Summary
Plants have a broad capacity to regenerate damaged organs. The study of wounding in multiple developmental systems has uncovered many of the molecular properties underlying plants' competence for regeneration at the local cellular level. However, in nature, wounding is rarely localized to one place, and plants need to coordinate regeneration responses at multiple tissues with environmental conditions and their physiological state. Here, we review the evidence for systemic signals that regulate regeneration on a plant‐wide level. We focus on the role of auxin and sugars as short‑ and long‐range signals in natural wounding contexts and discuss the varied origin of these signals in different regeneration scenarios. Together, this evidence calls for a broader, system‐wide view of plant regeneration competence.
Keywords: adventitious roots, auxin, graft junction, regeneration, sugar transport
| Contents | ||
|---|---|---|
| Summary | 408 | |
| I. | Introduction | 408 |
| II. | Auxin as a systemic regeneration signal | 409 |
| III. | The role of sugars in regeneration | 410 |
| IV. | Conclusions | 411 |
| Acknowledgements | 411 | |
| References | 411 |
I. Introduction
Outside laboratory settings, plants rarely grow undisturbed, and physical damage is a ubiquitous part of their lives. Recovery from damage is a complex response involving wound closure, preparation of the defense systems for invasion, nutrients rerouting, and activation of tissue‐specific repair mechanisms. In certain scenarios, wounding triggers restorative growth, a response ascribed to the ‘regeneration competence’ of the injured tissue. The competence to regenerate in plants is broad but limited by developmental stage and physiological conditions. Thus, dissection of the root meristem near its tip results in root meristem reformation, but the meristem fails to regenerate when a similar injury is inflicted at a more proximal position (Sena et al., 2009; Durgaprasad et al., 2019). Similarly, dissection of a young leaf tip triggers growth at its base to compensate for the missing leaf tissues, whereas older leaves lose the competence to initiate such growth (Sena et al., 2009; Kuchen et al., 2012). Young shoots or leaves removed from their mother plant generate wound‐induced shoot‐borne (or ‘adventitious’) roots to replace the missing root system, but such capacity diminishes in older shoots or detached leaves (Steffens & Rasmussen, 2016; Li et al., 2020). The molecular definition of ‘regeneration competence’ is still unclear, but it is usually ascribed to a certain autonomous cell state, either epigenetic (He et al., 2012; Chen et al., 2016; Hernández‐Coronado et al., 2022), transcriptional (Sena et al., 2009; Durgaprasad et al., 2019), or hormonal (Ikeuchi et al., 2019).
Most experimental setups studying plant wound‐response focus on a single wound, but wounding in nature is often a whole‐plant affair and systemic signals may be involved in coordinating and prioritizing wound repair. Recent high‐resolution studies of plant regeneration have begun to uncover the contribution of local vs systemic signals to plant regeneration; key amongst these is the phytohormone auxin (Canher et al., 2020; Hoermayer et al., 2020; Matosevich et al., 2020), and evidence from cuttings calls for a more holistic, whole‐plant view, of plant regeneration that goes beyond local tissue competence (Druege et al., 2019). Here, we discuss the systemic control of plant regenerative response. Many factors, including jasmonic acid and cytokinin, control tissue regeneration and can move systemically through the plant (Ikeuchi et al., 2019; Zhang et al., 2019). However, local vs systemic effects of many such factors are often difficult to untangle. In this piece, we focus on two signals, auxin and sugars, whose systemic effects on regeneration have been studied in most detail (summarized in Fig. 1). We limit ourselves to natural, or close‐to‐natural, wounding contexts. Though much has been learned about regeneration from tissue‐culture experiments, the nature of these artificial systems often precludes distinction between systemic and local activity.
Fig. 1.
