between both exercise intensity and exercise duration is important in determining the plasma IL‐6 response to a work bout.
1.4. IL‐6 and glycogen availability
Carbohydrate supplementation during exercise has been shown to attenuate IL‐6 production, 54 whereas depletion of muscle glycogen stores leads to an amplification of the IL‐6 response. Steensberg et al. (2001) found that prior depletion of skeletal muscle glycogen led to increases in both IL‐6 mRNA expression within myocytes and IL‐6 release into the circulation. 55 An editorial that accompanied this article stated the following: “the IL‐6 response may be a signal indicating that muscle glycogen stores are reaching critically low levels and that the active muscles' reliance on blood glucose as a source of energy is on the increase”. 56 As glycogen depletion is associated with both exercise duration and intensity, it may be that glycogen content within the skeletal muscle is the major biochemical determinant of IL‐6 production. Indeed, we know that muscle glycogen stores can modify the metabolic signaling response to exercise. For example, low muscle glycogen amplifies the exercise response of AMPK (which, as stated above, is linked to IL‐6 expression/release 57 ), increasing both its activity and its translocation to the nucleus. 58 Similarly, low‐glycogen conditions increase p38 MAPK activity in the nucleus, an effect that has been associated with increased expression of IL‐6 mRNA. 59
1.5. IL‐6 and training status
A period of endurance training reduces the magnitude of the IL‐6 response to an exercise bout of the same absolute intensity, which is likely explainable by reduced rates of muscle glycogen utilization due to greater mitochondrial enzyme activity. 60 However, training does not appear to alter the IL‐6 response to the same relative intensity; Fischer et al. (2004) found that the plasma IL‐6 response to 3 hours of dynamic two‐legged knee extension performed at 50% of Pmax was unchanged following 10 weeks of endurance training, despite absolute workloads being 44% higher post‐training. Interestingly, training led to significant blunting of post‐exercise increases in IL‐6 mRNA expression (87‐fold increase to eightfold increase after training). Muscle glycogen concentrations at the end of the 3‐hour exercise bout were not different following the training period. However, pre‐exercise muscle glycogen levels were 74% higher following the training period, which the authors suggest could have reduced the metabolic challenge in the early period of the exercise bout and helped to explain the reduced mRNA expression. 61
Despite the longitudinal findings of Croft et al. (2009), 60 cross‐sectional research suggests that trained individuals may produce more IL‐6 than untrained, even when working at the same relative intensity. 62 This echoes the observations of Ostrowski et al. (2000) who saw the highest post‐marathon IL‐6 concentrations in the fastest runners, 50 suggesting that the absolute metabolic rate within the working muscles may also influence IL‐6 production. Further, Antunes et al. (2018) found that the magnitude of the IL‐10 (an anti‐inflammatory cytokine produced downstream of IL‐6) response to exercise was positively associated with exercise performance in spite of a fixed relative exercise intensity. 63 Thus, the literature suggests that training status may have a meaningful influence on the IL‐6 response to exercise, although this has less of an effect compared to exercise intensity, duration, or muscle glycogen status.
1.6. IL‐6 and exercise mode
The highest post‐exercise plasma concentrations of IL‐6 are typically reported following running rather than cycling or knee‐extensor exercise. 14 Direct comparisons between cycling and running at similar exercise intensities also find higher post‐exercise IL‐6 levels following running. 64 This may be due to the greater amount of muscle mass that is recruited with running, meaning that more muscle fibers will be stimulated to produce IL‐6. On the contrary, the greater IL‐6 concentrations we see following running may be a result of eccentric muscle contraction, resulting in increased muscle damage and IL‐6 leaking into the circulation. It could also be that the increased muscle damage recruits leukocytes to the area and these release IL‐6 as part of an inflammatory response. The later argument is supported by the correlation between creatine kinase (a marker of muscle damage) and IL‐6 levels following a 160‐km ultramarathon. 65
Taken together, therefore, these data suggest that the IL‐6 response to exercise is significantly elevated with reduced glycogen stores; and that numerous factors such as training status, diet, and the duration, mode, and relative intensity of the exercise may impact on the extent to which this occurs during exercise.
1.7. Mechanistic links between exercise and IL‐6 production
While it is well established that exercise duration, intensity, and glycogen depletion are all factors which mediate the release of IL‐6 stores from skeletal muscle fibers, the molecular mechanism(s) through which IL‐6 production occurs are less clear. It has been proposed that lactate production within the skeletal muscle is a mediator of the IL‐6 response to exercise. Hojman et al (2019) found strong associations between plasma lactate and plasma IL‐6 concentrations during a 2‐hr high‐intensity cycling protocol (R2 = 0.70, p < 0.001). 66 The authors also used a mouse model to show that injecting lactate into resting skeletal muscle resulted in a fivefold increase in plasma IL‐6 and a 27‐fold increase in intramuscular IL‐6 mRNA expression. 66 It was suggested that lactate induces the exocytosis of IL‐6 from intracellular vesicles within skeletal muscle fibers. 66 Lactate production may be able to explain the IL‐6 response to high‐intensity exercise; however, it is unclear how this fits with the large increases in IL‐6 that are found in response to long‐duration exercise at intensities below the lactate threshold 40 where muscle lactate is not elevated above resting levels. 67 Additionally, the lactate hypothesis fails to explain the amplifying effect of glycogen depletion on IL‐6 production, with a low‐glycogen state impairing carbohydrate metabolism via glycolysis. 68
An alternative mechanism for IL‐6 production may be through the actions of AMPK. Exercise‐associated increases in AMPK activity within skeletal muscle have been shown to correlate with the magnitude of IL‐6 production. 69 However, the literature is conflicting as to the direction of the IL‐6/AMPK relationship. Exposing in vivo mouse muscle fibers to AICAR (an AMPK activator) causes the exocytosis of IL‐6 vesicles. 70 Yet, IL‐6 has also been demonstrated to increase AMPK activation, possibly through increasing the concentration of cAMP, in rat muscle in vitro. 71 Activation of AMPK occurs when cells are in low‐energy state (i.e., when ATP is depleted), which is similar to the conditions under which IL‐6 is produced by myocytes. AMPK activation is also known to trigger other metabolic actions within myocytes such as the translocation of GLUT4. 72 Thus, regardless of whether AMPK is an activator of IL‐6 or vice versa, these two signaling molecules appear to be mechanistically linked.
Other possible mechanisms have also been suggested. One such possibility is excitation‐transcription coupling, 73 in which myocyte depolarization leads to the secretion of ATP into the extracellular space. The excreted ATP then binds to purinergic receptors (P2Y) within the sarcolemma which causes a transient “slow” increase in cytosolic and nuclear Ca2+ via inositol trisphosphate‐dependent mechanisms. (This is a distinct process from the “fast” Ca2+ spikes that occur as part of excitation‐contraction coupling as mediated by dihydropyridine and ryanodine receptors 74 ). The Ca2+ slow component, which results in Ca2+ accumulation within the nucleus, activates transcription factors such as AP‐1 and NF‐κB, which trigger IL‐6 mRNA expression alongside transcription of many other genes. 73 Once this mRNA is translated to functional IL‐6, it is stored in vesicles within the cytoplasm until stimulated for release through muscle contraction. 70
Finally, it should also be noted that IL‐6 that has been released from skeletal muscle fibers can act in an autocrine manner, stimulating the further release of IL‐6 in a positive feedback loop. 73 This could relate to the observations that IL‐6 activates AMPK 71 which can in turn trigger the exocytosis of IL‐6 vesicles, 70 and may explain the exponential rise in plasma IL‐6 that occurs in response to exercise duration.
Thus, although further research is required to definitively identify the molecular mechanism(s) by which exercise is linked to increased production of IL‐6 in contracting muscle, metabolic challenge appears to be a common feature that links all of the proposed signaling pathways. Nevertheless, IL‐6 signaling's context‐dependent nature should be borne in mind when attempting to draw such an overarching conclusion—for example, muscle damage can stimulate IL‐6 release as a response to the mechanical stresses involved in exercise. 46 However, such IL‐6 release has been reported to largely derive from tendinous tissue (rather than skeletal myocytes), and to account for only a small proportion of total exercise‐associated IL‐6 production. 46 , 47
2. ACUTE ACTIONS OF IL‐6 DURING EXERCISE
Given that IL‐6 production appears to be elevated under conditions where metabolic substrate availability is challenged (i.e., prolonged and intense exercise with reduced glycogen availability), the primary role of IL‐6 in exercise may be to act as an energy sensor. In this regard, IL‐6 acts in autocrine, paracrine, and endocrine fashions to defend against this challenge and maintain the working muscles with energy substrates (see Figure 1).
FIGURE 1.