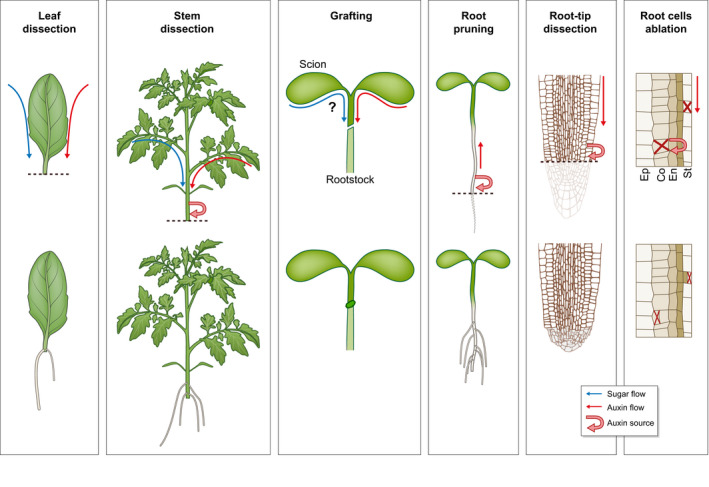
The origin of local and systemic signals in different regeneration scenarios. Arrows mark the flow of the signal (red, auxin; blue, sugars). Curved arrow marks site of synthesis. Co, cortex; En, endodermis; Ep, epidermis; St, stele.
II. Auxin as a systemic regeneration signal
The small molecule auxin is closely implicated in enabling regeneration, and it is used in controlling plant clonal propagation and tissue culture growth (Ikeuchi et al., 2019). It is produced in multiple places in the plant and is transported in a polar manner, mostly basipetally, to form short‑ and long‐range gradients within the plant (Robert & Friml, 2009; Zhao, 2018). The relationship between auxin gradients and regeneration response is likely an ancient trait. When the thallus of liverworts is dissected, a new meristem is initiated from the cut site of only one of the cut halves (Larue & Narayanaswami, 1957; Nishihama et al., 2015). This differential response is guided by depletion of the plant‐wide auxin gradient formed by the meristem (Ishida et al., 2022), and exogenous application of auxin inhibited the regeneration response (Larue & Narayanaswami, 1957).
The aerial parts of many plant species can form shoot‐borne roots from stems or leaves when these parts are removed. Many signals control this process, but auxin remains a key factor (Bellini et al., 2014). Auxin accumulation at the site of root initiation site was demonstrated for many species, and exogenous application of auxin, or auxin‐like molecules, is often sufficient to promote root initiation (Steffens & Rasmussen, 2016).
Current evidence suggests that most of the auxin activating wound‐induced root initiation is coming from remote tissues. When a rosette leaf of Arabidopsis was detached and placed on hormone‐free media, it initiates roots from its basal parts (Chen et al., 2014; Bustillo‐Avendaño et al., 2018). This process required the activation of auxin biosynthesis in the distal part of the detached leaf, away from the cut site, and disruption of auxin transport by application of N‐1‐naphthylphthalamic acid (NPA) inhibited root formation (Liu et al., 2014; Xu, 2018). Application of NPA also inhibited the formation of roots in stem cuttings of Petunia hybrida (Ahkami et al., 2013) and cut tomato and Arabidopsis hypocotyls (Sukumar et al., 2013; Alaguero‐Cordovilla et al., 2021), suggesting these restorative growth responses are also controlled by remote production of auxin.
Given that auxin can, and is often, synthesized near its site of action (Brumos et al., 2018; Zhao, 2018; Matosevich et al., 2020), it is not clear why auxin produced away from the site of injury should be required for wound repair. One possible hypothesis is that injury to part of the plant can prime the rest of it for regenerative growth. In this scenario, auxin can serve as a systemic signal that tunes the regenerative response to the environmental and physiological state of the plant (Mroue et al., 2017).
The systemic levels of auxin are responsive to damage and environmental conditions. Physical damage and increased temperature lead to increased synthesis of auxin in the leaf, which then spreads systemically throughout the plant (Machado et al., 2016; Serivichyaswat et al., 2022). This response is not limited to leaves, as damage to the Arabidopsis hypocotyl also increased the expression of the auxin‐responsive promoter DR5 in all aerial parts of the plant (Huang et al., 2020). This wound‐induced systemic increase in auxin is sensitive to physiological factors such as plant age. The expression of microRNA156‐targeted SQUAMOSA PROMOTER BINDING PROTEIN‐LIKE (SPL) increases with plant age (Wu et al., 2009). In cut leaves, these SPLs suppress the induction of auxin biosynthesis, leading to a reduced number of shoot‐borne roots. Exogenous auxin application restored root initiation in leaves with high SPL levels (Ye et al., 2020).
Consistent with systemic auxin acting as a priming or modulating agent during regeneration, different sources of auxin, either local or remote, are important in different scenarios of tissue regeneration. During shoot‐borne root initiation, NPA treatment does not abolish root initiation, and at least some of the auxin accumulation can be ascribed to local biosynthesis near the cut site, independent of the shoot‐derived auxin (Alaguero‐Cordovilla et al., 2021). In some cases, remote auxin is not required at all, such as during regeneration of the root meristem from dissection. Here, auxin signaling is induced in cells near the cut site that proliferate to replace the missing distal part of the meristem (Sena et al., 2009; Efroni et al., 2016). This accumulation results from the induction of auxin biosynthesis YUCCA genes at the cut root tip itself (Matosevich et al., 2020).
The systemic source of auxin required for regeneration may not even be the shoot. When the entire apical root meristem is removed (‘root pruning’), lateral roots are induced above the injury site. This response is the result of induction of YUCCA‐mediated auxin biosynthesis near the cut site, followed by acropetal (rather than shoot‐derived basipetal) transport to the root initiation site (Xu et al., 2017). Strikingly, the source of auxin during repair may differ even for adjacent tissues. Cell death in the root internal stele triggers auxin accumulation in adjacent endodermis cells, leading to restorative cell division. No induction of auxin biosynthesis genes could be detected near the dying cells, and in silico simulations are consistent with auxin accumulation resulting from dying cells blocking the basipetal auxin flow, although its ultimate source is unclear (Canher et al., 2020). By contrast, repair of ablated cells in external root tissues, which also results in increased auxin accumulation, occurred even when auxin biosynthesis and polar transport were chemically inhibited, suggesting auxin may be derived from internal stores (Hoermayer et al., 2020).
Another example of systemic auxin acting as a modulator of tissue repair is during the reconnection of severed vasculature in graft formation. In this case, wound healing initiates with callus growth at the site of injury, followed by the formation of new vasculature strands connecting the scion and rootstock across the graft junction. The activation of the auxin response machinery is required for the process (Melnyk et al., 2015). As it was demonstrated that regeneration of damaged vasculature is promoted by auxin application, it was generally thought that graft formation is promoted by shoot‐derived auxin (Wulf et al., 2019). Recent evidence, however, paints a more complex picture. Removal of the cotyledons and treatment with NPA led to reduced proliferation at the graft junction, but vasculature still regenerated at normal rates (Melnyk et al., 2015; Serivichyaswat et al., 2022). And while treatment with the auxin transport inhibitor 2,3,5‐triiodobenzoic acid could inhibit cell proliferation at the graft junction (Matsuoka et al., 2016), removal of both cotyledons could promote graft formation in Arabidopsis micrograft when low levels of sucrose were added, suggesting cotyledon‐derived auxin does not play a major role in vasculature regeneration (Marsch‐Martínez et al., 2013). Indeed, vascular reconnection in heterografts did not correlate with auxin flow (Wulf et al., 2019). However, a systemic increase in auxin level induced by elevated temperature had a promotive effect on proliferation at the graft site and connections were made faster (Serivichyaswat et al., 2022). Taken together, this suggests that though wound healing at the graft site probably relies on local auxin supply, it can be enhanced by remote signals influenced by environmental conditions.
It should be noted that auxin is involved in a plethora of plant developmental processes, and its effect on regeneration cannot be considered specific (Vanneste & Friml, 2009). Further, other remote signals may act in parallel or together with auxin to control growth and regeneration. Thus, removal of the shoot of Arabidopsis resulted in severe inhibition of lateral root initiation and regeneration of cut root apical meristems. However, exogenous auxin had only a very mild effect on lateral root initiation in shoot‐less plants and could not rescue root meristem regeneration at all, suggesting other long‐range factors may be at play (Reed et al., 1998; Matosevich et al., 2020).