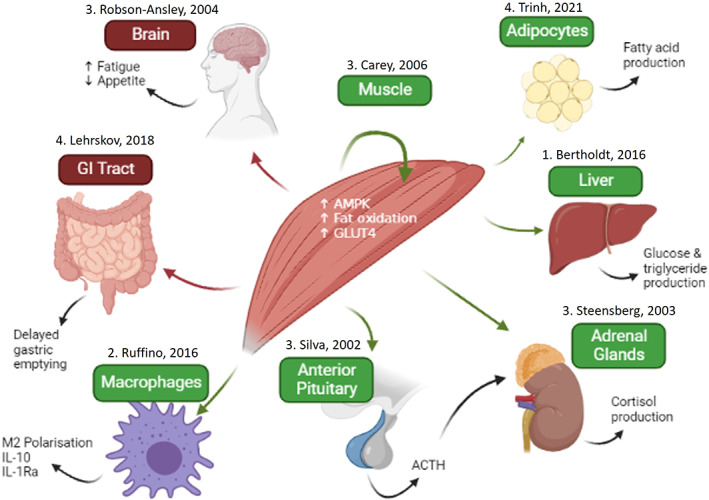
Actions of IL‐6 during and following exercise. Green represents classical signaling and red represents trans‐signaling as the likely mechanisms of action. Numbers (1–4) represent levels of evidence: 1—rodent studies, 2—human cell studies, 3—human infusion studies, and 4—human tocilizumab studies.
2.1. Autocrine/paracrine intramuscular actions of IL‐6 during exercise
Locally, IL‐6 promotes the translocation of the glucose transporter GLUT4 from intracellular vesicles to the plasma membrane of muscle fibers, as demonstrated using an exercising mouse model with IL‐6 neutralization. 75 This enables an increased glucose flux from the blood to the working skeletal muscle fibers. Meanwhile, IL‐6 has been shown to upregulate fatty acid oxidation and inhibit the lipogenic effects of insulin within isolated myocytes, 76 while IL‐6 infusions in humans at rest can also increase fatty acid oxidation and promote lipolysis of fat stores within skeletal muscle tissue in vivo. 77 If these experimental models accurately represent the actions of muscle‐derived IL‐6 during exercise, then this myokine may facilitate a decreased reliance upon glucose metabolism, which could be beneficial for example during long‐duration aerobic exercise. These local effects of IL‐6 appear to be mediated via AMPK activation, 78 which likely occurs via classical signaling due to the fact the skeletal muscle fibers express mbIL‐6R. Interestingly, given the anti‐inflammatory effects of exercise‐associated IL‐6 classical signaling described below, there is evidence that obesity and type 2 diabetes result in “IL‐6 resistance” with decreased levels of AMPK activation for a given IL‐6 exposure. 79 , 80
2.2. Endocrine actions of IL‐6 during exercise
IL‐6 can also act in an endocrine fashion to enable muscle‐organ crosstalk. 81 One such organ is adipose tissue, whose relevance to exercise‐associated health benefits derives from its role as a major source of many pro‐inflammatory mediators that drive chronic inflammation in physical inactivity‐related diseases such as type‐2 diabetes. 82 Infusion with recombinant human IL‐6 has been shown to promote lipolysis and lead to increased release of fatty acid into the bloodstream. 83 Conversely, blocking IL‐6 signaling, using the IL‐6R antibody tocilizumab, impairs fatty acid mobilization both at rest and during exercise 6 and impairs the reductions in visceral fat mass associated with a 12‐week exercise training program. 84 Adipocytes express mbIL‐6R, suggesting that IL‐6 is acting via classical signaling in this context. 85 We have previously reported that muscle‐derived IL‐6 secretion during exercise correlates with upregulation of anti‐inflammatory gene expression and also improved insulin sensitivity, over the course of an 8‐week walking program. 16 Thus, although such correlations do not necessarily imply causation, it is possible that in exercise IL‐6 may act to suppress the pro‐inflammatory actions of adipocytes, and to mobilize fatty acids that can then be used as metabolic substrates by the working muscles.
It is known that IL‐6 also acts as a messenger between skeletal muscle and the gut. Infusion with recombinant IL‐6 delays gastric emptying, leading to reductions in the postprandial peaks in glucose and insulin, while gastric emptying is delayed post‐exercise with tocilizumab treatment. 86 These actions of IL‐6 on the gut may be evolutionarily advantageous as it leads to a more prolonged absorption of glucose, which is therefore spared for later during the exercise bout in question. This may also have specific implications for the brain and nervous system which heavily rely on glucose metabolism. Hence, the actions of IL‐6 on the gut could be an anticipatory mechanism that favors the potential for long‐term glucose requirements over the immediate needs of the skeletal muscle and nervous system. Intestinal tissue has been shown to be responsive to both classical and trans‐signaling, although it has been suggested that the effects of IL‐6 predominantly occur via the trans‐signaling pathway. 87
IL‐6 has been linked to exercise‐associated perceptions of fatigue, 88 which suggests that IL‐6 signaling occurs within the central nervous system (CNS). Rat and mouse models have shown that, while the brain can produce IL‐6, 89 muscle‐derived IL‐6 can cross the blood–brain barrier during exercise, 90 and sIL‐6R expression has also been observed within the brain. 88 Thus, it seems plausible that IL‐6/sIL‐6R complexes may trigger trans‐signaling effects in the CNS during or following exercise. It is also possible that IL‐6 modulates CNS activity indirectly through acting on peripheral afferent neurons or via signaling in the circumventricular organs which lie outside the blood–brain barrier. 91 Accordingly, injection with recombinant IL‐6 and the increases in resting IL‐6 seen as a consequence of sleep deprivation have been shown to induce fatigue at rest and during exercise, and to impair 10‐km race performance in trained male runners. 8 , 92 , 93 Correlations have been observed between IL‐6, sIL‐6R, sgp130 and alterations in brain activity during exercise. 88 In line with this, we have reported correlations between sIL‐6R levels and self‐reported perceptions of fatigue, stress, mood, and upper respiratory tract infection prevalence in athletes during intense training blocks. 94 Outside of exercise, a link between the IL‐6 signaling axis and fatigue has been reported in cancer, depression, and Parkinson's disease. 95 , 96 , 97 This supports the proposal of a neuro‐inflammatory model of fatigue that could be common across disease and exercise. 88 Indeed, IL‐6‐induced fatigue from exercise appears to be an evolutionary mechanism that acts to limit the challenge to homeostasis during long and strenuous activity. 98 Thus, the effects of IL‐6 in the CNS (which are likely to be mediated largely via trans‐signaling) appear to contribute toward preservation of body homeostasis through conveying the sensation of fatigue during intense and prolonged exercise.
As well as the direct effects of IL‐6 signaling, IL‐6 can also induce indirect responses through upregulating secondary signaling molecules. Compared to other cytokines, IL‐6 demonstrates the earliest and most prominent exercise‐associated increases in plasma concentrations. 99 This initial IL‐6 peak induces subsequent secondary peaks post‐exercise (e.g., in IL‐10 and IL‐1Ra 100 ); this is likely achieved by evoking classical signaling responses via interactions with mbIL‐6R on the surface of monocyte–macrophages and various lymphocyte sub‐types. IL‐10 promotes monocyte–macrophage polarization into the anti‐inflammatory M2 phenotype rather than the pro‐inflammatory M1 phenotype, 101 while IL‐1Ra is a soluble cytokine receptor that binds to, and inhibits the actions of, the pro‐inflammatory cytokine IL‐1. 102 Additionally, tocilizumab infusions have also been shown to impair the normal recruitment of natural killer and dendritic cells into the systemic circulation during exercise, highlighting IL‐6's role in modulating the immune response to exercise. 103 Thus, as discussed earlier, an outcome of initial exercise‐associated IL‐6 production may ultimately be to trigger—via such secondary signaling mechanisms—a range of anti‐inflammatory downstream effects. 16
Finally, IL‐6 also interacts with the hypothalamic–pituitary–adrenal (HPA) axis to influence systemic stress responses. Multiple cell types within the HPA axis are sensitive to IL‐6 and possess membrane‐bound receptors for the cytokine. 104 Infusions with recombinant IL‐6 to achieve plasma concentrations similar to those observed after exercise result in increased cortisol production, 100 which likely occurs through IL‐6 both stimulating adrenocorticotropic hormone release from the pituitary, 105 and through directly acting in the adrenal glands 106 (see Figure 1). Although IL‐6 appears to modulate the HPA axis at rest 100 and during infection, 104 how much influence IL‐6 has on the HPA axis during exercise remains unclear. Nevertheless, the commonality of the actions of IL‐6 and cortisol (e.g., anti‐inflammatory effects, and upregulation of blood glucose) suggests that these two signaling molecules are mechanistically linked. Interestingly, the effect of IL‐6 on the HPA axis appears to be subject to a negative feedback loop, whereby adrenocorticotropic hormone and cortisol production are no longer upregulated with chronic IL‐6 exposure. 107 This may partly explain the reduced cortisol response to both absolute and relative workloads that can occur following an exercise training program. 108 Alternatively, it could reflect the dysregulation of the HPA axis which can occur as part of the “overtraining syndrome”, 109 in which the body's ability to recover from strenuous exercise is exceeded, resulting in sustained negative impacts on performance.