III. The role of sugars in regeneration
Apart from hormonal control, other systemic factors contribute to a coordinated wound response, an aspect that is mostly studied in the initiation of shoot‐borne roots. Sugars, produced in the shoot via photosynthesis and transported systemically through the plant via the phloem, were shown to affect regenerative responses. In hibiscus cuttings, leaf removal reduced the rooting capacity even when treated with auxin, but this could be rescued by sucrose supplementation to the cutting base (van Overbeek et al., 1946). The sucrose effect depended on adequate ammonium levels; indeed, the wound‐induced root primordium is also a sink for nitrogen‐based compounds and microelements (Svenson & Davies, 1995; Zerche et al., 2016).
Sucrose application promoted shoot‐borne roots in dark‐treated Arabidopsis seedlings or leaf explants (Takahashi et al., 2003; Chen et al., 2014) and rescued the reduction in shoot‐borne roots in shoot‐less cucumber seedlings (Qi et al., 2020). The sugars required for wound‐induced shoot‐borne root formation are unlikely to come from local stores, as dark pretreatment of cuttings, which drains internal carbohydrate stores, could even improve rooting when cuttings were returned to normal light regimes (Klopotek et al., 2010).
Carbohydrates are transported from leaves via the phloem, but it is unclear how they are recruited to wound‐induced root initiation sites. Some evidence, mainly from gene expression studies, suggests an active change in sugar transport may induce the formation of a new sink. In Arabidopsis, mechanical wounding upregulates the expression of the hexose transporter SUGAR TRANSPORTER4 (STP4) and the sucrose transporter SUCROSE PROTON SYMPORTER3 (SUC3) (Truernit et al., 1996; Meyer et al., 2004). Expression of sucrose exporters from the SUGARS WILL EVENTUALLY BE EXPORTED TRANSPORTERS (SWEET) family are induced during the formation of roots from detached leaves (Chen et al., 2012; Liu et al., 2022). In rice, herbivory feeding of leaves led to the upregulation of OsSUC4 (Chang et al., 2019). Fungi attack in Arabidopsis leaves increased STP4 expression and the sucrose hydrolysis activity of the membrane‐bound CELL‐WALL INVERTASE (CWINV) (Fotopoulos et al., 2003; Sauer, 2007). Transcriptome analysis of P. hybrida cuttings revealed a temporal upregulation of CWINV and STP near the site of injury, followed by an increase in hexose content, shortly before wound‐induced root emergence (Ahkami et al., 2009; Klopotek et al., 2016).
What regulates the induction of these sugar transport and metabolism genes is unknown. However, recently, a group of DOF (DNA‐binding with one finger) transcription factors was found to be induced by physical damage to the plant. Curiously, amongst the genes induced by these DOFs was a SWEET transporter (Zhang et al., 2022), suggesting they could also play a role in regulating sugar transport. Indeed, a mutant in a rice orthologue of the same DOFs was defective in sugar uptake and had reduced expression of SUC and SWEET genes (Wu et al., 2018). Though these correlative studies suggest a link between changes in carbohydrate metabolism, transport, and wound response, functional studies are required to determine their role in controlling tissue regeneration.
Sugar signaling may also play a role in controlling regenerative growth. The Arabidopsis gin‐2 mutant, defective in the sugar sensor hexokinase1, does not initiate wound‐induced roots in response to glucose and auxin application (Moore et al., 2003). The target of rapamycin (TOR) protein kinase, integrates environmental cues and cellular energy levels to developmental and metabolic responses (Dobrenel et al., 2016). Chemical inhibition (Deng et al., 2017) or genetic perturbation (Stitz et al., 2022) of TOR leads to a reduction in root initiation in potato wound‐induced roots or Arabidopsis lateral roots, respectively.
Signals rarely act on their own, and sugars and auxin have a reciprocal effect on one another, with a large overlap between glucose‑ and auxin‐responsive genes (Mishra et al., 2021). Consistently, there is an additive effect for these factors during rooting (van Overbeek et al., 1946; Calamar & De Klerk, 2002; Moore et al., 2003; Corrêa et al., 2005; Agulló‐Antón et al., 2011); at the moment, however, the nature of this interaction during regeneration is unclear.
IV. Conclusions
Studies of regeneration have mostly focused on uncovering the molecular mechanisms underlying tissue regeneration competence. By contrast, this review highlights the systemic and coordinated nature of the regeneration response. Though we have limited ourselves to just two mobile signals, many molecules and hormones are systemically transported in the plant and can potentially affect tissue repair. Although research of this topic is still at an early stage, future studies can provide a broader, plant‐wide view of the regeneration process and the factors controlling the decision of whether or not to regenerate.
Acknowledgements
IE is supported by an HHMI International Research Scholar Grant (55008730) and BSF (2019192). RM is a fellow of the Ariane de Rothschild Women Doctoral Program.
References
- Agulló‐Antón MÁ, Sánchez‐Bravo J, Acosta M, Druege U. 2011. Auxins or sugars: what makes the difference in the adventitious rooting of stored carnation cuttings? Journal of Plant Growth Regulation 30: 100–113. [Google Scholar]
- Ahkami AH, Lischewski S, Haensch KT, Porfirova S, Hofmann J, Rolletschek H, Melzer M, Franken P, Hause B, Druege U et al. 2009. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: involvement of wound response and primary metabolism. New Phytologist 181: 613–625. [DOI] [PubMed] [Google Scholar]
- Ahkami AH, Melzer M, Ghaffari MR, Pollmann S, Ghorbani Javid M, Shahinnia F, Hajirezaei MR, Druege U. 2013. Distribution of indole‐3‐acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 238: 499–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaguero‐Cordovilla A, Sánchez‐García AB, Ibáñez S, Albacete A, Cano A, Acosta M, Pérez‐Pérez JM. 2021. An auxin‐mediated regulatory framework for wound‐induced adventitious root formation in tomato shoot explants. Plant, Cell & Environment 44: 1642–1662. [DOI] [PubMed] [Google Scholar]
- Bellini C, Pacurar DI, Perrone I. 2014. Adventitious roots and lateral roots: similarities and differences. Annual Review of Plant Biology 65: 639–666. [DOI] [PubMed] [Google Scholar]
- Brumos J, Robles LM, Yun J, Vu TC, Jackson S, Alonso JM, Stepanova AN. 2018. Local auxin biosynthesis is a key regulator of plant development. Developmental Cell 47: 306–318. [DOI] [PubMed] [Google Scholar]
- Bustillo‐Avendaño E, Ibáñez S, Sanz O, Sousa Barros JA, Gude I, Perianez‐Rodriguez J, Micol JL, Del Pozo JC, Moreno‐Risueno MA, Pérez‐Pérez JM. 2018. Regulation of hormonal control, cell reprogramming, and patterning during de novo root organogenesis. Plant Physiology 176: 1709–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamar A, De Klerk GJ. 2002. Effect of sucrose on adventitious root regeneration in apple. Plant Cell, Tissue and Organ Culture 70: 207–212. [Google Scholar]
- Canher B, Heyman J, Savina M, Devendran A, Eekhout T, Vercauteren I, Prinsen E, Matosevich R, Xu J, Mironova V et al. 2020. Rocks in the auxin stream: wound‐induced auxin accumulation and ERF115 expression synergistically drive stem cell regeneration. Proceedings of the National Academy of Sciences, USA 117: 16667–16677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YA, Dai NC, Chen HJ, Tseng CH, Huang ST, Wang SJ. 2019. Regulation of rice sucrose transporter 4 gene expression in response to insect herbivore chewing. Journal of Plant Interactions 14: 525–532. [Google Scholar]
- Chen L, Tong J, Xiao L, Ruan Y, Liu J, Zeng M, Huang H, Wang JW, Xu L. 2016. YUCCA‐mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. Journal of Experimental Botany 67: 4273–4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L‐Q, Qu X‐Q, Hou B‐H, Sosso D, Osorio S, Fernie AR, Frommer WB. 2012. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335: 207–211. [DOI] [PubMed] [Google Scholar]
- Chen X, Qu Y, Sheng L, Liu J, Huang H, Xu L. 2014. A simple method suitable to study de novo root organogenesis. Frontiers in Plant Science 5: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa LDR, Paim DC, Schwambach J, Fett‐Neto AG. 2005. Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Plant Growth Regulation 45: 63–73. [Google Scholar]