It is important to note that despite the aforementioned actions of IL‐6 during exercise in humans, only in mice (to our knowledge) has inhibiting IL‐6 signaling been directly shown to impair exercise performance. 5 , 110 Given that inter‐species differences exist in IL‐6 signaling (and in exercise physiology in general), we should be cautious in extrapolating these findings to humans. Indeed, we have identified one IL‐6‐blockade study in obese humans that examined exercise capacity: In this case, improvements in VO2max following 12 weeks of training were not different between placebo and treatment with the IL‐6/IL‐6R blocker tocilizumab. 84 However, it should be noted both that the participants in this study were obese (with the authors suggesting that IL‐6 may act differently in lean and obese people), and that the actions of IL‐6 in defending muscle metabolic substrate availability are not likely to influence determinants of VO2max. Instead, IL‐6 is more likely to be an important factor in exercise in domains where glycogen depletion and central nervous system function are major fatigue modulators.
In summary, as shown in Figure 1, IL‐6, produced as a myokine by exercising muscles, acts both locally and systemically to maintain muscle fiber homeostasis during and following exercise, and to elicit responses that contribute to exercise‐associated health benefits in the context of chronic inflammatory physical inactivity‐related diseases such as type‐2 diabetes. This is achieved through effects such as increasing energy substrate availability, inducing fatigue to limit an excessive challenge to homeostasis, and promoting an anti‐inflammatory environment within the bloodstream and tissues. Nevertheless, the extent to which IL‐6 signaling is important for exercise performance in humans is currently unknown.
3. IL‐6 AND LONG‐TERM TRAINING
Epidemiological studies have found that resting IL‐6 levels are inversely related to habitual physical activity. 111 , 112 Similarly, taking part in an exercise training program typically leads to a reduction in resting IL‐6 levels, at least in inactive or recreationally active populations. 113 It should be noted that in a rested state, plasma IL‐6 levels do not reflect IL‐6 release from skeletal muscle, but instead reflects production and accumulation from other sources, including leukocytes and adipocytes. The IL‐6 that is released from these cell populations is thought to be indicative of the general chronic inflammatory status of an individual. 113 Indeed, reductions in resting IL‐6 seen in participants with chronic low‐level inflammation undertaking an aerobic exercise training program have been shown to be accompanied by decreases in other inflammatory markers, including C‐reactive protein and tumor necrosis factor‐alpha. 113
The IL‐6 response to training programs in those who are already well‐trained is less clear. Elite athletes who are free from inflammatory conditions typically have resting plasma IL‐6 levels of between 0.1 and 1.0 pg/ml, far below those observed in diseased populations. Such athletes may not have the capacity to reduce IL‐6 further and typically training‐induced reductions have not been observed in this population. 114 , 115 However, it has long been suggested that an elevation in cytokine levels and an accompanying inflammatory state may be a mechanism that leads to the overtraining syndrome. 9 In some—but not all 116 (see Halson and Jeukendrup, 2004)—cases, this is supported by studies in which individuals suffering from overtraining syndrome have elevated levels of IL‐6 and other pro‐inflammatory cytokines, 117 , 118 and short periods of highly intensified training resulted in increased resting IL‐6 levels and increased perceived fatigue. 119 , 120 This suggests that a “U” shaped relationship may exist between training load and basal IL‐6 levels, with non‐excessive training levels being associated with lower IL‐6 levels in either sedentary or excessively exercising individuals. If this is the case, then one may speculate that the IL‐6 response to a training program may be a biomarker of training effectiveness in individuals with underlying chronic inflammation, while also being a marker of an ability to tolerate training load in an athletic population.
Training status also seems to affect the IL‐6 response to an acute exercise bout. Croft et al. (2009) found that the increase in IL‐6 observed after a fixed absolute exercise bout was significantly reduced following 6 weeks of high‐intensity interval training in recreationally active males (a 3.3 pg.ml−1 increase pre‐training vs 1.7 pg.ml−1 post‐training). Lactate response and glycogen utilization during the criterion exercise bout were also significantly reduced while the participants additionally improved their VO2max and running performance. This study supports the notion that glycogen depletion and lactate production impact upon IL‐6 release from the working skeletal muscle. Increased mbIL‐6R surface expression in myocytes due to upregulation of the IL‐6R gene during training, and hence increased skeletal muscle sensitivity to IL‐6 classical signaling, may provide an additional driver for reduced exercise‐associated IL‐6 production in trained individuals. 12 Once again, one may speculate that such IL‐6 responses to a work bout of a fixed absolute intensity may be useful biomarkers for monitoring training adaptations surrounding glycogen storage, mbIL‐6R expression, and energy substrate utilization during submaximal exercise. For example, a reduction in the plasma IL‐6 response to a 2‐hour ride at 200 W performed before and after a training program would suggest a reduced metabolic strain on the working muscles and hence that the training promoted favorable adaptations. This may be of special significance for exercise practitioners where muscle biopsies and indirect calorimetry can be impractical.
IL‐6 signaling may also be important for long‐term adaptations to training. Tocilizumab impairs the increases in cardiac left ventricular mass found following 12 weeks of aerobic training compared to a placebo treatment in humans. 121 The acute increase in IL‐6 following a resistance training session is also predictive of the increase in muscle fiber cross‐sectional area following a 16‐week resistance training program. 122 Meanwhile, recombinant IL‐6 infusions in addition to an exercise program led to a greater increase in exercise performance compared to an exercise program, albeit in mice. 7 The additional IL‐6 appeared to act at the muscle level, with greater increases in levels of oxidative phosphorylation proteins but no difference in VO2max compared to exercise alone.
4. SIL‐6R RESPONSE TO EXERCISE
In contrast to IL‐6, the sIL‐6R response to acute exercise is still poorly understood. Immediately post‐exercise, blood‐borne levels of sIL‐6R have been reported to decrease, remain unchanged, or increased with respect to pre‐exercise baseline values (reviewed in Robson‐Ansley et al., 2010). These discrepancies are likely the result of small study numbers and sample sizes, different exercise protocols, and inconsistencies in controlling for changes in factors such as plasma volume. If there is an acute effect of exercise on sIL‐6R levels, it would appear to involve much smaller proportional changes than is the case for IL‐6. For example, one of the few studies that reported an increase in sIL‐6R levels and controlled for changes in plasma volume recorded a fivefold increase in IL‐6 but only a 1.2‐fold increase in sIL‐6R following a 1‐hour cycling bout. 123 This suggests that the primary mechanism through which exercise acutely modulates the IL‐6 signaling axis is through upregulating the generation and production of IL‐6 by skeletal muscle, and not through altering sIL‐6R levels.
In contrast to the uncertainty surrounding acute exercise, there appears to be a general consensus that long‐term training leads to reductions in plasma sIL‐6R levels. Silverman et al. (2009) reported that the addition of an exercise intervention to a 6‐month diet‐based weight‐loss program led to a significant reduction in sIL‐6R levels in obese and overweight post‐menopausal women compare to dieting alone. Interestingly, a weight loss program in isolation led to increases in sIL‐6R, despite reductions in body mass, BMI, and fat mass that were similar to the group that also took part in an exercise intervention. 124 This suggests that the mechanism responsible for sIL‐6R reductions is independent of changes in fat mass, which is of note as adipocytes produce IL‐6 and so could be hypothesized as playing a role in sIL‐6R regulation. Adamopoulos et al. (2002) also found that resting sIL‐6R levels were significantly reduced following a 12‐week exercise program in chronic heart failure patients (29.2 ± 3.0 ng/ml vs 34.0 ± 3.0 ng/ml, p < 0.01), with levels returning to baseline after 12 weeks of detraining (35.5 ± 3.0 ng/ml). 125 Interestingly, an age/gender‐matched control group that was disease‐free reported no reductions in sIL‐6R or any other inflammatory markers following the same 12‐week training program (5.8 ± 0.2 ng/ml vs. 5.7 ± 0.3 ng/ml). 125 This could be due to the control group already having much lower sIL‐6R levels at baseline or that the training stimulus (30 min of cycling at 60%–80% of heart rate max carried out 5 days per week) was insufficient to induce physiological changes in this population. 125 Work done in our research group with athletes suggests that the latter may be the case: In highly trained swimmers who were completing between 19 and 89 km/week, resting sIL‐6R levels were negatively associated with the previous week's training distance (r = −0.74, p = 0.005 94 ). Notably, those with higher levels of sIL‐6R had worse perceptions of moods and greater perceived stress, which could have resulted from increased IL‐6 trans‐signaling within the CNS (as described previously).
It would appear, then, that an appropriate training dose can reduce sIL‐6R levels in all individuals, regardless of training status. Such reductions have been tentatively attributed to exercise‐associated regulatory mechanisms controlling either expression/activation of proteases responsible for mbIL‐6R “shedding”, 126 or influx of leukocyte subpopulations (as sources of sIL‐6R “shedding”) into the vicinity of damaged muscle tissue post‐exercise. 13
Importantly, because training‐associated sIL‐6R changes appear to take place over a timescale of days or weeks, such changes are detectable via a program of weekly capillary blood sampling, whose relatively non‐invasive nature would not overly inconvenience the patient/participant. As sIL‐6R reductions would shift the balance of the IL‐6 signaling axis toward anti‐inflammatory (classical signaling) and away from pro‐inflammatory (trans‐signaling), this points toward a possible role for sIL‐6R as a relatively non‐invasive biomarker for training and health benefits in athletes and other participants in exercise. For example, reductions in sIL‐6R levels in a recreationally active individual may indicate favorable adaptations to a training program, with the balance shifting toward more classical and less trans‐signaling. On the contrary, an athlete with elevated levels of sIL‐6R despite performing a large amount of training could indicate a shift toward a pro‐inflammatory state as a result of increased trans‐signaling and a maladaptive response to their exercise program.