- Deng K, Dong P, Wang W, Feng L, Xiong F, Wang K, Zhang S, Feng S, Wang B, Zhang J et al. 2017. The TOR pathway is involved in adventitious root formation in Arabidopsis and potato. Frontiers in Plant Science 8: 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrenel T, Caldana C, Hanson J, Robaglia C, Vincentz M, Veit B, Meyer C. 2016. TOR signaling and nutrient sensing. Annual Review of Plant Biology 67: 261–285. [DOI] [PubMed] [Google Scholar]
- Druege U, Hilo A, Pérez‐Pérez JM, Klopotek Y, Acosta M, Shahinnia F, Zerche S, Franken P, Hajirezaei MR. 2019. Molecular and physiological control of adventitious rooting in cuttings: phytohormone action meets resource allocation. Annals of Botany 123: 929–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgaprasad K, Roy MV, Venugopal MA, Kareem A, Raj K, Willemsen V, Mähönen AP, Scheres B, Prasad K. 2019. Gradient expression of transcription factor imposes a boundary on organ regeneration potential in plants. Cell Reports 29: 453–463. [DOI] [PubMed] [Google Scholar]
- Efroni I, Mello A, Nawy T, Ip PL, Rahni R, Delrose N, Powers A, Satija R, Birnbaum KD. 2016. Root regeneration triggers an embryo‐like sequence guided by hormonal interactions. Cell 165: 1721–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotopoulos V, Gilbert MJ, Pittman JK, Marvier AC, Buchanan AJ, Sauer N, Hall JL, Williams LE. 2003. The monosaccharide transporter gene, AtSTP4, and the cell‐wall invertase, Atβfruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum . Plant Physiology 132: 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Chen X, Huang H, Xu L. 2012. Reprogramming of H3K27me3 is critical for acquisition of pluripotency from cultured Arabidopsis tissues. PLoS Genetics 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Coronado M, Dias Araujo PC, Ip P‐L, Nunes CO, Rahni R, Wudick MM, Lizzio MA, Feijó JA, Birnbaum KD. 2022. Plant glutamate receptors mediate a bet‐hedging strategy between regeneration and defense. Developmental Cell 57: 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoermayer L, Montesinos JC, Marhava P, Benková E, Yoshida S, Friml J. 2020. Wounding‐induced changes in cellular pressure and localized auxin signalling spatially coordinate restorative divisions in roots. Proceedings of the National Academy of Sciences, USA 117: 15322–15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Wang Y, Liu Y, Wang G, She X. 2020. Reactive oxygen species regulate auxin levels to mediate adventitious root induction in Arabidopsis hypocotyl cuttings. Journal of Integrative Plant Biology 62: 912–926. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Favero DS, Sakamoto Y, Iwase A, Coleman D, Rymen B, Sugimoto K. 2019. Molecular mechanisms of plant regeneration. Annual Review of Plant Biology 70: 377–406. [DOI] [PubMed] [Google Scholar]
- Ishida S, Suzuki H, Iwaki A, Kawamura S, Yamaoka S, Kojima M, Takebayashi Y, Yamaguchi K, Shigenobu S, Sakakibara H et al. 2022. Diminished auxin signaling triggers cellular reprogramming by inducing a regeneration factor in the liverwort Marchantia polymorpha . Plant and Cell Physiology 63: 384–400. [DOI] [PubMed] [Google Scholar]
- Klopotek Y, Franken P, Klaering HP, Fischer K, Hause B, Hajirezaei MR, Druege U. 2016. A higher sink competitiveness of the rooting zone and invertases are involved in dark stimulation of adventitious root formation in Petunia hybrida cuttings. Plant Science 243: 10–22. [DOI] [PubMed] [Google Scholar]
- Klopotek Y, Haensch KT, Hause B, Hajirezaei MR, Druege U. 2010. Dark exposure of petunia cuttings strongly improves adventitious root formation and enhances carbohydrate availability during rooting in the light. Journal of Plant Physiology 167: 547–554. [DOI] [PubMed] [Google Scholar]
- Kuchen EE, Fox S, Barbier de Reuille P, Kennaway R, Bensmihen S, Avondo J, Calder GM, Southam P, Robinson S, Bangham A. 2012. Generation of leaf shape through early patterns of growth and tissue polarity. Science 335: 1092–1096. [DOI] [PubMed] [Google Scholar]
- Larue CD, Narayanaswami S. 1957. Auxin inhibition in the liverwort Lunularia . New Phytologist 56: 61–70. [Google Scholar]
- Li H, Yao L, Sun L, Zhu Z. 2020. ETHYLENE INSENSITIVE 3 suppresses plant de novo root regeneration from leaf explants and mediates age‐regulated regeneration decline. Development 147: dev179457. [DOI] [PubMed] [Google Scholar]
- Liu J, Sheng L, Xu Y, Li J, Yang Z. 2014. WOX11 and 12 are involved in the first‐step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zhang Y, Fang X, Tran S, Zhai N, Yang Z, Guo F, Chen L, Yu J, Ison MS et al. 2022. Transcriptional landscapes of de novo root regeneration from detached Arabidopsis leaves revealed by time‐lapse and single‐cell RNA sequencing analyses. Plant Communications 3: 100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado RAR, Robert CAM, Arce CCM, Ferrieri AP, Xu S, Jimenez‐Aleman GH, Baldwin IT, Erb M. 2016. Auxin is rapidly induced by herbivore attack and regulates a subset of systemic, jasmonate‐dependent defenses. Plant Physiology 172: 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch‐Martínez N, Franken J, Gonzalez‐Aguilera KL, de Folter S, Angenent G, Alvarez‐Buylla ER. 2013. An efficient flat‐surface collar‐free grafting method for Arabidopsis thaliana seedlings. Plant Methods 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matosevich R, Cohen I, Gil‐Yarom N, Modrego A, Friedlander‐Shani L, Verna C, Scarpella E, Efroni I. 2020. Local auxin biosynthesis is required for root regeneration after wounding. Nature Plants 6: 1020–1030. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Sugawara E, Aoki R, Takuma K, Terao‐Morita M, Satoh S, Asahina M. 2016. Differential cellular control by cotyledon‐derived phytohormones involved in graft reunion of Arabidopsis hypocotyls. Plant and Cell Physiology 57: 2620–2631. [DOI] [PubMed] [Google Scholar]
- Melnyk CW, Schuster C, Leyser O, Meyerowitz EM. 2015. A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana . Current Biology 25: 1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Lauterbach C, Niedermeier M, Barth I, Sjolund RD, Sauer N. 2004. Wounding enhances expression of AtSUC3, a sucrose transporter from Arabidopsis sieve elements and sink tissues. Plant Physiology 134: 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BS, Sharma M, Laxmi A. 2021. Role of sugar and auxin crosstalk in plant growth and development. Physiologia Plantarum 174: e13546. [DOI] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J. 2003. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336. [DOI] [PubMed] [Google Scholar]
- Mroue S, Simeunovic A, Robert HS. 2017. Auxin production as an integrator of environmental cues for developmental growth regulation. Journal of Experimental Botany 69: 201–212. [DOI] [PubMed] [Google Scholar]
- Nishihama R, Ishizaki K, Hosaka M, Matsuda Y, Kubota A, Kohchi T. 2015. Phytochrome‐mediated regulation of cell division and growth during regeneration and sporeling development in the liverwort Marchantia polymorpha . Journal of Plant Research 128: 407–421. [DOI] [PubMed] [Google Scholar]
- van Overbeek J, Gordon SA, Gregory LE. 1946. An analysis of the function of the leaf in the process of root formation in cuttings. American Journal of Botany 33: 100–107. [Google Scholar]
- Qi X, Li Q, Shen J, Qian C, Xu X, Xu Q, Chen X. 2020. Sugar enhances waterlogging‐induced adventitious root formation in cucumber by promoting auxin transport and signalling. Plant, Cell & Environment 43: 1545–1557. [DOI] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK. 1998. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiology 118: 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert HS, Friml J. 2009. Auxin and other signals on the move in plants. Nature Chemical Biology 5: 325–332. [DOI] [PubMed] [Google Scholar]
- Sauer N. 2007. Molecular physiology of higher plant sucrose transporters. FEBS Letters 581: 2309–2317. [DOI] [PubMed] [Google Scholar]