5. EXERCISE AND THE SGP130 RESPONSE
Another important regulator of the IL‐6 signaling axis is sgp130, which acts to prevent excessive rates of trans‐signaling through binding to, and buffering of, IL‐6:sIL‐6R complexes. 25 Therefore, given the impact of exercise on IL‐6 and sIL‐6R, it is necessary to also consider the possibility that exercise may influence sgp130 signaling. Under normal conditions, plasma levels of sgp130 appear to be in excess of those of sIL‐6R, with serum concentration of up to 400 ng/ml. 127 This represents the capacity of sgp130 to act as a trans‐signaling buffer.
However, the sgp130 buffer system can be disturbed in certain conditions. 25 Recently, Bonomi et al. (2020) identified 26 SNPs in the gp130 gene that influenced serum sgp130 levels, of which two have been previously linked to inflammatory and cardiovascular conditions; however, regression analysis revealed that the 26 SNPs could only explain 11% of sgp130 variation, with no single SNP being responsible for more than 1%. 128 Following acute exercise, venous sgp130 levels have been reported as either slightly increased 123 , 129 or unchanged 130 compared to baseline, while we have reported no association between capillary blood sgp130 levels and the level of physical activity undertaken in the previous week. 34 It should be noted that neither of the studies that reported small increases in sgp130 controlled for the changes in plasma volume that can occur during exercise, and so it may be that their observations are an artifact of hemoconcentration. Similarly, the mechanism through which exercise could upregulate sgp130 is unclear. It appears that the majority, if not all, of the sgp130 that is present in the plasma derives from alternative spliced mRNA sequences, while shedding of membrane‐bound gp130 is unlikely. 131 Interestingly, alternative splicing gives rise to three different isoforms of sgp130 with molecular weights of 50, 90, and 110 kDa, 127 , 132 whose expression and capacity to block IL‐6 trans‐signaling varies by leukocyte type. 131 Hypothetically, then, exercise could alter the sgp130 buffer system through the recruitment or stimulation of immune cells to produce more sgp130, or through impacting upon the alternative splicing process and altering which sgp130 isoform is produced. Either of these effects could extend the capacity of the buffer system, and so decrease the fraction of newly produced IL‐6 involved in trans‐signaling.
However, to our knowledge, no reports exist of such effects occurring in those undertaking exercise. Therefore, further research is required to establish whether sgp130 fluctuations do indeed occur during exercise, and also whether there are changes in sgp130 isoform expression. Nevertheless, as shown in Table 1, it appears that plasma sgp130 levels demonstrate much less variability than that of either IL‐6 or sIL‐6R, 133 suggesting that these latter two molecules play larger roles in mediating the impact of exercise on the IL‐6 signaling axis.
6. INFLUENCE OF THE RS2228145 SNP
In contrast to the limited genetic influence on sgp130 levels (as described above), the rs2228145 SNP, an A → C missense mutation in the IL‐6R nucleotide sequence, can explain around half of the variation in plasma sIL‐6R levels within the population. 27 This SNP impacts upon a site adjacent to ADAM‐10/ADAM‐17 cleavage site on the mbIL‐6R protein (Valine356), with the resulting Aspartate→Alanine358 change increasing the susceptibility of mbIL‐6R to these proteases. Subsequently, sIL‐6R shedding is upregulated, with each inherited C allele associated with a ~ 50% increase in plasma sIL‐6R levels. 27 These increases appear to be of clinical significance, with rs2228145 genotype linked with several inflammatory conditions, including COVID‐19, rheumatoid arthritis, asthma, and non‐alcoholic steatohepatitis. 29 , 30 , 32 , 134
To our knowledge, only one study has explored the relationship between rs2228145 and the response to an exercise program. 135 In this study, AA homozygotes responded differently to a nutritional and exercise intervention compared to bearers of the CC genotype: Specifically, AA participants had reductions in waist circumference, triglycerides and fasting plasma glucose that were not evident in the CC group. Although this study was hindered by small sample sizes and not directly measuring plasma sIL‐6R levels, it does provide preliminary evidence that rs2228145 could moderate responsiveness to lifestyle interventions. 135 These findings suggest that CC homozygotes may not experience the same increase in IL‐6 classical signaling that accompanies an acute exercise bout, with higher plasma sIL‐6R levels prompting IL‐6 to either follow the trans‐signaling pathway or to be locked up within the sgp130 buffer system. If so, this may dampen the metabolic regulatory and anti‐inflammatory actions of IL‐6. In support of this hypothesis, our research group has recently found that individuals with the AA genotype had lower plasma sIL‐6R levels and appeared to take part in more physical activity compared to those with other genotypes. 34 This suggests that the differences in the IL‐6 signaling pathway that arise from the SNP may make physical activity less tolerable to those bearing the CC genotype, possibly through increased fatigue or alternatively because exercise has previously been an ineffective lifestyle intervention for them. As mentioned above, evidence from both our research group and that of Vargas et al 88 , 93 , 94 , 136 regarding the impact of sIL‐6R (and therefore trans‐signaling) on fatigue, mood, and other psychological parameters may provide a mechanism to underpin such a proposal. These findings suggest that the rs2228145 SNP may be of use as a novel biomarker for exercise tolerance, and hence potentially a useful tool to aid coaches and exercise providers in producing individualized training programs. However, more research is needed to confirm whether these observations can be repeated in larger cohorts of participants, and also exploring whether genetically‐influenced resistance to exercise programs can be overcome through adjusting exercise mode, intensity, and duration.
7. PERSPECTIVES
In this review, we have attempted to consolidate the current understanding of the IL‐6 signaling pathway and how it is influenced by both acute exercise and chronic training. Although many excellent reviews on individual molecules within the pathway already exist, 137 , 138 we hope to have provided a more integrated synthesis of the literature to enable a greater appreciation of how the balance between IL‐6, sIL‐6R, and sgp130 is crucial in determining signaling outcomes. We also hope that we have increased the awareness of the rs2228145 SNP which, although gaining wider interest in clinical circles, still appears to be underappreciated within the sports science community. The authors believe that the appropriate monitoring of the components of the IL‐6 signaling pathway and its relevant genetic variants could provide insightful information relevant to both health and athletic performance. Examples of such insights include assessments of inflammatory status, metabolic substrate utilization, prevalence of illness, training load tolerance, and submaximal fitness.
8. CONCLUSION
The IL‐6 signaling axis plays important roles in both influencing inflammatory status and contributing to the normal physiological, and potentially psychological, responses to exercise. IL‐6 is produced acutely by the working muscle, with the magnitude of this response relating to exercise intensity, exercise duration, and muscle glycogen content. This muscle‐derived IL‐6 acts with sIL‐6R and sgp130 to maintain, both locally and systemically, homeostasis through increasing energy substrate availability, preventing excessive physical activity through increasing perception of effort, and promoting an anti‐inflammatory milieu within the body. It may be of interest from a therapeutic perspective to note that long‐term training programs can help to lower chronic plasma IL‐6 and sIL‐6R levels, and so contribute to protection from, or even reversal of, the progression of chronic inflammatory diseases. Finally, the IL6‐R SNP rs2228145, which impacts on sIL‐6R levels, and thereby influences IL‐6 trans‐signaling and possibly reduces exercise tolerance, provides the prospect that genetic factors can also impact on the IL‐6 signaling axis and hence influence the body's response to exercise. In conclusion, therefore, we recommend that future research should look to further explore the impact of exercise on this signaling axis, and the consequences of this impact on personalized (and therefore genetics‐based) provision of exercise training programs, in the context of both physical inactivity‐related disease, and sporting performance.
FUNDING INFORMATION
Funding for this work was provided by the Knowledge Economy Skills Scholarship 2 East (KESS 2 East) scheme in association with partner organizations Sport Wales, Welsh Athletics, and Welsh Triathlon.
CONFLICT OF INTEREST
The authors declare no conflicts of interest. All material has been created by the authors.
Nash D, Hughes MG, Butcher L, et al. IL‐6 signaling in acute exercise and chronic training: Potential consequences for health and athletic performance. Scand J Med Sci Sports. 2023;33:4‐19. doi: 10.1111/sms.14241
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Rahn S, Becker‐Pauly C. Meprin and ADAM proteases as triggers of systemic inflammation in sepsis. FEBS Lett. 2022;596(5):534‐556. doi: 10.1002/1873-3468.14225 [DOI] [PubMed] [Google Scholar]
- 2. Dittmer D, Calzada‐Nova G, Velazquez‐Salinas L, Verdugo‐Rodriguez A, Rodriguez LL, Borca MV. The role of interleukin 6 during viral infections. Front Microbiol. 2019;10:01057. doi: 10.3389/fmicb.2019.01057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen Y, Qiao Y, Pan Y, et al. Cytokine correlation between serum interleukin‐6 level and type 1 diabetes mellitus: a systematic review and meta‐analysis. Cytokine. 2017;94:14‐20. doi: 10.1016/j.cyto.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 4. Starr ME, Evers BM, Saito H. Age‐associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL‐6. J Gerontol A Biol Sci Med Sci. 2009;64(7):723‐730. doi: 10.1093/gerona/glp046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fäldt J, Wernstedt I, Fitzgerald SM, Wallenius K, Bergström G, Jansson JO. Reduced exercise endurance in interleukin‐6‐deficient mice. Endocrinology. 2004;145(6):2680‐2686. doi: 10.1210/en.2003-1319 [DOI] [PubMed] [Google Scholar]
- 6. Trinh B, Peletier M, Simonsen C, et al. Blocking endogenous IL‐6 impairs mobilization of free fatty acids during rest and exercise in lean and obese men. Cell Rep Med. 2021;2(9):100396. doi: 10.1016/J.XCRM.2021.100396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leuchtmann AB, Furrer R, Steurer SA, et al. Interleukin‐6 potentiates endurance training adaptation and improves functional capacity in old mice. J Cachexia Sarcopenia Muscle. 2022;13:1164‐1176. doi: 10.1002/jcsm.12949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robson‐Ansley PJ, De Milander L, Collins M, Noakes TD. Acute interleukin‐6 administration impairs athletic performance in healthy, trained male runners. Can J Appl Physiol. 2004;29(4):411‐418. doi: 10.1139/h04-026 [DOI] [PubMed] [Google Scholar]
- 9. Smith LL. Cytokine Hypothesis of Overtraining: A Physiological Adaptation to Excessive Stress? Med Sci Sports Exerc. 2000;32:317‐331. [DOI] [PubMed] [Google Scholar]
- 10. Scheller J, Chalaris A, Schmidt‐arras D, Rose‐john S. The pro‐ and anti‐inflammatory properties of the cytokine interleukin‐6. Biochim Biophys Acta. 2011;1813:878‐888. doi: 10.1016/j.bbamcr.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 11. Kamimura D, Ishihara K, Hirano T. IL‐6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1‐38. doi: 10.1007/S10254-003-0012-2 [DOI] [PubMed] [Google Scholar]
- 12. Keller C, Steensberg A, Hansen AK, Fischer CP, Plomgaard P, Pedersen BK. Effect of exercise, training, and glycogen availability on IL‐6 receptor expression in human skeletal muscle. J Appl Physiol. 2005;99(6):2075‐2079. doi: 10.1152/japplphysiol.00590.2005 [DOI] [PubMed] [Google Scholar]
- 13. Robson‐Ansley P, Cockburn E, Walshe I, Stevenson E, Nimmo M. The effect of exercise on plasma soluble IL‐6 receptor concentration: a dichotomous response. Exerc Immunol Rev. 2010;16:56‐76. [PubMed] [Google Scholar]
- 14. Fischer CP. Interleukin‐6 in acute exercise and training: What is the biological relevance? Exerc Immunol Rev. 2006;12:6‐33. [PubMed] [Google Scholar]
- 15. Kelly M, Keller C, Avilucea PR, et al. AMPK activity is diminished in tissues of IL‐6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449‐454. doi: 10.1016/j.bbrc.2004.05.188 [DOI] [PubMed] [Google Scholar]
- 16. Ruffino JS, Davies NA, Morris K, et al. Moderate‐intensity exercise alters markers of alternative activation in circulating monocytes in females‐ a putative role for PPARgama. Eur J Appl Physiol. 2016;116(9):1671‐1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lantier L, Fentz J, Mounier R, et al. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J. 2014;28(7):3211‐3224. doi: 10.1096/FJ.14-250449 [DOI] [PubMed] [Google Scholar]
- 18. Petersen AMW, Pedersen BK. The role of IL‐6 in mediating the anti‐inflammatory effects of exercise. J Physiol Pharmacol. 2006;57(10):43‐51. [PubMed] [Google Scholar]
- 19. Lokau J, Agthe M, Garbers C. Generation of soluble Interleukin‐11 and Interleukin‐6 receptors: a crucial function for proteases during inflammation. Mediators Inflamm. 2016;2016:1‐10. doi: 10.1155/2016/1785021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peters M, Jacobs S, Ehlers M, et al. The function of the soluble interleukin 6 (IL‐6) receptor in vivo: sensitization of human soluble IL‐6 receptor transgenic mice towards IL‐6 and prolongation of the plasma half‐life of IL‐6. J Exp Med. 1996;183(4):1399‐1406. doi: 10.1084/jem.183.4.1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schumacher N, Meyer D, Mauermann A, et al. Shedding of endogenous Interleukin‐6 receptor (IL‐6R) is governed by a Disintegrin and metalloproteinase (ADAM) proteases while a full‐length IL‐6R isoform localizes to circulating microvesicles *. J Biol Chem. 2015;290(43):26059‐26071. doi: 10.1074/JBC.M115.649509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Briso EM, Dienz O, Rincon M. CD4 T cells IL‐6R ectodomain shedding in activated cutting edge: soluble IL‐6R is produced by. J Immunol Ref. 2008;180:7102‐7106. doi: 10.4049/jimmunol.180.11.7102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leggate M, Nowell MA, Jones SA, Nimmo MA. The response of interleukin‐6 and soluble interleukin‐6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress Chaperones. 2010;15(6):827‐833. doi: 10.1007/s12192-010-0192-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bin Dhuban K, Bartolucci S, D'Hennezel E, Piccirillo CA. Signaling through gp130 compromises suppressive function in human FOXP3+ regulatory T cells. Front Immunol. 2019;10(10):1532. doi: 10.3389/FIMMU.2019.01532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aparicio‐Siegmund S, Garbers Y, Flynn CM, et al. The IL‐6‐neutralizing sIL‐6R‐sgp130 buffer system is disturbed in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2019;317:E411‐E420. doi: 10.1152/ajpendo.00166.2019.-Serum [DOI] [PubMed] [Google Scholar]
- 26. Garbers C, Aparicio‐Siegmund S, Rose‐John S. The IL‐6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol. 2015;34:75‐82. doi: 10.1016/j.coi.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 27. Galicia JC, Tai H, Komatsu Y, Shimada Y, Akazawa K, Yoshie H. Polymorphisms in the IL‐6 receptor (IL‐6R) gene: strong evidence that serum levels of soluble IL‐6R are genetically influenced. Genes Immun. 2004;5(6):513‐516. doi: 10.1038/sj.gene.6364120 [DOI] [PubMed] [Google Scholar]
- 28. Rafiq S, Frayling TM, Murray A, et al. A common variant of the interleukin 6 receptor (IL‐6r) gene increases IL‐6r and IL‐6 levels, without other inflammatory effects. Genes Immun. 2007;8(7):552‐559. doi: 10.1038/sj.gene.6364414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Topchieva LV, Kurbatova IV, Dudanova OP, Sokolovskaya AA, Shipovskaya AA. IL6R Gene Polymorphic Variant rs2228145 ( C > A ) as a Marker of Genetic Liability to Nonalcoholic Steatohepatitis in the Russian Population of Karelia. Bull Exp Biol Med. 2018;165(1):64‐68. doi: 10.1007/s10517-018-4100-3 [DOI] [PubMed] [Google Scholar]
- 30. Ahmed S, Hussain S, Ammar A, Jahan S, Khaliq S, Kaul H. Interleukin 6 receptor (IL6‐R) gene polymorphisms underlie susceptibility to rheumatoid Arthritis. Clin Lab. 2017;63:1365‐1369. doi: 10.7754/Clin.Lab.2017.170216 [DOI] [PubMed] [Google Scholar]
- 31. Campos LP, Graciolo V, Sousa MM, et al. Polymorphisms rs1800795 of interleukin‐6 and rs2228145 of interleukin‐6 receptor genes in Euro‐ Brazilians with adult‐onset type 1 diabetes mellitus. Genet Mol Res. 2019;18(3):18260. [Google Scholar]
- 32. Bovijn J, Lindgren CM, Holmes MV. Genetic variants mimicking therapeutic inhibition of IL‐6 receptor signaling and risk of COVID‐19. Lancet Rheumatol. 2020;2(11):e658‐e659. doi: 10.1016/S2665-9913(20)30345-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferreira RC, Freitag DF, Cutler AJ, Howson JMM, Rainbow DB. Functional IL6R 358Ala allele impairs classical IL‐6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet. 2013;9(4):e1003444. doi: 10.1371/journal.pgen.1003444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Webb R, Early A, Scarlett B, et al. Provisional designation of the Interleukin‐6 receptor (IL‐6R) as a novel marker gene for exercise tolerance. Exerc Med. 2021;5:001. doi: 10.26644/em.2021.001 [DOI] [Google Scholar]