- Sena G, Wang X, Liu H‐Y, Hofhuis H, Birnbaum KD. 2009. Organ regeneration does not require a functional stem cell niche in plants. Nature 457: 1150–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serivichyaswat PT, Bartusch K, Leso M, Musseau C, Iwase A, Chen Y, Sugimoto K, Quint M, Melnyk CW. 2022. High temperature perception in leaves promotes vascular regeneration and graft formation in distant tissues. Development 149: dev200079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Rasmussen A. 2016. The physiology of adventitious roots. Plant Physiology 170: 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitz M, Kuster D, Reinert M, Schepetilnikov M, Berthet B, Janocha D, Artins A, Boix M, Henriques R, Pfeiffer A et al. 2022. TOR acts as metabolic gatekeeper for auxin‐dependent lateral root initiation in Arabidopsis thaliana . bioRxiv. doi: 10.1101/2022.03.29.486207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar P, Maloney GS, Muday GK. 2013. Localized induction of the ATP‐binding cassette B19 auxin transporter enhances adventitious root formation in Arabidopsis. Plant Physiology 162: 1392–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson SE, Davies FT. 1995. Change in tissue mineral elemental concentration during root initiation and development of poinsettia cuttings. HortScience 30: 617–619. [Google Scholar]
- Takahashi F, Sato‐Nara K, Kobayashi K, Suzuki M, Suzuki H. 2003. Sugar‐induced adventitious roots in Arabidopsis seedlings. Journal of Plant Research 116: 83–91. [DOI] [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illig J, Sauer N. 1996. The sink‐specific and stress‐regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 8: 2169–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Friml J. 2009. Auxin: a trigger for change in plant development. Cell 136: 1005–1016. [DOI] [PubMed] [Google Scholar]
- Wu G, Park MY, Conway SR, Wang J‐W, Weigel D, Poethig RS. 2009. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138: 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lee S‐K, Yoo Y, Wei J, Kwon S‐Y, Lee S‐W, Jeon J‐S, An G. 2018. Rice transcription factor OsDOF11 modulates sugar transport by promoting expression of sucrose transporter and SWEET genes. Molecular Plant 11: 833–845. [DOI] [PubMed] [Google Scholar]
- Wulf KE, Reid JB, Foo E. 2019. Auxin transport and stem vascular reconnection – has our thinking become canalized? Annals of Botany 123: 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Miao J, Yumoto E, Yokota T, Asahina M, Watahiki M. 2017. YUCCA9-mediated auxin biosynthesis and polar auxin transport synergistically regulate regeneration of root systems following root cutting. Plant and Cell Physiology 58: 1710–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. 2018. De novo root regeneration from leaf explants: wounding, auxin, and cell fate transition. Current Opinion in Plant Biology 41: 39–45. [DOI] [PubMed] [Google Scholar]
- Ye B‐B, Shang G‐D, Pan Y, Xu Z‐G, Zhou C‐M, Mao Y‐B, Bao N, Sun L, Xu T, Wang J‐W. 2020. AP2/ERF transcription factors integrate age and wound signals for root regeneration. Plant Cell 32: 226–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerche S, Haensch KT, Druege U, Hajirezaei MR. 2016. Nitrogen remobilisation facilitates adventitious root formation on reversible dark‐induced carbohydrate depletion in Petunia hybrida . BMC Plant Biology 16: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Matsuoka K, Kareem A, Robert M, Roszak P, Blob B, Bisht A, De Veylder L, Voiniciuc C, Asahina M et al. 2022. Cell‐wall damage activates DOF transcription factors to promote wound healing and tissue regeneration in Arabidopsis thaliana . Current Biology 32: 1883–1894. [DOI] [PubMed] [Google Scholar]
- Zhang G, Zhao F, Chen L, Pan Y, Sun L, Bao N, Zhang T, Cui C‐X, Qiu Z, Zhang Y. 2019. Jasmonate‐mediated wound signalling promotes plant regeneration. Nature Plants 5: 491–497. [DOI] [PubMed] [Google Scholar]
- Zhao Y. 2018. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annual Review of Plant Biology 69: 417–435. [DOI] [PubMed] [Google Scholar]