- 35. Gray SR, Clifford M, Lancaster R, Leggate M, Davies M, Nimmo MA. The response of circulating levels of the interleukin‐6/interleukin‐6 receptor complex to exercise in young men. Cytokine. 2009;47:98‐102. doi: 10.1016/j.cyto.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 36. Baran P, Hansen S, Waetzig GH, et al. The balance of interleukin (IL)‐6, IL‐6·soluble IL‐6 receptor (sIL‐6R), and IL‐6·sIL‐6R·sgp130 complexes allows simultaneous classic and trans‐signaling. J Biol Chem. 2018;293(18):6762‐6775. doi: 10.1074/jbc.RA117.001163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dekker M, Lee S, Hudson R, et al. An exercise intervention without weight loss decreases circulating interleukin‐6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism. 2007;56(3):332‐338. doi: 10.1016/J.METABOL.2006.10.015 [DOI] [PubMed] [Google Scholar]
- 38. Cui Y, Dai W, Li Y. Circulating levels of sgp130 and sex hormones in male patients with coronary atherosclerotic disease. Atherosclerosis. 2017;266:151‐157. doi: 10.1016/J.ATHEROSCLEROSIS.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 39. Steensberg A, Van HG, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin‐6 in contracting human skeletal muscles can account for the exercise‐induced increase in plasma interleukin‐6. J Physiol. 2000;529:237‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goussetis E, Spiropoulos A, Tsironi M, et al. Spartathlon, a 246 kilometer foot race: Effects of acute in flammation induced by prolonged exercise on circulating progenitor reparative cells. Blood Cells Mol Dis. 2009;42(3):294‐299. doi: 10.1016/j.bcmd.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 41. Hiscock N, Chan MHS, Bisucci T, Darby IA, Febbraio MA. Skeletal myocytes are a source of interleukin‐6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 2004;18(9):992‐994. doi: 10.1096/fj.03-1259fje [DOI] [PubMed] [Google Scholar]
- 42. Plomgaard P, Penkowa M, Pedersen BK. Fiber type specific expression of TNF‐alpha, IL‐6 and IL‐18 in human skeletal muscles. Exerc Immunol Rev. 2005;11:53‐63. [PubMed] [Google Scholar]
- 43. Fischer CP, Hiscock NJ, Penkowa M, et al. Supplementation with vitamins C and E inhibits the release of interleukin‐6 from contracting human skeletal muscle. J Physiol. 2004;558(2):633‐645. doi: 10.1113/jphysiol.2004.066779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Banzet S, Koulmann N, Simler N, et al. Fibre‐type specificity of interleukin‐6 gene transcription during muscle contraction in rat: association with calcineurin activity. J Physiol. 2005;566(3):839‐847. doi: 10.1113/JPHYSIOL.2005.089193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liang AP, Drazick AT, Gao H, Li Y. Skeletal muscle secretion of IL‐6 is muscle type specific: ex vivo evidence. Biochem Biophys Res Commun. 2018;505(1):146‐150. doi: 10.1016/J.BBRC.2018.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Langberg H, Olesen JL, Gemmer C, Kjær M. Substantial elevation of interleukin‐6 concentration in peritendinous tissue, in contrast to muscle, following prolonged exercise in humans. J Physiol. 2002;542(3):985‐990. doi: 10.1113/jphysiol.2002.019141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nybo L, Nielsen B, Pedersen BK, Møller K, Secher NH. Interleukin‐6 release from the human brain during prolonged exercise. J Physiol. 2002;542(3):991‐995. doi: 10.1113/jphysiol.2002.022285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 2007;103:1744‐1751. doi: 10.1152/japplphysiol.00679.2007 [DOI] [PubMed] [Google Scholar]
- 49. Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. A trauma‐like elevation of plasma cytokines in humans in response to treadmill running. J Physiol. 1998;513:889‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin‐6 in humans ‐ effect of intensity of exercise. Eur J Appl Physiol. 2000;83(6):512‐515. [DOI] [PubMed] [Google Scholar]
- 51. Cullen T, Thomas AW, Webb R, Hughes MG. Interleukin‐6 and associated cytokine responses to an acute bout of high‐intensity interval exercise: the effect of exercise intensity and volume. Appl Physiol Nutr Metab. 2016;41(8):803‐808. doi: 10.1139/apnm-2015-0640 [DOI] [PubMed] [Google Scholar]
- 52. Markovitch D, Tyrrell RM, Thompson D. Acute moderate‐intensity exercise in middle‐aged men has neither an anti‐ nor proinflammatory effect. J Appl Physiol. 2008;105(1):260‐265. doi: 10.1152/JAPPLPHYSIOL.00096.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim D‐Y, Seo B‐D, Kim D‐J. Effect of walking exercise on changes in cardiorespiratory fitness, metabolic syndrome markers, and high‐molecular‐weight adiponectin in obese middle‐aged women. J Phys Ther Sci. 2014;26:1723‐1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robson‐Ansley P, Walshe I, Ward D. The effect of carbohydrate ingestion on plasma interleukin‐6, hepcidin and iron concentrations following prolonged exercise. Cytokine. 2011;53(2):196‐200. doi: 10.1016/j.cyto.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 55. Steensberg A, Febbraio MA, Osada T, et al. Interleukin‐6 production in contracting human skeletal muscle is influenced by pre‐exercise muscle glycogen content. J Physiol. 2001;537(2):633‐639. doi: 10.1111/j.1469-7793.2001.00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gleeson M. Interleukins and Exercise. J Physiol. 2000;529:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Du J‐H, Xu N, Song Y, et al. AICAR stimulates IL‐6 production via p38 MAPK in cardiac fibroblasts in adult mice: a possible role for AMPK. Biochem Biophys Res Commun. 2005;337:1139‐1144. doi: 10.1016/j.bbrc.2005.09.174 [DOI] [PubMed] [Google Scholar]
- 58. Steinberg GR, Watt MJ, McGee S, et al. Reduced glycogen availability is associated with increased AMPKalpha2 activity, nuclear AMPKalpha2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Appl Physiol Nutr Metab. 2006;31(3):302‐312. doi: 10.1139/H06-003 [DOI] [PubMed] [Google Scholar]
- 59. Chan MHS, Mcgee SL, Watt MJ, Hargreaves M, Febbraio MA. Altering dietary nutrient intake that reduces glycogen content leads to phosphorylation of nuclear p38 MAP kinase in human skeletal muscle: association with IL‐6 gene transcription during contraction. FASEB J. 2004;18(14):1785‐1787. doi: 10.1096/fj.03-1039fje [DOI] [PubMed] [Google Scholar]
- 60. Croft L, Bartlett JD, MacLaren DPM, et al. High‐intensity interval training attenuates the exercise‐induced increase in plasma IL‐6 in response to acute exercise. Appl Physiol Nutr Metab. 2009;34(6):1098‐1107. doi: 10.1139/H09-117 [DOI] [PubMed] [Google Scholar]
- 61. Fischer CP, Plomgaard P, Hansen AK, Pilegaard H, Saltin B, Pedersen BK. Endurance training reduces the contraction‐induced interleukin‐6 mRNA expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287(6):1189‐1194. doi: 10.1152/ajpendo.00206.2004 [DOI] [PubMed] [Google Scholar]
- 62. Cipryan L. The effect of fitness level on cardiac autonomic regulation, IL‐6, total antioxidant capacity, and muscle damage responses to a single bout of high‐intensity interval training. J Sport Heal Sci. 2018;7(3):363‐371. doi: 10.1016/j.jshs.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Antunes BM, Campos EZ, Santos RVTD, et al. Anti‐inflammatory response to acute exercise is related with intensity and physical fitness. J Cell Biochem. 2018;120(3):27810. doi: 10.1002/jcb.27810 [DOI] [PubMed] [Google Scholar]
- 64. Nieman DC, Nehlsen‐Cannarella SL, Fagoaga OR, et al. Influence of mode and carbohydrate on the cytokine response to heavy exertion. Med Sci Sports Exerc. 1998;30(5):671‐678. doi: 10.1097/00005768-199805000-00005 [DOI] [PubMed] [Google Scholar]
- 65. Nieman DC, Dumke CL, Henson DA, McAnulty SR, Gross SJ, Lind RH. Muscle damage is linked to cytokine changes following a 160‐km race. Brain Behav Immun. 2005;19(5):398‐403. doi: 10.1016/J.BBI.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 66. Hojman P, Brolin C, Nørgaard‐Christensen N, et al. IL‐6 release from muscles during exercise is stimulated by lactate‐dependent protease activity. Am J Physiol Endocrinol Metab. 2019;316(5):E940‐E947. doi: 10.1152/ajpendo.00414.2018 [DOI] [PubMed] [Google Scholar]
- 67. Black MI, Jones AM, Blackwell JR, et al. Muscle metabolic and neuromuscular determinants of fatigue during cycling in different exercise intensity domains. J Appl Physiol. 2017;122:446‐459. doi: 10.1152/japplphysiol.00942.2016.-Lactate [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Margolis LM, Wilson MA, Whitney CC, et al. Exercising with low muscle glycogen content increases fat oxidation and decreases endogenous, but not exogenous carbohydrate oxidation. Metabolism. 2019;97:1‐8. doi: 10.1016/j.metabol.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 69. MacDonald CP, Wojtaszewski JF, Klarlund Pedersen B, Kiens B, Richter EA, Richter EA. Interleukin‐6 release from human skeletal muscle during exercise: relation to AMPK activity. J Appl Physiol. 2003;95:2273‐2277. doi: 10.1152/japplphysiol.00242.2003.-We [DOI] [PubMed] [Google Scholar]
- 70. Lauritzen HPMM, Brandauer J, Schjerling P, et al. Contraction and AICAR stimulate IL‐6 vesicle depletion from skeletal muscle fibers in vivo. Diabetes. 2013;62(9):3081‐3092. doi: 10.2337/db12-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kelly M, Gauthier MS, Saha AK, Ruderman NB. Activation of AMP‐activated protein kinase by interleukin‐6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes. 2009;58(9):1953‐1960. doi: 10.2337/db08-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. O'Neill HM, Maarbjerg SJ, Crane JD, et al. AMP‐activated protein kinase (AMPK) β1β2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci. 2011;108(38):16092‐16097. doi: 10.1073/pnas.1105062108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bustamante M, Fernández‐Verdejo R, Jaimovich E, Buvinic S. Electrical stimulation induces IL‐6 in skeletal muscle through extracellular ATP by activating Ca2+ signals and an IL‐6 autocrine loop. Am J Physiol Endocrinol Metab. 2014;306(8):E869‐E882. doi: 10.1152/ajpendo.00450.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Eltit JM, Garcí AA, Hidalgo J, et al. Membrane electrical activity elicits inositol 1,4,5‐trisphosphate‐dependent slow Ca2+ signals through a Gβγ/phosphatidylinositol 3‐kinase γ pathway in skeletal myotubes. J Biol Chem. 2006;281(17):12143‐12154. doi: 10.1074/jbc.M511218200 [DOI] [PubMed] [Google Scholar]
- 75. Ikeda S, Tamura Y, Kakehi S, Sanada H. Biochemical and biophysical research communications exercise‐induced increase in IL‐6 level enhances GLUT4 expression and insulin sensitivity in mouse skeletal muscle. Biochem Biophys Res Commun. 2016;473(4):947‐952. doi: 10.1016/j.bbrc.2016.03.159 [DOI] [PubMed] [Google Scholar]
- 76. Bruce CR, Dyck DJ. Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin‐6 and tumor necrosis factor. Am J Physiol Endocrinol Metab. 2004;287:616‐621. doi: 10.1152/ajpendo.00150 [DOI] [PubMed] [Google Scholar]
- 77. Wolsk E, Mygind H, Grøndahl TS, Pedersen BK, Van Hall G. IL‐6 selectively stimulates fat metabolism in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;299(5):832‐840. doi: 10.1152/AJPENDO.00328.2010 [DOI] [PubMed] [Google Scholar]
- 78. Carey AL, Steinberg GR, Macaulay SL, et al. Interleukin‐6 increases insulin‐stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP‐activated protein kinase. Diabetes. 2006;55:2688‐2697. doi: 10.2337/db05-1404 [DOI] [PubMed] [Google Scholar]
- 79. Sriwijitkamol A, Coletta DK, Wajcberg E, et al. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes a time‐course and dose‐response study. Diabetes. 2007;56:836‐848. doi: 10.2337/db06-1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Scheele C, Nielsen S, Kelly M, et al. Satellite cells derived from obese humans with type 2 diabetes and differentiated into myocytes in vitro exhibit abnormal response to IL‐6. PLoS One. 2012;7(6):e39657. doi: 10.1371/JOURNAL.PONE.0039657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Severinsen MCK, Pedersen BK. Muscle‐organ crosstalk: the emerging roles of myokines. Endocr Rev. 2020;41(4):594‐609. doi: 10.1210/endrev/bnaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Olefsky JM, Glass CK. Macrophages, Inflammation, and Insulin Resistance. Annu Rev Physiol. 2010;72:219‐246. doi: 10.1146/ANNUREV-PHYSIOL-021909-135846 [DOI] [PubMed] [Google Scholar]
- 83. Van HG, Steensberg A, Sacchetti M, et al. Interleukin‐6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88(7):3005‐3010. doi: 10.1210/jc.2002-021687 [DOI] [PubMed] [Google Scholar]
- 84. Wedell‐Neergaard AS, Lang Lehrskov L, Christensen RH, et al. Exercise‐induced changes in visceral adipose tissue mass are regulated by IL‐6 signaling: a randomized controlled trial. Cell Metab. 2019;29(4):844‐855.e3. doi: 10.1016/j.cmet.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 85. Sindhu S, Thomas R, Shihab P, Sriraman D, Behbehani K, Ahmad R. Obesity is a positive modulator of IL‐6R and IL‐6 expression in the subcutaneous adipose tissue: significance for metabolic inflammation. PLoS One. 2015;10(7):e0133494. doi: 10.1371/journal.pone.0133494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lehrskov LL, Lyngbaek MP, Soederlund L, et al. Interleukin‐6 delays gastric emptying in humans with direct effects on glycemic control. Cell Metab. 2018;27(6):1201‐1211.e3. doi: 10.1016/j.cmet.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 87. Aden K, Breuer A, Rehman A, et al. Classic IL‐6R signalling is dispensable for intestinal epithelial proliferation and repair. Oncogenesis. 2016;5:e270. doi: 10.1038/oncsis.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vargas N, Marino F. A neuroinflammatory model for acute fatigue during exercise. Sports Med. 2014;44(11):1479‐1487. doi: 10.1007/s40279-014-0232-4 [DOI] [PubMed] [Google Scholar]
- 89. Vallières L, Rivest S. Regulation of the genes encoding interleukin‐6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin‐1β. J Neurochem. 1997;69(4):1668‐1683. doi: 10.1046/j.1471-4159.1997.69041668.x [DOI] [PubMed] [Google Scholar]
- 90. Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin‐6 across the murine blood‐brain barrier. Neurosci Lett. 1994;179(1–2):53‐56. doi: 10.1016/0304-3940(94)90933-4 [DOI] [PubMed] [Google Scholar]
- 91. Roth J, Harré E‐M, Rummel C, Gerstberger R, Hübschle T. Signaling the brain in systemic inflammation: role of sensory circumventricular organs. Front Biosci. 2004;9(1):290‐300. [DOI] [PubMed] [Google Scholar]
- 92. Spa¨th‐Schwalbe E, Hansen K, Schmidt F, et al. Acute Effects of Recombinant Human Interleukin‐6 on Endocrine and Central Nervous Sleep Functions in Healthy Men. J Clin Endocrinol. 1998;83:1573‐1579. [DOI] [PubMed] [Google Scholar]
- 93. Cullen T, Thomas G, Wadley AJ. Sleep deprivation: cytokine and neuroendocrine effects on perception of effort. Med Sci Sports Exerc. 2019;52(4):909‐918. doi: 10.1249/MSS.0000000000002207 [DOI] [PubMed] [Google Scholar]
- 94. Cullen T, Thomas AW, Webb R, Phillips T, Hughes MG. SIL‐6R is related to weekly training mileage and psychological well‐being in athletes. Med Sci Sports Exerc. 2017;49(6):1176‐1183. doi: 10.1249/MSS.0000000000001210 [DOI] [PubMed] [Google Scholar]
- 95. Pereira JR, dos Santos LV, Santos RMS, et al. IL‐6 serum levels are elevated in Parkinson's disease patients with fatigue compared to patients without fatigue. J Neurol Sci. 2016;370:153‐156. doi: 10.1016/J.JNS.2016.09.030 [DOI] [PubMed] [Google Scholar]
- 96. Collado‐Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12(9):2759‐2766. doi: 10.1158/1078-0432.CCR-05-2398 [DOI] [PubMed] [Google Scholar]
- 97. Pedraz‐petrozzi B, Neumann E, Sammer G. Pro‐inflammatory markers and fatigue in patients with depression: a case‐control study. Sci Rep. 2020;10:9494. doi: 10.1038/s41598-020-66532-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Proschinger S, Freese J. Neuroimmunological and neuroenergetic aspects in exercise‐induced fatigue. Exerc Immunol Rev. 2019;25(1):8‐19. [PubMed] [Google Scholar]
- 99. Petersen AMW, Pedersen BK. The anti‐inflammatory effect of exercise. J Appl Physiol. 2005;98:1154‐1162. doi: 10.1152/japplphysiol.00164.2004 [DOI] [PubMed] [Google Scholar]
- 100. Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL‐6 enhances plasma IL‐1ra, IL‐10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285(2):433‐437. doi: 10.1152/ajpendo.00074.2003 [DOI] [PubMed] [Google Scholar]
- 101. Wang N, Liang H, Zen K, Lenz LL. Molecular mechanisms that influence the macrophage M1‐M2 polarization balance. Front Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Arend WP. The Balance between IL‐1 and IL‐1Ra in Disease. Cytokine Growth Factor Rev. 2002;13:323‐340. [DOI] [PubMed] [Google Scholar]
- 103. Lund Bay M, Heywood S, Wedell‐Neergaard A‐S, et al. Human immune cell mobilization during exercise: effect of IL‐6 receptor blockade. Exp Physiol. 2020;105:2086‐2098. doi: 10.1113/EP088864 [DOI] [PubMed] [Google Scholar]
- 104. Bethin KE, Vogt SK, Muglia LJ. Interleukin‐6 is an essential, corticotropin‐releasing hormone‐independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A. 2000;97(16):9317‐9322. doi: 10.1073/pnas.97.16.9317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mastorakos G, Chrousos GP, Weber JS. Recombinant Interleukin‐6 activates the hypothalamic‐pituitary‐adrenal Axis in humans. J Clin Endocrinol Metab. 1993;77:1690‐1694. [DOI] [PubMed] [Google Scholar]
- 106. Jenkins DE, Sreenivasan D, Carman F, Samal B, Eiden LE, Bunn SJ. Interleukin‐6‐mediated signaling in adrenal medullary chromaffin cells. J Neurochem. 2016;139(6):1138‐1150. doi: 10.1111/jnc.13870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Späth‐Schwalbe E, Born J, Schrezenmeier H, et al. Interleukin‐6 stimulates the hypothalamus‐pituitary‐adrenocortical axis in man. J Clin Endocrinol Metab. 1994;79(4):1212‐1214. doi: 10.1210/jcem.79.4.7962296 [DOI] [PubMed] [Google Scholar]
- 108. Hackney AC, Battaglini C, Evans ES. Cortisol, stress and adaptation during exercise training. Balt J Sport Heal Sci. 2018;3(70):34‐41. doi: 10.33607/bjshs.v3i70.485 [DOI] [Google Scholar]
- 109. Cadegiani FA, Kater CE. Hypothalamic‐pituitary‐adrenal (HPA) Axis functioning in overtraining syndrome: findings from endocrine and metabolic responses on overtraining syndrome (EROS)‐EROS‐HPA Axis. Sport Med Open. 2017;3(1):45. doi: 10.1186/s40798-017-0113-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chowdhury S, Schulz L, Palmisano B, et al. Muscle‐derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J Clin Invest. 2020;130(6):2888‐2902. doi: 10.1172/JCI133572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hamer M, Sabia S, Batty GD, et al. Physical activity and inflammatory markers over 10 years: follow‐up in men and women from the Whitehall II cohort study. Circulation. 2012;126:928‐933. doi: 10.1161/CIRCULATIONAHA.112.103879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vella CA, Allison MA, Cushman M, et al. Physical activity and adiposity‐related inflammation: the MESA. Med Sci Sports Exerc. 2017;49(5):915‐921. doi: 10.1249/MSS.0000000000001179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zheng G, Qiu P, Xia R, et al. Effect of aerobic exercise on inflammatory markers in healthy middle‐aged and older adults: a systematic review and meta‐analysis of randomized controlled trials. Front Aging Neurosci. 2019;11:98. doi: 10.3389/fnagi.2019.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Suzui M, Kawai T, Kimura H, et al. Natural killer cell lytic activity and CD56 dim and CD56 bright cell distributions during and after intensive training. J Appl Physiol. 2004;96:2167‐2173. doi: 10.1152/japplphysiol.00513.2003.-The [DOI] [PubMed] [Google Scholar]
- 115. Halson S, Jeukendrup A, Gleeson M. Immunological responses to overreaching in cyclists. Med Sci Sports Exerc. 2003;35(5):854‐861. doi: 10.1249/01.MSS.0000064964.80040.E9 [DOI] [PubMed] [Google Scholar]
- 116. Halson SL, Jeukendrup AE. Does overtraining exist? An analysis of overreaching and overtraining research. Sports Med. 2004;34(14):967‐981. doi: 10.2165/00007256-200434140-00003 [DOI] [PubMed] [Google Scholar]
- 117. Gholamnezhad Z, Boskabady MH, Hosseini M, Sankian M, Rad AK. Evaluation of immune response after moderate and overtraining exercise in wistar rat. Iran J Basic Med Sci. 2014;17(1):1‐8. [PMC free article] [PubMed] [Google Scholar]
- 118. Chung Y, Hsiao Y‐T, Huang W‐C, Grau M. Physiological and psychological effects of treadmill overtraining implementation. Biology (Basel). 2021;10:515. doi: 10.3390/biology10060515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mcclung JP, Martini S, Murphy NE, et al. Effects of a 7‐day military training exercise on inflammatory biomarkers, serum hepcidin, and iron status. Nutr J. 2013;12:141. doi: 10.1186/1475-2891-12-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Robson‐Ansley PJ, Andrew AE, Ae B, Gleeson M. Elevated plasma interleukin‐6 levels in trained male triathletes following an acute period of intense interval training. Eur J Appl Physiol. 2007;99:353‐360. doi: 10.1007/s00421-006-0354-y [DOI] [PubMed] [Google Scholar]
- 121. Højgaard Christensen R, Lang Lehrskov L, Wedell‐Neergaard A‐S, et al. Aerobic exercise induces cardiac fat loss and alters cardiac muscle mass through an Interleukin‐6 receptor‐dependent mechanism cardiac analysis of a double‐blind randomized controlled clinical trial in abdominally obese humans. Circulation. 2019;140:1684‐1686. doi: 10.1161/CIRCULATIONAHA.119.042287 [DOI] [PubMed] [Google Scholar]
- 122. Mitchell CJ, Churchward‐Venne TA, Bellamy L, Parise G, Baker SK. Muscular and systemic correlates of resistance training‐induced muscle hypertrophy. PLoS One. 2013;8(10):e78636. doi: 10.1371/journal.pone.0078636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gray SR, Robinson M, Nimmo MA. Response of plasma IL‐6 and its soluble receptors during submaximal exercise to fatigue in sedentary middle‐aged men. Cell Stress Chaperones. 2008;13:247‐251. doi: 10.1007/s12192-008-0019-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Silverman NE, Barbara AE, Ae JN, Ryan AS. Addition of aerobic exercise to a weight loss program increases BMD, with an associated reduction in inflammation in overweight postmenopausal women. Calcif Tissue Int. 2009;84:257‐265. doi: 10.1007/s00223-009-9232-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Adamopoulos S, Parissis J, Karatzas D, et al. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:653‐663. doi: 10.1016/S0735-1097(01)01795-8 [DOI] [PubMed] [Google Scholar]
- 126. Somineni HK, Boivin GP, Elased KM. Daily exercise training protects against albuminuria and angiotensin converting enzyme 2 shedding in db/db diabetic mice. J Endocrinol. 2014;221:235‐251. doi: 10.1530/JOE-13-0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Narazaki M, Yasukawa K, Saito T, et al. Soluble forms of the Interleukin‐6 signal‐transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane‐anchored gp130. Blood. 1993;82(4):1120‐1126. [PubMed] [Google Scholar]
- 128. Bonomi A, Veglia F, Baldassarre D, et al. Analysis of the genetic variants associated with circulating levels of sgp130. Results from the IMPROVE study. Genes Immun. 2020;21:100‐108. doi: 10.1038/s41435-019-0090-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Walshe I, Robson‐Ansley P, St Clair Gibson A, Lawrence C, Thompson KG, Ansley L. The reliability of the IL‐6, sIL‐6R and sgp130 response to a preloaded time trial. Eur J Appl Physiol. 2010;110:619‐625. doi: 10.1007/s00421-010-1548-x [DOI] [PubMed] [Google Scholar]
- 130. Patterson S, Reid S, Gray S, Nimmo M. The response of plasma interleukin‐6 and its soluble receptors to exercise in the cold in humans. J Sports Sci. 2008;26(9):927‐933. doi: 10.1080/02640410801885941 [DOI] [PubMed] [Google Scholar]
- 131. Wolf J, Waetzig GH, Chalaris A, et al. Different soluble forms of the interleukin‐6 family signal transducer gp130 fine‐tune the blockade of interleukin‐6 trans‐signaling. J Biol Chem. 2016;291(31):16186‐16196. doi: 10.1074/jbc.M116.718551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tanaka M, Kishimura M, Ozaki S, et al. Cloning of novel soluble gp130 and detection of its neutralizing autoantibodies in rheumatoid arthritis. J Clin Invest. 2000;106(1):137‐144. doi: 10.1172/JCI7479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Aziz N, Detels R, Quint JJ, Gjertson D, Ryner T, Butch AW. Biological variation of immunological blood biomarkers in healthy individuals and quality goals for biomarker tests. BMC Immunol. 2019;20(1):20‐33. doi: 10.1186/s12865-019-0313-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hawkins GA, Robinson MB, Hastie AT, et al. The IL6R variation asp 358 ala is a potential modifier of lung function in subjects with asthma. J Allergy Clin Immunol. 2012;130(9):510‐5.e1. doi: 10.1016/j.jaci.2012.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Vargas V, Bonatto S, Macagnan F, et al. Influence of the 48867A>C (Asp358Ala) IL6R polymorphism on response to a lifestyle modification intervention in individuals with metabolic syndrome. Genet Mol Res. 2013;12(3):3983‐3991. doi: 10.4238/2013.February.28.8 [DOI] [PubMed] [Google Scholar]
- 136. Vargas N, Marino F. Neuroinflammation, cortical activity, and fatiguing behaviour during self‐paced exercise. Pflugers Arch Eur J Physiol. 2018;470(2):413‐426. doi: 10.1007/s00424-017-2086-8 [DOI] [PubMed] [Google Scholar]
- 137. Ellingsgaard H, Hojman P, Pedersen BK. Exercise and health — emerging roles of IL‐6. Curr Opin Physiol. 2019;10:49‐54. doi: 10.1016/j.cophys.2019.03.009 [DOI] [Google Scholar]
- 138. Kistner TM, Pedersen BK, Lieberman DE. Interleukin 6 as an energy allocator in muscle tissue. Nat Metab. 2022;4:170‐179. doi: 10.1038/s42255-022-00538-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


